Published online Oct 28, 2022. doi: 10.35712/aig.v3.i4.105
Peer-review started: June 22, 2022
First decision: July 11, 2022
Revised: July 13, 2022
Accepted: October 26, 2022
Article in press: October 26, 2022
Published online: October 28, 2022
Processing time: 128 Days and 0.2 Hours
About 25% of the general population in Japan are reported to have nonalcoholic fatty liver disease (NAFLD). NAFLD and nonalcoholic steatohepatitis carry a risk of progressing further to hepatocellular carcinoma. The primary treatment for NAFLD is dietary therapy. Dietary counseling plays an essential role in dietary therapy. Although artificial intelligence (AI)-based nutrition management software applications have been developed and put into practical use in recent years, the majority focus on weight loss or muscle strengthening, and no software has been developed for patient use in clinical practice.
To examine whether effective dietary counseling is possible using AI-based nutrition management software.
NAFLD patients who had been assessed using an AI-based nutrition management software application (Calomeal) that automatically analyzed images of meals photographed by patients and agreed to receive dietary counseling were given dietary counseling. Blood biochemistry tests were performed before (baseline) and 6 mo after (6M follow-up) dietary counseling. After the dietary counseling, the patients were asked to complete a questionnaire survey.
A total of 29 patients diagnosed with NAFLD between August 2020 and March 2022 were included. There were significant decreases in liver enzyme and triglyceride levels at the 6M follow-up compared to baseline. The food analysis capability of the AI used by Calomeal in this study was 75.1%. Patient satisfaction with the AI-based dietary counselling was high.
AI-based nutrition management appeared to raise awareness of dietary habits among NAFLD patients. However, it did not directly alleviate the burden of registered dietitians, and impro
Core Tip: Use of artificial intelligence (AI)-based nutrition management software (Calomeal) appeared to raise awareness of nonalcoholic fatty liver disease patients’ dietary habits, and they showed significant decreases in liver enzyme and triglyceride levels at the 6-mo follow-up compared to baseline. The food analysis capability of the AI package used in this study was 75.1%, and patient satisfaction with the AI-based dietary counselling was high. However, due to the limitations of the food analysis capabilities of AI, it did not directly alleviate the burden of registered dietitians, and improvements in the analytical capabilities of AI are much anticipated.
- Citation: Kusano Y, Funada K, Yamaguchi M, Sugawara M, Tamano M. Dietary counseling based on artificial intelligence for patients with nonalcoholic fatty liver disease. Artif Intell Gastroenterol 2022; 3(4): 105-116
- URL: https://www.wjgnet.com/2644-3236/full/v3/i4/105.htm
- DOI: https://dx.doi.org/10.35712/aig.v3.i4.105
About 25% of the general population in Japan are reported to have nonalcoholic fatty liver disease (NAFLD), and this figure is expected to increase to 39.3% by 2030[1]. NAFLD includes nonalcoholic fatty liver (NAFL) and nonalcoholic steatohepatitis (NASH). NAFL and NASH carry a risk of progressing further to hepatocellular carcinoma. Although the risk of developing liver cancer from NAFL is low (0.44 per 1000 persons per year), the risk increases with the progression of liver pathology (5.29 per 1000 persons per year in patients with NASH and 0.45 to 22.6 per 1000 persons per year in patients with liver cirrhosis)[2,3].
The primary treatment for NAFLD is dietary therapy. Dietary counseling plays an essential role in dietary therapy. In dietary counseling, patients self-report all the food items that they consumed over the previous 1 wk, and during the interview, registered dietitians calculate the caloric and nutritive values of the food items. Limitations exist with patients’ memory certainty and the listening and calculation skills of the dietitians in the field. In recent years, pictures taken with digital cameras have been used in combination with interviews. Neither of these is very efficient, because dietary counseling begins when patients come in for their consultation, and the caloric and nutrient intakes are calculated based on patients’ recalled food items consumed in the past.
Artificial intelligence (AI)-based image analysis has penetrated deeply in our daily lives, and the field of medicine is no exception. In gastroenterology, it is used to diagnose colon polyps, Helicobacter pylori infection, and stomach cancer[4-8]. Although AI-based nutrition management software applications have been developed and put into practical use in recent years, the majority focus on weight loss or muscle strengthening, and no software has been developed for patient use in clinical practice.
Thus, the present study examined whether effective dietary counseling is possible using food intake data of NAFLD patients that had been automatically analyzed with an AI-based nutrition management software application.
This prospective study was conducted in compliance with the ethics guidelines of the 2008 Declaration of Helsinki. Approval was obtained from the Biomedical Ethics Committee of the authors’ affiliated hospital (No. 2014). Written informed consent was obtained from all patients.
