Published online Jun 8, 2024. doi: 10.37126/aige.v5.i2.91424
Revised: April 12, 2024
Accepted: May 6, 2024
Published online: June 8, 2024
Processing time: 134 Days and 16.9 Hours
Colorectal diseases are increasing due to altered lifestyle, genetic, and environmental factors. Colonoscopy plays an important role in diagnosis. Advances in colonoscope (ultrathin scope, magnetic scope, capsule) and technological gadgets (Balloon assisted scope, third eye retroscope, NaviAid G-EYE, dye-based chromoendoscopy, virtual chromoendoscopy, narrow band imaging, i-SCAN, etc.) have made colonoscopy more comfortable and efficient. Now in-vivo microscopy can be performed using confocal laser endomicroscopy, optical coherence tomography, spectroscopy, etc. Besides developments in diagnostic colonoscopy, therapeutic colonoscopy has improved to manage lower gastrointestinal tract bleeding, obstruction, perforations, resection polyps, and early colorectal cancers. The introduction of combined endo-laparoscopic surgery and robotic endoscopic surgery has made these interventions feasible. The role of artificial intelligence in the diagnosis and management of colorectal diseases is also increasing day by day. Hence, this article is to review cutting-edge developments in endoscopic principles for the management of colorectal diseases.
Core Tip: Diagnostic and therapeutic endoscopy is evolving in the management of colorectal diseases. The rigid scopes have given way to flexible and capsule scopes. Conventional white light endoscopes have been replaced by high-definition and modified image enhancements. Now, both diagnosis and in-vivo histology assessment could be feasible. Therapeutic endoscopy has evolved to manage selective colorectal disorders for which surgery was considered the standard of care in the past. The role of robotics and artificial intelligence is indispensable.
- Citation: Ghosh NK, Kumar A. Ultra-minimally invasive endoscopic techniques and colorectal diseases: Current status and its future. Artif Intell Gastrointest Endosc 2024; 5(2): 91424
- URL: https://www.wjgnet.com/2689-7164/full/v5/i2/91424.htm
- DOI: https://dx.doi.org/10.37126/aige.v5.i2.91424
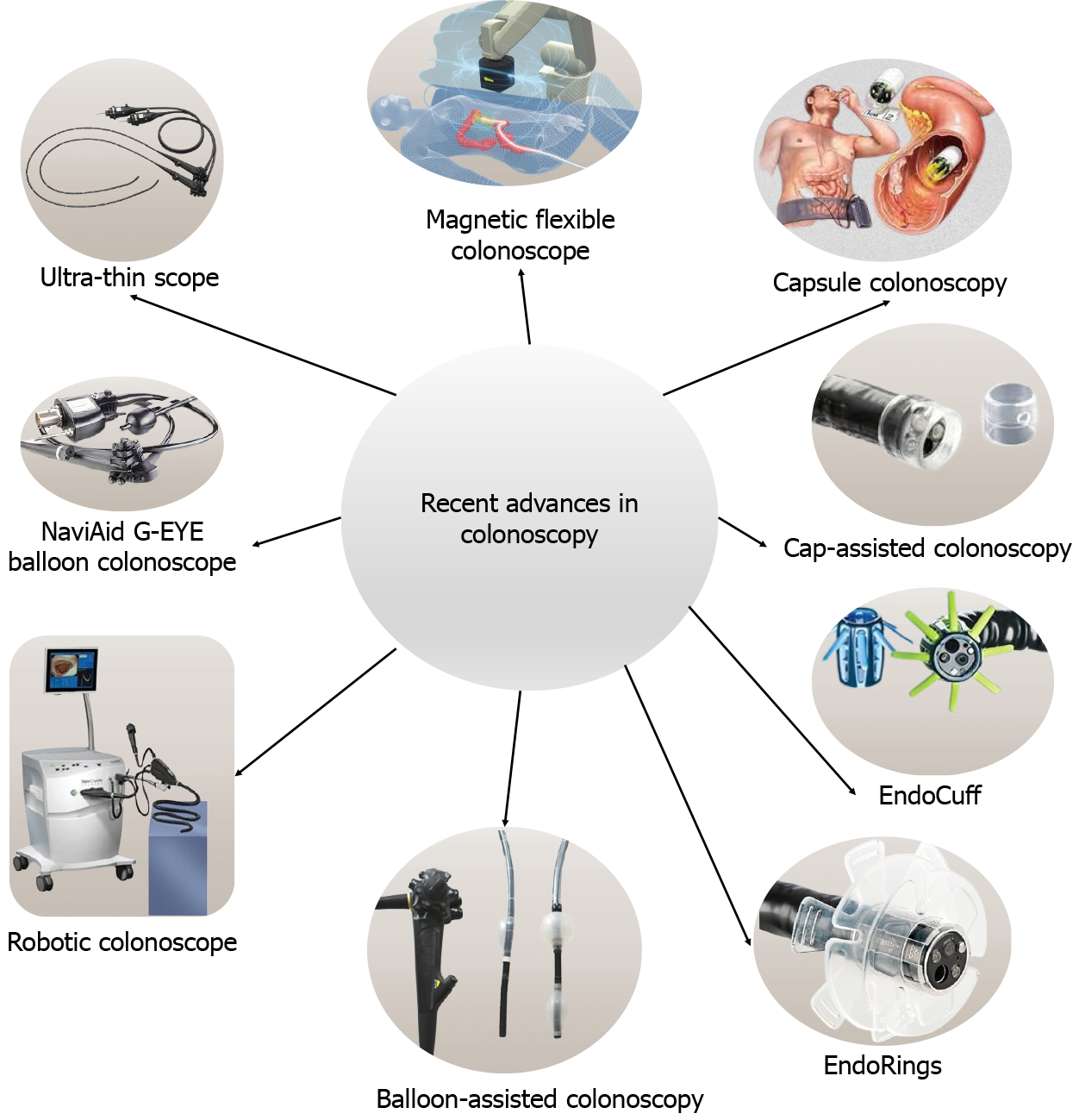
Diseases of the colorectum encompass various benign and malignant conditions with varied morbidity and mortality. Proximity to the anal opening makes the colon and rectum accessible to diagnostic and therapeutic colonoscopy. The first colonoscopy was developed from one made by Philipp Bozzini (1805), the father of endoscopy[1]. The initial colonoscopes were limited by less flexibility and poor illumination. Modern scopes facilitate cecal intubation, prevent loop formation, and patient satisfaction with fewer adverse events[2]. Thin-caliber colonoscopes, magnetic flexible colonoscopes, and capsule colonoscopes are getting popular due to lesser patient discomfort and better efficiency. The technical developments in colonoscopes include light sources, flexible and torque-stable shafts, fiber optics, four-way angulation control, charge-coupled devices for image sensing, and the ability to produce a high-quality digital image. Nevertheless, it is multifunctional as it can be used for suction, insufflation with air or water, and biopsy with a shaft. Presently, high-definition-white light endoscopy is the standard vision system[3] and the developments in this field are expanding (Table 1). Other advancements include endocuff vision, amplify EYE, and G-EYE devices to enhance the mucosa and improve the gaze of vision. The introduction of artificial intelligence (AI) algorithms has made endoscopists' jobs easier and more efficient for the detection of missing lesions which are prone to human errors.
| No. | Techniques | Technology | Clinical use diagnostic/therapeutic | Ref. |
| 1 | Chromoendoscopy | Real-time tissue enhancement using biocompatible dyes | Polyp and adenoma detection, differentiation of benign from malignant lesions, dysplasia associated with IBD | [111-114] |
| 2 | Narrow band imaging | Physical spectral filters generate narrow bands of 415 and 540 nm in center wavelength | Adenoma detection, dysplasia associated with IBD | [115,116] |
| 3 | Blue light imaging | Narrowed spectrum LED light of 410 nm and 450 nm enabling haemoglobin excitation and a positive mucosal contrast | Differentiation of non-neoplastic from neoplastic lesions, in vivo histology | [117-119] |
| 4 | Autofluorescence endoscopy | Fluorescence of fluorophores differentiate tissues | Adenoma detection, differentiation of non-neoplastic lesions from neoplastic lesions | [120-123] |
| 5 | Flexible spectral imaging color enhancement | This differentiation is based on the surface capillary pattern | Differentiation of non-neoplastic from neoplastic lesions, in vivo histology | [124-126] |
| 6 | Linked color imaging | Pre-processing narrow band LED radiation and post-processing color technology to separate imported colors into red, green, and blue what enhances color differences | Polyp detection | [127-129] |
| 7 | i-SCAN | Enhancement of the image contrast through a real-time post-processing algorithm, basing the different reflective properties of normal and abnormal mucosa | Adenoma detection, in vivo histology, disease activity in IBD, detection of neoplasia in IBD | [130-133] |
| 8 | i-SCAN OE | Incorporation of a digital pre-processor optical enhancement to improve visualization of mucosal vascular pattern | Detection of pre-malignant lesions | [134] |
Along with the diagnostic evolution of colonoscopes, the realm of therapeutic intervention is expanding. The first colonoscopic intervention was a polypectomy using loop snare-cautery[4]. Nowadays, therapeutic colonoscopy can be used for the management of lower gastrointestinal (GI) bleeders, colorectal strictures, malignant large bowel obstructions, colorectal perforations, foreign body removal, etc. The endoscopic resection of early colorectal cancer (CRC) becoming the standard of care as it is associated with fewer morbidity[5,6]. Hirschsprung’s disease where surgery was once considered the only treatment, nowadays per-rectal endoscopic myotomy has shown good results in a few case reports. Similarly for malignant colonic polyps, segmental colectomy was considered the standard of care, where endoscopic polypectomy is considered a reasonable treatment modality and recommended by NCCN guidelines[4]. Similarly, for post-colonoscopy perforation, surgical management was considered as the standard of treatment, with the advent of endoscopic skills, and gadgets like clips, it is possible to avoid surgery in these patients. In this article, we shall discuss the role of minimally invasive endoscopic techniques in the diagnosis and management of colorectal disorders and their prospects.
It’s a narrow caliber scope to reduce discomfort, require less sedation, and improve therapeutic efficacy. A randomized controlled trial (RCT) of 220 females in whom pain following colonoscopy was reported to be higher, has shown that ultra-thin colonoscopy was reportedly less painful than conventional colonoscopy[7]. It can be easily negotiated beyond the stenotic segment to assess the proximal bowel. In a prospective study on 100 stenotic CRC patients, Ito et al[8] found 65.5% synchronous lesions in the proximal colon. Magnetic flexible colonoscopy has a similar function as a conventional colonoscope; however, it is more patient-friendly due to less looping of the scope[9]. The mechanism includes a “front-pull” rather than a “push technique”. The forward mechanism prevents buckling of the instrument and resultant pain, and perforation. Capsule colonoscopy is a non-invasive method of screening; however, it requires aggressive bowel preparation as a small amount of debris can affect the capsule mobility. European Society of Gastrointestinal Endoscopy guideline recommends the use of second-generation capsules for screening of average risk of CRC where previous colonoscopy failed due to difficult scope progression[10].
The Third Eye Retroscope (TER; Avantis Medical Systems, Sunnyvale, CA, United States) is an optical technology to detect polyps located at the proximal folds and the flexures. In a pilot study of 29 human subjects, out of 38 polyps, 34 polyps could be detected with a forward scope whereas 4 additional polyps were detected using TER (11.8% increase in the yield)[11]. The newer version, Third Eye Panoramic (Avantis Medical Systems) uses a video cap containing two side viewing lenses fitted into the standard colonoscope limits the drawbacks of primitive TER i.e., technical learning curve, duration of the procedure, and cost[12].
The Fuse Full Spectrum Endoscopy® colonoscopy (EndoChoice Inc., Alpharetta, GA, United States) has a high resolution and 330° field of view. In a multicentre RCT, Fuse colonoscopy reported a significantly lower adenoma miss rate (7.5% vs 40.8%, P < 0.0001)[13]. Hence, the use of Fuse colonoscopy for colorectal screening and surveillance can be more useful. The extra wide-angle view colonoscope could detect significantly more polyps compared to the standard one (68% vs 51%, P < 0.0001)[14], and the detection of polyps behind the folds was also significant (62% vs 47%, P = 0.0009)[14].
The NaviAid G-EYE System (SMART Medical Systems Ltd, Ra’anana, Israel) comprises the G-EYE balloon colonoscope and the NaviAid SPARK2C inflation system which facilitates the straightening of haustral folds and flexures. In a RCT, compared to standard colonoscopy, NaviAid G-EYE balloon colonoscopy could detect an additional 17 (81%) adenomas among 106 patients[15]. Cap-assisted colonoscopy is used to improve the visualization of colonic epithelium. A meta-analysis including 12 studies (6185 patients) reported the detection of significantly more number of polyps [odds ratio (OR) = 1.13; P = 0.030), lower polyp miss rate (12.2% vs 28.6%), and higher cecal intubation rate (OR = 1.36, P = 0.020) with cap-assisted colonoscopy compared to conventional colonoscopy[16]. EndoCuff and EndoRings are similar to the cap-assisted colonoscopy, the device mounted over the scope helps to flatten the colonic folds during the withdrawal, hence, improving visualization. An RCT of 500 individuals reported a higher adenoma detection rate (ADR) with Endocuff-assisted colonoscopy compared to standard scope (35.4% vs 20.7%, P < 0.0001)[17]. A meta-analysis (6038 patients) comparing the Endocuff vs conventional colonoscopy demonstrated an increase in ADR [risk ratio (RR) = 1.18], especially in those endoscopists with an ADR < 35%, thus, improving the efficiency of novice endoscopists[18]. The EndoRings works with similar principles and an RCT (CLEVER Study) has shown a lower adenoma miss rate (10.4% vs 48.3%, P < 0.001) and a lower polyp miss rate (9.1% vs 52.8%, P < 0.001)[19].
Conventional white light endoscopy has several limitations which were minimized by various techniques. Chromoendoscopy uses various stains during endoscopy to enhance differences in the mucosa, and dysplastic or neoplastic lesions that are not apparent in white light. A prospective trial of targeted use of dye in patients with inflammatory bowel disease (IBD), demonstrated a significantly higher detection of dysplastic lesions than in standard colonoscopy[20]. Currently, SCENIC guidelines for the management of dysplasia in patients with IBD strongly recommend the use of chromoendoscopy[21].
The limitations of dye-based chromoendoscopy (operator dependency, time-consuming, lack of standardization of dilution methods, need for additional instruments, risk of met-hemoglobinuria, cost-effectiveness, risk of carcinogenesis, etc.) can be abrogated with the use of digital chromo-endoscopy including narrow-band imaging (NBI), Fuji intelligent chromo-endoscopy, i-SCAN, blue laser imaging, linked color imaging, etc.[22-25]. American Society for Gastrointestinal Endoscopy strongly recommends the use of advanced imaging techniques by expert endoscopists[26].
Various technical advancements can be used to assess the type of epithelium during colonoscopy. Confocal laser endomicroscopy can help to differentiate benign from malignant lesions, advanced adenomas, and adenomatous polyps with high sensitivity and specificity without histology[27]. Endocytoscopy involves staining the colorectal mucosa and visualization at a higher magnification and its accuracy in differentiating benign from malignant lesions was 94.1% compared to 96% on histopathology for differentiating neoplastic lesions from non-neoplastic lesions[28]. Optical coherence tomography is based on the use of infrared light to measure the optical reflectivity of tissue and hence; the differentiation of benign from malignant lesions in real-time based on microstructural images with high resolution[29]. Spectroscopy is based on the principles of light scattering and the vibrational and rotational aspects of molecules provide clues about the type of tissue (benign vs malignant, adenomatous vs non-adenomatous)[30]. A study has reported an accuracy of 83% to 88% in spectroscopy in differentiating benign from malignant lesions[31].
Since the inception of robotics, its use in the medical field has been growing. The unique locomotion and adaptability of the colorectum make it feasible to use these new-generation colonoscopes[32]. The robotic colonoscope is more comfortable and less painful to the patient[33]. Currently, only the Endotics system (Era Endoscopy, Cascina, Pisa, Italy) is available for use and it is based on an electro-pneumatic self-advancing locomotion mechanism. This colonoscope is controlled by a hand-held device and consists of a disposable scope (called E-Worm) and it advances using two clampers located at both ends of the scope. Repetitive movements of lengthening and shortening allow the locomotion of the probe as that of a worm which is generated by a hydro-pneumatic system inside the probe. The advantages of the current Endotics system include reduced pressure on the colonic wall during advancement, reduced pain, discomfort, learning curve, complications, and comparable adenoma detection to conventional colonoscopes[34-36]. However, it requires a higher degree of colon cleansing due to the small suction channel[36]. Small series have reported fewer cecal intubation rates[34,35]. Other robotic systems that are not available for clinical practice are NeoGuide Endoscopy System, Invendoscope SC40, Aer-O-Scope System, ColonoSight System, Robotic-assisted Colonoscopy Capsule, etc. To curb the drawbacks of the Robotic Colonoscope, Li et al[37] developed a visual servo-based semi-autonomous manipulation of an electromagnetic actuated soft-tethered colonoscope which will improve the autonomy of conventional robotic colonoscope, to decrease the user workload, and promote its use in clinical practice.
Mr. John McCarthy coined the term “Artificial intelligence (AI)”, and since then it has evolved and infiltrated into day-to-day life and facilitated diagnostic and therapeutic interventions of medicine[38]. It helps to increase the ADR (CADe; computer-aided detection), and characterization of the lesion (CADx; computer-aided diagnosis). In a study by Abdelrahim et al[39] AI technique can detect more polyps compared to endoscopists (for < 5 mm polyps, AI can detect 81.2% polyps vs endoscopist 66%, P < 0.001, for > 5 mm polyps, AI can detect 87.5% polyps vs 42.3% with endoscopists, P < 0.001). The CADx can provide histopathological diagnosis using NBI, linked-color imaging, blue-color imaging, magnifying endoscopy, confocal laser endoscopy, autofluorescence imaging, etc. A recent systematic review and meta-analysis of RCTs (33 trials involving 27404 patients) of AI-aided colonoscopy vs conventional colonoscopy showed a significantly higher polyp detection rate [RR = 1.238; 95% confidence interval (CI): 1.158-1.323], ADR (RR = 1.242; 95%CI: 1.159-1.332), polyps and adenoma per colonoscopy[40]. The role of AI in the management of IBD is immense. In cases of ulcerative colitis, it helps to assess endoscopic remission. In a systematic review and meta-analysis of 18 studies (13687 patients), the pooled sensitivity and specificity of diagnosis of endoscopic remission were 87% and 92%, respectively and the area under the curve (AUC) was 0.96 (95%CI: 0.94-0.97)[41]. In Crohn’s disease, an AI algorithm based on computed tomography enterography can be used to assess the bowel and mesentery, which can predict the time interval to surgery with an AUC of 0.896[42]. Table 2 shows various commercially available CAD systems. Though AI-based colonoscopy algorithms have shown the potential to expedite diagnosis and further therapeutic intervention, however, there are a few limitations including ethical challenges, privacy, transparency and trust, cybersecurity, responsibility, bias, and data quality assurance[43,44]. These technical and technological advancements have made colonoscopy more comfortable and efficient (Figure 1).
| No. | Product name | Company | Integration | CAD mode | Regulatory (country, year) | Clinical use |
| 1 | EndoBRAIN | Cybernet System Co. (Tokyo, Japan) | CF-290ECI, Olympus Co. | CADx | Japan, 2018 | Colorectal polyps endocytoscopy-computer-aided diagnosis |
| 2 | EndoBRAIN-EYE | Cybernet System Co. | Olympus colonoscopes | CADe | Japan, 2020 | Colorectal polyps endocytoscopy-computer-aided diagnosis |
| 3 | EndoBRAIN-PLUS | Cybernet System Co. | CF-290ECI, Olympus Co. | CADx | Japan, 2020 | Image enhanced colonoscopy |
| 4 | EndoBRAIN-UC | Cybernet System Co. | CF-290ECI, Olympus Co. | CADx | Japan, 2020 | Image enhanced colonoscopy |
| 5 | GI Genius | Medtronic Co. (Dublin, Ireland) | Multi vendors possible | CADe | United States, Europe, 2019 | Colorectal polyps |
| 6 | DISCOVERY | Pentax Medical Co. (Tokyo, Japan) | Pentax colonoscope | CADe | Europe, 2020 | Colorectal polyps |
| 7 | ENDO-AID | Olympus Co. (Tokyo, Japan) | Olympus colonoscopes | CADe | Europe, 2020 | Colorectal polyps, real-time aid, texture and color enhancement imaging |
| 8 | CAD-EYE | Fujifilm (Tokyo, Japan) | Fukifilm colonoscope | CADe CADx | Europe, Japan, 2020 | Colorectal polyps, real-time aid |
| 9 | EndoScreener | Shanghai Wision AI Co. (Shanghai, China) | Multi vendors | CADe | Europe, United States, 2021 | Colorectal polyps |
| 10 | WISE VISION | NEC Co. (Tokyo, Japan) | Multi vendors | CADe | Japan, Europe, 2020 | Colorectal polyps |
Colonoscopy has evolved from a diagnostic modality to interventions, for which surgery was once considered the standard of care. The first colonoscopic intervention was a polypectomy performed in the year 1969 using a loop snare-cautery device[45]. Colonoscopy has its place in interventions including localization of lower GI bleeding and management, dilatation of colorectal stenosis, management of colorectal perforation, foreign body removal, and many more[5,6].
The indications for surgery in lower GI bleeding are decreasing as 80% of lower GI bleeding resolves spontaneously and the majority of the rest can be managed endoscopically or through intervention radiology[46]. Most of the patients with lower GI bleed are at high risk for surgical intervention and the mortality rate for emergency surgery is nearly 10%[47]. Endoscopic treatment is commonly used are diverticular bleeding and angiodysplasias. The treatment options include clipping, band ligation, endoscopic detachable snare ligation, thermal cauterization, and epinephrine injection[48-50]. Epinephrine injection has a 20% rebleeding rate[51]. A clip can be applied to the neck of the diverticulum to stop bleeding[51]. Many studies have shown the superiority of band ligation for controlling diverticular bleeding and can be performed safely by trainees[52]. Similarly, in a meta-analysis of 384 colonic diverticular patients, band ligation was more efficacious than clips[50]. Colonic angiodysplasia accounts for 3%-15% of lower GI bleeding and it increases with age, argon plasma coagulation is the preferred treatment and reported efficacy in various studies[53].
Therapeutic colonoscopy can be used to manage lower GI obstruction. In cases of malignant colorectal obstruction metallic stent has shown its use[54]. In a meta-analysis of 12 studies, metallic stenting has shown a higher primary anastomosis rate, lower complications, and no difference in mortality when compared to emergency surgery[55]. Decompressive colonoscopy can be used for the management of pseudo-obstruction (Ogilvie syndrome)[56,57]. Colorectal stenosis is amenable to pneumatic dilatation. In a study of 40 patients with postoperative colorectal anastomotic site stricture, the restructure rate following pneumatic dilatation was 12.5% after a median follow-up of 5 years[58]. For ileocolic stricture in Crohn’s disease surgical resection is associated with more complications (32.2% vs 4.7%; P < 0.0001), however, there is less requirement for secondary surgery and longer surgery-free survival (11.1 ± 0.6 vs 5.4 ± 0.6 years; P < 0.001)[59]. Recently, endoscopic incision has shown potential use for anastomotic stricture dilatation[60].
Endoscopic access to sub-epithelial space is limited, however, recent developments in third-space endoscopy have expanded the use of endoscopic techniques in the diagnosis and management of mesenchymal tissues of the GI tract. Inoue et al[61] reported the first case of per-oral endoscopic myotomy for the treatment of achalasia cardia. Kulkarni et al[62] reported a case of per-rectal endoscopic myotomy in a 19-year-old girl for Hirschprung’s disease with clinical and manometry improvement at 9 months follow-up.
For endoscopically detected non-pedunculated colorectal polyps > 10 mm the conventional endoscopic mucosal resection (CEMR) is considered as an effective method with a technical success rate between 90% and 100%[63]. In conventional EMR, a cushion is created between the lesion and the muscle layer with a submucosal injection which decreases the risk of thermal injury and perforation, however, it increases the technical difficulties[63-66]. The risk of recurrence following CEMR is between 15%-50%[67]. To curtail the limitations of CEMR, Binmoeller et al[68] proposed an alternative way or EMR, underwater EMR (UEMR). Water emersion has certain advantages including reduction of haustral folds making easy snare capture[69]. Lenz et al[70] compared CEMR with UEMR in an RCT and UMER reduced the recurrence rate from 15% with CEMR to 2%.
Endoscopic submucosal dissection (ESD) is considered a better alternative to EMR with higher curative resection and lower recurrence rates[71-74]. However, it is performed using one endoscope which limits traction during the procedure leading to technical challenges. The recent development of traction wire (ProdiGI Traction Wire®; Medtronic, Minneapolis, MN) can provide continuous traction throughout the procedure making it easier[75]. Another development is the use of double-scope ESD, where 2 separate endoscopes were used for separate traction and cutting, being used for upper GI and colorectal lesions[76,77]. Several modifications of traction during ESD include clip with line method, spring-and-loop with clip, clip and snare method, and magnetic method. Endoscopic full-thickness resection (EFTR) is indicated in adenomas, intramucosal carcinomas, and limited submucosal invasion (< 1000 μm below the muscularis mucosa) without any evidence of lymphovascular invasion. Other indications include technical difficulty in ESD and carcinoid tumors. Authors have reported that in the presence of fibrosis in the case of right-sided colonic lesions, recurrent lesions, or giant pedunculated polyps, EFTR remains a good treatment option[78-80]. The resection can be purely endoscopic or laparoscopic-assisted and closure of the defect can be performed using a clip, endo-loop, or a full-thickness resection device. However, these are technically challenging and yet to be used in humans.
Flexible endoluminal robotics helps to overcome the limitations of endoscopic resection by providing multidirectional traction using two robotic arms[81]. To meet the requirements various modified flexible endoluminal robots have been designed i.e., EndoMaster EASESystem (EndoMaster Pte Ltd, Singapore), Flex Robotic System (Medrobotics, Raynham, MA, United States), EndoLuminal Surgical System (ELS, ColubrisMX, Houston, TX, United States), flexible auxiliary single-arm transluminal endoscopic robot (FASTER system), etc. Preclinical studies on animal models have shown the utility of the EndoMaster EASE system in performing colonic ESD[82]. The Flex Robotic System was initially designed for trans-oral minimal access surgeries and it can be used for colorectal lesions extending up to 25 cm from the anal verge[83]. Fok et al[84] have reported a case series of rectal polyps at a mean distance of 8.3 cm from the anal verge, resected using Flex Robotic System. The ELS flexible robotic system uses wristed instruments and an endoscope and this platform is similar to the current robotic surgical system[85]. Compared to the EndoMaster EASE system, it can accommodate a 6-mm endoscope with 3 degrees of freedom, 2 instrument channels, 2 insufflation channels, and 1 working channel[85]. It can be used to perform endorectal surgery up to 55 cm from the anal verge. Pre-clinical studies on ex vivo resection in the porcine colon using an ELS flexible system have been performed[85]. The FASTER system uses a module attached to the distal tip of the endoscope which is a robotic arm with a grasper with 4 degrees of freedom[86]. It is mostly used for gastric lesions in pre-clinical studies and is yet to be applied to the colorectum[86].
Surgical re-exploration was considered the standard procedure for post-surgical complications even for small anastomotic leaks. However, with the use of various modalities i.e., clips, stents, and EndoSPONGEs, these morbid re-explorations could be avoided and a delay in adjuvant treatment could be minimized[87]. Anastomotic leak following restorative colorectal resection is the most feared complication as it can lead to morbidity and mortality with a reported incidence of 1%-19% among various studies[88-90]. Construction of a diverting stoma decreases morbidity following the leak, however, it doesn’t prevent a leak[91,92]. Radiological drainage is sometimes challenging due to the anatomical location of purulent fluid in the deep pelvis. The use of an Endo-SPONGE can be useful in these conditions which consists of a polyurethane sponge placed trans-anally into the leak cavity using a flexible sigmoidoscope. It drains the collection and helps to promote granulation tissue formation. The sponge can be cut into various sizes to allow it to fit into the cavity and it is changed every 48-72 h till the cavity heals[93]. However, the efficacy is better for acute leaks (75%) compared to chronic leaks (38%)[94]. Another method for closure of anastomotic dehiscence is the OverStitch™ suturing system described by Kantsevoy and Thuluvath[95] for the closure of post-polyethylene glycol tube removal long-standing gastrocutaneous fistula. Though it seems appealing, it is technically challenging in locations like the right colon due to poor angulation, margin maneuverability, and incompatibility with colonoscopy[96]. Endoscopic closure of small GI perforations has been known since 1997, however, the newly developed Over the scope clip® system (OVESCO Endoscopy AG, Tuebingen, Germany) which consists of larger endo-clips can be used for colorectal anastomotic leaks. Radziunas et al[97] reported faster healing of post-LAR fistula using OESCO clips. Other methods of management of anastomotic leaks are the use of covered stents and fibrin glue. Though studies have reported positive outcomes with endoscopic techniques, however, it is important to realize that patient selection is paramount in treatment decision-making.
Colorectal anastomotic site stricture dilatation can be performed using a Hegar dilator or a balloon dilator. The use of endoscopic electro-incision can be performed by experienced endoscopists experienced in per-oral endoscopic myotomies. The endoscopic incision has shown 100% effectiveness at a follow-up of 33.75 months by Acar et al[98]. The reported incidence of perforation is also less in electroincision compared to bougie dilatation (0.1%-0.4% vs 4%)[99].
Trans-anal resection of rectal lesions has the risk of chronic pain after coccygectomy, rectal-cutaneous fistulae, wound infection, anal stenosis, and fecal incontinence[100,101]. Presently, there are trans-anal endoscopic microsurgery, trans-anal endoscopic operation, and transanal minimally invasive surgery and the basic difference among them is the platform and instruments used to perform local excision. These techniques are used for the excision of benign rectal polyps, and malignant lesions as a part of multimodality treatment of early rectal cancers, and in patients who are deemed unfit for radical surgery. A multicenter trial (The CARTS study) has assessed the role of long-course chemoradiotherapy followed by trans-anal endoscopic surgery after 6-8 wk for T1-3, N0 rectal cancers and reported a pathological complete response of 55%; however, local recurrence developed in 8.5%[102]. Similarly, The ACOSOG Z6041 study reported a 3-year survival rate of 88.2% for T2N0 rectal cancers for neoadjuvant treatment followed by transanal surgery[103]. Recently published GRECCAR 2 trial comparing total mesorectal excision and local excision in patients with < 4 cm T2-T3 rectal cancers with good response to treatment following neoadjuvant treatment showed no difference in local recurrence after long-term follow-up (local excision: 84% vs total mesorectal excision: 82%, P = 0.85)[104]. The upcoming STAR-TREC trial can answer the feasibility of wait and watch vs local excision vs total mesorectal excision for early rectal cancer (cT1-3b N0 tumors, ≤ 40 mm in diameter) following chemoradiotherapy[105]. The ASCRS recommends local excision for T1No rectal cancers and selective neoadjuvant treatment followed by local excision for T2 rectal cancers[106].
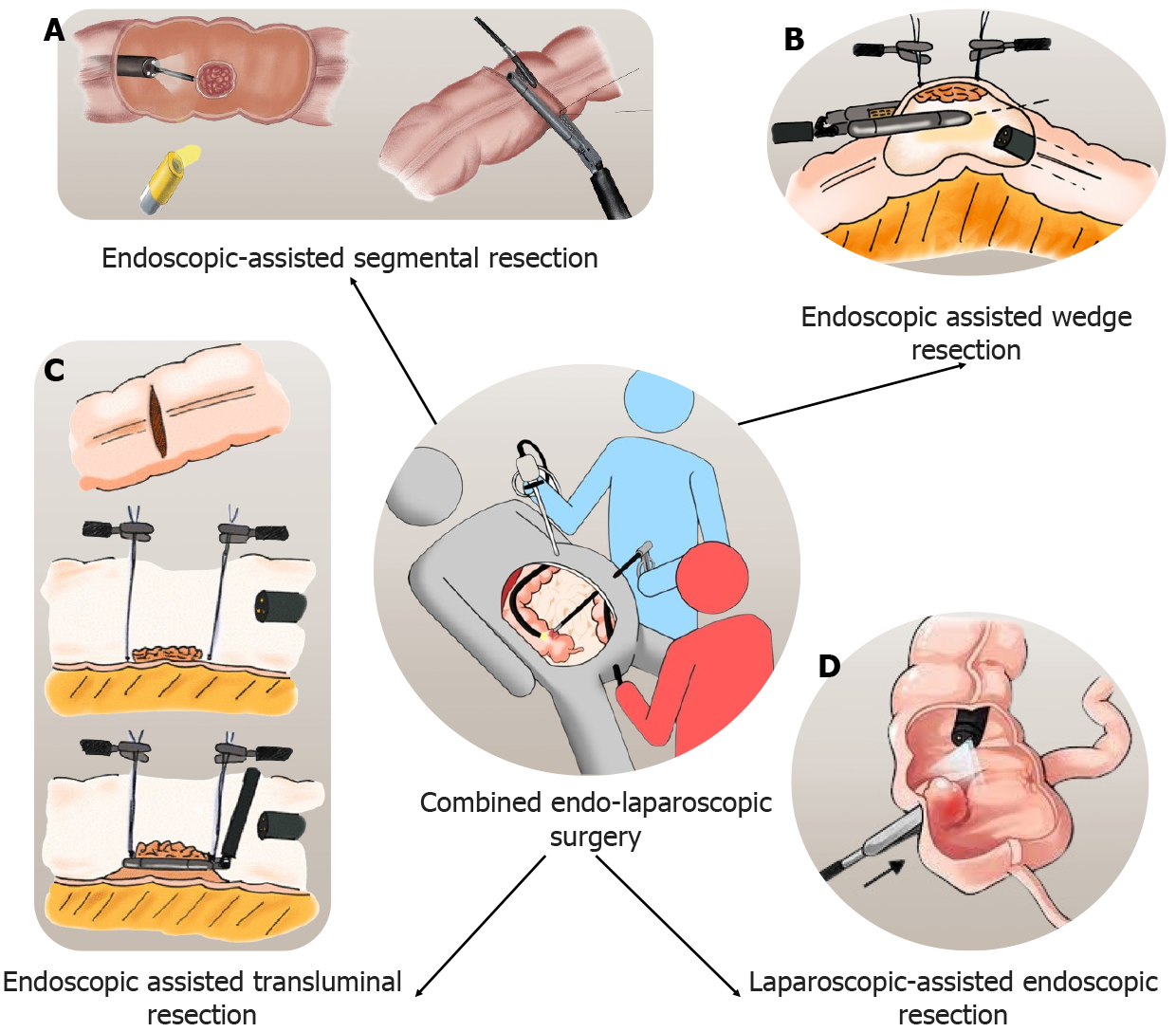
Polypectomy of adenomatous polyps is considered for secondary prevention of CRCs. However, the resection of some polyps becomes technically challenging when they are flat or located behind colonic folds. Laparoscopy can help endoscopic polypectomy, known as combined endo-laparoscopic surgery (CELS). It starts with the creation of pneumoperitoneum and abdominal exploration. All necessary adhesiolysis is performed to aid colonoscopy. It can be performed in various ways as described below. The laparoscopic-assisted endoscopic resection (LAER) is laparoscopic manipulation of the colonic segment to facilitate intraluminal exposure of polyp and endoscopic removal. Another method is endoscopic assisted wedge resection (EAWR) where after endoscopic localization of the polyp, excision is performed with a laparoscopic linear stapling device. The endoscopic assisted transluminal resection (EATR) is used for lesions located near the mesentery. In this method, a small colotomy is performed after localization during colonoscopy. The lesion is elevated and divided using a linear stapling device. Closure of colotomy is performed with laparoscopic sutures or staplers. The endoscopic-assisted segmental resection is used for lesions not amenable to LAER, EAWR, or EATR. After colonoscopic localization, laparoscopic segmental resection is performed. Figure 2 shows different types of CELS. Studies comparing surgical resection with CELS reported quicker procedures and fewer complications[107-109]. No long-term studies are reporting the outcome of CELS for colonic malignancy, the upcoming LIMERIC-II trial will help to know the long-term oncological outcome of CELS for malignant lesions[110]. Table 3 shows studies reporting a comparison of CELS with surgical resection.
| No. | Number of patients | Complications (%) | Length of stay (d) | Procedure time (min) | Cost (dollars)/others | Ref. |
| 1 | 23 SR; 23 CELS | CELS: 13; SR: 47.8 | CELS: 4; SR: 7 | CELS: 135; SR: 200 | - | Golda et al[107], 2022 |
| 2 | 11 CELS; 11 SR | CELS: 0; SR: 9 | CELS: 0-3; SR: 2-5 | CELS: 66.73; SR: 204.7 | CELS: 5523.29; SR: 12626.33 | Jayaram et al[108], 2019 |
| 3 | 5 CELS; 9 SR | CELS: 0; SR: 33.3 | CELS: 1; SR: 5 | CELS: 159; SR: 205 | - | Lee et al[109], 2013 |
As we have discussed, many acute, chronic, benign, and malignant conditions of colorectum can be managed endoscopically for which surgery was considered the standard of care, however, it was associated with morbidity. Studies comparing endoscopic vs surgical management in colorectal malignancies are shown in Table 4. Overall, the 5-year overall survival is better with surgical resection compared to endoscopic polypectomy, however, in high-risk patients, still endoscopic polypectomy is reasonable considering post-operative complications following surgery. In cases of malignant obstruction, both metallic stenting and emergency surgery had similar survival; however, surgery had more complications. Hence, patient selection is paramount for treatment decision-making.
| No. | Patients | Diagnosis | Complications (%) | Outcome (surgery vs endoscopy) | Ref. |
| 1 | Of 2077 patients: Surgical resection (1340), endoscopic polypectomy (737) | Malignant polyp | Surgery: 8.8. Endoscopy: 1.4 | 1-yr OS (92% vs 75%); 5-yr OS (88% vs 62%) | Cooper et al[136], 2012 |
| 2 | Of 13157 patients: Surgical resection (11113), endoscopic polypectomy (2044) | Malignant polyp | - | Right colon polyps: < 20 mm polyp: 94.5% vs 94.3% (P = 0.9); 20-39 mm: 91.8% vs 74.2% (P < 0.01); > 40 mm: 92.4% vs 60% (P < 0.01) | Gangireddy et al[137], 2018 |
| 3 | Of 31062 patients: Surgical resection (28469), endoscopic polypectomy (2593) | Malignant polyp | - | 5-yr OS: 86.1% vs 80.6% (P < 0.05) | Lowe et al[138], 2020 |
| 4 | Of 12 studies (meta-analysis) | Malignant obstruction | SEMS: 32.7. ES: 48.2 (RR = 0.61, 95%CI: 0.41-0.91) | Mortality (RR = 1.06, 95%CI: 0.55-2.04). Successful primary anastomosis (SEMS: 69.75% vs ES: 55.07%) (RR = 1.26, 95%CI: 1.01-1.57) | Cirocchi et al[55], 2021 |
| 5 | Of 167 patients: SEMS (115), ES (52) | Malignant obstruction | SEMS: 20. ES: 30.8 | 5-yr OS: 85.6 vs 82.6% (P = 0.68) | Kim et al[54], 2023 |
The twentieth century was credited with the research on the development and refinement of colonoscopes for better efficacy and fewer complications. Therapeutic endoscopy, as a subspeciality, subsequently came up in the later part of the century but with limited application in colorectal diseases. The incorporation of technology, tools, and techniques in the colonoscopes including AI has added another dimension to it. This newer version of scopes has resulted in quicker and more efficient diagnoses as compared to conventional colonoscopy with more accuracy, minimal discomfort, and wide therapeutic applications. Endo cytology is improving day by day, which can quicken the diagnosis of malignant pathology at an earlier stage. Robotic endoscopes, an upcoming tool may be used as a surgical robot to curtail the technical limitations of conventional scopes with broad indications and application in colorectal diseases. These innovations and newer versions of scope may avoid surgical intervention in select colorectal diseases in addition to its role in early and accurate diagnosis.
Minimally invasive techniques for the diagnosis and treatment of colorectal diseases are ongoing and allow earlier diagnosis and treatment with better outcomes. Ultra-minimally invasive colonoscopes present today are a result of thinner versions, flexibility, better vision systems, and mucosal enhancement tools. The use of AI has resulted in the minimization of human errors and improvement in the diagnostic yield. Development in smart glass has a further chance of improvement in diagnosis and intraprocedural professional guidance. Due to these newer innovations and newer versions of scope therapeutic colorectal intervention has increased. These minimally invasive colonoscopes loaded with gadgets and multiprong applications are being used both for benign (bleeding, obstruction, perforation) and malignant diseases (EMR, ESD, FTER). The use of robotic endoscopes with flexible instruments can be an emerging alternative to surgery in some selected diseases in the form of endorectal surgeries.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Health care sciences and services
Country of origin: India
Peer-review report’s classification
Scientific Quality: Grade D
Novelty: Grade C
Creativity or Innovation: Grade C
Scientific Significance: Grade C
P-Reviewer: Seow-Choen F, Singapore S-Editor: Wang JJ L-Editor: A P-Editor: Wang JL
| 1. | Ramai D, Zakhia K, Etienne D, Reddy M. Philipp Bozzini (1773-1809): The earliest description of endoscopy. J Med Biogr. 2018;26:137-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Gerges C, Neumann H Sr, Ishaq S, Sivanathan V, Galle PR, Neuhaus H, Neumann H. Evaluation of a novel colonoscope offering flexibility adjuster - a retrospective observational study. Therap Adv Gastroenterol. 2021;14:17562848211013494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 3. | Tziatzios G, Gkolfakis P, Lazaridis LD, Facciorusso A, Antonelli G, Hassan C, Repici A, Sharma P, Rex DK, Triantafyllou K. High-definition colonoscopy for improving adenoma detection: a systematic review and meta-analysis of randomized controlled studies. Gastrointest Endosc. 2020;91:1027-1036.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 4. | Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Farkas L, Garrido-Laguna I, Grem JL, Gunn A, Hecht JR, Hoffe S, Hubbard J, Hunt S, Johung KL, Kirilcuk N, Krishnamurthi S, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Nurkin S, Overman MJ, Parikh A, Patel H, Pedersen K, Saltz L, Schneider C, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Gregory KM, Gurski LA. Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:329-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1054] [Cited by in RCA: 939] [Article Influence: 234.8] [Reference Citation Analysis (16)] |
| 5. | Hammami A, Elloumi H, Bouali R. Clinical practice standards for colonoscopy. Tunis Med. 2021;99:952-960. [PubMed] |
| 6. | Serur A, Rhee R, Ramjist J. Current Nonoperative Therapeutic Interventions for Lower Gastrointestinal Hemorrhage. Clin Colon Rectal Surg. 2020;33:22-27. [PubMed] [DOI] [Full Text] |
| 7. | Hamada Y, Tanaka K, Katsurahara M, Horiki N, Yamada R, Tsuboi J, Nakamura M, Tamaru S, Yamada T, Takei Y. Efficacy of a small-caliber colonoscope for pain in female patients during unsedated colonoscopy: a randomized controlled study. Endosc Int Open. 2021;9:E1055-E1061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Ito S, Hotta K, Imai K, Kishida Y, Takizawa K, Kakushima N, Kawata N, Yoshida M, Yabuuchi Y, Ishiwatari H, Matsubayashi H, Shiomi A, Ono H. Ultrathin colonoscopy can improve complete preoperative colonoscopy for stenotic colorectal cancer: Prospective observational study. Dig Endosc. 2021;33:621-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Zhang K, Yuan Q, Zhu S, Xu D, An Z. Is Unsedated Colonoscopy Gaining Ground Over Sedated Colonoscopy? J Natl Med Assoc. 2018;110:143-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Spada C, Hassan C, Galmiche JP, Neuhaus H, Dumonceau JM, Adler S, Epstein O, Gay G, Pennazio M, Rex DK, Benamouzig R, de Franchis R, Delvaux M, Devière J, Eliakim R, Fraser C, Hagenmuller F, Herrerias JM, Keuchel M, Macrae F, Munoz-Navas M, Ponchon T, Quintero E, Riccioni ME, Rondonotti E, Marmo R, Sung JJ, Tajiri H, Toth E, Triantafyllou K, Van Gossum A, Costamagna G; European Society of Gastrointestinal Endoscopy. Colon capsule endoscopy: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2012;44:527-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 167] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 11. | Triadafilopoulos G, Li J. A pilot study to assess the safety and efficacy of the Third Eye retrograde auxiliary imaging system during colonoscopy. Endoscopy. 2008;40:478-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Rubin M, Lurie L, Bose K, Kim SH. Expanding the view of a standard colonoscope with the Third Eye Panoramic cap. World J Gastroenterol. 2015;21:10683-10687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Gralnek IM, Siersema PD, Halpern Z, Segol O, Melhem A, Suissa A, Santo E, Sloyer A, Fenster J, Moons LM, Dik VK, D'Agostino RB Jr, Rex DK. Standard forward-viewing colonoscopy versus full-spectrum endoscopy: an international, multicentre, randomised, tandem colonoscopy trial. Lancet Oncol. 2014;15:353-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 171] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 14. | Uraoka T, Tanaka S, Matsumoto T, Matsuda T, Oka S, Moriyama T, Higashi R, Saito Y. A novel extra-wide-angle-view colonoscope: a simulated pilot study using anatomic colorectal models. Gastrointest Endosc. 2013;77:480-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Gralnek IM. Emerging technological advancements in colonoscopy: Third Eye® Retroscope® and Third Eye® Panoramic(TM) , Fuse® Full Spectrum Endoscopy® colonoscopy platform, Extra-Wide-Angle-View colonoscope, and NaviAid(TM) G-EYE(TM) balloon colonoscope. Dig Endosc. 2015;27:223-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Westwood DA, Alexakis N, Connor SJ. Transparent cap-assisted colonoscopy versus standard adult colonoscopy: a systematic review and meta-analysis. Dis Colon Rectum. 2012;55:218-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Floer M, Biecker E, Fitzlaff R, Röming H, Ameis D, Heinecke A, Kunsch S, Ellenrieder V, Ströbel P, Schepke M, Meister T. Higher adenoma detection rates with endocuff-assisted colonoscopy - a randomized controlled multicenter trial. PLoS One. 2014;9:e114267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 18. | Triantafyllou K, Gkolfakis P, Tziatzios G, Papanikolaou IS, Fuccio L, Hassan C. Effect of Endocuff use on colonoscopy outcomes: A systematic review and meta-analysis. World J Gastroenterol. 2019;25:1158-1170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Dik VK, Gralnek IM, Segol O, Suissa A, Belderbos TD, Moons LM, Segev M, Domanov S, Rex DK, Siersema PD. Multicenter, randomized, tandem evaluation of EndoRings colonoscopy--results of the CLEVER study. Endoscopy. 2015;47:1151-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 20. | Marion JF, Waye JD, Present DH, Israel Y, Bodian C, Harpaz N, Chapman M, Itzkowitz S, Steinlauf AF, Abreu MT, Ullman TA, Aisenberg J, Mayer L; Chromoendoscopy Study Group at Mount Sinai School of Medicine. Chromoendoscopy-targeted biopsies are superior to standard colonoscopic surveillance for detecting dysplasia in inflammatory bowel disease patients: a prospective endoscopic trial. Am J Gastroenterol. 2008;103:2342-2349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 208] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 21. | Laine L, Kaltenbach T, Barkun A, McQuaid KR, Subramanian V, Soetikno R; SCENIC Guideline Development Panel. SCENIC international consensus statement on surveillance and management of dysplasia in inflammatory bowel disease. Gastroenterology. 2015;148:639-651.e28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 390] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 22. | Trivedi PJ, Braden B. Indications, stains and techniques in chromoendoscopy. QJM. 2013;106:117-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 23. | Konijeti GG, Shrime MG, Ananthakrishnan AN, Chan AT. Cost-effectiveness analysis of chromoendoscopy for colorectal cancer surveillance in patients with ulcerative colitis. Gastrointest Endosc. 2014;79:455-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 24. | Olliver JR, Wild CP, Sahay P, Dexter S, Hardie LJ. Chromoendoscopy with methylene blue and associated DNA damage in Barrett's oesophagus. Lancet. 2003;362:373-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 184] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 25. | ASGE Technology Committee; Manfredi MA, Abu Dayyeh BK, Bhat YM, Chauhan SS, Gottlieb KT, Hwang JH, Komanduri S, Konda V, Lo SK, Maple JT, Murad FM, Siddiqui UD, Wallace MB, Banerjee S. Electronic chromoendoscopy. Gastrointest Endosc. 2015;81:249-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 26. | ASGE Technology Committee; Abu Dayyeh BK, Thosani N, Konda V, Wallace MB, Rex DK, Chauhan SS, Hwang JH, Komanduri S, Manfredi M, Maple JT, Murad FM, Siddiqui UD, Banerjee S. ASGE Technology Committee systematic review and meta-analysis assessing the ASGE PIVI thresholds for adopting real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc. 2015;81:502.e1-502.e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 246] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 27. | Shahid MW, Buchner AM, Heckman MG, Krishna M, Raimondo M, Woodward T, Wallace MB. Diagnostic accuracy of probe-based confocal laser endomicroscopy and narrow band imaging for small colorectal polyps: a feasibility study. Am J Gastroenterol. 2012;107:231-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 28. | Mori Y, Kudo S, Ikehara N, Wakamura K, Wada Y, Kutsukawa M, Misawa M, Kudo T, Kobayashi Y, Miyachi H, Yamamura F, Ohtsuka K, Inoue H, Hamatani S. Comprehensive diagnostic ability of endocytoscopy compared with biopsy for colorectal neoplasms: a prospective randomized noninferiority trial. Endoscopy. 2013;45:98-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 29. | Wang C, Zhang Q, Wu X, Tang T, Liu H, Zhu SW, Gao BZ, Yuan XC. Quantitative diagnosis of colorectal polyps by spectral domain optical coherence tomography. Biomed Res Int. 2014;2014:570629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Kallaway C, Almond LM, Barr H, Wood J, Hutchings J, Kendall C, Stone N. Advances in the clinical application of Raman spectroscopy for cancer diagnostics. Photodiagnosis Photodyn Ther. 2013;10:207-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 110] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 31. | Li X, Yang T, Li S. Discrimination of serum Raman spectroscopy between normal and colorectal cancer using selected parameters and regression-discriminant analysis. Appl Opt. 2012;51:5038-5043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 32. | Trecca A, Catalano F, Bella A, Borghini R. Robotic colonoscopy: efficacy, tolerability and safety. Preliminary clinical results from a pilot study. Surg Endosc. 2020;34:1442-1450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Kim HG. Painless Colonoscopy: Available Techniques and Instruments. Clin Endosc. 2016;49:444-448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Cosentino F, Tumino E, Passoni GR, Morandi E, Capria A. Functional evaluation of the endotics system, a new disposable self-propelled robotic colonoscope: in vitro tests and clinical trial. Int J Artif Organs. 2009;32:517-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 35. | Tumino E, Sacco R, Bertini M, Bertoni M, Parisi G, Capria A. Endotics system vs colonoscopy for the detection of polyps. World J Gastroenterol. 2010;16:5452-5456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 48] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 36. | Tumino E, Parisi G, Bertoni M, Bertini M, Metrangolo S, Ierardi E, Cervelli R, Bresci G, Sacco R. Use of robotic colonoscopy in patients with previous incomplete colonoscopy. Eur Rev Med Pharmacol Sci. 2017;21:819-826. [PubMed] |
| 37. | Li Y, Ng WY, Li W, Huang Y, Zhang H, Xian Y, Li J, Sun Y, Chiu PWY, Li Z. Towards Semi-Autonomous Colon Screening Using an Electromagnetically Actuated Soft-Tethered Colonoscope Based on Visual Servo Control. IEEE Trans Biomed Eng. 2024;71:77-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 38. | Kaul V, Enslin S, Gross SA. History of artificial intelligence in medicine. Gastrointest Endosc. 2020;92:807-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 307] [Article Influence: 61.4] [Reference Citation Analysis (1)] |
| 39. | Abdelrahim M, Saiga H, Maeda N, Hossain E, Ikeda H, Bhandari P. Automated sizing of colorectal polyps using computer vision. Gut. 2022;71:7-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 40. | Zhang Y, Zhang X, Wu Q, Gu C, Wang Z. Artificial Intelligence-Aided Colonoscopy for Polyp Detection: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. J Laparoendosc Adv Surg Tech A. 2021;31:1143-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 41. | Lv B, Ma L, Shi Y, Tao T. A systematic review and meta-analysis of artificial intelligence-diagnosed endoscopic remission in ulcerative colitis. iScience. 2023;26:108120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 42. | Ruiqing L, Jing Y, Shunli L, Jia K, Zhibo W, Hongping Z, Keyu R, Xiaoming Z, Zhiming W, Weiming Z, Tianye N, Yun L. A Novel Radiomics Model Integrating Luminal and Mesenteric Features to Predict Mucosal Activity and Surgery Risk in Crohn's Disease Patients: A Multicenter Study. Acad Radiol. 2023;30 Suppl 1:S207-S219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 43. | Jeyaraman M, Balaji S, Jeyaraman N, Yadav S. Unraveling the Ethical Enigma: Artificial Intelligence in Healthcare. Cureus. 2023;15:e43262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 44] [Reference Citation Analysis (0)] |
| 44. | Khan B, Fatima H, Qureshi A, Kumar S, Hanan A, Hussain J, Abdullah S. Drawbacks of Artificial Intelligence and Their Potential Solutions in the Healthcare Sector. Biomed Mater Devices. 2023;1-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 105] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 45. | Lee A, Tutticci N. Enhancing polyp detection: technological advances in colonoscopy imaging. Transl Gastroenterol Hepatol. 2021;6:61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 46. | Barnert J, Messmann H. Management of lower gastrointestinal tract bleeding. Best Pract Res Clin Gastroenterol. 2008;22:295-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 47. | Farrell JJ, Friedman LS. Review article: the management of lower gastrointestinal bleeding. Aliment Pharmacol Ther. 2005;21:1281-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 117] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 48. | Strate LL, Gralnek IM. ACG Clinical Guideline: Management of Patients With Acute Lower Gastrointestinal Bleeding. Am J Gastroenterol. 2016;111:459-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 298] [Article Influence: 33.1] [Reference Citation Analysis (2)] |
| 49. | Kouanda AM, Somsouk M, Sewell JL, Day LW. Urgent colonoscopy in patients with lower GI bleeding: a systematic review and meta-analysis. Gastrointest Endosc. 2017;86:107-117.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 50. | Ishii N, Omata F, Nagata N, Kaise M. Effectiveness of endoscopic treatments for colonic diverticular bleeding. Gastrointest Endosc. 2018;87:58-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 51. | Soetikno R, Ishii N, Kolb JM, Hammad H, Kaltenbach T. The Role of Endoscopic Hemostasis Therapy in Acute Lower Gastrointestinal Hemorrhage. Gastrointest Endosc Clin N Am. 2018;28:391-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 52. | Ishii N, Setoyama T, Deshpande GA, Omata F, Matsuda M, Suzuki S, Uemura M, Iizuka Y, Fukuda K, Suzuki K, Fujita Y. Endoscopic band ligation for colonic diverticular hemorrhage. Gastrointest Endosc. 2012;75:382-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 53. | ASGE Standards of Practice Committee; Pasha SF, Shergill A, Acosta RD, Chandrasekhara V, Chathadi KV, Early D, Evans JA, Fisher D, Fonkalsrud L, Hwang JH, Khashab MA, Lightdale JR, Muthusamy VR, Saltzman JR, Cash BD. The role of endoscopy in the patient with lower GI bleeding. Gastrointest Endosc. 2014;79:875-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 144] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 54. | Kim EM, Park JH, Kim BC, Son IT, Kim JY, Kim JW. Self-expandable metallic stents as a bridge to surgery in obstructive right- and left-sided colorectal cancer: a multicenter cohort study. Sci Rep. 2023;13:438. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 55. | Cirocchi R, Arezzo A, Sapienza P, Crocetti D, Cavaliere D, Solaini L, Ercolani G, Sterpetti AV, Mingoli A, Fiori E. Current Status of the Self-Expandable Metal Stent as a Bridge to Surgery Versus Emergency Surgery in Colorectal Cancer: Results from an Updated Systematic Review and Meta-Analysis of the Literature. Medicina (Kaunas). 2021;57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 56. | Jetmore AB, Timmcke AE, Gathright JB Jr, Hicks TC, Ray JE, Baker JW. Ogilvie's syndrome: colonoscopic decompression and analysis of predisposing factors. Dis Colon Rectum. 1992;35:1135-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 88] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 57. | Geller A, Petersen BT, Gostout CJ. Endoscopic decompression for acute colonic pseudo-obstruction. Gastrointest Endosc. 1996;44:144-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 88] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 58. | Chan RH, Lin SC, Chen PC, Lin WT, Wu CH, Lee JC, Lin BW. Management of colorectal anastomotic stricture with multidiameter balloon dilation: long-term results. Tech Coloproctol. 2020;24:1271-1276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 59. | Lan N, Stocchi L, Ashburn JH, Hull TL, Steele SR, Delaney CP, Shen B. Outcomes of Endoscopic Balloon Dilation vs Surgical Resection for Primary Ileocolic Strictures in Patients With Crohn's Disease. Clin Gastroenterol Hepatol. 2018;16:1260-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 60. | Lee TG, Yoon SM, Lee SJ. Endoscopic radial incision and cutting technique for treatment-naive stricture of colorectal anastomosis: Two case reports. World J Gastrointest Surg. 2020;12:460-467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 61. | Inoue H, Minami H, Kobayashi Y, Sato Y, Kaga M, Suzuki M, Satodate H, Odaka N, Itoh H, Kudo S. Peroral endoscopic myotomy (POEM) for esophageal achalasia. Endoscopy. 2010;42:265-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1168] [Cited by in RCA: 1228] [Article Influence: 81.9] [Reference Citation Analysis (1)] |
| 62. | Kulkarni A, Dubewar S, Gawande A, Bhaware B, Mukewar S. Long per-rectal endoscopic myotomy for a case of Hirchsprung's disease. VideoGIE. 2023;8:242-244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 63. | Chien HC, Uedo N, Hsieh PH. Comparison of underwater and conventional endoscopic mucosal resection for removing sessile colorectal polyps: a propensity-score matched cohort study. Endosc Int Open. 2019;7:E1528-E1536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 64. | Choi AY, Moosvi Z, Shah S, Roccato MK, Wang AY, Hamerski CM, Samarasena JB. Underwater versus conventional EMR for colorectal polyps: systematic review and meta-analysis. Gastrointest Endosc. 2021;93:378-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 65. | Lenz L, Di Sena V, Nakao FS, Andrade GP, Rohr MR, Ferrari AP Jr. Comparative results of gastric submucosal injection with hydroxypropyl methylcellulose, carboxymethylcellulose and normal saline solution in a porcine model. Arq Gastroenterol. 2010;47:184-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 66. | Lenz L, Martins B, Kawaguti FS, Tellian A, Pennachi CMPS, Sorbello M, Gusmon C, Paulo GA, Uemura R, Geiger S, Lima MS, Safatle-Ribeiro A, Baba E, Hashimoto CL, Maluf-Filho F, Ribeiro U Jr. UNDERWATER ENDOSCOPIC MUCOSAL RESECTION FOR NON-PEDUNCULATED COLORECTAL LESIONS. A PROSPECTIVE SINGLE-ARM STUDY. Arq Gastroenterol. 2020;57:193-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 67. | Spadaccini M, Fuccio L, Lamonaca L, Frazzoni L, Maselli R, Di Leo M, Galtieri PA, Craviotto V, D'Amico F, Hassan C, Repici A. Underwater EMR for colorectal lesions: a systematic review with meta-analysis (with video). Gastrointest Endosc. 2019;89:1109-1116.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 68. | Binmoeller KF, Weilert F, Shah J, Bhat Y, Kane S. "Underwater" EMR without submucosal injection for large sessile colorectal polyps (with video). Gastrointest Endosc. 2012;75:1086-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 273] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 69. | Binmoeller KF, Hamerski CM, Shah JN, Bhat YM, Kane SD, Garcia-Kennedy R. Attempted underwater en bloc resection for large (2-4 cm) colorectal laterally spreading tumors (with video). Gastrointest Endosc. 2015;81:713-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 70. | Lenz L, Martins B, Andrade de Paulo G, Kawaguti FS, Baba ER, Uemura RS, Gusmon CC, Geiger SN, Moura RN, Pennacchi C, Simas de Lima M, Safatle-Ribeiro AV, Hashimoto CL, Ribeiro U, Maluf-Filho F. Underwater versus conventional EMR for nonpedunculated colorectal lesions: a randomized clinical trial. Gastrointest Endosc. 2023;97:549-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 71. | Mejia Perez LK, Yang D, Draganov PV, Jawaid S, Chak A, Dumot J, Alaber O, Vargo JJ, Jang S, Mehta N, Fukami N, Chua T, Gabr M, Kudaravalli P, Aihara H, Maluf-Filho F, Ngamruengphong S, Pourmousavi Khoshknab M, Bhatt A. Endoscopic submucosal dissection vs endoscopic mucosal resection for early Barrett's neoplasia in the West: a retrospective study. Endoscopy. 2022;54:439-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 72. | Han C, Sun Y. Efficacy and safety of endoscopic submucosal dissection versus endoscopic mucosal resection for superficial esophageal carcinoma: a systematic review and meta-analysis. Dis Esophagus. 2021;34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 73. | Yang D, Othman M, Draganov PV. Endoscopic Mucosal Resection vs Endoscopic Submucosal Dissection For Barrett's Esophagus and Colorectal Neoplasia. Clin Gastroenterol Hepatol. 2019;17:1019-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 74. | Zhang Y, Ding H, Chen T, Zhang X, Chen WF, Li Q, Yao L, Korrapati P, Jin XJ, Zhang YX, Xu MD, Zhou PH. Outcomes of Endoscopic Submucosal Dissection vs Esophagectomy for T1 Esophageal Squamous Cell Carcinoma in a Real-World Cohort. Clin Gastroenterol Hepatol. 2019;17:73-81.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 75. | Joseph A, Kahaleh M, Li AA, Haber GB, Kedia P, Makiguchi ME, Sharma NR, Hwang JH, Chak A, Al-Taee AM, Braun D, Mok S, Mehta NA, Gorgun E, Vargo J, Abe S, Saito Y, Stevens T, Bhatt A. Initial Multicenter Experience of Traction Wire Endoscopic Submucosal Dissection. TIGE. 2023;25:21-29. [DOI] [Full Text] |
| 76. | Ahn JY, Choi KD, Lee JH, Choi JY, Kim MY, Choi KS, Kim DH, Song HJ, Lee GH, Jung HY, Kim JH, Baek S. Is transnasal endoscope-assisted endoscopic submucosal dissection for gastric neoplasm useful in training beginners? A prospective randomized trial. Surg Endosc. 2013;27:1158-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 77. | Ebigbo A, Tziatzios G, Gölder SK, Probst A, Messmann H. Double-endoscope assisted endoscopic submucosal dissection for treating tumors in rectum and distal colon by expert endoscopists: a feasibility study. Tech Coloproctol. 2020;24:1293-1299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 78. | Isomoto H, Nishiyama H, Yamaguchi N, Fukuda E, Ishii H, Ikeda K, Ohnita K, Nakao K, Kohno S, Shikuwa S. Clinicopathological factors associated with clinical outcomes of endoscopic submucosal dissection for colorectal epithelial neoplasms. Endoscopy. 2009;41:679-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 161] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 79. | Yoshida N, Wakabayashi N, Kanemasa K, Sumida Y, Hasegawa D, Inoue K, Morimoto Y, Kashiwa A, Konishi H, Yagi N, Naito Y, Yanagisawa A, Yoshikawa T. Endoscopic submucosal dissection for colorectal tumors: technical difficulties and rate of perforation. Endoscopy. 2009;41:758-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 86] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 80. | Choi YS, Lee JB, Lee EJ, Lee SH, Suh JP, Lee DH, Kim DS, Youk EG. Can endoscopic submucosal dissection technique be an alternative treatment option for a difficult giant (≥ 30 mm) pedunculated colorectal polyp? Dis Colon Rectum. 2013;56:660-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 81. | Saito Y, Sumiyama K, Chiu PW. Robot assisted tumor resection devices. Expert Rev Med Devices. 2017;14:657-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 82. | Chiu PWY, Ho KY, Phee SJ. Colonic endoscopic submucosal dissection using a novel robotic system (with video). Gastrointest Endosc. 2021;93:1172-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 83. | Turiani Hourneaux de Moura D, Aihara H, Jirapinyo P, Farias G, Hathorn KE, Bazarbashi A, Sachdev A, Thompson CC. Robot-assisted endoscopic submucosal dissection versus conventional ESD for colorectal lesions: outcomes of a randomized pilot study in endoscopists without prior ESD experience (with video). Gastrointest Endosc. 2019;90:290-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 84. | Fok KY, Arabaci F, Barto W. Flexible robotic transanal resection of rectal polyps - A case series. Int J Med Robot. 2022;18:e2413. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 85. | Atallah S, Sanchez A, Bianchi E, Larach SW. Envisioning the future of colorectal surgery: preclinical assessment and detailed description of an endoluminal robotic system (ColubrisMX ELS). Tech Coloproctol. 2021;25:1199-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 86. | Ji R, Yang JL, Yang XX, Fu SC, Li LX, Li YQ, Zuo XL. Simplified robot-assisted endoscopic submucosal dissection for esophageal and gastric lesions: a randomized controlled porcine study (with videos). Gastrointest Endosc. 2022;96:140-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 87. | Simpson GS, Smith R, Sutton P, Shekouh A, McFaul C, Johnson M, Vimalachandran D. The aetiology of delay to commencement of adjuvant chemotherapy following colorectal resection. Int J Surg Oncol. 2014;2014:670212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 88. | Platell C, Barwood N, Dorfmann G, Makin G. The incidence of anastomotic leaks in patients undergoing colorectal surgery. Colorectal Dis. 2007;9:71-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 188] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 89. | Branagan G, Finnis D; Wessex Colorectal Cancer Audit Working Group. Prognosis after anastomotic leakage in colorectal surgery. Dis Colon Rectum. 2005;48:1021-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 341] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 90. | McDermott FD, Heeney A, Kelly ME, Steele RJ, Carlson GL, Winter DC. Systematic review of preoperative, intraoperative and postoperative risk factors for colorectal anastomotic leaks. Br J Surg. 2015;102:462-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 581] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 91. | Matthiessen P, Hallböök O, Rutegård J, Simert G, Sjödahl R. Defunctioning stoma reduces symptomatic anastomotic leakage after low anterior resection of the rectum for cancer: a randomized multicenter trial. Ann Surg. 2007;246:207-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 861] [Cited by in RCA: 793] [Article Influence: 44.1] [Reference Citation Analysis (0)] |
| 92. | Wong NY, Eu KW. A defunctioning ileostomy does not prevent clinical anastomotic leak after a low anterior resection: a prospective, comparative study. Dis Colon Rectum. 2005;48:2076-2079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 176] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 93. | Mussetto A, Arena R, Buzzi A, Fuccio L, Dari S, Brancaccio ML, Triossi O. Long-term efficacy of vacuum-assisted therapy (Endo-SPONGE(®)) in large anastomotic leakages following anterior rectal resection. Ann Gastroenterol. 2017;30:649-653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 94. | van Koperen PJ, van Berge Henegouwen MI, Rosman C, Bakker CM, Heres P, Slors JF, Bemelman WA. The Dutch multicenter experience of the endo-sponge treatment for anastomotic leakage after colorectal surgery. Surg Endosc. 2009;23:1379-1383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 116] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 95. | Kantsevoy SV, Thuluvath PJ. Successful closure of a chronic refractory gastrocutaneous fistula with a new endoscopic suturing device (with video). Gastrointest Endosc. 2012;75:688-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 96. | Ge PS, Thompson CC. The Use of the Overstitch to Close Perforations and Fistulas. Gastrointest Endosc Clin N Am. 2020;30:147-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 97. | Radziunas G, Dulskas A, Aliosin O, Lunevicius R, Samalavicius NE. The over-the-scope clipping system for treatment of chronic coloenteric fistula: a case report. World J Surg Oncol. 2015;13:219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 98. | Acar T, Aslan F, Acar N, Kamer E, Ünsal B, Hacıyanlı M. Role of endoscopic interventions and electroincision in benign anastomotic strictures following colorectal surgery. Turk J Gastroenterol. 2019;30:673-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 99. | Lew RJ, Kochman ML. A review of endoscopic methods of esophageal dilation. J Clin Gastroenterol. 2002;35:117-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 170] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 100. | Hargrove WC 3rd, Gertner MH, Fitts WT Jr. The Kraske operation for carcinoma of the rectum. Surg Gynecol Obstet. 1979;148:931-933. [PubMed] |
| 101. | Thompson BW, Tucker WE. Transsphincteric approach to lesions of the rectum. South Med J. 1987;80:41-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 102. | Verseveld M, de Graaf EJ, Verhoef C, van Meerten E, Punt CJ, de Hingh IH, Nagtegaal ID, Nuyttens JJ, Marijnen CA, de Wilt JH; CARTS Study Group. Chemoradiation therapy for rectal cancer in the distal rectum followed by organ-sparing transanal endoscopic microsurgery (CARTS study). Br J Surg. 2015;102:853-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 138] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 103. | Garcia-Aguilar J, Renfro LA, Chow OS, Shi Q, Carrero XW, Lynn PB, Thomas CR Jr, Chan E, Cataldo PA, Marcet JE, Medich DS, Johnson CS, Oommen SC, Wolff BG, Pigazzi A, McNevin SM, Pons RK, Bleday R. Organ preservation for clinical T2N0 distal rectal cancer using neoadjuvant chemoradiotherapy and local excision (ACOSOG Z6041): results of an open-label, single-arm, multi-institutional, phase 2 trial. Lancet Oncol. 2015;16:1537-1546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 249] [Cited by in RCA: 293] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 104. | Rullier E, Vendrely V, Asselineau J, Rouanet P, Tuech JJ, Valverde A, de Chaisemartin C, Rivoire M, Trilling B, Jafari M, Portier G, Meunier B, Sieleznieff I, Bertrand M, Marchal F, Dubois A, Pocard M, Rullier A, Smith D, Frulio N, Frison E, Denost Q. Organ preservation with chemoradiotherapy plus local excision for rectal cancer: 5-year results of the GRECCAR 2 randomised trial. Lancet Gastroenterol Hepatol. 2020;5:465-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 167] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 105. | Bach SP; STAR-TREC Collaborative. Can we Save the rectum by watchful waiting or TransAnal surgery following (chemo)Radiotherapy versus Total mesorectal excision for early REctal Cancer (STAR-TREC)? Protocol for the international, multicentre, rolling phase II/III partially randomized patient preference trial evaluating long-course concurrent chemoradiotherapy versus short-course radiotherapy organ preservation approaches. Colorectal Dis. 2022;24:639-651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 42] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 106. | You YN, Hardiman KM, Bafford A, Poylin V, Francone TD, Davis K, Paquette IM, Steele SR, Feingold DL; On Behalf of the Clinical Practice Guidelines Committee of the American Society of Colon and Rectal Surgeons. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Management of Rectal Cancer. Dis Colon Rectum. 2020;63:1191-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 216] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 107. | Golda T, Lazzara C, Sorribas M, Soriano A, Frago R, Alrasheed A, Kreisler E, Biondo S. Combined endoscopic-laparoscopic surgery (CELS) can avoid segmental colectomy in endoscopically unremovable colonic polyps: a cohort study over 10 years. Surg Endosc. 2022;36:196-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 108. | Jayaram A, Barr N, Plummer R, Yao M, Chen L, Yoo J. Combined endo-laparoscopic surgery (CELS) for benign colon polyps: a single institution cost analysis. Surg Endosc. 2019;33:3238-3242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 109. | Lee MK, Chen F, Esrailian E, Russell MM, Sack J, Lin AY, Yoo J. Combined endoscopic and laparoscopic surgery may be an alternative to bowel resection for the management of colon polyps not removable by standard colonoscopy. Surg Endosc. 2013;27:2082-2086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 110. | Hanevelt J, Huisman JF, Leicher LW, Lacle MM, Richir MC, Didden P, Geesing JMJ, Smakman N, Droste JSTS, Ter Borg F, Talsma AK, Schrauwen RWM, van Wely BJ, Schot I, Vermaas M, Bos P, Sietses C, Hazen WL, Wasowicz DK, Ploeg DE, Ramsoekh D, Tuynman JB, Alderlieste YA, Renger RJ, Schreuder RM, Bloemen JG, van Lijnschoten I, Consten ECJ, Sikkenk DJ, Schwartz MP, Vos A, Burger JPW, Spanier BWM, Knijn N, de Vos Tot Nederveen Cappel WH, Moons LMG, van Westreenen HL. Limited wedge resection for T1 colon cancer (LIMERIC-II trial) - rationale and study protocol of a prospective multicenter clinical trial. BMC Gastroenterol. 2023;23:214. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 111. | Jang JY. The Past, Present, and Future of Image-Enhanced Endoscopy. Clin Endosc. 2015;48:466-475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 112. | Buchner AM. The Role of Chromoendoscopy in Evaluating Colorectal Dysplasia. Gastroenterol Hepatol (N Y). 2017;13:336-347. [PubMed] |
| 113. | Kiesslich R, Fritsch J, Holtmann M, Koehler HH, Stolte M, Kanzler S, Nafe B, Jung M, Galle PR, Neurath MF. Methylene blue-aided chromoendoscopy for the detection of intraepithelial neoplasia and colon cancer in ulcerative colitis. Gastroenterology. 2003;124:880-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 616] [Cited by in RCA: 557] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 114. | Repici A, Wallace MB, East JE, Sharma P, Ramirez FC, Bruining DH, Young M, Gatof D, Irene Mimi Canto M, Marcon N, Cannizzaro R, Kiesslich R, Rutter M, Dekker E, Siersema PD, Spaander M, Kupcinskas L, Jonaitis L, Bisschops R, Radaelli F, Bhandari P, Wilson A, Early D, Gupta N, Vieth M, Lauwers GY, Rossini M, Hassan C. Efficacy of Per-oral Methylene Blue Formulation for Screening Colonoscopy. Gastroenterology. 2019;156:2198-2207.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 115. | McGill SK, Evangelou E, Ioannidis JP, Soetikno RM, Kaltenbach T. Narrow band imaging to differentiate neoplastic and non-neoplastic colorectal polyps in real time: a meta-analysis of diagnostic operating characteristics. Gut. 2013;62:1704-1713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 127] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 116. | Hewett DG, Kaltenbach T, Sano Y, Tanaka S, Saunders BP, Ponchon T, Soetikno R, Rex DK. Validation of a simple classification system for endoscopic diagnosis of small colorectal polyps using narrow-band imaging. Gastroenterology. 2012;143:599-607.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 414] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 117. | Yoshida N, Hisabe T, Inada Y, Kugai M, Yagi N, Hirai F, Yao K, Matsui T, Iwashita A, Kato M, Yanagisawa A, Naito Y. The ability of a novel blue laser imaging system for the diagnosis of invasion depth of colorectal neoplasms. J Gastroenterol. 2014;49:73-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 118. | Yoshida N, Yagi N, Inada Y, Kugai M, Okayama T, Kamada K, Katada K, Uchiyama K, Ishikawa T, Handa O, Takagi T, Konishi H, Kokura S, Yanagisawa A, Naito Y. Ability of a novel blue laser imaging system for the diagnosis of colorectal polyps. Dig Endosc. 2014;26:250-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 119. | Ito R, Ikematsu H, Murano T, Shinmura K, Kojima M, Kumahara K, Furue Y, Sunakawa H, Minamide T, Sato D, Yamamoto Y, Takashima K, Yoda Y, Hori K, Yano T. Diagnostic ability of Japan Narrow-Band Imaging Expert Team classification for colorectal lesions by magnifying endoscopy with blue laser imaging versus narrow-band imaging. Endosc Int Open. 2021;9:E271-E277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 120. | Zhao ZY, Guan YG, Li BR, Shan YQ, Yan FH, Gao YJ, Wang H, Lou Z, Fu CG, Yu ED. Detection and miss rates of autofluorescence imaging of adenomatous and polypoid lesions during colonoscopy: a systematic review and meta-analysis. Endosc Int Open. 2015;3:E226-E235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 121. | Moriichi K, Fujiya M, Sato R, Watari J, Nomura Y, Nata T, Ueno N, Maeda S, Kashima S, Itabashi K, Ishikawa C, Inaba Y, Ito T, Okamoto K, Tanabe H, Mizukami Y, Saitoh Y, Kohgo Y. Back-to-back comparison of auto-fluorescence imaging (AFI) versus high resolution white light colonoscopy for adenoma detection. BMC Gastroenterol. 2012;12:75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 122. | Takeuchi Y, Sawaya M, Oka S, Tamai N, Kawamura T, Uraoka T, Ikematsu H, Moriyama T, Arao M, Ishikawa H, Ito Y, Matsuda T. Efficacy of autofluorescence imaging for flat neoplasm detection: a multicenter randomized controlled trial (A-FLAT trial). Gastrointest Endosc. 2019;89:460-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 123. | Kuiper T, van den Broek FJ, Naber AH, van Soest EJ, Scholten P, Mallant-Hent RCh, van den Brande J, Jansen JM, van Oijen AH, Marsman WA, Bergman JJ, Fockens P, Dekker E. Endoscopic trimodal imaging detects colonic neoplasia as well as standard video endoscopy. Gastroenterology. 2011;140:1887-1894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 124. | Longcroft-Wheaton GR, Higgins B, Bhandari P. Flexible spectral imaging color enhancement and indigo carmine in neoplasia diagnosis during colonoscopy: a large prospective UK series. Eur J Gastroenterol Hepatol. 2011;23:903-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 125. | Pham NB, Vu KT, Nguyen NH, Doan HT, Tran TT. Magnifying Chromoendoscopy with Flexible Spectral Imaging Color Enhancement, Indigo Carmine, and Crystal Violet in Predicting the Histopathology of Colorectal Polyps: Diagnostic Value in a Scare-Setting Resource. Gastroenterol Res Pract. 2022;2022:6402904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 126. | dos Santos CE, Lima JC, Lopes CV, Malaman D, Salomão AD, Garcia AC, Teixeira CR. Computerized virtual chromoendoscopy versus indigo carmine chromoendoscopy combined with magnification for diagnosis of small colorectal lesions: a randomized and prospective study. Eur J Gastroenterol Hepatol. 2010;22:1364-1371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 127. | Yoshida N, Naito Y, Murakami T, Hirose R, Ogiso K, Inada Y, Dohi O, Kamada K, Uchiyama K, Handa O, Konishi H, Siah KTH, Yagi N, Fujita Y, Kishimoto M, Yanagisawa A, Itoh Y. Linked color imaging improves the visibility of colorectal polyps: a video study. Endosc Int Open. 2017;5:E518-E525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 128. | Yoshida N, Hisabe T, Ikematsu H, Ishihara H, Terasawa M, Inaba A, Sato D, Cho H, Ego M, Tanaka Y, Yasuda R, Inoue K, Murakami T, Inada Y, Itoh Y, Saito Y. Comparison Between Linked Color Imaging and Blue Laser Imaging for Improving the Visibility of Flat Colorectal Polyps: A Multicenter Pilot Study. Dig Dis Sci. 2020;65:2054-2062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 129. | Suzuki S, Aniwan S, Chiu HM, Laohavichitra K, Chirapongsathorn S, Yamamura T, Kuo CY, Yoshida N, Ang TL, Takezawa T, Rerknimitr R, Ishikawa H, Gotoda T; ATLAS Trial Group. Linked-Color Imaging Detects More Colorectal Adenoma and Serrated Lesions: An International Randomized Controlled Trial. Clin Gastroenterol Hepatol. 2023;21:1493-1502.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 130. | Aziz M, Ahmed Z, Haghbin H, Pervez A, Goyal H, Kamal F, Kobeissy A, Nawras A, Adler DG. Does i-scan improve adenoma detection rate compared to high-definition colonoscopy? A systematic review and meta-analysis. Endosc Int Open. 2022;10:E824-E831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 131. | Kidambi TD, Terdiman JP, El-Nachef N, Singh A, Kattah MG, Lee JK. Effect of I-scan Electronic Chromoendoscopy on Detection of Adenomas During Colonoscopy. Clin Gastroenterol Hepatol. 2019;17:701-708.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 132. | Lee JS, Jeon SW, Kwon YH. Comparative Study of Narrow-Band Imaging and i-scan for Predicting the Histology of Intermediate-to-Large Colorectal Polyps: A Prospective, Randomized Pilot Study. Clin Endosc. 2021;54:881-887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 133. | González-Bernardo O, Riestra S, Vivas S, de Francisco R, Pérez-Martínez I, Castaño-García A, Jiménez-Beltrán V, Rollé V, Suárez P, Suárez A. Chromoendoscopy With Indigo Carmine vs Virtual Chromoendoscopy (iSCAN 1) for Neoplasia Screening in Patients With Inflammatory Bowel Disease: A Prospective Randomized Study. Inflamm Bowel Dis. 2021;27:1256-1262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 134. | Nardone OM, Iacucci M. Image-Enhanced Endoscopy in the Surveillance of Colitis-Associated Neoplasia. Gastrointest Endosc Clin N Am. 2022;32:845-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 135. | Kamitani Y, Nonaka K, Isomoto H. Current Status and Future Perspectives of Artificial Intelligence in Colonoscopy. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 136. | Cooper GS, Xu F, Barnholtz Sloan JS, Koroukian SM, Schluchter MD. Management of malignant colonic polyps: a population-based analysis of colonoscopic polypectomy versus surgery. Cancer. 2012;118:651-659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 137. | Gangireddy VGR, Coleman T, Kanneganti P, Talla S, Annapureddy AR, Amin R, Parikh S. Polypectomy versus surgery in early colon cancer: size and location of colon cancer affect long-term survival. Int J Colorectal Dis. 2018;33:1349-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 138. | Lowe D, Saleem S, Arif MO, Sinha S, Brooks G. Role of Endoscopic Resection Versus Surgical Resection in Management of Malignant Colon Polyps: a National Cancer Database Analysis. J Gastrointest Surg. 2020;24:177-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |