Copyright
©2014 Baishideng Publishing Group Inc.
World J Gastroenterol. Jul 28, 2014; 20(28): 9217-9228
Published online Jul 28, 2014. doi: 10.3748/wjg.v20.i28.9217
Published online Jul 28, 2014. doi: 10.3748/wjg.v20.i28.9217
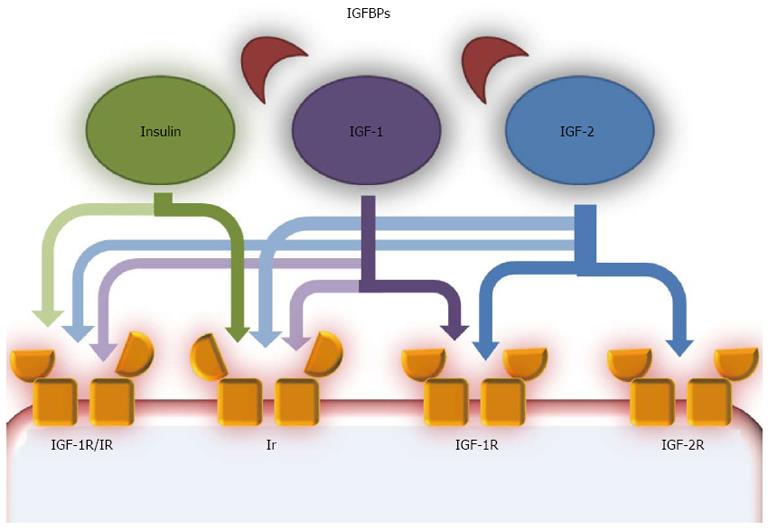
Figure 1 Insulin growth factor pathway scheme.
The insulin-like growth factor (IGF) pathway is composed of several components. IGF ligands are IGF-1 and IGF-2, two peptides that share high similarities with insulin. A family of carrier proteins, called insulin-like growth factor binding proteins (IGFBPs), bind IGF-1 and IGF-2 in the blood. IGF-1 and IGF-2 act as autocrine, paracrine and endocrine growth factors and are mostly produced in the liver, especially IGF-1 in the postnatal era and IGF-2 during fetal development. They can act on various receptors, but have higher affinity with IGF-1R, a tyrosine kinase receptor structurally similar to the insulin receptor (Ir). IGF-1 can also bind Ir, but with lower affinity than IGF-1R, while IGF-2 binds Ir only during fetal development. IGF-2R is structurally similar to IGF-1R, but binds only IGF-2 and most probably acts on this growth hormone with an inhibitory effect, as a clearance site. Another receptor is a hybrid receptor consisting of insulin and IGF-1R hemireceptors which preferentially binds IGF-1, while insulin does not have optimal binding[70].
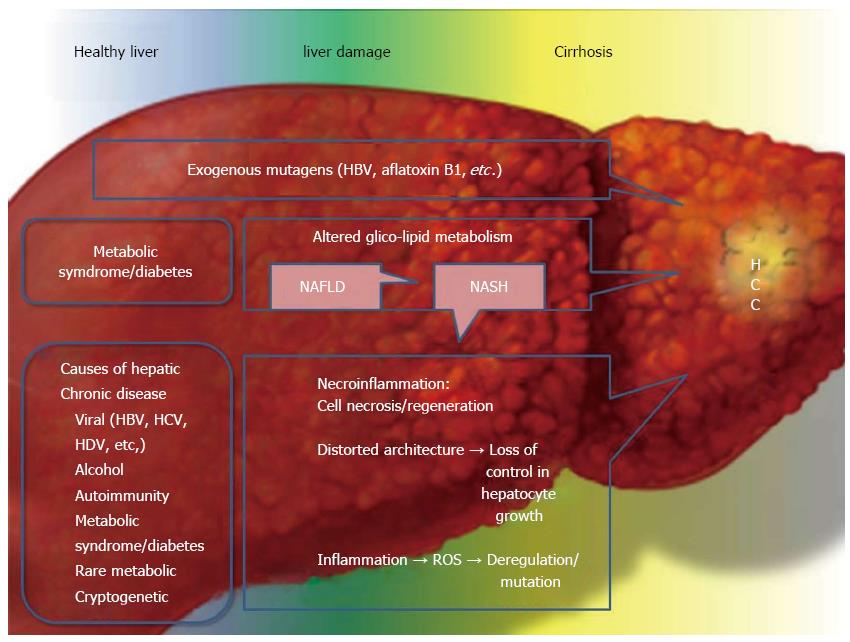
Figure 2 Main causes and concomitant causes of hepatocellular carcinoma, focusing on the metabolic syndrome and type 2 diabetes mellitus.
Hepatocellular carcinoma (HCC) development is often the final step in liver damage during chronic liver disease. Classically the step between a “normal” liver and HCC is liver cirrhosis. Liver cirrhosis causes disruption of the delicate and complex hepatic architecture. The formation of cirrhotic nodules has, per se, an important role in HCC development as the hepatic architecture is chronically replaced by fibrous tissue. This process is on the one hand, an attempt to repair the liver damage, and on the other hand, it leads to an increased hepatocellular turn-over with impaired vascularization, increased necrosis, apoptosis and inflammation. The final step is loss of control and the formation of malignant hepatic cells. It is easily understood that almost all causes of chronic liver damage, resulting in cirrhosis, can develop HCC. Recently type 2 diabetes (T2DM), MS and obesity have been identified as risk factors for HCC in the presence or absence of cirrhosis. In summary, these diseases are able to increase and accelerate the chronic necroinflammatory process present in cirrhotic liver, but their action is also important in the first steps of chronic liver damage. The altered metabolism of gluco-lipids leads to fat accumulation in the liver, causing non-alcoholic fatty liver disease (NAFLD). Fat is an important inducer of inflammation and can alter the normal cellular turn-over acting both locally in the liver and systemically via paracrine, autocrine and endocrine actions. Nonalcoholic steatohepatitis (NASH) is an eventual evolution of NAFLD and it is possible that not only NASH, with necroinflammation leading to cirrhosis, but also “simple” fatty liver can induce cancer development, as a first step, in the liver. This point merits further investigation. A separate discussion needs to be included for the so called “mutagens”. HBV and aflatoxin β1 are the two most important mutagens involved in HCC development and in the non-cirrhotic liver. HBV: Hepatitis B virus; HCV: Hepatitis C virus; HDV: Hepatitis D virus.
-
Citation: Scalera A, Tarantino G. Could metabolic syndrome lead to hepatocarcinoma
via non-alcoholic fatty liver disease? World J Gastroenterol 2014; 20(28): 9217-9228 - URL: https://www.wjgnet.com/1007-9327/full/v20/i28/9217.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i28.9217