Copyright
©2011 Baishideng Publishing Group Co.
World J Gastroenterol. Dec 28, 2011; 17(48): 5305-5309
Published online Dec 28, 2011. doi: 10.3748/wjg.v17.i48.5305
Published online Dec 28, 2011. doi: 10.3748/wjg.v17.i48.5305
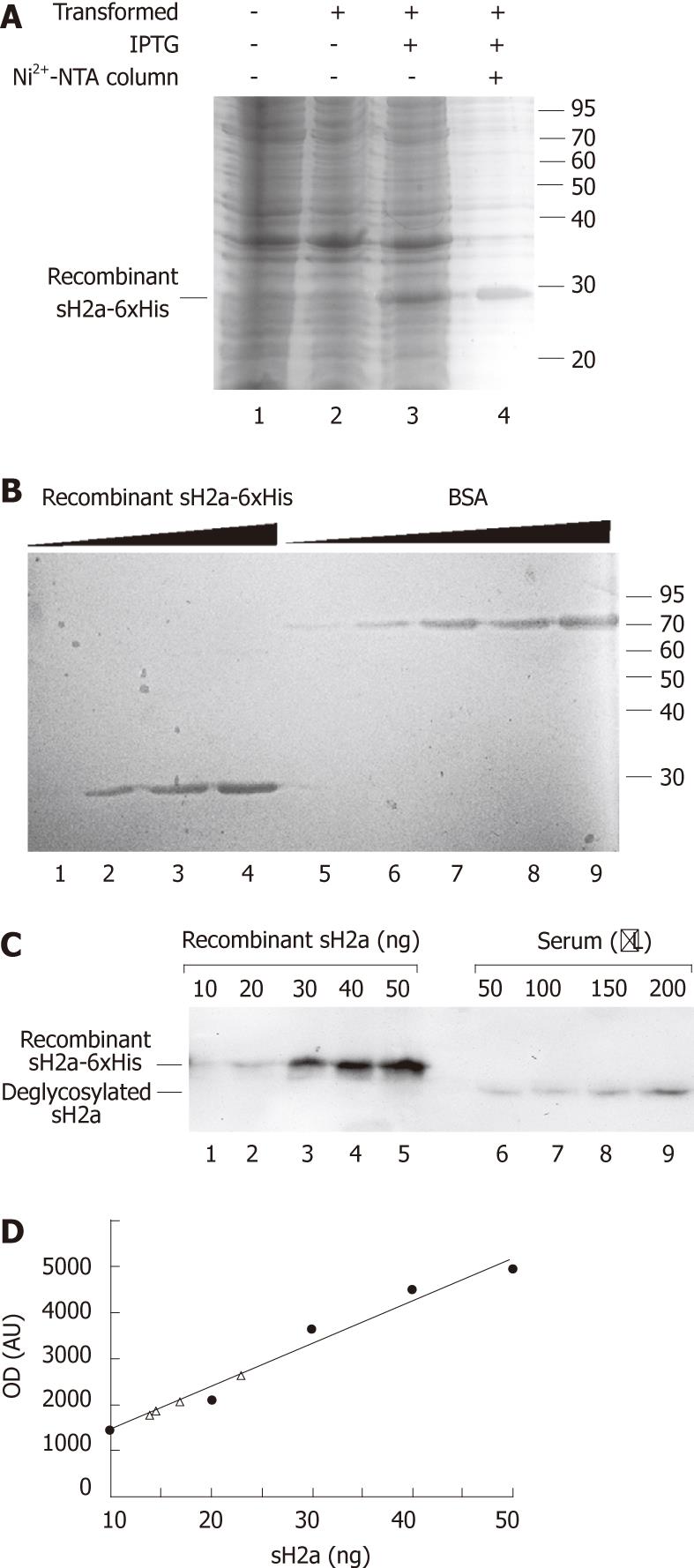
Figure 2 Recombinant sH2a concentration in serum.
A: Escherichia coli Rosetta DE3 pLysS were either left untransformed or transformed by heat shock with a plasmid carrying 6xHis-tagged sH2a and induced with 0.3 mmol isopropyl β-D-1-thiogalactopyranosid as explained in Materials and Methods. Cell lysates were treated with 6 mol guanidinium-hydrochloride, dyalized and run on 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), comparing with an aliquot of tagged sH2a purified on a Ni2+-NTA-agarose column (lane 4). The gel was stained with imperial blue; B: Increasing amounts of recombinant affinity purified 6xHis-tagged sH2a (lanes 1-4 correspond to 127, 635, 1270 and 1905 ng) were compared with increasing amounts of bovine serum albumin (lanes 5-9 are 100, 200, 500, 1000 and 2000 ng) on 12% SDS-PAGE, stained with Imperial blue; C and D: The indicated increasing concentrations of recombinant 6xHis-tagged sH2a were run on SDS-PAGE after immunoprecipitation with B9 antibody (lanes 1-5) and compared with B9-immunoprecipitates from increasing volumes of normal human serum treated with N-glycosidase-F (lanes 6-9) and analyzed by immunoblot as in Figure 1, except that detection was done using the electrochemiluminescence procedure. The immunoblot was quantified and the graph (D) shows a curve of recombinant sH2a (full circles) and extrapolation of the concentration of sH2a in 50, 100, 150 and 200 μL of serum (triangles). Shown is an immunoblot representative of three repeat experiments.
- Citation: Benyair R, Kondratyev M, Veselkin E, Tolchinsky S, Shenkman M, Lurie Y, Lederkremer GZ. Constant serum levels of secreted asialoglycoprotein receptor sH2a and decrease with cirrhosis. World J Gastroenterol 2011; 17(48): 5305-5309
- URL: https://www.wjgnet.com/1007-9327/full/v17/i48/5305.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i48.5305