Copyright
©2013 Baishideng Publishing Group Co.
World J Gastroenterol. Mar 28, 2013; 19(12): 1861-1876
Published online Mar 28, 2013. doi: 10.3748/wjg.v19.i12.1861
Published online Mar 28, 2013. doi: 10.3748/wjg.v19.i12.1861
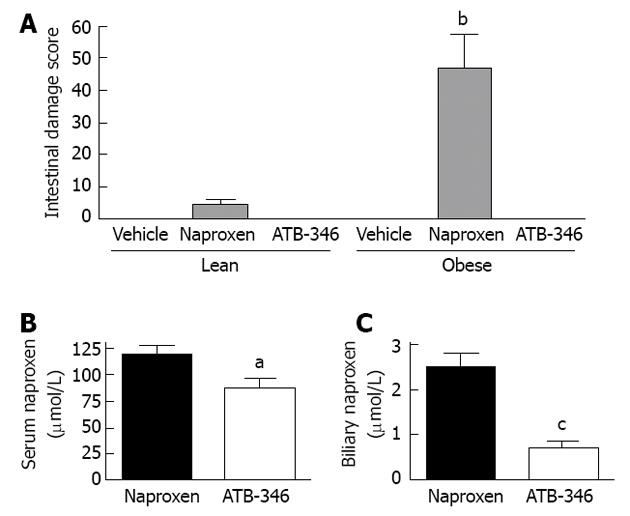
Figure 4 Intestinal safety and altered biliary excretion of ATB-346.
A: ATB-346 is a hydrogen sulfide-releasing derivative of naproxen[38].When administered to obese Zucker rats, twice-daily for 4.5 d at 10 mg/kg, naproxen induced small intestinal damage that was significantly more severe in the obese rats (bP < 0.01). However, at an equimolar dose, ATB-346 did not induce intestinal damage in lean or obese rats[175]; B: The serum levels of naproxen in normal rats after 4.5 d of twice-daily administration of ATB-346 were marginally, but significantly (aP < 0.05) lower than those in rats treated with an equimolar dose of naproxen; C: Biliary levels of naproxen in rats treated with ATB-346 (as above) were markedly reduced compared to those in rats treated with an equimolar dose of naproxen (cP < 0.001).The data shown in this graph are from Blackler et al[175].
- Citation: Wallace JL. Mechanisms, prevention and clinical implications of nonsteroidal anti-inflammatory drug-enteropathy. World J Gastroenterol 2013; 19(12): 1861-1876
- URL: https://www.wjgnet.com/1007-9327/full/v19/i12/1861.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i12.1861