Published online Jun 28, 2021. doi: 10.37126/aige.v2.i3.63
Peer-review started: April 29, 2021
First decision: May 19, 2021
Revised: May 29, 2021
Accepted: June 23, 2021
Article in press: June 23, 2021
Published online: June 28, 2021
Processing time: 67 Days and 22.6 Hours
Artificial intelligence (AI) is a branch of computer science. As a new technological science, it mainly develops and expands human intelligence through the research of intelligence theory, methods and technology. In the medical field, AI has bright application prospects (for example: imaging, diagnosis and treatment). The exploration of robotic gastroscopy and colonoscopy systems is not only a bold attempt, but also an inevitable trend of AI in the development of digestive endoscopy in the future. Based on the current research findings, this article summarizes the research progress of colonoscopy, and looking forward for the application of AI in colonoscopy.
Core Tip: Artificial intelligence is a new technological science that studies and develops theories, methods, technologies and application systems for simulating and expanding human intelligence. This article will systematically review the exploration and application of artificial intelligence technology in colonoscopy, and look forward to the development direction of intelligent colonoscopy.
- Citation: Wang RG. Progress and prospects of artificial intelligence in colonoscopy. Artif Intell Gastrointest Endosc 2021; 2(3): 63-70
- URL: https://www.wjgnet.com/2689-7164/full/v2/i3/63.htm
- DOI: https://dx.doi.org/10.37126/aige.v2.i3.63
Artificial intelligence (AI) is a new technological science that studies and develops theories, methods, technologies and application systems for simulating and expanding human intelligence. It relates to many fields, for instance, computer science, cybernetics, information theory, and neuroscience. The first AI seminar at Dartmouth College in 1956 marked the birth of the AI, but the development of AI has experienced several ups and downs. AI has achieved results both theoretically and practically in these cycles. It has made solid progress in the world, especially when scientists made breakthrough progress in deep learning.
In its more than 60 years of development, AI has been used in computer vision, natural language processing, data mining, automatic speech recognition. The applications of intelligent robot, automatic programming, and expert systems are becoming increasingly mature, making AI one of the three cutting-edge technologies in the 21st century.
AI is hailed as the stethoscope of the 21st century[1]. With the strengthening of people's health awareness, preventive and precise treatments have been paid more attention at the same time. The improvement of medical standards and the improvement of medical equipment have made the process of patients' visits produce increasingly medical data. Image recognition, speech/semantic recognition, and expert system have received more and more attention in the medical field, smart medical products have gradually emerged[2-4]. A large amount of image data and diagnostic data are used to simulate the mind and diagnostic process of medical experts especially in the field of medical image recognition, AI is expected to partially replace traditional empirical diagnosis so as to provide a more reliable diagnosis and treatment plan.
In recent years, the incidence of colorectal adenoma, colorectal cancer, and inflammatory bowel disease has increased significantly[5-7], causing great harm to human's health. Colonoscopy is the first choice for the diagnosis and treatment of colorectal diseases. It can not only intuitively judge the nature of the lesion, but also obtain biopsy specimens for pathological diagnosis. Colonoscopy is of great significance, especially in preventing and treating colorectal cancer, as it can be used to screen and follow up high-risk groups in patients who are asymptomatic. We can greatly reduce the incidence of colorectal cancer by adopting corresponding treatments according to the condition, and achieve the purpose of primary prevention. Even if colorectal lesions develop to the early stage of cancer, the 5-year survival rate of endoscopic treatment can still exceed 90%[6].
Studies have found that gradual expansion of colorectal cancer screening in asymptomatic populations and the early diagnosis promotion have extremely important socio-economic significance[8-10]. The popularization of colonoscopy screening among high-risk populations is restricted by the hard operation, excessive physical exertion, and limitation of technical inheritance, which has caused bottlenecks. At this time, the development and maturity of AI technology provides new ideas and possibilities for breaking through these bottlenecks.
According to the anatomical characteristics of the intestine, the ascending colon, descending colon and upper rectum, which are straighter and smaller in extension, are generally easier to pass with colonoscopy. However, the transverse colon and sigmoid colon are in a free state, with longer mesentery and larger mobility, which can easily cause loops. Common types of loops in the sigmoid colon include N loops, α loops, reverse α loops, and atypical loops, while the common types of loops in the transverse colon include deep loops/dangling loops, deep large γ loops, and inverted splenic loops[11]. Usually, the time for a skilled endoscopist to enter the cecum is about 4-6 minutes, but someone who have difficulty in this process may not be able to reach it, even if the operation time is more than 1 h[12].
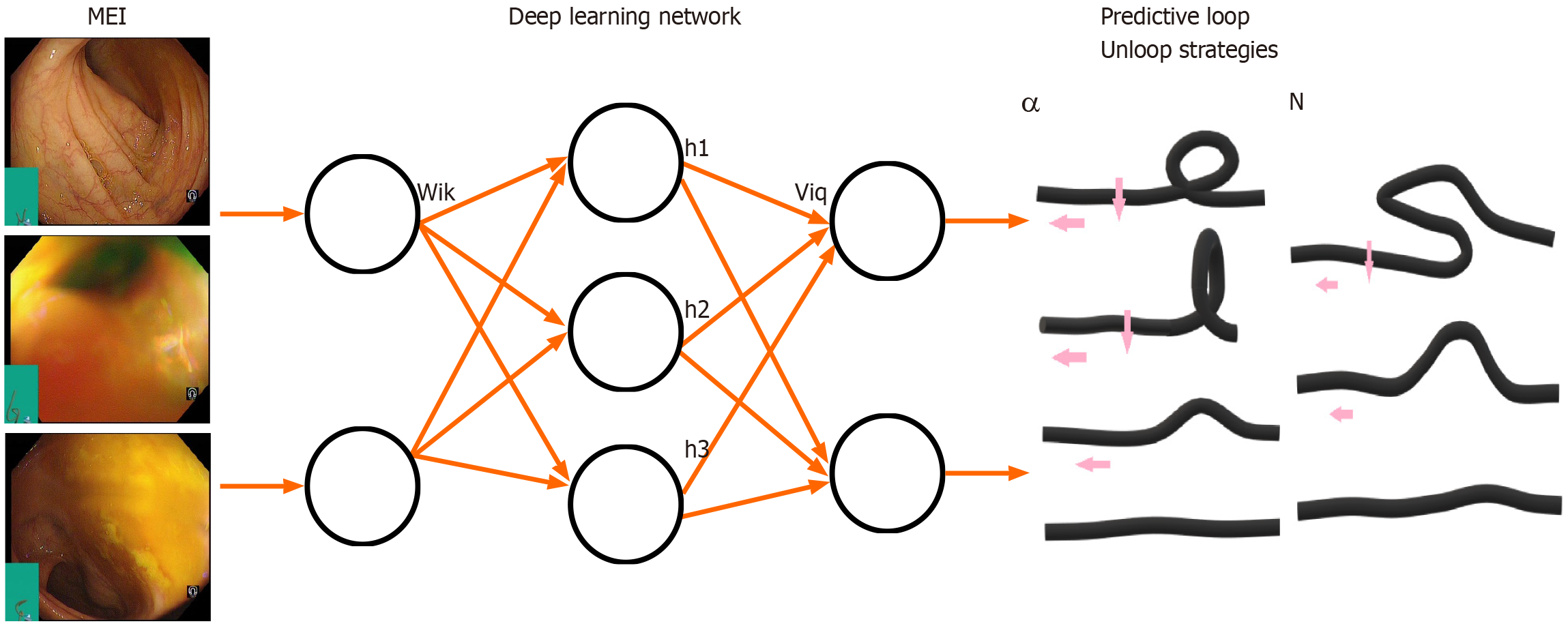
In view of the factors of patients who develop a loop during colonoscopy, experts have conducted many studies which found that factors including long-term constipation, abdominal surgery history, female, body mass index is lower or higher than normal, the volume of visceral fat tissue is low and the proficiency of colonoscopy directly affect the formation of intestinal loops[13] (Figure 1).
The successful removal of the loop is key for a colonoscopy to reach the cecum, and it is necessary for the endoscopist to be able to observe and monitor the shape of colonoscopy in order to overcome this technical difficulty. With the continuous advancement of colonoscopy accessories, magnetic endoscopic imaging (MEI), a real-time three-dimensional imaging colonoscopy-assisted positioning technology, has become an effective tool for observing the shape of the colonoscopy in human body[14]. There is a meta-analysis that summarizes 8 randomized controlled trials and contains 2967 patients which compares cecal intubation rates and times, sedation dose, abdominal pain scores and the use of ancillary maneuvers between MEI and standard colonoscopy. The conclusion is that compared with traditional technique, MEI has an advantages in cecal intubation rate, but MEI did not have any distinct advantages for cecal intubation time and lower pain scores[15] (DW1). The variable stiffness of the colonoscopy body, flexible tubing, and Responsive Insertion Technology (RIT)[16,17] make the inspection equipment more maneuverable. Prieto-de-Frías et al[17] and Pasternak et al[18] studied the application of RIT technology in reducing discomfort and pain during colonoscopy insertion. The results showed that the RIT group shortened the cecal intubation time, decrease intestinal loop formation, lower manual pressure of abdomen and decrease discomfort or pain of patients. Although RIT technology has shown good application prospects, it still relies on the experience of unwinding of endoscopists, some examinations are time-consuming and patients cannot achieve a good medical result.
MEI and RIT technology are an improvement of traditional colonoscopy in response to the actual problems in the endoscopy process. AI can explore the images of MEI technology in guiding colonoscopy. Applying deep learning to analyze a large number of unloop images, it is possible in the future to form a complete set of loop prediction and unlooping strategies system. The RIT technology can automatically adjust the bending angle of the intestinal cavity by sensing the degree of curvature of the endoscopic body, and minimize the formation of acute angles. These measures help to reduce the traction of the colonoscopy on the mesentery and the damage to the intestinal mucosa, and achieve the purpose of reducing the pain and injury of the patient during the colonoscopy. In general, MEI and RIT technologies provide useful explorations for the gradual migration of colonoscopy from artificial to intelligent (DW2).
Traditional research methods have limitations, such as multi-factors, complex variables, interrelationships, descriptive difficulties and quantitative mechanisms. It is urgent to introduce new ideas and methods to solve these problems. It can be described with a simplified model by demonstrating whether the colonoscopy is looped, and providing the corresponding unlooping strategy, as we mentioned above. The operation of the colonoscopy handle and insertion part by the endoscopist can be regarded as an input function. Analyze the correspondence between the data of the input function under the loop condition and the corresponding results of loop and unloop in a large number of cases, also fitting the unloop strategy function to assist the doctor in decision-making through the intelligent system. MEI and other technologies can display the posture of the colonoscopy in the intestine in real time, and wearable pressure sensor device can generate a series of mechanical data. A specific neural network model can be constructed to synthesize a loop-free strategy function by analyzing large amounts of data. We look forward to the AI-assisted system will be able to realize a loopless and painless colonoscopy in the future (DW3).
Smart medicine is the application of AI to improve the ability of medical services, which is the trend of future medical advancement. Smart medical care is to create a regional medical information platform for health records and use advanced Internet of Things technology to realize the interaction among patient-medical staff, institutions and equipment for achieving informatization gradually. Intelligent medicine cannot be separated from AI technology. On the basis of digital medicine, internet medicine and mobile medicine, smart medicine is gradually taking shape.
The emergence of smart medicine provides a new feasible path to solve the outstanding problems that restrict the medical development. Intelligent medical care plays an important role in science, it not only changes the traditional diagnosis and treatment methods but also improves the accuracy and efficiency, in addition, it relies on the advanced algorithms and powerful computing power of AI technology to significantly increase the success rate of medical innovation research and development and shorten time. In addition, smart medicine can also solve social problems, such as insufficient medical resources, unbalanced regional distribution, costs, personalized medical services, and respond to aging and chronic disease diagnosis and treatment needs. With the development of smart medical technology, AI can completely assist doctors in such arduous diagnoses in future, for example pathological diagnosis, laboratory test diagnosis, and imaging diagnosis.
Regarding the colonoscopy continuum robot-assisted system, some scholars have studied structural design, passability, compliance control based on force perception, and multi-motor control system design. Lee et al[19] proposed a caterpillar-like flexible self-propelled colonoscopy robot, which can effectively corner bends and conducted clinical trials, while Breedveld proposed a colonoscopy robot movement method based on a rollable doughnut[20]. Scholars research on the relevant working environment and clinical experiment results of the colonoscopy continuum robot assistance system, the flexible arbitrary bending of the colonoscopy assistance system, the exploration of the biomimetic and the continuum robot design, which are the most irreplaceable (DW4) part of the robot-assisted colonoscopy system, its structure and design provide an important reference.
In December 1998, the first Da Vinci Robot-Assisted Surgery System came out. In June 2000, the Da Vinci Robot-Assisted Surgery System became the first automatic mechanical system approved by the Food and Drug Administration for laparoscopic surgery. At present, the system is widely used. In 2017, the flexible endoscopy manipulation robot developed by the General Hospital of the Chinese People's Liberation Army successfully carried out clinical applications. It surpassed the traditional endoscopy operation method in terms of coordinated operation of multiple degrees of freedom of the endoscopy and quantitative display of operating parameters, and laid the foundation for high-quality standardized operation and internet medical treatment.
The research on small soft robots with multi-mode motion published by Hu has attracted widespread attention[21]. The article pointed out that the soft robot has bright prospects in the fields of bioengineering and minimally invasive treatment. They have greater potential to achieve high maneuverability through multi-channel motion because small soft robots have a higher degree of freedom than rigid robots. We can expect that these small flexible robots are equipped with camera devices to produce soft motion which is similar to worms that can move in the human digestive tract and has better control and operability than the magnetic-control capsule endoscopy.
At present, there are no reports on the use of flexible endoscopic robots for endoscopic treatment, and the author believes that the reason is that endoscopic treatment is different from examination. Endoscopic treatment have higher requirements for the operation technology, including horizontal and vertical joint movement of the endoscope handle to achieve rotation, control colonoscopy and handle strength during the treatment (DW5). The grasp of the patients’ breathing and coordination with its movement are relatively subtle that are difficult to achieve at this stage. However, with the accumulation of quantitatively analyzed endoscopic operation data and the construction of software endoscopic operation strategy functions, combined with powerful algorithms and machine learning, AI will continue to improve the existing colonoscopy equipment, accessories and instruments in the future. At the same time, it may partly replace manual labor, reduce medical costs and improve efficiency.
With the progress of colonoscopy operation technology and endoscopic imaging technology, especially magnifying endoscopy has achieved remarkable results in the detection of fine structure on the surface of colorectal tumors. It should be pointed out that the development of electronic staining endoscopy is extremely rapid, such as narrowband imaging technology (NBI), flexible spectral imaging color enhancement technology (FICE) and i-Scan digital contrast technology (iSCAN), etc. (DW6). These imaging technologies can highlight the mucosal surface structure or capillary morphology by switching between different wavelengths of light, clearly observe the boundary and scope of the lesion, and obtain a visual effect similar to chromoendoscopy.
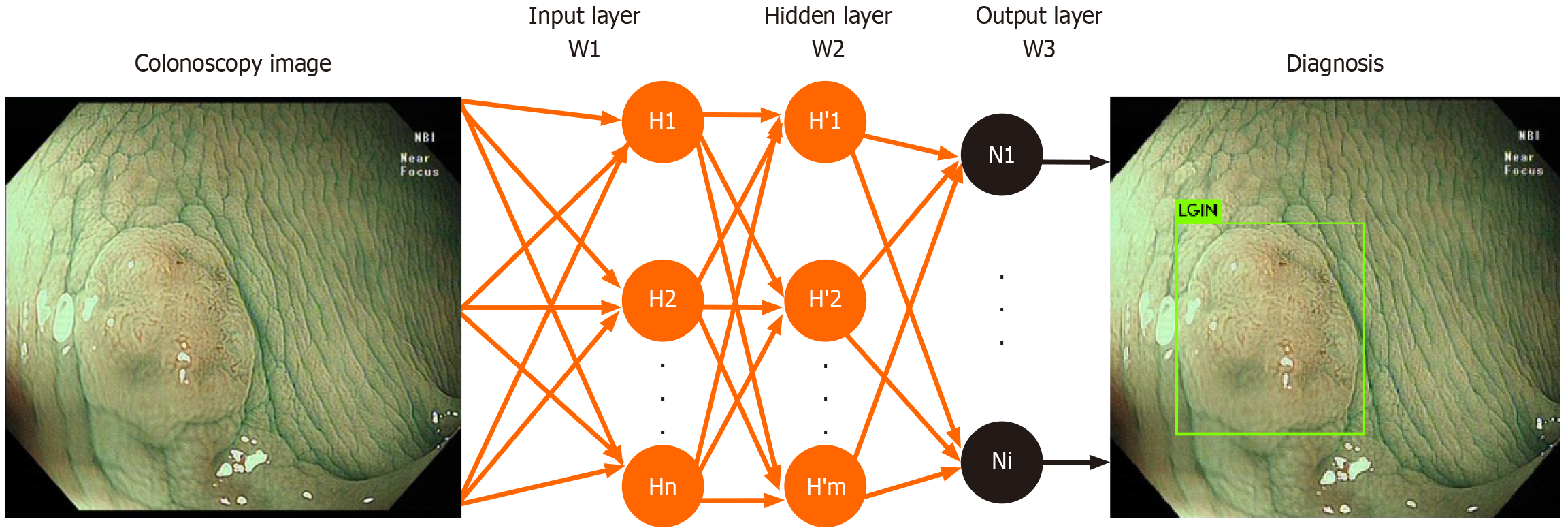
Depth research for colonoscopy image recognition has already started, using specific data sets and special deep learning network structure models to establish a labeled colonic lesion image data set to provide technical support for intelligent image recognition of colonoscopy images. Computer-aided diagnosis analysis used for accurately classify neoplastic/hyperplastic, adenoma/non-adenomas colorectal polyps found that the system have a classification accuracy rate above 90%, and the diagnosis time required is decreased compared with endoscopy experts and non-experts[4,22-24].
The dynamic recognition system decomposes the real-time video of the colonoscopy into a continuous picture. The deep learning neural network is used for the recognition of the marked images, and the fine recognition of each image is carried out to realize the purpose of automatically discovering and classifying the lesions. Mori et al[25] used deep learning models to analyze colonoscopy videos to classify adenomatous and hyperplastic polyps in real time, the results find that the accuracy of the AI model is 94%, the sensitivity and the specificity is 98% and 83% respectively (Figure 2).
We expect that AI combined with white light, chromoendoscopy and magnifying endoscopy will greatly reduce the time spent on diagnosis and treatment in the future, thereby providing great help for the clinical and scientific research of gastrointestinal diseases.
In recent years, the rapid development of capsule endoscopy technology, especially the appearance of magnetron capsule endoscopy, which has realized the controllability of the capsule endoscopy on some extent. The emergence of capsule endoscopy has made up for the insufficiency of gastroscopy and colonoscopy, the patients acceptance is high because of the whole examination process is painless. Nowadays, the application of capsule endoscopy is mostly focused on discovery of small bowel disease, for example bleeding.
AI is widely used in capsule endoscopy technology. The pixels are grouped by super pixel segmentation, the red ratio in the RGB space is used to extract the features of each super pixel, and these things are input into Support Vector Machines (SVM) for classification for intelligent recognition of capsule endoscopic bleeding. The specificity of the experimental results is 83%-98%, and the sensitivity is 94%-99%[26,27].
In order to identify polyps in capsule endoscopy images, Yuan and Meng[28] proposed a new complex feature learning method, which is a stacked sparse autoencoder with image manifold constraint. This method introduces multiple image constraints force images in the same category to share similar learning features and keep them, so the learned features retain a large number of differences and small internal differences in the images. The results show that the average overall recognition accuracy of this method is 98%, and could be further utilized in the clinical trials to help physicians from the tedious image reading work.
The development of depth research has enabled AI to achieve fruitful results in many aspects. However, there is no major breakthrough in the theory that AI follows, and the methods from supervised learning to unsupervised learning are still being explored. Therefore, looking for in-depth theoretical explanations is an important issue that must be solved in the development of the studies. In addition, deep learning generally requires a large amount of data, but not all applications have the conditions for it. Therefore, how to realize traditional knowledge expression and data-driven knowledge learning is an important research direction in the future. Furthermore, the neural network model needs to be adapted to transfer the learned knowledge to new conditions and environments in order to acquire the ability to solve many practical problems from a small number of learning samples. Finally, the method of machine learning is determined according to the functional relationship between the data and the target, a "deep forest" learning method, with a comparable setting proposed by Zhou and Feng[29], achieved a considerable or even better than deep neural networks.
In the field of colonoscopy image recognition, experts and scholars have made very useful explorations on the intelligent recognition of colorectal lesions, but most of them are limited to judge colorectal polyps. To achieve the integration of doctors and patients with auxiliary examination equipment, it is necessary to further expand the colorectal lesion image data set and the types of diseases involved. It must be pointed out that the endoscopic manifestations of colorectal diseases are various, the same disease often manifests differences in different periods and different diseases have very little difference in a specific period, and pathological diagnosis is still the gold standard.
In short, AI in colonoscopy has significant social benefits and bright application prospects, and it is foreseeable that smart medicine is an inevitable trend in medical development. Based on previous research, integrating colonoscopy’s loop factors, unlooping strategies, active lesion capture and recognition, and assistive robotics technology, we have reason to believe that the future smart colonoscopy system will bring a revolution, and promote the diagnosis and treatment of colorectal diseases, especially the widespread development of colorectal cancer screening for the benefit of mankind.
In the writing process of this article, I have adopted the opinions of Dr. Jiang X, the chief physician of the Department of Gastroenterology of Beijing Tsinghua Chang Gung Hospital, and the postdoctoral fellow of iCenter Liang X of Tsinghua University. I would like to express my sincere thanks!
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: Chinese Anti-Cancer Association, No. M167106008M.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bedny I S-Editor: Gao CC L-Editor: Filipodia P-Editor: Wang LYT
| 1. | Meskó B, Drobni Z, Bényei É, Gergely B, Győrffy Z. Digital health is a cultural transformation of traditional healthcare. Mhealth. 2017;3:38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 253] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 2. | Leachman SA, Merlino G. Medicine: The final frontier in cancer diagnosis. Nature. 2017;542:36-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Furiasse N, Thomas JD. Automated Algorithmic Software in Echocardiography: Artificial Intelligence? J Am Coll Cardiol. 2015;66:1467-1469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Zhang R, Zheng Y, Mak TW, Yu R, Wong SH, Lau JY, Poon CC. Automatic Detection and Classification of Colorectal Polyps by Transferring Low-Level CNN Features From Nonmedical Domain. IEEE J Biomed Health Inform. 2017;21:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 168] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 5. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11444] [Cited by in RCA: 13212] [Article Influence: 1468.0] [Reference Citation Analysis (3)] |
| 6. | DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, Alteri R, Robbins AS, Jemal A. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64:252-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1980] [Cited by in RCA: 2190] [Article Influence: 199.1] [Reference Citation Analysis (0)] |
| 7. | Zhang YZ, Li YY. Inflammatory bowel disease: pathogenesis. World J Gastroenterol. 2014;20:91-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 751] [Cited by in RCA: 1042] [Article Influence: 94.7] [Reference Citation Analysis (18)] |
| 8. | Lejeune C, Sassi F, Ellis L, Godward S, Mak V, Day M, Rachet B. Socio-economic disparities in access to treatment and their impact on colorectal cancer survival. Int J Epidemiol. 2010;39:710-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 9. | Borowski DW, Cawkwell S, Zaidi SM, Toward M, Maguire N, Gill TS. Primary care referral practice, variability and socio-economic deprivation in colorectal cancer. Colorectal Dis. 2016;18:1072-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Solmi F, Von Wagner C, Kobayashi LC, Raine R, Wardle J, Morris S. Decomposing socio-economic inequality in colorectal cancer screening uptake in England. Soc Sci Med. 2015;134:76-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Chan WK, Saravanan A, Manikam J, Goh KL, Mahadeva S. Appointment waiting times and education level influence the quality of bowel preparation in adult patients undergoing colonoscopy. BMC Gastroenterol. 2011;11:86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 12. | Zhao SB, Yang X, Fang J, Wang SL, Gu L, Xia T, Su XJ, Wang D, Li ZS, Bai Y. Effect of left lateral tilt-down position on cecal intubation time: a 2-center, pragmatic, randomized controlled trial. Gastrointest Endosc. 2018;87:852-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Moon SY, Kim BC, Sohn DK, Han KS, Kim B, Hong CW, Park BJ, Ryu KH, Nam JH. Predictors for difficult cecal insertion in colonoscopy: The impact of obesity indices. World J Gastroenterol. 2017;23:2346-2354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Bruce M, Choi J. Detection of endoscopic looping during colonoscopy procedure by using embedded bending sensors. Med Devices (Auckl). 2018;11:171-191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Chen Y, Duan YT, Xie Q, Qin XP, Chen B, Xia L, Zhou Y, Li NN, Wu XT. Magnetic endoscopic imaging vs standard colonoscopy: meta-analysis of randomized controlled trials. World J Gastroenterol. 2013;19:7197-7204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Shah SG, Saunders BP. Aids to insertion: magnetic imaging, variable stiffness, and overtubes. Gastrointest Endosc Clin N Am. 2005;15:673-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Prieto-de-Frías C, Muñoz-Navas M, Carretero C, Carrascosa J, Betés MT, de-la-Riva S, Herraiz MT, Súbtil JC. Comparative study of a responsive insertion technology (RIT) colonoscope vs a variable-stiffness colonoscope. Rev Esp Enferm Dig. 2013;105:208-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Pasternak A, Szura M, Solecki R, Matyja M, Szczepanik A, Matyja A. Impact of responsive insertion technology (RIT) on reducing discomfort during colonoscopy: randomized clinical trial. Surg Endosc. 2017;31:2247-2254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Lee D, Joe S, Choi J, Lee BI, Kim B. An elastic caterpillar-based self-propelled robotic colonoscope with high safety and mobility. Mechatronics. 2016;39:54-62. [DOI] [Full Text] |
| 20. | Rösch T, Adler A, Pohl H, Wettschureck E, Koch M, Wiedenmann B, Hoepffner N. A motor-driven single-use colonoscope controlled with a hand-held device: a feasibility study in volunteers. Gastrointest Endosc. 2008;67:1139-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 21. | Hu W, Lum GZ, Mastrangeli M, Sitti M. Small-scale soft-bodied robot with multimodal locomotion. Nature. 2018;554:81-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 988] [Cited by in RCA: 859] [Article Influence: 122.7] [Reference Citation Analysis (0)] |
| 22. | Chen PJ, Lin MC, Lai MJ, Lin JC, Lu HH, Tseng VS. Accurate Classification of Diminutive Colorectal Polyps Using Computer-Aided Analysis. Gastroenterology. 2018;154:568-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 276] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 23. | Byrne MF, Chapados N, Soudan F, Oertel C, Linares Pérez ML, Kelly R, Iqbal N, Chandelier F, Rex DK. Su1614 Artificial Intelligence (AI) in Endoscopy--Deep Learning for Optical Biopsy of Colorectal Polyps in Real-Time on Unaltered Endoscopic Videos. Gastrointest Endosc. 2017;85:AB364-AB365. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Komeda Y, Handa H, Watanabe T, Nomura T, Kitahashi M, Sakurai T, Okamoto A, Minami T, Kono M, Arizumi T, Takenaka M, Hagiwara S, Matsui S, Nishida N, Kashida H, Kudo M. Computer-Aided Diagnosis Based on Convolutional Neural Network System for Colorectal Polyp Classification: Preliminary Experience. Oncology. 2017;93 Suppl 1:30-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 129] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 25. | Mori Y, Kudo SE, Misawa M, Saito Y, Ikematsu H, Hotta K, Ohtsuka K, Urushibara F, Kataoka S, Ogawa Y, Maeda Y, Takeda K, Nakamura H, Ichimasa K, Kudo T, Hayashi T, Wakamura K, Ishida F, Inoue H, Itoh H, Oda M, Mori K. Real-Time Use of Artificial Intelligence in Identification of Diminutive Polyps During Colonoscopy: A Prospective Study. Ann Intern Med. 2018;169:357-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 355] [Article Influence: 50.7] [Reference Citation Analysis (1)] |
| 26. | Hassan AR, Haque MA. Computer-aided gastrointestinal hemorrhage detection in wireless capsule endoscopy videos. Comput Methods Programs Biomed. 2015;122:341-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 27. | Xu W, Yan G, Wang Z, Liu G, Kuang S, Zhao S. [A method for bleeding detection in endoscopy images using SVM]. Zhongguo Yiliao Qixie Zazhi. 2015;39:9-12. [PubMed] |
| 28. | Yuan Y, Meng MQ. Deep learning for polyp recognition in wireless capsule endoscopy images. Med Phys. 2017;44:1379-1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 99] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 29. | Zhou ZH, Feng J. Deep forest. Nat Sci Rev. 2019;6:74-86. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 168] [Article Influence: 24.0] [Reference Citation Analysis (1)] |