Published online Feb 28, 2022. doi: 10.37126/aige.v3.i1.1
Peer-review started: December 27, 2021
First decision: January 26, 2022
Revised: February 14, 2022
Accepted: February 24, 2022
Article in press: February 24, 2022
Published online: February 28, 2022
Processing time: 59 Days and 14.4 Hours
Artificial intelligence (AI) is a branch of computer science that develops intelligent machines. In recent years, medicine has been contemplated with this recent modality to aid in the diagnosis of diseases in several specialties, including gastroenterology and gastrointestinal endoscopy. This new technology has superior ability to perform tasks mimicking human behavior and can identify possible pathological alterations, such as pre-malignant lesions and dysplasia, precursor lesions of colorectal cancer (CRC), and support medical decision-making. CRC is among the three most prevalent cancer types, and the second most common cause of cancer-related deaths worldwide; in addition, it is a leading cause of death in patients with inflammatory bowel disease (IBD). Patients with IBD tend to have greater inflammatory cell activity in the intestinal mucosa, which can favor cell proliferation and CRC development. AI can contribute to the detection of pre-neoplastic lesions in patients at risk of CRC development, such as those with extensive IBD or when additional CRC risk factors, such as smoking, are present. In fact, AI systems could improve all aspects of care related to both the detection of pre-malignant and malignant lesions and the screening of patients with IBD. In this review, we aimed to show the benefits and innovations of AI in the screening of CRC in patients with IBD. The promising applications of AI have the potential to revolutionize clinical practice and gastrointestinal endoscopy, especially in at-risk patients, such as those with IBD.
Core Tip: Artificial intelligence (AI) is a promising technology in various areas of medicine. Recently, AI-assisted endoscopy has emerged with rapid dissemination and has favored the identification of complications in patients with inflammatory bowel disease (IBD), such as colorectal cancer (CRC). In this review, we discuss the benefits and innovations of AI for CRC screening in patients with IBD. The promising applications of AI have the potential to revolutionize clinical practice and gastrointestinal endoscopy.
- Citation: Marques KF, Marques AF, Lopes MA, Beraldo RF, Lima TB, Sassaki LY. Artificial intelligence in colorectal cancer screening in patients with inflammatory bowel disease. Artif Intell Gastrointest Endosc 2022; 3(1): 1-8
- URL: https://www.wjgnet.com/2689-7164/full/v3/i1/1.htm
- DOI: https://dx.doi.org/10.37126/aige.v3.i1.1
Artificial intelligence (AI) is a branch of computer science that seeks to develop programmed machines to perform tasks that mimic rational human behavior through algorithms. AI can help in the prevention, diagnosis, and treatment of many diseases[1] and can be applied to diverse medical specialties, such as radiology, pathology, ophthalmology, dermatology, gastroenterology and gastrointestinal endoscopy[2].
Inflammatory bowel disease (IBD) is an immune-mediated condition encompassing Crohn's disease, ulcerative colitis, and indeterminate colitis and can lead to the development of complications compromising the patients’ quality of life[3]. Colorectal cancer (CRC) is one of the leading causes of death in patients with IBD, with a mortality rate of 10%-15%[4]. Patients with IBD tend to have greater action of inflammatory cells in the intestinal mucosa, favoring cell proliferation and CRC development[5]. In this scenario, AI can contribute to the detection of pre-neoplastic lesions in patients at risk of CRC development, such as those with extensive disease and in the presence of other CRC risk factors, such as smoking. Given the growing importance and application of AI in gastroenterology and gastrointestinal endoscopy, the aim of the present study was to review the role of AI in IBD, particularly in CRC screening in these patients.
An electronic search of the literature was performed using MEDLINE (PubMed) from 2010 to December 2021. Only articles published in English language were included. Keywords used in the search were artificial intelligence, inflammatory bowel disease, ulcerative colitis, Crohn's disease, colorectal cancer.
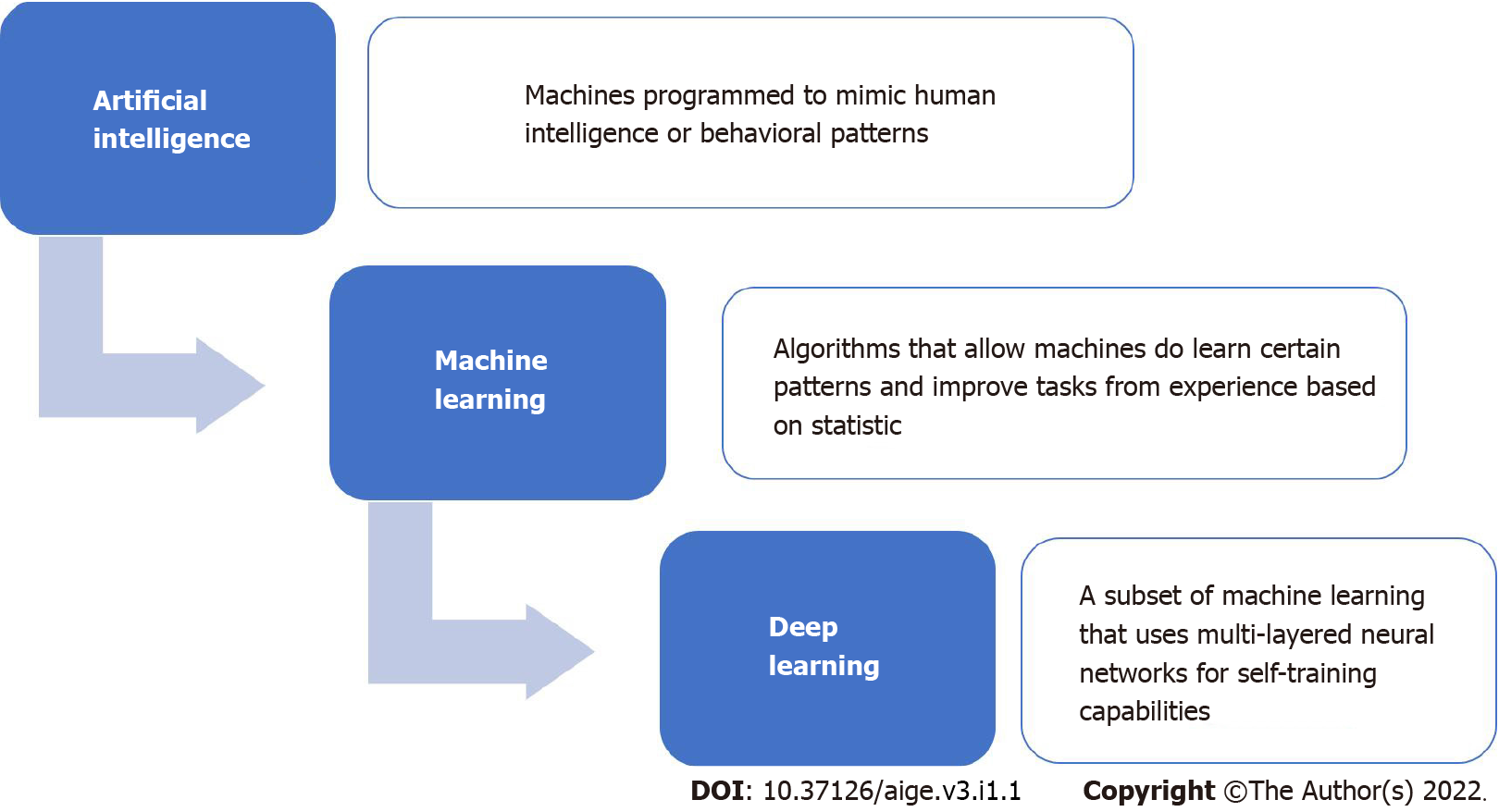
AI is the ability that allows machines to imitate intelligent human behavior[6]. “Machine learning” is an aspect of AI in which computer algorithms apply statistical learning models based on data imputation[7]. For example, supervised classification algorithms can recognize the presence or absence of polyps during colonoscopy. The concept of “deep learning” has recently emerged in machine learning, in which a deep neural network is used, inspired by the brain of mammals, presenting several layers of interconnected artificial neurons[8]. First-layer neurons transmit data and reference values to the next neuron layer, forming a complex algorithmic network. This process can mimic, for example, the visual cortex receiving pre-synaptic signals from the retina, and the mapping of parts of an image and extraction of their characteristics, which allows for real-time classification of images, along with detection and characterization of lesions in endoscopic procedures. Studies have already shown the benefits of this technology in colonoscopy, such as reducing the time to remove the device, improving the ability to predict histological diagnosis during the examination, and reducing the time needed to establish the diagnosis of the lesion[9]. A brief schematic describing AI, machine learning, and deep learning is shown in Figure 1.
In the field of gastrointestinal endoscopy, AI technology contributes to the diagnosis and treatment of different types of intestinal lesions, from benign polyps to CRC[10]. One of the features of AI in endoscopic examinations is the identification and characterization of gastrointestinal polyps, which can detect and grade dysplasia. Currently, computer-aided diagnosis (CADe) technology is available in some endoscopy centers and has been gaining popularity in the scientific community. The CADe system assesses four pillars of quality in endoscopic examinations: Visible surface area on the monitor, colon distension (allowing greater surface visibility), conditions of preparation, and clarity of the current vision. Through this assessment, it generates scores that can be compared with those of specialist endoscopists[11].
Using the CADe system, it is possible to identify and automatically distinguish benign and malignant polyps that are transmitted on the monitor, which can be visually underestimated by the endoscopist, resulting in a higher rate of detection and characterization of the adenoma in question. Another advantage of in AI examinations is the reduction of unnecessary polypectomies of non-neoplastic polyps[11]. This is possible through the so-called optical biopsy, which allows the visualization and histological verification of the polyp in real time, preventing biopsies of low-risk hyperplastic lesions and reducing costs with histological examinations and complications related to the procedure. However, despite the existence of this new approach, some health professionals and patients have been against not forwarding the material for histological analysis[12].
Currently, it is known that several AI algorithms have been developed to work in real time during colonoscopy, alerting the endoscopist about the presence of polyps through the emission of sound or visual signals. Karnes et al[13] developed an adenoma detection model using images from 8641 colonoscopies. To improve the efficiency of image classification, convolutional neural networks have been developed (deep learning). Its accuracy has reached 96.4% at a maximum rate of 170 images per second. Through AI, the endoscopist aids in the detection of polyps, serving as a second pair of eyes, more sophisticated, and with greater sensitivity through the use of high-precision machines[14]. Repici et al[15] performed a multicenter randomized trial using the CADe system. In this study, the adenoma detection rate in the CADe group was higher than that in the control group (54.8% vs 40.4%; P < 0.001).
Ishiyama et al[16] comments on the challenges encountered when using CADe. This method is known to be more effective in detecting lesions in the right colon because the distal part of the colon, especially the sigmoid colon, may have some blind spots, reducing the efficiency of the CADe system. The sigmoid colon is not fixed; instead, it presents sharp angulation points, such as the sigmoid-descending junction, which result in blind spots that increase the risk of missing lesions, especially small polyps. On the other hand, the transverse and descending colon have more superficial folds, allowing lesions to be more easily detected. To improve the effectiveness of CADe, techniques such as cap-assisted colonoscopy and ultra-wide vision colonoscopy are needed to enhance the visualization of the aforementioned mucosal areas.
Another study evaluated the use of a commercially available AI system (GI-Genius; Medtronic)[17]. High-definition white light colonoscopies of 840 patients were analyzed and 2684 histologically proven polyps were detected. In total, 1.5 million video images of the polyps were manually recorded from different angles, and the ability of the AI to virtually identify these lesions was assessed. In most cases, the AI reaction time in polyp detection was faster than that of the endoscopists, anticipating the diagnosis of the lesion[17].
AI can also be applied in the exams of video capsule endoscopy to facilitate visualization of lesions, reducing not only examination time but also labor and allowing for a thorough review of these images, improving the detection of neoplastic lesions and reducing human error[7].
The advantages of using AI compared to traditional endoscopy are mainly related to the reduction of costs and risks inherent to the endoscopic procedure, such as unnecessary polypectomies and histological analyses of lesions that lack potential for malignancy, in addition to shorter examination times. Furthermore, several studies have already demonstrated the benefits of using AI in all fields of digestive endoscopy. In the esophagus, AI can be applied in the diagnosis of Barrett's esophagus and in the diagnosis, prognosis, and evaluation of response to treatment of esophageal tumors[18]. In the stomach, AI can help in the detection of gastric cancer, as well as in the prognosis of patients undergoing chemotherapy[18]. In the lower gastrointestinal tract, its main indication has been in the detection of pre-neoplastic lesions and, more recently, in IBD[18].
The application of AI has been gaining strong influence in the field of IBD in recent years. Indeed, AI has been used to assess the genomic environment, build predictive models for the risk of developing IBD, and increase the accuracy of disease diagnosis[19]. Also, with this new technology it is possible to analyze endoscopic images and identify patterns of disease severity, allowing a better classification compared to disease severity assessed purely by endoscopy[19].
Maeda et al[20] reported a patient with IBD who benefited from the use of high-definition endoscopy devices with AI. Two dysplastic lesions were demarcated in the sigmoid colon, and further histological analysis confirmed the presence of atypical tubular glands with low-grade dysplasia[20].
Endocytoscopy is a new high-magnification endoscopic method designed for improved vivo assessment of lesions found in the gastrointestinal tract[21]. Maeda et al[20] applied endocytoscopy to analyze histological inflammation in patients with ulcerative colitis. With this tool, an AI model was developed to recognize persistent histological inflammation with a specificity of 97% and sensitivity of 74%[20]. Mossotto et al[22] presented a model that used histological and endoscopic data to differentiate pediatric IBD between ulcerative colitis and Crohn's disease. The accuracy of this method was 82.7%, and the presence of ileal disease was the most important factor in the classification of the disease[22].
In the future, the application of AI could revolutionize the entire management of patients with IBD, from predicting the risk of developing the disease to choosing the best therapeutic strategy for each patient. AI can help to create prediction models of disease development risk, based on data such as the presence of genetic and environmental risk factors, as well as characteristics of the intestinal microbiota and the immune response of each individual. Regarding the diagnosis of IBD, AI can assist with algorithms based on the presence of genetic mutations, presence of signs and symptoms, results of biochemical and serological exams, fecal biomarkers, endoscopic and histological characteristics, and presence of changes in radiological exams, in addition to facilitating the differentiation between ulcerative colitis and Crohn's disease. With regard to treatment, the application of AI can help in choosing the best therapeutic strategy for each disease phenotype. In addition, it can help in deciding the most suitable drug for each patient based on the severity and extent of their disease, presence of disease complications, presence of poor prognosis risk factors, and taking into consideration the drugs’ mechanism of action together with the inflammatory and genetic profile of each patient.
Colorectal cancer (CRC) stands out for being among the three most prevalent cancers and the second most common cause of cancer deaths worldwide[23]. Examinations such as colonoscopy are able to detect and remove pre-neoplastic lesions and, thus, prevent the development of CRC in some patients[9]. Detection of adenomas during colonoscopy is dependent on the examining endoscopist, with studies reporting a variation of 7%-53% among different physicians[9]. The marked difference in this rate has been attributed to the endoscopist's previous experience, the resection technique used and the adequate surveillance of suspicious lesions [24]. Failure to detect neoplastic lesions can be associated with the development of CRC in the interval between two colonoscopies[9]. AI has emerged in the field of gastrointestinal endoscopy to increase the detection rates of pre-neoplastic lesions.
In sporadic CRC, tumor progression begins with the mutation of antigen-presenting cells (APC) and the accumulation of β-catenin to induce hyperplastic epithelium, followed by K-ras mutation and adenoma formation, and finally culminating in CRC with the p53 gene mutation[25]. The pathogenesis of CRC in IBD has not been fully elucidated, but intestinal inflammation associated with the presence of cytokines and free radicals is believed to be a conducive environment for the development of low- and high-grade dysplasia and, consequently, cancer[25]. Despite having genetic alterations similar to sporadic CRC, neoplastic lesions seem to occur in a shorter time in patients with IBD and in a different sequence, with p53 mutated early and APC and GSK3β mutations occurring later[26].
CRC in patients with IBD is preceded by unequivocal neoplastic epithelial changes, which are considered dysplasia[27]. Studies have shown an increased risk of dysplasia and CRC of up to 19 times more than that in the population without IBD[1] Riddell et al[28] developed a dysplasia classification system including low-grade dysplasia, and high-grade dysplasia. When the distinction between dysplastic and non-dysplastic atypia or associated inflammatory changes is not made by the pathologist, the sample will be classified as undefined for dysplasia[27]. The primary objective of endoscopic surveillance is the early discovery of dysplasia.
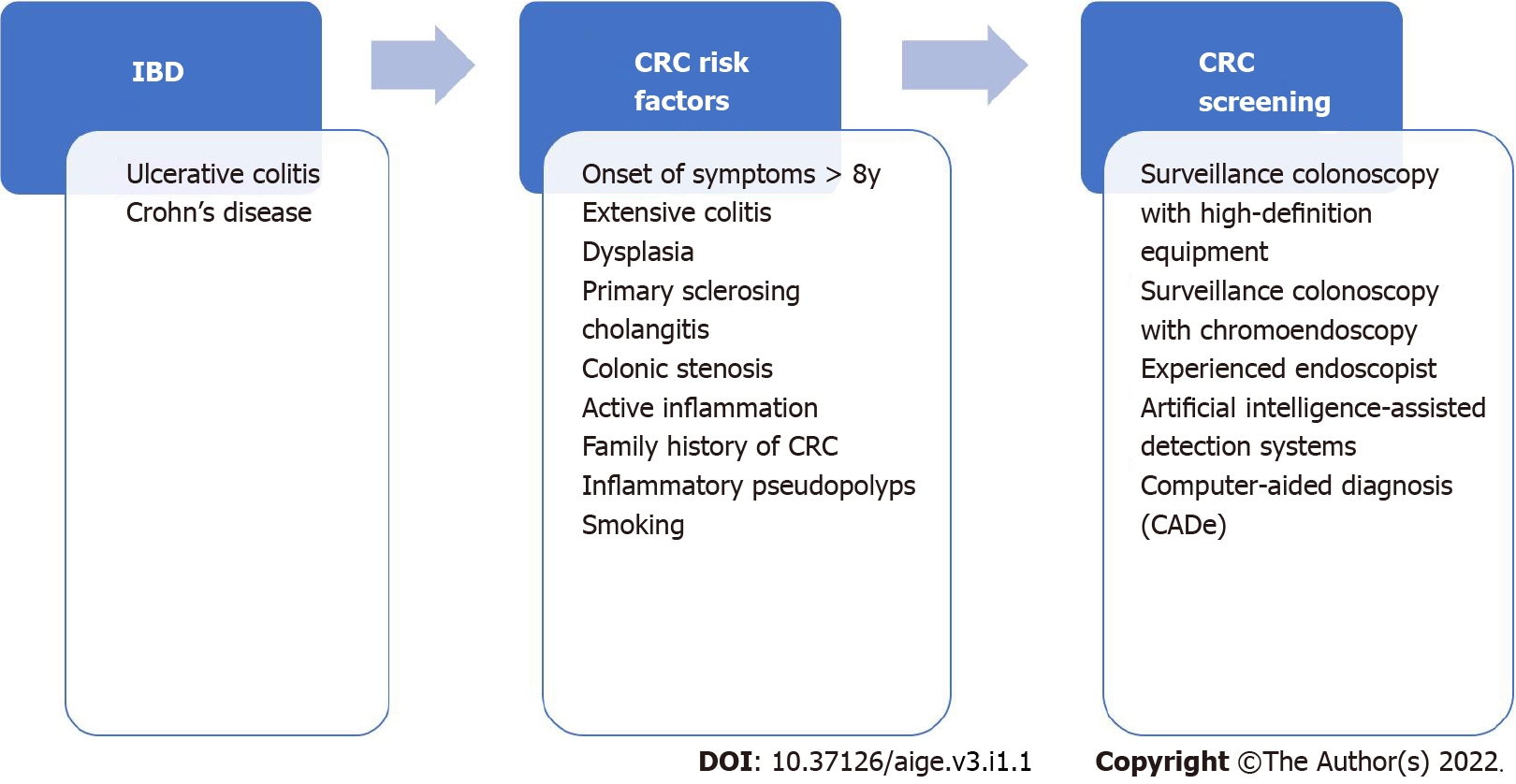
According to the ECCO-ESGAR guidelines (2019)[29], ileocolonoscopic examination for CRC screening should be performed 8 years after the onset of symptoms in patients with IBD. Thus, it is possible to reassess the extent of the disease and exclude possible dysplasia. It should also be routinely performed in patients with perianal Crohn's disease to assess the extent of disease, i.e., severity of luminal inflammation, and to exclude complications such as strictures and cancer[29]. Regarding endoscopic surveillance, high-risk patients (presence of intestinal stenosis or presence of dysplasia detected within the last 5 years, concomitant primary sclerosing cholangitis, and extensive colitis with severe active inflammation) should undergo annual colonoscopy surveillance[29].
Patients with intermediate risk factors (extensive colitis with mild or moderate active inflammation, post-inflammatory polyps, or a family history of CRC in a first-degree relative diagnosed at age 50 or older) should undergo surveillance scheduled for 2-3 years[29]. Patients without intermediate or high-risk features should undergo surveillance colonoscopy scheduled for 5 years[29]. It is also important to mention that patients with colonic stenosis detected within 5 years should be considered at high risk of CRC and should receive surveillance colonoscopy annually[29].
The SCENIC Consensus[30], which is the international consensus on surveillance and management of dysplasia in IBD, has developed screening recommendations for CRC and follow-up after removal of endoscopically resectable dysplastic polypoid lesions. Surveillance colonoscopy is recommended instead of colectomy in these cases, and the consensus also recommends the use of high-definition equipment because it provides image signals with higher pixel density[30]. This was reinforced by the study by Subramanian et al[31], in which examinations performed with high-definition equipment detected twice as much as dysplasia compared with standard-definition colonoscopies. If surveillance is performed with standard-definition colonoscopy, chromoendoscopy, which consists of applying dye throughout the colon that provides contrast enhancement to improve visualization of the epithelial surface, is recommended over white light colonoscopy[30]. In contrast, screening for endoscopically invisible dysplasia (confirmed by a pathologist), referral to an endoscopist experienced in surveillance of IBD using chromoendoscopy with high-definition colonoscopy has been suggested[30]. Figure 2 illustrates the indications of CRC screening in patients with IBD and the recommended methods to perform the surveillance colonoscopy.
Despite the advent of high-definition colonoscopes and chromoendoscopy, the development of the integration of AI-assisted detection systems into conventional colonoscopy began due to the high mortality attributed to neoplasia in patients with IBD[1]. Studies have shown machines capable of assisting in the differentiation of neoplasms associated with colitis, sporadic colorectal adenomas, and non-neoplastic lesions[1]. In view of the increased risk of developing CRC, automated real-time polyp detection systems can significantly reduce missed diagnosis rates and help endoscopists detect polyps in real time[10]. With the intention of improving adenoma detection rates, computer algorithms can accurately detect and localize the presence of premalignant lesions[32] through a Convolutional Neural Network; that is, a type of particular multilayer artificial neural network that is highly efficient for image classification and can detect changes in the colonic mucosa[10].
Although patients with IBD, especially those with extensive colitis, are at higher risk of developing CRC than the general population, there is little evidence of AI application in CRC surveillance or improved models that favor the detection of risk in patients with IBD. Most of the studies that analyzed the detection of polyps excluded patients with IBD[19]. Future studies are necessary to validate these findings in independent cohorts and to determine whether the application of these models will improve the detection of precancerous lesions and the disease prognosis in patients with IBD.
The use of AI can promote numerous benefits in medicine, especially in the field of digestive endoscopy. Early detection of pre-neoplastic lesions allows for immediate intervention and prevention of progression to more severe phenotypes, such as CRC. The benefits for patients with IBD go beyond CRC screening and include the identification and characterization of inflammation, recurrence pattern, mucosal healing, and recognition of a worrisome lesions. Future studies related to AI are expected to add clinical information, such as prediction of disease complications as well as models to predict the best drugs for each patient according to their inflammatory profile and response to previous treatments. Moreover, AI can help in the IBD diagnosis using combinations of symptoms and biomarkers, in addition to genetic and microbiota data, and can also help differentiating Crohn’s disease from ulcerative colitis. Despite advances in this area, AI technology was not designed to replace human intelligence but rather to improve the detection of lesions. To this end, the combination of the expertise of endoscopists with AI is essential for its successful application in clinical practice. Another limitation worth mentioning is that currently the use of AI is not widely available; it is, however, expected to be applied in the future for colonoscopy and optical biopsy or endocytoscopy. It is also expected that there will be greater accessibility and availability of AI, not only for patients with IBD, but also for the general population.
Provenance and peer review: Invited article; externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gao W, Goli A, Hanada E, Wang P S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Kohli A, Holzwanger EA, Levy AN. Emerging use of artificial intelligence in inflammatory bowel disease. World J Gastroenterol. 2020;26:6923-6928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 2. | Kulkarni S, Seneviratne N, Baig MS, Khan AHA. Artificial Intelligence in Medicine: Where Are We Now? Acad Radiol. 2020;27:62-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 136] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 3. | Flynn S, Eisenstein S. Inflammatory Bowel Disease Presentation and Diagnosis. Surg Clin North Am. 2019;99:1051-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 245] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 4. | Stidham RW, Higgins PDR. Colorectal Cancer in Inflammatory Bowel Disease. Clin Colon Rectal Surg. 2018;31:168-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 203] [Article Influence: 29.0] [Reference Citation Analysis (1)] |
| 5. | Keller DS, Windsor A, Cohen R, Chand M. Colorectal cancer in inflammatory bowel disease: review of the evidence. Tech Coloproctol. 2019;23:3-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 174] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 6. | Stafford IS, Kellermann M, Mossotto E, Beattie RM, MacArthur BD, Ennis S. A systematic review of the applications of artificial intelligence and machine learning in autoimmune diseases. NPJ Digit Med. 2020;3:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 127] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 7. | Sundaram S, Choden T, Mattar MC, Desai S, Desai M. Artificial intelligence in inflammatory bowel disease endoscopy: current landscape and the road ahead. Ther Adv Gastrointest Endosc. 2021;14:26317745211017809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | van der Sommen F, de Groof J, Struyvenberg M, van der Putten J, Boers T, Fockens K, Schoon EJ, Curvers W, de With P, Mori Y, Byrne M, Bergman JJGHM. Machine learning in GI endoscopy: practical guidance in how to interpret a novel field. Gut. 2020;69:2035-2045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 86] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 9. | Parsa N, Byrne MF. Artificial intelligence for identification and characterization of colonic polyps. Ther Adv Gastrointest Endosc. 2021;14:26317745211014698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Mitsala A, Tsalikidis C, Pitiakoudis M, Simopoulos C, Tsaroucha AK. Artificial Intelligence in Colorectal Cancer Screening, Diagnosis and Treatment. A New Era. Curr Oncol. 2021;28:1581-1607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 131] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 11. | Kudo SE, Mori Y, Misawa M, Takeda K, Kudo T, Itoh H, Oda M, Mori K. Artificial intelligence and colonoscopy: Current status and future perspectives. Dig Endosc. 2019;31:363-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 83] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 12. | Glover B, Teare J, Patel N. The Status of Advanced Imaging Techniques for Optical Biopsy of Colonic Polyps. Clin Transl Gastroenterol. 2020;11:e00130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Karnes WE, Alkayali T, Mittal M, Patel A, Kim J, Chang KJ, Ninh AQ, Urban G, Baldi P. Su1642 automated polyp detection using deep learning: leveling the field. Gastrointest Endosc. 85:AB376-AB377. [DOI] [Full Text] |
| 14. | Abadir AP, Ali MF, Karnes W, Samarasena JB. Artificial Intelligence in Gastrointestinal Endoscopy. Clin Endosc. 2020;53:132-141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 15. | Repici A, Badalamenti M, Maselli R, Correale L, Radaelli F, Rondonotti E, Ferrara E, Spadaccini M, Alkandari A, Fugazza A, Anderloni A, Galtieri PA, Pellegatta G, Carrara S, Di Leo M, Craviotto V, Lamonaca L, Lorenzetti R, Andrealli A, Antonelli G, Wallace M, Sharma P, Rosch T, Hassan C. Efficacy of Real-Time Computer-Aided Detection of Colorectal Neoplasia in a Randomized Trial. Gastroenterology. 2020;159:512-520.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 393] [Article Influence: 78.6] [Reference Citation Analysis (0)] |
| 16. | Ishiyama M, Kudo SE, Misawa M, Mori Y, Maeda Y, Ichimasa K, Kudo T, Hayashi T, Wakamura K, Miyachi H, Ishida F, Itoh H, Oda M, Mori K. Impact of the clinical use of artificial intelligence-assisted neoplasia detection for colonoscopy: a large-scale prospective, propensity score-matched study (with video). Gastrointest Endosc. 2022;95:155-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 17. | Hassan C, Wallace MB, Sharma P, Maselli R, Craviotto V, Spadaccini M, Repici A. New artificial intelligence system: first validation study versus experienced endoscopists for colorectal polyp detection. Gut. 2020;69:799-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 123] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 18. | Pecere S, Milluzzo SM, Esposito G, Dilaghi E, Telese A, Eusebi LH. Applications of Artificial Intelligence for the Diagnosis of Gastrointestinal Diseases. Diagnostics (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Gubatan J, Levitte S, Patel A, Balabanis T, Wei MT, Sinha SR. Artificial intelligence applications in inflammatory bowel disease: Emerging technologies and future directions. World J Gastroenterol. 2021;27:1920-1935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 49] [Cited by in RCA: 74] [Article Influence: 18.5] [Reference Citation Analysis (1)] |
| 20. | Maeda Y, Kudo SE, Ogata N, Misawa M, Mori Y, Mori K, Ohtsuka K. Can artificial intelligence help to detect dysplasia in patients with ulcerative colitis? Endoscopy. 2021;53:E273-E274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 21. | Abad MRA, Shimamura Y, Fujiyoshi Y, Seewald S, Inoue H. Endocytoscopy: technology and clinical application in upper gastrointestinal tract. Transl Gastroenterol Hepatol. 2020;5:28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Mossotto E, Ashton JJ, Coelho T, Beattie RM, MacArthur BD, Ennis S. Classification of Paediatric Inflammatory Bowel Disease using Machine Learning. Sci Rep. 2017;7:2427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 112] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 23. | Morán A, Ortega P, de Juan C, Fernández-Marcelo T, Frías C, Sánchez-Pernaute A, Torres AJ, Díaz-Rubio E, Iniesta P, Benito M. Differential colorectal carcinogenesis: Molecular basis and clinical relevance. World J Gastrointest Oncol. 2010;2:151-158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Corley DA, Jensen CD, Marks AR, Zhao WK, Lee JK, Doubeni CA, Zauber AG, de Boer J, Fireman BH, Schottinger JE, Quinn VP, Ghai NR, Levin TR, Quesenberry CP. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;370:1298-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1251] [Cited by in RCA: 1561] [Article Influence: 141.9] [Reference Citation Analysis (0)] |
| 25. | Marion JF, Sands BE. The SCENIC consensus statement on surveillance and management of dysplasia in inflammatory bowel disease: praise and words of caution. Gastroenterology. 2015;148:462-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 26. | Foersch S, Neurath MF. Colitis-associated neoplasia: molecular basis and clinical translation. Cell Mol Life Sci. 2014;71:3523-3535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 27. | Clarke WT, Feuerstein JD. Colorectal cancer surveillance in inflammatory bowel disease: Practice guidelines and recent developments. World J Gastroenterol. 2019;25:4148-4157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 91] [Cited by in RCA: 120] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 28. | Riddell RH, Goldman H, Ransohoff DF, Appelman HD, Fenoglio CM, Haggitt RC, Ahren C, Correa P, Hamilton SR, Morson BC. Dysplasia in inflammatory bowel disease: standardized classification with provisional clinical applications. Hum Pathol. 1983;14:931-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1348] [Cited by in RCA: 1214] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 29. | Maaser C, Sturm A, Vavricka SR, Kucharzik T, Fiorino G, Annese V, Calabrese E, Baumgart DC, Bettenworth D, Borralho Nunes P, Burisch J, Castiglione F, Eliakim R, Ellul P, González-Lama Y, Gordon H, Halligan S, Katsanos K, Kopylov U, Kotze PG, Krustinš E, Laghi A, Limdi JK, Rieder F, Rimola J, Taylor SA, Tolan D, van Rheenen P, Verstockt B, Stoker J; European Crohn’s and Colitis Organisation [ECCO] and the European Society of Gastrointestinal and Abdominal Radiology [ESGAR]. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. 2019;13:144-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1242] [Cited by in RCA: 1169] [Article Influence: 194.8] [Reference Citation Analysis (0)] |
| 30. | Laine L, Kaltenbach T, Barkun A, McQuaid KR, Subramanian V, Soetikno R; SCENIC Guideline Development Panel. SCENIC international consensus statement on surveillance and management of dysplasia in inflammatory bowel disease. Gastroenterology. 2015;148:639-651.e28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 390] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 31. | Subramanian V, Ramappa V, Telakis E, Mannath J, Jawhari AU, Hawkey CJ, Ragunath K. Comparison of high definition with standard white light endoscopy for detection of dysplastic lesions during surveillance colonoscopy in patients with colonic inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:350-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 32. | Nogueira-Rodríguez A, Domínguez-Carbajales R, López-Fernández H, Iglesias Á, Cubiella J, Fdez-Riverola F, Reboiro-Jato M, Glez-Peña D. Deep Neural Networks Approaches for Detecting and Classifying Colorectal Polyps. Neurocomputing. 2021;423:721-734. [RCA] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |