Published online Apr 28, 2021. doi: 10.37126/aige.v2.i2.25
Peer-review started: March 5, 2021
First decision: March 14, 2021
Revised: March 17, 2021
Accepted: April 20, 2021
Article in press: April 20, 2021
Published online: April 28, 2021
Processing time: 53 Days and 22.2 Hours
Artificial intelligence (AI) has been widely involved in every aspect of healthcare in the preclinical stage. In the digestive system, AI has been trained to assist auxiliary examinations including histopathology, endoscopy, ultrasonography, computerized tomography, and magnetic resonance imaging in detection, diagnosis, classification, differentiation, prognosis, and quality control. In the field of endoscopy, the application of AI, such as automatic detection, diagnosis, classification, and invasion depth, in early gastrointestinal (GI) cancers has received wide attention. There is a paucity of studies of AI application on common GI benign diseases based on endoscopy. In the review, we provide an overview of AI applications to endoscopy on common GI benign diseases including in the esophagus, stomach, intestine, and colon. It indicates that AI will gradually become an indispensable part of normal endoscopic detection and diagnosis of common GI benign diseases as clinical data, algorithms, and other related work are constantly repeated and improved.
Core Tip: In endoscopy, the application of artificial intelligence in early gastrointestinal cancer has been widely concerned. We provide a general conclusion of artificial intelligence endoscopy applications in common gastrointestinal benign diseases, such as Barrett’s esophagus, atrophic gastritis, and colonic polyp. Studies indicate high accuracies and efficiencies. Further related work is needed to boost the real application of artificial intelligence in common gastrointestinal benign diseases in the future.
- Citation: Yang H, Hu B. Application of artificial intelligence to endoscopy on common gastrointestinal benign diseases. Artif Intell Gastrointest Endosc 2021; 2(2): 25-35
- URL: https://www.wjgnet.com/2689-7164/full/v2/i2/25.htm
- DOI: https://dx.doi.org/10.37126/aige.v2.i2.25
Artificial intelligence (AI) is essentially a process of learning human thinking and transferring human experience based on mathematics and statistics. Iteration of algorithm, rising data, and improving computing power are cores of AI. Machine learning (ML) is a subset of AI[1], and deep learning is a subset of ML to realize ML[2], where multiple algorithms are structured together in complex layers. Artificial neural networks are one of the most common algorithms of AI[3]. Convolutional neural networks (CNNs) are a kind of supervised deep learning algorithm[4]. Its modified format is defined as deep convolutional neural networks[5]. Recognizing images based on artificial neural networks/CNNs promotes AI penetrating in medicine. Computer-aided diagnosis (CAD) systems are designed to interpret medical images using advances of AI from ML to deep learning[6].
In the field of gastroenterology, diseases of the liver, pancreases, and full digestive tract have been involved. Examples include a deep learning model based on computed tomography images to stage liver fibrosis, a deep learning model constructed to differentiate between precancerous lesions and pancreatic cancers, and a deep learning model used in endoscopy to detect early gastrointestinal (GI) cancers. A study covered five kinds of gastric diseases and showed the diagnostic specificity of the CNNs was higher than that of the endoscopists for early gastric cancer and high-grade intraepithelial neoplasia images (91.2% vs 86.7%). The diagnostic accuracy of the CNNs was close to those of the endoscopists for lesion-free, early gastric cancer and high-grade intraepithelial neoplasia, peptic ulcer (PU), advanced gastric cancer (GC), and gastric submucosal tumor images. The CNNs had an image recognition time of 42 s for all the test set images[7]. In this review, the application and research of AI on common GI benign lesions based on endoscopy were concluded.
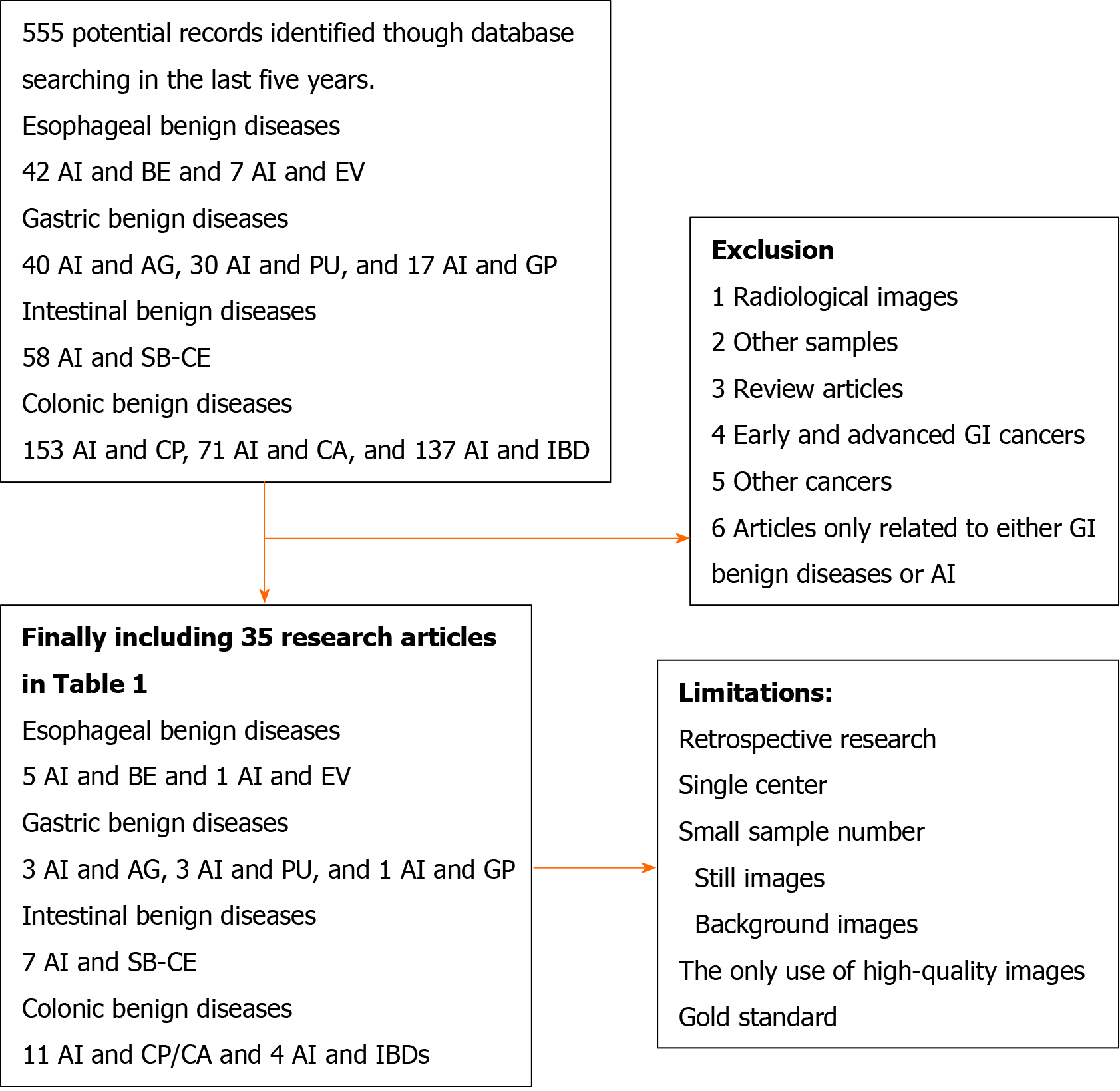
This review aimed to make a qualitative only review of the application of AI on common GI benign diseases. We searched the PubMed database for articles that were published in the last 5 years using the term combinations of artificial intelligence and common GI benign lesions [Barrett’s esophagus (BE), esophageal varices (EV), atrophic gastritis (AG), PU, gastric polyp, small bowel capsule endoscopy, colonic polyp/adenoma, and inflammatory bowel diseases (IBDs)]. Articles based on radiological images or other samples, review articles, research articles of early or advanced GI cancers or other cancers, and articles only related to either GI benign diseases or AI were excluded. Two authors independently extracted data. Any disagreement was resolved by discussion until consensus was reached or by consulting a third author. Endoscopic-related results were qualitatively concluded in Table 1. The flowchart was presented in Figure 1.
| Ref. | Aim and disease | Prospective/retrospective | AI method | Endoscopy image | Training dataset | Validation dataset | Result sensitivity | Result specificity | Result accuracy/AUC |
| Esophageal benign diseases | |||||||||
| de Groof et al[12] | Detecting Barrett’sneoplasia | Retrospective | CAD | WLI images | 40 images | A leave one out cross validation | 92% | 95% | 85%1 |
| Jisu et al[39] | Distinguishing BE | Retrospective | CNNs | Endomicroscopic images | 262 images | Image distortion methods | 80.77%1 | ||
| Ebigbo et al[40] | Distinguishing BE | Retrospective | CNNs (ResNet) | WLI images | 129 images | 62 images | 83.7% | 100.0% | 89.9%1 |
| Sehgal et al[41] | Detecting dysplasia in BE | Retrospective | ML (decision trees) | Video recordings(AAC) | 40 patients with NDBE and DBE | 97% | 88% | 92%1 | |
| de Groof et al[14] | Detecting Barrett’sneoplasia | Retrospective | CNN (CAD (ResNet-UNet)) | WLI images | 494364 images | 1704 images (early stage neoplasia in BE and NDBE from 669 patients) | 90% | 88% | 89%1 |
| Dong et al[16] | Screening high risk EV | Retrospective | ML (Random forest) | 238 patients | 109 patients | Training set (0.84); Validation set (0.82) | |||
| Gastric benign diseases | |||||||||
| Zhang et al[42] | Diagnosing CAG | Retrospective | CNNs (DenseNet) | WLI images | 5470 images | Five-fold cross validation | 94.5% | 94.0% | 94.2%1 |
| Guimarães et al[43] | DiagnosingCAG | Retrospective | CNNs (VGG16) | WLI images | 200 images | 70 images(ten-fold cross validation) | 93%1/0.98 | ||
| Horiuchi et al[44] | Differentiating CAG | Retrospective | CNNs (GoogLeNet) | ME-NBI images | 1078 images | 107 images | 95.4% | 71.0% | 85.3%1/0.85 |
| Zhang et al[7] | Diagnosing PU | Retrospective | CNNs (ResNet34) | WLI images | 4200 images | 228 images | 78.9% | 88.4% | 86.4%1 |
| Lee et al[45] | Differentiating PU | Retrospective | CNNs (ResNet-50/ Inception v3/VGG16 model) | WLI images | 200 images | 20 images | 92.6%1/85.24%1/91.2%1 | ||
| Namikawa et al[46] | Classifying gastriccancers and ulcers | Retrospective | CNNs (SSD) | WLI/NBI/chromoendoscopy images | 373 images | 720 images | 93.3% | 99.0% | 93.3 %1 |
| Zhang et al[26] | Detecting GP | Retrospective | CNNs (SSD-GPNet) | WLI images | 404 images | 50 images | 93.92%1 | ||
| Intestinal benign diseases | |||||||||
| Hwang et al[29] | Classifying hemorrhagic and ulcerations | Retrospective | CNNs (VGGNet) | Capsule endoscopy | 7556 images | 5760 images | Model 1 vs Model 2; 97.61% vs 95.07% | Model 1 vs Model 2; 96.04% vs 98.18% | Model 1 vs Model 2; 96.83%1 vs 96.62%1 |
| Aoki et al[47] | Detecting erosions and ulcerations | Retrospective | CNNs (SSD) | Capsule endoscopy | 5360 images | 10440 images | 88.2% | 90.9% | 90.8%1/0.958 |
| Aoki et al[48] | Detecting erosions and ulcerations | Retrospective | CNNs (SSD) | Capsule endoscopy | 20 videos | ||||
| Ding et al[49] | Detecting small bowel diseases | Retrospective | CNNs (ResNet) | Capsule endoscopy | 158235 images | 5000 patients | 99.88% per patient99.90% per lesion | 100% per patient100 % per lesion | |
| Fan et al[50] | Detecting erosions and ulcerations | Retrospective | CNNs (AlexNet) | Capsule endoscopy | Ulcer 2000; Erosion 2720 | Ulcer 500; Erosion 690 | Ulcer: 96.80%; Erosion: 93.67% | Ulcer: 94.79%; Erosion: 95.98% | Ulcer: 95.16%1; Erosion: 95.34%1/0.98 |
| Leenhardt et al[51] | Detecting small bowel angiectasia | Retrospective | CNNs | Capsule endoscopy | 300 videos with angiectasia | 300 videos with angiectasia | 100% | 96% | |
| Tsuboi et al[52] | Detecting small bowel angiectasia | Retrospective | CNNs (SSD) | Capsule endoscopy | 141 patients | 28 patients | 98.8% | 98.4% | 0.998 |
| Colonic benign diseases | |||||||||
| Lui et al[34] | Detecting missed colonic lesions | Retrospective and prospective | R-FCN (ResNet101) | Endoscopic videos (WLI) | 52 videos | Real-time AI detected at least 1 missed adenoma in 14 patients (26.9%) and increased the total number of adenomas detected by 23.6%. | |||
| Rodriguez-Diaz et al[53] | Histologically classifying CP | Retrospective | CAD | NBI | 745 images +65000 images | 96% | 84% | ||
| Komeda et al[54] | Diagnosing CP | Retrospective | CNNs-CAD | WLI/NBI/ chromoendoscopy images | 1200 images | 10-fold cross validation | 75.1%1 | ||
| Akbari et al[55] | Classifying CP | Retrospective | FCNs | WLI images | 200 images | 300 images | |||
| Chen et al[56] | Classifying diminutive CP | Retrospective | DCNNs-CAD | NBI images | 96 images + 188 images | 96.3% | 78.1% | ||
| Gong et al[57] | Detecting CA | Prospective | DCNNs | WLI images | DCNNs system (n = 355) or unassisted (control) colonoscopy (n = 349) | 58 (16%) of 35527 (8%) of 349 | |||
| Byrne et al[58] | Differentiating adenomatous and hyperplastic polyps | Retrospective | DCNNs | Videos and NBI images | 223 polyp videos | 40 videos | 98% | 83% | |
| Mori et al[59] | Identifying diminutive CP | Prospective | CAD | NBI/stained images | 791 consecutive patients undergoing colonoscopy and 23 endoscopists | Pathologic prediction rate of 98.1%1 | |||
| Misawa et al[60] | DetectingCP | Retrospective | CAD | WLI images | 105 positive and 306 negative videos | 50 positive and 85 negative videos | 90.0% | 63.3% | 76.5%1 |
| Taunk et al[61] | Classifying polyp histology | Retrospective | CAD | pCLE images | 125 images | 189 images | 95% | 94% | 94%1 |
| Wang et al[62] | Detecting CA | Prospective | CAD | WLI images | 484 patients in the CADe group and 478 in the sham group | 165 (34%) of 484; 132 (28%) of 478 | |||
| Tong et al[63] | Differentiating UC, CD, and ITB | Retrospective | CNNs/RF | WLI images | 6399 consecutive patients (5128 UC, 875 CD and 396 ITB) | RF (UC 97%, CD 65%, and ITB 68%); CNN (UC 99%, CD 87%, and ITB 52%) | RF (UC 97%, CD 53%, and ITB 76%); CNN (UC 97%, CD 83%, and ITB 81%) | RF (UC 0.97, CD 0.58, and ITB 0.72); CNN (UC 0.98, CD 0.85, and ITB 0.63) | |
| Ozawa et al[36] | Diagnosing UC | Retrospective | CAD | WLI images | 26304 images | 3981 images | 0.86 (Mayo 0); 0.98 (Mayo 0–1) | ||
| Stidham et al[37] | Grading the severity of ulcerative colitis | Retrospective | CNNs | WLI images | 2465 patients | 308 patients | 83.0% | 96.0% | 0.966 |
| Maeda et al[38] | Identifying histologic inflammation associated with UC | Retrospective | CAD | Endocytoscopic images | 87 patients | 100 patients | 74% | 97% | 91%1 |
Initially, a total of 555 articles were identified. After manually screening and reading, only research articles related to the application of AI to common GI benign lesions (BE, EV, AG, PU, gastric polyp, small bowel capsule endoscopy, colonic polyp/adenoma, and IBDs) based on different endoscopic images or tissue slides from endoscopic biopsies were included. Finally, 35 studies were tabulated in Table 1. Six studies demonstrated the application of AI on esophageal benign diseases (5 BE and 1 EV). Seven studies were about gastric benign diseases (3 AG, 3 PU, and 1 polyp). Seven studies were about intestinal diseases. Fifteen studies were about colonic benign diseases (11 polyp/adenoma and 4 IBDs).
BE is a precursor to esophageal adenocarcinoma. Intestinal metaplasia and gastric metaplasia are two pathological subclasses of BE. Intestinal metaplasia can progress to esophageal cancer. The ablation of dysplastic BE will reduce the risk of progression to cancer[8]. Endoscopic surveillance, including white-light imaging (WLI), narrow-band imaging, and chromoendoscopy, is performed to detect dysplasia in BE. Approximately 5% of the esophageal mucosa is found at risk by random biopsies sample[9].
Recently, AI has been applied in some studies of BE. For example, CAD based on deep learning and different algorithms trained by WLI and endomicroscopic images to detect, diagnose, and distinguish BE with achievable results (the accuracy from 80.77% to 92%, specificity from 88% to 100%, and sensitivity from 83.7% to 97%) (Table 1). On pathology, CAD with wide area transepithelial sampling could increase the detection of high-grade dysplasia/esophageal adenocarcinoma (absolute increase: 14.4%)[10]. Deep convolutional neural networks were used in the whole-slide tissue histopathology images-based diagnosis of dysplastic and non-dysplastic BE[11]. Moreover, distinguishing BE adenocarcinoma by AI methods has been studied based on different endoscopic images such as WLI and volumetric laser endomicroscopic images with accuracy from 88% to 92%, specificity from 88% to 93%, and sensitivity from 90% to 95%[12-14].
As another common esophageal benign disease, EV are associated with cirrhosis and portal hypertension, and variceal hemorrhage is a substantial cause of mortality[15]. However, related AI research is limited. A score system based on ML was built on the data of 238 patients with cirrhosis to reliably identify patients with varices that needed treatments and achieved an area under the curve (AUC) from 0.75 to 0.84 in different groups[16]. Another study of the index of spleen volume-to-platelet ratio based on deep learning-measured spleen volume on computed tomography to assess high-risk varices in B-viral compensated cirrhosis had a sensitivity of 69.4% and specificity of 78.5%[17]. There is little research of AI on esophagitis, although it is also a common esophageal disease associated with BE and esophageal cancer.
Gastritis, peptic ulcer, polyp and adenoma, and vascular lesion are common gastric benign diseases. The detection and diagnosis of these lesions account for a large part of daily endoscopic work. If AI can be applied in this field, then the rate of detection and accuracy will be improved. Moreover, the rapid identification of simple lesions can fill the lack of endoscopists and reduce the workload.
Early diagnosis of chronic AG, a precancerous lesion, is important to prevent the occurrence and development of GC. AI-assisted detection and diagnosis has been related to endoscopic images (Table 1), histological images[18,19], and X-ray images[20,21]. The accuracy was from 85.3% to 94.2%, the specificity was from 71% to 94%, and the sensitivity was from 94.5% to 95.4%. Helicobacter pylori infection, as a dominant cause of chronic AG and GC, has also been detected via AI methods based on endoscopic images, such as CNNs (GoogLeNet) and CNNs (ResNet-50 model), which achieved an accuracy up to 93.8% in a considerably short time of less than 200 s[22-24].
A CNN method was constructed to diagnose PU and differentiate GC from PU mainly based on WLI, narrow-band imaging, and chromoendoscopic images with an accuracy from 85.2% to 93.3%, specificity from 88.4% to 99%, and sensitivity from 78.9% to 93.3% (Table 1). In addition, a ML model was built on six parameters, such as age and the presence of PU, to predict recurrent ulcer bleeding within 1 year with an AUC of 0.775 and an accuracy of 84.3%[25].
There were only a few applications of AI on detecting gastric hyperplastic polyps and adenomas. A 93.92% accuracy was achieved when detecting polyps by CNNs (SSD-GPNet) based on WLI images[26]. A CNN method was trained to detect adenomas and showed an AUC of 0.99 based on histopathology whole-slide images[27]. Research and application of AI on gastric benign lesions are limited, although these diseases make up a considerable part of daily work. Some of them are usually prone to severe outcomes and risks despite the relative ease to diagnose. Indeed, the study of AI on this aspect will assist endoscopists to improve early detection rates and bring the opportunity of early treatment to benefit patients.
The application of AI in small bowel diseases has been concentrated on capsule endoscopy. It includes image enhancement using ML algorithms to reduce artifact interference as well as three-dimensional luminal map reconstruction and localization[28]. AI-assisted capsule endoscopy in detecting ulcer, erosion, bleeding, polyps, parasite, diverticulum, and angiectasia with an accuracy more than 90.0%, specificity from 90.9% to 100%, and sensitivity from 88.2% to 100% in a short time (about 6 min) (Table 1). Furthermore, a gradient class activation map was used to visualize and detect lesions by CNNs-VGGNet to improve the classification and localization[29]. In addition, a CNN method based on conventional abdominal radiographs was trained to detect high-grade small bowel obstruction with an AUC of 0.84, a sensitivity of 83.8%, and a specificity of 68.1%[30]. In another study, it achieved an AUC of 0.971, a sensitivity of 91.4%, and a specificity of 91.9% using region-based CNNs[31]. The limited research indicated CNNs could recognize specific images among a large variety with high efficiency and accuracy. The application of AI will relieve the clinical workload as capsule endoscopy reading is a time-consuming process.
A 1.0% increase of adenoma detection rate has been associated with a 3.0% decrease in the risk of interval colorectal cancer[32]. To improve colorectal polyp and adenoma detection, AI has been widely applied in the detection, real-time histological classification, segmentation, localization, and distinguishing of diminutive polyps and adenomas based on different methods trained by videos and images in retrospective or prospective and in multicenter or single center clinical trials (Table 1). Deep learning was also used to automatically classify colorectal polyps on histopathologic slides[33]. For the internal evaluation, the accuracy of the deep CNN method was 93.5%, which was comparable to the pathologists accuracy of 91.4%. On the external test, it achieved an accuracy of 87.0%, which was comparable to the pathologists accuracy of 86.6%. The application of AI in colorectal polyps has gained more concerns and practice, and it is deeper and closer to the clinical use to further increase the detection rate of polyps. For example, real-time AI detected at least one missed adenoma in 14 patients (26.9%) and increased the total number of adenomas detected by 23.6%[34].
AI methods have been trained in grading endoscopic disease severity of patients with ulcerative colitis and in predicting remission in patients with moderate to severe Crohn’s disease[35]. For example, a CNN-CAD system based on GoogLeNet was robustly promising to identify normal mucosa (Mayo 0) and mucosal healing state with an accuracy of 0.86 of Mayo 0 and of 0.98 of Mayo 0-1[36]. Another similar system could differentiate remission (Mayo 0 or 1) from moderate or severe disease (Mayo 2 or 3) with an AUC of 0.966, a specificity of 96.0%, and a sensitivity of 83.0%[37]. A CAD was constructed to identify the presence of histologic inflammation associated with ulcerative colitis using endocytoscopy with an accuracy of 91%, a specificity of 97%, and a sensitivity 74%[38] (Table 1).
We summarized the application and research of AI on common GI benign diseases. Limited studies are promising as most of the studies showed comparatively high accuracies and efficiencies. As studies of AI application on gastroenterology continue to increase, there are several areas of interest that will hold significant value in the future. First, the technical integration of AI systems will be important to optimize clinical workflow. New AI applications can easily “read in” data from a video input, allowing the systems to use the data for training and real time decision support. Second, AI systems will continue to expand the clinical applications. Some promising studies have demonstrated how AI can improve our performance on clinical tasks such as polyp identification, detection of small bowel bleeding, and endoscopic recognition of Helicobacter pylori infection, etc. More research, especially randomized controlled trials, on how to train and validate up-to-date algorithms will be continued on the present work to find more precise methods and identify new clinical tasks after practice. Third, further research will be needed to describe the most effective training methods for physician practices beginning to adopt AI technology because AI will be an indispensable helper of normal endoscopic detection and diagnosis of common GI benign lesions in the future.
Although AI is a relatively new technology, it has the potential to ease the daily workload of radiologists, pathologists, and sonographers. In endoscopy, AI related to early GI cancers and precancerous lesions has garnered more research than common GI benign diseases, despite the latter occupying a large proportion of daily work and being easier to detect and diagnose than early cancers. If models and diagnosing routes based on AI targeted at common GI benign diseases are well developed, then it will bring great benefits to patients and endoscopists, especially in primary hospitals where medical resources are lacking and core work is mainly focused on early diagnosis and treatment of common GI benign diseases. Furthermore, AI methods and technology targeted at common benign diseases will be easier for endoscopists to adopt professional education. More research is needed to overcome the challenges of integrating AI into the detection of common GI benign diseases by endoscopy, but the future is promising.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Azimi P S-Editor: Wang JL L-Editor: Filipodia P-Editor: Wang LL
| 1. | Bi Q, Goodman KE, Kaminsky J, Lessler J. What is Machine Learning? Am J Epidemiol. 2019;188:2222-2239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 116] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 2. | LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521:436-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36149] [Cited by in RCA: 20025] [Article Influence: 2002.5] [Reference Citation Analysis (0)] |
| 3. | Zador AM. A critique of pure learning and what artificial neural networks can learn from animal brains. Nat Commun. 2019;10:3770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 178] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 4. | Mieloszyk RJ, Bhargava P. Convolutional Neural Networks: The Possibilities are Almost Endless. Curr Probl Diagn Radiol. 2018;47:129-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | O'Toole AJ, Castillo CD, Parde CJ, Hill MQ, Chellappa R. Face Space Representations in Deep Convolutional Neural Networks. Trends Cogn Sci. 2018;22:794-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 6. | Ahmad OF, Soares AS, Mazomenos E, Brandao P, Vega R, Seward E, Stoyanov D, Chand M, Lovat LB. Artificial intelligence and computer-aided diagnosis in colonoscopy: current evidence and future directions. Lancet Gastroenterol Hepatol. 2019;4:71-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 126] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 7. | Zhang L, Zhang Y, Wang L, Wang J, Liu Y. Diagnosis of gastric lesions through a deep convolutional neural network. Dig Endosc. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Soh YSA, Lee YY, Gotoda T, Sharma P, Ho KY; Asian Barrett's Consortium. Challenges to diagnostic standardization of Barrett's esophagus in Asia. Dig Endosc. 2019;31:609-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | ASGE Technology Committee. , Thosani N, Abu Dayyeh BK, Sharma P, Aslanian HR, Enestvedt BK, Komanduri S, Manfredi M, Navaneethan U, Maple JT, Pannala R, Parsi MA, Smith ZL, Sullivan SA, Banerjee S. ASGE Technology Committee systematic review and meta-analysis assessing the ASGE Preservation and Incorporation of Valuable Endoscopic Innovations thresholds for adopting real-time imaging-assisted endoscopic targeted biopsy during endoscopic surveillance of Barrett's esophagus. Gastrointest Endosc 2016; 83: 684-98. e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 148] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 10. | Vennalaganti PR, Kaul V, Wang KK, Falk GW, Shaheen NJ, Infantolino A, Johnson DA, Eisen G, Gerson LB, Smith MS, Iyer PG, Lightdale CJ, Schnoll-Sussman F, Gupta N, Gross SA, Abrams J, Haber GB, Chuttani R, Pleskow DK, Kothari S, Goldblum JR, Zhang Y, Sharma P. Increased detection of Barrett's esophagus-associated neoplasia using wide-area trans-epithelial sampling: a multicenter, prospective, randomized trial. Gastrointest Endosc. 2018;87:348-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 11. | Sali R, Moradinasab N, Guleria S, Ehsan L, Fernandes P, Shah TU, Syed S, Brown DE. Deep Learning for Whole-Slide Tissue Histopathology Classification: A Comparative Study in the Identification of Dysplastic and Non-Dysplastic Barrett's Esophagus. J Pers Med. 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | de Groof J, van der Sommen F, van der Putten J, Struyvenberg MR, Zinger S, Curvers WL, Pech O, Meining A, Neuhaus H, Bisschops R, Schoon EJ, de With PH, Bergman JJ. The Argos project: The development of a computer-aided detection system to improve detection of Barrett's neoplasia on white light endoscopy. United European Gastroenterol J. 2019;7:538-547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 86] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 13. | Swager AF, van der Sommen F, Klomp SR, Zinger S, Meijer SL, Schoon EJ, Bergman JJGHM, de With PH, Curvers WL. Computer-aided detection of early Barrett's neoplasia using volumetric laser endomicroscopy. Gastrointest Endosc. 2017;86:839-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 102] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 14. | de Groof AJ, Struyvenberg MR, van der Putten J, van der Sommen F, Fockens KN, Curvers WL, Zinger S, Pouw RE, Coron E, Baldaque-Silva F, Pech O, Weusten B, Meining A, Neuhaus H, Bisschops R, Dent J, Schoon EJ, de With PH, Bergman JJ. Deep-Learning System Detects Neoplasia in Patients With Barrett's Esophagus With Higher Accuracy Than Endoscopists in a Multistep Training and Validation Study With Benchmarking. Gastroenterology 2020; 158: 915-929. e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 222] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 15. | Kovacs TOG, Jensen DM. Varices: Esophageal, Gastric, and Rectal. Clin Liver Dis. 2019;23:625-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 16. | Dong TS, Kalani A, Aby ES, Le L, Luu K, Hauer M, Kamath R, Lindor KD, Tabibian JH. Machine Learning-based Development and Validation of a Scoring System for Screening High-Risk Esophageal Varices. Clin Gastroenterol Hepatol 2019; 17: 1894-1901. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 17. | Lee CM, Lee SS, Choi WM, Kim KM, Sung YS, Lee S, Lee SJ, Yoon JS, Suk HI. An index based on deep learning-measured spleen volume on CT for the assessment of high-risk varix in B-viral compensated cirrhosis. Eur Radiol. 2021;31: 3355-3365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 18. | Steinbuss G, Kriegsmann K, Kriegsmann M. Identification of Gastritis Subtypes by Convolutional Neuronal Networks on Histological Images of Antrum and Corpus Biopsies. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Martin DR, Hanson JA, Gullapalli RR, Schultz FA, Sethi A, Clark DP. A Deep Learning Convolutional Neural Network Can Recognize Common Patterns of Injury in Gastric Pathology. Arch Pathol Lab Med. 2020;144:370-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 20. | Li Z, Togo R, Ogawa T, Haseyama M. Chronic gastritis classification using gastric X-ray images with a semi-supervised learning method based on tri-training. Med Biol Eng Comput. 2020;58:1239-1250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Kanai M, Togo R, Ogawa T, Haseyama M. Chronic atrophic gastritis detection with a convolutional neural network considering stomach regions. World J Gastroenterol. 2020;26:3650-3659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 22. | Shichijo S, Nomura S, Aoyama K, Nishikawa Y, Miura M, Shinagawa T, Takiyama H, Tanimoto T, Ishihara S, Matsuo K, Tada T. Application of Convolutional Neural Networks in the Diagnosis of Helicobacter pylori Infection Based on Endoscopic Images. EBioMedicine. 2017;25:106-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 235] [Cited by in RCA: 181] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 23. | Zheng W, Zhang X, Kim JJ, Zhu X, Ye G, Ye B, Wang J, Luo S, Li J, Yu T, Liu J, Hu W, Si J. High Accuracy of Convolutional Neural Network for Evaluation of Helicobacter pylori Infection Based on Endoscopic Images: Preliminary Experience. Clin Transl Gastroenterol. 2019;10:e00109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 24. | Bang CS, Lee JJ, Baik GH. Artificial Intelligence for the Prediction of Helicobacter Pylori Infection in Endoscopic Images: Systematic Review and Meta-Analysis Of Diagnostic Test Accuracy. J Med Internet Res. 2020;22:e21983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 25. | Wong GL, Ma AJ, Deng H, Ching JY, Wong VW, Tse YK, Yip TC, Lau LH, Liu HH, Leung CM, Tsang SW, Chan CW, Lau JY, Yuen PC, Chan FK. Machine learning model to predict recurrent ulcer bleeding in patients with history of idiopathic gastroduodenal ulcer bleeding. Aliment Pharmacol Ther. 2019;49:912-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (1)] |
| 26. | Zhang X, Chen F, Yu T, An J, Huang Z, Liu J, Hu W, Wang L, Duan H, Si J. Real-time gastric polyp detection using convolutional neural networks. PLoS One. 2019;14:e0214133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 27. | Iizuka O, Kanavati F, Kato K, Rambeau M, Arihiro K, Tsuneki M. Deep Learning Models for Histopathological Classification of Gastric and Colonic Epithelial Tumours. Sci Rep. 2020;10:1504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 215] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 28. | Turan M, Almalioglu Y, Araujo H, Konukoglu E, Sitti M. A non-rigid map fusion-based direct SLAM method for endoscopic capsule robots. Int J Intell Robot Appl. 2017;1:399-409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 29. | Hwang Y, Lee HH, Park C, Tama BA, Kim JS, Cheung DY, Chung WC, Cho YS, Lee KM, Choi MG, Lee S, Lee BI. Improved classification and localization approach to small bowel capsule endoscopy using convolutional neural network. Dig Endosc. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 30. | Cheng PM, Tejura TK, Tran KN, Whang G. Detection of high-grade small bowel obstruction on conventional radiography with convolutional neural networks. Abdom Radiol (NY). 2018;43:1120-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 31. | Cheng PM, Tran KN, Whang G, Tejura TK. Refining Convolutional Neural Network Detection of Small-Bowel Obstruction in Conventional Radiography. AJR Am J Roentgenol. 2019;212:342-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 32. | Corley DA, Levin TR, Doubeni CA. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;370:2541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 33. | Wei JW, Suriawinata AA, Vaickus LJ, Ren B, Liu X, Lisovsky M, Tomita N, Abdollahi B, Kim AS, Snover DC, Baron JA, Barry EL, Hassanpour S. Evaluation of a Deep Neural Network for Automated Classification of Colorectal Polyps on Histopathologic Slides. JAMA Netw Open. 2020;3:e203398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 34. | Lui TKL, Hui CKY, Tsui VWM, Cheung KS, Ko MKL, Foo DCC, Mak LY, Yeung CK, Lui TH, Wong SY, Leung WK. New insights on missed colonic lesions during colonoscopy through artificial intelligence-assisted real-time detection (with video). Gastrointest Endosc 2021; 93: 193-200. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 35. | Waljee AK, Wallace BI, Cohen-Mekelburg S, Liu Y, Liu B, Sauder K, Stidham RW, Zhu J, Higgins PDR. Development and Validation of Machine Learning Models in Prediction of Remission in Patients With Moderate to Severe Crohn Disease. JAMA Netw Open. 2019;2:e193721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 36. | Ozawa T, Ishihara S, Fujishiro M, Saito H, Kumagai Y, Shichijo S, Aoyama K, Tada T. Novel computer-assisted diagnosis system for endoscopic disease activity in patients with ulcerative colitis. Gastrointest Endosc 2019; 89: 416-421. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 147] [Article Influence: 24.5] [Reference Citation Analysis (1)] |
| 37. | Stidham RW, Liu W, Bishu S, Rice MD, Higgins PDR, Zhu J, Nallamothu BK, Waljee AK. Performance of a Deep Learning Model vs Human Reviewers in Grading Endoscopic Disease Severity of Patients With Ulcerative Colitis. JAMA Netw Open. 2019;2:e193963. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 174] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 38. | Maeda Y, Kudo SE, Mori Y, Misawa M, Ogata N, Sasanuma S, Wakamura K, Oda M, Mori K, Ohtsuka K. Fully automated diagnostic system with artificial intelligence using endocytoscopy to identify the presence of histologic inflammation associated with ulcerative colitis (with video). Gastrointest Endosc. 2019;89:408-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 164] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 39. | Jisu Hong, Bo-Yong Park, Hyunjin Park. Convolutional neural network classifier for distinguishing Barrett's esophagus and neoplasia endomicroscopy images. Annu Int Conf IEEE Eng Med Biol Soc. 2017;2017:2892-2895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 40. | Ebigbo A, Mendel R, Probst A, Manzeneder J, Prinz F, de Souza LA Jr, Papa J, Palm C, Messmann H. Real-time use of artificial intelligence in the evaluation of cancer in Barrett's oesophagus. Gut. 2020;69:615-616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 124] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 41. | Sehgal V, Rosenfeld A, Graham DG, Lipman G, Bisschops R, Ragunath K, Rodriguez-Justo M, Novelli M, Banks MR, Haidry RJ, Lovat LB. Machine Learning Creates a Simple Endoscopic Classification System that Improves Dysplasia Detection in Barrett's Oesophagus amongst Non-expert Endoscopists. Gastroenterol Res Pract. 2018;2018:1872437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 42. | Zhang Y, Li F, Yuan F, Zhang K, Huo L, Dong Z, Lang Y, Zhang Y, Wang M, Gao Z, Qin Z, Shen L. Diagnosing chronic atrophic gastritis by gastroscopy using artificial intelligence. Dig Liver Dis. 2020;52:566-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 43. | Guimarães P, Keller A, Fehlmann T, Lammert F, Casper M. Deep-learning based detection of gastric precancerous conditions. Gut. 2020;69:4-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 44. | Horiuchi Y, Aoyama K, Tokai Y, Hirasawa T, Yoshimizu S, Ishiyama A, Yoshio T, Tsuchida T, Fujisaki J, Tada T. Convolutional Neural Network for Differentiating Gastric Cancer from Gastritis Using Magnified Endoscopy with Narrow Band Imaging. Dig Dis Sci. 2020;65:1355-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 94] [Article Influence: 18.8] [Reference Citation Analysis (1)] |
| 45. | Lee JH, Kim YJ, Kim YW, Park S, Choi YI, Park DK, Kim KG, Chung JW. Spotting malignancies from gastric endoscopic images using deep learning. Surg Endosc. 2019;33:3790-3797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 46. | Namikawa K, Hirasawa T, Nakano K, Ikenoyama Y, Ishioka M, Shiroma S, Tokai Y, Yoshimizu S, Horiuchi Y, Ishiyama A, Yoshio T, Tsuchida T, Fujisaki J, Tada T. Artificial intelligence-based diagnostic system classifying gastric cancers and ulcers: comparison between the original and newly developed systems. Endoscopy. 2020;52:1077-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 47. | Aoki T, Yamada A, Aoyama K, Saito H, Tsuboi A, Nakada A, Niikura R, Fujishiro M, Oka S, Ishihara S, Matsuda T, Tanaka S, Koike K, Tada T. Automatic detection of erosions and ulcerations in wireless capsule endoscopy images based on a deep convolutional neural network. Gastrointest Endosc 2019; 89: 357-363. e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 174] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 48. | Aoki T, Yamada A, Aoyama K, Saito H, Fujisawa G, Odawara N, Kondo R, Tsuboi A, Ishibashi R, Nakada A, Niikura R, Fujishiro M, Oka S, Ishihara S, Matsuda T, Nakahori M, Tanaka S, Koike K, Tada T. Clinical usefulness of a deep learning-based system as the first screening on small-bowel capsule endoscopy reading. Dig Endosc. 2020;32:585-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 75] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 49. | Ding Z, Shi H, Zhang H, Meng L, Fan M, Han C, Zhang K, Ming F, Xie X, Liu H, Liu J, Lin R, Hou X. Gastroenterologist-Level Identification of Small-Bowel Diseases and Normal Variants by Capsule Endoscopy Using a Deep-Learning Model. Gastroenterology 2019; 157: 1044-1054. e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 208] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 50. | Fan S, Xu L, Fan Y, Wei K, Li L. Computer-aided detection of small intestinal ulcer and erosion in wireless capsule endoscopy images. Phys Med Biol. 2018;63:165001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 90] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 51. | Leenhardt R, Vasseur P, Li C, Saurin JC, Rahmi G, Cholet F, Becq A, Marteau P, Histace A, Dray X; CAD-CAP Database Working Group. A neural network algorithm for detection of GI angiectasia during small-bowel capsule endoscopy. Gastrointest Endosc. 2019;89:189-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 148] [Article Influence: 24.7] [Reference Citation Analysis (1)] |
| 52. | Tsuboi A, Oka S, Aoyama K, Saito H, Aoki T, Yamada A, Matsuda T, Fujishiro M, Ishihara S, Nakahori M, Koike K, Tanaka S, Tada T. Artificial intelligence using a convolutional neural network for automatic detection of small-bowel angioectasia in capsule endoscopy images. Dig Endosc. 2020;32:382-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 102] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 53. | Rodriguez-Diaz E, Baffy G, Lo WK, Mashimo H, Vidyarthi G, Mohapatra SS, Singh SK. Real-time artificial intelligence-based histologic classification of colorectal polyps with augmented visualization. Gastrointest Endosc. 2021;93:662-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 54. | Komeda Y, Handa H, Watanabe T, Nomura T, Kitahashi M, Sakurai T, Okamoto A, Minami T, Kono M, Arizumi T, Takenaka M, Hagiwara S, Matsui S, Nishida N, Kashida H, Kudo M. Computer-Aided Diagnosis Based on Convolutional Neural Network System for Colorectal Polyp Classification: Preliminary Experience. Oncology. 2017;93 Suppl 1:30-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 129] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 55. | Akbari M, Mohrekesh M, Nasr-Esfahani E, Soroushmehr SMR, Karimi N, Samavi S, Najarian K. Polyp Segmentation in Colonoscopy Images Using Fully Convolutional Network. Annu Int Conf IEEE Eng Med Biol Soc. 2018;2018:69-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 56. | Chen PJ, Lin MC, Lai MJ, Lin JC, Lu HH, Tseng VS. Accurate Classification of Diminutive Colorectal Polyps Using Computer-Aided Analysis. Gastroenterology. 2018;154:568-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 276] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 57. | Gong D, Wu L, Zhang J, Mu G, Shen L, Liu J, Wang Z, Zhou W, An P, Huang X, Jiang X, Li Y, Wan X, Hu S, Chen Y, Hu X, Xu Y, Zhu X, Li S, Yao L, He X, Chen D, Huang L, Wei X, Wang X, Yu H. Detection of colorectal adenomas with a real-time computer-aided system (ENDOANGEL): a randomised controlled study. Lancet Gastroenterol Hepatol. 2020;5:352-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 265] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 58. | Byrne MF, Chapados N, Soudan F, Oertel C, Linares Pérez M, Kelly R, Iqbal N, Chandelier F, Rex DK. Real-time differentiation of adenomatous and hyperplastic diminutive colorectal polyps during analysis of unaltered videos of standard colonoscopy using a deep learning model. Gut. 2019;68:94-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 363] [Cited by in RCA: 411] [Article Influence: 68.5] [Reference Citation Analysis (0)] |
| 59. | Mori Y, Kudo SE, Misawa M, Saito Y, Ikematsu H, Hotta K, Ohtsuka K, Urushibara F, Kataoka S, Ogawa Y, Maeda Y, Takeda K, Nakamura H, Ichimasa K, Kudo T, Hayashi T, Wakamura K, Ishida F, Inoue H, Itoh H, Oda M, Mori K. Real-Time Use of Artificial Intelligence in Identification of Diminutive Polyps During Colonoscopy: A Prospective Study. Ann Intern Med. 2018;169:357-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 355] [Article Influence: 50.7] [Reference Citation Analysis (1)] |
| 60. | Misawa M, Kudo SE, Mori Y, Cho T, Kataoka S, Yamauchi A, Ogawa Y, Maeda Y, Takeda K, Ichimasa K, Nakamura H, Yagawa Y, Toyoshima N, Ogata N, Kudo T, Hisayuki T, Hayashi T, Wakamura K, Baba T, Ishida F, Itoh H, Roth H, Oda M, Mori K. Artificial Intelligence-Assisted Polyp Detection for Colonoscopy: Initial Experience. Gastroenterology 2018; 154: 2027-2029. e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 269] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 61. | Taunk P, Atkinson CD, Lichtenstein D, Rodriguez-Diaz E, Singh SK. Computer-assisted assessment of colonic polyp histopathology using probe-based confocal laser endomicroscopy. Int J Colorectal Dis. 2019;34:2043-2051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 62. | Wang P, Liu X, Berzin TM, Glissen Brown JR, Liu P, Zhou C, Lei L, Li L, Guo Z, Lei S, Xiong F, Wang H, Song Y, Pan Y, Zhou G. Effect of a deep-learning computer-aided detection system on adenoma detection during colonoscopy (CADe-DB trial): a double-blind randomised study. Lancet Gastroenterol Hepatol. 2020;5:343-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 294] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 63. | Tong Y, Lu K, Yang Y, Li J, Lin Y, Wu D, Yang A, Li Y, Yu S, Qian J. Can natural language processing help differentiate inflammatory intestinal diseases in China? BMC Med Inform Decis Mak. 2020;20:248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |