Published online Aug 28, 2021. doi: 10.37126/aige.v2.i4.149
Peer-review started: May 9, 2021
First decision: May 19, 2021
Revised: May 20, 2021
Accepted: July 19, 2021
Article in press: July 19, 2021
Published online: August 28, 2021
Processing time: 119 Days and 15.6 Hours
Traditional endoscopic techniques for Barrett’s esophagus (BE) surveillance relied on factor of probability as endoscopists performed cumbersome random biopsies of low yield. Optical coherence tomography (OCT) is a novel technique based on tissue light interference and is set to break conventional barriers. OCT was initially introduced in ophthalmology but was soon adopted by other areas of medicine. When applied to endoscopy, OCT can render images of the superficial layers of the gastrointestinal tract and is highly sensitive in detecting dysplasia in BE. Volumetric laser endomicroscopy is a second generation OCT endoscope device which is able to identify buried glands after ablation. Addition of artificial intelligence to OCT has rendered it more productive. The newer additions to OCT such as angiogram and laser marking will increase the accuracy of investigation. In spite of the few inevitable drawbacks associated with the technology, it presently outperforms all newer endoscopic techniques for the surveillance of BE.
Core Tip: Surveillance of Barrett’s esophagus for dysplasia is a long-debated and intensively researched topic. Optical coherence tomography (OCT) is a breakthrough technology in the medical field that enables the visualization of the layers of a structure in an office setting. The application of artificial intelligence (AI) to OCT endoscopy is the latest addition to the armamentarium of endoscopists. AI-based diagnostic algorithm scores are proven to be better than clinical scores. The accuracy of AI-based system is enhanced further by using color coding software and convolutional neural networks. Multi-center randomized control trials validating these technologies is the need of the hour.
- Citation: Gupta N, Yelamanchi R, Agrawal H, Agarwal N. Role of optical coherence tomography in Barrett’s esophagus. Artif Intell Gastrointest Endosc 2021; 2(4): 149-156
- URL: https://www.wjgnet.com/2689-7164/full/v2/i4/149.htm
- DOI: https://dx.doi.org/10.37126/aige.v2.i4.149
Barrett’s esophagus (BE) is defined as columnar metaplasia (or intestinal metaplasia, as some authorities prefer to call it) of the stratified squamous epithelium, lining the lower end of the esophagus[1]. It occurs due to chronic exposure of the distal eso
Once the diagnosis of BE is made based on endoscopy, the endoscopist evaluates its extent as per the Prague C and M classification. All cases of BE should be biopsied at multiple levels as per the Seattle biopsy protocol to identify the presence of dysplasia or adenocarcinoma, which is the main concern. Traditional endoscopic techniques relied on the chance factor as endoscopists performed random cumbersome biopsies of low yield. The early diagnosis of esophageal neoplasia is important because it helps to initiate curative therapies for cancer. This has directed the path of research to identify newer techniques and technologies to increase the accuracy of biopsies during endoscopy[4]. Optical coherence tomography (OCT) is one of such techniques which is set to break conventional barriers.
Humans are prone to do errors due to fatigue, increased workload and working environment. The use of artificial intelligence (AI) has grown rapidly in the past few decades from using technology to perform simple household tasks to piloting aircraft. AI is also adopted into the medical field in the form of surgical robots in the last decade. The application of AI to endoscopy is widely researched as newer technologies of endoscopy are being developed. The purpose of this narrative review is to enlighten the readers about the principles of OCT and its application to BE and the use of AI in the OCT endoscopy.
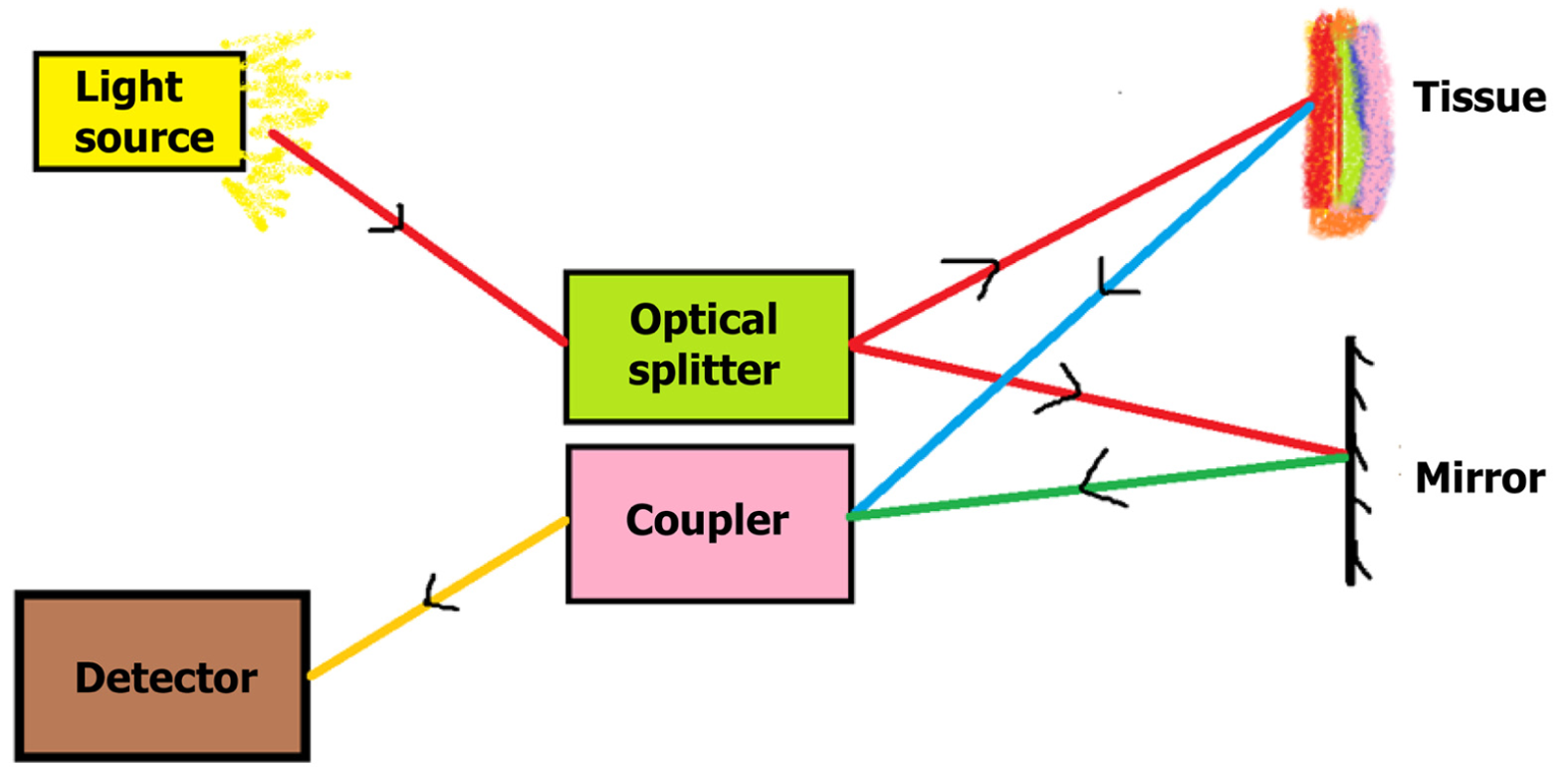
OCT is an imaging modality based on light interference. It is used to produce cross-sectional images of a structure based on the differential properties of various layers with respect to light refraction[5]. The basic setup of OCT consists of a light source which is a low-coherence semiconductor super-luminescent diode. The light is split into two beams by an optical splitter: A reference beam and a sample beam. The reference beam is reflected back by a mirror, while the sample beam is focused onto the tissue to be imaged. Based on the refractory properties of the layers of the tissue, the sample beam is variably reflected back. The reflected light from the reference and sample beams are coupled in a coupler, producing interference patterns which are analyzed, after which a cross-sectional image is created (Figure 1). The axial resolution of OCT will depend on the spectral band of the light source with large spectral bands having better resolution[5]. The transverse resolution is independent of axial resolution and will depend on the numerical aperture of the lens through which the light beam passes[5].
The conventional OCT technology is based on the time-domain (TD-OCT) concept in which variations in the time of the travelled beams of light are analyzed to form an image with the help of moving mirrors. The technology has now evolved into the Fourier-domain (FD-OCT) which uses static mirrors so an image is formed based on the modulations in the source spectrum. The FD-OCT has higher image acquisition speeds than TD-OCT. The resolution of FD-OCT is 1-3 μm, which is far better than the 10 μm resolution of TD-OCT. The FD-OCT is based on either charge-coupled device-based image acquisition (spectral-domain OCT) or photodetector-based image detection with longer wavelengths of the light source (swept-source OCT)[6]. The swept-source OCT has better resolution and twice the image acquisition speed compared to spectral-domain OCT[6].
OCT was initially introduced in ophthalmology as a method to visualize the layers of the retina but it was soon adopted into other areas of medicine. Nevertheless, the utility of OCT is still only the “tip of the iceberg” with its vast potential yet to be unleashed. When applied to endoscopy, OCT is able to render images of the superficial layers of the gastrointestinal tract. OCT can be combined with either a forward-viewing endoscope or a side-viewing endoscope, with the forward-viewing endoscope enabling the sampling of the desired tissue[7]. There are two main types of OCT endoscopes: The proximal scanning rotating endoscope, which is less expensive but has lower capture speed, and the distal scanning endoscope, which comes with a micromotor, acquires images at a much higher speed but comes at a cost higher than the proximal scanning endoscope[7].
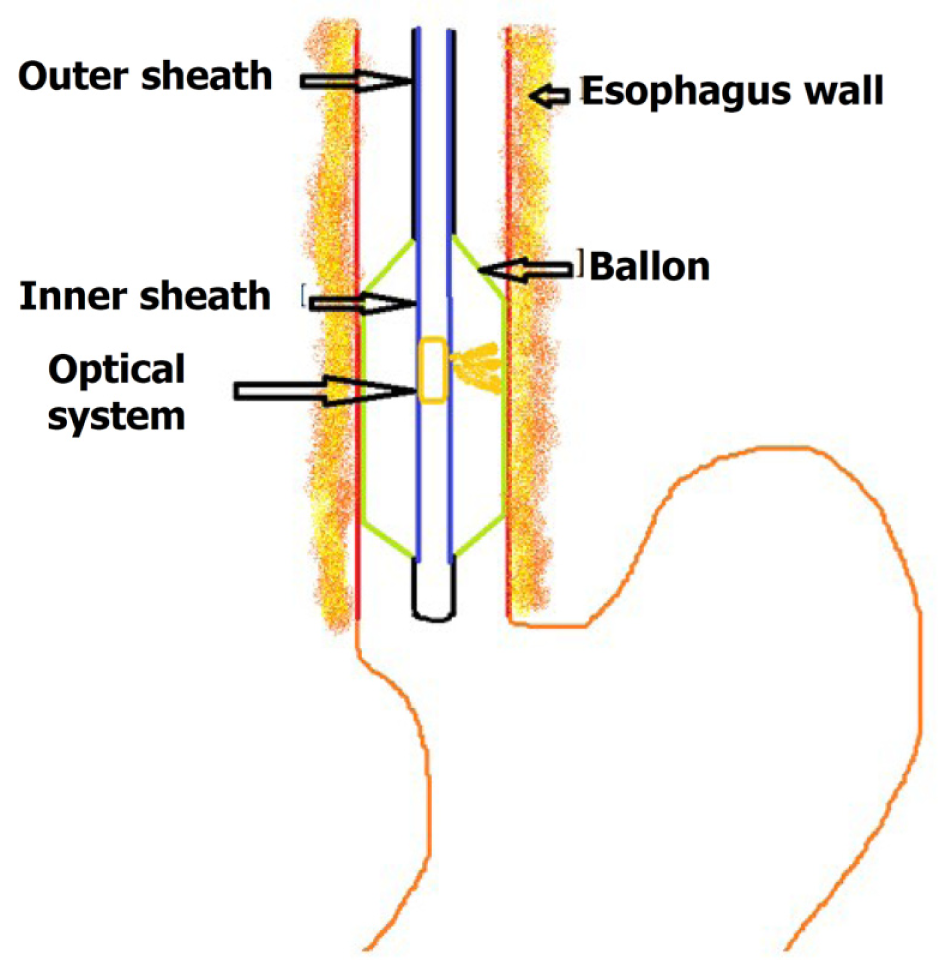
Volumetric laser endomicroscopy (VLE) is a second generation OCT endoscope device presently used for imaging[8] (Figure 2). It uses balloon centered imaging probes for imaging with a high axial resolution of 7 μm and a depth of 3 mm, which is 10 times greater compared to the standard endoscopic ultrasound[9]. It images the esophagus in six-centimeter intervals and is quite fast in image acquisition compared to the conventional OCT. It images about 1200 cross-sectional areas in the six cm span which are reconstructed. The application of VLE in BE is mainly to diagnose suspicious areas of mucosal abnormalities and in the post-treatment surveillance of BE and early neoplastic lesions.
The absence of layering, surface maturation, and gland maturation are the three independent predictive factors for dysplasia in OCT imaging. The surface maturation is assessed in terms of the surface OCT signal, which if equal or stronger than the sub-surface signal, is predictive of dysplasia. Gland maturation is assessed in terms of the number of abnormal glands identified in imaging with more than five glands predictive of dysplasia.
AI is based on computer algorithms which provide result based on the received input. The algorithms are created based on previous OCT images which are correlated with histological diagnosis. The AI system has been automated to evolve with time, based on its previous results just as a human brain which is known as machine learning. Machine learning may be supervised, semi-supervised or unsupervised. Hence, AI is said to as good as a human brain and sometimes even better. Swager et al[10] created an AI-based VLE prediction score using multivariable logistic regression analysis of 60 VLE images[10]. The components of the score were: the lack of layering of superficial layers, higher surface intensity than sub-surface intensity, and the number of abnormal glands (Table 1). A cut-off score of ≥ 8 was predicative of dysplasia with a sensitivity and specificity of 83% and 71% respectively[10]. This VLE prediction score based on computer-based VLE diagnostic algorithm (VLE-DA) was more sensitive (86%) and specific (88%) than the clinical VLE predication score[10-12]. The components of VLE-DA are listed in Table 1.
| VLE prediction score | ||
| Parameter | Score | |
| Layering | Layering present-more than 50% | 0 |
| Layering present–less than 50% | 8 | |
| Surface signal | Surface signal < subsurface signal | 0 |
| Surface signal = subsurface signal | 6 | |
| Surface signal > subsurface signal | 8 | |
| Abnormal glands | 0-5 | 0 |
| > 5 | 5 | |
| VLE-diagnostic algorithm | ||
| Mucosal layer partial effacement | Abnormal glands > 5 | Dysplasia |
| Abnormal glands ≤ 5 | Non-dysplasia | |
| Mucosal layer complete effacement | Surface intensity > subsurface intensity | Dysplasia |
| Surface intensity ≤ subsurface intensity | Non-dysplasia | |
The traditional OCT criteria were found to be 97% sensitive and 93% specific when applied to BE surveillance prospectively in a study by Poneros et al[13] in 2001. The accuracy of OCT in diagnosing dysplasia in BE was about 78% in a double-blinded study by Isenberg et al[14] in 2005. The utility of OCT in diagnosing dysplasia was also confirmed in a study by Evans et al[15] using the dysplasia index which was 83% sensitive and 75% specific[15]. Chen et al[16] used ultra-high-resolution OCT for diagnosing dysplasia and adenocarcinoma with an accuracy of 83.3% and 100% respectively[16]. The utility of ultra-high-resolution OCT was also confirmed in the study by Cobb et al[17].
The imaging capability of three-dimensional OCT is faster than conventional OCT. Its utility was proved in the study by Adler et al[18]. VLE has been found to be more sensitive and specific than random blind biopsies as per Seattle protocol. The role of VLE was initially proved in a study by Vakoc et al[19], while in a study by Trindade et al[20] five out of six patients were upstaged due to the diagnosis of dysplasia which was missed by conventional endoscopy and narrow band imaging[19,20]. The sensitivity and specificity of VLE in diagnosing dysplasia was 86% and 88% in a study by Leggett et al[11]. In a study by Jain et al[21], VLE was compared with histology; the sensitivity in diagnosing BE-related dysplasia was 50% and specificity was 47.1%[21]. The false negative rate was 2.9%. Even though the specificity was low in the study, it is far better than the random biopsies. In a systematic review by Kohli et al[22], the sensitivity and specificity of OCT in diagnosing dysplasia and early malignancy was in the ranges of 68%-83% and 75%-82% respectively[22].
A variety of ablation therapies such as radiofrequency ablation, cryoablation, laser ablation, photodynamic therapy, etc. are used for the treatment of high-grade BE dysplasia and insitu carcinoma. One of the main disadvantages of these procedures is the occurrence of buried glands or subsquamous glandular structures[23,24]. These glands, present beneath the epithelium, may undergo dysplastic changes and turn malignant, but are not visualized on routine endoscopy as the surface epithelium appears normal. OCT is one of the few techniques able to diagnose buried glands[25]. The sensitivity and specificity in identifying buried glands in post-treatment BE using VLE was shown to be 92.3% and 23.8% in a study by Jain et al[21]. However, in the study by Swager et al[26], most of the subsquamous glandular structures identified on OCT were histologically normal[26]. The role of OCT in post-ablative surveillance was also proved in a study by Benjamin et al[27].
Doppler-OCT is useful in detecting the changes in the sub-mucosal micro-vascular network, which further improves the accuracy of OCT. Doppler-OCT is also used to detect the change in the vascular pattern during post-photodynamic therapy for BE. Doppler-OCT helps to monitor the dose of photodynamic therapy[28,29].
As neoplasia is associated with neovascularization, this is one of the features used to distinguish benign epithelium form malignancy. OCT angiography is used to image the subsurface vasculature without the need for any contrast and is useful in diagnosing neoplasia[30]. The changes in the OCT signal caused by the movement of erythrocytes are quantified by calculating the decorrelation. However, this makes the OCT signal susceptible to artifacts due to respiratory and cardiac movements.
As a balloon is used to augment the scanning speed in VLE, simultaneous sampling of mucosa is not possible. The biopsy taken from the mucosa may not be the original mucosa intended on imaging. This disadvantage is overcome by using laser marking along with VLE. The laser fiber is used for creating point coagulation spots which act as markers for biopsy after the scan[31,32]. Simultaneous laser coagulation along with OCT is also possible[32].
The addition of deep learning to AI-based OCT systems further improved the accuracy of prediction of BE related dysplasia. Deep learning is one kind of machine learning where multiple diagnostic algorithms are layered to form a convolutional neural network just as a human brain. The output from one layer is fed to the next layer which further processes it and feeds it to the next layer to produce a refined output[33]. Deep learning also increases the speed of processing the images.
Trindade et al[34] used an AI-based new software termed intelligent real-time image segmentation for BE surveillance. The software provided color codes based on the degree of dysplasia using the previously mentioned VLE prediction features[34]. A multi-center randomized control trial with trial number NCT03814824 is going on, validating the above software, the results of which are awaited.
VLE has been proved to be better than confocal laser endomicroscopy (CLE), which is one of the emerging endoscopic imaging techniques for BE and associated dysplasia. The sensitivity and specificity of VLE using VLE-DA were higher than CLE in a study by Leggett et al[11]. CLE is also disadvantageous as it requires injection of contrast into the blood and a limited field of view and imaging depth[35]. Endoscopic ultrasound is an excellent imaging modality for assessing the depth of tumor involvement. However, its accuracy is lower in differentiating early invasive carcinoma (T1 and T2). In a study by Kahn et al[36], VLE showed good results in differentiating T1a lesions from T1b lesions[36].
All technologies have one or more drawbacks and OCT is no exception. The main drawback of OCT is the absence of real-time imaging, as it is the case with other imaging modalities. Even the fastest OCT technology and probes require seconds to process the reflected waves. VLE requires balloon apposition and although perfect apposition is theoretically possible, it is rare in reality. The mucous layer on the surface epithelium, the contractions of the esophagus, and the presence of blood interfere with the close approximation resulting in artifacts. Simultaneous biopsy is not possible during imaging in VLE probes, which may pose a difficulty in biopsying the originally identified area. Movement artifacts are common in Doppler-OCT and OCT angiography. Unlike endoscopic ultrasound, OCT cannot be used to image the deeper tissues. Finally, cost is one of the main limiting factors for the widespread usage in all institutes.
The application of AI to OCT requires inputs from a large number of experts with expertise in this new technology who are fewer at present. The accuracy of the AI systems is based on the data fed which requires advanced imaging techniques and higher quality images. As AI and machine learning require input from humans it may be the victim of human errors during data input. Much of the knowledge of AI in OCT is based on pilot studies and case series. The number of randomized control trials and multi-center trials are very less due to concerns raised by ethical committees.
Surveillance of BE for dysplasia is a long-debated and intensively researched topic. OCT is a breakthrough technology in the medical field that enables the visualization of the layers of a structure in an office setting. The application of OCT to endoscopy is the latest addition to the armamentarium of endoscopists. Even though earlier OCT instruments were slow to image tissues, the newer AI-based technologies are fast enough to add only a few minutes to the conventional endoscopy time and are highly accurate compared to clinical diagnosis. OCT is highly sensitive in detecting dysplasia in BE. Even though the specificity in diagnosing dysplasia is lower, it is far more efficient than the conventional blind biopsy protocol. An especially important feature is the ability of VLE to identify buried glands after ablation. The newer additions to OCT, such as angiogram and laser marking, will help to increase the accuracy of the investigation. The AI software systems and deep learning systems are evolving over time. However, the utility of AI to BE surveillance is still at its bud stage. In spite of the few unavoidable drawbacks associated with the technology, AI-based OCT system is presently the most promising of all newer endoscopic techniques for the surveillance of BE.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Morozov S S-Editor: Fan JR L-Editor: A P-Editor: Zhang YL
| 1. | Naini BV, Souza RF, Odze RD. Barrett's Esophagus: A Comprehensive and Contemporary Review for Pathologists. Am J Surg Pathol. 2016;40:e45-e66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 2. | Dam AN, Klapman J. A narrative review of Barrett's esophagus in 2020, molecular and clinical update. Ann Transl Med. 2020;8:1107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Qumseya BJ, Bukannan A, Gendy S, Ahemd Y, Sultan S, Bain P, Gross SA, Iyer P, Wani S. Systematic review and meta-analysis of prevalence and risk factors for Barrett's esophagus. Gastrointest Endosc. 2019;90:707-717.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 89] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 4. | Sharma P. New endoscopic techniques for detecting dysplasia in barrett esophagus. Gastroenterol Hepatol (NY). 2008;4:460-461. [PubMed] |
| 5. | Popescu DP, Choo-Smith LP, Flueraru C, Mao Y, Chang S, Disano J, Sherif S, Sowa MG. Optical coherence tomography: fundamental principles, instrumental designs and biomedical applications. Biophys Rev. 2011;3:155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 102] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 6. | Gabriele ML, Wollstein G, Ishikawa H, Kagemann L, Xu J, Folio LS, Schuman JS. Optical coherence tomography: history, current status, and laboratory work. Invest Ophthalmol Vis Sci. 2011;52:2425-2436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 168] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 7. | Gora MJ, Suter MJ, Tearney GJ, Li X. Endoscopic optical coherence tomography: technologies and clinical applications [Invited]. Biomed Opt Express. 2017;8:2405-2444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 168] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 8. | Wolfsen HC. Volumetric Laser Endomicroscopy in Patients With Barrett Esophagus. Gastroenterol Hepatol (NY). 2016;12:719-722. [PubMed] |
| 9. | Rodriguez MAC, de Moura DTH, Ribeiro IB, Bernardo WM, Morita FHA, Marques SB, Sakai P, de Moura EGH. Volumetric laser endomicroscopy and optical coherence tomography in Barrett's esophagus: a systematic review and meta-analysis. Endosc Int Open. 2019;7:E1078-E1091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Swager AF, Tearney GJ, Leggett CL, van Oijen MGH, Meijer SL, Weusten BL, Curvers WL, Bergman JJGHM. Identification of volumetric laser endomicroscopy features predictive for early neoplasia in Barrett's esophagus using high-quality histological correlation. Gastrointest Endosc. 2017;85:918-926.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 11. | Leggett CL, Gorospe EC, Chan DK, Muppa P, Owens V, Smyrk TC, Anderson M, Lutzke LS, Tearney G, Wang KK. Comparative diagnostic performance of volumetric laser endomicroscopy and confocal laser endomicroscopy in the detection of dysplasia associated with Barrett's esophagus. Gastrointest Endosc. 2016;83:880-888.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 90] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 12. | Struyvenberg MR, van der Sommen F, Swager AF, de Groof AJ, Rikos A, Schoon EJ, Bergman JJ, de With PHN, Curvers WL. Improved Barrett's neoplasia detection using computer-assisted multiframe analysis of volumetric laser endomicroscopy. Dis Esophagus. 2020;33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Poneros JM, Brand S, Bouma BE, Tearney GJ, Compton CC, Nishioka NS. Diagnosis of specialized intestinal metaplasia by optical coherence tomography. Gastroenterology. 2001;120:7-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 183] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 14. | Isenberg G, Sivak MV Jr, Chak A, Wong RC, Willis JE, Wolf B, Rowland DY, Das A, Rollins A. Accuracy of endoscopic optical coherence tomography in the detection of dysplasia in Barrett's esophagus: a prospective, double-blinded study. Gastrointest Endosc. 2005;62:825-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 127] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 15. | Evans JA, Poneros JM, Bouma BE, Bressner J, Halpern EF, Shishkov M, Lauwers GY, Mino-Kenudson M, Nishioka NS, Tearney GJ. Optical coherence tomography to identify intramucosal carcinoma and high-grade dysplasia in Barrett's esophagus. Clin Gastroenterol Hepatol. 2006;4:38-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 37] [Reference Citation Analysis (0)] |
| 16. | Chen Y, Aguirre AD, Hsiung PL, Desai S, Herz PR, Pedrosa M, Huang Q, Figueiredo M, Huang SW, Koski A, Schmitt JM, Fujimoto JG, Mashimo H. Ultrahigh resolution optical coherence tomography of Barrett's esophagus: preliminary descriptive clinical study correlating images with histology. Endoscopy. 2007;39:599-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 108] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 17. | Cobb MJ, Hwang JH, Upton MP, Chen Y, Oelschlager BK, Wood DE, Kimmey MB, Li X. Imaging of subsquamous Barrett's epithelium with ultrahigh-resolution optical coherence tomography: a histologic correlation study. Gastrointest Endosc. 2010;71:223-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 18. | Adler DC, Zhou C, Tsai TH, Lee HC, Becker L, Schmitt JM, Huang Q, Fujimoto JG, Mashimo H. Three-dimensional optical coherence tomography of Barrett's esophagus and buried glands beneath neosquamous epithelium following radiofrequency ablation. Endoscopy. 2009;41:773-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Vakoc BJ, Shishko M, Yun SH, Oh WY, Suter MJ, Desjardins AE, Evans JA, Nishioka NS, Tearney GJ, Bouma BE. Comprehensive esophageal microscopy by using optical frequency-domain imaging (with video). Gastrointest Endosc. 2007;65:898-905. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 123] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 20. | Trindade AJ, George BJ, Berkowitz J, Sejpal DV, McKinley MJ. Volumetric laser endomicroscopy can target neoplasia not detected by conventional endoscopic measures in long segment Barrett's esophagus. Endosc Int Open. 2016;4:E318-E322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Jain D, Fatima S, Jain S, Singhal S. Volumetric Laser Endomicroscopy for Barrett's Esophagus - Looking at the Fine Print. J Gastrointestin Liver Dis. 2017;26:291-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Kohli DR, Schubert ML, Zfass AM, Shah TU. Performance characteristics of optical coherence tomography in assessment of Barrett's esophagus and esophageal cancer: systematic review. Dis Esophagus. 2017;30:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Castela J, Serrano M, Ferro SM, Pereira DV, Chaves P, Pereira AD. Buried Barrett's Esophagus with High-Grade Dysplasia after Radiofrequency Ablation. Clin Endosc. 2019;52:269-272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Spechler SJ. Buried (but not dead) Barrett's metaplasia: tales from the crypts. Gastrointest Endosc. 2012;76:41-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Zhou C, Tsai TH, Lee HC, Kirtane T, Figueiredo M, Tao YK, Ahsen OO, Adler DC, Schmitt JM, Huang Q, Fujimoto JG, Mashimo H. Characterization of buried glands before and after radiofrequency ablation by using 3-dimensional optical coherence tomography (with videos). Gastrointest Endosc. 2012;76:32-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 26. | Swager AF, Boerwinkel DF, de Bruin DM, Faber DJ, van Leeuwen TG, Weusten BL, Meijer SL, Bergman JJ, Curvers WL. Detection of buried Barrett's glands after radiofrequency ablation with volumetric laser endomicroscopy. Gastrointest Endosc. 2016;83:80-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 27. | Benjamin T, Shakya S, Thota PN. Feasibility of volumetric laser endomicroscopy in Barrett's esophagus with dysplasia and in post-ablation surveillance. J Gastrointestin Liver Dis. 2016;25:407-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Standish BA, Yang VX, Munce NR, Wong Kee Song LM, Gardiner G, Lin A, Mao YI, Vitkin A, Marcon NE, Wilson BC. Doppler optical coherence tomography monitoring of microvascular tissue response during photodynamic therapy in an animal model of Barrett's esophagus. Gastrointest Endosc. 2007;66:326-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Li H, Standish BA, Mariampillai A, Munce NR, Mao Y, Chiu S, Marcon NE, Wilson BC, Vitkin A, Yang VX. Feasibility of interstitial Doppler optical coherence tomography for in vivo detection of microvascular changes during photodynamic therapy. Lasers Surg Med. 2006;38:754-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 30. | Lee HC, Ahsen OO, Liang K, Wang Z, Figueiredo M, Giacomelli MG, Potsaid B, Huang Q, Mashimo H, Fujimoto JG. Endoscopic optical coherence tomography angiography microvascular features associated with dysplasia in Barrett's esophagus (with video). Gastrointest Endosc. 2017;86:476-484.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 31. | Beaudette K, Baac HW, Madore WJ, Villiger M, Godbout N, Bouma BE, Boudoux C. Laser tissue coagulation and concurrent optical coherence tomography through a double-clad fiber coupler. Biomed Opt Express. 2015;6:1293-1303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 32. | Uno K, Koike T, Shimosegawa T. Recent development of optical coherence tomography for preoperative diagnosis of esophageal malignancies. World J Gastrointest Endosc. 2015;7:872-880. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | de Groof AJ, Struyvenberg MR, Fockens KN, van der Putten J, van der Sommen F, Boers TG, Zinger S, Bisschops R, de With PH, Pouw RE, Curvers WL, Schoon EJ, Bergman JJGHM. Deep learning algorithm detection of Barrett's neoplasia with high accuracy during live endoscopic procedures: a pilot study (with video). Gastrointest Endosc. 2020;91:1242-1250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 89] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 34. | Trindade AJ, McKinley MJ, Fan C, Leggett CL, Kahn A, Pleskow DK. Endoscopic Surveillance of Barrett's Esophagus Using Volumetric Laser Endomicroscopy With Artificial Intelligence Image Enhancement. Gastroenterology. 2019;157:303-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 35. | Tsai TH, Fujimoto JG, Mashimo H. Endoscopic Optical Coherence Tomography for Clinical Gastroenterology. Diagnostics (Basel). 2014;4:57-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 36. | Kahn A, Kamboj AK, Muppa P, Sawas T, Lutzke LS, Buras MR, Golafshar MA, Katzka DA, Iyer PG, Smyrk TC, Wang KK, Leggett CL. Staging of T1 esophageal adenocarcinoma with volumetric laser endomicroscopy: a feasibility study. Endosc Int Open. 2019;7:E462-E470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |