Published online Jun 28, 2021. doi: 10.37126/aige.v2.i3.89
Peer-review started: June 2, 2021
First decision: June 18, 2021
Revised: June 20, 2021
Accepted: June 28, 2021
Article in press: June 28, 2021
Published online: June 28, 2021
Processing time: 33 Days and 18.6 Hours
The application of artificial intelligence (AI) using deep learning and machine learning approaches in modern medicine is rapidly expanding. Within the field of Gastroenterology, AI is being evaluated across a breadth of clinical and diagnostic applications including identification of pathology, differentiation of disease processes, and even automated procedure report generation. Many pancreatic pathologies can have overlapping features creating a diagnostic dilemma that provides a window for AI-assisted improvement in current evaluation and diagnosis, particularly using endoscopic ultrasound. This topic highlight will review the basics of AI, history of AI in gastrointestinal endoscopy, and prospects for AI in the evaluation of autoimmune pancreatitis, pancreatic ductal adenocarcinoma, chronic pancreatitis and intraductal papillary mucinous neoplasm.
Core Tip: Artificial intelligence is an emerging diagnostic tool that may further aid clinicians in the current evaluation of diseases of the pancreas.
- Citation: Mankoo R, Ali AH, Hammoud GM. Use of artificial intelligence in endoscopic ultrasound evaluation of pancreatic pathologies. Artif Intell Gastrointest Endosc 2021; 2(3): 89-94
- URL: https://www.wjgnet.com/2689-7164/full/v2/i3/89.htm
- DOI: https://dx.doi.org/10.37126/aige.v2.i3.89
Artificial intelligence (AI) has emerged as a mechanism to assist clinicians, particularly in the analysis and interpretation of clinical data such as radiologic images and pathology. In general, AI encompasses the use of computer algorithms and learning models designed to complete undertakings that typically require conscious human processing[1]. For pattern recognition in images, a deep neural network learns multiple representations of the input images at different levels of abstractions. Subsets of AI include machine learning (support vector machine algorithms, artificial neural networks) and direct learning (convolutional neural networks, recurrent neural networks[1-3]. Deep learning has shown great promise in healthcare applications ranging from early detection of cancers to predicting disease survivability. The overarching goal of AI in medicine has been to decrease inter-operator variability while improving diagnostic accuracy and real-time decision making[4]. The application of AI in Gastroenterology has largely been focused on endoscopy, ranging from the detection and classification of colon polyps, to the diagnosis of esophageal and gastric cancer[1,3]. However, more recently there has been further evaluation of the role of AI in biliopancreatic endoscopy, including improved endoscopic ultrasound (EUS) differentiation between pancreatic ductal adenocarcinoma (PDAC) and other pancreatic pathologies such as autoimmune pancreatitis (AIP), chronic pancreatitis (CP) and cystic pancreas lesions such as intraductal papillary mucinous neoplasm (IPMN). This “topic highlight” will focus on the potential use of AI in the EUS evaluation of pancreatic conditions.
Early studies on the application of AI in GI endoscopy dating back to the 1990s-2000s were focused on aiding the detection and classification of colorectal polyps to improve adenoma detection rates and decrease interval colon cancers[5-8]. Additional studies have used AI to help diagnose inflammatory bowel disease and predict histologic inflammation during colonoscopy evaluation[9,10], as well as grade bowel preparation[11]. The use of AI in upper endoscopy has been assessed in the identification and labeling of basic anatomic structures with automatic image capture[12], diagnosis of Helicobacter pylori infection[13], identification of gastric and esophageal cancer[14], as well as diagnosis of dysplasia in Barrett’s esophagus[15]. With regards to capsule endoscopy, existing technology within current software platforms allows for removal of redundant or uninformative images and identifies potential images of bleeding through color detection, while more recent studies are looking into the use of AI to identify other small bowel pathologies[16]. PDAC and AIP are diseases with a highly analogous visual presentation that are difficult to distinguish by imaging. AI systems have been developed to aid EUS evaluation of pancreatic lesions with the particular goal of distinguishing pancreatic cancer from other pancreatic pathologies including CP and AIP[17-19].
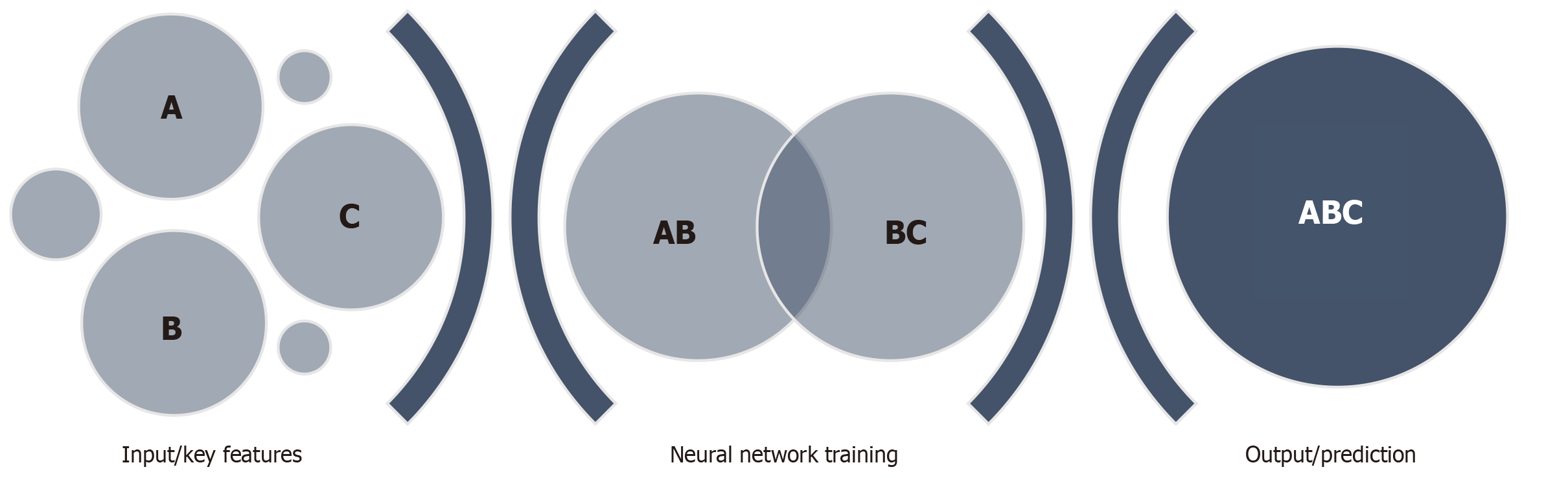
The use of AI in pancreaticobiliary endoscopy is still in its infancy, therefore there is a paucity of literature related to EUS evaluation of pancreatic conditions using AI-based systems. However, the need for improved diagnostic evaluation of pancreatic conditions including AIP, PDAC, CP and pancreatic cystic lesions, provides an exciting niche for further research. AI has previously been applied in EUS differentiation of pancreatic cystic lesions and pancreatic tumors, thereby offering the capability of earlier and more accurate diagnosis. Both conventional machine learning and deep learning architectures have been used. A convolutional neural network (CNN) is a deep learning algorithm developed based on the concepts of visual tasks and signaling. In building a CNN for EUS, initial image data is collected and labeled based on the findings, these images are then entered as input and filtered through a multi-layer deep learning program which allows the system to learn key features of the provided EUS images. Multiple rounds of this process allow for the formation of a neural network where the system can then apply the previously learned features in analyzing novel images (Figure 1).
To identify relevant literature on this topic, we searched the PubMed database through our institution’s library for articles combining the terms “autoimmune pancreatitis”, “pancreatic adenocarcinoma”, “chronic pancreatitis”, “intraductal papillary mucinous neoplasm”, “artificial intelligence”, and “endoscopic ultrasound”.
Autoimmune pancreatitis is an inflammatory condition of the pancreas commonly associated with a constellation of findings referred to as immunoglobulin G4-related disease. AIP is characterized radiologically/endoscopically by diffuse or focal enlargement of the pancreas parenchyma and diffuse irregular narrowing of the main pancreatic duct, histologically by pancreatic fibrosis and lymphoplasmacytic infiltration, and serologically by increased levels of serum gamma globulin, including immunoglobulin G4 (IgG4)[20,21]. The diagnosis of AIP can be challenging due to the overlap of clinical, laboratory and imaging findings with those of PDAC[22-24]. Studies have shown that 2%-5% of patients who undergo pancreatic resection of suspected cancer are found to have AIP on histopathologic evaluation, and instead of receiving highly effective immunosuppressive therapy such as corticosteroids, these patients are left to manage the morbidity associated with an invasive surgery[25,26]. While EUS remains the preeminent diagnostic tool in evaluating pancreatic diseases, the yield of needle aspiration/biopsy techniques can be inconclusive or non-specific, creating a diagnostic dilemma that may ultimately delay or compromise patient care[25-28].
In late 2020, Marya et al[22] published novel research on the development of EUS-based AI to improve the diagnosis of AIP. Using a CNN built from a large collection of EUS images and videos (583 patients: 146 AIP, 292 PDAC, 72 CP, 73 normal pancreas), their team sought to develop a reliable, real-time method of distinguishing AIP from PDAC on EUS evaluation. Going one step further, they also used occlusion heatmapping to identify key sonographic features of AIP compared to PDAC, further strengthening the utility of their model. On combined still image and continuous video image analysis, the developed CNN was able to distinguish AIP from PDAC with 90% sensitivity and 87% specificity; and distinguish AIP from all other studied diagnoses (PDAC, CP, normal pancreas) with 90% sensitivity and 78% specificity. On continuous video image analysis, the developed CNN was able to successfully differentiate AIP from PDAC with a sensitivity of 90% and specificity of 93%; and differentiate AIP from all other studied diagnoses with a sensitivity of 90% and specificity of 85%. Furthermore, occlusion heatmap evaluation showed that “enhanced hyperechoic interfaces between pancreas parenchyma and pancreas duct/vessels” were predictive of AIP, and “post-acoustic enhancement deep to a dilated pancreas duct” was more commonly associated with PDAC. In addition, the study evaluated the accuracy of diagnosis between the CNN and a group of expert endosonographers, showing that the CNN correctly diagnosed AIP with a sensitivity of 88.2% and specificity of 82.5%, while expert endosonographers correctly diagnosed AIP with a sensitivity of 53.8% and specificity of 86.7%. Overall, this study serves as a model for the application of AI in the EUS evaluation of pancreatic pathologies including AIP.
CP is an irreversible fibro-inflammatory condition caused by recurrent or persistent pancreatic parenchymal injury[29]. The diagnosis of CP is often made by analyzing a patient’s risk factors, radiographic imaging results and direct/indirect pancreatic function laboratory tests. EUS-guided tissue acquisition still serves as the gold standard for CP diagnosis when less invasive tools are inconclusive, however, studies have found similar sensitivities and specificities in the diagnosis of CP using EUS, MRI or CT[30]. This again identifies another diagnostic dilemma for which AI may serve a role to improve diagnostic accuracy, thereby improving patient care and outcomes.
Computer aided diagnosis based on digital image analysis (DIA) was initially utilized in a small study attempting to differentiate between focal, pseudotumorous pancreatitis and pancreatic malignancy with an overall diagnostic accuracy of 89%[31]. In 2008, Săftoiu et al developed a neural network to differentiate between CP and pancreatic malignancy through imaging features of EUS-elastography, further expanding to include the evaluation of contrast-enhanced EUS images in 2015[19]. Their initial system was able to differentiate between malignant and benign pancreatic masses with a sensitivity of 91.4%, specificity of 87.9% and accuracy of 89.7%. Das et al[32] used DIA of the spatial distribution of pixels on EUS images to create a neural network that could differentiate PDAC and CP with a 93% accuracy. In 2013, Zhu et al[33] published data on the use of a support vector machine predictive model to differentiate PDAC and CP based on EUS images which achieved a diagnostic accuracy of 94%. Overall, these studies provide positive reinforcement to the notion that AI can improve EUS differentiation of pancreatic malignancy from other pathologies including CP.
With the increasing detection of pancreatic cystic lesions on cross-sectional imaging, IPMNs have become an important pancreatic pathology given their potential for malignant transformation[34]. Early resection of IPMNs, particularly those with high grade dysplasia limit the progression to PDAC. International consensus guidelines for IPMN management have identified high risk stigmata (i.e., obstructive jaundice) and worrisome features (size > 3 cm, enhancing mural nodule < 5 mm, thickened cyst wall, MPD > 5-9 mm, abrupt change in MPD diameter) of malignancy associated with IPMN[34]. However, the use of these features alone to differentiate benign vs malignant IPMN leaves room for improvement, particularly through the use of AI-assisted EUS evaluation. In 2019, Kuwahara et al[35] performed a retrospective single-center study that developed an EUS-based CNN to differentiate benign vs malignant IPMNs. Their model identified malignant IPMNs with a diagnostic accuracy of 94%, compared to the human pre-operative diagnosis control group based on consensus guidelines which had an accuracy of 56%. While further research in this area is needed, the overarching theme of improved diagnostic accuracy when AI is applied to EUS evaluation of pancreatic disease appears to be evident.
The diagnosis of pancreatic lesions can be difficult, often stemming from the overlap of features found in benign lesions with those found in PDAC. The development of improved diagnostic tools to differentiate PDAC from other pancreatic lesions presents an opportunity for significant impact on the overall care of patients with pancreatic disease. More robust studies are needed to validate the current available research, namely in the form of prospective, multicenter studies which may further determine the generalizability of current models and the overall, real-time clinical application of these AI systems. It should be noted that standardization of endoscopic image capture and reporting may better help facilitate future interdisciplinary work in this field[36,37]. While the use of AI to evaluate the pancreas appears to be in its early stages, the potential for AI-assisted EUS assessment provides an exciting and promising future for the diagnosis and management of pancreatic lesions.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Byeon H, Imai Y S-Editor: Liu M L-Editor: A P-Editor: Wang LYT
| 1. | Pannala R, Krishnan K, Melson J, Parsi MA, Schulman AR, Sullivan S, Trikudanathan G, Trindade AJ, Watson RR, Maple JT, Lichtenstein DR. Artificial intelligence in gastrointestinal endoscopy. VideoGIE. 2020;5:598-613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 2. | LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521:436-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36149] [Cited by in RCA: 20052] [Article Influence: 2005.2] [Reference Citation Analysis (0)] |
| 3. | Abadir AP, Ali MF, Karnes W, Samarasena JB. Artificial Intelligence in Gastrointestinal Endoscopy. Clin Endosc. 2020;132-141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 4. | Akshintala VS, Khashab MA. Artificial intelligence in pancreaticobiliary endoscopy. J Gastroenterol Hepatol. 2021;36:25-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 5. | Fernández-Esparrach G, Bernal J, López-Cerón M, Córdova H, Sánchez-Montes C, Rodríguez de Miguel C, Sánchez FJ. Exploring the clinical potential of an automatic colonic polyp detection method based on the creation of energy maps. Endoscopy. 2016;48:837-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 87] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 6. | Tajbakhsh N, Gurudu SR, Liang J. Automated Polyp Detection in Colonoscopy Videos Using Shape and Context Information. IEEE Trans Med Imaging. 2016;35:630-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 276] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 7. | Takemura Y, Yoshida S, Tanaka S, Onji K, Oka S, Tamaki T, Kaneda K, Yoshihara M, Chayama K. Quantitative analysis and development of a computer-aided system for identification of regular pit patterns of colorectal lesions. Gastrointest Endosc. 2010;72:1047-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 8. | Tischendorf JJ, Gross S, Winograd R, Hecker H, Auer R, Behrens A, Trautwein C, Aach T, Stehle T. Computer-aided classification of colorectal polyps based on vascular patterns: a pilot study. Endoscopy. 2010;42:203-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 9. | Maeda Y, Kudo SE, Mori Y, Misawa M, Ogata N, Sasanuma S, Wakamura K, Oda M, Mori K, Ohtsuka K. Fully automated diagnostic system with artificial intelligence using endocytoscopy to identify the presence of histologic inflammation associated with ulcerative colitis (with video). Gastrointest Endosc. 2019;89:408-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 165] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 10. | Takenaka K, Ohtsuka K, Fujii T, Negi M, Suzuki K, Shimizu H, Oshima S, Akiyama S, Motobayashi M, Nagahori M, Saito E, Matsuoka K, Watanabe M. Development and Validation of a Deep Neural Network for Accurate Evaluation of Endoscopic Images From Patients With Ulcerative Colitis. Gastroenterology. 2020;158:2150-2157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 181] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 11. | Zhou J, Wu L, Wan X, Shen L, Liu J, Zhang J, Jiang X, Wang Z, Yu S, Kang J, Li M, Hu S, Hu X, Gong D, Chen D, Yao L, Zhu Y, Yu H. A novel artificial intelligence system for the assessment of bowel preparation (with video). Gastrointest Endosc 2020; 91: 428-435. e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 103] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 12. | Wu L, Zhang J, Zhou W, An P, Shen L, Liu J, Jiang X, Huang X, Mu G, Wan X, Lv X, Gao J, Cui N, Hu S, Chen Y, Hu X, Li J, Chen D, Gong D, He X, Ding Q, Zhu X, Li S, Wei X, Li X, Wang X, Zhou J, Zhang M, Yu HG. Randomised controlled trial of WISENSE, a real-time quality improving system for monitoring blind spots during esophagogastroduodenoscopy. Gut. 2019;68:2161-2169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 244] [Cited by in RCA: 209] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 13. | Zheng W, Zhang X, Kim JJ, Zhu X, Ye G, Ye B, Wang J, Luo S, Li J, Yu T, Liu J, Hu W, Si J. High Accuracy of Convolutional Neural Network for Evaluation of Helicobacter pylori Infection Based on Endoscopic Images: Preliminary Experience. Clin Transl Gastroenterol. 2019;10:e00109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 14. | Luo H, Xu G, Li C, He L, Luo L, Wang Z, Jing B, Deng Y, Jin Y, Li Y, Li B, Tan W, He C, Seeruttun SR, Wu Q, Huang J, Huang DW, Chen B, Lin SB, Chen QM, Yuan CM, Chen HX, Pu HY, Zhou F, He Y, Xu RH. Real-time artificial intelligence for detection of upper gastrointestinal cancer by endoscopy: a multicentre, case-control, diagnostic study. Lancet Oncol. 2019;20:1645-1654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 253] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 15. | de Groof AJ, Struyvenberg MR, van der Putten J, van der Sommen F, Fockens KN, Curvers WL, Zinger S, Pouw RE, Coron E, Baldaque-Silva F, Pech O, Weusten B, Meining A, Neuhaus H, Bisschops R, Dent J, Schoon EJ, de With PH, Bergman JJ. Deep-Learning System Detects Neoplasia in Patients With Barrett's Esophagus With Higher Accuracy Than Endoscopists in a Multistep Training and Validation Study With Benchmarking. Gastroenterology 2020; 158: 915-929. e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 222] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 16. | Ding Z, Shi H, Zhang H, Meng L, Fan M, Han C, Zhang K, Ming F, Xie X, Liu H, Liu J, Lin R, Hou X. Gastroenterologist-Level Identification of Small-Bowel Diseases and Normal Variants by Capsule Endoscopy Using a Deep-Learning Model. Gastroenterology 2019; 157: 1044-1054. e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 208] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 17. | Zhang MM, Yang H, Jin ZD, Yu JG, Cai ZY, Li ZS. Differential diagnosis of pancreatic cancer from normal tissue with digital imaging processing and pattern recognition based on a support vector machine of EUS images. Gastrointest Endosc. 2010;72:978-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 18. | Zhu J, Wang L, Chu Y, Hou X, Xing L, Kong F, Zhou Y, Wang Y, Jin Z, Li Z. A new descriptor for computer-aided diagnosis of EUS imaging to distinguish autoimmune pancreatitis from chronic pancreatitis. Gastrointest Endosc 2015; 82: 831-836. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Săftoiu A, Vilmann P, Gorunescu F, Gheonea DI, Gorunescu M, Ciurea T, Popescu GL, Iordache A, Hassan H, Iordache S. Neural network analysis of dynamic sequences of EUS elastography used for the differential diagnosis of chronic pancreatitis and pancreatic cancer. Gastrointest Endosc. 2008;68:1086-1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 174] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 20. | Chari ST, Smyrk TC, Levy MJ, Topazian MD, Takahashi N, Zhang L, Clain JE, Pearson RK, Petersen BT, Vege SS, Farnell MB. Diagnosis of autoimmune pancreatitis: the Mayo Clinic experience. Clin Gastroenterol Hepatol. 2006;4:1010-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 722] [Cited by in RCA: 657] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 21. | Chari ST, Takahashi N, Levy MJ, Smyrk TC, Clain JE, Pearson RK, Petersen BT, Topazian MA, Vege SS. A diagnostic strategy to distinguish autoimmune pancreatitis from pancreatic cancer. Clin Gastroenterol Hepatol. 2009;7:1097-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 224] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 22. | SARLES H, SARLES JC, MURATORE R, GUIEN C. Chronic inflammatory sclerosis of the pancreas--an autonomous pancreatic disease? Am J Dig Dis. 1961;6:688-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 496] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 23. | Comings DE, Skubi KB, Van Eyes J, Motulsky AG. Familial multifocal fibrosclerosis. Findings suggesting that retroperitoneal fibrosis, mediastinal fibrosis, sclerosing cholangitis, Riedel's thyroiditis, and pseudotumor of the orbit may be different manifestations of a single disease. Ann Intern Med. 1967;66:884-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 268] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 24. | Gardner TB, Levy MJ, Takahashi N, Smyrk TC, Chari ST. Misdiagnosis of autoimmune pancreatitis: a caution to clinicians. Am J Gastroenterol. 2009;104:1620-1623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Marya NB, Powers PD, Chari ST, Gleeson FC, Leggett CL, Abu Dayyeh BK, Chandrasekhara V, Iyer PG, Majumder S, Pearson RK, Petersen BT, Rajan E, Sawas T, Storm AC, Vege SS, Chen S, Long Z, Hough DM, Mara K, Levy MJ. Utilisation of artificial intelligence for the development of an EUS-convolutional neural network model trained to enhance the diagnosis of autoimmune pancreatitis. Gut. 2021;70:1335-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 85] [Article Influence: 21.3] [Reference Citation Analysis (1)] |
| 26. | Hewitt MJ, McPhail MJ, Possamai L, Dhar A, Vlavianos P, Monahan KJ. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: a meta-analysis. Gastrointest Endosc. 2012;75:319-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 510] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 27. | Ishikawa T, Kawashima H, Ohno E, Suhara H, Hayashi D, Hiramatsu T, Matsubara H, Suzuki T, Kuwahara T, Ishikawa E, Shimoyama Y, Kinoshita F, Hirooka Y, Fujishiro M. Usefulness of endoscopic ultrasound-guided fine-needle biopsy for the diagnosis of autoimmune pancreatitis using a 22-gauge Franseen needle: a prospective multicenter study. Endoscopy. 2020;52:978-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 28. | Nishimori I, Tamakoshi A, Otsuki M; Research Committee on Intractable Diseases of the Pancreas; Ministry of Health, Labour, and Welfare of Japan. Prevalence of autoimmune pancreatitis in Japan from a nationwide survey in 2002. J Gastroenterol. 2007;42 Suppl 18:6-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 127] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 29. | Gardner TB, Adler DG, Forsmark CE, Sauer BG, Taylor JR, Whitcomb DC. ACG Clinical Guideline: Chronic Pancreatitis. Am J Gastroenterol. 2020;115:322-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 206] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 30. | Issa Y, Kempeneers MA, van Santvoort HC, Bollen TL, Bipat S, Boermeester MA. Diagnostic performance of imaging modalities in chronic pancreatitis: a systematic review and meta-analysis. Eur Radiol. 2017;27:3820-3844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 31. | Norton ID, Zheng Y, Wiersema MS, Greenleaf J, Clain JE, Dimagno EP. Neural network analysis of EUS images to differentiate between pancreatic malignancy and pancreatitis. Gastrointest Endosc. 2001;54:625-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 76] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 32. | Das A, Nguyen CC, Li F, Li B. Digital image analysis of EUS images accurately differentiates pancreatic cancer from chronic pancreatitis and normal tissue. Gastrointest Endosc. 2008;67:861-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 81] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 33. | Zhu M, Xu C, Yu J, Wu Y, Li C, Zhang M, Jin Z, Li Z. Differentiation of pancreatic cancer and chronic pancreatitis using computer-aided diagnosis of endoscopic ultrasound (EUS) images: a diagnostic test. PLoS One. 2013;8:e63820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (2)] |
| 34. | Tanaka M, Fernández-Del Castillo C, Kamisawa T, Jang JY, Levy P, Ohtsuka T, Salvia R, Shimizu Y, Tada M, Wolfgang CL. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology. 2017;17:738-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 868] [Cited by in RCA: 1157] [Article Influence: 144.6] [Reference Citation Analysis (1)] |
| 35. | Kuwahara T, Hara K, Mizuno N, Okuno N, Matsumoto S, Obata M, Kurita Y, Koda H, Toriyama K, Onishi S, Ishihara M, Tanaka T, Tajika M, Niwa Y. Usefulness of Deep Learning Analysis for the Diagnosis of Malignancy in Intraductal Papillary Mucinous Neoplasms of the Pancreas. Clin Transl Gastroenterol. 2019;10:1-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 128] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 36. | Kuwahara T, Hara K, Mizuno N, Haba S, Okuno N, Koda H, Miyano A, Fumihara D. Current status of artificial intelligence analysis for endoscopic ultrasonography. Dig Endosc. 2021;33:298-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 37. | Ahmad OF, Stassen P, Webster GJ. Artificial intelligence in biliopancreatic endoscopy: Is there any role? Best Pract Res Clin Gastroenterol. 2021;52-53:101724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |