Published online Sep 28, 2020. doi: 10.35711/aimi.v1.i3.87
Peer-review started: August 23, 2020
First decision: September 13, 2020
Revised: September 22, 2020
Accepted: September 23, 2020
Article in press: September 23, 2020
Published online: September 28, 2020
Processing time: 35 Days and 13.6 Hours
In this editorial, we discussed the current research status of artificial intelligence (AI) in Oncology, reviewing the basics of machine learning (ML) and deep learning (DL) techniques and their emerging applications on clinical and imaging cancer workflow. The growing amounts of available “big data” coupled to the increasing computational power have enabled the development of computer-based systems capable to perform advanced tasks in many areas of clinical care, especially in medical imaging. ML is a branch of data science that allows the creation of computer algorithms that can learn and make predictions without prior instructions. DL is a subgroup of artificial neural network algorithms configurated to automatically extract features and perform high-level tasks; convolutional neural networks are the most common DL models used in medical image analysis. AI methods have been proposed in many areas of oncology granting promising results in radiology-based clinical applications. In detail, we explored the emerging applications of AI in oncological risk assessment, lesion detection, characterization, staging, and therapy response. Critical issues such as the lack of reproducibility and generalizability need to be addressed to fully implement AI systems in clinical practice. Nevertheless, AI impact on cancer imaging has been driving the shift of oncology towards a precision diagnostics and personalized cancer treatment.
Core Tip: Advanced computational systems and availability of multi-dimensional data have led the possibility of artificial intelligence (AI) consisting of machine learning (ML) and deep learning (DL) algorithms to be implemented in healthcare data analysis, with reliable results in the oncology field and particularly in diagnostic imaging tasks. Supervised algorithms are the most common ML models used in medical image analysis, while convolutional neural networks are the main DL approach. AI-based models have demonstrated outperforming results in oncological risk assessment, lesion detection, segmentation, characterization, staging, and therapy response. Growing emerging evidence supports the leading role of AI in all cancer imaging pathways from screening programs to diagnostic and prognostic tasks, boosting the paradigm of precision medicine.
- Citation: Verde F, Romeo V, Stanzione A, Maurea S. Current trends of artificial intelligence in cancer imaging. Artif Intell Med Imaging 2020; 1(3): 87-93
- URL: https://www.wjgnet.com/2644-3260/full/v1/i3/87.htm
- DOI: https://dx.doi.org/10.35711/aimi.v1.i3.87
In this new era of health-related technology and medical advances, artificial intelligence (AI) has put down roots making it possible to teach computers to do an intelligence human task, thus emerging as a problem-solving tool in data analysis and improving many aspects of clinical care[1,2].
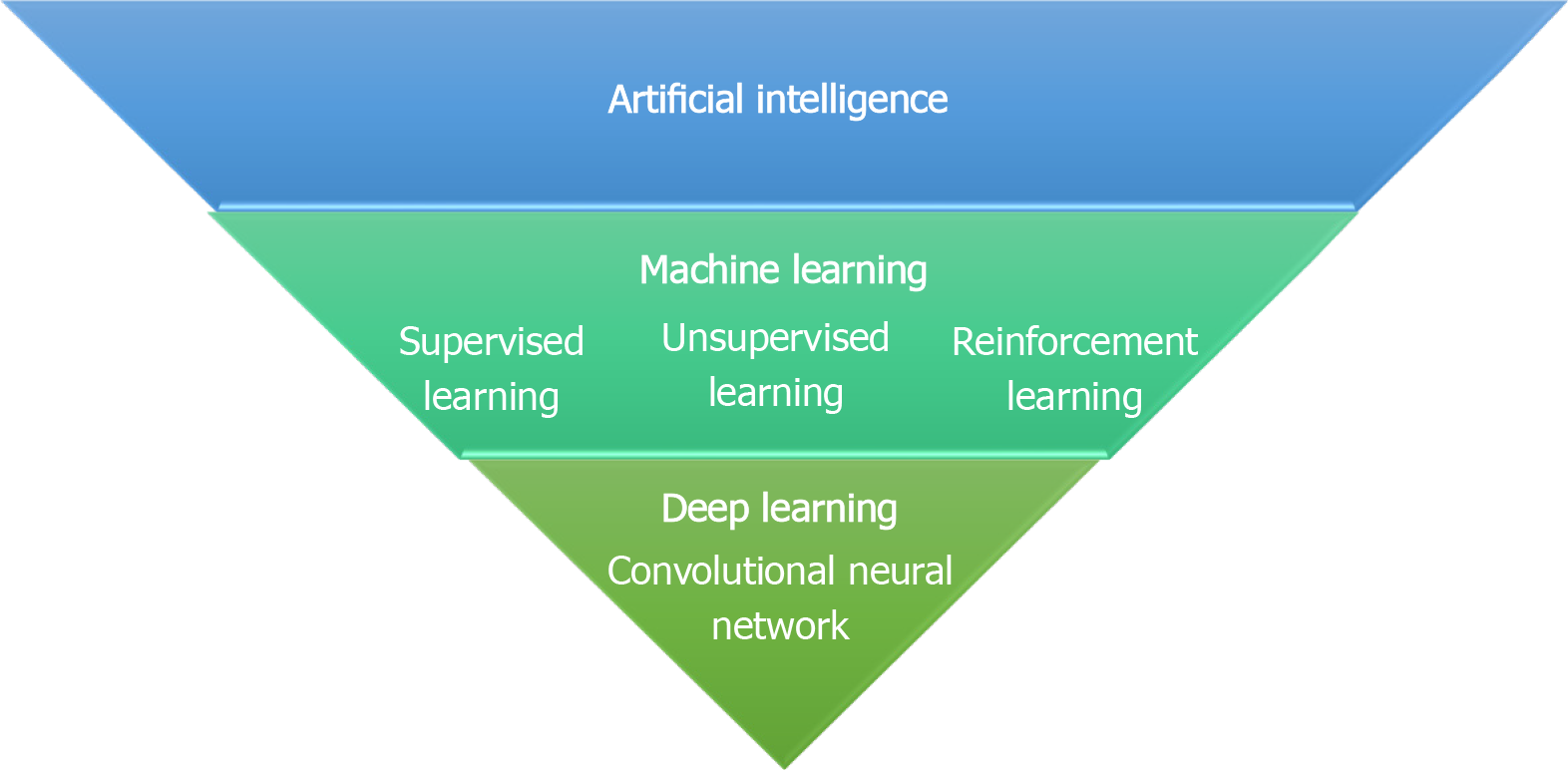
Machine learning (ML) is a subset of AI that develops computer algorithms to make predictions or decision tasks without prior explicit programmed rules. ML algorithms use iterative static methods learning from “training” data to progressively improve the model performance over time. Based on the type of learning, ML is generally divided in (1) supervised learning, which uses labelled training data to map the expected outputs; (2) unsupervised learning, which deploys unlabelled data to learn new patterns; and (3) reinforcement learning, considered as a subfield of ML using reinforcement tools in a dynamic setting[3] (Figure 1). The supervised method is the most used ML technique in medical imaging applications and, relying on the relationship between input features and expected outcomes, the ML algorithms are grouped into three broad categories: Linear, Nonlinear and Ensemble, as described in Table 1. Furthermore, based on the data features exploited by the algorithms, ML can be applied to handcrafted features as predefined features in the data set, or to non-handcrafted features, involving raw data as part of the learning process[4]. Deep learning (DL), is a subgroup of ML techniques using non-handcrafted features and it is composed of artificial neural networks (ANN) modelled as neuron multi-layered networks allowing to automatically extract features without prior labelling and perform high-level tasks[5]. The most common ANN used in medical image analysis is based on convolutional architecture [convolutional neural networks (CNN)], consisting of hidden multi-layers that compute and filter high dimensional data to obtain the correct outputs, such as detection and characterization of tumoral lesions on imaging examinations[6].
| ML technique | ML alghoritms | Description |
| Linear | (1) Linear regression; and (2) Logistic regression | Linear methods are used to modelling the relationship between the dependent variable and one or more independent variables |
| Nonlinear | (1) Naive Bayes; (2) Decision tree; (3) k-Nearest Neighbors; (4) Support vector machines; and (5) Neural network | Nonlinear approaches are used to produce predictive insights depending on nonlinear relationships in experimental data |
| Ensemble | (1) Random forest; (2) Bootstrap aggregation; and (3) Stacked generalization | Ensemble techniques stack multiple models in order to improve prediction robustness and provide more accurate predictions than any individual model |
AI-based approaches have been investigated in many fields of oncology, from imaging to histopathological and molecular diagnosis. Indeed, encouraging results have been obtained in cancer imaging, especially in screening environments. Among the different available imaging modalities, computed tomography (CT) and magnetic resonance imaging (MRI) are the most widely employed due to their prominent role in oncologic patients for staging, treatment monitoring and follow-up. Moreover, the introduction of advanced imaging techniques such as perfusion CT, MRI and MRI-diffusion-weighted imaging could provide the addition of functional over morphological data to further characterize tumor phenotype and behavior. Of note, radiology and oncology share the need for precision diagnosis and prediction models, by using cross-valuable multiple parameters from medical images and clinical data. The current applications of AI in cancer imaging include the optimization of the clinical-radiological workflow (patient screening, image acquisition) and also more specific image-based tasks (cancer detection, characterization, and treatment monitoring).
In the next sections we introduce the possible applications of AI in oncology imaging (Table 2).
| Clinical application | Oncologic field | Imaging modality | AI technique |
| Clinical-radiological workflow | Breast cancer[9] | Mammography | ML |
| Image acquisition[10,11] | CT, MRI | DL | |
| Cancer detection | Breast cancer[12,13] | Mammography | DL |
| Lung cancer[14] | X-Ray, CT | DL | |
| Tumor segmentation | Breast Cancer[17,18] | MRI | DL |
| Tumor characterization | Adrenal cancer[20] | MRI | ML |
| Renal cancer[21] | MRI | ML | |
| Lung cancer[22] | CT | ML | |
| Tumor staging | Head and neck cancer[23] | CT | ML |
| Endometrial cancer[24] | MRI | ML | |
| Treatment monitoring | Breast cancer [26] | MRI | ML |
AI techniques can enable the aggregation of clinical and imaging data to improve screening programs’ efficiency, due to the possibility to analyse a large volume of different types of data including clinical risk factors, genetic data, and imaging examinations. Breast cancer surely represents a leading area of AI development, in particular in screening practices as demonstrated by recent studies that explored the impact on clinical practice of ML model in identifying individuals at increased risk of breast cancer[7,8]. Indeed, a recent study of Ming et al[9] investigated the performance of different ML-based techniques in predicting breast cancer risk using clinical and genetic risk factors in comparison to the Breast and Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm (BOADICEA) risk prediction model; decision ML-based models yielded better results in classifying cancer from non-cancer cases and increased the predictive accuracy by 20%-25% including equal risk factors used in the BOADICEA model. Moreover, considerable differences between the BOADICEA and risk-based ML models were observed in terms of classification for mammography surveillance according to the Swiss Surveillance Protocol, confirming the feasibility of ML prediction models in the clinical-imaging decision workup[9].
Regarding imaging acquisition and pre-processing, DL methods have shown an important impact on the reduction of radiation dose in CT examinations[10] and have been used for improving magnetic resonance imaging quality with the potential to decrease acquisition time[11].
Recent evidences of AI applications include breast cancer detection in mammography, tomosynthesis, and MRI as well as identification of CT lung nodes, brain tumors, and prostate cancer on MRI.
Among these, mammographic detection of breast cancer represents a challenging image analysis task because breast cancer could be masked by healthy breast tissue. In a recent study[12], a DL AI system provided by Google Health company outperformed the radiologists involved in the mammographic screening from multiple centres in the United Kingdom (UK) and United States (US). The AI system yielded absolute reductions of 1.2% and 2.7% in false-positive and false-negative rates, respectively, in the UK test set and 5.7% and 9.4% in the US dataset. Moreover, the AI system exceeded the average performance of six expert radiologists who interpreted a sample of 500 randomly selected cases in a controlled study[12]. Similarly, Rodríguez-Ruiz et al[13] demonstrated that radiologists improved their diagnostic performance in detecting breast cancer on screening mammography examinations with the use of a DL-based AI system. In detail, the authors observed that radiologists improved their average area under the receiver operating characteristic curve (AUC) from 0.87 to 0.89 (P = 0.002).
In the field of lung cancer, recent research showed that a DL automatic detection algorithm achieved higher performance than the radiologist group in the detection of malignant pulmonary nodules on chest radiographs; moreover, radiologists' performance improved when DL algorithm was used as a second reader[14].
Segmentation represents one of the most challenging tasks of oncological image analysis and AI algorithms have allowed the development of systems that can enable automatic tumor segmentation. Recently, DL networks, such as CNN, have been applied in segmentation tasks gaining accurate results regarding radiotherapy treatment planning, volume measurements and, monitoring disease progression[15,16]. Indeed, increasing evidence in recent literature has highlighted the high performance of DL models in performing fully automated whole-breast segmentation to obtain reliable and robust methods for quantitative imaging analysis[17,18].
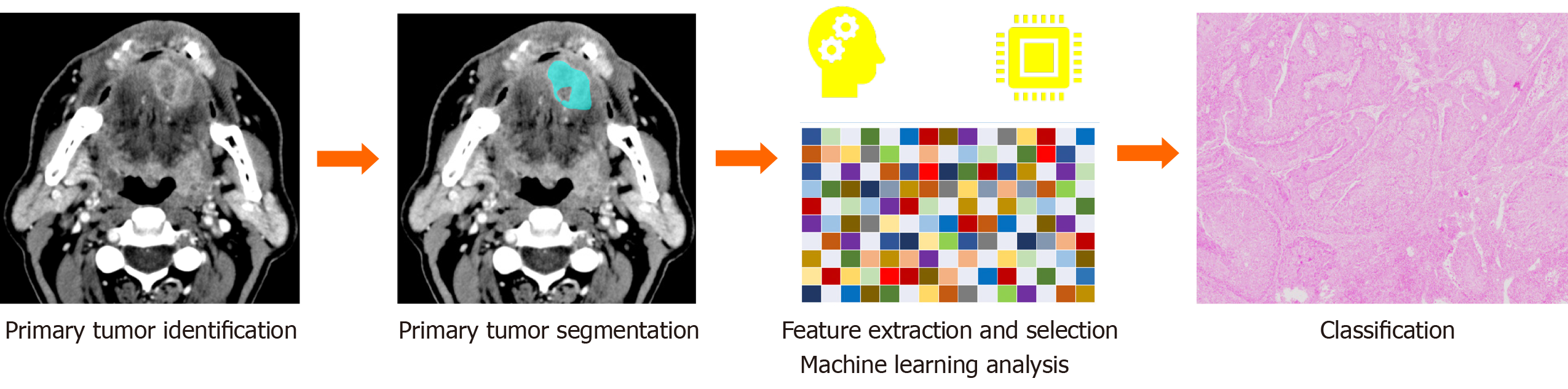
High-performance levels of AI algorithms in handling multi-dimensional data have allowed extracting and analyzing radiomic biomarkers reflecting image tumor heterogeneity thus empowering precision diagnosis and staging in cancer imaging[19] (Figure 2). For instance, with our research group, we used a combined model of MRI radiomic features and ML analysis to differentiate typical and atypical adenomas from non-adenoma adrenal lesions, which showed a better performance than the radiologist assessment[20]. Further, our group assessed the usefulness of an ML-based radiomic approach applied to MR imaging to differentiate high- from low-grade clear cell renal cell carcinoma achieving accuracy greater than 90%[21]. Reliable results and robust evidence have been providing in lung cancer diagnosis as showed in a recent work of Beig et al[22], which proposed a radiomic-based ML algorithm using non-contrast lung CT to distinguish non-small cell lung cancer adenocarcinomas from benign granuloma, resulting with outperforming results of AI system in comparison to the radiologists’ evaluation (accuracy = 75% vs 61%).
Staging represents a crucial point of the oncological workflow to delineate the most appropriate treatment in a personalized and precision way. In this view, recent pilot studies have been carried out on the staging of primary tumor size, lymph nodes involvement, and distant metastasis[23]. For example, our group investigated the clinical feasibility of a combined approach of radiomics and ML-based on MR images for the identification of deep myometrial invasion in endometrial cancer in a clinical context; indeed, the integration of the developed ML algorithm improved radiologist accuracy from 82% to 100%[24].
Temporal follow-up of tumors as wells as the treatment response are active fields of research of AI technology to find accurate models for evaluation of efficient anticancer therapies that increase the progression-free survival of patients. In this regard, excellent results have been obtained using AI radiomics MRI-based models in predicting survival and recurrence-free survival in breast cancer[25]. Moreover, the development of AI models has been explored in breast cancer imaging to assess predictive image-based phenotypes for precision medicine, in particular to predict the response to neoadjuvant chemotherapy (NAC). In a recent study, Sutton et al[26] explored the usefulness of a combined radiomics MRI-based and molecular subtype-based ML model in assessing the complete pathological response (pCR) to NAC; their AI model accurately predicted pCR on MRI with an AUC of 0.88 and showed that the performance in predicting pCR increased when radiomics features were combined with molecular subtype in comparison of the solely molecular subtype results[26].
AI techniques still have to face some issues to be incorporated in clinical practice. Of note, large datasets containing annotated images are needed for training of DL algorithms, but standardized imaging workflow lacks[15]. Complex AI functions are not easily interpreted by healthcare providers, and this “black box” nature could affect the acceptance of AI programs, also from the ethical and legal points of view[27]. Moreover, variability across multi-center and multi-vendor should be addressed with future studies sharing more reliable and robust validation[28].
Despite these drawbacks, AI incorporation into cancer imaging has been boosting the shift of oncology towards a precision diagnostics and personalized cancer treatment. Indeed, as previously discussed, recent literature evidence pointed out the emerging role of AI in supporting all cancer imaging pathways from screening programs to diagnostic and prognostic tasks, offering new methods to increase radiologists’ performance in order to improve oncological care environment. Furthermore, the integration of AI into molecular pathological epidemiology can aid in developing pathologic signatures to stratify patients’ risk and predict biological tumor behavior by using clinical, radiological and pathological data[29]. In the future, collaborations between different expertise figures, such as oncologists, epidemiologists, radiologists, pathologists and data scientists, should be encouraged to achieve harmonized and integrated development of AI systems in cancer research.
We have provided an overview of AI integration in cancer imaging, focusing on the basics of this disruptive technology and on the significant results obtained in improving clinical and radiological workup for oncological patients. Although, more evidence is still demanding the outlook of AI in cancer imaging remains bright.
Manuscript source: Invited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Abdel Razek AAK, Ogino S, Ren J, Wang RF S-Editor: Wang JL L-Editor: A P-Editor: Li JH
| 1. | Lakhani P, Prater AB, Hutson RK, Andriole KP, Dreyer KJ, Morey J, Prevedello LM, Clark TJ, Geis JR, Itri JN, Hawkins CM. Machine Learning in Radiology: Applications Beyond Image Interpretation. J Am Coll Radiol. 2018;15:350-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 144] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 2. | Verde F, Stanzione A, Romeo V, Cuocolo R, Maurea S, Brunetti A. Could Blockchain Technology Empower Patients, Improve Education, and Boost Research in Radiology Departments? J Digit Imaging. 2019;32:1112-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Choy G, Khalilzadeh O, Michalski M, Do S, Samir AE, Pianykh OS, Geis JR, Pandharipande PV, Brink JA, Dreyer KJ. Current Applications and Future Impact of Machine Learning in Radiology. Radiology. 2018;288:318-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 488] [Article Influence: 69.7] [Reference Citation Analysis (0)] |
| 4. | Goldenberg SL, Nir G, Salcudean SE. A new era: artificial intelligence and machine learning in prostate cancer. Nat Rev Urol. 2019;16:391-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 248] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 5. | Yang YJ, Bang CS. Application of artificial intelligence in gastroenterology. World J Gastroenterol. 2019;25:1666-1683. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 211] [Cited by in RCA: 160] [Article Influence: 26.7] [Reference Citation Analysis (5)] |
| 6. | Chartrand G, Cheng PM, Vorontsov E, Drozdzal M, Turcotte S, Pal CJ, Kadoury S, Tang A. Deep Learning: A Primer for Radiologists. Radiographics. 2017;37:2113-2131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 689] [Article Influence: 98.4] [Reference Citation Analysis (0)] |
| 7. | Sheth D, Giger ML. Artificial intelligence in the interpretation of breast cancer on MRI. J Magn Reson Imaging. 2020;51:1310-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 106] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 8. | Ming C, Viassolo V, Probst-Hensch N, Chappuis PO, Dinov ID, Katapodi MC. Machine learning techniques for personalized breast cancer risk prediction: comparison with the BCRAT and BOADICEA models. Breast Cancer Res. 2019;21:75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 9. | Ming C, Viassolo V, Probst-Hensch N, Dinov ID, Chappuis PO, Katapodi MC. Machine learning-based lifetime breast cancer risk reclassification compared with the BOADICEA model: impact on screening recommendations. Br J Cancer. 2020;123:860-867. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 10. | Greffier J, Hamard A, Pereira F, Barrau C, Pasquier H, Beregi JP, Frandon J. Image quality and dose reduction opportunity of deep learning image reconstruction algorithm for CT: a phantom study. Eur Radiol. 2020;30:3951-3959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 217] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 11. | Montagnon E, Cerny M, Cadrin-Chênevert A, Hamilton V, Derennes T, Ilinca A, Vandenbroucke-Menu F, Turcotte S, Kadoury S, Tang A. Deep learning workflow in radiology: a primer. Insights Imaging. 2020;11:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 92] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 12. | McKinney SM, Sieniek M, Godbole V, Godwin J, Antropova N, Ashrafian H, Back T, Chesus M, Corrado GC, Darzi A, Etemadi M, Garcia-Vicente F, Gilbert FJ, Halling-Brown M, Hassabis D, Jansen S, Karthikesalingam A, Kelly CJ, King D, Ledsam JR, Melnick D, Mostofi H, Peng L, Reicher JJ, Romera-Paredes B, Sidebottom R, Suleyman M, Tse D, Young KC, De Fauw J, Shetty S. International evaluation of an AI system for breast cancer screening. Nature. 2020;577:89-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1364] [Cited by in RCA: 1188] [Article Influence: 237.6] [Reference Citation Analysis (0)] |
| 13. | RodrÃguez-Ruiz A, Krupinski E, Mordang JJ, Schilling K, Heywang-Köbrunner SH, Sechopoulos I, Mann RM. Detection of Breast Cancer with Mammography: Effect of an Artificial Intelligence Support System. Radiology. 2019;290:305-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 295] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 14. | Nam JG, Park S, Hwang EJ, Lee JH, Jin KN, Lim KY, Vu TH, Sohn JH, Hwang S, Goo JM, Park CM. Development and Validation of Deep Learning-based Automatic Detection Algorithm for Malignant Pulmonary Nodules on Chest Radiographs. Radiology. 2019;290:218-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 328] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 15. | Cuocolo R, Caruso M, Perillo T, Ugga L, Petretta M. Machine Learning in oncology: A clinical appraisal. Cancer Lett. 2020;481:55-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 112] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 16. | Deig CR, Kanwar A, Thompson RF. Artificial Intelligence in Radiation Oncology. Hematol Oncol Clin North Am. 2019;33:1095-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Zhang L, Mohamed AA, Chai R, Guo Y, Zheng B, Wu S. Automated deep learning method for whole-breast segmentation in diffusion-weighted breast MRI. J Magn Reson Imaging. 2020;51:635-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Zhang Y, Chen JH, Chang KT, Park VY, Kim MJ, Chan S, Chang P, Chow D, Luk A, Kwong T, Su MY. Automatic Breast and Fibroglandular Tissue Segmentation in Breast MRI Using Deep Learning by a Fully-Convolutional Residual Neural Network U-Net. Acad Radiol. 2019;26:1526-1535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 19. | Gitto S, Cuocolo R, Albano D, Chianca V, Messina C, Gambino A, Ugga L, Cortese MC, Lazzara A, Ricci D, Spairani R, Zanchetta E, Luzzati A, Brunetti A, Parafioriti A, Sconfienza LM. MRI radiomics-based machine-learning classification of bone chondrosarcoma. Eur J Radiol. 2020;128:109043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 20. | Romeo V, Maurea S, Cuocolo R, Petretta M, Mainenti PP, Verde F, Coppola M, Dell'Aversana S, Brunetti A. Characterization of Adrenal Lesions on Unenhanced MRI Using Texture Analysis: A Machine-Learning Approach. J Magn Reson Imaging. 2018;48:198-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 21. | Stanzione A, Ricciardi C, Cuocolo R, Romeo V, Petrone J, Sarnataro M, Mainenti PP, Improta G, De Rosa F, Insabato L, Brunetti A, Maurea S. MRI Radiomics for the Prediction of Fuhrman Grade in Clear Cell Renal Cell Carcinoma: a Machine Learning Exploratory Study. J Digit Imaging. 2020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 22. | Beig N, Khorrami M, Alilou M, Prasanna P, Braman N, Orooji M, Rakshit S, Bera K, Rajiah P, Ginsberg J, Donatelli C, Thawani R, Yang M, Jacono F, Tiwari P, Velcheti V, Gilkeson R, Linden P, Madabhushi A. Perinodular and Intranodular Radiomic Features on Lung CT Images Distinguish Adenocarcinomas from Granulomas. Radiology. 2019;290:783-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 239] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 23. | Romeo V, Cuocolo R, Ricciardi C, Ugga L, Cocozza S, Verde F, Stanzione A, Napolitano V, Russo D, Improta G, Elefante A, Staibano S, Brunetti A. Prediction of Tumor Grade and Nodal Status in Oropharyngeal and Oral Cavity Squamous-cell Carcinoma Using a Radiomic Approach. Anticancer Res. 2020;40:271-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 24. | Stanzione A, Cuocolo R, Del Grosso R, Nardiello A, Romeo V, Travaglino A, Raffone A, Bifulco G, Zullo F, Insabato L, Maurea S, Mainenti PP. Deep Myometrial Infiltration of Endometrial Cancer on MRI: A Radiomics-Powered Machine Learning Pilot Study. Acad Radiol. 2020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 61] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 25. | Reig B, Heacock L, Geras KJ, Moy L. Machine learning in breast MRI. J Magn Reson Imaging. 2020;52:998-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 96] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 26. | Sutton EJ, Onishi N, Fehr DA, Dashevsky BZ, Sadinski M, Pinker K, Martinez DF, Brogi E, Braunstein L, Razavi P, El-Tamer M, Sacchini V, Deasy JO, Morris EA, Veeraraghavan H. A machine learning model that classifies breast cancer pathologic complete response on MRI post-neoadjuvant chemotherapy. Breast Cancer Res. 2020;22:57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 27. | Carter SM, Rogers W, Win KT, Frazer H, Richards B, Houssami N. The ethical, legal and social implications of using artificial intelligence systems in breast cancer care. Breast. 2020;49:25-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 122] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 28. | Thrall JH, Li X, Li Q, Cruz C, Do S, Dreyer K, Brink J. Artificial Intelligence and Machine Learning in Radiology: Opportunities, Challenges, Pitfalls, and Criteria for Success. J Am Coll Radiol. 2018;15:504-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 312] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 29. | Ogino S, Nowak JA, Hamada T, Milner DA Jr, Nishihara R. Insights into Pathogenic Interactions Among Environment, Host, and Tumor at the Crossroads of Molecular Pathology and Epidemiology. Annu Rev Pathol. 2019;14:83-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 176] [Article Influence: 29.3] [Reference Citation Analysis (0)] |