Copyright
©The Author(s) 2023.
World J Gastroenterol. Jul 7, 2023; 29(25): 3964-3983
Published online Jul 7, 2023. doi: 10.3748/wjg.v29.i25.3964
Published online Jul 7, 2023. doi: 10.3748/wjg.v29.i25.3964
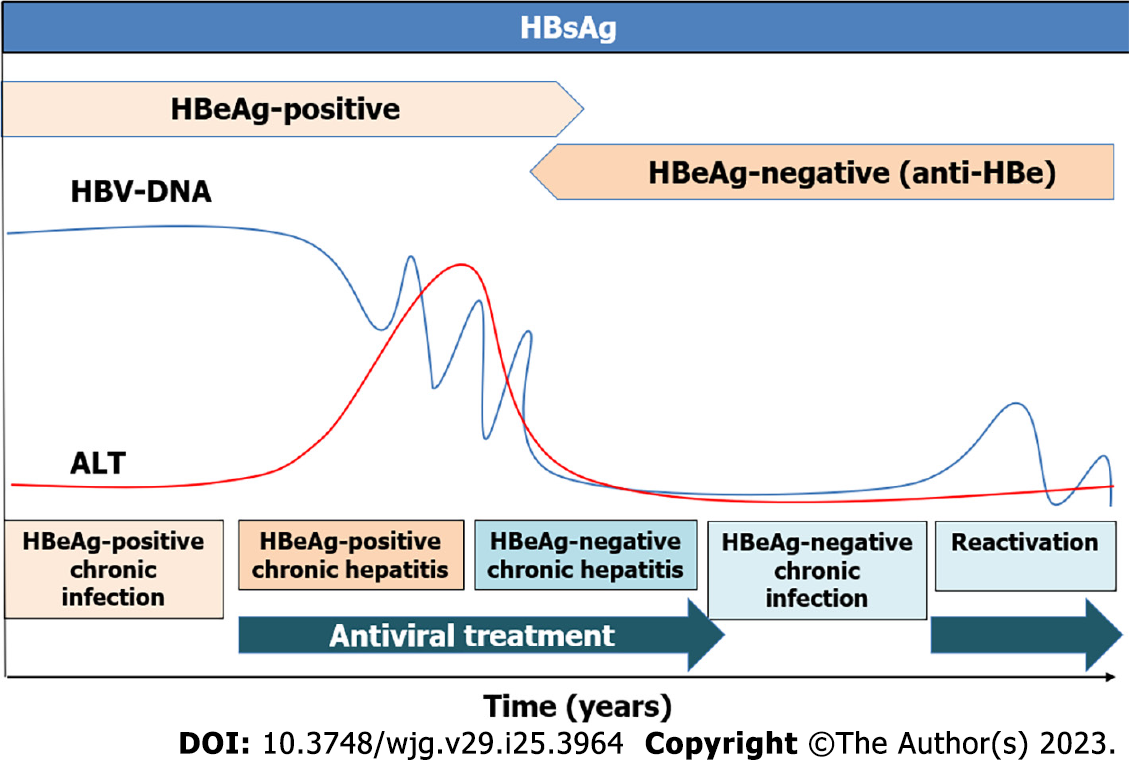
Figure 1 Phases of hepatitis B virus infection.
The persistence of hepatitis B virus surface antigen longer than six months is considered a marker for chronic hepatitis B infection. The HB e antigen (HBeAg)-positive chronic infection occurs in younger patients with high viral load and normal liver function tests. In contrast, HBeAg-negative chronic infection is characterized by low levels of hepatitis B virus-DNA with normal liver function and without fibrosis. Generally, treatment is not recommended in these two phases. Chronic hepatitis is defined by the presence of transaminases and high viral load that can induce liver fibrosis and risk of developing hepatocellular carcinoma, being antiviral treatment recommended[9-11]. HBV: Hepatitis B virus; HBeAg: Hepatitis B e antigen; ALT: Alanine aminotransferase.
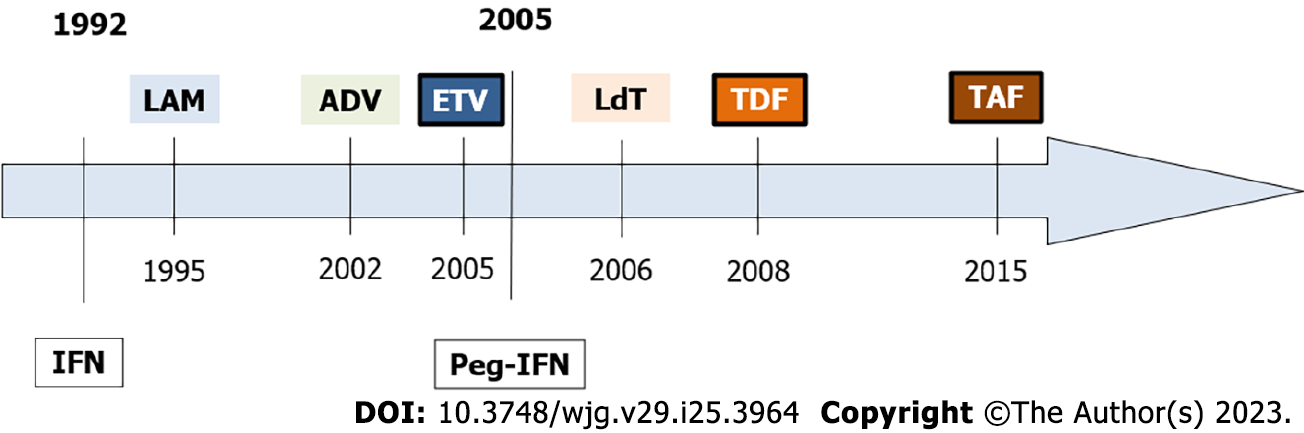
Figure 2 Hepatitis B virus antiviral treatment history.
The figure shows the United States Food and Drug Administration approval dates for hepatitis B virus drugs. IFN: Interferon; Peg-IFN: Pegylated interferon; LAM: Lamivudine; ADV: Adefovir; TDF: Tenofovir; ETV: Entecavir; LdT: Telbivudine; TAF: Tenofovir alafenamide.
- Citation: Broquetas T, Carrión JA. Past, present, and future of long-term treatment for hepatitis B virus. World J Gastroenterol 2023; 29(25): 3964-3983
- URL: https://www.wjgnet.com/1007-9327/full/v29/i25/3964.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i25.3964