Published online Apr 28, 2022. doi: 10.35712/aig.v3.i2.28
Peer-review started: December 21, 2021
First decision: March 12, 2022
Revised: March 28, 2022
Accepted: April 19, 2022
Article in press: April 19, 2022
Published online: April 28, 2022
Processing time: 129 Days and 18 Hours
Colorectal cancer is one of the most common neoplasia with an high risk to metastatic spread. Improving medical and surgical treatment is moving along with improving the precision of diagnosis and patient's assessment, the latter two aided more and more with the use of artificial intelligence (AI). The management of colorectal liver metastasis is multidisciplinary, and surgery is the main option. After the diagnosis, a surgical assessment of the patient is fundamental. Reaching a R0 resection with a proper remnant liver volume can be done using new techniques involving also artificial intelligence. Considering the recent application of artificial intelligence as a valid substitute for liver biopsy in chronic liver diseases, several authors tried to apply similar techniques to pre-operative imaging of liver metastasis. Radiomics showed good results in identifying structural changes in a unhealthy liver and in evaluating the prognosis after a liver resection. Recently deep learning has been successfully applied in estimating the remnant liver volume before surgery. Moreover AI techniques can help surgeons to perform an early diagnosis of neoplastic relapse or a better differentiation between a colorectal metastasis and a benign lesion. AI could be applied also in the histopathological diagnostic tool. Although AI implementation is still partially automatized, it appears faster and more precise than the usual diagnostic tools and, in the short future, could become the new gold standard in liver surgery.
Core Tip: Colon cancer is one of the most frequent cancers that unfortunately has a high risk of metastatic spread especially to the liver. The treatment of liver metastases is multidisciplinary, but surgery remains undoubtedly the main act. The results in the treatment of liver metastases have improved significantly over the years, but we continue to seek further paths of improvement. A new path, to which we currently entrust many hopes, is that of artificial intelligence, which could bring revolutionary solutions both in the diagnosis of liver metastases, and as a useful guide for surgical techniques. The purpose of this article is to summarize the latest news reported in the literature and possible research developments on this topic.
- Citation: Tonini V, Vigutto G, Donati R. Liver surgery for colorectal metastasis: New paths and new goals with the help of artificial intelligence. Artif Intell Gastroenterol 2022; 3(2): 28-35
- URL: https://www.wjgnet.com/2644-3236/full/v3/i2/28.htm
- DOI: https://dx.doi.org/10.35712/aig.v3.i2.28
Nowadays, colorectal cancer is one of the most common neoplasia in Western countries and among the main causes of death for oncologic diseases[1,2]. Between 30% and 50% of patients with colorectal cancer will develop liver metastasis during their life and surgical resection remains a fundamental treatment[1,2]. The improvement of surgical techniques, along with the use of newer and better schemes of chemotherapy, will increase the chances of a longer disease free survival for these patients[3]. Meanwhile, artificial intelligence (AI) is infiltrating healthcare exponentially and it has already been applied to several fields related to gastroenterology and hepatology[4,5].
The treatment of colorectal metastasis is generally multidisciplinary, involving many professional figures and multiples pathways[1,2]. Discussing other therapies, such as chemotherapy or radiotherapy, is beyond the scope of this article.
Surgical treatment always goes with hepatic resection[1]. All metastatic patients need to undergo several pre-operative exams for a better definition of the disease and its extent: a thoraco-abdominal contrast-enhanced CT scan and/or a contrast-enhanced MRI[1,6]. The use of routine PET/CT scan remains controversial[1,7]. The main goals during the assessment are evaluating the extent of the hepatic disease and searching for any extra hepatic localization of disease, the latter one is an exclusion criteria for any kind of hepatic resection[1,8].
Once surgery is considered, the assessment becomes more operative: new main goals are estimating how complex is performing a R0 resection and evaluating the liver remnant volume[1]. Clearly, a R0 resection should be achieved to increase the disease free survival and the overall survival, but the well-known 1cm border of healthy tissue is now reconsidered due to the increasing effectiveness of chemotherapy and the complexity of the resection[1,9,10]. At the same time, the size of the remnant liver must be evaluated with a three dimensional CT volumetry and it should be more than 20% in a healthy liver, more than 30% in post- systemic chemotherapy liver and more than 40% in a cirrhotic liver[1,11]. In case of an insufficient liver remnant volume, a portal vein embolization can be considered to increase the size to the residual liver[1,12], while, in case of bilateral lesions with a majority of them in one lobe, a two-stage hepatectomy with or without contralateral limited resections can be done[1,13]. Finally, a mini invasive approach should be considered if the surgeon is experienced in these techniques, considering the well-known advantages of mini invasive approaches[14].
The recent advent of artificial intelligence has changed the paradigm in the field of medical imaging interpretation together with radiomics. Artificial intelligence is a discipline that aims at mimicking the function of human brain in solving complex problems using computers. Machine learning and deep learning are branches of AI in which machines are thought how to learn from data using analytical models and algorithms. While machine learning methods usually require less computation on the computer side and more human intervention, deep learning may involve a huge amount of information (from which stems the adjective “deep”) and thus requires high performance computers, but less or no human intervention.
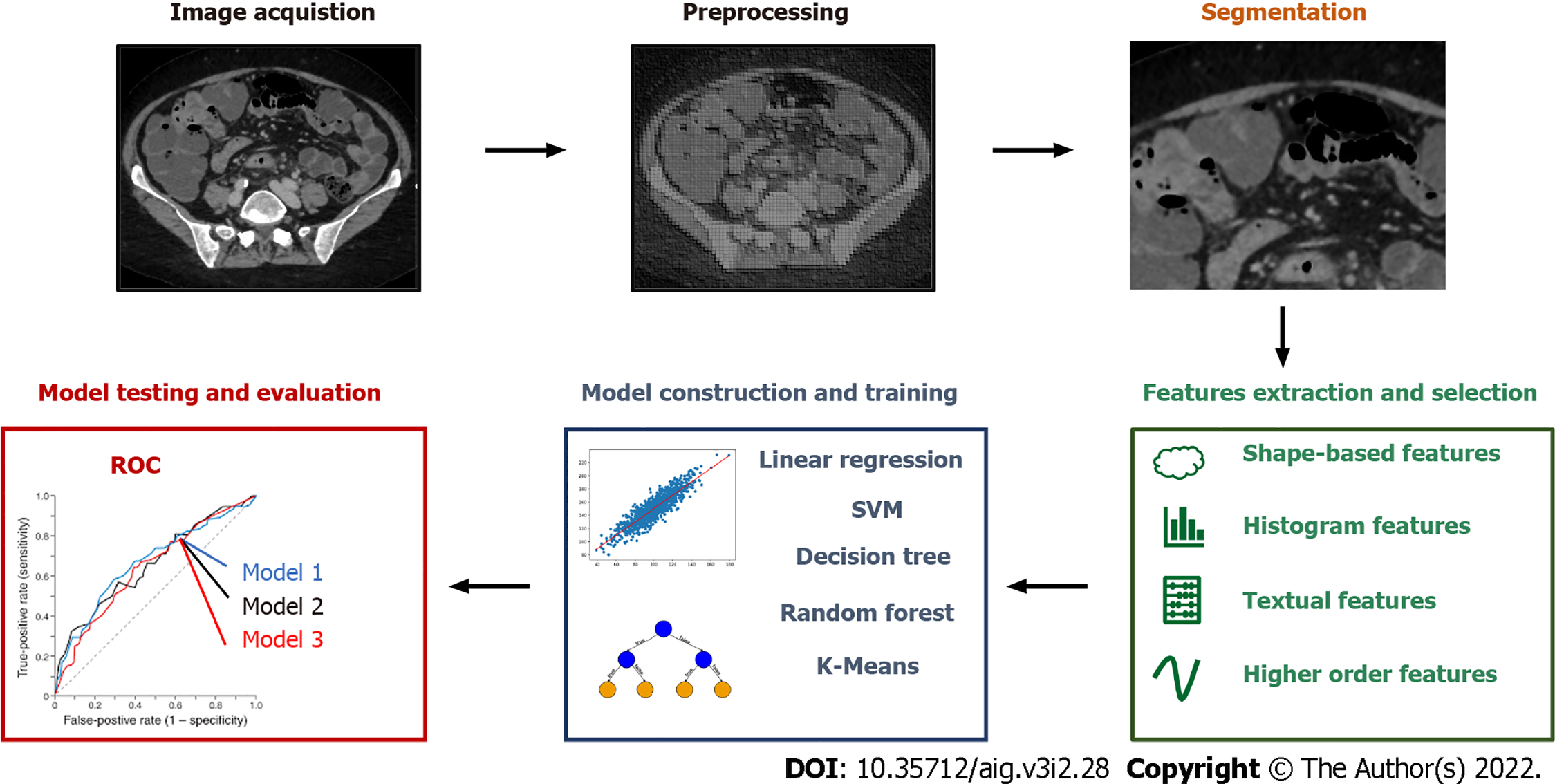
Radiomics is a tool for extensive extraction of quantitative features from medical imaging[4] and can be applied to ultrasound (US), magnetic resonance imaging (MRI), positron emission tomography (PET) and computed tomography (CT). The science of radiomics has taken advantage of machine learning with great benefit for medicine in general. The large amount of information provided by radiomics together with the improvements in AI have given raise to new methods of reading and interpreting medical images. Experts in different domains have now the opportunity to make less challenging the hard task of interpreting images thanks to this machine-aided approach. As shown in Figure 1 the workflow of conventional radiomics and AI applied to medical imaging is split in image acquisition, preprocessing, segmentation, features extraction and selection, model construction and training, model testing and evaluation. In conventional radiomics, one of the main prerequisites during the phase of image acquisition and preprocessing is a certain degree of standardization of the processes, in order to obtain a database with images that have comparable characteristics. Images segmentation consists in locating lesions manually or with the aid of a computer, in order to identify the region of interest o volumes of interests. Feature extraction and selection is a crucial step in machine learning paradigms in order to obtain a subset of quantitative parameters that are given as inputs to train the analytical model. In case of radiomics, these can be shape-based features (e.g. size, shape, location), histogram features (or others first-order features like standard deviation and variance), textual features (e.g. tumor heterogeneity) and other higher order features extracted with wavelet transforms or Laplacian filters. In the phase of model construction, it is important to choose the analytical engine that gives the best results in term of performance in relation to the selected features. To do so, several models can be chosen and then tested such as linear regression, support vector machines, decision tree, random forest, K-Means. The evaluation of the models and the assessment of their performance is inferred from indicators and methods such as the receiver operating characteristic, nomograms and the decision curve analysis.
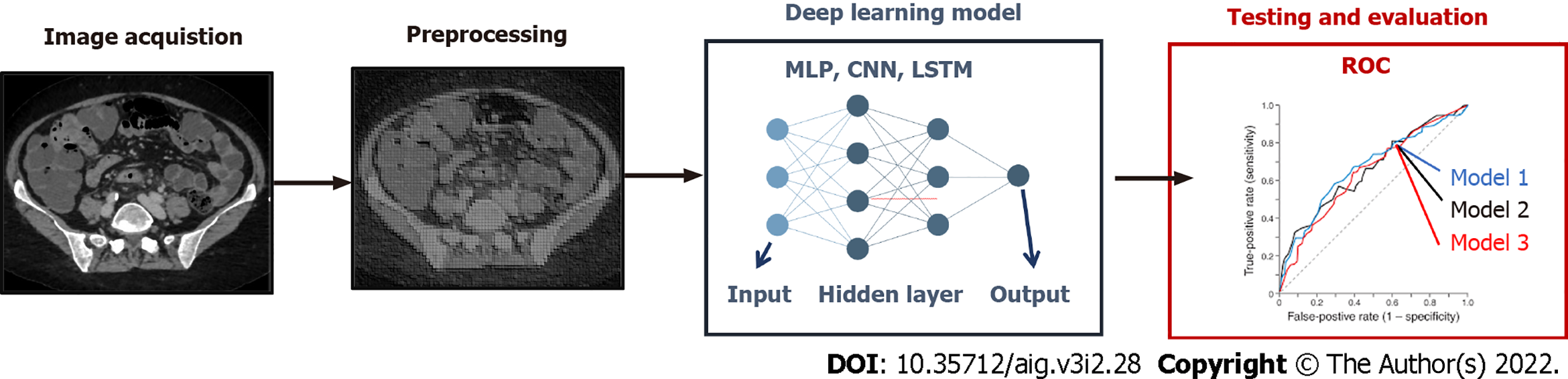
Whereas conventional radiomics is still a widely used approach in medical image analysis, in recent years, deep learning has been introduced in the clinical practice thanks to its promising results[7]. This technique can reach high levels of performance while not requiring manual human intervention in the phases of image segmentation and features extraction (Figure 2). In this paradigm, features are in fact automatically selected by a neural network to maximize the performance of the algorithm (called “backpropagation algorithm”). However, a larger amount of data (e.g. of number of medical images) is commonly needed to train the neural network models using backpropagation. Among the most popular techniques are multilayer perceptron networks, convolutional neural network, long short-term memory recurrent neural networks. Such as in conventional radiomics, different deep learning techniques can be applied to the input data in order to obtain the best performance.
Recently, artificial intelligence was applied to various fields in medicine, including general surgery and hepatology[4,5], as seen in Table 1. Decharatanachart et al[4] published a meta-analysis on AI supported imaging and standard liver biopsy. They showed a similar prediction rate for liver cirrhosis without the risk of complications of a biopsy and without the usual interpretation bias of ultrasonography. Meanwhile, Christou et al[5] focused more on the possibility of integrating diagnosis and management in several gastroenterological diseases, such as inflammatory bowel disease (IBD), Helicobacter pylori infection and gastric cancer, and several hepatic diseases, such as HCV infection and cirrhosis[5]. On one hand, they described how the use of machine learning and CAD can increase sensibility and specificity of a standard endoscopic or radiologic exam; on the other hand they describe the limitations of AI[5].
| Ref. | Type of paper | Main topic | AI implementation |
| Decharatanachart et al[4], 2021 | Meta-analysis | Chronic liver diseases | Diagnosis and staging of liver fibrosis without biopsy |
| Christou et al[5], 2021 | Review | IBD, GI bleeding and chronic liver diseases | Increasing accuracy of gold standard diagnostic exams |
| Park et al[15], 2020 | Review | Liver diseases | Staging of liver disease and prognosis after liver resection or chemotherapy |
| Wang et al[16], 2012 | Survey | Liver imaging | Diagnosis of structural changes in healthy liver |
| Shan et al[19], 2019 | Research article | Liver imaging (CT) | Prediction of early recurrence after HCC resection/RF |
| Hu et al[18], 2019 | Research article | Liver imaging (US) | Evaluating microvascular invasion in HCC |
| Iranmanesh et al[19], 2014 | Research article | Liver imaging (CT) | Evaluating portal pressure without invasive methods |
| Wang et al[23], 2019 | Research article | Liver imaging (CT/MRI) | Using liver segmentation to an automatized liver biometry |
| Fang et al[21], 2020 | Research article | Liver imaging | Using liver segmentation to more accurate localization of a hepatic lesion |
| Winkel et al[22], 2020 | Comparative study | Liver imaging | Comparing a fully automated liver segmentation to a manual one |
| Zhou et al[23], 2019 | Review | Liver imaging | Detecting hepatic lesions, characterized them and evaluate a response after treatment |
| Yasaka et al[24], 2018 | Retrospective study | Liver imaging (CT) | Differentiation between benign and malignant hepatic lesions |
| Guo et al[25], 2018 | Research article | Liver imaging (US) | Differentiation between benign and malignant hepatic lesions |
| Schmauch et al[26], 2019 | Research article | Liver imaging (US) | Differentiation between benign and malignant hepatic lesions |
| Tiyarattanachai et al[27], 2021 | Retrospective study | Liver imaging (US) | Detect and diagnose hepatic lesions |
| Perez et al[28], 2020 | Review | HCC | Improving diagnosis and evaluation after ancillary treatments |
| Vivanti et al[29], 2017 | Research article | Liver neoplasia | Evaluating post chemotherapy response |
| Li et al[30], 2015 | Research article | Liver imaging (CT) | Differentiation between benign and malignant hepatic lesions |
| Hamm et al[31], 2019 | Research article | Liver imaging (MRI) | Differentiation between benign and malignant hepatic lesions |
| Zhang et al[32], 2018 | Research article | HCC | Differentiation between healthy and tumoral tissue in patient's liver |
| Preis et al[33], 2011 | Research article | Liver imaging (PET) | Differentiation between benign and malignant hepatic lesions |
| Chen et al[34], 2020 | Review | Liver surgery | Implementation in pre and post operative care |
| Nakayama et al[35], 2017 | Retrospective study | Liver surgery | Use of 3D modeling to improve hepatice resection |
| Zhang et al[36], 2018 | Prospective study | Liver surgery | Diagnosis and treatment of perihilar CCC |
| Vorontsov et al[37], 2019 | Retrospective study | Liver surgery | Improving CRM identification and segmentation |
| Chartrand et al[39], 2017 | Comparative study | Liver imaging | Improving liver segmentation and volumetry |
| Cancian et al[40], 2021 | Research article. | Liver pathology | Better assessment pf tumor microenvironment |
One the of the main application of AI in liver surgery is in the pre-operative imaging. Park et al[15] described the use of radiomics and deep learning in liver diseases. Radiomics appears to be an effective way to analyse the structural changes of an unhealthy liver, comparable to the standard techniques like biopsies[15,16]. Furthermore, radiomics is already in use for determining the prognosis after surgical resection or radiofrequency[17] for hepatocellular carcinoma, especially related to micro vascular invasion[15,18]. Deep learning finds its best application in liver segmentation, where it is fundamental in estimating the liver remnant volume and the fat ratio in post chemotherapy liver[15,19,20]. Fang et al[21] focused on the implementation of deep learning in CT-guided biopsy to obtain a better localization of the lesion. In addition they presented a basic algorithm that could offer good results. At the same time, Winkel et al[22] compared manual segmentation and automatic segmentation with the use of deep learning showing a similar efficacy of the automatic segmentation with a faster elaboration of the images.
Focusing on focal liver lesions, Zhou et al[23] illustrated a 5 categories classification based on dynamic contrast-enhanced CT scan with a deep learning software: applying this classification, the radiologist would be able to make a diagnosis between a carcinoma and a benign lesion without biopsy[23,24]. They reported the application of deep learning to a contrast-enhanced ultrasonography (CEUS) to better distinguish between a benign and malignant lesion of the liver, showing again a better performance using AI techniques compared to the conventional technique[23,25]. Schmauch et al[26] presented a glimpse of future implementations of the standard ultrasonography where the use of a deep learning technique could drastically improve the diagnostic value of a widespread imaging such as US. Similarly, Tiyarattanachai et al[27] implemented a deep learning software for the US reporting a better outcome both in prevention and diagnosis of a focal liver lesion. Closely related to our main topic, Perez et al[28] proposed a review on the management of hepatocellular carcinoma using AI for diagnosis, treatment and prognosis. Combining the US deep learning software[26] and the contrast-enhanced CT scan deep learning software[24,29,30], the clinician can reach a diagnosis on a focal liver lesion without the use of liver biopsy; in case of more doubts, a deep learning MRI software[31,32] and a deep learning PET software[33] are under external verification, but they appears promising.
Another main application of AI in liver surgery is the pre-operative patient assessment. The second part of the paper of Perez et al[28] described how the combined effort of US, CT, MRI scan and deep learning software increase the precision of the hepatic resection and the early recognition of a relapse. Beside the use of AI in the diagnosis, Chen et al[34] described the intra-operative advantages of using 3D rendering of the patient’s liver to study and apply the best approach for a liver resection and, at the same time, to keep the same 3D model during the operation for a more intuitive way to reach the aforementioned R0 resection[34-36].
About colorectal liver metastasis, Voronstov et al[37] proposed a CT-based deep learning software to automatize and improve the recognition of metastasis rather than benign focal liver lesions. Detection performance of the software was still lower for lesion smaller than 10 mm, but it became more precise for lesions between 10 and 20 mm[37]. Manual liver segmentation was still more accurate for lesions smaller than 10mm, but it reached the same value for lesions greater than 10 mm and it was more efficient in lesions greater than 20 mm; the same results appeared considering lesion-volume estimation[37]. The authors also stated that all software calculations for an automatized or semi-automatized recognition and evaluation of metastasis is a significantly faster procedure than the usual manual one, as expected[37-39].
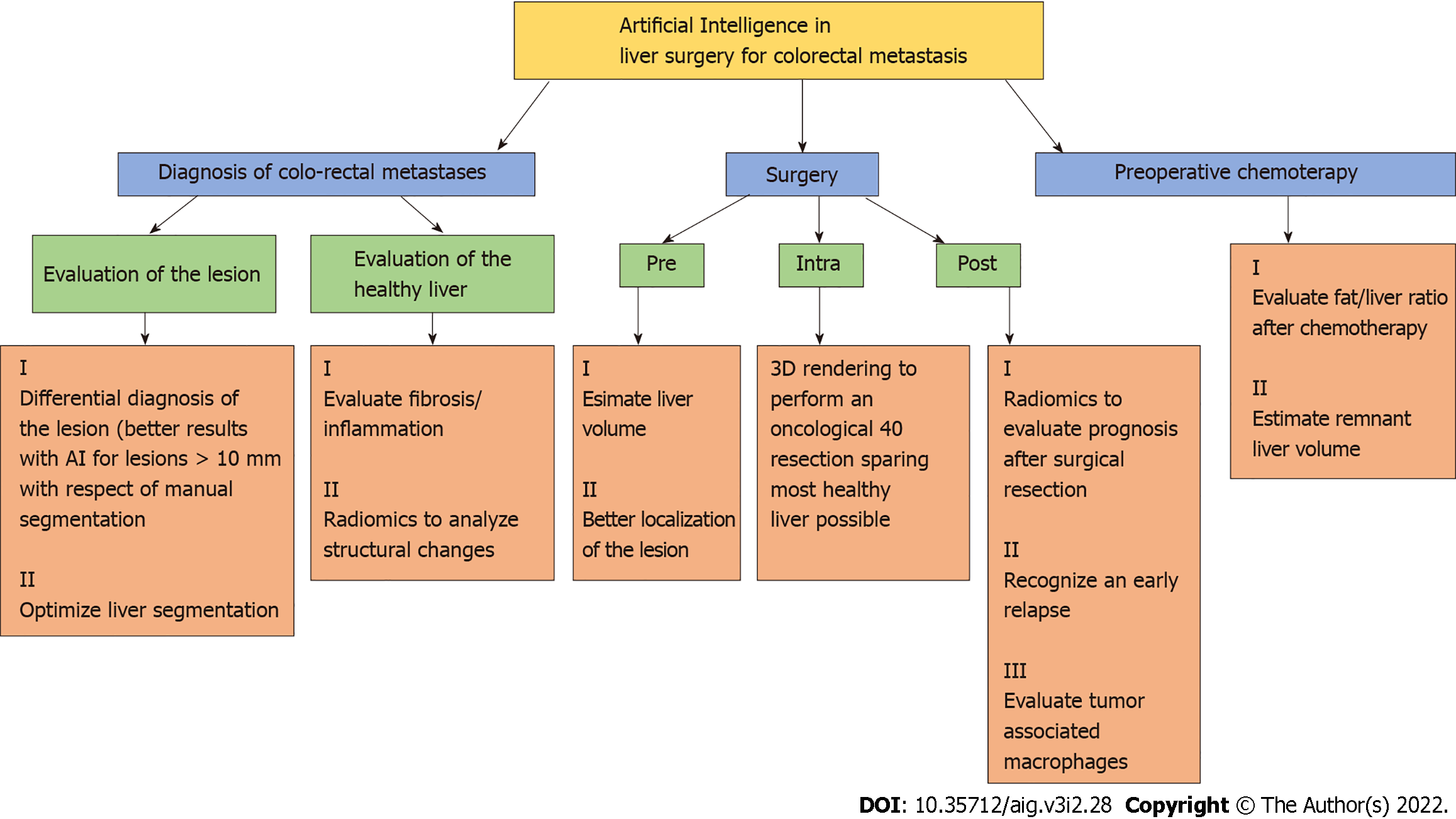
Within the same sphere, Cancian et al[40] focused on the analysis of the tumor microenvironment using a deep learning technique to evaluate the morphology of tumor associated macrophages. The same group recently described how different macrophages’ morphologies are associated with different outcomes and therapeutic responses in colorectal liver metastasis[41], so they developed a pipeline using a CAD tool to process faster the histopathological slides. Although the pipeline is still under verification for a fully automatic application, a combined use of a manual and automatic approach showed a better and faster identification of macrophages' morphologies[40,41]. In Figure 3 are shown in a schematic manner the main tools of AI in diagnosis and treatment of colo-rectal liver metastases.
Artificial intelligence and deep learning offer new hopes in diagnosis and therapy of the liver metastasis. Therefore new promising research directions open up in this field, that must be confirmed with larger studies in the future.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Da Costa AC, United Kingdom; Karamarkovic AR, Serbia S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Dhir M, Sasson AR. Surgical Management of Liver Metastases From Colorectal Cancer. J Oncol Pract. 2016;12:33-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 2. | Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier AM. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg. 2006;244:254-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 826] [Cited by in RCA: 1009] [Article Influence: 53.1] [Reference Citation Analysis (0)] |
| 3. | Folprecht G. Liver Metastases in Colorectal Cancer. Am Soc Clin Oncol Educ Book. 2016;35:e186-e192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Decharatanachart P, Chaiteerakij R, Tiyarattanachai T, Treeprasertsuk S. Application of artificial intelligence in chronic liver diseases: a systematic review and meta-analysis. BMC Gastroenterol. 2021;21:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 5. | Christou CD, Tsoulfas G. Challenges and opportunities in the application of artificial intelligence in gastroenterology and hepatology. World J Gastroenterol. 2021;27:6191-6223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (8)] |
| 6. | van Kessel CS, Buckens CF, van den Bosch MA, van Leeuwen MS, van Hillegersberg R, Verkooijen HM. Preoperative imaging of colorectal liver metastases after neoadjuvant chemotherapy: a meta-analysis. Ann Surg Oncol. 2012;19:2805-2813. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 120] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 7. | Moulton CA, Gu CS, Law CH, Tandan VR, Hart R, Quan D, Fairfull Smith RJ, Jalink DW, Husien M, Serrano PE, Hendler AL, Haider MA, Ruo L, Gulenchyn KY, Finch T, Julian JA, Levine MN, Gallinger S. Effect of PET before liver resection on surgical management for colorectal adenocarcinoma metastases: a randomized clinical trial. JAMA. 2014;311:1863-1869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 113] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 8. | Ekberg H, Tranberg KG, Andersson R, Lundstedt C, Hägerstrand I, Ranstam J, Bengmark S. Determinants of survival in liver resection for colorectal secondaries. Br J Surg. 1986;73:727-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 338] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 9. | Dhir M, Lyden ER, Wang A, Smith LM, Ullrich F, Are C. Influence of margins on overall survival after hepatic resection for colorectal metastasis: a meta-analysis. Ann Surg. 2011;254:234-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Pawlik TM, Scoggins CR, Zorzi D, Abdalla EK, Andres A, Eng C, Curley SA, Loyer EM, Muratore A, Mentha G, Capussotti L, Vauthey JN. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005;241:715-722, discussion 722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 843] [Cited by in RCA: 811] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 11. | Ribero D, Chun YS, Vauthey JN. Standardized liver volumetry for portal vein embolization. Semin Intervent Radiol. 2008;25:104-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 12. | van Lienden KP, van den Esschert JW, de Graaf W, Bipat S, Lameris JS, van Gulik TM, van Delden OM. Portal vein embolization before liver resection: a systematic review. Cardiovasc Intervent Radiol. 2013;36:25-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 345] [Cited by in RCA: 333] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 13. | Lam VW, Laurence JM, Johnston E, Hollands MJ, Pleass HC, Richardson AJ. A systematic review of two-stage hepatectomy in patients with initially unresectable colorectal liver metastases. HPB (Oxford). 2013;15:483-491. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 137] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 14. | Ye SP, Qiu H, Liao SJ, Ai JH, Shi J. Mini-invasive vs open resection of colorectal cancer and liver metastases: A meta-analysis. World J Gastroenterol. 2019;25:2819-2832. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | Park HJ, Park B, Lee SS. Radiomics and Deep Learning: Hepatic Applications. Korean J Radiol. 2020;21:387-401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 95] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 16. | Wang S, Summers RM. Machine learning and radiology. Med Image Anal. 2012;16:933-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 352] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 17. | Shan QY, Hu HT, Feng ST, Peng ZP, Chen SL, Zhou Q, Li X, Xie XY, Lu MD, Wang W, Kuang M. CT-based peritumoral radiomics signatures to predict early recurrence in hepatocellular carcinoma after curative tumor resection or ablation. Cancer Imaging. 2019;19:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 130] [Article Influence: 21.7] [Reference Citation Analysis (1)] |
| 18. | Hu HT, Wang Z, Huang XW, Chen SL, Zheng X, Ruan SM, Xie XY, Lu MD, Yu J, Tian J, Liang P, Wang W, Kuang M. Ultrasound-based radiomics score: a potential biomarker for the prediction of microvascular invasion in hepatocellular carcinoma. Eur Radiol. 2019;29:2890-2901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 141] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 19. | Iranmanesh P, Vazquez O, Terraz S, Majno P, Spahr L, Poncet A, Morel P, Mentha G, Toso C. Accurate computed tomography-based portal pressure assessment in patients with hepatocellular carcinoma. J Hepatol. 2014;60:969-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 20. | Wang K, Mamidipalli A, Retson T, Bahrami N, Hasenstab K, Blansit K, Bass E, Delgado T, Cunha G, Middleton MS, Loomba R, Neuschwander-Tetri BA, Sirlin CB, Hsiao A; members of the NASH Clinical Research Network. Automated CT and MRI Liver Segmentation and Biometry Using a Generalized Convolutional Neural Network. Radiol Artif Intell. 2019;1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 21. | Fang X, Xu S, Wood BJ, Yan P. Deep learning-based liver segmentation for fusion-guided intervention. Int J Comput Assist Radiol Surg. 2020;15:963-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Winkel DJ, Weikert TJ, Breit HC, Chabin G, Gibson E, Heye TJ, Comaniciu D, Boll DT. Validation of a fully automated liver segmentation algorithm using multi-scale deep reinforcement learning and comparison versus manual segmentation. Eur J Radiol. 2020;126:108918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 23. | Zhou LQ, Wang JY, Yu SY, Wu GG, Wei Q, Deng YB, Wu XL, Cui XW, Dietrich CF. Artificial intelligence in medical imaging of the liver. World J Gastroenterol 2019; 25: 672-682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 192] [Cited by in RCA: 138] [Article Influence: 23.0] [Reference Citation Analysis (8)] |
| 24. | Yasaka K, Akai H, Abe O, Kiryu S. Deep Learning with Convolutional Neural Network for Differentiation of Liver Masses at Dynamic Contrast-enhanced CT: A Preliminary Study. Radiology. 2018;286:887-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 393] [Article Influence: 49.1] [Reference Citation Analysis (0)] |
| 25. | Guo LH, Wang D, Qian YY, Zheng X, Zhao CK, Li XL, Bo XW, Yue WW, Zhang Q, Shi J, Xu HX. A two-stage multi-view learning framework based computer-aided diagnosis of liver tumors with contrast enhanced ultrasound images. Clin Hemorheol Microcirc. 2018;69:343-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 26. | Schmauch B, Herent P, Jehanno P, Dehaene O, Saillard C, Aubé C, Luciani A, Lassau N, Jégou S. Diagnosis of focal liver lesions from ultrasound using deep learning. Diagn Interv Imaging. 2019;100:227-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 104] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 27. | Tiyarattanachai T, Apiparakoon T, Marukatat S, Sukcharoen S, Geratikornsupuk N, Anukulkarnkusol N, Mekaroonkamol P, Tanpowpong N, Sarakul P, Rerknimitr R, Chaiteerakij R. Development and validation of artificial intelligence to detect and diagnose liver lesions from ultrasound images. PLoS One. 2021;16:e0252882. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (1)] |
| 28. | Jiménez Pérez M, Grande RG. Application of artificial intelligence in the diagnosis and treatment of hepatocellular carcinoma: A review. World J Gastroenterol. 2020;26:5617-5628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 52] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (2)] |
| 29. | Vivanti R, Szeskin A, Lev-Cohain N, Sosna J, Joskowicz L. Automatic detection of new tumors and tumor burden evaluation in longitudinal liver CT scan studies. Int J Comput Assist Radiol Surg. 2017;12:1945-1957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 30. | Li W, Jia F, Hu Q. Automatic Segmentation of Liver Tumor in CT Images with Deep Convolutional Neural Networks. J Comput Commun. 2015;3:146-151. |
| 31. | Hamm CA, Wang CJ, Savic LJ, Ferrante M, Schobert I, Schlachter T, Lin M, Duncan JS, Weinreb JC, Chapiro J, Letzen B. Deep learning for liver tumor diagnosis part I: development of a convolutional neural network classifier for multi-phasic MRI. Eur Radiol. 2019;29:3338-3347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 207] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 32. | Zhang F, Yang J, Nezami N, Laage-Gaupp F, Chapiro J, De Lin M, Duncan J. Liver Tissue Classification Using an Auto-context-based Deep Neural Network with a Multi-phase Training Framework. Patch Based Tech Med Imaging (2018). 2018;11075:59-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 33. | Preis O, Blake MA, Scott JA. Neural network evaluation of PET scans of the liver: a potentially useful adjunct in clinical interpretation. Radiology. 2011;258:714-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Chen H, He Y, Jia W. Precise hepatectomy in the intelligent digital era. Int J Biol Sci. 2020;16:365-373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 35. | Nakayama K, Oshiro Y, Miyamoto R, Kohno K, Fukunaga K, Ohkohchi N. The Effect of Three-Dimensional Preoperative Simulation on Liver Surgery. World J Surg. 2017;41:1840-1847. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 36. | Zhang J, Qiao QL, Guo XC, Zhao JX. Application of three-dimensional visualization technique in preoperative planning of progressive hilar cholangiocarcinoma. Am J Transl Res. 2018;10:1730-1735. [PubMed] |
| 37. | Vorontsov E, Cerny M, Régnier P, Di Jorio L, Pal CJ, Lapointe R, Vandenbroucke-Menu F, Turcotte S, Kadoury S, Tang A. Deep Learning for Automated Segmentation of Liver Lesions at CT in Patients with Colorectal Cancer Liver Metastases. Radiol Artif Intell. 2019;1:180014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (1)] |
| 38. | Boykov YY, Jolly MP. Interactive graph cuts for optimal boundary & region segmentation of objects in N-D images. International Conference on Computer Vision. 2001; 105–112. |
| 39. | Chartrand G, Cresson T, Chav R, Gotra A, Tang A, De Guise JA. Liver Segmentation on CT and MR Using Laplacian Mesh Optimization. IEEE Trans Biomed Eng. 2017;64:2110-2121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 40. | Cancian P, Cortese N, Donadon M, Di Maio M, Soldani C, Marchesi F, Savevski V, Santambrogio MD, Cerina L, Laino ME, Torzilli G, Mantovani A, Terracciano L, Roncalli M, Di Tommaso L. Development of a Deep-Learning Pipeline to Recognize and Characterize Macrophages in Colo-Rectal Liver Metastasis. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 41. | Donadon M, Torzilli G, Cortese N, Soldani C, Di Tommaso L, Franceschini B, Carriero R, Barbagallo M, Rigamonti A, Anselmo A, Colombo FS, Maggi G, Lleo A, Cibella J, Peano C, Kunderfranco P, Roncalli M, Mantovani A, Marchesi F. Macrophage morphology correlates with single-cell diversity and prognosis in colorectal liver metastasis. J Exp Med. 2020;217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 120] [Article Influence: 24.0] [Reference Citation Analysis (0)] |