Published online Feb 28, 2022. doi: 10.35711/aimi.v3.i1.8
Peer-review started: January 6, 2022
First decision: February 10, 2022
Revised: February 12, 2022
Accepted: February 21, 2022
Article in press: February 21, 2022
Published online: February 28, 2022
Processing time: 52 Days and 9.2 Hours
For many years, ultrasound was thought to have no indications in pulmonary imaging because lungs are filled with air, creating no acoustic mismatch, as encountered by ultrasound wave beam. Lung ultrasound (LUS) was started in adult critical care settings to detect pleural effusion and acquired more indications over time. In the neonatal intensive care unit (NICU), the use of chest ultrasound has gained more attention during the last two decades. Being a radiation-free, bedside, rapid, and handy tool, LUS started to replace chest X-rays in NICU. Using LUS depends upon understanding the nature of normal lungs and the changes induced by different diseases. With the help of LUS, an experienced neonatologist can detect many of the respiratory problems so fast that interventional therapy can be introduced as early as possible. LUS can diagnose pleural effusion, pneumothorax, pneumonia, transient tachypnoea of the newborn, respiratory distress syndrome, pulmonary atelectasis, meconium aspiration syndrome, bronchopulmonary dysplasia, and some other disorders with very high accuracy. LUS will be helpful in initial diagnosis, follow-up, and predicting the need for further procedures such as mechanical ventilation, diuretic therapy, surfactant therapy, etc. There are some limitations to using LUS in some respiratory disorders such as bullae, interstitial emphysema, and other conditions. This review will highlight the importance of LUS, its uses, and limitations.
Core Tip: Lung ultrasound is a valuable imaging procedure in neonatal respiratory care. It helps diagnose many respiratory disorders with excellent accuracy and safety. Some limitations are experienced for its use, but its benefits are more.
- Citation: Bediwy AS, Al-Biltagi M, Nazeer JA, Saeed NK. Chest ultrasound in neonates: What neonatologists should know. Artif Intell Med Imaging 2022; 3(1): 8-20
- URL: https://www.wjgnet.com/2644-3260/full/v3/i1/8.htm
- DOI: https://dx.doi.org/10.35711/aimi.v3.i1.8
Lung diseases are the most common reasons of respiratory distress in newborn, leading in some instances to respiratory failure; even may end with death. Mortality caused by neonatal respiratory problems was estimated to be 11% in the United States and 32% in China[1,2]. Thus, neonatologists need to identify the etiology and pathology of lung disease causing respiratory problems. Since the sixties of the last century, applying point-of-care ultrasound (POCUS) in neonates was first illustrated, with growing interest with several applications to be used over the past two decades[3,4]. Lung ultrasound started in adult critical care medicine to diagnose various lung and pleural problems. Then in the early nineties, chest ultrasound was suggested to diagnose neonatal respiratory distress syndrome (RDS). Since then, pediatric and neonatal ultrasound of the lung has developed rapidly[5]. After that, several indications were introduced for the lung ultrasound in neonates as transient tachypnoea of the newborn (TTN), neonatal pneumonia, pneumothorax, and meconium aspiration syndrome (MAS) with high specificity and sensitivity[6-10]. Neonatal lung ultrasound (LUS) is an easy bedside procedure with no radiation exposure and can be done serially in neonates[11,12]. LUS can differentiate neonatal respiratory diseases and predict neonatal morbidity[3]. Because of its advantages, LUS aids in distinguishing the various causes of neonatal respiratory failure and guides the management[3,13]. Another advantage of performing LUS in the neonatal intensive care unit (ICU) is the immediate interpretation by the neonatologist with a more accurate diagnosis aiding to start a precise and rapid therapeutic intervention[13]. Although the European Resuscitation Council guidelines recommend utilizing LUS to confirm the placement of the endotracheal tube (ETT) diagnose cardiac tamponade, pneumothorax, and pneumonia, the use of LUS is still not routinely taught in neonatology training programs around the world[14].
There was a notable increase in publications on the use of LUS in both adults and neonates during the last fifteen years. The successful establishment of LUS programs in some neonatal intensive care units (NICU) resulted in a significant reduction in chest radiograms and, subsequently, radiation exposure to patients[12]. One study showed that the risk of cancer occurrence in infants receiving a single small dose of radiation was two to three times higher than the average population and was six to nine times higher than the risk from an exposure of a 60-year-old patient[15]. The POCUS Working Group of the European Society of Paediatric and Neonatal Intensive Care issued evidence-based guidelines on POCUS for neonates and children in 2020[16]. Because it costs less than chest radiology, being radiation-free with higher sensitivity for diagnosing small lesions close to the pleural surface, LUS has been widely used in NICUs. Recently, it has been the most preferred radiological intervention for diagnosing many diseases in the neonatal ICU as RDS, TTN, pneumothorax, MAS, pleural effusions, and neonatal pneumonia than the chest X-ray[17]. LUS is beneficial in the initial diagnosis, follow-up, and assessing the need for further procedures such as mechanical ventilation. Every neonatologist needs to know LUS and get training courses for this unique safe technique.
Ultrasound imaging uses one principle; an interface reflects the ultrasound wave between the different media with various acoustic absorption and impedance[18]. Ultrasound is of limited use in normal well-aerated lungs as there is no acoustic discrepancy in the ultrasound beam as it confronts air[19]. LUS is very useful in neonates because of the thin chest wall and less ossification of the bony thoracic cage[11,20]. A high-frequency linear probe is preferred to perform LUS in neonates because of the relatively thinner chest walls and smaller thoraxes. This high-frequency probe gives a better image quality and allows visualization of the entire lung surface[21]. The high-frequency probe gives a good resolution with penetration to a superficial depth. We use probes with higher frequencies in preterm neonates, for example micro-linear probes with a small footprint (like a hockey stick). An operator with high experience may use different probe types[22]. Different ultrasound modes can be used for LUS. 2-Dimensional brightness (B-mode) and motion (M-mode), and the color doppler to estimate blood flow[23].
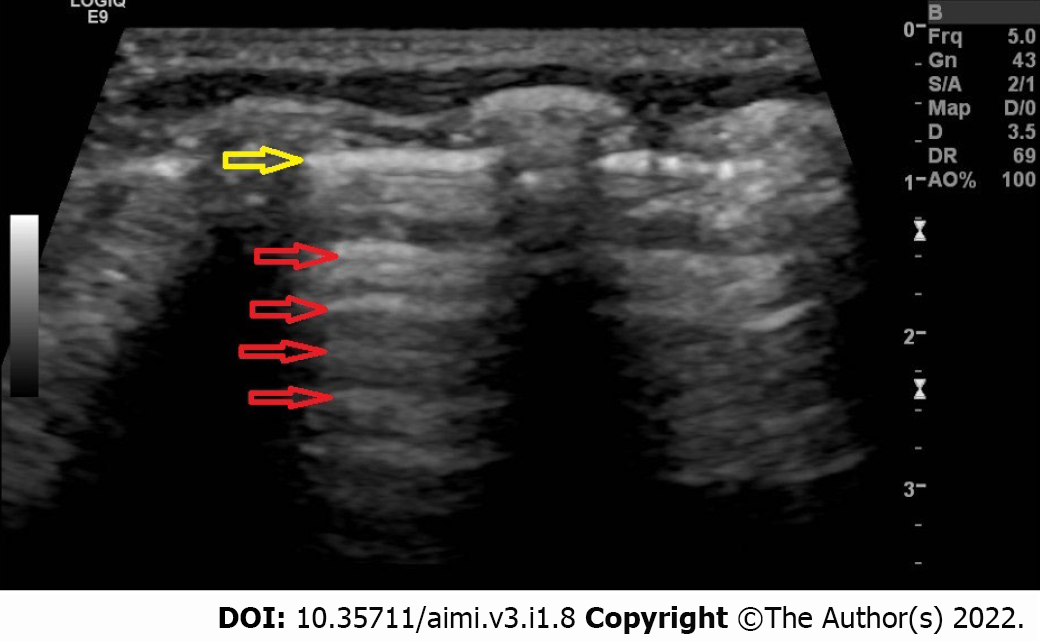
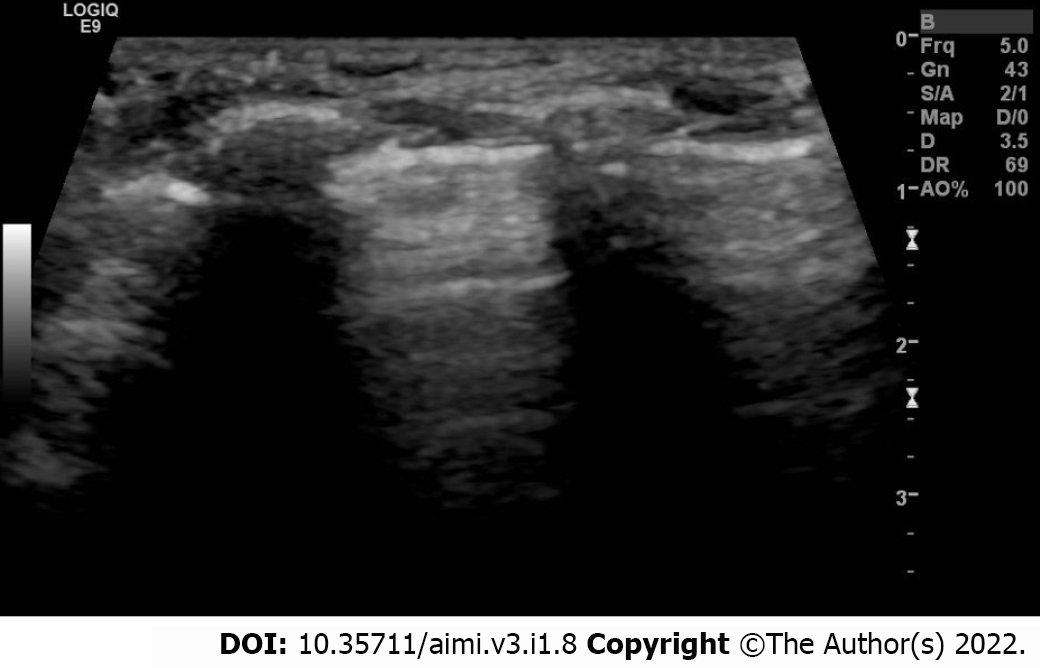
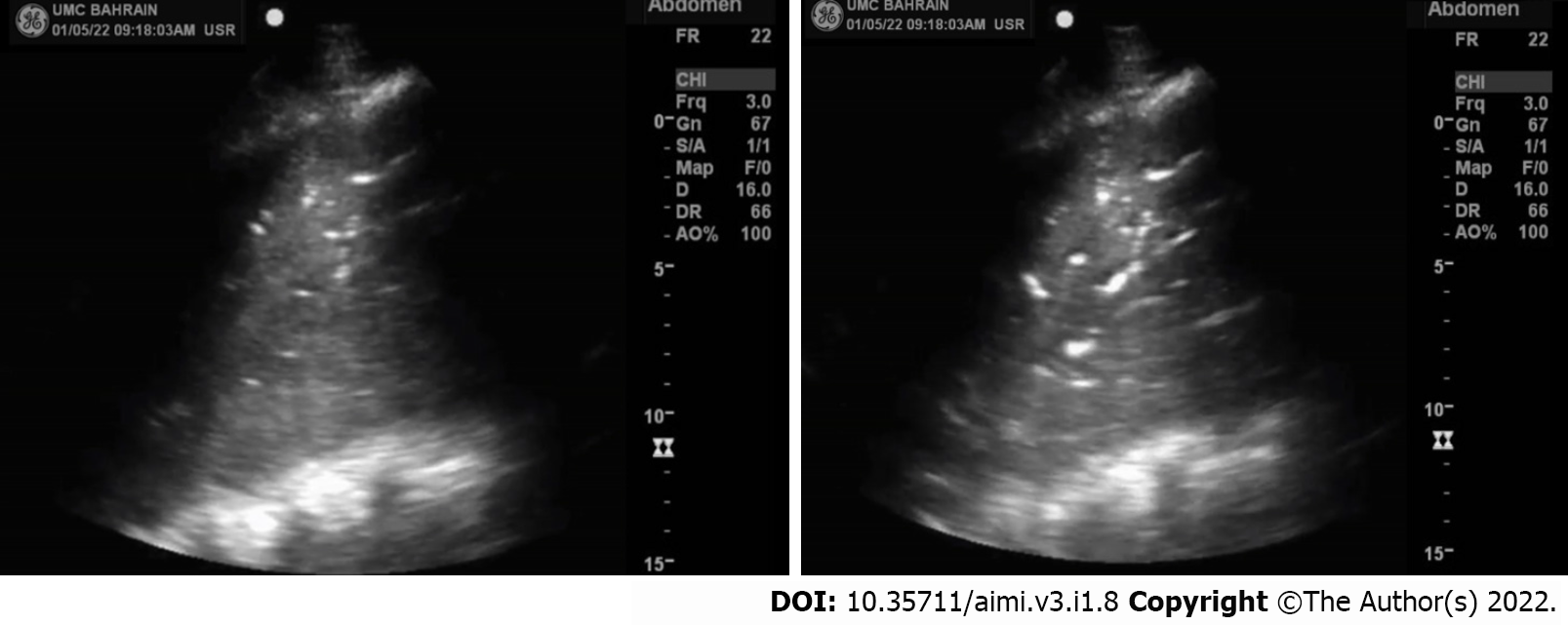
To perform lung ultrasound in neonates we perform it in the lateral, supine, or prone position. Each chest side hemithorax is divided into three areas: Posterior, anterior, and lateral, by the posterior and anterior axillary lines. We can perform longitudinal and transverse scans in all areas to directly identify the ribs, subcutaneous tissue, pleural line, and recognize the lung sliding to indirectly assess the lung tissue[21]. To evaluate or interpret the LUS images, we should understand some terms such as pleural line, A-lines, B-lines, lung sliding and acoustic shadowing artifacts (rib shadow). The pleural line (Figure 1) represents the lung's outer surface, including the visceral and parietal pleura. The pleural line is a regular and smooth hyperechoic line, moving to and fro with respiration. We can clearly visualize the pleural lines in neonates even without pleural or pulmonary pathology. It becomes apparent after birth following the first few breaths[24]. The Bat sign (Figure 2) represents a normal lung surface and is identified by visualizing the bright lateral pleural line (visceral and parietal) and the dark "bat wings" of the two adjacent ribs on each side. In the presence of lung or pleural diseases, the pleural line may become thick and coarse compared to the thin and regular hyperechoic pleural line shape in the healthy lung.
The A-lines are a group of parallel flat lines, occurring at regular distances below and in parallel with the pleural line. They represent a significant alteration in acoustic impedance at the pleuropulmonary line creating horizontal artifacts[25]. A-lines are echo artifacts reflected from the pleural line. They are visualized as hyperechoic, horizontal lines, occurring at equal spaces and extending deeply into the two-Dimensional image. The acoustic shadowing of the ribs represents an artifact arising from the ribs, shown by an anechoic area underneath the ribs and extending deeply into the two-dimensional image and disrupting the A-lines[26]. When the air content of the lung decreases as in subpleural interstitial edema, there will be an acoustic mismatch generated by the ultrasound wave between the fluid interface surrounded by air. This change will be reflected repeatedly at the deeper zones[21,27] and creates vertical artifacts called B-lines. These B-lines correlate with the pulmonary interstitial fluid content. The number of these lines increases with reducing the air content. B-lines or comet tail artifacts represent reverberation artifacts that are laser-like, hyperechoic, shadows that arise from this pleural line extending to the edge of the screen with coinciding movement with respiration. They can be caused by interstitial edema or interlobar septal pulmonary scarring[11,20]. The presence of multiple B-lines indicates alveolar interstitial edema[28,29]. Proof of compact coalesced B-lines in the lung denotes a serious form of the alveolar-interstitial syndrome, called "white lung". It is normal to visualize B-lines in healthy neonatal lungs. Their number will decrease with the baby's growth until being non-visualized at the age of 6 mo in a healthy infant[30,31]. Serial ultrasound imaging is advised to differentiate between standard B lines visualized during the neonatal period from pathological B-lines. If B lines increase, being more compact and coalesced, they will be more pathological. The denser the B-lines are, the more likely they are due to underlying lung pathology.
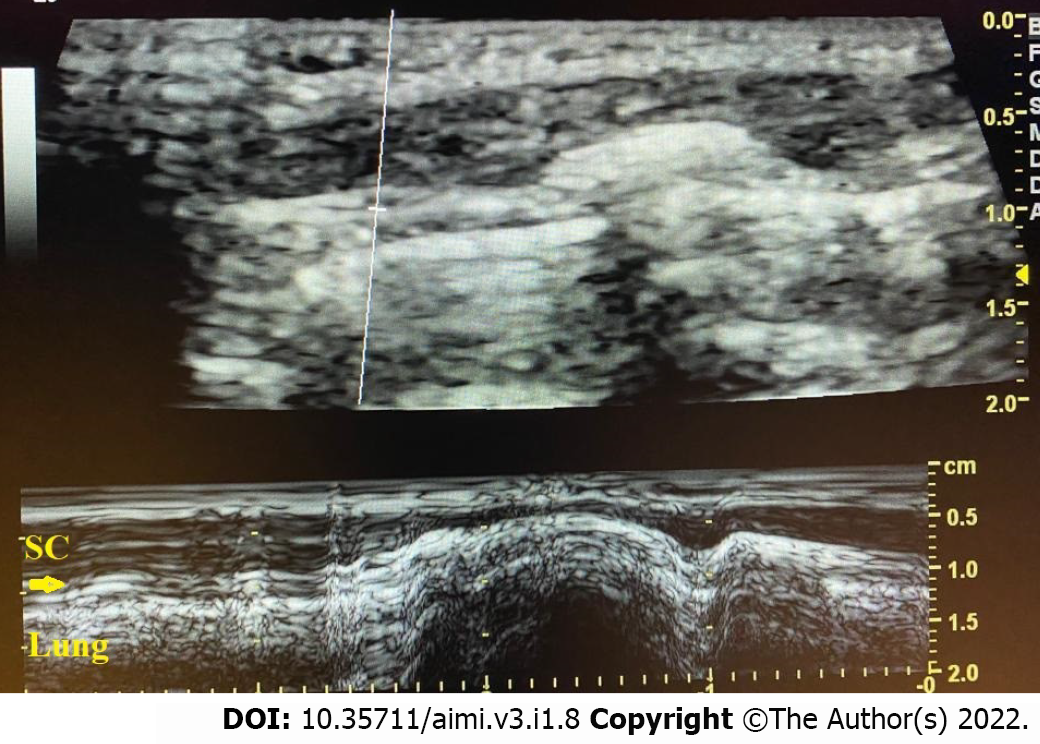
Lung sliding (Figure 3) represents the to-and-fro movement of the parietal and visceral pleura (pleural line) with respiratory movements and could be seen in B-mode and M-mode. Lung sliding visualized in B-mode is known as the movement of marching ants alongside the pleural line with respiration while, in M-mode, we can see lung sliding as the seashore sign in which the non-moving structures above the pleural line correspond to the sea, and the movement underneath the pleural line induces some irregularities simulating a sandy shore (Figure 4)[21,26]. Sometimes, the lung sliding is absent, which indicates a problem in the pleuropulmonary interface that can be observed in pneumothorax, complete atelectasis, pleuropulmonary pathology, and severe hyperinflation that could be seen in cases of foreign body aspiration[32]. Neonatal LUS scores provide a standardized approach to assess pulmonary pathology in the neonate, and evaluation of the disease progression is a semi-quantitative way[3,33-36]. Practically, the score of LUS is frequently assessed by six chest regions over the anterior and lateral zones of the chest. Early after birth, gravity plays a significant role, giving a slight distinction between the dependent and non-dependent lung zones[37]. For each zone, the score will range from 0 to 3. Thus, the total score will be between 0 and 18. Different neonatal pulmonary and pleural diseases have different numbers of B-lines and subpleural lung consolidations per each zone, which can help distinguish each of them[33]. A recent study proved that using more lung zones (10 or even 12 zones) in the first few days after birth did not result in better accuracy for diagnosis and management of bronchopulmonary dysplasia when compared to the standard six zones approach[38].
Neonatal LUS has a broad spectrum of clinical uses nowadays. The guidelines made by the POCUS working group of the European Society of Paediatric and Neonatal Intensive Care in 2020[16] stated that there was reasonable evidence (level B evidence) for neonatal LUS use in cases of transient tachypnoea of the newborn (TTN), respiratory distress syndrome (RDS), pneumothorax, and pleural effusions (with the advantage of guiding the thoracentesis). In some other diseases, the level of evidence was less (level C), such as pulmonary edema and atelectasis. Different algorithms were suggested for neonatal
LUS in the neonate can detect even small volumes of pleural effusion very efficiently and can be used to guide pleural fluid aspiration[42]. In the B-mode, fluid is usually anechoic, sometimes with hepatization of the lung parenchyma. We can see the sinusoid sign in-M mode with the visceral line moving towards the pleural line during respiration. Colour doppler is not commonly used in these cases but can differentiate between echogenic and solid collections inside the effusion[21,26].
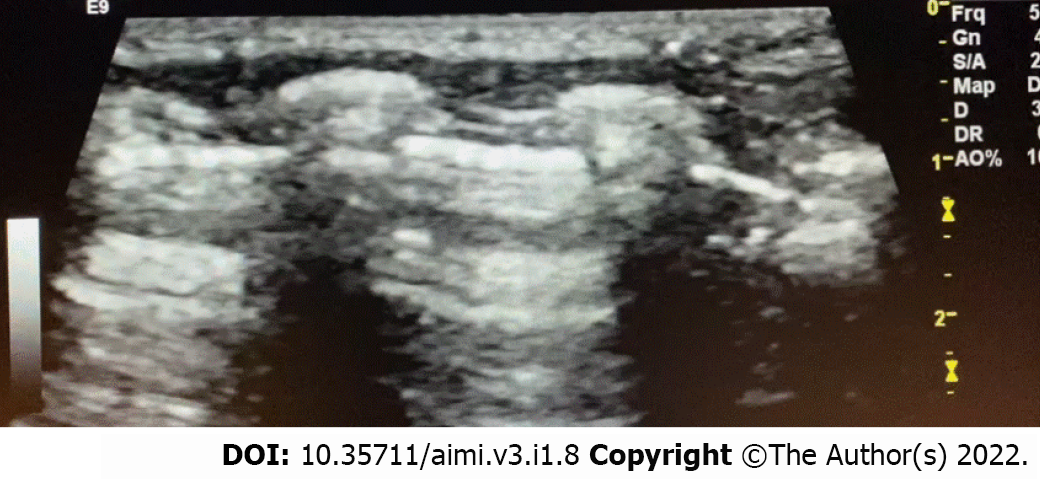
Pneumonia is a severe neonatal disease that carries a high risk of morbidity and mortality, with about one million neonatal deaths yearly and about 10% of the worldwide child mortality[43]. Many pathogens are causing pneumonia in the neonates, such as bacteria, fungi, and viruses. Pneumonia can be acquired after birth or even during the intrauterine period[44]. The pathology includes epithelial injury of airways and alveoli, leakage of protein fluid (exudate), and interstitial edema of the alveoli. Clinical presentations are usually nonspecific and can be indistinguishable from RDS or TTN. Besides the laboratory workup, LUS can help in diagnosis. LUS in neonatal pneumonia cases shows pulmonary consolidation areas with irregular margins surrounding multiple B-lines. Other LUS findings that could present in pneumonia include an invisible pleural line on the affected part of the lung and absent lung sliding. Sometimes we can observe a dynamic air bronchogram, moving with respiration (Figure 5), especially in extensive areas of consolidation, which indicates the patency of airways (thus excluding atelectasis)[45]. In one study on forty cases of neonatal pneumonia vs forty neonates without pulmonary diseases, the authors found that LUS was a reliable method to diagnose neonatal pneumonia. They recommended routine use of LUS in the NICU[46]. A meta-analysis reviewed eight studies found that LUS has excellent sensitivity (96%) and specificity (93%) for the diagnosis of pneumonia in children, and the study recommended LUS as an alternative tool in such cases with no radiation exposure[47].
RDS or hyaline membrane disease is a significant reason for NICU admission and neonatal death. It primarily happens in preterm babies as about 70% of cases are seen in neonates born before 28 wk of pregnancy, and 15%-30% of cases occur in neonates 32-36 wk of gestation[48]. Pulmonary surfactant deficiency is significant in the pathogenesis of RDS. Type II pneumocytes produce pulmonary surfactants. One of their essential functions is to reduce the surface tension in the alveoli preventing the end-expiratory collapse of the alveoli, which requires more work of breathing to re-open in the next respiratory cycle. Affected patients present with respiratory distress and failure within 4-6 h postpartum and, in many cases, require mechanical ventilation[21,26]. LUS in RDS cases shows compact B-lines that coalesce together, giving the appearance of an echographic white lung, a thickened and irregular pleural line, and multiple areas of subpleural pulmonary consolidation (reflecting the presence of alveolar collapse). In one study, these ultrasonic features showed both sensitivity and specificity of 100% for RDS diagnosis[49]. In another study involving 59 neonates having clinical features suggestive of RDS, only 23 of them had actual RDS. In that study, the sensitivity of LUS was 95.6% (in comparison to 91.3 for chest X-ray), and the specificity was 94.4% (it was 84.2% in chest X-ray)[50]. LUS appearance of RDS is, sometimes, not symmetrical in the same or both lungs. Due to gravity issues, these features are usually found in the posterior parts of the chest because of the supine position acquired by the baby most of the time. So, it is crucial to examine the posterior chest in neonates not to miss these signs[51].
The treatment of choice in cases of RDS is the administration of surfactant and supported ventilation as needed. Neonatal LUS is able to expect the requirement for giving surfactant therapy and possibility of mechanical ventilation. One study showed that the presence of white lung signs in neonatal respiratory distress anticipated the need for intubation and mechanical ventilation with good sensitivity and specificity (88.9%, 100%, respectively)[52]. Another study showed that the lung ultrasound score in the first few hours after birth significantly correlates with the oxygenation condition (oxygen indices) in neonates and revealed adequate reliability to predict the requirement for surfactant therapy in premature infants[37]. Two more studies showed that the accuracy of LUS was higher than the fraction of inspired oxygen (FiO2) in predicting the need for surfactant administration in premature babies[53,54]. A recently published trial showed a significant ability of LUS in predicting the need for surfactant compared to FiO2 as a guide for that[55]. The concept of Echography-guided Surfactant THERapy, which uses the LUS score to direct surfactant therapy, resulted in an earlier intake of surfactant, which reduced the duration of invasive mechanical ventilation without any additional cost[56-58].
Atelectasis is a collapse of a part of the lung parenchyma causing impairment of gas exchange. It can be caused by either airway obstruction, lung compression (by pulmonary or extrapulmonary lesion), or alveolar collapse due to increased surface tension of the alveolar wall. The most common mechanism of atelectasis in neonates is airway obstruction by thick mucus, meconium, or foreign particles. Atelectasis is usually associated with some other respiratory disorders[59]. Additionally, right upper lobe collapse in an intubated and mechanically ventilated baby can occur because of traumatic damage to the airway mucosa of the right-sided bronchi[60].
LUS can demonstrate atelectasis as an area of consolidation with the anechoic border and A-lines disruption[20,61]. Complete collapse leads to the absence of lung sliding and lung hepatization[11,61]. In severe atelectasis, lung pulse signs can be noticed in LUS, in which the collapsed part of the lung is pulsating with heartbeats[61]. Static air bronchogram can be observed with atelectasis, and this is different from dynamic air bronchograms (in pneumonia) that move with respiration, although differentiating between them is often challenging and requires an experienced sonographer[46,62]. Also, atelectasis in many cases is indistinguishable from pleural effusion in chest X-ray, but with LUS, it is easy to distinguish. One study showed that the sensitivity of LUS for diagnosing lung atelectasis was 100% vs 75% of chest X-rays (CT was the reference procedure in this study)[61]. Another study showed that the accuracy of LUS for diagnosis of post-anesthesia atelectasis in children was 88%, with a sensitivity of 89% and specificity of 88% (using magnetic resonance imaging as reference)[63].
The incidence of pneumothorax in neonates is about 1%-2%, but this rate is much more in neonates on mechanical ventilation, reaching up to 30%[64]. Tension pneumothorax is mainly encountered in neonates on mechanical ventilation either due to the original disease (as meconium aspiration or ball-valve obstruction of airways causing air trapping and rupture of alveoli) or due to iatrogenic causes such as birth trauma or improper suctioning techniques[65]. LUS signs of a pneumothorax include absent lung sliding, absent B-lines, and the existence of lung point. The absence of lung sliding and B-lines can be explained by accumulation of air in the pleural cavity, preventing the movement of the visceral pleura. It is worth noting that any disease that interrupts the visceral and parietal pleural interface will also cause absent lung sliding. The lung point sign is an area identified where parietal and visceral pleura separate[66]. This sign may be lacking in large tension pneumothorax[67,68].
Many studies showed the usefulness of LUS to detect pneumothorax. One study showed sensitivity and specificity to be 96.7% and 100%, respectively[69]. Another study showed the superiority of LUS over chest X-rays in diagnosing pneumothorax[66]. Another large multi-center study found that LUS is a safe and effective tool to identify serious pneumothorax and assist to manage chest drainage without doing chest X-rays. That study also showed that LUS has sensitivity, specificity, positive predictive value, and negative predictive value reaching up to 100% in diagnosing pneumothorax[8]. Another study compared three imaging techniques for the diagnosis of pneumothorax. It showed that LUS had 100% sensitivity and specificity, chest X-ray had 96% sensitivity and 100% specificity, while chest transillumination had 87% sensitivity and 96% specificity[67].
TTN, or the so-called "wet lung", is considered the most common reason of neonatal respiratory distress. TTN is usually a mild disorder, caused by a delay in the fetal lung fluid clearance (most of the fluid is removed by vaginal squeezing of the chest during labor, while the lymphatics system and pulmonary circulation clear the remaining fluid after being transported to lung interstitium)[70]. So, prematurity and elective cesarean sections are the main precipitating factors[72]. The condition usually resolves spontaneously within 24 h after birth but in a few cases may persist for several days. LUS can distinguish TTN from RDS by identifying B-lines' number and site[6,7]. In TTN cases, there are bilateral symmetric B-lines with a regular pleural line. Severe TTN presents as a white lung. LUS has high specificity but low sensitivity for the diagnosis of TTN. The double lung point sign represents the area between the upper and lower lung zones at which we can distinguish spaced-out B-lines next to confluent B-lines. So, double lung point can be considered a demarcation point of echogenic differences in the lung field[6,7,68].
The double lung point additionally occurs during the diseases recovery phase, such as severe TTN, RDS, and pneumonia[6] Sometimes a mixed RDS/TTN pattern can be identified when the baby has reduced reabsorption of the lung fluid and relative surfactant deficiency. This pattern can be recognized using the LUS score[72]. One prospective cohort study on 59 neonates with respiratory distress found that sensitivity and specificity of LUS for TTN diagnosis were 93.3% and 96.5%, respectively. These values were better than those observed in chest X-rays (89.4% and 91.3%, respectively)[50]. Another recent meta-analysis concluded that LUS has excellent specificity and sensitivity for diagnosing TTN[73]. Studies also showed that LUS could diagnose TTN and predict which neonate may need a higher level of care[74].
Bronchopulmonary dysplasia (BPD) is a common complication related to prematurity and is one of the common complications of RDS. BPD is associated with required respiratory support and/or oxygen supplement at 36 wk corrected gestation. It associates with long-term morbidity and even mortality in some cases[75]. In BPD, structural lung abnormalities, immature biochemical pathways, and oxidant injuries are associated with repeated pulmonary infections and poor nutrition, leading to impaired cardiopulmonary function[76]. LUS features of BPD include thickened coarse pleural lines, subpleural consolidations, and B-lines. According to the severity of inter-lobar septal scarring and interstitial edema, B-lines can be scattered or diffuse. LUS score can help diagnose BPD severity[77] and guide the management, including diuretics use[36].
LUS score can predict the development of BPD in some studies. In a multi-center cohort study, authors found that LUS score on day seven and day fourteen correlates with the oxygenation indices and predicts BPD occurrence when adjusted for gestation and sex[38]. In another cohort study, LUS was done on days 3, 7, and 14 in neonates born before 29 wk gestation[78]. This study showed that the LUS score was higher in neonates who later developed BPD on all-time points, with an LUS score of more than ten on day seven having the highest sensitivity and specificity.
MAS is due to intra-uterine aspiration of meconium-contaminated amniotic fluid into the newborn airways due to fetal hypoxia, acidosis, or infection[79]. Meconium obstructs the airways and induces surfactant dysfunction, chemical pneumonitis, and secondary infection. These will lead to hypoxia due to ventilation/perfusion mismatch[80]. Neonates with MAS have yellowish greenish (meconium stained) skin, umbilical cord, and nails, and signs of respiratory distress. It may develop immediately after birth. MAS is a specific type of pneumonia. So, its LUS features are like pneumonia, giving the features of irregular subpleural consolidations with coalescent B-lines. These features are usually unilateral[81]. Some studies showed the usefulness of LUS for diagnosing MAS in neonates[9,82]. However, LUS should be correlated to the clinical circumstances and physical examination.
Table 1 summarizes lung ultrasound appearance in different neonatal lung diseases compared to chest X-rays.
| Disease | Chest X-ray | Lung ultrasound |
| Pleural effusion | Homogenous opacity obliterating costophrenic and cardiophrenic angles | B-mode: Fluid is anechoic, sometimes ± hepatization of the lung parenchyma. M-mode: The sinusoid sign with the visceral line moving towards the pleural line during respiration |
| Pneumonia | Homogeneous opacities that can be patchy or lobar in distribution | Consolidation areas with irregular margins surrounding multiple B-lines. Invisible pleural line on the affected area. Sometimes: Dynamic air bronchogram |
| RDS | Alveolar shadowing (ground glass) with air bronchogram | Compact coalescent B-lines (white lung). Thickened, irregular pleural line. Multiple areas of sub-pleural consolidation |
| Atelectasis | Area of opacity in the lung with features of volume loss as shifting of mediastinum to the same side, pulled fissure, etc. | Area of consolidation with anechoic clear border and disrupted A-lines. Static air bronchogram. Complete collapse leads to the absence of lung sliding, lung hepatization, and lung pulse signs |
| Pneumothorax | Jet black translucency with collapsed lung and sometimes mediastinal shift to the other side | Absent lung sliding, absent B-lines, and the presence of lung point |
| TTN | Interstitial oedema predominantly in the peri-hilar region (wet silhouette) | Double lung point sign. B-lines. In severe cases: (white lung) |
| BPD | Ill-defined diffuse reticular markings with circular lucent areas in between and hyperinflated lung | Thickened coarse pleural linesSubpleural areas of consolidation. B-lines |
| MAS | Patchy consolidation | Same as pneumonia |
LUS can be used to assess lung recruitment with positive end-expiratory pressure without the need for exposure to ionizing radiation by doing CT chest[83]. LUS can also effectively monitor bronchoalveolar lavage in neonates with atelectasis, with an efficacy approaching 93%[84]. Another application of interest that was seen in some studies is the use of LUS to assess the position of the ETT in the trachea by measuring the space between the ETT distal end and the aortic arch apex[85] or the space between ETT distal end and the superior edge of the right pulmonary artery[86]. We can achieve this technique by utilising either a phase array probe (while doing the high parasternal view) or a linear probe (in the midsagittal view). Another study reported the use of LUS to immediately confirm the proper ETT position during neonatal resuscitation. This study used a linear probe in the transverse position[87].
Another critical use of ultrasounds is evaluation of vocal cord function. One study displayed that utilising high-frequency linear hockey stick probe in a transverse position over the middle of the neck could identify the presence of vocal cord paresis post-operatively (after aortic arch repair) with high sensitivity and specificity is compared to flexible fibreoptic endoscopy[88]. LUS has also been utilized to evaluate the diaphragm[89,90]. A recent study used LUS and diaphragmatic shortening fraction, a known way of assessing adult diaphragm function, to evaluate diaphragm in neonates. This study found that the diaphragmatic shortening fraction could be assessed in neonates[91]. LUS has also been suggested as a modality to follow asymptomatic CPAMs, but more studies are needed to stabilize this indication[92,93].
Although LUS is a very effective and safe imaging technique in neonates, we should consider the clinical finding of each case. Moreover, according to the application of LUS, and some problems in actual clinical practice, LUS has some limitations in some pulmonary conditions. For example, as mentioned above, the diagnosis of CPAMs using LUS is still not standardized, and many studies must be done in this context. Some cases of CPAMs can be detected in utero using ultrasound as the fetal lung is filled with fluid. On the contrary, due to air-filled neonatal lungs, their diagnosis by LUS in the neonatal period seems to be difficult because these lesions are usually away from the chest wall. Thus, lesions that are located away from the pleura could not be visualized by LUS[92].
LUS cannot identify some specific lesions because of the influence of gas in front of the lesion. When the acoustic beam of ultrasound encounters gas, it will be reflected ultimately. So, cases of pulmonary bullae cannot be visualized by LUS because of the large amount of gas in the bulla reflecting the acoustic beam of ultrasound. Similarly, the presence of subcutaneous emphysema or pneumomediastinum will affect the results of LUS due to the same reasons described above. Although LUS is a handy tool to diagnose pneumothorax, it cannot measure the size due to the total reflection caused by the gas[66].
Consequently, we need more studies to quantify the size of pneumothorax using LUS. Pulmonary interstitial emphysema is another condition that LUS cannot diagnose. In a published case study, the authors used LUS to follow-up localized interstitial emphysema. The infant presented again with tachypnoea after being treated with continuous positive airway pressure for three days. The chest computed tomography revealed localized interstitial emphysema of the left upper lobe, whereas LUS did not show this lesion[94]. We emphasized that using LUS is potentially harmful without adequate expertise. It may not provide definite diagnostic information and may allow over trust in the procedure, which could have profound legal implications and not address the underlying lesions. The misuse of artifacts as a diagnostic tool should be abandoned. Lung ultrasound imaging is advantageous when definite imaging is possible, even in the newborn.
Lung ultrasound is a valuable imaging tool frequently used in neonatal respiratory care. It helps diagnose many respiratory disorders with excellent accuracy and safety with no radiation risk. Lung ultrasound is operator dependent and needs adequate experience to achieve good results. Some limitations are encountered for its use, but its benefits are more.
We thank the anonymous referees for their useful suggestions.
Provenance and peer review: Invited article; externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pediatrics
Country/Territory of origin: Egypt
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Haurylenka D, Trovato G S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Angus DC, Linde-Zwirble WT, Clermont G, Griffin MF, Clark RH. Epidemiology of neonatal respiratory failure in the United States: projections from California and New York. Am J Respir Crit Care Med. 2001;164:1154-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 119] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 2. | Qian L, Liu C, Zhuang W, Guo Y, Yu J, Chen H, Wang S, Lin Z, Xia S, Ni L, Liu X, Chen C, Sun B; Chinese Collaborative Study Group for Neonatal Respiratory Diseases. Neonatal respiratory failure: a 12-month clinical epidemiologic study from 2004 to 2005 in China. Pediatrics. 2008;121:e1115-e1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Raimondi F, Yousef N, Migliaro F, Capasso L, De Luca D. Point-of-care lung ultrasound in neonatology: classification into descriptive and functional applications. Pediatr Res. 2021;90:524-531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 144] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 4. | Mongodi S, Santangelo E, De Luca D, Rovida S, Corradi F, Volpicelli G, Gargani L, Bouhemad B, Mojoli F. Quantitative Lung Ultrasound: Time for a Consensus? Chest. 2020;158:469-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Avni EF, Braude P, Pardou A, Matos C. Hyaline membrane disease in the newborn: diagnosis by ultrasound. Pediatr Radiol. 1990;20:143-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Liu J, Chen XX, Li XW, Chen SW, Wang Y, Fu W. Lung Ultrasonography to Diagnose Transient Tachypnea of the Newborn. Chest. 2016;149:1269-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 7. | Raimondi F, Yousef N, Rodriguez Fanjul J, De Luca D, Corsini I, Shankar-Aguilera S, Dani C, Di Guardo V, Lama S, Mosca F, Migliaro F, Sodano A, Vallone G, Capasso L. A Multicenter Lung Ultrasound Study on Transient Tachypnea of the Neonate. Neonatology. 2019;115:263-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 8. | Raimondi F, Rodriguez Fanjul J, Aversa S, Chirico G, Yousef N, De Luca D, Corsini I, Dani C, Grappone L, Orfeo L, Migliaro F, Vallone G, Capasso L; Lung Ultrasound in the Crashing Infant (LUCI) Protocol Study Group. Lung Ultrasound for Diagnosing Pneumothorax in the Critically Ill Neonate. J Pediatr. 2016;175:74-78.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 126] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 9. | Piastra M, Yousef N, Brat R, Manzoni P, Mokhtari M, De Luca D. Lung ultrasound findings in meconium aspiration syndrome. Early Hum Dev. 2014;90 Suppl 2:S41-S43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 98] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 10. | Corsini I, Parri N, Gozzini E, Coviello C, Leonardi V, Poggi C, Giacalone M, Bianconi T, Tofani L, Raimondi F, Dani C. Lung Ultrasound for the Differential Diagnosis of Respiratory Distress in Neonates. Neonatology. 2019;115:77-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 11. | Ammirabile A, Buonsenso D, Di Mauro A. Lung Ultrasound in Pediatrics and Neonatology: An Update. Healthcare (Basel). 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Escourrou G, De Luca D. Lung ultrasound decreased radiation exposure in preterm infants in a neonatal intensive care unit. Acta Paediatr. 2016;105:e237-e239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 13. | Okbay Gunes A, Karadag N, Cakir H, Hakyemez Toptan H, Karatekin G. The Associations Between Lung Ultrasonography Scores in the First Day of Life and Clinical Outcomes: Authors' Reply. J Ultrasound Med. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Van de Voorde P, Turner NM, Djakow J, de Lucas N, Martinez-Mejias A, Biarent D, Bingham R, Brissaud O, Hoffmann F, Johannesdottir GB, Lauritsen T, Maconochie I. European Resuscitation Council Guidelines 2021: Paediatric Life Support. Resuscitation. 2021;161:327-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 208] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 15. | Hall EJ. Lessons we have learned from our children: cancer risks from diagnostic radiology. Pediatr Radiol. 2002;32:700-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 275] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 16. | Singh Y, Tissot C, Fraga MV, Yousef N, Cortes RG, Lopez J, Sanchez-de-Toledo J, Brierley J, Colunga JM, Raffaj D, Da Cruz E, Durand P, Kenderessy P, Lang HJ, Nishisaki A, Kneyber MC, Tissieres P, Conlon TW, De Luca D. International evidence-based guidelines on Point of Care Ultrasound (POCUS) for critically ill neonates and children issued by the POCUS Working Group of the European Society of Paediatric and Neonatal Intensive Care (ESPNIC). Crit Care. 2020;24:65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 314] [Cited by in RCA: 356] [Article Influence: 71.2] [Reference Citation Analysis (1)] |
| 17. | Retraction. Immunization with pseudotype baculovirus expressing envelope protein of Japanese encephalitis virus elicits protective immunity in mice by Yaoming Li, Jing Ye, Shengbo Cao, Shaobo Xiao, Qian Zhao, Xueqin Liu, Meilin Jin, Huanchun Chen. J Gene Med. 2009;11:454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 18. | Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5281] [Cited by in RCA: 5685] [Article Influence: 157.9] [Reference Citation Analysis (0)] |
| 19. | Gargani L. Lung ultrasound: a new tool for the cardiologist. Cardiovasc Ultrasound. 2011;9:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 216] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 20. | Kurepa D, Zaghloul N, Watkins L, Liu J. Neonatal lung ultrasound exam guidelines. J Perinatol. 2018;38:11-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 93] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 21. | Liang HY, Liang XW, Chen ZY, Tan XH, Yang HH, Liao JY, Cai K, Yu JS. Ultrasound in neonatal lung disease. Quant Imaging Med Surg. 2018;8:535-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 22. | Gomond-Le Goff C, Vivalda L, Foligno S, Loi B, Yousef N, De Luca D. Effect of Different Probes and Expertise on the Interpretation Reliability of Point-of-Care Lung Ultrasound. Chest. 2020;157:924-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 23. | Demi L, van Hoeve W, van Sloun RJG, Soldati G, Demi M. Determination of a potential quantitative measure of the state of the lung using lung ultrasound spectroscopy. Sci Rep. 2017;7:12746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 24. | Blank DA, Rogerson SR, Kamlin COF, Fox LM, Lorenz L, Kane SC, Polglase GR, Hooper SB, Davis PG. Lung ultrasound during the initiation of breathing in healthy term and late preterm infants immediately after birth, a prospective, observational study. Resuscitation. 2017;114:59-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 25. | Lichtenstein DA, Mezière GA, Lagoueyte JF, Biderman P, Goldstein I, Gepner A. A-lines and B-lines: lung ultrasound as a bedside tool for predicting pulmonary artery occlusion pressure in the critically ill. Chest. 2009;136:1014-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 274] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 26. | Ruoss JL, Bazacliu C, Cacho N, De Luca D. Lung Ultrasound in the Neonatal Intensive Care Unit: Does It Impact Clinical Care? Children (Basel). 2021;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Jambrik Z, Monti S, Coppola V, Agricola E, Mottola G, Miniati M, Picano E. Usefulness of ultrasound lung comets as a nonradiologic sign of extravascular lung water. Am J Cardiol. 2004;93:1265-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 417] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 28. | Lichtenstein D, Mézière G, Biderman P, Gepner A, Barré O. The comet-tail artifact. An ultrasound sign of alveolar-interstitial syndrome. Am J Respir Crit Care Med. 1997;156:1640-1646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 751] [Cited by in RCA: 702] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 29. | Volpicelli G, Mussa A, Garofalo G, Cardinale L, Casoli G, Perotto F, Fava C, Frascisco M. Bedside lung ultrasound in the assessment of alveolar-interstitial syndrome. Am J Emerg Med. 2006;24:689-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 392] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 30. | Aichhorn L, Küng E, Habrina L, Werther T, Berger A, Urlesberger B, Schwaberger B. The Role of Lung Ultrasound in the Management of the Critically Ill Neonate-A Narrative Review and Practical Guide. Children (Basel). 2021;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 31. | Buonsenso D, Soldati G, Curatola A, Morello R, De Rose C, Vacca ME, Lazzareschi I, Musolino AM, Valentini P. Lung Ultrasound Pattern in Healthy Infants During the First 6 Months of Life. J Ultrasound Med. 2020;39:2379-2388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 32. | Lovrenski J, Vilotijević Dautović G, Lovrenski A. Reduced or Absent "Lung Sliding" - A Novel Lung Ultrasound Sign of Pediatric Foreign Body Aspiration. J Ultrasound Med. 2019;38:3079-3082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 33. | Raimondi F, Migliaro F, Corsini I, Meneghin F, Dolce P, Pierri L, Perri A, Aversa S, Nobile S, Lama S, Varano S, Savoia M, Gatto S, Leonardi V, Capasso L, Carnielli VP, Mosca F, Dani C, Vento G, Lista G. Lung Ultrasound Score Progress in Neonatal Respiratory Distress Syndrome. Pediatrics. 2021;147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 79] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 34. | Oulego-Erroz I, Alonso-Quintela P, Terroba-Seara S, Jiménez-González A, Rodríguez-Blanco S. Early assessment of lung aeration using an ultrasound score as a biomarker of developing bronchopulmonary dysplasia: a prospective observational study. J Perinatol. 2021;41:62-68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 35. | Alonso-Ojembarrena A, Serna-Guerediaga I, Aldecoa-Bilbao V, Gregorio-Hernández R, Alonso-Quintela P, Concheiro-Guisán A, Ramos-Rodríguez A, de Las Heras-Martín M, Rodeño-Fernández L, Oulego-Erroz I. The Predictive Value of Lung Ultrasound Scores in Developing Bronchopulmonary Dysplasia: A Prospective Multicenter Diagnostic Accuracy Study. Chest. 2021;160:1006-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 36. | Alonso-Ojembarrena A, Lechuga-Sancho AM, Morales-Arandojo P, Acuñas-Soto S, López-de-Francisco R, Lubián-López SP. Lung ultrasound score and diuretics in preterm infants born before 32 weeks: A pilot study. Pediatr Pulmonol. 2020;55:3312-3318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 37. | Brat R, Yousef N, Klifa R, Reynaud S, Shankar Aguilera S, De Luca D. Lung Ultrasonography Score to Evaluate Oxygenation and Surfactant Need in Neonates Treated With Continuous Positive Airway Pressure. JAMA Pediatr. 2015;169:e151797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 312] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 38. | Loi B, Vigo G, Baraldi E, Raimondi F, Carnielli VP, Mosca F, De Luca D. Lung Ultrasound to Monitor Extremely Preterm Infants and Predict Bronchopulmonary Dysplasia. A Multicenter Longitudinal Cohort Study. Am J Respir Crit Care Med. 2021;203:1398-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 102] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 39. | Yousef N, Singh Y, De Luca D. "Playing it SAFE in the NICU" SAFE-R: a targeted diagnostic ultrasound protocol for the suddenly decompensating infant in the NICU. Eur J Pediatr. 2022;181:393-398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 40. | Liu J, Copetti R, Sorantin E, Lovrenski J, Rodriguez-Fanjul J, Kurepa D, Feng X, Cattaross L, Zhang H, Hwang M, Yeh TF, Lipener Y, Lodha A, Wang JQ, Cao HY, Hu CB, Lyu GR, Qiu XR, Jia LQ, Wang XM, Ren XL, Guo JY, Gao YQ, Li JJ, Liu Y, Fu W, Wang Y, Lu ZL, Wang HW, Shang LL. Protocol and Guidelines for Point-of-Care Lung Ultrasound in Diagnosing Neonatal Pulmonary Diseases Based on International Expert Consensus. J Vis Exp. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (1)] |
| 41. | Liu J, Guo G, Kurepa D, Volpicelli G, Sorantin E, Lovrenski J, Alonso-Ojembarrena A, Hsieh KS, Lodha A, Yeh TF, Jagła M, Shah H, Yan W, Hu CB, Zhou XG, Guo RJ, Cao HY, Wang Y, Zong HF, Shang LL, Ma HR, Liu Y, Fu W, Shan RY, Qiu RX, Ren XL, Copetti R, Rodriguez-Fanjul J, Feletti F; Society of Pediatrics, Asia-Pacific Health Association; the Division of Critical Ultrasound, Pediatric Society of Asia-Pacific Health Association; the Critical Ultrasound Group of Neonatal Specialty Committee, the Cross-Straits Medicine Exchange Association as well as the World Interactive Network Focused On Critical Ultrasound China Branch. Specification and guideline for technical aspects and scanning parameter settings of neonatal lung ultrasound examination. J Matern Fetal Neonatal Med. 2022;35:1003-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 42. | Soni NJ, Franco R, Velez MI, Schnobrich D, Dancel R, Restrepo MI, Mayo PH. Ultrasound in the diagnosis and management of pleural effusions. J Hosp Med. 2015;10:811-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 131] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 43. | Duke T. Neonatal pneumonia in developing countries. Arch Dis Child Fetal Neonatal Ed. 2005;90:F211-F219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 100] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 44. | Nissen MD. Congenital and neonatal pneumonia. Paediatr Respir Rev. 2007;8:195-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 45. | Copetti R, Cattarossi L. Ultrasound diagnosis of pneumonia in children. Radiol Med. 2008;113:190-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 213] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 46. | Liu J, Liu F, Liu Y, Wang HW, Feng ZC. Lung ultrasonography for the diagnosis of severe neonatal pneumonia. Chest. 2014;146:383-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 124] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 47. | Pereda MA, Chavez MA, Hooper-Miele CC, Gilman RH, Steinhoff MC, Ellington LE, Gross M, Price C, Tielsch JM, Checkley W. Lung ultrasound for the diagnosis of pneumonia in children: a meta-analysis. Pediatrics. 2015;135:714-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 299] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 48. | Hjalmarson O. Epidemiology and classification of acute, neonatal respiratory disorders. A prospective study. Acta Paediatr Scand. 1981;70:773-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 140] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 49. | Copetti R, Cattarossi L, Macagno F, Violino M, Furlan R. Lung ultrasound in respiratory distress syndrome: a useful tool for early diagnosis. Neonatology. 2008;94:52-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 198] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 50. | Vergine M, Copetti R, Brusa G, Cattarossi L. Lung ultrasound accuracy in respiratory distress syndrome and transient tachypnea of the newborn. Neonatology. 2014;106:87-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 51. | Lovrenski J. Lung ultrasonography of pulmonary complications in preterm infants with respiratory distress syndrome. Ups J Med Sci. 2012;117:10-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 52. | Raimondi F, Migliaro F, Sodano A, Ferrara T, Lama S, Vallone G, Capasso L. Use of neonatal chest ultrasound to predict noninvasive ventilation failure. Pediatrics. 2014;134:e1089-e1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 112] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 53. | Badurdeen S, Kamlin COF, Rogerson SR, Kane SC, Polglase GR, Hooper SB, Davis PG, Blank DA. Lung ultrasound during newborn resuscitation predicts the need for surfactant therapy in very- and extremely preterm infants. Resuscitation. 2021;162:227-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 54. | De Martino L, Yousef N, Ben-Ammar R, Raimondi F, Shankar-Aguilera S, De Luca D. Lung Ultrasound Score Predicts Surfactant Need in Extremely Preterm Neonates. Pediatrics. 2018;142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 185] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 55. | Raimondi F, Migliaro F, Corsini I, Meneghin F, Pierri L, Salomè S, Perri A, Aversa S, Nobile S, Lama S, Varano S, Savoia M, Gatto S, Leonardi V, Capasso L, Carnielli VP, Mosca F, Dani C, Vento G, Dolce P, Lista G. Neonatal Lung Ultrasound and Surfactant Administration: A Pragmatic, Multicenter Study. Chest. 2021;160:2178-2186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 56. | De Luca D, Autilio C, Pezza L, Shankar-Aguilera S, Tingay DG, Carnielli VP. Personalized Medicine for the Management of RDS in Preterm Neonates. Neonatology. 2021;118:127-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 57. | Spila-Alegiani S, Da Cas R, Giambi C, Raschetti R, Salmaso S. [Human papillomavirus vaccine register]. Recenti Prog Med. 2013;104:262-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 58. | De Luca D, Yousef N. Pharmaceutical Expenditure Is Unchanged with Ultrasound-Guided Surfactant Administration. Am J Perinatol. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 59. | Peroni DG, Boner AL. Atelectasis: mechanisms, diagnosis and management. Paediatr Respir Rev. 2000;1:274-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 60. | Whitfield JM, Jones MD Jr. Atelectasis associated with mechanical ventilation for hyaline membrane disease. Crit Care Med. 1980;8:729-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 61. | Liu J, Chen SW, Liu F, Li QP, Kong XY, Feng ZC. The diagnosis of neonatal pulmonary atelectasis using lung ultrasonography. Chest. 2015;147:1013-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 62. | Öktem A, Zenciroğlu A, Üner Ç, Aydoğan S, Dilli D, Okumuş N. Efficiency of Lung Ultrasonography in the Diagnosis and Follow-up of Viral Pneumonia in Newborn. Am J Perinatol. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 63. | Acosta CM, Maidana GA, Jacovitti D, Belaunzarán A, Cereceda S, Rae E, Molina A, Gonorazky S, Bohm SH, Tusman G. Accuracy of transthoracic lung ultrasound for diagnosing anesthesia-induced atelectasis in children. Anesthesiology. 2014;120:1370-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 126] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 64. | Apiliogullari B, Sunam GS, Ceran S, Koc H. Evaluation of neonatal pneumothorax. J Int Med Res. 2011;39:2436-2440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 65. | Wyatt TH. Pneumothorax in the neonate. J Obstet Gynecol Neonatal Nurs. 1995;24:211-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 66. | Fei Q, Lin Y, Yuan TM. Lung Ultrasound, a Better Choice for Neonatal Pneumothorax: A Systematic Review and Meta-analysis. Ultrasound Med Biol. 2021;47:359-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 67. | Cattarossi L, Copetti R, Brusa G, Pintaldi S. Lung Ultrasound Diagnostic Accuracy in Neonatal Pneumothorax. Can Respir J. 2016;2016:6515069. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 68. | Copetti R, Cattarossi L. The 'double lung point': an ultrasound sign diagnostic of transient tachypnea of the newborn. Neonatology. 2007;91:203-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 156] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 69. | Dahmarde H, Parooie F, Salarzaei M. Accuracy of Ultrasound in Diagnosis of Pneumothorax: A Comparison between Neonates and Adults-A Systematic Review and Meta-Analysis. Can Respir J. 2019;2019:5271982. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 70. | Yurdakök M. Transient tachypnea of the newborn: what is new? J Matern Fetal Neonatal Med. 2010;23 Suppl 3:24-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 71. | Guglani L, Lakshminrusimha S, Ryan RM. Transient tachypnea of the newborn. Pediatr Rev. 2008;29:e59-e65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 72. | Machado AL, Bochio BC, Wady AF, Jorge JH, Canevarolo SV Jr, Vergani CE. Impact strength of denture base and reline acrylic resins: An in vitro study. J Dent Biomech. 2012;3:1758736012459535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 73. | He L, Sun Y, Sheng W, Yao Q. Diagnostic performance of lung ultrasound for transient tachypnea of the newborn: A meta-analysis. PLoS One. 2021;16:e0248827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 74. | Raimondi F, Migliaro F, Sodano A, Umbaldo A, Romano A, Vallone G, Capasso L. Can neonatal lung ultrasound monitor fluid clearance and predict the need of respiratory support? Crit Care. 2012;16:R220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 75. | Kewitz G, Wudel S, Hopp H, Hopfenmüller W, Vogel M, Roots I. Below median birth weight in appropriate-for-gestational-age preterm infants as a risk factor for bronchopulmonary dysplasia. J Perinat Med. 2008;36:359-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 76. | McEvoy CT, Jain L, Schmidt B, Abman S, Bancalari E, Aschner JL. Bronchopulmonary dysplasia: NHLBI Workshop on the Primary Prevention of Chronic Lung Diseases. Ann Am Thorac Soc. 2014;11 Suppl 3:S146-S153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 187] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 77. | Liu J, Chen SW, Liu F, Wang Y, Kong XY, Li QP, Huang JJ. BPD, Not BPD, or iatrogenic BPD: findings of lung ultrasound examinations. Medicine (Baltimore). 2014;93:e133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 78. | Mohamed A, Mohsen N, Diambomba Y, Lashin A, Louis D, Elsayed Y, Shah PS. Lung Ultrasound for Prediction of Bronchopulmonary Dysplasia in Extreme Preterm Neonates: A Prospective Diagnostic Cohort Study. J Pediatr. 2021;238:187-192.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 79. | Poggi SH, Ghidini A. Pathophysiology of meconium passage into the amniotic fluid. Early Hum Dev. 2009;85:607-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 80. | Chettri S, Bhat BV, Adhisivam B. Current Concepts in the Management of Meconium Aspiration Syndrome. Indian J Pediatr. 2016;83:1125-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 81. | Cattarossi L. Lung ultrasound: its role in neonatology and pediatrics. Early Hum Dev. 2013;89 Suppl 1:S17-S19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 82. | Liu J, Cao HY, Fu W. Lung ultrasonography to diagnose meconium aspiration syndrome of the newborn. J Int Med Res. 2016;44:1534-1542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 83. | Sameshima YT, Lourenço de Almeida JF, Silva MM, Remondini R, Haddad LB, Neto MJ, Buarque de Gusmão Funari M. Ultrasound-guided lung recruitment in a 3-month-old infant with acute respiratory distress syndrome. Ultrasound Q. 2014;30:301-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 84. | Liu J, Ren XL, Fu W, Liu Y, Xia RM. Bronchoalveolar lavage for the treatment of neonatal pulmonary atelectasis under lung ultrasound monitoring. J Matern Fetal Neonatal Med. 2017;30:2362-2366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 85. | Chowdhry R, Dangman B, Pinheiro JM. The concordance of ultrasound technique versus X-ray to confirm endotracheal tube position in neonates. J Perinatol. 2015;35:481-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 86. | Zaytseva A, Kurepa D, Ahn S, Weinberger B. Determination of optimal endotracheal tube tip depth from the gum in neonates by X-ray and ultrasound. J Matern Fetal Neonatal Med. 2020;33:2075-2080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 87. | Takeuchi S, Arai J. Ultrasonographic confirmation of tracheal intubation for congenital chylothorax. Pediatr Int. 2018;60:308-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 88. | Lee MGY, Millar J, Rose E, Jones A, Wood D, Luitingh TL, Zannino D, Brink J, Konstantinov IE, Brizard CP, d'Udekem Y. Laryngeal ultrasound detects a high incidence of vocal cord paresis after aortic arch repair in neonates and young children. J Thorac Cardiovasc Surg. 2018;155:2579-2587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 89. | Pitt B, Filippatos G, Agarwal R, Anker SD, Bakris GL, Rossing P, Joseph A, Kolkhof P, Nowack C, Schloemer P, Ruilope LM; FIGARO-DKD Investigators. Cardiovascular Events with Finerenone in Kidney Disease and Type 2 Diabetes. N Engl J Med. 2021;385:2252-2263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 860] [Article Influence: 215.0] [Reference Citation Analysis (0)] |
| 90. | Llamas-Álvarez AM, Tenza-Lozano EM, Latour-Pérez J. Diaphragm and Lung Ultrasound to Predict Weaning Outcome: Systematic Review and Meta-Analysis. Chest. 2017;152:1140-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 134] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 91. | Alonso-Ojembarrena A, Ruiz-González E, Estepa-Pedregosa L, Armenteros-López AI, Segado-Arenas A, Lubián-López SP. Reproducibility and reference values of diaphragmatic shortening fraction for term and premature infants. Pediatr Pulmonol. 2020;55:1963-1968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 92. | Yousef N, Mokhtari M, Durand P, Raimondi F, Migliaro F, Letourneau A, Tissières P, De Luca D. Lung Ultrasound Findings in Congenital Pulmonary Airway Malformation. Am J Perinatol. 2018;35:1222-1227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 93. | Adin ME. Ultrasound as a screening tool in the follow-up of asymptomatic congenital cystic adenomatoid malformation. Ultrasound. 2016;24:175-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 94. | Balcells C, Del Río R, Riaza L, Rebollo M, Rodriguez-Fanjul J, Camprubí M. Lung ultrasound: a useful tool for the follow-up of neonatal localized interstitial emphysema. J Pediatr. 2015;166:1543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |