Published online Jun 8, 2024. doi: 10.37126/aige.v5.i2.90723
Revised: April 10, 2024
Accepted: April 19, 2024
Published online: June 8, 2024
Processing time: 150 Days and 15.3 Hours
The sphere of artificial intelligence (AI) is ever expanding. Applications for clinical practice have been emerging over recent years. Although its uptake has been most prominent in endoscopy, this represents only one aspect of holistic patient care. There are a multitude of other potential avenues in which gastro
Core Tip: We reviewed the role of artificial intelligence (AI) in colorectal cancer as a whole. Divided into pre-operative, intra-operative and post-operative, we identified a number of adjuncts that can help the clinician. Endoscopic detection and diagnosis are two of the mainstays of AI in this field, however there are a number of other adjuncts that can help surgeons intraoperatively. These include identification of anatomy, and assessment of blood supply. Moreover, post-operative efficiency improvements with histopathological analysis can aid the clinician. Overall, these adjuncts are aimed at augmenting the clinicians experience to improve outcomes.
- Citation: Lingam G, Shakir T, Kader R, Chand M. Role of artificial intelligence in colorectal cancer. Artif Intell Gastrointest Endosc 2024; 5(2): 90723
- URL: https://www.wjgnet.com/2689-7164/full/v5/i2/90723.htm
- DOI: https://dx.doi.org/10.37126/aige.v5.i2.90723
Colorectal cancer (CRC) is the third most common cancer and the second most common cause of cancer-related deaths worldwide[1,2]. Approximately 21% of CRC cases are diagnosed at stage IV, or with metastatic disease[3]. Of note, the five-year survival rate declines from 80%-90% in patients with local disease compared to 12.5% for those diagnosed with distant-stage disease[4].
Developments in the diagnosis and management of CRC have led to decreases in overall mortality; most notably through screening[5,6]. A recent multi-centre European study of 84585 patients identified the risk of CRC was reduced in screened patients, with a relative risk reduction of 18%[7]. Additionally, further advances have been made regarding novel diagnostic markers and their use for risk stratification and early detection[8].
Surgery is currently the gold standard for deeply invasive CRC, but this remains a management option with significant potential morbidity and mortality[9]. Operative risks include anastomotic leak, ureteral injuries, haemorrhage, injury to surrounding organs, and ileus. The primary treatment for unresectable metastatic disease is systemic therapy; chemotherapy, immunotherapy or biologic therapy[10]. Again, this management option is plagued with potential complications and side effects. These risks need to be ameliorated if patient outcomes are to be improved.
Despite the advent of novel techniques and treatment options, the morbidity and mortality related to CRC remains. Thus, the idiom of “prevention is better than cure” holds true. The digital surgery revolution has enabled a multitude of adjuncts to improve all aspects of surgical care. Artificial intelligence (AI) represents just one of these. AI is defined as the ability of digital computing or a computer-controlled robot to perform tasks commonly associated with intelligent beings[11]. AI first aided in healthcare in the 1950s when physicians first used the technology to improve their diagnoses[12]. This includes but is not limited to: The use of AI in analysing medical imaging, supporting clinician decision making by analysing large quantities of data, tailoring personalised treatment plans and remote monitoring of patient data[13-15]. Since then, several studies have demonstrated its use within the healthcare setting; with many studies reporting AI performance to be comparable, if not exceeding humans[16].
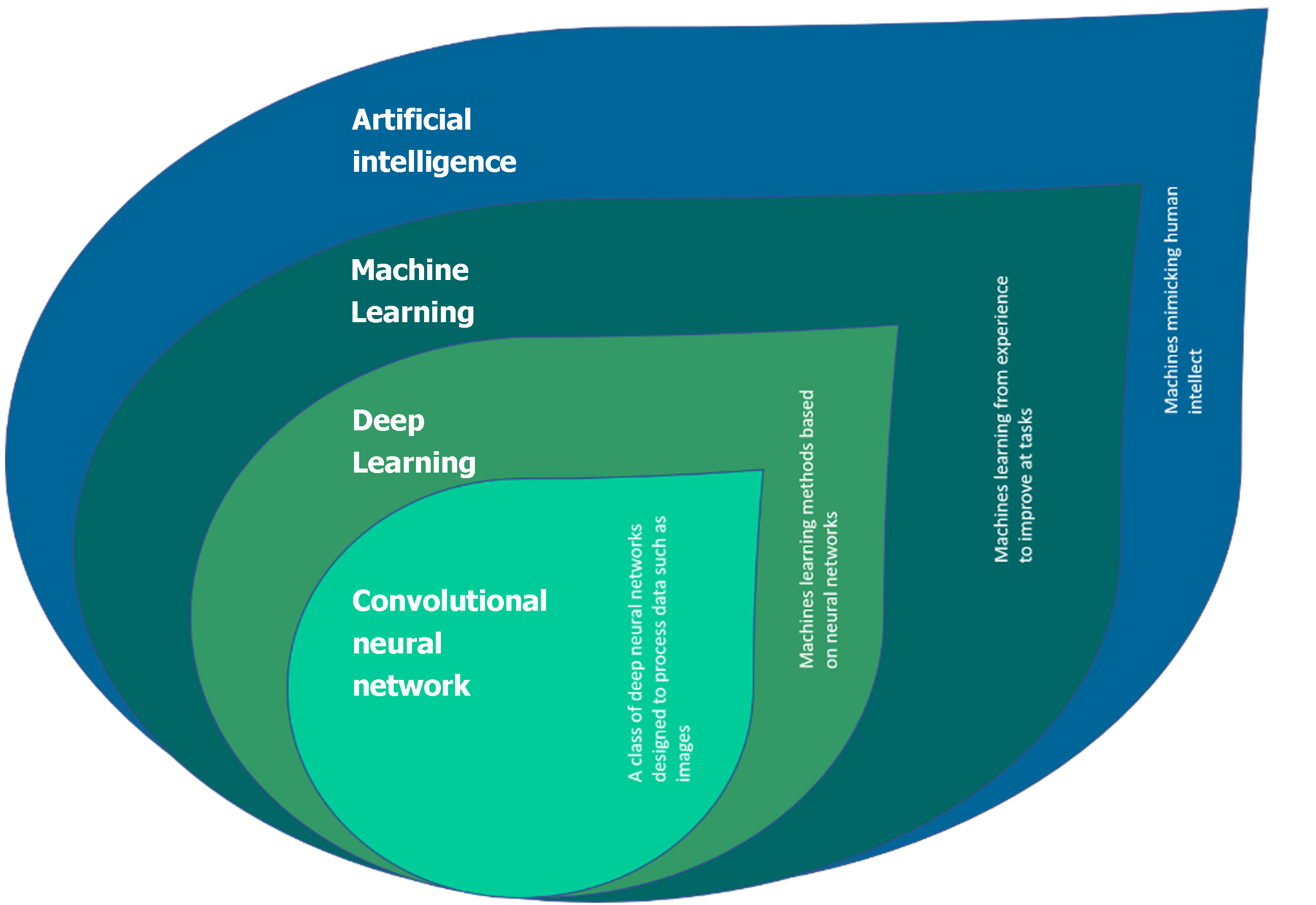
AI is not a singular component but in fact a collection of technologies (Figure 1). Not all forms of AI are relevant to healthcare. The most salient forms are outlined below.
Machine learning is one of the most common forms of AI[16]. The technology aims to marry data models, with the system learning from trained examples. Within healthcare the most common application of machine learning is precision medicine[16]. For example, predicting and tailoring treatment regimens based upon bespoke patient characteristics[17]. More complex forms of machine learning involve deep learning or neural network models.
Deep learning is based on models with less assumptions about the raw data; it is therefore more adept at handling complex data[18]. It is composed of a multi-layer neural network architecture which learns representations of data with multiple levels of abstraction, without the need for human-crafted feature extraction[19-21]. An artificial neural network (ANN) is an algorithm that is inspired by human biology. Every ANN contains nodes that communicate with other nodes via connections (analogous to cell bodies connecting via axons)[22,23]. A convolutional neural network (CNN) is a type of neural network that distinguishes itself with superior performance for image or audio data[21]. Early clinical applications in the translation of CNNs include computer-aided detection (CADe) and diagnosis (CADx) of colorectal polyps during colonoscopy, with multiple regulatory approved algorithms available in the United Kingdom, European and Asian market[21].
Deep learning and ANN models are increasingly being used throughout the field of medicine. The most prominent use being the application within radiology for the detection of basic pathology and pre-cancerous lesions[24,25].
Natural language processing (NLP) in its simplest form is teaching a computer how to read[26]. Traditional NLP applies grammatical rules to interpret meaning from text. The main applications of NLP in healthcare includes the understanding of clinical documentation. For example, preparing radiology reports, transcribing patient interactions and analysing clinical notes. NLP in combination with machine learning has even been used to help perform systematic reviews[27]. Perhaps the most well- known NLP system is “Chat GPT4” (Generative Pre-trained Transformer 4). This is a generic AI large language model open to public use, providing human-like conversation. Recently, a research team from Copenhagen built upon this model to create a specialty-specific clinical AI model. They were able to demonstrate that “Gastro GPT”, outperforms generic models in overall utility and completing clinical tasks[28].
Developments in all these aspects of AI may aid with ultimately providing the ability to perform autonomous surgery[29]. Nonetheless, presently AI is being utilised in all aspects of surgical care: Pre-operative, intra-operative and post-operative. This article will review the current applications in the field of colorectal cancer.
A series of broad scoping and focused searches were conducted in Medline (via OVID), CINAHL Complete (via EBSCO), Cochrane Database of Systematic Reviews, and ScienceDirect, Academic Search Index and Directory of Open Access Journals (via EBSCO Discovery Service). The search strategy employed controlled vocabulary subject headings pertinent to each database, and free text keyword searches of title and abstract fields. Synonyms, truncation and spelling variations were used to retrieve records relating to colorectal cancer, colonoscopy, artificial intelligence, imaging overlay, pre- and post-operative care, individualised medicine, and prognoses.
Results were reviewed and records meeting the inclusion criteria were selected for full-text retrieval. Inclusion criteria included: English language articles, randomised controlled trials (RCTs), cohort studies, qualitative research and publications after the year 2000.
The advent of the colonoscopy screening programme in Europe has led to a significant decrease in CRC-related mortality. This is in part due to the earlier detection and resection of neoplastic polyps. Colonoscopy remains the gold standard for both screening and surveillance of CRC[30]. However, detection of polyps during colonoscopy remains user dependent. A meta-analysis of 43 publications and more than 15000 colonoscopies reported a human miss rate of 26% for adenomas, 9% for advanced adenomas and 27% for serrated polyps[31]. The use of CADe systems has provided a promising avenue for improving polyp detection rates. A meta-analysis including 5 RCTs reported a significant improvement of pooled adenoma detection rate (ADR) to 36.6% from 25.2% in the control group (P < 0.01)[32]. Another RCT of over 600 patients concluded similarly that ADR was improved with use of CADe, but also additionally commented that this improvement was seen across both expert and non-expert endoscopists[33].
AI in colonoscopy has progressed significantly, with recent improvements in translational aspects. Initial work was centered on using hand-crafted annotations to automated polyp classification using existing human derived classification. One of the first applications of this was for Kudo pit patterns from as early as 2010. Takemura et al[34] developed a partially automated software application capable of distinguishing polyps based upon colour differences on borders of polyps and mucosa, with a 98.5% accuracy. However, the main limitation to clinical translation was the computational power required and therefore the program’s ability to function in real-time.
The emergence of deep-learning and CNN’s addressed issues relating to computational power and processing time. The Paris classification was modelled using deep learning methods from colonoscopy videos to reach a mean 89% accuracy, surpassing all reported rates from the literature[35]. Similar results were achieved for Narrow-band Imaging International Colorectal Endoscopic (NICE) and Japan Narrow-band Imaging Expert Team classifications, whereby CNN’s were employed. These are similar to ANN, however focus mainly on image processing. Deep metric learning was used to train the system with approximately 4000 polyp images, which were then tested against two experts. With respect to the NICE classification of type I-III lesions, the system was able to produce a mean accuracy level of 94%[36].
However, a real breakthrough may be on the horizon with novel classification methods. AI has the ability to classify polyps histologically (without human derived classification systems), termed CADx[37]. A recent study comparing CADx to expert endoscopists reported a sensitivity for diminutive polyps of 91% vs 42% respectively[38]. Significant improvements were also seen in negative predictive values and overall accuracy indicating the potential to outperform endoscopists.
Multimodal data categorisation has enabled not only surface risk analysis of polyps, but also the incorporation of patient demographic data and colonic location into creating a novel colorectal cancer invasion calculation. This represents significant strides in augmenting real-life practice, as management differs greatly for benign and malignant polyps – namely endoscopic resection versus formal bowel resection. Lu et al[39] trained their system with over 300000 images and benchmarked this against two expert endoscopists. More notably, the system has also been trained and tested using real time video data. Accuracy reached up 93.8%, with the system outperforming 5 out of 7 junior endoscopists. Whilst there have been significant technological advances, reviews have reported limitations[40]. This includes the lack of high quality RCT’s impact upon the clinical usefulness of this data and the failure to improve non-expert’s optical diagnosis performance when supported with CADx. Future work may help to elucidate the aspects which will augment and aid the clinician’s practice.
Despite being the gold standard, colonoscopy is not always possible. As the global population ages, we are faced with increasing demands on healthcare, including the need for non-invasive diagnostic tools. Virtual colonoscopy (VC) or computed tomography colonography (CTC) is a commonly used alternative when colonoscopy is not possible. This is often due to a patient’s poor baseline health but can also be used if colonoscopy fails. Common reasons for failure include: Poor bowel prep, stricturing disease or patient discomfort. AI based polyp detection frameworks have also been proposed for VC. Similarly, with respect to colonoscopy, the most promising areas appear to be in the increased detection of smaller polyps[41,42]. A study evaluating 104 CTC records reported their prospective clinical performance data with a computer aided detection system[41]. This had per-polyp sensitivities of 91.5% for adenomas 10mm or larger, and 82.1% for adenomas 6-9 mm. Common reasons for computer-aided system misses include: Adherent contrast medium, flat adenomas and adenomas adjacent to normal colonic folds[41].
Another less invasive alternative to colonoscopy is colon capsule endoscopy (CCE). This involves a patient swallowing a camera capsule, with the device relaying image and video data back to the clinician. There are several limitations to this diagnostic procedure including poor luminal views, inability to perform therapeutic procedures and the subjectivity of image interpretation. However, a key barrier to its use is the length of time it takes for endoscopists to interpret the images. Some studies have shown that AI can aid in the reduction of reading times for CCE. A study of twenty videos reported that their CADe system reduced expert endoscopist reading time from 12.2 min to 3.1 min in the intervention group, without reducing the pathology detection rate[43].
Lymph node involvement in CRC plays a key role in management pathways and disease-free survival. Currently CRC is typically staged with contrast enhanced computer tomography (CT), with the addition of magnetic resonance imaging (MRI) in those with rectal cancer. In patients with locally advanced tumours, the current recommendation for rectal cancer is neoadjuvant chemoradiotherapy[44]. However due to the poor accuracy of lymph node staging in CRC, neoadjuvant treatment is not favoured[45]. The accuracy of lymph node staging is affected by multiple factors including poor quality images, experience of radiologist and patient related factors. Two published series reported a 70% or less accuracy for diagnosing lymph node metastasis on both CT and MRI[46,47]. With this significant need for improvement in the accuracy of lymph node staging, the hope remains that AI has a role to play.
Radiomics is a method of extracting regions of interest from diagnostic images. This in combination with deep learning algorithms have shown there to be benefit during pre-operative lymph node staging in CRC. A systematic review and meta-analysis aimed to evaluate the diagnostic accuracy of AI models in the detection of lymph node metastasis on pre-operative staging imaging for CRC[48]. They concluded that both radiomics models and deep learning models have the ability to predict lymph node metastasis more accurately than radiologists. This meta-analysis reported with regards to rectal cancer, there was a per-patient area under the receiver operating characteristic curve (AUROC) of 0.917 for deep learning models versus the radiologists who had an AUROC of 0.688. Similar results were seen in colorectal cancer with a per-patient AUROC of 0.727 vs 0.676 for radiomics and radiologists respectively. Another study of 545 patients evaluated pre-operative MRI imaging with Faster Region-based Convolutional Neural Network; an advanced deep learning system[49]. Again, they were able to show AI models are reliable tools for predicting metastatic lymph nodes pre-operatively[49].
The majority of CRC patients will undergo surgery. This involves surgical resection of the cancer-plagued bowel, often with an anastomosis to re-establish continuity. Inadequate formation or poor healing of this anastomosis can lead to a major complication known as an anastomotic leak (AL). AL rates vary depending on several factors but can be as high as 30%[50].
Sufficient blood supply is a key component of tissue healing including anastomotic healing. Traditionally the perfusion of bowel has been measured via human inspection. This may include assessment of bowel discolouration, noting of bleeding edges, identification of palpable vasculature and intestinal peristalsis. This is a highly subjective technique and been proven to be unreliable[51].
The recent advances in visualisation systems have driven the field towards more objective measures which augment the intraoperative views. This includes techniques such as fluorescence imaging and laser speckle contrast imaging (LSCI).
Indocyanine green (ICG) is the most widely used fluorescent dye for intraoperative perfusion assessment[52]. ICG binds to plasma proteins and is then eliminated via the biliary system (with no enterohepatic recirculation). It fluoresces when activated with near infra-red or laser systems[53]. Intraoperatively, ICG is injected with the visual luminescence used to help assess perfusion of the anastomosis. A meta-analysis including over 3000 patients showed the use of ICG was associated with lower anastomotic leakage rate (OR = 0.31), postoperative complications (OR = 0.70) and reoperation rate (OR = 0.334)[54].
ICG has also been widely studied across other surgical domains including lymph node detection, extrahepatic biliary anatomy identification and ureter identification. As aforementioned, ureteric injury during colonic surgery is a significant risk. The incidence of injury is noted to be as high as 1.95%[55]. They are associated with higher mortality, morbidity and increased hospital stay[56]. Traditionally identification of ureters has been performed via visual inspection of peristalsis (Kelly’s Sign). However, this can be affected by several factors including; incorrect plane of dissection, adipose tissue and adhesions. In higher risk cases, it is generally accepted that indwelling ureteral stents can be used. The evidence for stenting decreasing ureteric injury remains insufficient[57]. This has led to rise of fluorescence imaging in intra-operative ureter detection. Injection of ICG via a cystoscopy-guided ureteric catheter allows ICG to bind to the ureteric epithelium[58]. This technique has been proven to be safe and useful in several small studies[55].
Since the introduction of total mesorectal excision (TME) local recurrence rates have fallen dramatically. Globally TME is the accepted gold-standard treatment in those with rectal cancer[59]. Lateral pelvic lymph node dissection (LPLND) refers to the removal of the nodes along the common iliac, internal iliac and obturator arteries. Management of disease within in this area varies worldwide. Generally, Western nations have adopted neo-adjuvant chemotherapy whilst Eastern countries have opted for LPLND without neo-adjuvant therapy[60]. In fact, the Japanese Society for Cancer of the Colon and Rectum guidelines state that even when pre-operative imaging does not detect lateral lymph node metastasis, LPLND should still be performed[61].
ICG fluorescence imaging has been proven to be a useful tool for identifying lymphatic drainage and colorectal resections are no exception. Zhou et al[62] concluded that ICG enhanced imaging is a feasible and convenient to guide LPLND. Additionally, this series of 42 patients showed a significantly lower intraoperative blood loss (55.8 mL vs 108.0 mL, P = 0.003) and a greater number of harvested lateral pelvic lymph nodes (11.5 vs 7.1, P = 0.017)[62].
However, the use of ICG fluorescence imaging is not without its limitations. Overall, it is still considered a subjective tool. Furthermore, it requires the use of a fluorophore which has been known to cause anaphylaxis. This has allowed newer visualisation techniques to break through including LSCI.
‘Speckles’ refer to the pattern created (with dark and bright areas) when an object is illuminated by laser light[63]. LSCI uses this concept to be able to detect tissue blood flow and therefore tissue perfusion via laser scatter. This culminates in a colour heatmap of real-time blood flow. The advantages of LSCI over ICG fluorescence is the lack of fluorophore needed, the ability for repeat use on demand and no delay between contrast injection and perfusion display. The main disadvantage is poor tissue depth perception, with the perfusion visualisation limited to the most superficial 1-2 mm of tissue[64]. A case- control study of 27 patients aimed to validate the use of LSCI in assessing bowel perfusion as well as assessing improvement in patient outcomes with regards to AL rate[65,66]. They reported 0% of patients (n = 27) undergoing LSCI perfusion assessment developed an anastomotic leak, however 18.5% (n = 27) of the patients in the control group did.
Despite being in its relative infancy, LSCI has potential future applications. Integration with AI to provided objective measurements, and analysis of extracellular matrix stiffness[67] provide an insight into development opportunities. However, a lack of high quality RCT’s limit its current clinical application.
CRC cannot be classified as one disease but rather a collection of neoplastic diseases with huge heterogeneity in biology[68]. Therefore, factors such as tumour grade, lymphovascular invasion, and microsatellite status are pertinent in guiding the treatment and therefore prognosis of the disease. AI can be broadly useful in two main domains of pathology: Clinical application and research[69]. AI can support pathologists in quantitative repetitive tasks. An example of this is the detection and quantification of mitotic figures. Mitotic count is a vital histopathological parameter for the prognostication of malignant diseases[70]. However, the quantification is often fraught with discrepancies due to inter and intra-observer bias. A multi-centre study conducted in the United States compared computer-assisted mitotic count analysis to unaided mitotic count analysis[70]. They found that the interobserver consistency for the mitotic count was significantly increased with computer aid (interobserver correlation coefficient 0.92 vs 0.70 in the unaided group)[70].
Additionally, AI can be used as a quality control measure to provide a second opinion for pathologists[69]. A multi-centre study of 740 patients found that in second-opinion cases, discrepancies were identified in 14.1% of patients, of which 4.1% had a resulting change in care[71]. In the research domain of pathology, AI can analyse large complex datasets which can lead to individualised treatment. For example, an American study of over 1000 patients evaluated a multistain deep learning model which was used to determine the Almmunoscore[72]. This model outperformed other clinical, immune and molecular based parameters.
Similarly, to pathology, AI has a significant role to play in the reduction of time spent on routine tasks. The amount of radiological data is growing at a disproportionate rate to the amount of trained-readers[73]. In fact, McDonald et al calculated that the average radiologist must interpret one image every 3-4 seconds in an 8 h workday to keep up with demands.
AI algorithms, in particular deep learning models have shown remarkable progress in image-recognition tasks[74]. Traditionally, radiologists visually assess images using a combination of education and experience, to report their findings. However, this can be subjective and relies on the ability of the observers’ pattern recognition. In contrast, AI is able to utilise vast quantities of data and complex patterns to provide a quantitative assessment[74].
Furthermore, the use of AI models has been shown to increase the accuracy of radiological reports. Jerebko et al[75] designed a system with the aim of increasing the sensitivity and reducing the number of false positive findings with computed tomographic colonography. They reported a 36% reduction in false positive rate and improved sensitivity of 6.9%.
Enhanced recovery after surgery pathways have been shown to reduce the length of hospital stay and post- operative complications in patients undergoing elective colorectal surgery[76]. The protocol encompasses a multi-disciplinary approach with advice for patient management at three key stages: Preoperative, perioperative and post-operative. A key component of the post-operative phase is early mobilisation. Unfortunately, this can often be hindered by simple logistics, such as the need for patient observations in the inpatient setting. The advent of ‘wearables’ has significantly impacted healthcare by enabling remote monitoring of patients. Examples include rings, wristbands, and body suits that can detect a patient’s heart rate, temperature, oxygen saturations, glucose level, motion data and electrocardiography[77]. This will allow patients to be ambulated quicker post operatively, increase the flow of patients through the hospital, and have financial implications. Additionally, data generated from wearables, in combination with machine learning, has been able to predict disease progression in neurological disorder up to 4 times more precisely than traditional methods[74].
AI has the potential to augment all aspects of surgery and CRC: Pre-operatively, intra-operatively and post-operatively (Table 1). Comparable and improved results have been seen compared with human recognition of polyps during colonoscopy. Laser speckle contrast imaging in combination with AI has the potential to be a safe and effective method of assessment and diagnosis. Cancer surveillance can be streamlined and become more accurate with pathological and radiological augmentations. We may not have even realised the tremendous potential of this exponentially evolving field. Exciting times for the future of surgery and colorectal cancer exist, with innumerable benefits for the patient journey.
| Pre-operative | Intra-operative | Post-operative |
| Colonoscopic detection: Human derived classification; Novel computer aided detection/diagnosis | Indocyanine green: Ureteric identification; Perfusion assessment | Surveillance imaging |
| Virtual colonoscopy | Laser speckle contrast imaging | Pathological quantification |
| Capsule endoscopy | Remote patient monitoring | |
| Lymph node metastasis |
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country of origin: United Kingdom
Peer-review report’s classification
Scientific Quality: Grade B
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Sun X, China S-Editor: Liu JH L-Editor: A P-Editor: Cai YX
| 1. | Quero G, Mascagni P, Kolbinger FR, Fiorillo C, De Sio D, Longo F, Schena CA, Laterza V, Rosa F, Menghi R, Papa V, Tondolo V, Cina C, Distler M, Weitz J, Speidel S, Padoy N, Alfieri S. Artificial Intelligence in Colorectal Cancer Surgery: Present and Future Perspectives. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Baidoun F, Elshiwy K, Elkeraie Y, Merjaneh Z, Khoudari G, Sarmini MT, Gad M, Al-Husseini M, Saad A. Colorectal Cancer Epidemiology: Recent Trends and Impact on Outcomes. Curr Drug Targets. 2021;22:998-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 156] [Article Influence: 39.0] [Reference Citation Analysis (2)] |
| 3. | Robinson JR, Newcomb PA, Hardikar S, Cohen SA, Phipps AI. Stage IV colorectal cancer primary site and patterns of distant metastasis. Cancer Epidemiol. 2017;48:92-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 4. | Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1848] [Cited by in RCA: 2073] [Article Influence: 188.5] [Reference Citation Analysis (0)] |
| 5. | Rawla P, Sunkara T, Barsouk A. Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Prz Gastroenterol. 2019;14:89-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 444] [Cited by in RCA: 1068] [Article Influence: 178.0] [Reference Citation Analysis (1)] |
| 6. | Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3058] [Cited by in RCA: 3306] [Article Influence: 413.3] [Reference Citation Analysis (3)] |
| 7. | Bretthauer M, Løberg M, Wieszczy P, Kalager M, Emilsson L, Garborg K, Rupinski M, Dekker E, Spaander M, Bugajski M, Holme Ø, Zauber AG, Pilonis ND, Mroz A, Kuipers EJ, Shi J, Hernán MA, Adami HO, Regula J, Hoff G, Kaminski MF; NordICC Study Group. Effect of Colonoscopy Screening on Risks of Colorectal Cancer and Related Death. N Engl J Med. 2022;387:1547-1556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 407] [Article Influence: 135.7] [Reference Citation Analysis (1)] |
| 8. | Zygulska AL, Pierzchalski P. Novel Diagnostic Biomarkers in Colorectal Cancer. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 135] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 9. | Angelucci GP, Sinibaldi G, Orsaria P, Arcudi C. Morbidity and Mortality after Colorectal Surgery for Cancer. Surg Sci. 2013;4:520-524. [DOI] [Full Text] |
| 10. | Biller LH, Schrag D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA. 2021;325:669-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 1436] [Article Influence: 359.0] [Reference Citation Analysis (0)] |
| 11. | Copeland BJ. "Artificial intelligence". Encyclopedia Britannica, April 10, 2024. Accessed 16 April 2024. Available from: https://www.britannica.com/technology/artificial-intelligence. |
| 12. | Secinaro S, Calandra D, Secinaro A, Muthurangu V, Biancone P. The role of artificial intelligence in healthcare: a structured literature review. BMC Med Inform Decis Mak. 2021;21:125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 243] [Cited by in RCA: 214] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 13. | Putra RH, Doi C, Yoda N, Astuti ER, Sasaki K. Current applications and development of artificial intelligence for digital dental radiography. Dentomaxillofac Radiol. 2022;51:20210197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 73] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 14. | Yang X, Wang Y, Byrne R, Schneider G, Yang S. Concepts of Artificial Intelligence for Computer-Assisted Drug Discovery. Chem Rev. 2019;119:10520-10594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 440] [Article Influence: 73.3] [Reference Citation Analysis (0)] |
| 15. | Burton RJ, Albur M, Eberl M, Cuff SM. Using artificial intelligence to reduce diagnostic workload without compromising detection of urinary tract infections. BMC Med Inform Decis Mak. 2019;19:171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 16. | Davenport T, Kalakota R. The potential for artificial intelligence in healthcare. Future Healthc J. 2019;6:94-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1432] [Cited by in RCA: 1127] [Article Influence: 187.8] [Reference Citation Analysis (0)] |
| 17. | Lee SI, Celik S, Logsdon BA, Lundberg SM, Martins TJ, Oehler VG, Estey EH, Miller CP, Chien S, Dai J, Saxena A, Blau CA, Becker PS. A machine learning approach to integrate big data for precision medicine in acute myeloid leukemia. Nat Commun. 2018;9:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 143] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 18. | Panch T, Szolovits P, Atun R. Artificial intelligence, machine learning and health systems. J Glob Health. 2018;8:020303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 191] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 19. | Chan HP, Samala RK, Hadjiiski LM, Zhou C. Deep Learning in Medical Image Analysis. Adv Exp Med Biol. 2020;1213:3-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 328] [Article Influence: 65.6] [Reference Citation Analysis (0)] |
| 20. | LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521:436-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36149] [Cited by in RCA: 20047] [Article Influence: 2004.7] [Reference Citation Analysis (0)] |
| 21. | Kader R, Hadjinicolaou AV, Georgiades F, Stoyanov D, Lovat LB. Optical diagnosis of colorectal polyps using convolutional neural networks. World J Gastroenterol. 2021;27:5908-5918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (3)] |
| 22. | Ramesh AN, Kambhampati C, Monson JR, Drew PJ. Artificial intelligence in medicine. Ann R Coll Surg Engl. 2004;86:334-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 390] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 23. | Choi RY, Coyner AS, Kalpathy-Cramer, J, Chiang MF. Introduction to machine learning, neural networks, and deep learning. Transl Vision Sci Technol. 2020;9:14. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 257] [Reference Citation Analysis (2)] |
| 24. | Fakoor R, Ladhak F, Nazi A, Huber M. Using deep learning to enhance cancer diagnosis and classification. ICML. 2013;28:3937-3949. |
| 25. | McBee MP, Awan OA, Colucci AT, Ghobadi CW, Kadom N, Kansagra AP, Tridandapani S, Auffermann WF. Deep Learning in Radiology. Acad Radiol. 2018;25:1472-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 257] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 26. | Van Vleck TT, Farrell D, Chan L. Natural Language Processing in Nephrology. Adv Chronic Kidney Dis. 2022;29:465-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 27. | Spinelli A, Carrano FM, Laino ME, Andreozzi M, Koleth G, Hassan C, Repici A, Chand M, Savevski V, Pellino G. Artificial intelligence in colorectal surgery: an AI-powered systematic review. Tech Coloproctol. 2023;27:615-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 28. | GastroGPT Outperforms General Models in GI Clinical Tasks. Medscape. [Online] October 19, 2023. [Cited: December 1, 2023]. Available from: https://www.medscape.com/viewarticle/997542?form=fpf. |
| 29. | Gumbs AA, Frigerio I, Spolverato G, Croner R, Illanes A, Chouillard E, Elyan E. Artificial Intelligence Surgery: How Do We Get to Autonomous Actions in Surgery? Sensors (Basel). 2021;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 30. | Allescher HD, Weingart V. Optimizing Screening Colonoscopy: Strategies and Alternatives. Visc Med. 2019;35:215-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Zhao S, Wang S, Pan P, Xia T, Chang X, Yang X, Guo L, Meng Q, Yang F, Qian W, Xu Z, Wang Y, Wang Z, Gu L, Wang R, Jia F, Yao J, Li Z, Bai Y. Magnitude, Risk Factors, and Factors Associated With Adenoma Miss Rate of Tandem Colonoscopy: A Systematic Review and Meta-analysis. Gastroenterology. 2019;156:1661-1674.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 375] [Article Influence: 62.5] [Reference Citation Analysis (0)] |
| 32. | Hassan C, Spadaccini M, Iannone A, Maselli R, Jovani M, Chandrasekar VT, Antonelli G, Yu H, Areia M, Dinis-Ribeiro M, Bhandari P, Sharma P, Rex DK, Rösch T, Wallace M, Repici A. Performance of artificial intelligence in colonoscopy for adenoma and polyp detection: a systematic review and meta-analysis. Gastrointest Endosc. 2021;93:77-85.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 307] [Article Influence: 76.8] [Reference Citation Analysis (1)] |
| 33. | Repici A, Spadaccini M, Antonelli G, Correale L, Maselli R, Galtieri PA, Pellegatta G, Capogreco A, Milluzzo SM, Lollo G, Di Paolo D, Badalamenti M, Ferrara E, Fugazza A, Carrara S, Anderloni A, Rondonotti E, Amato A, De Gottardi A, Spada C, Radaelli F, Savevski V, Wallace MB, Sharma P, Rösch T, Hassan C. Artificial intelligence and colonoscopy experience: lessons from two randomised trials. Gut. 2022;71:757-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 134] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 34. | Takemura Y, Yoshida S, Tanaka S, Onji K, Oka S, Tamaki T, Kaneda K, Yoshihara M, Chayama K. Quantitative analysis and development of a computer-aided system for identification of regular pit patterns of colorectal lesions. Gastrointest Endosc. 2010;72:1047-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 35. | Krenzer A, Heil S, Fitting D, Matti S, Zoller WG, Hann A, Puppe F. Automated classification of polyps using deep learning architectures and few-shot learning. BMC Med Imaging. 2023;23:59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 36. | Okamoto Y, Yoshida S, Izakura S, Katayama D, Michida R, Koide T, Tamaki T, Kamigaichi Y, Tamari H, Shimohara Y, Nishimura T, Inagaki K, Tanaka H, Yamashita K, Sumimoto K, Oka S, Tanaka S. Development of multi-class computer-aided diagnostic systems using the NICE/JNET classifications for colorectal lesions. J Gastroenterol Hepatol. 2022;37:104-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 37. | Hassan C, Balsamo G, Lorenzetti R, Zullo A, Antonelli G. Artificial Intelligence Allows Leaving-In-Situ Colorectal Polyps. Clin Gastroenterol Hepatol. 2022;20:2505-2513.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 61] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 38. | Hossain E, Abdelrahim M, Tanasescu A, Yamada M, Kondo H, Yamada S, Hamamoto R, Marugame A, Saito Y, Bhandari P. Performance of a novel computer-aided diagnosis system in the characterization of colorectal polyps, and its role in meeting Preservation and Incorporation of Valuable Endoscopic Innovations standards set by the American Society of Gastrointestinal Endoscopy. DEN Open. 2023;3:e178. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 39. | Real-time automated diagnosis of colorectal cancer invasion depth using a deep learning model with multimodal data (with video). Lu ZH, Xu YM, Yao LW, Zhou W, Gong W, Yang GH, Guo MW, Guo MW, Zhang BP, Huang X, He CP, Zhou R, Deng YC, Yu HG. Gastrointest Endosc. 2022;95. [RCA] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 40. | Shakir T, Kader R, Bhan C, Chand M. AI in colonoscopy-detection and characterisation of malignant polyps. Art Int Surg. 2023;3:186-194. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 41. | Summers RM, Handwerker LR, Pickhardt PJ, Van Uitert RL, Deshpande KK, Yeshwant S, Yao J, Franaszek M. Performance of a previously validated CT colonography computer-aided detection system in a new patient population. AJR Am J Roentgenol. 2008;191:168-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 42. | Alkabbany I, Ali AM, Mohamed M, Elshazly SM, Farag A. An AI-Based Colonic Polyp Classifier for Colorectal Cancer Screening Using Low-Dose Abdominal CT. Sensors (Basel). 2022;22. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 43. | Aoki T, Yamada A, Aoyama K, Saito H, Fujisawa G, Odawara N, Kondo R, Tsuboi A, Ishibashi R, Nakada A, Niikura R, Fujishiro M, Oka S, Ishihara S, Matsuda T, Nakahori M, Tanaka S, Koike K, Tada T. Clinical usefulness of a deep learning-based system as the first screening on small-bowel capsule endoscopy reading. Dig Endosc. 2020;32:585-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 75] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 44. | Arnold CR, Mangesius J, Jager R, Ganswindt U. Neoadjuvant chemoradiotherapy in rectal cancer. Mag Eur Med Onc. 2020;13:329-333. [DOI] [Full Text] |
| 45. | Benson AB, Venook AP, Al-Hawary MM, Cederquist L, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Engstrom PF, Garrido-Laguna I, Grem JL, Grothey A, Hochster HS, Hoffe S, Hunt S, Kamel A, Kirilcuk N, Krishnamurthi S, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Murphy JD, Nurkin S, Saltz L, Sharma S, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Wuthrick E, Gregory KM, Freedman-Cass DA. NCCN Guidelines Insights: Colon Cancer, Version 2.2018. J Natl Compr Canc Netw. 2018;16:359-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 635] [Cited by in RCA: 685] [Article Influence: 97.9] [Reference Citation Analysis (1)] |
| 46. | Iannicelli E, Di Renzo S, Ferri M, Pilozzi E, Di Girolamo M, Sapori A, Ziparo V, David V. Accuracy of high-resolution MRI with lumen distention in rectal cancer staging and circumferential margin involvement prediction. Korean J Radiol. 2014;15:37-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 47. | Fernandez LM, Parlade AJ, Wasser EJ, Dasilva G, de Azevedo RU, Ortega CD, Perez RO, Habr-Gama A, Berho M, Wexner SD. How Reliable Is CT Scan in Staging Right Colon Cancer? Dis Colon Rectum. 2019;62:960-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 48. | Bedrikovetski S, Dudi-Venkata NN, Kroon HM, Seow W, Vather R, Carneiro G, Moore JW, Sammour T. Artificial intelligence for pre-operative lymph node staging in colorectal cancer: a systematic review and meta-analysis. BMC Cancer. 2021;21:1058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 95] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 49. | Ding L, Liu G, Zhang X, Liu S, Li S, Zhang Z, Guo Y, Lu Y. A deep learning nomogram kit for predicting metastatic lymph nodes in rectal cancer. Cancer Med. 2020;9:8809-8820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 50. | Hajjar R, Gonzalez E, Fragoso G, Oliero M, Alaoui AA, Calvé A, Vennin Rendos H, Djediai S, Cuisiniere T, Laplante P, Gerkins C, Ajayi AS, Diop K, Taleb N, Thérien S, Schampaert F, Alratrout H, Dagbert F, Loungnarath R, Sebajang H, Schwenter F, Wassef R, Ratelle R, Debroux E, Cailhier JF, Routy B, Annabi B, Brereton NJB, Richard C, Santos MM. Gut microbiota influence anastomotic healing in colorectal cancer surgery through modulation of mucosal proinflammatory cytokines. Gut. 2023;72:1143-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 51] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 51. | Urbanavičius L, Pattyn P, de Putte DV, Venskutonis D. How to assess intestinal viability during surgery: A review of techniques. World J Gastrointest Surg. 2011;3:59-69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 142] [Cited by in RCA: 159] [Article Influence: 11.4] [Reference Citation Analysis (1)] |
| 52. | Degett TH, Andersen HS, Gögenur I. Indocyanine green fluorescence angiography for intraoperative assessment of gastrointestinal anastomotic perfusion: a systematic review of clinical trials. Langenbecks Arch Surg. 2016;401:767-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 141] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 53. | Morales-Conde S, Licardie E, Alarcón I, Balla A. Indocyanine green (ICG) fluorescence guide for the use and indications in general surgery: recommendations based on the descriptive review of the literature and the analysis of experience. Cir Esp (Engl Ed). 2022;100:534-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 54. | Lin J, Zheng B, Lin S, Chen Z, Chen S. The efficacy of intraoperative ICG fluorescence angiography on anastomotic leak after resection for colorectal cancer: a meta-analysis. Int J Colorectal Dis. 2021;36:27-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 55. | Peltrini R, Podda M, Castiglioni S, Di Nuzzo MM, D'Ambra M, Lionetti R, Sodo M, Luglio G, Mucilli F, Di Saverio S, Bracale U, Corcione F. Intraoperative use of indocyanine green fluorescence imaging in rectal cancer surgery: The state of the art. World J Gastroenterol. 2021;27:6374-6386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 56. | Halabi WJ, Jafari MD, Nguyen VQ, Carmichael JC, Mills S, Pigazzi A, Stamos MJ. Ureteral injuries in colorectal surgery: an analysis of trends, outcomes, and risk factors over a 10-year period in the United States. Dis Colon Rectum. 2014;57:179-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 57. | Croghan SM, Zaborowski A, Mohan HM, Mulvin D, McGuire BB, Murphy M, Galvin DJ, Lennon G, Quinlan D, Winter DC. The sentinel stent? A systematic review of the role of prophylactic ureteric stenting prior to colorectal resections. Int J Colorectal Dis. 2019;34:1161-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 58. | Frangioni JV. In vivo near-infrared fluorescence imaging. Curr Opin Chem Biol. 2003;7:626-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1974] [Cited by in RCA: 1865] [Article Influence: 88.8] [Reference Citation Analysis (0)] |
| 59. | Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet. 1986;1:1479-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1867] [Cited by in RCA: 1914] [Article Influence: 49.1] [Reference Citation Analysis (0)] |
| 60. | Ogura A, van Oostendorp S, Kusters M. Neoadjuvant (chemo)radiotherapy and Lateral Node Dissection: Is It Mutually Exclusive? Clin Colon Rectal Surg. 2020;33:355-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 61. | Hashiguchi Y, Muro K, Saito Y, Ito Y, Ajioka Y, Hamaguchi T, Hasegawa K, Hotta K, Ishida H, Ishiguro M, Ishihara S, Kanemitsu Y, Kinugasa Y, Murofushi K, Nakajima TE, Oka S, Tanaka T, Taniguchi H, Tsuji A, Uehara K, Ueno H, Yamanaka T, Yamazaki K, Yoshida M, Yoshino T, Itabashi M, Sakamaki K, Sano K, Shimada Y, Tanaka S, Uetake H, Yamaguchi S, Yamaguchi N, Kobayashi H, Matsuda K, Kotake K, Sugihara K, Japanese Society for Cancer of the Colon and Rectum. Japanese society for cancer of the colon and rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol. 2020;25:1-42. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1024] [Cited by in RCA: 1311] [Article Influence: 262.2] [Reference Citation Analysis (1)] |
| 62. | Zhou SC, Tian YT, Wang XW, Zhao CD, Ma S, Jiang J, Li EN, Zhou HT, Liu Q, Liang JW, Zhou ZX, Wang XS. Application of indocyanine green-enhanced near-infrared fluorescence-guided imaging in laparoscopic lateral pelvic lymph node dissection for middle-low rectal cancer. World J Gastroenterol. 2019;25:4502-4511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 63] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 63. | Draijer M, Hondebrink E, van Leeuwen T, Steenbergen W. Review of laser speckle contrast techniques for visualizing tissue perfusion. Lasers Med Sci. 2009;24:639-651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 228] [Cited by in RCA: 202] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 64. | Liu YZ, Shah SK, Sanders CM, Nwaiwu CA, Dechert AF, Mehrotra S, Schwaitzberg SD, Kim PCW, Wilson EB. Utility and usability of laser speckle contrast imaging (LSCI) for displaying real-time tissue perfusion/blood flow in robot-assisted surgery (RAS): comparison to indocyanine green (ICG) and use in laparoscopic surgery. Surg Endosc. 2023;37:4803-4811. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 65. | Kojima S, Sakamoto T, Matsui Y, Nambu K, Masamune K. Clinical efficacy of bowel perfusion assessment during laparoscopic colorectal resection using laser speckle contrast imaging: A matched case-control study. Asian J Endosc Surg. 2020;13:329-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 66. | Kojima S, Sakamoto T, Nagai Y, Matsui Y, Nambu K, Masamune K. Laser Speckle Contrast Imaging for Intraoperative Quantitative Assessment of Intestinal Blood Perfusion During Colorectal Surgery: A Prospective Pilot Study. Surg Innov. 2019;26:293-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 67. | Chao PY, Liu WW, You SF, Li PC. Shear Wave Elasticity Measurements of Three-Dimensional Cancer Cell Cultures Using Laser Speckle Contrast Imaging. Sci Rep. 2018;8:14470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 68. | Sagaert X, Vanstapel A, Verbeek S. Tumor Heterogeneity in Colorectal Cancer: What Do We Know So Far? Pathobiology. 2018;85:72-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 113] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 69. | Schüffler P, Steiger K, Weichert W. How to use AI in pathology. Gene Chromosome Canc. 2023;62. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 70. | Bertram CA, Aubreville M, Donovan TA, Bartel A, Wilm F, Marzahl C, Assenmacher CA, Becker K, Bennett M, Corner S, Cossic B, Denk D, Dettwiler M, Gonzalez BG, Gurtner C, Haverkamp AK, Heier A, Lehmbecker A, Merz S, Noland EL, Plog S, Schmidt A, Sebastian F, Sledge DG, Smedley RC, Tecilla M, Thaiwong T, Fuchs-Baumgartinger A, Meuten DJ, Breininger K, Kiupel M, Maier A, Klopfleisch R. Computer-assisted mitotic count using a deep learning-based algorithm improves interobserver reproducibility and accuracy. Vet Pathol. 2022;59:211-226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 71. | Johnson SM, Samulski TD, O'Connor SM, Smith SV, Funkhouser WK, Broaddus RR, Calhoun BC. Clinical and Financial Implications of Second-Opinion Surgical Pathology Review. Am J Clin Pathol. 2021;156:559-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 72. | Foersch S, Glasner C, Woerl AC, Eckstein M, Wagner DC, Schulz S, Kellers F, Fernandez A, Tserea K, Kloth M, Hartmann A, Heintz A, Weichert W, Roth W, Geppert C, Kather JN, Jesinghaus M. Multistain deep learning for prediction of prognosis and therapy response in colorectal cancer. Nat Med. 2023;29:430-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 105] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 73. | McDonald RJ, Schwartz KM, Eckel LJ, Diehn FE, Hunt CH, Bartholmai BJ, Erickson BJ, Kallmes DF. The effects of changes in utilization and technological advancements of cross-sectional imaging on radiologist workload. Acad Radiol. 2015;22:1191-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 278] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 74. | Hosny A, Parmar C, Quackenbush J, Schwartz LH, Aerts HJWL. Artificial intelligence in radiology. Nat Rev Cancer. 2018;18:500-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1552] [Cited by in RCA: 1852] [Article Influence: 264.6] [Reference Citation Analysis (2)] |
| 75. | Jerebko AK, Malley JD, Franaszek M, Summers RM. Multiple neural network classification scheme for detection of colonic polyps in CT colonography data sets. Acad Radiol. 2003;10:154-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 73] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 76. | Cavallaro P, Bordeianou L. Implementation of an ERAS Pathway in Colorectal Surgery. Clin Colon Rectal Surg. 2019;32:102-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 103] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 77. | Dunn J, Runge R, Snyder M. Wearables and the medical revolution. Per Med. 2018;15:429-448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 474] [Cited by in RCA: 271] [Article Influence: 38.7] [Reference Citation Analysis (0)] |