Published online Apr 28, 2022. doi: 10.35713/aic.v3.i2.17
Peer-review started: December 31, 2021
First decision: March 12, 2022
Revised: March 27, 2022
Accepted: April 20, 2022
Article in press: April 20, 2022
Published online: April 28, 2022
Processing time: 118 Days and 4.3 Hours
Gastric cancer (GC) is a major cancer worldwide, with high mortality and morbidity. Endoscopy, important for the early detection of GC, requires trained skills, high-quality technologies, surveillance and screening programs. Early diagnosis allows a better prognosis, through surgical or curative endoscopic therapy. Magnified endoscopy with virtual chromoendoscopy remarkably improve the detection of early gastric cancer (EGC) when endoscopy is performed by expert endoscopists. Artificial intelligence (AI) has also been introduced to GC diagnostics to increase diagnostic efficiency. AI improves the early detection of gastric lesions because it supports the non-expert and experienced endoscopist in defining the margins of the tumor and the depth of infiltration. AI increases the detection rate of EGC, reduces the rate of missing tumors, and characterizes EGCs, allowing clinicians to make the best therapeutic decision, that is, one that ensures curability. AI has had a remarkable evolution in medicine in recent years, moving from the research phase to clinical practice. In addition, the diagnosis of GC has markedly progressed. We predict that AI will allow great evolution in the diagnosis and treatment of EGC by overcoming the variability in performance that is currently a limitation of chromoendoscopy.
Core Tip: Early diagnosis and treatment of gastric cancer (GC) can benefit from the introduction of artificial intelligence (AI) into endoscopic diagnostics of the upper digestive tract. AI improves endoscopic diagnosis because it overcomes the difficulty of diagnosis linked to the experience of the endoscopist. Improving endoscopic diagnosis will allow for better treatment, which is more likely to be curative, with submucosal endoscopic dissection or surgery. However, because research advances in this area continue to be rapid, prospective multicenter studies are needed on the application of AI to the diagnosis of early GC.
- Citation: Panarese A. Usefulness of artificial intelligence in early gastric cancer. Artif Intell Cancer 2022; 3(2): 17-26
- URL: https://www.wjgnet.com/2644-3228/full/v3/i2/17.htm
- DOI: https://dx.doi.org/10.35713/aic.v3.i2.17
Gastric cancer (GC), the fourth leading cause of cancer in men and seventh in women, is still third for cancer-related deaths worldwide[1]. It’s 5-year survival rate is less than 40%[2] and its prognosis is related to the stage at the time of detection. The 5-year survival rate of patients with early gastric cancer (EGC) is 91.5%, whereas it is 16.4% for patients in the advanced stage[2-4]. The screening programs are cost effective in high-incidence regions[1,5] and advanced endoscopic technologies allow endoscopists to diagnose EGC[6-8]; however, optical diagnosis requires a period of training[9].
Recently, the practice of medicine has changed with the development of artificial intelligence (AI) based on image recognition with deep learning (DL) using the convolutional neural network (CNN), which, in upper endoscopy, is trained with endoscopic images and detects GC accurately[10-14]. Several AI-assisted CNN computer-aided diagnosis (CAD) systems have been built, with diagnostic precision in the detection of GC based on different types of endoscopic images. AI helps endoscopists to achieve the accuracy needed for GC screening, surveillance of precancerous, as well as for detecting the depth of invasion of gastric lesions, and when applied to radiological imaging techniques, lymph node and peritoneal metastasis[11-14].
While computed tomography, endoscopic ultrasound, and positron emission tomography are important for the diagnosis and staging of advanced GC, endoscopy plays an essential role in the early detection of EGC, as it allows the gastric mucosa to be examined directly. Endoscopy with targeted biopsies is the gold standard method for diagnosing EGC, and the accurate diagnosis of EGC through endoscopic imaging is a primary goal for improving the poor prognosis of patients[4,15-17]. Although the quality and accuracy of endoscopic detection are variable between centers and endoscopists, endoscopy is crucial because many early-stage tumors (i.e. intramucosal cancer) can be resected endoscopically in a curative manner, with an excellent prognosis at 5 years[4,18,19].
Unfortunately, few endoscopists are experts in advanced endoscopic imaging, and diagnostic accuracy depends largely on the clinical experience of the experts and is influenced by multiple factors, such as training and technologies[9,20]. Ultimately, early diagnosis and curative treatment are important for prognosis but can be difficult to achieve depending on the endoscopist[10,21]. The false negative rate of GC detected by esophagogastroduodenoscopy is 4.6-25.8[22-24], with higher values for inexperienced endoscopists[9,25]. The diagnostic capacity of endoscopists, due to the endoscopic appearance of EGC, which is usually very subtle, varies widely with regard to the differentiation between GC and gastritis, the prediction of the horizontal extension of GC and the depth of invasion[26].
As lesions of the gastric mucosa develop according to the Correa cascade, from atrophy to intestinal metaplasia, intraepithelial neoplasia and invasive neoplasia[27,28]; improving the accuracy of endoscopic diagnosis of precancerous lesions and EGC through screening and surveillance programs, is useful to reduce the incidence and mortality of GC[29-31]. The standard modality for the detection of EGC is endoscopy with white light imaging (WLI), but its overall sensitivity is not satisfactory (40%-60%)[32]. Magnified endoscopy (ME) with image-enhanced endoscopy techniques such as narrow-band imaging (NBI; Olympus Co., Tokyo, Japan), flexible spectral imaging color enhancement (FICE; Fujifilm Co., Tokyo, Japan), and blue laser imaging (BLI; Fujifilm), improve the accuracy of the detection of gastric lesions[26,33,34]. In particular, ME-NBI, the most frequent technology used in AI studies, achieves significantly better sensitivity, specificity, and accuracy than WLI, facilitating examination of the glandular epithelium in the stomach by observing the microvascular architecture and structure of the microsurface[32,35-39].
However, the virtual chromoendoscopic diagnosis of EGC requires considerable skill and experience[9,38,40,41]. The diagnostic effectiveness of endoscopists non yet trained in differentiating EGC from non-cancerous lesions with ME-NBI is disappointing[9,36,41]. Optical diagnosis can improve with AI-assisted CNN, which has been mainly applied to ME-NBI[14].
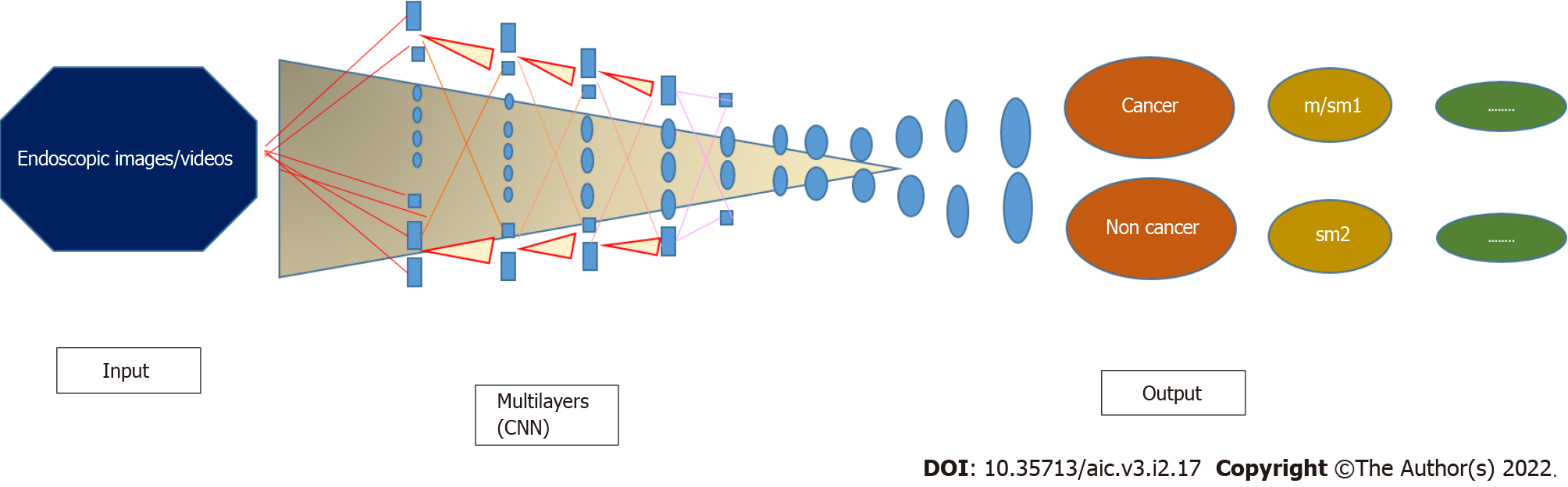
AI, which mimics human cognitive function[42] with its efficient computational power and learning capabilities, can be applied to GC because it processes and analyzes large amounts of data with systems that classify and recognize lesion images without the need to write complicated image processing algorithms[43]. Therefore, AI could help gastroenterologists in clinical diagnosis and decision-making. Technically, the DL method approximates complex information using a multilayer system (e.g., CNN), in which neural layers connect only to the next layer (Figure 1), overcoming the limitation of the "black box" of previous systems because it shows the reasons for the decisions made[44]. Over the years, new CNN-based systems have been introduced to analyze lesions of the gastric mucosa, using higher quality images and image selection strategies based on evidence from previous experiences. CNN systems in the initial training phase take a few hours to generate the identification system, which can then be used repeatedly; and has a good adaptability as it can be used on multiple platforms for the real-time analysis of JPEG images or video captured by chromoendoscopy. Magnifying chromoendoscopic images can improve the speed and accuracy of CNN diagnostics compared to conventional endoscopy alone[45,46]. Typically, training images are judged by experienced endoscopists and pathologically confirmed, and only endoscopic and chromoendoscopic images with appropriate magnification and typical manifestation for learning the CNN model are selected.
In recent studies, other important outcomes have been added to the main outcome to establish endoscopic resectability, namely the identification of the margins and depth of the lesion[47-49]. Gastric tumors of differentiated intramucous type (m) or infiltrating only the superficial layer of the submucosal (≤ 500 μm: Sm1) can be resected endoscopically, while those that deeply invade the submucosal (> 500 μm: Sm2) are surgically resected because of the risk of lymph node and distant metastases. The optical differentiation between m/Sm1 and Sm2 is often difficult[19].
Using PubMed, Embase, Web of Science, and Cochrane Library databases to search the literature on CAD systems for the diagnosis of EGC, we identified 26 relevant physician-initiated studies through November 2021. Table 1 summarizes the main characteristics of the studies (two single-center prospective[50,51], two multicenter prospective[49,52], and twenty-two retrospective[14,45-48,53-69]): Study design; endoscopic modality; main study aim; and subjects/lesions/images for validation. Table 2 describes the endpoints of the studies.
| Ref. | Study design | Endoscopic modality | Main study aim | Subjects for validation |
| Kubota et al[53], 2012 | Retrospective | WLI | Prediction of invasion depth | 344 patients |
| Miyaki et al[63], 2013 | Retrospective | ME-FICE | Differentiation of cancerous areas from non-cancerous areas | 46 patients |
| Miyaki et al[64], 2015 | Retrospective | ME-BLI | Differentiation of cancerous areas from non-cancerous areas | 95 patients |
| Kanesaka et al[65], 2018 | Retrospective | ME-NBI | Delineation of cancerous areas | 81 images |
| Hirasawa et al[14], 2018 | Retrospective | WLI, CE, NBI | Delineation of cancer | 69 patients |
| Zhu et al[54], 2019 | Retrospective | WLI, NBI | Prediction of invasion depth | 203 lesions |
| Cho et al[50], 2019 | Prospective validation dataset | WLI | Differentiation of cancerous areas from non-cancerous areas | 200 patients |
| Ishioka et al[55], 2019 | Retrospective | WLI | Detection of GC | 62 patients |
| Yoon et al[56], 2019 | Retrospective | WLI | Detection of GC | 800 patients |
| Tang et al[57], 2020 | Retrospective | WLI | Differentiation of cancerous areas from non-cancerous areas | 279 patients |
| Namikawa et al[58], 2020 | Retrospective | WLI | Differentiation of cancerous areas from non-cancerous areas | 220 lesions |
| Li et al[66], 2020 | Retrospective | ME-NBI | Detection of cancer | 341 images |
| An et al[62], 2020 | Retrospective | WLI, CE, ME-NBI | Delineation of EGC margins | 355 images |
| Horiuki et al[67], 2020 | Retrospective | ME-NBI | Differentiation of cancerous areas from non-cancerous areas | 258 images |
| Nagao et al[45], 2020 | Retrospective | WLI, CE, NBI | Prediction of invasion depth of GC | 1084 GC |
| Wu et al[52], 2021 | Prospective | WLI | Detection of Blind spotsAnd early gastric cancer | 1050 patients |
| Ueyama et al[59], 2021 | Retrospective | ME-NBI | Differentiation of cancerous areas from non-cancerous areas | 2300 images |
| Ling et al[48], 2021 | Retrospective | ME-NBI | Differentiation status and margins for EGC | 139 + 58 + 87 EGCs |
| Ikenoyama et al[46], 2021 | Retrospective | WLI, CE, NBI | Detection of cancer | 140 lesions |
| Hu et al[68], 2021 | Retrospective | ME-NBI | Detection of cancer | 295 lesions |
| Oura et al[60], 2021 | Retrospective | WLI | Missing GC and point out low-quality images | 855 lesions + 50 lesions |
| Zhang et al[61], 2021 | Retrospective | WLI | Detection of cancer | 1091 images |
| Wu et al[51], 2021 | Prospective | WLI | Screening gastric lesions | 10000 patients |
| Hamada et al[69], 2022 | Retrospective | WLI, CE, BLI | Depth of invasion of EGC | 68 patients |
| Nam et al[47], 2022 | Retrospective | WLI | Lesion detection, differentiation and depth | 1366 patients |
| Wu et al[49], 2022 | Prospective | ME-NBI | GC and EGC detection, EGC invasion depth and differentiation status |
| Ref. | Main outcome |
| [45,53,54,69] | Accuracy rate of diagnosing the depth of wall invasion of gastric cancer |
| [64] | Detection rate of gastric cancer |
| [63] | Identification rate of cancerous lesions, reddened lesions and surrounding tissue |
| [48,62,65] | Detection rate of early gastric cancer and its margins |
| [14] | Identification rate of gastric cancer and gastric ulcer |
| [50] | Identification rate of advanced gastric cancer, early gastric cancer, high grade dysplasia, low grade dysplasia and non-neoplasm |
| [46,51,55,57,59,60,66,68] | Detection rate of early gastric cancer |
| [56] | Detection rate of early gastric cancer and its localization. Accuracy rate of diagnosing the depth of wall invasion of gastric cancer |
| [58] | Identification rate of early gastric cancer, advanced gastric cancer and benign gastric ulcer |
| [67] | Identification rate of early gastric cancer and gastritis |
| [52] | Identification rate of early gastric cancer and number of blind spots |
| [61] | Identification rate of early gastric cancer and other gastric lesions (high grade dysplasia, peptic ulcer, advanced gastric cancer, gastric submucosal tumors and normal gastric mucosa) |
| [47] | Identification rate of early gastric cancer, advanced gastric cancer and benign gastric ulcer. Accuracy rate of diagnosing the depth of wall invasion of gastric cancer |
| [49] | Detection rate of early gastric cancer. Accuracy rate of diagnosing the depth of wall invasion of gastric cancer |
Selected studies included a diagnostic test on the application of AI in endoscopy for the diagnosis of EGC; the absolute numbers of true-positive, false-negative, true-negative and false-positive; clear information about data and number of images; the description of the algorithms and the process applied to the EGC diagnosis.
To form a training dataset, 11 studies used only WLI images[47,50-53,55-58,60,61], 9 only virtual chromoendoscopy images[48-49,59,63-68], 1 only WLI and chromoendoscopy images[54], and 5 WLI, chromoendoscopy and NBI images[14,45,46,62,69]. The identified studies were largely published in the last 3 years.
Overall, current CNN systems work quite well in detecting the endoscopic/chromoendoscopic characteristics of EGC and other gastric lesions and could provide diagnostic support to experienced and non-expert endoscopists in future practice. AI-assisted CNN CAD systems can avoid subjectivity during the processing and diagnosis of endoscopic/chromoendoscopic images; moreover, in the screening of GC, they work as a “confirmer” or “corrector,” providing a second opinion to reduce the diagnostic errors committed by endoscopists and suggesting optimal treatment. Current studies by Asian authors[54,59] confirm that CAD systems detect EGCs and estimate the depth of infiltration and extension, overcoming the problem of operator training and the subjectivity of diagnosis. Moreover, if the first studies report comparable results between experts and CAD systems, the most recent ones show that AI has reached a sensitivity even higher than that of experts, with similar specificity[46]. Over time, images used for CAD system training have improved and, at present, advanced training strategies and videos are being used.
Namikawa et al[58] first reported the usefulness of AI systems in GC detection, developing the “original convolutional neural network (O-CNN),” with a relatively low positive predictive value (PPV). The same authors developed an advanced AI-based diagnostic system, “advanced CNN (A-CNN)”, by adding a new training dataset to the O-CNN and evaluated its applicability for the classification of GC and gastric ulcer. The diagnostic performance of A-CNN was evaluated retrospectively using an independent validation dataset and compared to that of the O-CNN by estimating the overall accuracy of the classification. The sensitivity, specificity, and PPV rates of A-CNN for the classification of GC at the lesion level were 99.0%, 93.3%, and 92.5%, respectively, and 93.3%, 99.0%, and 99.1% for the classification of gastric ulcers. The overall accuracy of O-CNN and A-CNN in the classification of GC and gastric ulcer was 45.9% (GC: 100%, gastric ulcer 0.8%) and 95.9% (GC: 99.0%, gastric ulcer 93.3%), respectively, at the lesion level. The A-CNN system can effectively classify GC and gastric ulcer. Yu et al[36] explored the diagnostic capacity of the CNN system with ME-NBI to distinguish EGC from gastritis. CNN accuracy with ME-NBI images was 85.3% (220 of 258 images correctly diagnosed). Rates of sensitivity, specificity, PPV, and negative predictive value (NPV) were 95.4%, 71.0%, 82.3%, and 91.7%, respectively. In total, 7 of 151 EGC images were identified as gastritis, while 31 of the 107 gastritis images were recognized as EGC. The overall test speed was 51.83 images/s (0.02 s/image). CNN with ME-NBI can differentiate between EGC and gastritis with high sensitivity and NPV in a short period of time. Thus, the A-CNN system can complement current clinical practice of diagnosis with ME-NBI.
Nam et al[47] have developed and validated CNN-based AI models for lesion detection, differential diagnosis (AI-DDx), and depth of invasion (AI-ID; pT1a vs pT1b among EGC). AI-DDx is comparable to experts and outperforms novice and intermediate endoscopists in the differential diagnosis of gastric mucosal lesions. AI-ID performs better than endoscopic ultrasound to assess depth of invasion. Ling et al[48] have developed a system to identify in real time with precision with ME-NBI the state of differentiation and delineate the margins of the EGC, fundamental to determine a surgical strategy and achieve the curative resection. In the unprocessed videos of EGC, the system obtained a real-time diagnosis of EGC differentiation and its margins ME-NBI endoscopy. This system has achieved higher performance than experts and has been successfully tested in real EGC videos.
Zhu et al[54] represented a further step forward because they developed an algorithm capable of differentiating lesions with Sm2 invasion depth from m/Sm1. AI has presented 76% sensitivity and 96% specificity in identifying “Sm2 or deeper” cancers, resulting in significantly higher sensitivity and specificity than those achieved through visual inspection of endoscopists. The specificity of 96% could minimize the overdiagnosis of invasion, which would contribute to a reduction of unnecessary surgeries for m/Sm1 cancers.
Wu et al[52], in a prospective multicenter randomized controlled trial, developed a CNN system to monitor blind spots during esophagogastroduodenoscopy, updating the previous system (ENDOANGEL), verifying efficacy in improving endoscopy quality, and pretesting performance in detecting EGC.
Ultimately, AI is even superior to endoscopists experienced in identifying and classifying ECC, eliminates interobserver variability, and can train inexperienced endoscopists. Yet, it must optimize the ability to recognize all lesions (PPV) and not interpret the inflammatory or benign aspects of the mucosa as neoplastic (NPV). Over time, CAD systems have improved image selection strategies with strict criteria, using high-quality data and videos, and eliminating overlearning and misdiagnosis. Videos improve the performance of AI[55] because they represent real-life scenarios, and compared to static images improve PPV and NPV. Regarding the selection of images, gastritis, that is, the presence of inflammation, reduces the performance of AI[14] and endoscopists[70]. The small (diameter ≤ 5 mm) and depressed EGCs, difficult to distinguish from gastritis even for experienced endoscopists, influence the rate of false negatives; and gastritis with redness, atrophy and intestinal metaplasia affects the rate of false positives. In dedicated studies, CAD systems detect Helicobacter pylori (H. pylori) infection (sensitivity 89%, specificity 87% and diagnostic time 194 s)[71,72], but, regarding the diagnosis of EGC with AI sistems, we propose to evaluate the gastric mucosa after the eradication of H. pylori to reduce the intensity of redness of gastritis.
Integrating in appropriate algorithms, through the intersection of engineering and medical expertise, high-quality image sets, poor images, and images from regular sites, will increase clinical effectiveness. Moreover, the products obtained through collaboration among centers specialized in the diagnosis and treatment of gastric lesions are reproducible and the limitation in applying AI to the diagnosis of EGC is the acquisition of new technologies, which requires investment. Finally, prospective multicenter trials are needed.
The application of AI to the clinical practice of the upper digestive tract increases the rate of EGC compared to all GCs, exceeding the subjectivity of the diagnosis and reducing the chance of missing EGCs. AI recognizes those lesions that not even the most experienced endoscopists can detect, as if “illuminating” the images with its third artificial eye. Of course, AI increases the accuracy of endoscopic diagnosis of EGC, especially when combined with the experience of endoscopists. However, since its introduction in this field is very recent, the results in clinical practice must be further validated, considering all possible aspects, both technical and technological concerning endoscopy, and organizational ones.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cheng H, China; Kawabata H, Japan; Luo W, China; Yu W, China S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, Znaor A, Bray F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941-1953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3585] [Cited by in RCA: 4896] [Article Influence: 699.4] [Reference Citation Analysis (1)] |
| 2. | Song Z, Wu Y, Yang J, Yang D, Fang X. Progress in the treatment of advanced gastric cancer. Tumour Biol. 2017;39:1010428317714626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 644] [Article Influence: 80.5] [Reference Citation Analysis (0)] |
| 3. | Nishizawa T, Yahagi N. Long-Term Outcomes of Using Endoscopic Submucosal Dissection to Treat Early Gastric Cancer. Gut Liver. 2018;12:119-124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 4. | Young E, Philpott H, Singh R. Endoscopic diagnosis and treatment of gastric dysplasia and early cancer: Current evidence and what the future may hold. World J Gastroenterol. 2021;27:5126-5151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 56] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (3)] |
| 5. | Zhang X, Li M, Chen S, Hu J, Guo Q, Liu R, Zheng H, Jin Z, Yuan Y, Xi Y, Hua B. Endoscopic Screening in Asian Countries Is Associated With Reduced Gastric Cancer Mortality: A Meta-analysis and Systematic Review. Gastroenterology. 2018;155:347-354.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 225] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 6. | Yao K, Takaki Y, Matsui T, Iwashita A, Anagnostopoulos GK, Kaye P, Ragunath K. Clinical application of magnification endoscopy and narrow-band imaging in the upper gastrointestinal tract: new imaging techniques for detecting and characterizing gastrointestinal neoplasia. Gastrointest Endosc Clin N Am. 2008;18:415-433, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 7. | Osawa H, Yamamoto H, Miura Y, Yoshizawa M, Sunada K, Satoh K, Sugano K. Diagnosis of extent of early gastric cancer using flexible spectral imaging color enhancement. World J Gastrointest Endosc. 2012;4:356-361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Kimura-Tsuchiya R, Dohi O, Fujita Y, Yagi N, Majima A, Horii Y, Kitaichi T, Onozawa Y, Suzuki K, Tomie A, Okayama T, Yoshida N, Kamada K, Katada K, Uchiyama K, Ishikawa T, Takagi T, Handa O, Konishi H, Kishimoto M, Naito Y, Yanagisawa A, Itoh Y. Magnifying Endoscopy with Blue Laser Imaging Improves the Microstructure Visualization in Early Gastric Cancer: Comparison of Magnifying Endoscopy with Narrow-Band Imaging. Gastroenterol Res Pract. 2017;2017:8303046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Wagner A, Zandanell S, Kiesslich T, Neureiter D, Klieser E, Holzinger J, Berr F. Systematic Review on Optical Diagnosis of Early Gastrointestinal Neoplasia. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Esteva A, Kuprel B, Novoa RA, Ko J, Swetter SM, Blau HM, Thrun S. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5683] [Cited by in RCA: 5355] [Article Influence: 669.4] [Reference Citation Analysis (0)] |
| 11. | Ehteshami Bejnordi B, Veta M, Johannes van Diest P, van Ginneken B, Karssemeijer N, Litjens G, van der Laak JAWM; the CAMELYON16 Consortium, Hermsen M, Manson QF, Balkenhol M, Geessink O, Stathonikos N, van Dijk MC, Bult P, Beca F, Beck AH, Wang D, Khosla A, Gargeya R, Irshad H, Zhong A, Dou Q, Li Q, Chen H, Lin HJ, Heng PA, Haß C, Bruni E, Wong Q, Halici U, Öner MÜ, Cetin-Atalay R, Berseth M, Khvatkov V, Vylegzhanin A, Kraus O, Shaban M, Rajpoot N, Awan R, Sirinukunwattana K, Qaiser T, Tsang YW, Tellez D, Annuscheit J, Hufnagl P, Valkonen M, Kartasalo K, Latonen L, Ruusuvuori P, Liimatainen K, Albarqouni S, Mungal B, George A, Demirci S, Navab N, Watanabe S, Seno S, Takenaka Y, Matsuda H, Ahmady Phoulady H, Kovalev V, Kalinovsky A, Liauchuk V, Bueno G, Fernandez-Carrobles MM, Serrano I, Deniz O, Racoceanu D, Venâncio R. Diagnostic Assessment of Deep Learning Algorithms for Detection of Lymph Node Metastases in Women With Breast Cancer. JAMA. 2017;318:2199-2210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1871] [Cited by in RCA: 1556] [Article Influence: 194.5] [Reference Citation Analysis (0)] |
| 12. | Bi WL, Hosny A, Schabath MB, Giger ML, Birkbak NJ, Mehrtash A, Allison T, Arnaout O, Abbosh C, Dunn IF, Mak RH, Tamimi RM, Tempany CM, Swanton C, Hoffmann U, Schwartz LH, Gillies RJ, Huang RY, Aerts HJWL. Artificial intelligence in cancer imaging: Clinical challenges and applications. CA Cancer J Clin. 2019;69:127-157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 848] [Cited by in RCA: 818] [Article Influence: 136.3] [Reference Citation Analysis (3)] |
| 13. | Szegedy C, Liu W, Jia Y, Sermanent P, Reed SE, Anguelov D, Erhan D, Vanhoucke V, Rabinovich A. Going deeper with convolutions. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition 2015; 1-x9. |
| 14. | Hirasawa T, Aoyama K, Tanimoto T, Ishihara S, Shichijo S, Ozawa T, Ohnishi T, Fujishiro M, Matsuo K, Fujisaki J, Tada T. Application of artificial intelligence using a convolutional neural network for detecting gastric cancer in endoscopic images. Gastric Cancer. 2018;21:653-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 568] [Cited by in RCA: 426] [Article Influence: 60.9] [Reference Citation Analysis (0)] |
| 15. | Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016;388:2654-2664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1282] [Cited by in RCA: 1468] [Article Influence: 163.1] [Reference Citation Analysis (0)] |
| 16. | Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev. 2014;23:700-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1159] [Cited by in RCA: 1327] [Article Influence: 120.6] [Reference Citation Analysis (0)] |
| 17. | Kono Y, Kanzaki H, Iwamuro M, Kawano S, Kawahara Y, Okada H. Reality of Gastric Cancer in Young Patients: The Importance and Difficulty of the Early Diagnosis, Prevention and Treatment. Acta Med Okayama. 2020;74:461-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 18. | Draganov PV, Wang AY, Othman MO, Fukami N. AGA Institute Clinical Practice Update: Endoscopic Submucosal Dissection in the United States. Clin Gastroenterol Hepatol. 2019;17:16-25.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 319] [Article Influence: 53.2] [Reference Citation Analysis (0)] |
| 19. | Ono H, Yao K, Fujishiro M, Oda I, Uedo N, Nimura S, Yahagi N, Iishi H, Oka M, Ajioka Y, Fujimoto K. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer (second edition). Dig Endosc. 2021;33:4-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 308] [Article Influence: 77.0] [Reference Citation Analysis (0)] |
| 20. | Yamazato T, Oyama T, Yoshida T, Baba Y, Yamanouchi K, Ishii Y, Inoue F, Toda S, Mannen K, Shimoda R, Iwakiri R, Fujimoto K. Two years' intensive training in endoscopic diagnosis facilitates detection of early gastric cancer. Intern Med. 2012;51:1461-1465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Barbour JA, O'Toole P, Suzuki N, Dolwani S. Learning endoscopic submucosal dissection in the UK: Barriers, solutions and pathways for training. Frontline Gastroenterol. 2021;12:671-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Menon S, Trudgill N. How commonly is upper gastrointestinal cancer missed at endoscopy? Endosc Int Open. 2014;2:E46-E50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 230] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 23. | Hosokawa O, Hattori M, Douden K, Hayashi H, Ohta K, Kaizaki Y. Difference in accuracy between gastroscopy and colonoscopy for detection of cancer. Hepatogastroenterology. 2007;54:442-444. [PubMed] |
| 24. | Raftopoulos SC, Segarajasingam DS, Burke V, Ee HC, Yusoff IF. A cohort study of missed and new cancers after esophagogastroduodenoscopy. Am J Gastroenterol. 2010;105:1292-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 25. | Yoshimizu S, Hirasawa T, Horiuchi Y, Omae M, Ishiyama A, Yoshio T, Tsuchida T, Fujisaki J. Differences in upper gastrointestinal neoplasm detection rates based on inspection time and esophagogastroduodenoscopy training. Endosc Int Open. 2018;6:E1190-E1197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Yao K, Uedo N, Kamada T, Hirasawa T, Nagahama T, Yoshinaga S, Oka M, Inoue K, Mabe K, Yao T, Yoshida M, Miyashiro I, Fujimoto K, Tajiri H. Guidelines for endoscopic diagnosis of early gastric cancer. Dig Endosc. 2020;32:663-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 132] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 27. | Correa P, Piazuelo MB. The gastric precancerous cascade. J Dig Dis. 2012;13:2-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 521] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 28. | Kodama M, Murakami K, Okimoto T, Abe H, Sato R, Ogawa R, Mizukami K, Shiota S, Nakagawa Y, Soma W, Arita T, Fujioka T. Histological characteristics of gastric mucosa prior to Helicobacter pylori eradication may predict gastric cancer. Scand J Gastroenterol. 2013;48:1249-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 29. | Lee KJ, Inoue M, Otani T, Iwasaki M, Sasazuki S, Tsugane S; JPHC Study Group. Gastric cancer screening and subsequent risk of gastric cancer: a large-scale population-based cohort study, with a 13-year follow-up in Japan. Int J Cancer. 2006;118:2315-2321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 148] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 30. | Park SY, Jeon SW, Jung MK, Cho CM, Tak WY, Kweon YO, Kim SK, Choi YH. Long-term follow-up study of gastric intraepithelial neoplasias: progression from low-grade dysplasia to invasive carcinoma. Eur J Gastroenterol Hepatol. 2008;20:966-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 31. | de Vries AC, van Grieken NC, Looman CW, Casparie MK, de Vries E, Meijer GA, Kuipers EJ. Gastric cancer risk in patients with premalignant gastric lesions: a nationwide cohort study in the Netherlands. Gastroenterology. 2008;134:945-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 585] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 32. | Ezoe Y, Muto M, Uedo N, Doyama H, Yao K, Oda I, Kaneko K, Kawahara Y, Yokoi C, Sugiura Y, Ishikawa H, Takeuchi Y, Kaneko Y, Saito Y. Magnifying narrowband imaging is more accurate than conventional white-light imaging in diagnosis of gastric mucosal cancer. Gastroenterology. 2011;141:2017-2025.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 283] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 33. | Zhou F, Wu L, Huang M, Jin Q, Qin Y, Chen J. The accuracy of magnifying narrow band imaging (ME-NBI) in distinguishing between cancerous and noncancerous gastric lesions: A meta-analysis. Medicine (Baltimore). 2018;97:e9780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 34. | Dohi O, Yagi N, Yoshida S, Ono S, Sanomura Y, Tanaka S, Naito Y, Kato M. Magnifying Blue Laser Imaging vs Magnifying Narrow-Band Imaging for the Diagnosis of Early Gastric Cancer: A Prospective, Multicenter, Comparative Study. Digestion. 2017;96:127-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 35. | Fujiyoshi MRA, Inoue H, Fujiyoshi Y, Nishikawa Y, Toshimori A, Shimamura Y, Tanabe M, Ikeda H, Onimaru M. Endoscopic Classifications of Early Gastric Cancer: A Literature Review. Cancers (Basel). 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 36. | Yu H, Yang AM, Lu XH, Zhou WX, Yao F, Fei GJ, Guo T, Yao LQ, He LP, Wang BM. Magnifying narrow-band imaging endoscopy is superior in diagnosis of early gastric cancer. World J Gastroenterol. 2015;21:9156-9162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (1)] |
| 37. | Yao K. Clinical Application of Magnifying Endoscopy with Narrow-Band Imaging in the Stomach. Clin Endosc. 2015;48:481-490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 38. | Ang TL, Fock KM, Teo EK, Tan J, Poh CH, Ong J, Ang D. The diagnostic utility of narrow band imaging magnifying endoscopy in clinical practice in a population with intermediate gastric cancer risk. Eur J Gastroenterol Hepatol. 2012;24:362-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 39. | Ang TL, Pittayanon R, Lau JY, Rerknimitr R, Ho SH, Singh R, Kwek AB, Ang DS, Chiu PW, Luk S, Goh KL, Ong JP, Tan JY, Teo EK, Fock KM. A multicenter randomized comparison between high-definition white light endoscopy and narrow band imaging for detection of gastric lesions. Eur J Gastroenterol Hepatol. 2015;27:1473-1478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 40. | Yao K, Uedo N, Muto M, Ishikawa H, Cardona HJ, Filho ECC, Pittayanon R, Olano C, Yao F, Parra-Blanco A, Ho SH, Avendano AG, Piscoya A, Fedorov E, Bialek AP, Mitrakov A, Caro L, Gonen C, Dolwani S, Farca A, Cuaresma LF, Bonilla JJ, Kasetsermwiriya W, Ragunath K, Kim SE, Marini M, Li H, Cimmino DG, Piskorz MM, Iacopini F, So JB, Yamazaki K, Kim GH, Ang TL, Milhomem-Cardoso DM, Waldbaum CA, Carvajal WAP, Hayward CM, Singh R, Banerjee R, Anagnostopoulos GK, Takahashi Y. Development of an E-learning System for the Endoscopic Diagnosis of Early Gastric Cancer: An International Multicenter Randomized Controlled Trial. EBioMedicine. 2016;9:140-147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 41. | Shibagaki K, Ishimura N, Yuki T, Taniguchi H, Aimi M, Kobayashi K, Kotani S, Yazaki T, Yamashita N, Tamagawa Y, Mishiro T, Ishihara S, Yasuda A, Kinshita Y. Magnification endoscopy in combination with acetic acid enhancement and narrow-band imaging for the accurate diagnosis of colonic neoplasms. Endosc Int Open. 2020;8:E488-E497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 42. | Russel S, Norvig P. Artificial Intelligence: A Modern Approach. 2th ed. Pearson Education, 2003. |
| 43. | Litjens G, Kooi T, Bejnordi BE, Setio AAA, Ciompi F, Ghafoorian M, van der Laak JAWM, van Ginneken B, Sánchez CI. A survey on deep learning in medical image analysis. Med Image Anal. 2017;42:60-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5573] [Cited by in RCA: 4932] [Article Influence: 616.5] [Reference Citation Analysis (0)] |
| 44. | Yeung S, Downing NL, Fei-Fei L, Milstein A. Bedside Computer Vision - Moving Artificial Intelligence from Driver Assistance to Patient Safety. N Engl J Med. 2018;378:1271-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 45. | Nagao S, Tsuji Y, Sakaguchi Y, Takahashi Y, Minatsuki C, Niimi K, Yamashita H, Yamamichi N, Seto Y, Tada T, Koike K. Highly accurate artificial intelligence systems to predict the invasion depth of gastric cancer: efficacy of conventional white-light imaging, nonmagnifying narrow-band imaging, and indigo-carmine dye contrast imaging. Gastrointest Endosc. 2020;92:866-873.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 46. | Ikenoyama Y, Hirasawa T, Ishioka M, Namikawa K, Yoshimizu S, Horiuchi Y, Ishiyama A, Yoshio T, Tsuchida T, Takeuchi Y, Shichijo S, Katayama N, Fujisaki J, Tada T. Detecting early gastric cancer: Comparison between the diagnostic ability of convolutional neural networks and endoscopists. Dig Endosc. 2021;33:141-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 103] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 47. | Nam JY, Chung HJ, Choi KS, Lee H, Kim TJ, Soh H, Kang EA, Cho SJ, Ye JC, Im JP, Kim SG, Kim JS, Chung H, Lee JH. Deep learning model for diagnosing gastric mucosal lesions using endoscopic images: development, validation, and method comparison. Gastrointest Endosc. 2022;95:258-268.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 48. | Ling T, Wu L, Fu Y, Xu Q, An P, Zhang J, Hu S, Chen Y, He X, Wang J, Chen X, Zhou J, Xu Y, Zou X, Yu H. A deep learning-based system for identifying differentiation status and delineating the margins of early gastric cancer in magnifying narrow-band imaging endoscopy. Endoscopy. 2021;53:469-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 49. | Wu L, Wang J, He X, Zhu Y, Jiang X, Chen Y, Wang Y, Huang L, Shang R, Dong Z, Chen B, Tao X, Wu Q, Yu H. Deep learning system compared with expert endoscopists in predicting early gastric cancer and its invasion depth and differentiation status (with videos). Gastrointest Endosc. 2022;95:92-104.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 42] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 50. | Cho BJ, Bang CS, Park SW, Yang YJ, Seo SI, Lim H, Shin WG, Hong JT, Yoo YT, Hong SH, Choi JH, Lee JJ, Baik GH. Automated classification of gastric neoplasms in endoscopic images using a convolutional neural network. Endoscopy. 2019;51:1121-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 96] [Article Influence: 16.0] [Reference Citation Analysis (1)] |
| 51. | Wu L, Xu M, Jiang X, He X, Zhang H, Ai Y, Tong Q, Lv P, Lu B, Guo M, Huang M, Ye L, Shen L, Yu H. Real-time artificial intelligence for detecting focal lesions and diagnosing neoplasms of the stomach by white-light endoscopy (with videos). Gastrointest Endosc. 2022;95:269-280.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 42] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 52. | Wu L, He X, Liu M, Xie H, An P, Zhang J, Zhang H, Ai Y, Tong Q, Guo M, Huang M, Ge C, Yang Z, Yuan J, Liu J, Zhou W, Jiang X, Huang X, Mu G, Wan X, Li Y, Wang H, Wang Y, Chen D, Gong D, Wang J, Huang L, Li J, Yao L, Zhu Y, Yu H. Evaluation of the effects of an artificial intelligence system on endoscopy quality and preliminary testing of its performance in detecting early gastric cancer: a randomized controlled trial. Endoscopy. 2021;53:1199-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 100] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 53. | Kubota K, Kuroda J, Yoshida M, Ohta K, Kitajima M. Medical image analysis: computer-aided diagnosis of gastric cancer invasion on endoscopic images. Surg Endosc. 2012;26:1485-1489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
| 54. | Zhu Y, Wang QC, Xu MD, Zhang Z, Cheng J, Zhong YS, Zhang YQ, Chen WF, Yao LQ, Zhou PH, Li QL. Application of convolutional neural network in the diagnosis of the invasion depth of gastric cancer based on conventional endoscopy. Gastrointest Endosc. 2019;89:806-815.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 231] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 55. | Ishioka M, Hirasawa T, Tada T. Detecting gastric cancer from video images using convolutional neural networks. Dig Endosc. 2019;31:e34-e35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 56. | Yoon HJ, Kim S, Kim JH, Keum JS, Oh SI, Jo J, Chun J, Youn YH, Park H, Kwon IG, Choi SH, Noh SH. A Lesion-Based Convolutional Neural Network Improves Endoscopic Detection and Depth Prediction of Early Gastric Cancer. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 91] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 57. | Tang D, Wang L, Ling T, Lv Y, Ni M, Zhan Q, Fu Y, Zhuang D, Guo H, Dou X, Zhang W, Xu G, Zou X. Development and validation of a real-time artificial intelligence-assisted system for detecting early gastric cancer: A multicentre retrospective diagnostic study. EBioMedicine. 2020;62:103146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 58. | Namikawa K, Hirasawa T, Nakano K, Ikenoyama Y, Ishioka M, Shiroma S, Tokai Y, Yoshimizu S, Horiuchi Y, Ishiyama A, Yoshio T, Tsuchida T, Fujisaki J, Tada T. Artificial intelligence-based diagnostic system classifying gastric cancers and ulcers: comparison between the original and newly developed systems. Endoscopy. 2020;52:1077-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 59. | Ueyama H, Kato Y, Akazawa Y, Yatagai N, Komori H, Takeda T, Matsumoto K, Ueda K, Hojo M, Yao T, Nagahara A, Tada T. Application of artificial intelligence using a convolutional neural network for diagnosis of early gastric cancer based on magnifying endoscopy with narrow-band imaging. J Gastroenterol Hepatol. 2021;36:482-489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 89] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 60. | Oura H, Matsumura T, Fujie M, Ishikawa T, Nagashima A, Shiratori W, Tokunaga M, Kaneko T, Imai Y, Oike T, Yokoyama Y, Akizue N, Ota Y, Okimoto K, Arai M, Nakagawa Y, Inada M, Yamaguchi K, Kato J, Kato N. Development and evaluation of a double-check support system using artificial intelligence in endoscopic screening for gastric cancer. Gastric Cancer. 2022;25:392-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 61. | Zhang L, Zhang Y, Wang L, Wang J, Liu Y. Diagnosis of gastric lesions through a deep convolutional neural network. Dig Endosc. 2021;33:788-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 62. | An P, Yang D, Wang J, Wu L, Zhou J, Zeng Z, Huang X, Xiao Y, Hu S, Chen Y, Yao F, Guo M, Wu Q, Yang Y, Yu H. A deep learning method for delineating early gastric cancer resection margin under chromoendoscopy and white light endoscopy. Gastric Cancer. 2020;23:884-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 63. | Miyaki R, Yoshida S, Tanaka S, Kominami Y, Sanomura Y, Matsuo T, Oka S, Raytchev B, Tamaki T, Koide T, Kaneda K, Yoshihara M, Chayama K. Quantitative identification of mucosal gastric cancer under magnifying endoscopy with flexible spectral imaging color enhancement. J Gastroenterol Hepatol. 2013;28:841-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 64. | Miyaki R, Yoshida S, Tanaka S, Kominami Y, Sanomura Y, Matsuo T, Oka S, Raytchev B, Tamaki T, Koide T, Kaneda K, Yoshihara M, Chayama K. A computer system to be used with laser-based endoscopy for quantitative diagnosis of early gastric cancer. J Clin Gastroenterol. 2015;49:108-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 65. | Kanesaka T, Lee TC, Uedo N, Lin KP, Chen HZ, Lee JY, Wang HP, Chang HT. Computer-aided diagnosis for identifying and delineating early gastric cancers in magnifying narrow-band imaging. Gastrointest Endosc. 2018;87:1339-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 129] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 66. | Li L, Chen Y, Shen Z, Zhang X, Sang J, Ding Y, Yang X, Li J, Chen M, Jin C, Chen C, Yu C. Convolutional neural network for the diagnosis of early gastric cancer based on magnifying narrow band imaging. Gastric Cancer. 2020;23:126-132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 137] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 67. | Horiuchi Y, Aoyama K, Tokai Y, Hirasawa T, Yoshimizu S, Ishiyama A, Yoshio T, Tsuchida T, Fujisaki J, Tada T. Convolutional Neural Network for Differentiating Gastric Cancer from Gastritis Using Magnified Endoscopy with Narrow Band Imaging. Dig Dis Sci. 2020;65:1355-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 94] [Article Influence: 18.8] [Reference Citation Analysis (1)] |
| 68. | Hu H, Gong L, Dong D, Zhu L, Wang M, He J, Shu L, Cai Y, Cai S, Su W, Zhong Y, Li C, Zhu Y, Fang M, Zhong L, Yang X, Zhou P, Tian J. Identifying early gastric cancer under magnifying narrow-band images with deep learning: a multicenter study. Gastrointest Endosc. 2021;93:1333-1341.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 69. | Hamada K, Kawahara Y, Tanimoto T, Ohto A, Toda A, Aida T, Yamasaki Y, Gotoda T, Ogawa T, Abe M, Okanoue S, Takei K, Kikuchi S, Kuroda S, Fujiwara T, Okada H. Application of convolutional neural networks for evaluating the depth of invasion of early gastric cancer based on endoscopic images. J Gastroenterol Hepatol. 2022;37:352-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 70. | Panarese A, Galatola G, Armentano R, Pimentel-Nunes P, Ierardi E, Caruso ML, Pesce F, Lenti MV, Palmitessa V, Coletta S, Shahini E. Helicobacter pylori-induced inflammation masks the underlying presence of low-grade dysplasia on gastric lesions. World J Gastroenterol. 2020;26:3834-3850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 71. | Shichijo S, Nomura S, Aoyama K, Nishikawa Y, Miura M, Shinagawa T, Takiyama H, Tanimoto T, Ishihara S, Matsuo K, Tada T. Application of Convolutional Neural Networks in the Diagnosis of Helicobacter pylori Infection Based on Endoscopic Images. EBioMedicine. 2017;25:106-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 235] [Cited by in RCA: 181] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 72. | Itoh T, Kawahira H, Nakashima H, Yata N. Deep learning analyzes Helicobacter pylori infection by upper gastrointestinal endoscopy images. Endosc Int Open. 2018;6:E139-E144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 130] [Article Influence: 18.6] [Reference Citation Analysis (0)] |