Published online Jun 28, 2020. doi: 10.35711/aimi.v1.i1.40
Peer-review started: May 28, 2020
First decision: June 5, 2020
Revised: June 8, 2020
Accepted: June 12, 2020
Article in press: June 12, 2020
Published online: June 28, 2020
Processing time: 42 Days and 14.9 Hours
Acute pancreatitis is a common acute inflammatory disease involving the pancreas and peripancreatic tissues or remote organs. The revised Atlanta classification 2012 of acute pancreatitis divides patients into mild, moderately severe and severe groups. Major changes of the classification include acute fluid collection terminology. However, some inappropriate terms of the radiological diagnosis reports in the daily clinical work or available literature may still be found. The aim of this review article is: to present an image-rich overview of different morphologic characteristics of the early-stage (within 4 wk after symptom onset) local complications associated with acute pancreatitis by computed tomography or magnetic resonance imaging; to clarify confusing imaging concepts for pancreatic fluid collections and underline standardised reporting nomenclature; to assist communication among treating physicians; and to facilitate the implications for clinical management decision-making.
Core tip: To our best of knowledge, this is the first pictorial review that determines the spectrum of magnetic resonance imaging features in patients with acute pancreatitis of distinct early acute necrotic collection compared with acute peripancreatic fluid collection.
- Citation: Xiao B. Acute pancreatitis: A pictorial review of early pancreatic fluid collections. Artif Intell Med Imaging 2020; 1(1): 40-49
- URL: https://www.wjgnet.com/2644-3260/full/v1/i1/40.htm
- DOI: https://dx.doi.org/10.35711/aimi.v1.i1.40
Acute pancreatitis is a common digestive disease, which is related to an acute onset of epigastric pain with/without nausea and vomiting. Cholelithiasis, alcoholism and hyperlipidaemia are the most widely recognised etiological factors in acute pancreatitis patients[1]. Clinically, physicians often make the accurate diagnosis based on clinical manifestations and biochemical parameters (sufficiently elevated serum lipase or amylase) for the majority of patients with acute pancreatitis[1]. Indeed, the routine medical imaging for this disease is unwarranted. However, the natural history and consequences of critically ill patients (particularly moderately severe or severe acute pancreatitis) can result in a variety of local complications[2]. These developments thus prompt imaging to detect clinical complications.
Imaging approaches, especially computed tomography (CT) and magnetic resonance imaging (MRI), are valuable in detecting local complications associated with acute pancreatitis in both the early-phase and the late-phase of disease. With the increasing application of the revised Atlanta classification criteria 2012[2], radiologists play a crucial role in relevant imaging diagnosis, scientific research and multidisci-plinary team communication. Although this classification updates the definitions of acute pancreatitis and many pancreatitis-associated complications, some inappropriate terms of the radiological diagnosis reports in the daily clinical work or available literature may still be found.
Therefore, the purpose of this pictorial article is: To serve as an image-rich overview of different morphologic characteristics of the early-stage (within 4 wk after symptom onset) local complications associated with acute pancreatitis by CT or MR; to clarify confusing imaging concepts and enable standardised reporting nomenclature; to assist communication among treating physicians; and to facilitate the implications for treatments.
In general, acute pancreatitis is classically divided into two types: Interstitial oedematous pancreatitis and necrotising pancreatitis. Clinically, the majority of patients with acute pancreatitis are present as interstitial oedematous pancreatitis. They have diffuse or localised enlargement of the pancreas owing to inflammatory oedema. On the other hand, necrotising pancreatitis accounts for 20%-30% of acute pancreatitis patients[3-5], and it is subdivided into three subtypes on the basis of contrast-enhanced CT according to the new Atlanta classification[2]: (1) Combined pancreatic necrosis and peripancreatic necrosis (most common, approximately 75% of all necrotising pancreatitis); (2) Peripancreatic tissue necrosis alone (less common, with an incidence of approximately 20%)[6]; and (3) Pancreatic parenchymal necrosis alone (rare, with an incidence of only 5%)[7-9]. For radiologists, it is crucial to make a distinction between interstitial oedematous pancreatitis and necrotising pancreatitis in diagnosing acute pancreatitis. The correct diagnosis of imaging type can assist in the recognition of subsequent pancreatitis-related complications and application of the proper terminology.
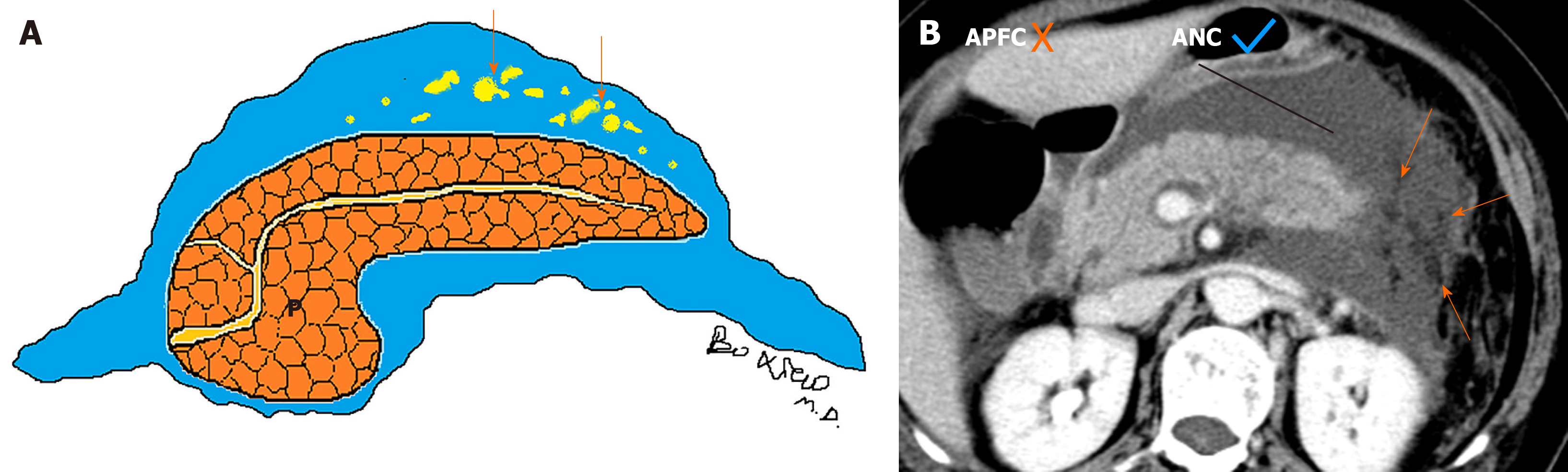
Currently, there are two diagnostic terms for local complications in the early stage of acute pancreatitis: Acute peripancreatic fluid collections (APFCs) and acute necrotic collections (ANCs)[2]. On the one hand, the characteristics of acute peripancreatic fluid collections include: (1) Arising from interstitial oedematous pancreatitis; (2) Nonencapsulated collections (lack of a well-defined capsule/definable wall); (3) Collection age within the initial 4 wk after symptom onset; (4) Peripancreatic location (surrounding or adjacent to pancreas); and (5) A homogeneous or simple fluid appearance (containing purely fluid) (Figure 1)[7-9].
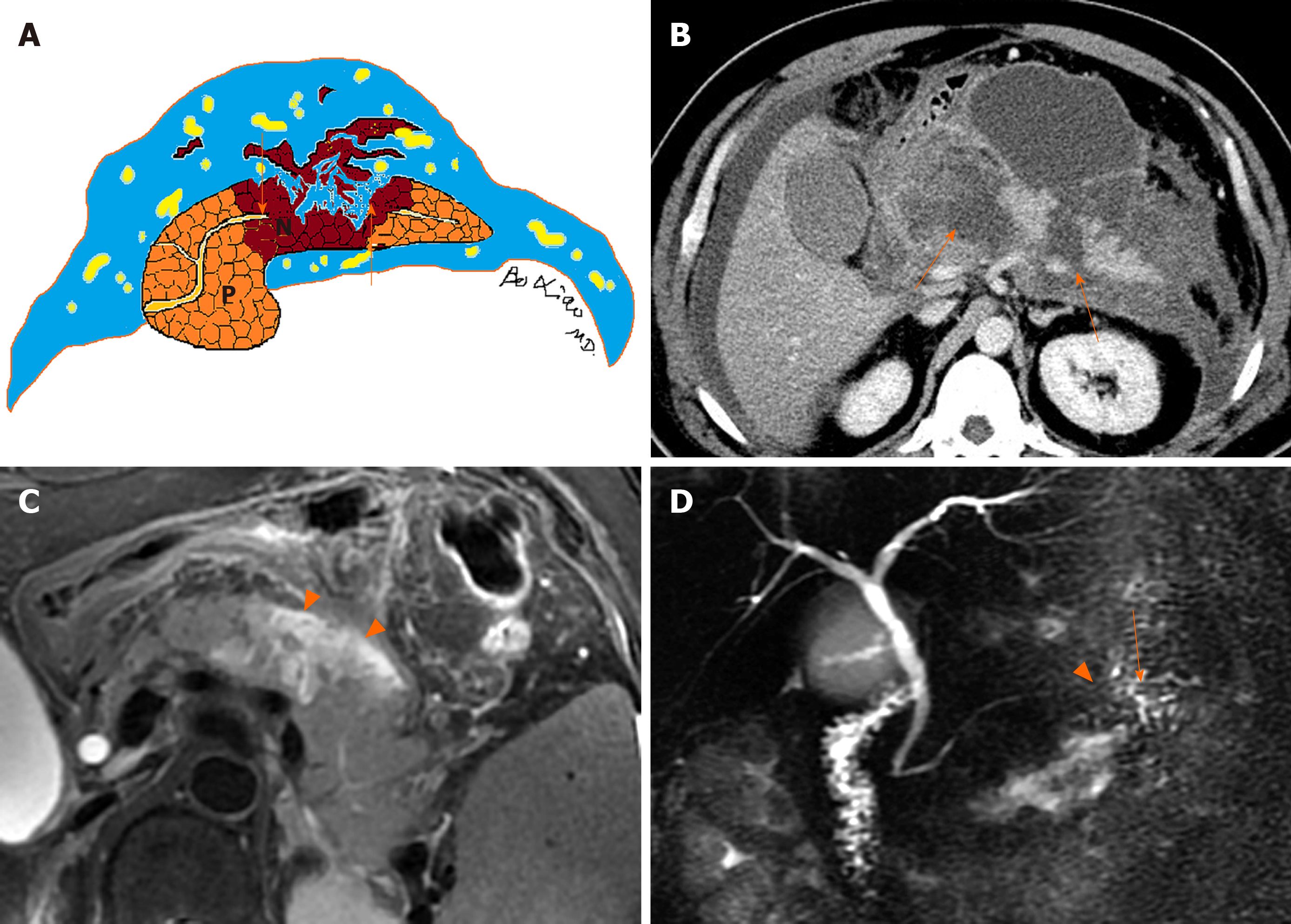
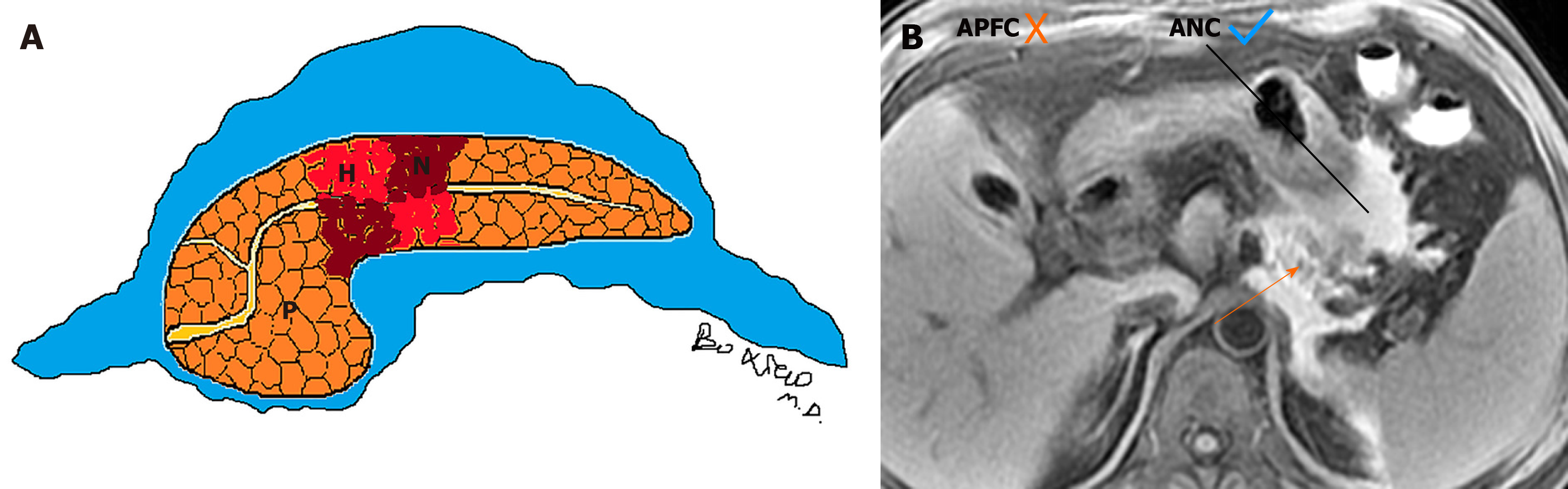
On the other hand, the characteristics of acute necrotic collections include: (1) Occurring only in the setting of necrotising pancreatitis; (2) Collections without an encapsulating capsule or over time with poorly organised wall, (3) Collection age within the first 4 wk of this disease; (4) Peripancreatic and pancreatic different locations (surrounding the pancreas or intrapancreatic extension or both); and (5) A heterogeneous appearance due to containing variable amounts of inflammatory fluid and liquefied or nonliquefied necrotic debris (Figure 2)[7-10].
On CT/MRI, acute peripancreatic fluid collections are predominantly localised in peripancreatic areas (e.g., the lesser sac) and the retroperitoneal spaces (e.g., left anterior pararenal space) or peripancreatic fascial planes. They exhibit variable shape and size but mostly present as a uniform linear or strip-shaped liquid appearance[3-6]. In addition, the volume of APFCs is relatively smaller due to fluid generally confined by a simple retroperitoneum space and/or normal peripancreatic fascial planes (Figure 3).
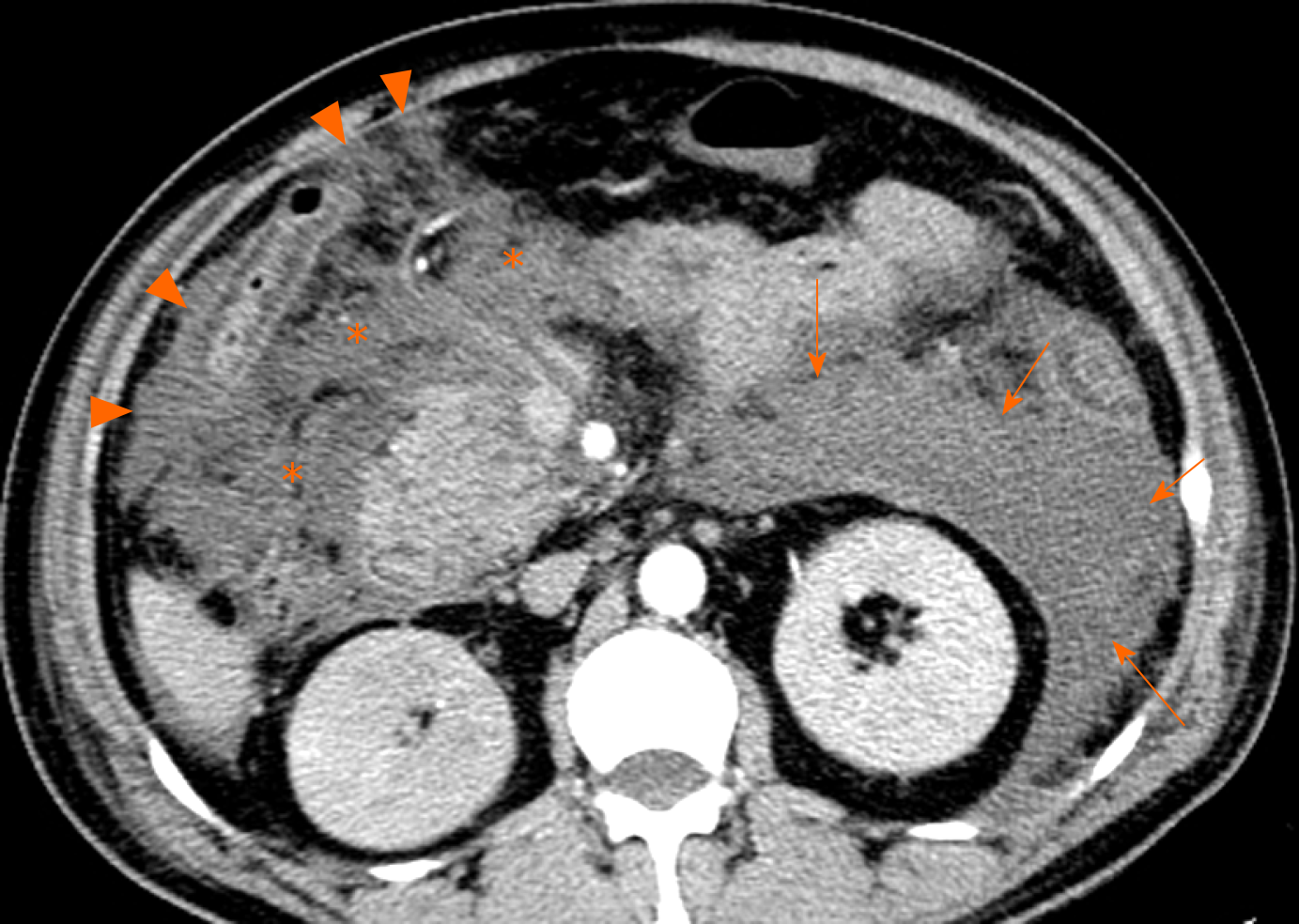
On the other side, acute necrotic collections often break through the limitation of the interfascial planes and can affect multiple retroperitoneal spaces, interfascial planes, subperitoneal spaces and other abdominal spaces. In fact, they are most frequently situated in the lesser sac and the anterior pararenal spaces, followed by transverse mesocolon, mesenteric root, and thereafter, gastrohepatic, gastrosplenic and gastrocolic ligaments[11]. Furthermore, these collections may additionally involve the remote regions, such as the pelvic sidewalls and mediastinum. Thus, acute necrotic collections are generally numerous (multiple), irregular and loculated, and the volume of effusion often appears larger than that of acute peripancreatic fluid collections (Figure 4)[9-11].
Acute peripancreatic fluid collections have homogeneous fluid appearances. They are uniformly hypoattenuating on CT and T1 hypointense and T2 hyperintense on MRI. After intravenous contrast-material administration, acute peripancreatic fluid collections are not enhancing owing to the pure fluid nature[8-10].
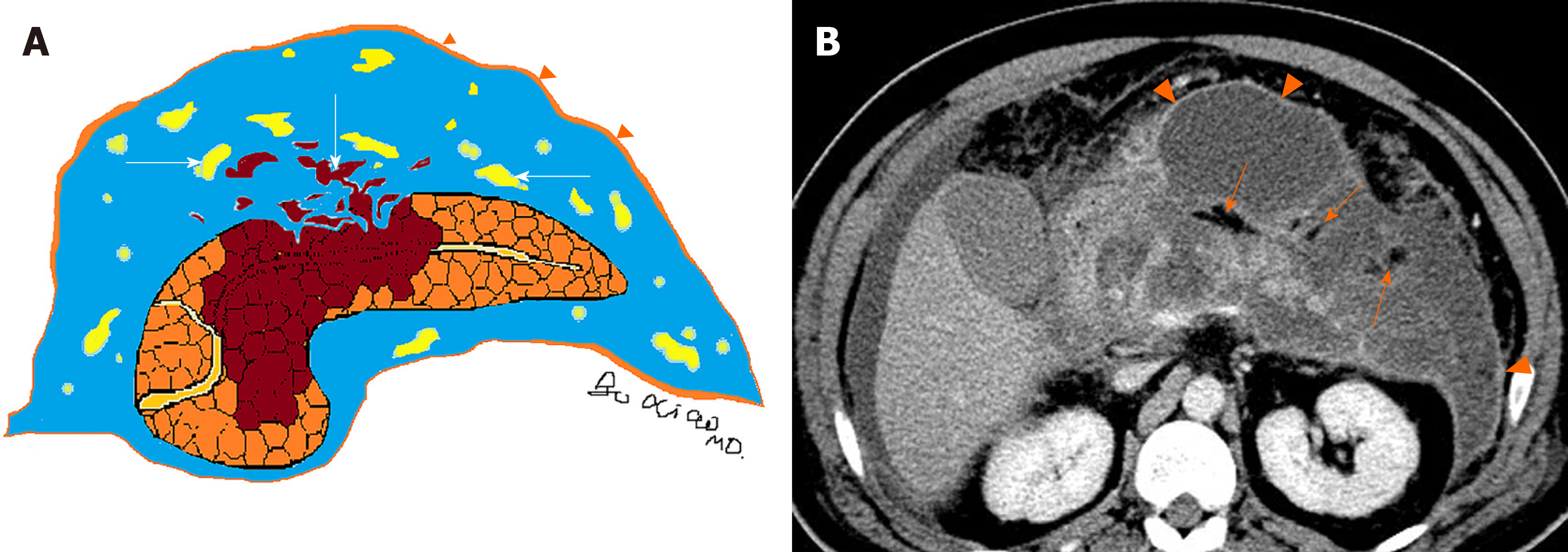
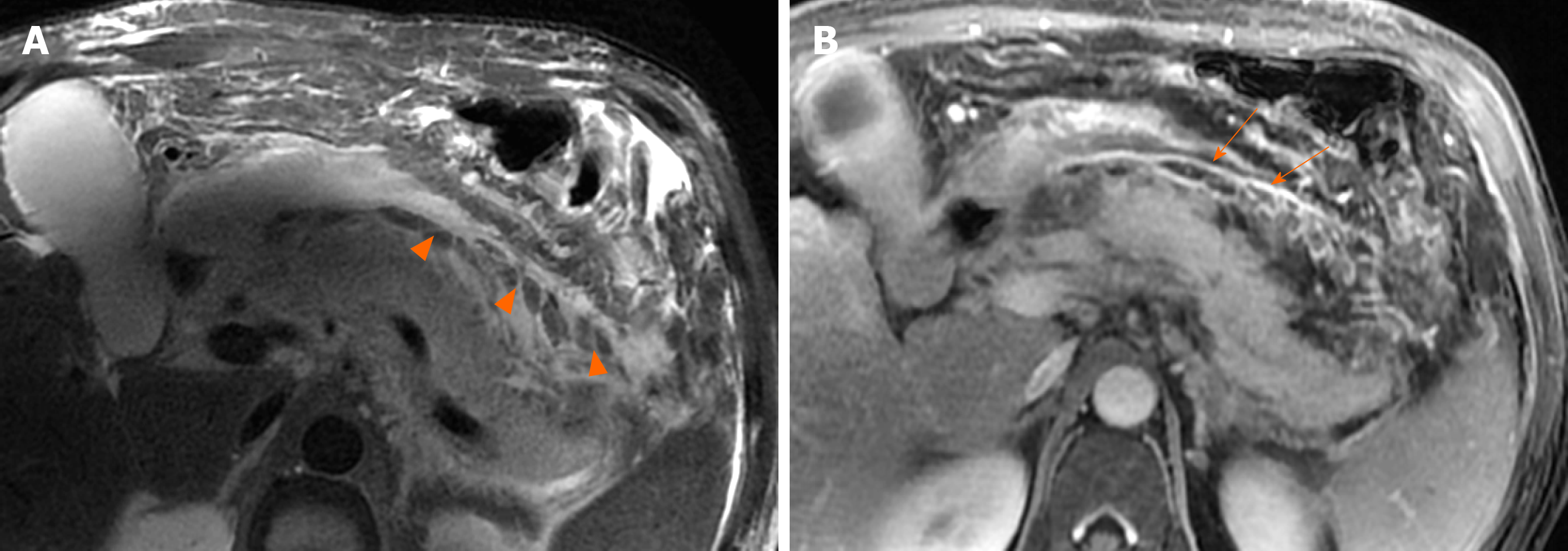
In contrast, acute necrotic collections are heterogeneous (Figure 5). There are relatively hyperdense materials (necrotic fragments of pancreas) and/or markedly hypodense fat globules (peripancreatic fat) among hypodense fluid on CT[6-8]. Similarly, there are varying degrees of round, patchy, strip-shaped T2-hypointense components (adipose fragments or necrotic pancreatic tissue) among T2-hyperintense fluid on MR images[3-6]. During contrast-enhanced CT/MRI, the internal necrotic debris or trapped fat within acute necrotic collections often does not show enhancement (Figure 5), while the immature, fibrous granulation tissue wall of these collections may be detectable as slight to confluent enhancement (Figure 6).
In general, (peri)pancreatic fluid collections may also be associated with a variety of complications, which can make the clinical condition more complex. After conservative treatments, acute peripancreatic fluid collections are often absorbed quickly with rare follow-up complications. Most of these patients are discharged within 1-2 wk after admission[7-9].
Clinically, necrotising pancreatitis with acute necrotic collection is mainly seen in patients with moderate severe acute pancreatitis and severe acute pancreatitis, with a longer disease course (lasting several weeks or months). Consequently, secondary infectious complications are more likely to occur in acute necrotic collections, compared with acute peripancreatic fluid collections[9-11]. Infection should be suspected when there are secondary clinical signs of sepsis, such as a new occurrence of fever and leucocytosis[8-10]. On CT images, the sign of multiple extraluminal gas or a gas-fluid level in the peripancreatic zones and retroperitoneal spaces is highly suggestive of acute necrotic collections complicated by infection (Figure 7). If clinical manifestations are concordant or needle-guided aspiration confirms the development of infection, then these collections should be classified as infected acute necrotic collections[9-11]. In this setting, percutaneous aspiration or drain insertion can be performed for the treatment of an infected collection[11-13].
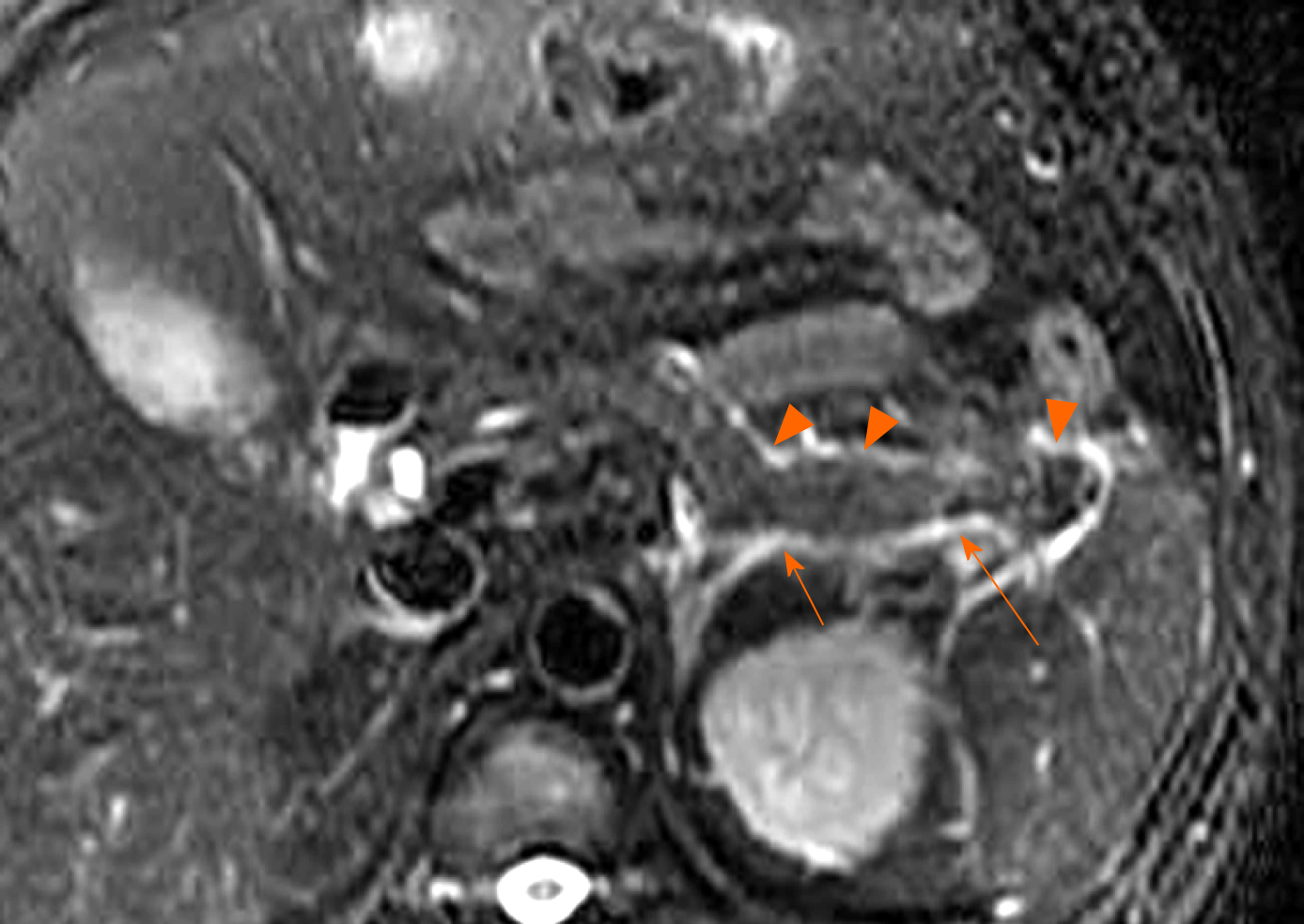
Moreover, when necrotising pancreatitis affects a large area of intraparenchymal pancreas, it involves the main pancreatic duct (necrosis of the pancreatic duct). Over time, the pancreatic duct rupture or disrupted integrity of the pancreatic duct accompanied with the intrapancreatic acute necrotic collections (liquefied pancreatic tissue) can result in the formation of “disconnected duct syndrome” (Figure 8)[11,14]. As for this condition, a collection communication with main pancreatic duct is usually evident on MRI and MR cholangiopancreatography (Figure 8). This syndrome may alter treatment but does not affect acute necrotic collection classification, and these patients often require surgical management for a complete recovery[12-15].
Depending on the revised Atlanta classification and our clinical practice, common terminology misuse conditions in the daily imaging reports are summarised as follows: (1) On CT/MRI, no necrosis finding was observed in the pancreatic parenchyma, which was assumed to be “interstitial oedematous pancreatitis.” Then the accumulation of fluid in the peripancreatic regions may be misinterpreted as “acute peripancreatic fluid collection.” However, a pitfall is probably present because we may ignore the presence of small “adipose tissue debris” in peripancreatic collections (Figure 9). At this point, it should be diagnosed as “necrotising pancreatitis (peripancreatic necrotic type),” due to necrotic adipose fragments around the pancreas. Therefore, the nomenclature of the collection is referred to as an “acute necrotic collection” (Figure 9)[8-11]; (2) On CT/MRI images, when a peripancreatic or retroperitoneal homogeneous collection with uniform fluid density or signal intensity was seen, it may be misinterpreted as an “acute peripancreatic fluid collection.” Instead, if a peripancreatic collection was secondary to known pancreatic parenchymal necrosis and/or haemorrhage, the correct diagnosis term for the collection should be “acute necrotic collection,” even if it is radiologically homogeneous and contains no nonliquefied component (Figure 10)[4-8]; and (3) As aforementioned content, it may be easier to diagnose a peripancreatic homogeneous collection as “acute peripancreatic fluid collection.” However, if a collection is involving the pancreas parenchyma, a correct term for the surrounding fluid should be diagnosed as “acute necrotic collection” (Figure 11), regardless of the nature of fluid density or signal intensity. In another word, any collection involving the pancreas parenchyma should be determined as necrotising pancreatitis[8-12].
Although there are many differences between acute peripancreatic fluid collections and acute necrotic collections, in the clinical practice it is sometimes difficult to accurately distinguish acute peripancreatic fluid collections from acute necrotic collections on CT at the early stage of acute pancreatitis (especially within 2 d of symptom onset). The reasons may be related to the following factors: (1) It may allow sufficient time for completed necrosis of the pancreas and/or peripancreatic fat (findings of solid necrotic materials to liquefy over several days). Thus, heterogeneous contents may not be found within the early-phase fluid. This also explains why an early contrast-enhanced CT may underestimate the eventual degree of (peri)pancreatic necrosis. In this setting, further CT studies after an interval of between 5 d and 7 d should be performed[2-5]; and (2) Due to the low contrast resolution of CT, it is difficult to detect a small amount of heterogeneous contents. For this purpose, MRI may be required for this distinction because it is very sensitive to the detection of internal architecture of collections (even a small area of heterogeneous debris)[6-9]. Moreover, MRI diffusion weighted imaging combined with ADC value measurement is helpful for the differential diagnosis of interstitial oedematous pancreatitis and necrotising pancreatitis[4-6]. Whether diffusion weighted imaging is also valuable for the early differential diagnosis of acute peripancreatic fluid collection and acute necrotic collection may become a direction of future research.
To sum up, the natural history and consequences of different pancreatic and peripancreatic collections are now better described and understood. The differential diagnosis of these collections within 4 wk of symptom onset is succinctly summarised in Table 1. The accurate description of pancreatitis-associated collections, including location (pancreatic, peripancreatic, others), the presence of contents (liquid, solid, gas), the thickness of collection wall (thin, thick) and the presence or absence of infectious findings will facilitate the radiologic reports in daily practice. Finally, radiologists should be fully aware of the standardised imaging nomenclature on the basis of associated morphologic descriptions. It is necessary for accurate documen-tation and reporting of academic research, and it is also important to direct implications of care plans for patients with acute pancreatitis.
| Key points | Acute peripancreatic fluid collection | Acute necrotic collection |
| Clinical severity | Mostly mild acute pancreatitis | Moderately severe acute pancreatitis or severe acute pancreatitis |
| Management algorithm | Conservative treatment (usually resolves spontaneously without intervention) | Likely increased morbidity and intervention rates (drainage or surgical treatment) |
| Course and prognosis | The hospital stay is usually about one week after onset; a good prognosis | Hospitalization often lasts from weeks to months; increased infection and mortality rates |
| CT/MRI imaging pattern | Occurs only in the setting of interstitial oedematous pancreatitis | Occurs in the setting of acute necrotising pancreatitis (including peripancreatic necrosis only) |
| Location and number of collections on CT/MRI | Mostly confined to simple retroperitoneal space or interfascial plane | Mostly in transabdominal-pelvic cavities and multiple spaces or interfascial planes |
| Shape, size, edge | Linear/strip-shaped, a small amount of collections, clear edge | Large patchy-shaped, a large amount of collections, unclear or irregular edge |
| Density/intense, enhancement characteristics | Homogeneous low density/hypointense T1 hyperintense T2 signal; no enhancement | Mixed features, mainly low density/hypointense T1 /hyperintense T2 signal, containing low density fat/fat signal intensity and low density or hypointense pancreas fragments; fragments are not enhancing |
| Secondary or concomitant signs | Rare | Frequent secondary infection with “bubble sign” (caused by infection itself or intestinal fistula with adjacent intestine); when a large area of intrapancreatic collections is present, “pancreatic duct disruption syndrome” may occur (further invasive operation is often required) |
Manuscript source: Invited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gokce E S-Editor: Wang JL L-Editor: Filipodia E-Editor: Xing YX
| 1. | Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144:1252-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1151] [Cited by in RCA: 1343] [Article Influence: 111.9] [Reference Citation Analysis (0)] |
| 2. | Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4932] [Cited by in RCA: 4315] [Article Influence: 359.6] [Reference Citation Analysis (45)] |
| 3. | Thoeni RF. The revised Atlanta classification of acute pancreatitis: its importance for the radiologist and its effect on treatment. Radiology. 2012;262:751-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 265] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 4. | Xiao B, Xu HB, Jiang ZQ, Zhang J, Zhang XM. Current concepts for the diagnosis of acute pancreatitis by multiparametric magnetic resonance imaging. Quant Imaging Med Surg. 2019;9:1973-1985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Sun H, Zuo HD, Lin Q, Yang DD, Zhou T, Tang MY, Wáng YXJ, Zhang XM. MR imaging for acute pancreatitis: the current status of clinical applications. Ann Transl Med. 2019;7:269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 6. | Meyrignac O, Lagarde S, Bournet B, Mokrane FZ, Buscail L, Rousseau H, Otal P. Acute Pancreatitis: Extrapancreatic Necrosis Volume as Early Predictor of Severity. Radiology. 2015;276:119-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 7. | Zhao K, Adam SZ, Keswani RN, Horowitz JM, Miller FH. Acute Pancreatitis: Revised Atlanta Classification and the Role of Cross-Sectional Imaging. AJR Am J Roentgenol. 2015;205:W32-W41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 8. | Murphy KP, O'Connor OJ, Maher MM. Updated imaging nomenclature for acute pancreatitis. AJR Am J Roentgenol. 2014;203:W464-W469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Türkvatan A, Erden A, Türkoğlu MA, Seçil M, Yüce G. Imaging of acute pancreatitis and its complications. Part 2: complications of acute pancreatitis. Diagn Interv Imaging. 2015;96:161-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Bollen TL. Acute pancreatitis: international classification and nomenclature. Clin Radiol. 2016;71:121-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 11. | Foster BR, Jensen KK, Bakis G, Shaaban AM, Coakley FV. Revised Atlanta Classification for Acute Pancreatitis: A Pictorial Essay. Radiographics. 2016;36:675-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 135] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 12. | Sarathi Patra P, Das K, Bhattacharyya A, Ray S, Hembram J, Sanyal S, Dhali GK. Natural resolution or intervention for fluid collections in acute severe pancreatitis. Br J Surg. 2014;101:1721-1728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 13. | Hollemans RA, Bakker OJ, Boermeester MA, Bollen TL, Bosscha K, Bruno MJ, Buskens E, Dejong CH, van Duijvendijk P, van Eijck CH, Fockens P, van Goor H, van Grevenstein WM, van der Harst E, Heisterkamp J, Hesselink EJ, Hofker S, Houdijk AP, Karsten T, Kruyt PM, van Laarhoven CJ, Laméris JS, van Leeuwen MS, Manusama ER, Molenaar IQ, Nieuwenhuijs VB, van Ramshorst B, Roos D, Rosman C, Schaapherder AF, van der Schelling GP, Timmer R, Verdonk RC, de Wit RJ, Gooszen HG, Besselink MG, van Santvoort HC; Dutch Pancreatitis Study Group. Superiority of Step-up Approach vs Open Necrosectomy in Long-term Follow-up of Patients With Necrotizing Pancreatitis. Gastroenterology. 2019;156:1016-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 150] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 14. | Fischer TD, Gutman DS, Hughes SJ, Trevino JG, Behrns KE. Disconnected pancreatic duct syndrome: disease classification and management strategies. J Am Coll Surg. 2014;219:704-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 15. | van Brunschot S, van Grinsven J, van Santvoort HC, Bakker OJ, Besselink MG, Boermeester MA, Bollen TL, Bosscha K, Bouwense SA, Bruno MJ, Cappendijk VC, Consten EC, Dejong CH, van Eijck CH, Erkelens WG, van Goor H, van Grevenstein WMU, Haveman JW, Hofker SH, Jansen JM, Laméris JS, van Lienden KP, Meijssen MA, Mulder CJ, Nieuwenhuijs VB, Poley JW, Quispel R, de Ridder RJ, Römkens TE, Scheepers JJ, Schepers NJ, Schwartz MP, Seerden T, Spanier BWM, Straathof JWA, Strijker M, Timmer R, Venneman NG, Vleggaar FP, Voermans RP, Witteman BJ, Gooszen HG, Dijkgraaf MG, Fockens P; Dutch Pancreatitis Study Group. Endoscopic or surgical step-up approach for infected necrotising pancreatitis: a multicentre randomised trial. Lancet. 2018;391:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 471] [Article Influence: 67.3] [Reference Citation Analysis (0)] |