Patients clinically diagnosed with NAFLD between August 2020 and March 2022 were included as subjects. NAFLD was diagnosed when fatty liver was observed in patients with an alcohol consumption equivalent to ≤ 30 g of ethanol per day in men and ≤ 20 g of ethanol per day in women[9]. Fatty liver was diagnosed when the following three criteria were confirmed on abdominal ultrasound examination: (1) Increased hepatic echogenicity; (2) positive liver-kidney contrast; and (3) deep ultrasound atten
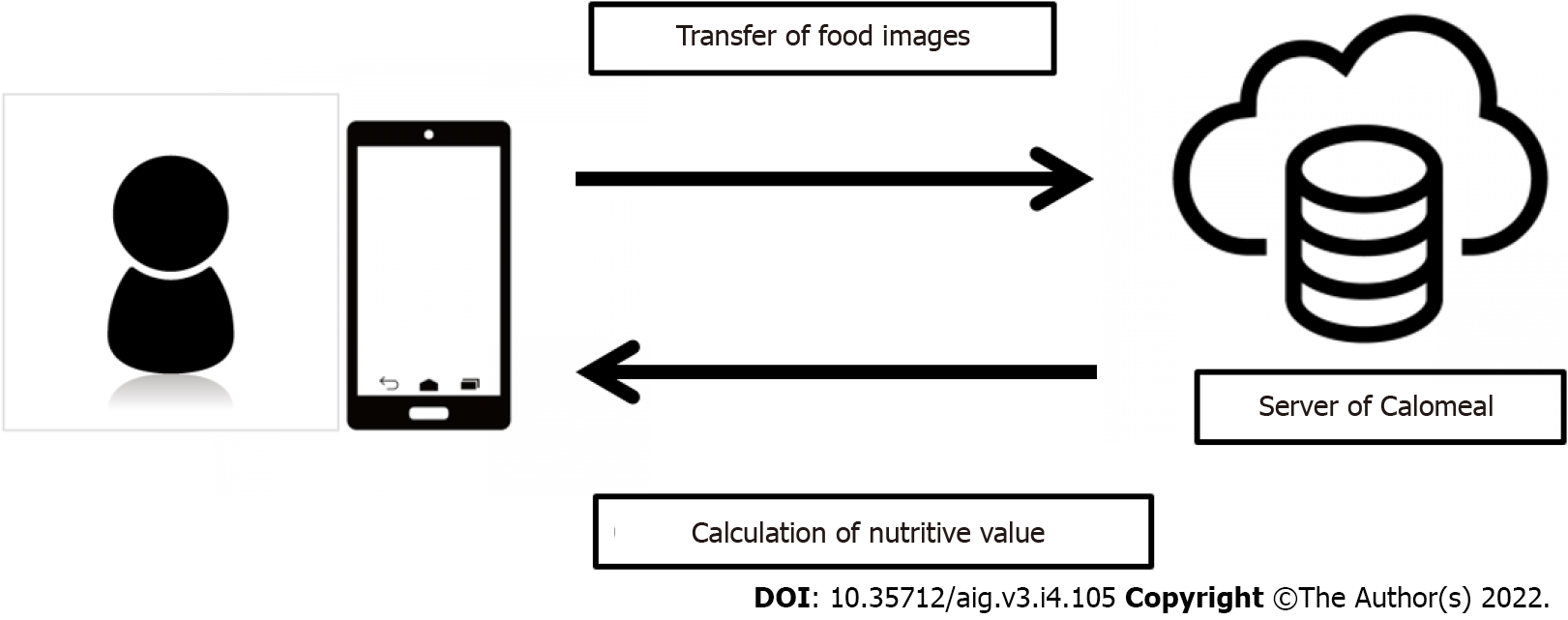
“Calomeal” is a software application developed by Life Log Technology, Inc. (Tokyo, Japan) that can easily record and manage one’s daily meals and physical activity. It has been available in Japan since December 2015. The "Calomeal" software is commercially available, and anyone can purchase it. When a user takes a picture of a meal using devices such as smartphones, the cloud-based AI calculates the nutritive values of the food in the photograph, and the results are sent to the user’s device (Figure 1). The AI of Calomeal was compiled using machine learning of food image data collected from major restaurant chains, food manufacturers, and everyday home-cooked meals. It can identify approximately 18000 food images. In addition to total caloric intake, Calomeal simultaneously calculates the nutritive values of proteins, lipids, carbohydrates, glucides, dietary fiber, and salt content.
This study used a customized version of Calomeal that was developed in cooperation with Life Log Technology. The following three areas were customized: (1) Changing the device from a smartphone to an iPad Mini; (2) simplifying food selection and offering more detailed options on consumed quantities; and (3) redirecting the AI analysis results to the study’s dedicated personal computer and not to the patients’ devices.
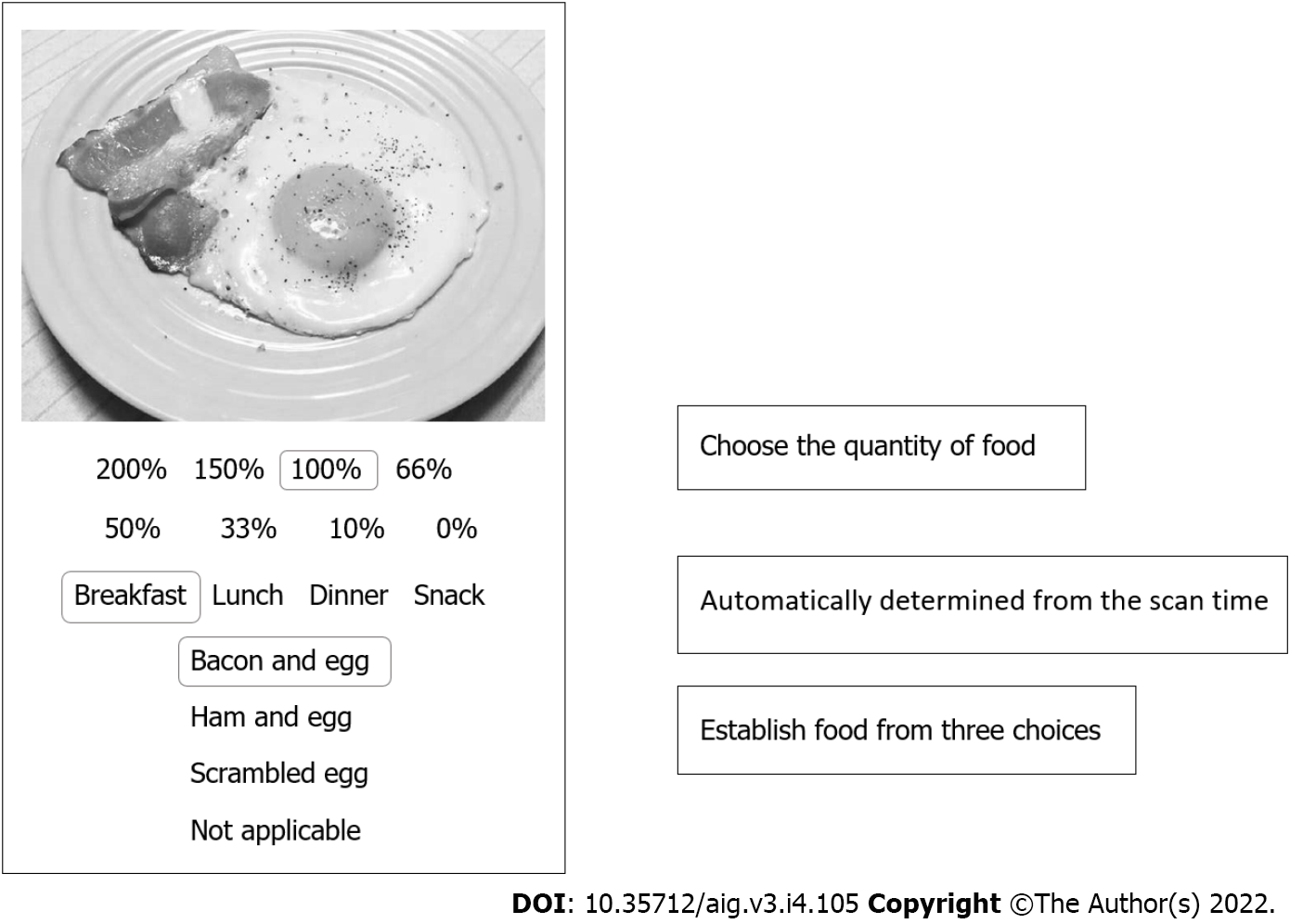
More specifically, since some NAFLD patients were older, iPad Minis were used as the device instead of smartphones because iPad Minis have larger display screens (Figure 2). When patients took pictures of a food item, the AI suggested the name of three possible food choices. The patients then selected from the three choices the food item that they consumed. When none of the offered three choices matched, “no suitable choice” was selected. Next, the patients chose the quantity of food that they consumed from the list of options. Eight options were available. Setting 100% as the normal serving size, the options were as follows: 200%, 150%, 100%, 66% (about two-thirds), 50%, 33% (about one-third), 10% (about one bite), and 0% (although a picture was taken, no food was consumed). AI calculated the nutritive values once these tasks were completed. The analysis results were forwarded to the study’s dedicated personal computer managed by the authors (Figure 3). Breakfast, lunch, dinner, or snacks were automatically determined based on the time when the foods were photographed.
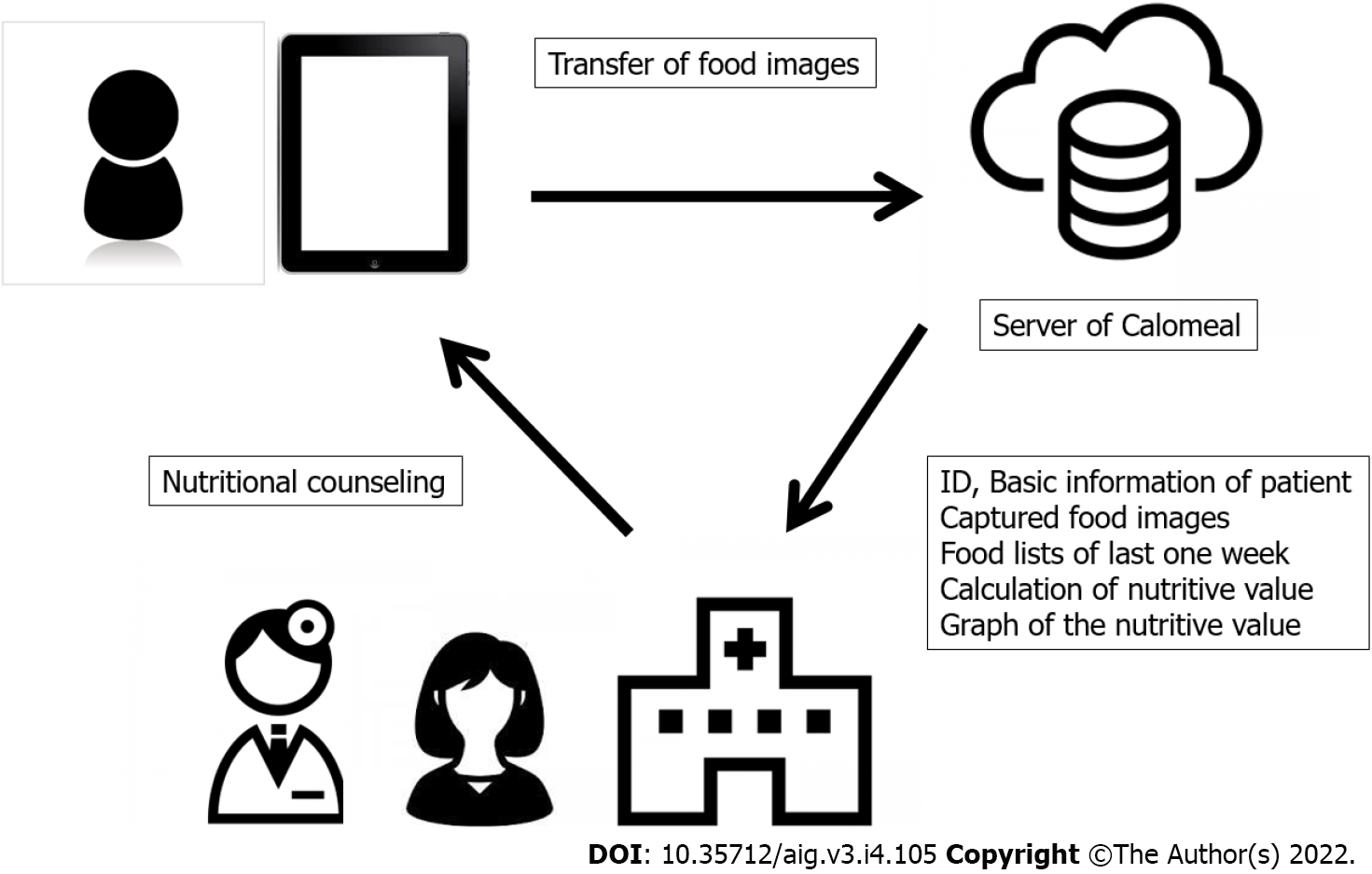
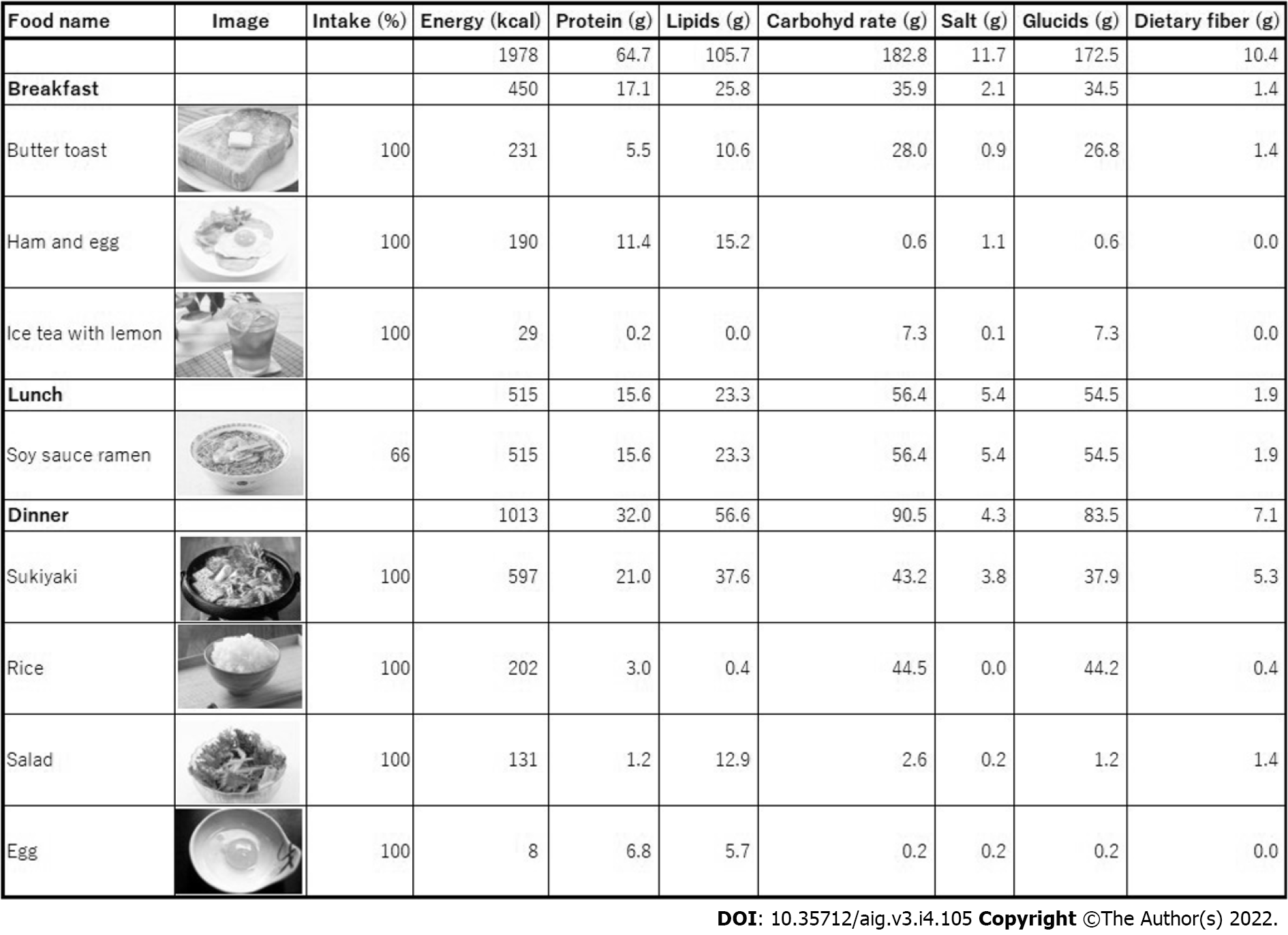
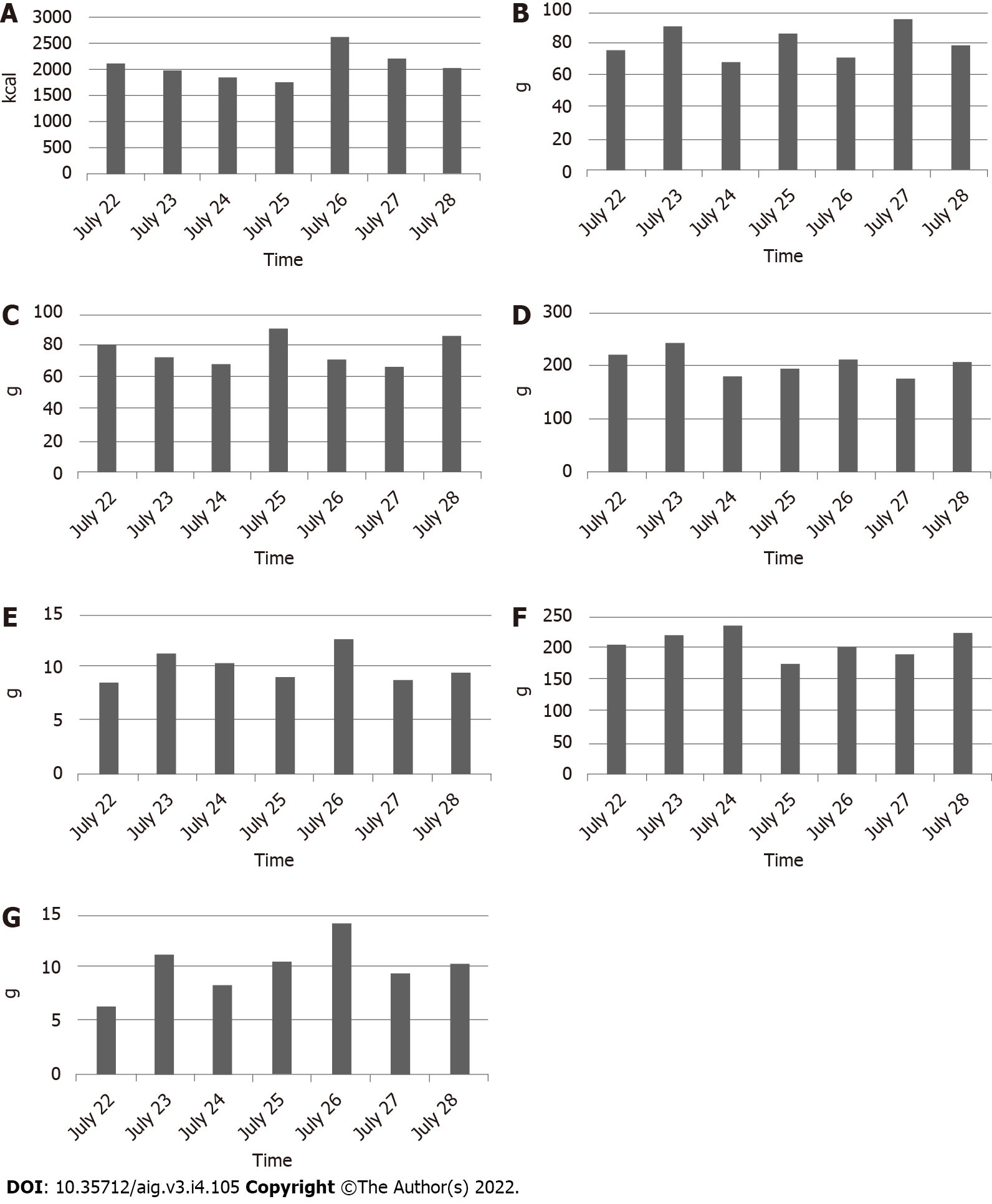
After photographing one week’s worth of pictures of meals, the patients came in for their dietary counseling session. By the time of the patients’ visits, the AI had prepared a list of a patient’s one week’s worth of photographed foods (Figure 4) and bar charts showing the amounts of caloric, protein, lipid, carbohydrate, glucide, dietary fiber, and salt intakes (Figure 5). Registered dietitians participating in this study conducted dietary counseling while presenting the abovementioned data.
The photographed food images for which the patients could not find a suitable choice at the time of taking the picture were examined by the registered dietitians before the patients’ visits, and after checking the photographed images, the registered dietitians entered the correct item name.
Around dozens of pieces of photograph data for one food (or one product) are prepared for machine learning. These photographs are learned by deep neural network, and foods (or a product) are analyzed by the pattern of the color and form.
The nutrient of common foods was calculated based on "standard table of food composition" announced by Japanese Ministry of Education, Culture, Sports, Science and Technology by dietitian of Life Log Technology company. The nutrient of foods of major restaurant chains and food manufacturers was calculated using the data published in the home page of each company. When the nutrient was not announced in home page of the company, the dietitian calculated the nutrient from announced raw materials.
Of all the patients enrolled in the study, patients who agreed to receive dietary counseling were given dietary counseling using Calomeal. Blood biochemistry tests were performed before (baseline) and 6 mo after (6M follow-up) dietary counseling. Aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ-glutamyltransferase (GGT), total cholesterol (T-cho), and triglyceride (TG) levels were compared between the baseline and 6M follow-up.
Body weight of all patients was compared between the baseline and 6M follow-up.
As noted earlier, the AI used in the present study was capable of analyzing approximately 18000 meal items. However, since most of these items were collected from menus of major restaurant chains and food manufacturers, its ability to identify everyday home-cooked meals was unknown. The AI’s ability to analyze food images was evaluated by calculating the percentage (%) of unidentified foods (i.e., foods for which the results of the AI’s automated analysis did not match the foods actually consumed by the patients, thereby categorized under “no suitable choice”) among all food items.
In addition, the time spent by registered dietitians before the patient visits in entering the correct food names of the unidentified “no suitable choice” food items was also measured.
After the dietary counseling, subjects in the Calomeal Group were asked to complete a questionnaire survey consisting of the following questions:
Question 1: Were you glad that you were given an AI-based dietary counseling session? (Yes or No).
Question 2: Did you find the dietary counseling rewarding? (Yes or No).
Question 3: Have you become more conscious of improving your dietary lifestyle? (Yes or No).
Question 4: Would you like to receive another AI-based dietary counseling session? (Yes or No).
Continuous data, such as those from the blood biochemistry tests, are shown as the mean ± standard deviation. The paired Wilcoxon test was used to test the difference in each parameter between the start of observation (baseline) and after 6 mo (6M follow-up). P < 0.05 was considered significant.
There were 29 patients who agreed and received dietary counseling using Calomeal. The operation of Calomeal using iPads was accepted by all 29 patients. Table 1 shows the patients’ characteristics. The patients had been taking all of these medications before starting this study, and no new drugs were initiated after the start of the observation in this study.
| Male/Female | 16/13 |
| Age (yr) | 56.4 ± 14.3 |
| Body weight (kg) | 74.1 ± 13.1 |
| BMI (kg/m2) | 28.4 ± 5.2 |
| Metabolic diseases | |
| Diabetes mellitus (Yes/No) | 10/19 |
| Dyslipidemia (Yes/No) | 14/15 |
| Concomitant drugs | |
| SGLT2 inhibitor | 2 |
| DPP-4 inhibitor | 3 |
| Thiazolidinedione | |
| GLP-1 agonist | 1 |
| Statin | 7 |
| Bezafibrate | |
| Pemafibrate | |
| EPA and DHA preparation | 1 |
| AST (U/L) | 50.2 ± 33.4 |
| ALT (U/L) | 53.8 ± 39.0 |
| GGT (U/L) | 80.3 ± 84.3 |
| T-B (mg/dL) | 1.3 ± 0.9 |
| Alb (mg/dL) | 4.2 ± 0.6 |
| eGFR (mL/min) | 71.1 ± 11.5 |
| HbA1c (%) | 6.4 ± 0.8 |
| T-cho (mg/dL) | 191.3 ± 35.0 |
| TG (mg/dL) | 126.5 ± 57.8 |
| WBC (103/μL) | 5.6 ± 1.5 |
| Hb (g/dL) | 14.5 ± 1.7 |
| Plts (104/μL) | 17.7 ± 7.4 |
Table 2 shows the AST, ALT, GGT, hemoglobin A1c (HbA1c), T-cho, and TG levels and body weight at baseline and 6M follow-up. AST, ALT, GGT, and TG levels were significantly lower (P = 0.0088, 0.0133, 0.0494, and 0.0246, respectively) at the 6M follow-up compared to the levels at baseline. Body weight was significantly lower (P = 0.0472) at the 6M follow-up compared to that at baseline.
| Baseline | 6M Follow-up | P value | |
| AST (U/L) | 50.2 ± 33.4 | 34.7 ± 14.7 | 0.0088 |
| ALT (U/L) | 53.8 ± 39.0 | 35.3 ± 16.8 | 0.0113 |
| GGT (U/L) | 80.3 ± 84.3 | 66.3 ± 82.9 | 0.0494 |
| HbA1c (%) | 6.4 ± 0.8 | 6.2 ± 0.6 | 0.27832 |
| T-cho (mg/dL) | 191.3 ± 35.0 | 189 ± 34.3 | 0.2109 |
| TG (mg/dL) | 126.5 ± 57.8 | 104.1 ± 60.6 | 0.0426 |
| Body weight (kg) | 74.0 ± 13.1 | 71.2 ± 12.3 | 0.0472 |
Table 3 shows the total number of food items photographed in 1 wk of all patients in the Calomeal Group, the number of food items categorized under “no suitable choice”, and the food analysis capability of the AI. The mean number of total photographed food items was 62.6 (20 to 104), the mean number of food items categorized under “no suitable choice” was 15.0 (1 to 32), and the mean analysis capability was 75.1% (51.5 to 98.6%). Before dietary counseling sessions, registered dietitians spent on average 25.9 min (4.5 to 67.0 min) identifying the food items categorized under “no suitable choice.”
| Case | All foods | No of candidates | Analysis rate (%) | Time for confirmation (min) |
| 1 | 57 | 13 | 77.2 | 25.0 |
| 2 | 31 | 2 | 93.5 | 4.5 |
| 3 | 90 | 31 | 65.6 | 67.0 |
| 4 | 40 | 7 | 82.5 | 20.5 |
| 5 | 74 | 20 | 73.0 | 31.0 |
| 6 | 69 | 1 | 98.6 | 6.0 |
| 7 | 24 | 3 | 87.5 | 9.5 |
| 8 | 20 | 9 | 55.0 | 12.0 |
| 9 | 47 | 9 | 80.9 | 28.5 |
| 10 | 87 | 29 | 66.7 | 35.5 |
| 11 | 26 | 12 | 53.8 | 14.0 |
| 12 | 35 | 8 | 77.1 | 10.0 |
| 13 | 85 | 25 | 70.6 | 36.5 |
| 14 | 95 | 8 | 91.6 | 8.5 |
| 15 | 53 | 15 | 71.7 | 20.0 |
| 16 | 84 | 32 | 61.9 | 39.5 |
| 17 | 77 | 13 | 83.1 | 14.0 |
| 18 | 51 | 7 | 86.3 | 13.0 |
| 19 | 33 | 16 | 51.5 | 20.0 |
| 20 | 103 | 9 | 91.3 | 19.5 |
| 21 | 32 | 6 | 81.3 | 5.5 |
| 22 | 68 | 25 | 63.2 | 32.5 |
| 23 | 88 | 30 | 65.9 | 50.0 |
| 24 | 41 | 18 | 56.1 | 66.0 |
| 25 | 74 | 22 | 70.3 | 36.0 |
| 26 | 104 | 16 | 84.6 | 34.0 |
| 27 | 84 | 22 | 73.8 | 47.5 |
| 28 | 83 | 13 | 84.3 | 25.5 |
| 29 | 61 | 13 | 78.7 | 20.5 |
| Average | 62.6 | 15.0 | 75.1 | 25.9 |
Table 4 shows the findings of the responses to the questionnaire survey. When the patients were asked “Were you glad that you were given an AI-based dietary counseling session?” in Question 1, all 29 patients said “Yes.” When the patients were asked “Did you find the dietary counseling rewarding?” in Question 2, 15 of the 29 patients responded “Yes.” When the patients were asked “Have you become more conscious of improving your dietary lifestyle after the dietary counseling session?” in Question 3, all 29 patients responded “Yes.” When the patients were asked “Would you like to receive another AI-based dietary counseling session?” in Question 4, four of the 29 patients responded “No.”
| Question | Yes | No |
| 1 | 29 | 0 |
| 2 | 15 | 14 |
| 3 | 29 | 0 |
| 4 | 25 | 29 |
AI helps diagnose NAFLD through its use in diagnostic imaging procedures such as ultrasound, computed tomography, and magnetic resonance imaging and pathological diagnostic procedures[10-12]. However, there have been no reports of using AI in nutritional therapies and dietary counseling for NAFLD. Thus, the authors focused on this aspect and planned the present study.
There is a long history of using AI in food analysis. In 1983, Chen et al[13] reported that the caloric intake of foods could be calculated using a small wearable computer that can be attached to clothing like a tin badge. This small computer called the eButton is being used for the healthy transformation of homemade foods[14,15]. When the eButton is used solely for identifying foods, the sensitivity is 85.0% and the specificity is 85.8%. Since the small eButton can be worn by attaching it to clothing, privacy is maintained[13]; however, its widespread use by the general public may be difficult because it is a purpose-built computer.
In Japan, there are five types of nutrition management software applications that can be downloaded on smartphones. Although there may be some differences in their analysis capability, all of them are very easy to use[16]. Of these software applications, Oka et al[17] used Asken for dietary counseling of type 2 diabetes mellitus patients and reported its benefit. Of these five software applications, the present study focused on Calomeal. The main reason for choosing Calomeal was its ease of operation.
In the present study, significant decreases in AST, ALT, GGT, and TG levels and body weight were observed at the 6M follow-up compared to the levels at baseline. However, there is an important limitation of this study. The present study did not compare dietary counseling using conventional methods with dietary counseling using Calomeal. The reason was that, in contrary to the authors’ expectations, fewer patients gave consent for dietary counseling after being diagnosed with NAFLD during the study period. Thus, it cannot be asserted that this study’s dietary counseling using Calomeal is superior to conventional methods. This issue needs to be addressed in future research.
The food analysis capability of the AI used by Calomeal in this study was 75.1%. The food analysis capability using Life Log Technology’s own data was reported to be 85.7%, which showed a discrepancy with the present study’s findings. One possible reason may be the small percentage of data for everyday home-cooked meals, since the food items registered in Calomeal’s AI consisted primarily of major restaurant chain menus, food manufacturers’ items, and convenience store products. In the present study, NAFLD patients, especially elderly patients, often consumed unique homemade dishes whose food names were difficult to identify just by looking at the pictures.
In other words, there were various daily diets, especially the cooking methods of family diets cannot be unified. These made food illegible and energy intake difficult to calculate. This can lead to significant error in the study.
Assuming that a patient eats four dish items per meal, three meals a day, this means that 4 × 3 × 7 = 84 items should have been photographed in 1 wk. However, as shown in Table 2, there was a large difference among patients, some with very few photographed food items (20 items) and some who had photographed many food items (104 items). The following reasons were given: (1) Patients could not take pictures while they were dining out because they were conscious of their surroundings; (2) patients could not bring the iPads to work; and (3) Japanese “teishoku” set lunches often plate various dishes on one plate, and some patients took pictures of the whole plate as one photograph. In the case of (3), the AI could not analyze multiple food items at once. This issue was reported to Life Log Technology as feedback, and the Calomeal software at the time of writing this paper had already taken this into account.
In the present study, the names of all food images that patients selected as “no suitable choice” on the screen were examined and entered individually by the registered dietitians. This procedure, which on average took 25.9 min (4.5 to 67.0 min), was conducted before the dietary counseling sessions. Since the duration of one dietary counseling session was 30 min, this means that preparation required about the same amount of time. However, when meeting the patient face-to-face, a list of food items pho
The questionnaire results showed that all of the respondents said that they were glad to have undergone dietary counseling using Calomeal. Similarly, they all responded that they had become aware of their dietary habits. On the other hand, 15 of 29 patients found the content of the dietary counseling rewarding. The majority of patients in the Calomeal Group in this study had a good command of their smartphones, and these patients had free access to the internet. Perhaps there was a lack of originality in the hospital-based dietary counseling, since nutritional information, to some extent, is available when one searches the internet. When the patients were asked whether they would like to receive dietary counseling again using Calomeal, four patients responded “No.” During outpatient follow-up interviews, all four patients commented that they “did not want to bring the devices out of their homes or to work.” This issue could be resolved by changing the device to a smartphone.
When an AI-based nutrition management software application automatically analyzed images of meals photographed by NAFLD patients, there were significant decreases in AST, ALT, GGT, and TG levels after 6 mo (6M follow-up). Thus, this method appeared to raise awareness of dietary habits of NAFLD patients. On the other hand, due to the limitations of the food analysis capabilities of AI, it did not directly alleviate the burden of registered dietitians, and improvements in the analytical capabilities of AI are much anticipated.
Approximately 27000 people a year die from liver cancer in Japan. Liver cancer from non-viral liver disease increases while cancerogenesis from viral liver decreases. In the non-viral liver disease, nonalcoholic fatty liver disease (NAFLD) increases in particular. Therefore, carcinogenesis restraint from NAFLD is urgent business to reduce liver cancer death. Diet therapy is the first choice for the treatment of NAFLD and nutrition education for this purpose becomes extremely important.
The authors paid attention to the nutrition education using the artificial intelligence and led to the idea of this study using the application software called the "Calomeal". The authors have the patients understand the importance of the diet by performing the nutrition education using the artificial intelligence for the NAFLD patients and want to help inhibit the cancerogenesis from NAFLD. A study on optimization of the nutrition education using the artificial intelligence (AI) for NAFLD is the attempt that leads the world and thinks with pioneer positioning of the future health promotion medical care.
Patients clinically diagnosed with NAFLD between August 2020 and March 2022 were included as subjects. "Calomeal" as a software application developed by Life Log Technology, Inc. (Tokyo, Japan) was used for the nutrition education. Blood biochemistry tests were performed before (baseline) and 6 mo after (6M follow-up) dietary counseling. After the dietary counseling, the patients were asked to complete a questionnaire survey.
There were significant decreases in liver enzyme and triglyceride levels at the 6M follow-up compared to baseline. The food analysis capability of the AI used by Calomeal in this study was 75.1%. Patient satisfaction with the AI-based dietary counselling was high.
The authors have the patients understand the importance of the diet because the NAFLD patients receive a nutrition education using the artificial intelligence, and the purpose of this study is to carry a help of the cancerogenesis restraint.
When an AI-based nutrition management software application automatically analyzed images of meals photographed by NAFLD patients, liver function was improved significantly. On the other hand, due to the limitations of the food analysis capabilities of AI, improvements in the analytical capabilities of AI are much anticipated.
The direction of future research is nutrition education using more advanced artificial intelligence to inhibit the carcinogenesis from NAFLD.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): E, E, E
P-Reviewer: Liu Y, China; MORYA AK, India; Yang JS, China; Zhang LL, China S-Editor: Liu JH L-Editor: Wang TQ P-Editor: Liu JH
| 1. | Ito T, Ishigami M, Zou B, Tanaka T, Takahashi H, Kurosaki M, Maeda M, Thin KN, Tanaka K, Takahashi Y, Itoh Y, Oniki K, Seko Y, Saruwatari J, Kawanaka M, Atsukawa M, Hyogo H, Ono M, Ogawa E, Barnett SD, Stave CD, Cheung RC, Fujishiro M, Eguchi Y, Toyoda H, Nguyen MH. The epidemiology of NAFLD and lean NAFLD in Japan: a meta-analysis with individual and forecasting analysis, 1995-2040. Hepatol Int. 2021;15:366-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 87] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 2. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7533] [Article Influence: 837.0] [Reference Citation Analysis (0)] |
| 3. | Amarapurkar DN, Dharod M, Gautam S, Patel N. Risk of development of hepatocellular carcinoma in patients with NASH-related cirrhosis. Trop Gastroenterol. 2013;34:159-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Misawa M, Kudo SE, Mori Y, Cho T, Kataoka S, Yamauchi A, Ogawa Y, Maeda Y, Takeda K, Ichimasa K, Nakamura H, Yagawa Y, Toyoshima N, Ogata N, Kudo T, Hisayuki T, Hayashi T, Wakamura K, Baba T, Ishida F, Itoh H, Roth H, Oda M, Mori K. Artificial Intelligence-Assisted Polyp Detection for Colonoscopy: Initial Experience. Gastroenterology. 2018;154:2027-2029.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 269] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 5. | Nakashima H, Kawahira H, Kawachi H, Sakaki N. Artificial intelligence diagnosis of Helicobacter pylori infection using blue laser imaging-bright and linked color imaging: a single-center prospective study. Ann Gastroenterol. 2018;31:462-468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (1)] |
| 6. | Bang CS, Lee JJ, Baik GH. Artificial Intelligence for the Prediction of Helicobacter Pylori Infection in Endoscopic Images: Systematic Review and Meta-Analysis Of Diagnostic Test Accuracy. J Med Internet Res. 2020;22:e21983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 7. | Jin P, Ji X, Kang W, Li Y, Liu H, Ma F, Ma S, Hu H, Li W, Tian Y. Artificial intelligence in gastric cancer: a systematic review. J Cancer Res Clin Oncol. 2020;146:2339-2350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 8. | Joseph J, LePage EM, Cheney CP, Pawa R. Artificial intelligence in colonoscopy. World J Gastroenterol. 2021;27:4802-4817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Watanabe S, Hashimoto E, Ikejima K, Uto H, Ono M, Sumida Y, Seike M, Takei Y, Takehara T, Tokushige K, Nakajima A, Yoneda M, Saibara T, Shiota G, Sakaida I, Nakamuta M, Mizuta T, Tsubouchi H, Sugano K, Shimosegawa T; Japanese Society of Gastroenterology; Japan Society of Hepatology. Evidence-based clinical practice guidelines for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. J Gastroenterol. 2015;50:364-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 157] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 10. | Decharatanachart P, Chaiteerakij R, Tiyarattanachai T, Treeprasertsuk S. Application of artificial intelligence in chronic liver diseases: a systematic review and meta-analysis. BMC Gastroenterol. 2021;21:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 11. | Su TH, Wu CH, Kao JH. Artificial intelligence in precision medicine in hepatology. J Gastroenterol Hepatol. 2021;36:569-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 12. | Wong GL, Yuen PC, Ma AJ, Chan AW, Leung HH, Wong VW. Artificial intelligence in prediction of non-alcoholic fatty liver disease and fibrosis. J Gastroenterol Hepatol. 2021;36:543-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 13. | Chen HC, Jia W, Yue Y, Li Z, Sun YN, Fernstrom JD, Sun M. Model-based measurement of food portion size for image-based dietary assessment using 3D/2D registration. Meas Sci Technol. 2013;24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Jia W, Li Y, Qu R, Baranowski T, Burke LE, Zhang H, Bai Y, Mancino JM, Xu G, Mao ZH, Sun M. Automatic food detection in egocentric images using artificial intelligence technology. Public Health Nutr. 2019;22:1168-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 15. | Raber M, Baranowski T, Crawford K, Sharma SV, Schick V, Markham C, Jia W, Sun M, Steinman E, Chandra J. The Healthy Cooking Index: Nutrition Optimizing Home Food Preparation Practices across Multiple Data Collection Methods. J Acad Nutr Diet. 2020;120:1119-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Shinozaki N, Murakami K. Evaluation of the Ability of Diet-Tracking Mobile Applications to Estimate Energy and Nutrient Intake in Japan. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 17. | Oka R, Nomura A, Yasugi A, Kometani M, Gondoh Y, Yoshimura K, Yoneda T. Study Protocol for the Effects of Artificial Intelligence (AI)-Supported Automated Nutritional Intervention on Glycemic Control in Patients with Type 2 Diabetes Mellitus. Diabetes Ther. 2019;10:1151-1161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |