Published online Oct 28, 2021. doi: 10.35713/aic.v2.i5.51
Peer-review started: October 12, 2021
First decision: October 20, 2021
Revised: October 22, 2021
Accepted: October 27, 2021
Article in press: October 27, 2021
Published online: October 28, 2021
Processing time: 15 Days and 10.2 Hours
Acute kidney injury (AKI) has serious consequences on the prognosis of patients undergoing liver transplantation (LT) for liver cancer and cirrhosis. Artificial neural network (ANN) has recently been proposed as a useful tool in many fields in the setting of solid organ transplantation and surgical oncology, where patient prognosis depends on a multidimensional and nonlinear relationship between variables pertaining to the surgical procedure, the donor (graft characteristics), and the recipient comorbidities. In the specific case of LT, ANN models have been developed mainly to predict survival in patients with cirrhosis, to assess the best donor-to-recipient match during allocation processes, and to foresee postoperative complications and outcomes. This is a specific opinion review on the role of ANN in the prediction of AKI after LT for liver cancer and cirrhosis, highlighting potential strengths of the method to forecast this serious postoperative complication.
Core Tip: This opinion review aims to explore the potential benefits of artificial neural network models in predicting the occurrence of acute kidney injury in the postoperative period of liver transplantation for cirrhosis and hepatocellular carcinoma.
- Citation: Bredt LC, Peres LAB. Artificial neural network for prediction of acute kidney injury after liver transplantation for cirrhosis and hepatocellular carcinoma. Artif Intell Cancer 2021; 2(5): 51-59
- URL: https://www.wjgnet.com/2644-3228/full/v2/i5/51.htm
- DOI: https://dx.doi.org/10.35713/aic.v2.i5.51
Liver transplantation (LT) is the best treatment option for patients with early stages of hepatocellular carcinoma (HCC) and cirrhosis[1-4]. Mainly, the use of LT depends on maintaining a balance between patient-specific survival benefit, the availability of alternative treatment modalities[5,6], and the equitable distribution of donor organs[5,7-12]. Current selection criteria aim to avoid transplant futility by excluding patients at a high risk of tumor recurrence[10,11]. Selecting patients with HCC within Milan criteria has been shown to provide excellent patient outcomes[13-15].
Among the possible complications related to LT for cirrhosis and HCC, acute kidney injury (AKI) is a common complication, with extremely variable reported incidence rates (4% to 94%)[16-22], and is associated with several immediate complications, including volume overload, metabolic acidosis and electrolyte disturbances. Although most patients eventually recover after an episode of AKI, many patients may not return to baseline renal function, and the occurrence of AKI has been shown to be an independent risk factor for the development of chronic kidney disease and death, as well as for the reduction of survival rates of liver receptors[23]. In addition, transplant patients who require temporary renal replacement therapy (RRT) have a prolonged hospital stay, with subsequent need for more resources and higher costs related to LT[24].
Artificial neural network (ANN) is commonly used to solve complex problems, where the behavior of variables is not rigorously known. One of its main characteristics is the ability to learn through examples and generalize the information learned, generating a non-linear model, making its application in spatial analysis very efficient[25]. ANN can be an alternative with high performance to the logistic regression (LR) model, where the relative risk term is parameterized by an ANN instead of regression, enabling the application of deep learning. ANN models have been developed mainly to predict survival in patients with cirrhosis, to assess the best donor-to-recipient match during allocation processes, and to foresee postoperative complications and outcomes[26-32], but studies evaluating such a promising tool, as ANN, for predicting AKI following LT for cirrhosis and HCC, are scarce.
The multifactorial origin of AKI after LT makes it complex to predict which candidate for the procedure has an increased risk of this complication[33,34]. In the face of this complexity, ANN would be a very reliable prognostic tool for AKI risk assessment, enabling, therefore, early or even prophylactic therapies for AKI, improving patients outcomes[35]. This is a specific opinion review on the role of ANN in the prediction of AKI after LT for liver cancer and cirrhosis, highlighting potential strengths of the method to forecast this serious postoperative complication.
The etiology of AKI after LT is multifactorial and not fully understood, with several risk factors related to the organ receptor[20,22,24,35], graft-related characteristics[36], and finally some perioperative have been identified over the past few years[20,33,34]. Similarly, the use of postoperative nephrotoxic immunosuppression can further provoke or aggravate kidney damage[20].
Based on these risk factors, various models have been developed using LR for predicting AKI after LT. However, because several of these models address postoperative parameters, their utility in predictive modeling appears to be of questionable relevance. Regardless of the variability of the triggering factors, it is of fundamental importance to identify patients at risk ideally by the set of preoperative clinical assessment and complementary information of the intraoperative period, thus enabling the adoption of preventive measures or early therapies for AKI, such as reduced doses and postponing postoperative patients immunosuppression, and also early RRT, thus reducing mortality and accelerated recovery of renal function[20].
Among the potential AKI predictors that can be evaluated at the time of transplant indication, the severity of the recipient’s liver disease stands out[20-37], expressed by the Model for End-Stage Liver Disease (MELD) score. The MELD score determines the allocation of the organ prioritizing the "sickest first" patient, with high values of the score conferring a greater risk for the occurrence of ARF after TH, thus reflecting an interrelationship between liver and renal functions in cirrhotic patients[38]. Similarly, another predictors related to the recipient have been identified, such as high levels of pre-transplant serum creatinine, high body mass index (BMI) of the recipient (BMI values above 30 kg/m2), and the presence of pre-existing diabetes mellitus[33,35,37].
In addition to the clinical characteristics of the recipient, there are predictive factors of AKI that are related to the functional quality of the graft. The first situation refers to the modality of TH performed, as living-donor LT, in general, offers a graft that is functionally superior to deceased-donor LT, where the critical clinical conditions of the donor confer a greater potential risk to the occurrence of postoperative AKI[20]. Moreover, "marginal grafts" from "extended criteria donors" have increasingly been used, including steatotic grafts, grafts from clinically critical donors, grafts with high ischemia time, both “warm ischemia time” and “cold ischemia time”[20,37,39].
There are some intraoperative events that can be crucial for the occurrence of AKI. The main factor concerns the occurrence of intraoperative arterial hypotension (IOAH) with consequent renal hypoperfusion during LT[22]. Patients undergoing LT often experience IOAH as a result of several factors, including the duration of surgery, the severity of bleeding, the severity of post-reperfusion syndrome of the graft, and the severity of liver disease[33,35,39]. On some occasions, this renal hypoperfusion occurs in patients with previous renal dysfunction[34], and can often be aggravated by the deleterious renal effects of blood transfusion[22,34,37] and the use of vasoactive drugs in the intraoperative period[40].
An ANN lies under the umbrella of reinforcement machine learning, and comprises ‘units’ arranged in a series of layers, each of which connects to layers on either side. ANNs are inspired by biological systems, such as the brain, and how they process information. The original concept of ANNs is derived from neurobiological models. ANNs are massively parallel, computer-intensive and data-driven algorithmic system that is composed of multitude of highly interconnected nodes (neurons). Each elementary node of a neural network is able to receive an input from external sources, according to the relative importance and different weight, which transforms into an output signal to other nodes by different activation function[25].
In terms of topology, to implement an ANN, different variables must be defined, among which: (1) the number of nodes in the input layer (such variable corresponds to the number of variables that will be used to feed the neural network, being normally the variables of greater importance for the problem under study); (2) the number of hidden layers and the number of neurons to be placed in these layers; and (3) the number of neurons in the output layer[41].
The process of learning of an ANN is a process where free parameters are adapted through a process of stimulation by the environment in which the network is inserted. With this, the type of learning is determined based on the way in which the modification of the parameters takes place. In summary, there is the following sequence of events: (1) the neural network is stimulated by an environment; (2) the neural network undergoes modifications in its free parameters as a result of this stimulation; and (3) the neural network responds in a new way to the environment, due to changes in its internal structure[25].
Considering the interactions of linked nodes, an output obtained from one node can serve as an input for other nodes, and the conversion of inputs into outputs is activated by virtue of certain transforming function that is typically monotone. The specified working function depends on parameters determined for the training set of inputs and outputs. The network architecture is the organization of nodes and the types of connections permitted. The nodes are arranged in a series of layers with connections between nodes in different layers, but not between nodes in the same layer[42].
ANNs can be classified into feedforward and feedback networks categories, and back-propagation updating algorithm with adjustment of connection weights between the neurons during the training process, is a widely used feedforward networks. Feedforward networks is included within the supervised learning network, essentially using a gradient descent-training algorithm[43,44].
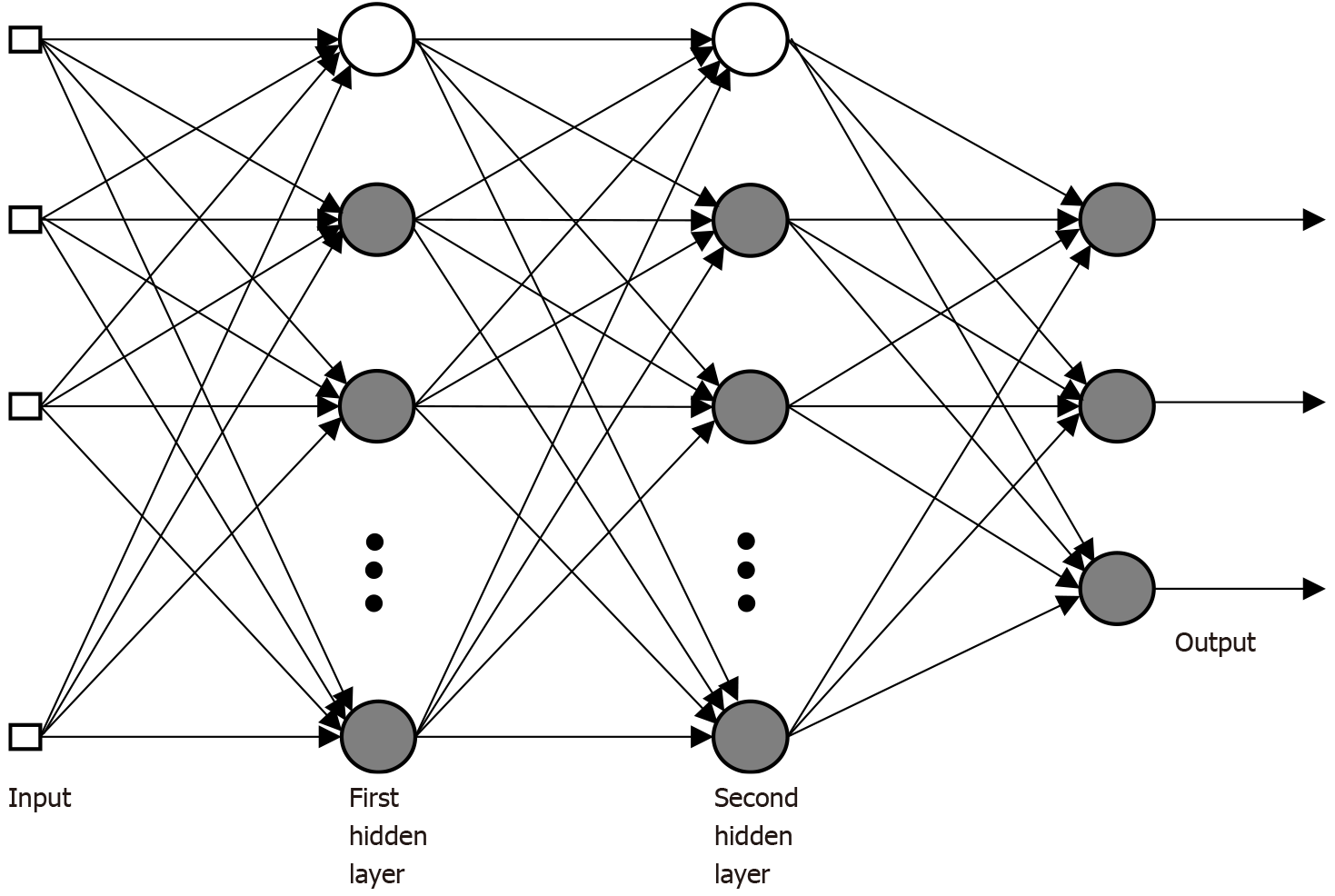
The perceptron, introduced by Rosenblatt in 1958, is a simple form of RNA whose main application is in pattern classification problems. The single-layer perceptron is only capable of classifying linearly separable patterns. In practice, the problem to be worked on does not admit an exact linear separation, making it necessary to use a multilayer perceptron. Multilayer perceptron (MLP)-type architectures are the most used and known artificial neural models. An MLP network is subdivided into layers: input layer, intermediate or hidden layer(s) and output layer. In the multilayer ANN architecture, inputs are extended from the input layer to the output layer, passing through one or more hidden layers. In this same sense, a multilayer neural network is typically composed of aligned layers of neurons. The input layer distributes the input information to the hidden layer(s) of the network. At the output layer, the solution to the problem is obtained. Hidden layers are intermediate layers, whose function is to separate the input and output layers. Neurons in one layer are connected only to neurons in the immediately posterior layer, with no feedback or connections between neurons in the same layer. Also, characteristically, the layers are fully connected[45].
In Figure 1 it is possible to observe an MLP-type architecture with two intermediate layers. The presented network has all connections, which means that a neuron in any layer of the network is connected to all other neurons in the previous layer. Signals flow through the network positively, from left to right, layer by layer.
The learning process of MLP networks by back-propagation consists of two steps: propagation and back-propagation. In the propagation step, an activation pattern is applied to the nodes of the network’s input layer and its effect propagates through the network, layer by layer. In the last layer, a set of outputs is produced, configured as the real network response. In the and back-propagation step, all synaptic weights are adjusted according to an error correction rule. The error signal is propagated backwards through the network, against the direction of the synaptic connections, the synaptic weights being adjusted to make the actual response of the network approach the desired response, in a statistical sense[25]. An important characteristic of MLP networks is the non-linearity of neuron outputs. This nonlinearity is obtained using a sigmoid-type function as an activation function, usually the logistic function[25].
Over the past two decades, machine learning algorithms have been increasingly applied for cancer diagnosis, prognostication, and treatment outcome prediction[46-49]. For example, recently, an MLA approach based on a random forest workflow has been developed by a group in Germany to predict disease-free survival after liver resection for HCC[50].
Studies regarding ANNs in the field of LT for cirrhosis and HCC, researchers[26-31] have already conducted studies with LR models and ANN for the prediction of survival of these patients (Table 1). In 1992, Doyle et al[26] introduced a 10 feed forward back-propagation ANN model to predict LT survival. Marsh et al[27] presented a three layer feed forward fully connected ANN model to predict the survival analysis and time to recurrence of HCC after LT. Parmanto et al[28] conducted a study with time series sequence of medical data of patients that undergone LT with ANNs using back-propagation through time algorithm, and their results were compared with 6-fold cross validation. Cucchetti et al[29] proposed an ANN survival prognosis model for patients with cirrhosis at a LT unit, and proved that ANN is better than MELD for this proposal. Zhang et al[30] proposed a MLP model of patients with cirrhosis and compared the performance of the model with MELD and Sequential Organ Failure Assessment score. In 2013, Cruz et al[31] conducted a study with radial basis function ANNs using multi-objective evolutionary algorithm in order to match the donor-recipient pairs.
| Ref. | Year | Model and endpoint |
| Doyle et al[26] | 1992 | 10 feed forward back-propagation ANN model to predict LT survival |
| Marsh et al[27] | 1997 | ANN for survival analysis and time to recurrence of HCC after LT |
| Parmanto et al[28] | 2001 | Back-propagation through time ANN algorithm to predict outcomes after LT |
| Cucchetti et al[29] | 2007 | ANN for survival prognosis of patients with cirrhosis |
| Zhang et al[30] | 2012 | MLP model for predicting outcomes of patients with cirrhosis and compared the performance with MELD and SOFA scores |
| Cruz et al[31] | 2013 | Radial basis function ANNs using multi-objective evolutionary algorithm to match the donor-recipient pairs |
| Lee et al[52] | 2018 | Compared the performance of ML approaches (decision tree, random forest, gradient boosting machine, support vector machine, naïve Bayes, MLP, and deep belief networks) with that of LR analysis to predict AKI after LT for cirrhosis and HCC (49%) |
| He et al[53] | 2021 | LR analysis as a conventional model, and random forest, support vector machine, classical decision tree, and conditional inference tree algorithms to predict AKI after LT for cirrhosis and HCC (40.7%) |
The results of the researchers above demonstrate that the ANNs predictive models can be capable of using live data of cirrhotic patients with or without HCC, and perform both diagnostic and predictive tasks[32]. Because of the simplicity in structure, ability to do parallel processing tasks, having long term memory, having fault tolerant ability and getting collective output, ANN models can do better than LR models[51].
In the specific scenario of AKI after LT for cirrhosis and HCC, in 2018, Lee et al[52] compared the performance of machine learning approaches with that of LR analysis to predict AKI after LT for cirrhosis and up to 49% of total patients with HCC. This huge analysis of 1211 patients adopted preoperative and intraoperative input variables. The primary outcome was postoperative AKI defined by Acute Kidney Injury Network criteria. The following machine learning techniques were used: decision tree, random forest, gradient boosting machine, support vector machine, naïve Bayes, MLP, and deep belief networks. These techniques were compared with LR analysis regarding the area under the receiver operating characteristic (AUROC). AKI incidence was 30.1%. The performance in terms of AUROC was best in gradient boosting machine among all analyses to predict AKI of all stages (0.90, 95%CI: 0.86–0.93), and decision tree and random forest techniques showed moderate performance (AUROC 0.86 and 0.85, respectively). The AUROC of the MLP was 0.64 (0.59–0.69), vector machine was 0.62 (0.57–0.67), naïve Bayes was 0.60 (0.54–0.65), and deep belief network was 0.59 (0.53–0.64). The AUROC of LR analysis was 0.61 (95%CI: 0.56–0.66), concluding that MLP model showed best performance than LR analysis, with a slight higher, but significant, AUROC.
He et al[53] evaluated a total of 493 patients (40.7% of patients with HCC) with donation after cardiac death LT. In this study, AKI was defined according to the clinical practice guidelines of Kidney Disease Improving Global Outcomes, and the clinical data of patients with AKI and without AKI were compared through LR analysis as a conventional model, and four predictive machine learning models were developed using random forest, support vector machine, classical decision tree, and conditional inference tree algorithms. The predictive power of these models was then evaluated using the AUROC. The reported incidence of AKI was 35.7% (176/493) during the follow-up period. Compared with the non-AKI group, the AKI group showed a remarkably lower survival rate (P < 0.001). The random forest model demonstrated the highest prediction accuracy of 0.79 with AUROC of 0.850 (95%CI: 0.794–0.905), which was significantly higher than the AUCs of the other machine learning algorithms and LR models (P < 0.001).
As the standard ANN workflow involves model performance monitoring and re-training to account for model drift, a multidisciplinary partnership between clinicians and data scientists is required, with a commitment to the curation and iterative maintenance of datasets to allow for the development of meaningful decision-support tools[54]. This process should involve, first and foremost, a robust, consistent, and objective means of collecting data. The data in the case of postoperative AKI, are mainly laboratorial and clinicopathologic characteristics from electronic medical records, and clinicians and surgeons must to establish interdisciplinary partnerships that strive towards a common goal and synergism. For instance, clinicians and surgeons help provide a clinically relevant outcome, and data scientists can identify the optimal methodology to make predictions for the outcome based on the available data.
The reported high incidence of AKI after LT for cirrhosis and HCC in numerous studies highlights the importance of this issue. The prediction of this complication may provide a focus for further research, mainly in the development of ANNs predictive models that may be applied immediately after LT.
ANNs are essentially a large number of interconnected processing elements, working in unison to solve specific problems, and its use for this specific purpose is directly related to the efficiency with which it provides responses close to real output data. ANN methods may provide feasible tools for forecasting AKI after LT in this population, and perhaps provide a high-performance predictive model that may ultimately improve perioperative management of these patients at risk for this serious complication.
Manuscript source: Invited manuscript
Specialty type: Transplantation
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Song B S-Editor: Wang JL L-Editor: A P-Editor: Wang JL
| 1. | Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6573] [Article Influence: 469.5] [Reference Citation Analysis (1)] |
| 2. | Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2800] [Cited by in RCA: 4105] [Article Influence: 586.4] [Reference Citation Analysis (6)] |
| 3. | Bruix J, Reig M, Sherman M. Evidence-Based Diagnosis, Staging, and Treatment of Patients With Hepatocellular Carcinoma. Gastroenterology. 2016;150:835-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1024] [Cited by in RCA: 1275] [Article Influence: 141.7] [Reference Citation Analysis (2)] |
| 4. | European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4059] [Cited by in RCA: 4520] [Article Influence: 347.7] [Reference Citation Analysis (2)] |
| 5. | Johnson RJ, Bradbury LL, Martin K, Neuberger J; UK Transplant Registry. Organ donation and transplantation in the UK-the last decade: a report from the UK national transplant registry. Transplantation. 2014;97 Suppl 1:S1-S27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 151] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 6. | Neuberger J, James O. Guidelines for selection of patients for liver transplantation in the era of donor-organ shortage. Lancet. 1999;354:1636-1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 128] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 7. | Kwong A, Kim WR, Lake JR, Smith JM, Schladt DP, Skeans MA, Noreen SM, Foutz J, Miller E, Snyder JJ, Israni AK, Kasiske BL. OPTN/SRTR 2018 Annual Data Report: Liver. Am J Transplant. 2020;20 Suppl s1:193-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 313] [Article Influence: 62.6] [Reference Citation Analysis (0)] |
| 8. | Freeman RB, Edwards EB, Harper AM. Waiting list removal rates among patients with chronic and malignant liver diseases. Am J Transplant. 2006;6:1416-1421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 165] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 9. | Northup PG, Intagliata NM, Shah NL, Pelletier SJ, Berg CL, Argo CK. Excess mortality on the liver transplant waiting list: unintended policy consequences and Model for End-Stage Liver Disease (MELD) inflation. Hepatology. 2015;61:285-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 157] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 10. | Mehta N, Dodge JL, Goel A, Roberts JP, Hirose R, Yao FY. Identification of liver transplant candidates with hepatocellular carcinoma and a very low dropout risk: implications for the current organ allocation policy. Liver Transpl. 2013;19:1343-1353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 124] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 11. | Mazzaferro V, Sposito C, Coppa J, Miceli R, Bhoori S, Bongini M, Camerini T, Milione M, Regalia E, Spreafico C, Gangeri L, Buzzoni R, de Braud FG, De Feo T, Mariani L. The Long-Term Benefit of Liver Transplantation for Hepatic Metastases From Neuroendocrine Tumors. Am J Transplant. 2016;16:2892-2902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 146] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 12. | Yeh H, Smoot E, Schoenfeld DA, Markmann JF. Geographic inequity in access to livers for transplantation. Transplantation. 2011;91:479-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 139] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 13. | Mazzaferro V. Squaring the circle of selection and allocation in liver transplantation for HCC: An adaptive approach. Hepatology. 2016;63:1707-1717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 104] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 14. | Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, Camerini T, Roayaie S, Schwartz ME, Grazi GL, Adam R, Neuhaus P, Salizzoni M, Bruix J, Forner A, De Carlis L, Cillo U, Burroughs AK, Troisi R, Rossi M, Gerunda GE, Lerut J, Belghiti J, Boin I, Gugenheim J, Rochling F, Van Hoek B, Majno P; Metroticket Investigator Study Group. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1572] [Article Influence: 92.5] [Reference Citation Analysis (1)] |
| 15. | Mazzaferro V, Sposito C, Zhou J, Pinna AD, De Carlis L, Fan J, Cescon M, Di Sandro S, Yi-Feng H, Lauterio A, Bongini M, Cucchetti A. Metroticket 2.0 Model for Analysis of Competing Risks of Death After Liver Transplantation for Hepatocellular Carcinoma. Gastroenterology. 2018;154:128-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 476] [Article Influence: 68.0] [Reference Citation Analysis (0)] |
| 16. | Hamada M, Matsukawa S, Shimizu S, Kai S, Mizota T. Acute kidney injury after pediatric liver transplantation: incidence, risk factors, and association with outcome. J Anesth. 2017;31:758-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Chae MS, Lee N, Park DH, Lee J, Jung HS, Park CS, Choi JH, Hong SH. Influence of oxygen content immediately after graft reperfusion on occurrence of postoperative acute kidney injury in living donor liver transplantation. Medicine (Baltimore). 2017;96:e7626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Mizota T, Hamada M, Matsukawa S, Seo H, Tanaka T, Segawa H. Relationship Between Intraoperative Hypotension and Acute Kidney Injury After Living Donor Liver Transplantation: A Retrospective Analysis. J Cardiothorac Vasc Anesth. 2017;31:582-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Kim WH, Lee HC, Lim L, Ryu HG, Jung CW. Intraoperative Oliguria with Decreased SvO₂ Predicts Acute Kidney Injury after Living Donor Liver Transplantation. J Clin Med. 2018;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Kalisvaart M, Schlegel A, Umbro I, de Haan JE, Polak WG, IJzermans JN, Mirza DF, Perera MTP, Isaac JR, Ferguson J, Mitterhofer AP, de Jonge J, Muiesan P. The AKI Prediction Score: a new prediction model for acute kidney injury after liver transplantation. HPB (Oxford). 2019;21:1707-1717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 21. | Zhou J, Zhang X, Lyu L, Ma X, Miao G, Chu H. Modifiable risk factors of acute kidney injury after liver transplantation: a systematic review and meta-analysis. BMC Nephrol. 2021;22:149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 22. | Joosten A, Lucidi V, Ickx B, Van Obbergh L, Germanova D, Berna A, Alexander B, Desebbe O, Carrier FM, Cherqui D, Adam R, Duranteau J, Saugel B, Vincent JL, Rinehart J, Van der Linden P. Intraoperative hypotension during liver transplant surgery is associated with postoperative acute kidney injury: a historical cohort study. BMC Anesthesiol. 2021;21:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 23. | Chawla LS, Bellomo R, Bihorac A, Goldstein SL, Siew ED, Bagshaw SM, Bittleman D, Cruz D, Endre Z, Fitzgerald RL, Forni L, Kane-Gill SL, Hoste E, Koyner J, Liu KD, Macedo E, Mehta R, Murray P, Nadim M, Ostermann M, Palevsky PM, Pannu N, Rosner M, Wald R, Zarbock A, Ronco C, Kellum JA; Acute Disease Quality Initiative Workgroup 16. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol. 2017;13:241-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 645] [Cited by in RCA: 1055] [Article Influence: 131.9] [Reference Citation Analysis (0)] |
| 24. | Hobson C, Ozrazgat-Baslanti T, Kuxhausen A, Thottakkara P, Efron PA, Moore FA, Moldawer LL, Segal MS, Bihorac A. Cost and Mortality Associated With Postoperative Acute Kidney Injury. Ann Surg. 2015;261:1207-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 294] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 25. | Haykin S. Neural networks: Principles and Practice. New York: Bookman, 2001. |
| 26. | Doyle HR, Dvorchik I, Mitchell S, Marino IR, Ebert FH, McMichael J, Fung JJ. Predicting outcomes after liver transplantation. A connectionist approach. Ann Surg. 1994;219:408-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 55] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Marsh JW, Dvorchik I, Subotin M, Balan V, Rakela J, Popechitelev EP, Subbotin V, Casavilla A, Carr BI, Fung JJ, Iwatsuki S. The prediction of risk of recurrence and time to recurrence of hepatocellular carcinoma after orthotopic liver transplantation: a pilot study. Hepatology. 1997;26:444-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 153] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 28. | Parmanto B, Doyle HR. Recurrent neural networks for predicting outcomes after liver transplantation: representing temporal sequence of clinical observations. Methods Inf Med. 2001;40:386-391. [PubMed] |
| 29. | Cucchetti A, Vivarelli M, Heaton ND, Phillips S, Piscaglia F, Bolondi L, La Barba G, Foxton MR, Rela M, O'Grady J, Pinna AD. Artificial neural network is superior to MELD in predicting mortality of patients with end-stage liver disease. Gut. 2007;56:253-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | Zhang M, Yin F, Chen B, Li YP, Yan LN, Wen TF, Li B. Pretransplant prediction of posttransplant survival for liver recipients with benign end-stage liver diseases: a nonlinear model. PLoS One. 2012;7:e31256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Cruz-Ramírez M, Hervás-Martínez C, Fernández JC, Briceño J, de la Mata M. Predicting patient survival after liver transplantation using evolutionary multi-objective artificial neural networks. Artif Intell Med. 2013;58:37-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 32. | Dreiseitl S, Ohno-Machado L. Logistic regression and artificial neural network classification models: a methodology review. J Biomed Inform. 2002;35:352-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1196] [Cited by in RCA: 774] [Article Influence: 33.7] [Reference Citation Analysis (1)] |
| 33. | Park MH, Shim HS, Kim WH, Kim HJ, Kim DJ, Lee SH, Kim CS, Gwak MS, Kim GS. Clinical Risk Scoring Models for Prediction of Acute Kidney Injury after Living Donor Liver Transplantation: A Retrospective Observational Study. PLoS One. 2015;10:e0136230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 34. | Zongyi Y, Baifeng L, Funian Z, Hao L, Xin W. Risk factors of acute kidney injury after orthotopic liver transplantation in China. Sci Rep. 2017;7:41555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 35. | Utsumi M, Umeda Y, Sadamori H, Nagasaka T, Takaki A, Matsuda H, Shinoura S, Yoshida R, Nobuoka D, Satoh D, Fuji T, Yagi T, Fujiwara T. Risk factors for acute renal injury in living donor liver transplantation: evaluation of the RIFLE criteria. Transpl Int. 2013;26:842-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 36. | Jochmans I, Meurisse N, Neyrinck A, Verhaegen M, Monbaliu D, Pirenne J. Hepatic ischemia/reperfusion injury associates with acute kidney injury in liver transplantation: Prospective cohort study. Liver Transpl. 2017;23:634-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 37. | Thongprayoon C, Kaewput W, Thamcharoen N, Bathini T, Watthanasuntorn K, Lertjitbanjong P, Sharma K, Salim SA, Ungprasert P, Wijarnpreecha K, Kröner PT, Aeddula NR, Mao MA, Cheungpasitporn W. Incidence and Impact of Acute Kidney Injury after Liver Transplantation: A Meta-Analysis. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 92] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 38. | Tinti F, Umbro I, Meçule A, Rossi M, Merli M, Nofroni I, Corradini SG, Poli L, Pugliese F, Ruberto F, Berloco PB, Mitterhofer AP. RIFLE criteria and hepatic function in the assessment of acute renal failure in liver transplantation. Transplant Proc. 2010;42:1233-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 39. | Romano TG, Schmidtbauer I, Silva FM, Pompilio CE, D'Albuquerque LA, Macedo E. Role of MELD score and serum creatinine as prognostic tools for the development of acute kidney injury after liver transplantation. PLoS One. 2013;8:e64089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 40. | Wyssusek KH, Keys AL, Yung J, Moloney ET, Sivalingam P, Paul SK. Evaluation of perioperative predictors of acute kidney injury post orthotopic liver transplantation. Anaesth Intensive Care. 2015;43:757-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 41. | Santos AM, Seixas JM, Pereira BB, Medronho RA. Usando Redes Neurais Artificiais e Regressão Logística na predição da Hepatite A. Revista Brasileira de Epidemiologia. 2005;8:117-126. |
| 42. | Eyng E, Fileti AMF. Control of absorption columns in the bioethanol process: Influence of measurement uncertainties. Eng Appl Artif Intell. 2010;23:271-282. [DOI] [Full Text] |
| 43. | Eyng E, Silva FV, Palú F, Fileti AMF. Neural Network Based Control of an Absorption Column in the Process of Bioethanol Production. Braz Arch Biol Techn. 2009;52:961-972. [DOI] [Full Text] |
| 44. | Liew PL, Lee YC, Lin YC, Lee TS, Lee WJ, Wang W, Chien CW. Comparison of artificial neural networks with logistic regression in prediction of gallbladder disease among obese patients. Dig Liver Dis. 2007;39:356-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 45. | Minsky ML, Papert SA. Perceptrons. Cambridge: MIT Press, 1969. |
| 46. | Ivanics T, Patel MS, Erdman L, Sapisochin G. Artificial intelligence in transplantation (machine-learning classifiers and transplant oncology). Curr Opin Organ Transplant. 2020;25:426-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 47. | Kourou K, Exarchos TP, Exarchos KP, Karamouzis MV, Fotiadis DI. Machine learning applications in cancer prognosis and prediction. Comput Struct Biotechnol J. 2015;13:8-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1362] [Cited by in RCA: 1289] [Article Influence: 117.2] [Reference Citation Analysis (0)] |
| 48. | Singal AG, Mukherjee A, Elmunzer BJ, Higgins PD, Lok AS, Zhu J, Marrero JA, Waljee AK. Machine learning algorithms outperform conventional regression models in predicting development of hepatocellular carcinoma. Am J Gastroenterol. 2013;108:1723-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 203] [Article Influence: 16.9] [Reference Citation Analysis (1)] |
| 49. | Rajkomar A, Dean J, Kohane I. Machine Learning in Medicine. N Engl J Med. 2019;380:1347-1358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1274] [Cited by in RCA: 1599] [Article Influence: 266.5] [Reference Citation Analysis (0)] |
| 50. | Schoenberg MB, Bucher JN, Koch D, Börner N, Hesse S, De Toni EN, Seidensticker M, Angele MK, Klein C, Bazhin AV, Werner J, Guba MO. A novel machine learning algorithm to predict disease free survival after resection of hepatocellular carcinoma. Ann Transl Med. 2020;8:434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 51. | Chandra V, Girijadevi R, Nair AS, Pillai SS, Pillai RM. MTar: a computational microRNA target prediction architecture for human transcriptome. BMC Bioinformatics. 2010;11 Suppl 1:S2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 52. | Lee HC, Yoon SB, Yang SM, Kim WH, Ryu HG, Jung CW, Suh KS, Lee KH. Prediction of Acute Kidney Injury after Liver Transplantation: Machine Learning Approaches vs. Logistic Regression Model. J Clin Med. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 124] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 53. | He ZL, Zhou JB, Liu ZK, Dong SY, Zhang YT, Shen T, Zheng SS, Xu X. Application of machine learning models for predicting acute kidney injury following donation after cardiac death liver transplantation. Hepatobiliary Pancreat Dis Int. 2021;20:222-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 54. | Sendak M, Gao M, Nichols M, Lin A, Balu S. Machine Learning in Health Care: A Critical Appraisal of Challenges and Opportunities. EGEMS (Wash DC). 2019;7:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |