Copyright
©2007 Baishideng Publishing Group Co.
World J Gastroenterol. Sep 21, 2007; 13(35): 4673-4689
Published online Sep 21, 2007. doi: 10.3748/wjg.v13.i35.4673
Published online Sep 21, 2007. doi: 10.3748/wjg.v13.i35.4673
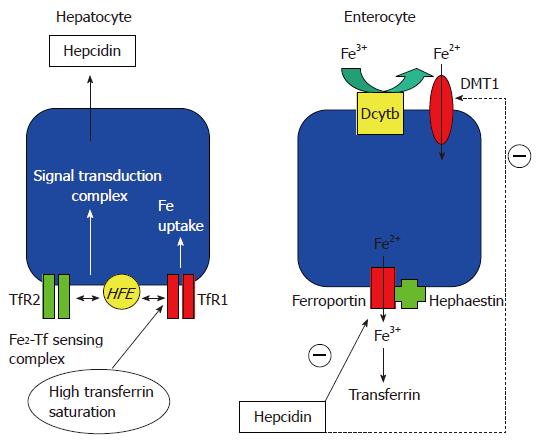
Figure 3 Current concepts regarding hepatic regulation of iron metabolism.
Left panel, hepatocyte. At normal transferrin saturation, TfR1 may sequester HFE[185]. At increased transferrin saturation, diferric transferrin competes with HFE for binding to TfR1[55]. Freed HFE is proposed to bind TfR2; the complex conveys transferrin saturation status via a cytoplasmic signal transduction complex, leading to synthesis and secretion of hepcidin. In HH, mutations in the genes encoding HFE, TfR2, haemojuvelin or hepcidin may all disrupt this sensing system, leading to deficient hepcidin production and iron overload[53]. Right panel, enterocyte. Circulating hepcidin may reduce iron absorption by interacting with ferroportin, causing its internalization and degradation[49] and/or by reducing DMT1 expression[50].
-
Citation: Sebastiani G, Walker AP.
HFE gene in primary and secondary hepatic iron overload. World J Gastroenterol 2007; 13(35): 4673-4689 - URL: https://www.wjgnet.com/1007-9327/full/v13/i35/4673.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i35.4673