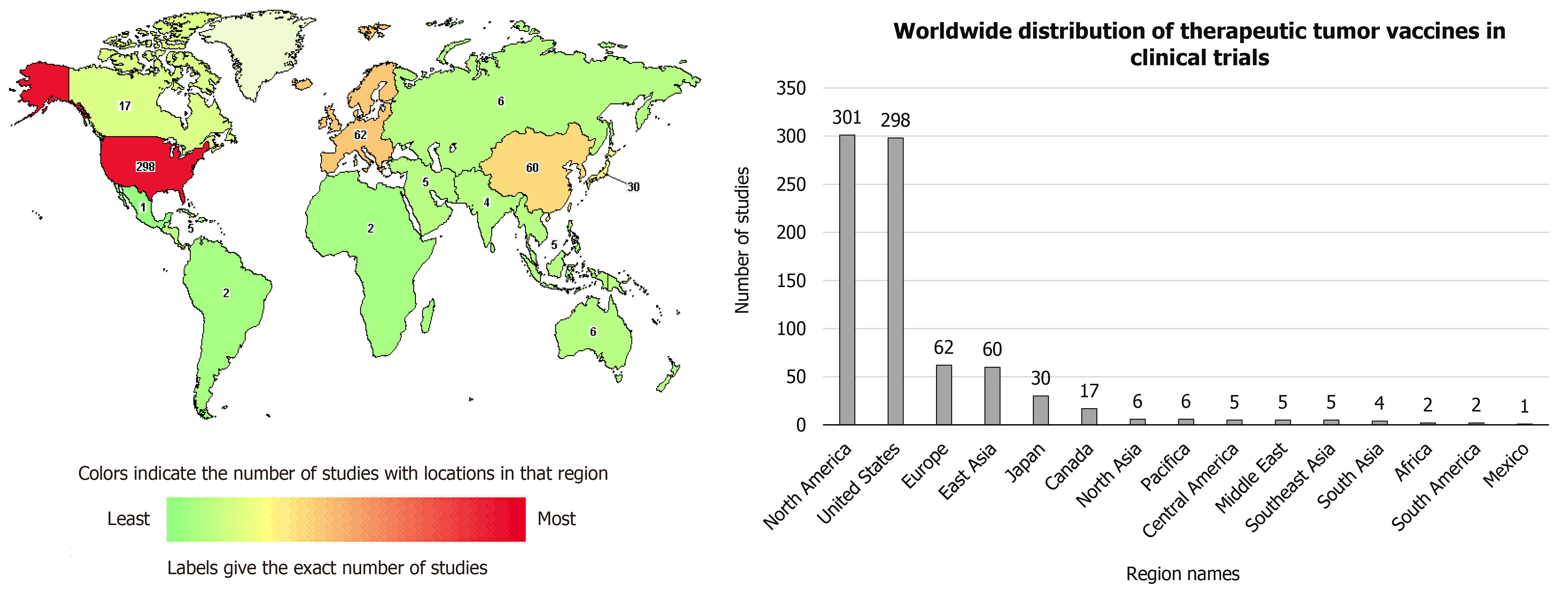
Published online Jun 28, 2021. doi: 10.35713/aic.v2.i3.25
Peer-review started: April 10, 2021
First decision: April 28, 2021
Revised: May 11, 2021
Accepted: June 22, 2021
Article in press: June 22, 2021
Published online: June 28, 2021
Processing time: 79 Days and 8.7 Hours
Malignant tumors are still a worldwide threat to human health. Tumor treatment strategies are constantly evolving, and the advent of tumor immunotherapy has brought up hope to many types of tumors, especially for those that are refractory to conventional therapies including surgery, radiotherapy, and chemotherapy. Tumor vaccines can initiate or amplify an anti-tumor immune response in tumor patients through active immunization, and therefore occupy an important position in tumor immunotherapy. The main types of tumor vaccines include tumor cell vaccines, dendritic cell vaccines, polypeptide vaccines and nucleic acid vaccines. Due to factors such as poor antigen selection and suppressive tumor microenvironment, earliest tumor vaccines on clinical trials failed to achieve satisfactory clinical effects. However, with the development of second-generation genome sequencing technologies and bioinformatics tools, it is possible to predict neoantigens generated by tumor-specific mutations and therefore prepare personalized vaccines. This article summarizes the global efforts in developing tumor vaccines and highlights several representative tumor vaccines in each category.
Core Tip: There are many advancements in the field of cancer immunotherapy in the past decade such as the application of immune checkpoint blockade and adoptive cell therapy. Tumor therapeutic vaccines have emerged as an additional effective treatment strategy due to their ability to trigger potent immune response. Typically, they are tumor cell vaccines, dendritic cell vaccines, peptide vaccines or nucleic acid vaccines. This article mainly reviews the current clinical status as well as research and development status of these four types of therapeutic tumor vaccines for those who are interested.
- Citation: Wei Q, Fang ZY, Zhang ZM, Zhang TF. Therapeutic tumor vaccines — a rising star to benefit cancer patients. Artif Intell Cancer 2021; 2(3): 25-41
- URL: https://www.wjgnet.com/2644-3228/full/v2/i3/25.htm
- DOI: https://dx.doi.org/10.35713/aic.v2.i3.25
Exploratory research on tumor vaccines can be traced back to 1891 when Dr. William B. Coley first proved that heat-inactivated Streptococcus pyogenes and Serratia marcescens (Coley toxin) are effective treatments for inoperable tumors[1]. Coley toxin is especially effective for osteosarcoma and soft tissue sarcoma, thus inspiring the subsequent development of various tumor vaccines. While Coley toxin has faded out of clinical application, its pioneering role cannot be erased. Therapeutic tumor vaccines represent a viable option for tumor immunotherapy, which aims to stimulate the patient's immune system to specifically kill tumor cells without damaging normal cells[2]. Therapeutic cancer vaccines are designed to induce enduring anti-tumor immunity, which enables active immunity to systematically prevent tumor recurrence or metastatic disease. Research on the exploration of approaches to therapeutic tumor vaccines has been ongoing and has been achieving varying degrees of success[3]. So far, the United States Food and Drug Administration (FDA) has approved the following two types of preventive tumor vaccines: Hepatitis B virus (HBV) vaccine-a recombinant HBV vaccine Recombivax HB® approved in 1983 and Engerix-B® approved in 1989, and human papillomavirus (HPV) vaccine: Recombinant HPV type 6, 11, 16, 18 (Gardasil®), recombinant HPV 9-valent vaccine (Gardasil® 9) and recombi
Compared with preventive tumor vaccines, therapeutic tumor vaccine development has lagged significantly. In terms of therapeutic tumor vaccines, the United States FDA so far only approved sipuleucel-T (Provenge®) in 2010 for the treatment of asymp
| Vaccine type | Disease | Combination | Phase | NCT ID | |
| Tumor cell vaccine | GVAX | Neuroblastoma. Pediatric Solid Tumor | Nivolumab. Ipilimumab | Phase I | NCT04239040 |
| Locally Advanced Pancreatic Ductal Adenocarcinoma | Nivolumab CCR2/CCR5 dual antagonist | Phase I; Phase II | NCT03767582 | ||
| Metastatic Pancreatic Adenocarcinoma | Epacadostat. Pembrolizumab CRS-207 CY | Phase II | NCT03006302 | ||
| Colorectal Cancer | Phase I | NCT01952730 | |||
| GVAX Pancreas Vaccine | Pancreatic Cancer | Cyclophosphamide Nivolumab | Phase II | NCT03161379 | |
| Pancreatic Cancer | Cyclophosphamide Nivolumab Urelumab | Phase I; Phase II | NCT02451982 | ||
| GM-CSF vaccine | Multiple Myeloma | Lenalidomide Prevnar13 | Phase II | NCT03376477 | |
| DC Vaccine | AST-VAC2 | NSCLC in the Advanced and Adjuvant Settings | Phase I | NCT03371485 | |
| MIDRIXNEO | NSCLC | Antigen-specific DTH. Control DTH | Phase I | NCT04078269 | |
| Autologous Dendritic Cell-Adenovirus CCL21 Vaccine | NSCLC Stage IV, IVA, IVB Lung Cancer AJCC v8 | Pembrolizumab | Phase I | NCT03546361 | |
| Autologous DCs: MESOVAX | Mesothelioma. Malignant PD-L1 Negative Advanced Cancer Progressive Disease | Pembrolizumab. Interleukin-2 | Phase I | NCT03546426 | |
| PEP-DC vaccine | Pancreatic Adenocarcinoma | Phase I | NCT04627246 | ||
| ME TARP vaccine | Prostate Cancer | Phase II | NCT02362451 | ||
| DC/AML Fusion Vaccine | Acute Myelogenous Leukemia | Decitabine | Phase I | NCT03679650 | |
| Acute Myelogenous Leukemia | Phase II | NCT03059485 | |||
| mDC3/8-KRAS Vaccine | Pancreatic Ductal Adenocarcinoma | Phase I | NCT03592888 | ||
| Autologous DC vaccine: RaC-Ad | Head Neck Tumors, Neuroendocrine Tumors, Soft Tissue Sarcoma Rare Cancer | Interleukin-2 | Phase II | NCT04166006 | |
| COREVAX-1 | Stage IV Colorectal Cancer Curative Resection | Interleukin-2 | Phase II | NCT02919644 | |
| Autologous DCs + Prevnar 13 | Stage III, IIIA, IIIB, IV, IVA, IVB Hepatocellular Carcinoma AJCC v8, Stage III, IIIA, IIIB, IV Intrahepatic Cholangiocarcinoma AJCC v8, Unresectable Hepatocellular Carcinoma, Unresectable Intrahepatic Cholangiocarcinoma | Radiation: External Beam Radiation Therapy | Early Phase I | NCT03942328 | |
| DC Tumor Cell Lysate Vaccine: ATL-DC | Recurrent Glioblastoma | Pembrolizumab poly-ICLC | Phase I | NCT04201873 | |
| Dendritic Cell/Tumor Fusion Vaccine | Glioblastoma, Neuroectodermal Tumors | Interleukin-12 Temozolomide | Phase I; Phase II | NCT04388033 | |
| DC1 Vaccine+ WOKVAC Vaccine | Female Breast Cancer, Male Breast Cancer, Stage I, II, III Breast Cancer, HER2-positive Breast Cancer | Phase II | NCT03384914 | ||
| neoantigen-primed DC vaccine | Gastric Cancer, Hepatocellular Carcinoma, NSCLC, Colon Rectal Cancer | Phase I | NCT04147078 | ||
| MG-7-DC vaccine | Later stage of gastric cancer | Sintilimab | Phase I; Phase II | NCT04567069 | |
| IKKb matured, RNA-loaded DC vaccine | Melanoma, Uveal Metastatic | Phase II | NCT04335890 | ||
| Peptide vaccine | UCPVax: VolATIL | Squamous Cell Carcinoma of the Head and Neck, Anal Canal Cancer, Cervical Cancer | Atezolizumab | Phase II | NCT03946358 |
| UCPVax-Glio | Glioblastoma | Phase I; Phase II | NCT04280848 | ||
| UCPVax | Metastatic NSCLC | Phase I; Phase II | NCT02818426 | ||
| MUC1 | NSCLC | PolyICLC | Phase I; Phase II | NCT01720836 | |
| SVN53-67/M57-KLH | Lung Atypical Carcinoid Tumor, Lung Typical Carcinoid Tumor, Metastatic Pancreatic Neuroendocrine Tumor | Incomplete Freund's Adjuvant Octreotide Acetate Sargramostim | Phase I | NCT03879694 | |
| NSABP FB-14/AE37 | Triple-negative Breast Cancer | Pembrolizumab | Phase II | NCT04024800 | |
| KRAS peptide vaccine | Colorectal Cancer, Pancreatic Cancer | Nivolumab Ipilimumab | Phase I | NCT04117087 | |
| da VINc/OTSGC-A24 | Gastric Cancer | Nivolumab Ipilimumab | Phase I | NCT03784040 | |
| ARG1-18, 19, 20 | NSCLC, Urothelial Carcinoma, Malignant Melanoma, Ovarian Cancer, Colorectal Cancer, Breast Cancer, Squamous Cell Carcinoma of the Head and Neck, Metastatic Cancer | Phase I | NCT03689192 | ||
| Personalized peptide vaccine | Stage IV, IVA, IVB Colorectal Cancer AJCC v7, Stage IV Pancreatic Cancer AJCC v6 and v7 | Imiquimod Pembrolizumab | Phase I | NCT02600949 | |
| WT1/NY-ESO-1 | Ovarian Cancer, Fallopian Tube Primary Peritoneal Cancer, Recurrent Ovarian Cancer | Nivolumab | Phase I | NCT02737787 | |
| IMU-131/HER-Vaxx | Gastrointestinal Neoplasms, Adenocarcinoma | Cisplatin and either Fluorouracil (5-FU) or Capecitabine or Oxaliplatin and capecitabine | Phase I; Phase II | NCT02795988 | |
| ESR1 | Breast Cancer | Phase I | NCT04270149 | ||
| DNAJB1-PRKACA | Fibrolamellar, Hepatocellular Carcinoma | Nivolumab Ipilimumab | Phase I | NCT04248569 | |
| H3.3K27M | Diffuse Intrinsic Pontine Glioma, Diffuse Midline Glioma, H3 K27M-Mutant | Nivolumab | Phase I; Phase II | NCT02960230 | |
| H2NVAC | Ductal Breast Carcinoma In Situ | Granulocyte Macrophage Colony Stimulating Fator | Phase I | NCT04144023 | |
| IDH1R132H/AMPLIFY-NEOVAC | Malignant Glioma | Avelumab | Phase I | NCT03893903 | |
| DNA Vaccine | pTVG-HP/pTVG-AR | CRPC, Metastatic Cancer | Pembrolizumab rhGM-CSF | Phase II | NCT04090528 |
| Mammaglobin-A | Breast Cancer | Phase I | NCT02204098 | ||
| pTVG-HP | Prostate Cancer | Nivolumab GM-CSF | Phase II | NCT03600350 | |
| pNGVL4a-Sig/E7(detox)/HSP70 | Cervical Cancer, Precancerous Condition, HPV Disease, Human Papilom-virus | Imiquimod | Phase I | NCT00788164 | |
| Salmonella oral vaccine | Relapsed Neuroblastoma | Lenalidomide | Early Phase I | NCT04049864 | |
The original tumor cell vaccine tends to fail to induce a strong immune response. In order to change this deficiency, molecular modification techniques have been employed to change the immune characteristics or genetic background of tumor cells to improve their immunogenicity and generate a stronger immune response. Tumor cell vaccine is a whole tumor cell vaccine containing a series of antigens prepared from surgically removed tumor tissues. The removed tumor tissues are minced to tumor cells which are usually inactivated by radiation in the laboratory so that they no longer have proliferative activity even after being imported into the human body. Tumor cell vaccines are basically divided into two types, namely autologous tumor cell vaccines and allogeneic tumor cell vaccines[7,8]. Autologous tumor cell vaccines are prepared by extracting tumor cells from the tumor tissues of patients receiving treatment. They have the advantages of carrying relatively complete known and unknown tumor antigens and not being restricted by major histocompatibility complex (MHC), thus avoiding the immune escape of tumor cells caused by the loss of certain antigens during the process of tumor progression. However, the vaccine made by inactivating tumor cells is extremely weak in immunogenicity and incapable of inducing sufficient anti-tumor immune effects. Allogeneic tumor cell vaccines are prepared using specific types of tumor cells from some other patients instead of the tumor cells from the patients receiving treatment themselves. These allogeneic tumor cell vaccines are more often used as off-the-shelf medicines. Some allogeneic tumor cell vaccines are prepared from mixed tumor cells extracted from tumor cells of several patients[8].
OncoVAX® is an autologous tumor cell vaccine developed using patients' autologous colorectal cancer cells and is used for adjuvant treatment of patients after colorectal cancer resection. The vaccine is a patient's autologous tumor cell vaccine that combines non-proliferative and non-tumorigenic autologous tumor cells with metabolic activity after irradiation and adjuvant of live attenuated TICE strain of bacillus Calmette-Guerin. The company Vaccinogen uses a patented method to extract and purify tumor cells from the resected colorectal cancer tissue, and then undergo radiation treatment, and then inoculate them to the patient to produce an effective and personalized immune response to the residual cancer cells that may still exist in the patient after the operation.
Vermorken et al[9] investigated the effect of OncoVAX® on 254 patients with stage II and III colon cancer in a randomized phase III clinical trial, and they published their results on the lancet. The patients were randomly divided into surgery group (control group, 126 cases) and surgery + vaccine group (treatment group, 128 cases). The median follow-up period was 5.3 years (8-107 mo). Among the tested patients, 65 patients relapsed, including 25 patients in the treatment group and 40 patients in the control group; the risk of recurrence of patients in the treatment group was reduced [risk ratio (RR) = 44%, 95% confidence interval (CI): 7%-66%, P = 0.023]. In the patient staging analysis, OncoVAX® had no significant effect on patients with stage III colon cancer, but it could significantly prolong the recurrence-free period of patients with stage II colon cancer (P = 0.011), and the overall risk of recurrence was reduced (RR = 61%, 95%CI: 18%-81%), the RFS of patients in the treatment group was significantly prolonged [the risk of recurrence or death was reduced (RR = 42%, 95%CI: 0%-68%, P = 0.032)].
5 clinical studies of OncoVAX®, including the study above, which established optimum dose and regimen, have been completed by 2014. 757 subjects with colorectal cancer, of which 720 had colon cancer, have been enrolled in OncoVAX® trials[10]. In addition, the results of the follow-up bioequivalent study (NCT00016133) involving 15 subjects with cGMP-level manufacturing standard concluded the immunogenicity of OncoVAX® was unaffected by the sterilization process[11]. OncoVAX® has reached a Special Protocol Assessment with the FDA and has been granted Fast Track status by the FDA. The phase IIIb clinical trial (NCT02448173) is under recruitment currently which is expected to be completed in July 2022.
Gemogenovatucel-T (FANG, Vigil™) is a whole autologous tumor cell vaccine developed by Gradalis Inc., which incorporates plasmid-encoded granulocyte-macrophage colony stimulating factor and a bifunctional small hairpin RNA interference vector targeting furin converting enzyme. Senzer et al[12] conducted a phase I clinical trial on patients with advanced tumors and demonstrated the long-term safety of the vaccine and the effect of inducing circulated and activated T cells against tumor cells during a 3-year follow-up.
Based on its safety, immunoeffectiveness, and suggested benefits previously verified, Nemunaitis et al[13] provided a follow-up study of a subset of 8 advanced hepatocellular carcinoma patients and demonstrated that no obvious toxicity was observed and a significant induction of systemic immune response. In the phase II clinical trial of patients with advanced ovarian cancer, the reaction with interferon-γ (IFN-γ) enzyme-linked immunospot assay (ELISPOT) before Gemogenovatucel-T vaccination serves as the baseline [negative rate: About 97% (30/31)]. In contrast, the IFN-γ ELISPOT reaction of the patient after vaccination was 100% (31/31) positive, and the circulating activated T cell population that induced by the autologous tumor cells was significantly expanded. In addition, the average RFS of the vaccinated group was 826 d with a median of 604 d, while the control group had an average RFS of 481 d with a median of 377 d (P = 0.033)[14].
Rocconi et al[15] has carried out a study (ClinicalTrials.gov, NCT02346747), in which 91 eligible patients with stage III or IV high-grade serous, endometrioid, or clear cell ovarian cancer were randomly assigned to receive Gemogenovatucel-T (n = 47) or placebo (n = 44). Recurrence-free survival was 11.5 mo (95%CI: 7.5-not reached) for patients assigned to Gemogenovatucel-T vs 8.4 mo (7.9-15.5) for patients assigned to placebo [hazard ratio (HR) 0.69, 90%CI: 0.44-1.07; one-sided P = 0.078]. According to the results, no grade 3 or 4 toxic events was reported among the Gemogenovatucel-T arm. Serious adverse events were reported in 4 patients in the placebo arm and 3 patients in the Gemogenovatucel-T arm. No treatment-related deaths occurred in either group[15].
Rocconi et al[16] posted the data of the double-blind, placebo-controlled trial in phase IIb. Patients were in complete response with Stage III/IV high grade serious, endometroid or clear cell ovarian cancer. Results demonstrated clinical benefit in homologous recombination proficient (HRP) ovarian cancer. RFS was improved with Vigil (n = 25) in HRP patients compared to placebo (n = 20) (HR = 0.386; 90%CI: 0.199- 0.750; P = 0.007), results were verified by Rhabdomyosarcoma 2-Associated Transcript (RMST) (P = 0.017). Similarly, OS benefit was observed in Vigil group compared to placebo (HR = 0.342; 90%CI: 0.141-0.832; P = 0.019). Results with OS were also verified with RMST (P = 0.008)[16].
Dendritic cell (DC) is widely recognized as the most powerful full-time antigen-presenting cell since its antigen-presenting ability is hundreds of times higher compared with other antigen presenting cells. The development of DC vaccines is still at an early stage, but a large amount of valuable experimental data has been obtained showing that DC exerts a powerful function in antigen presentation and initiating anti-tumor immunity. DC-based immunotherapy has been used to generate tumor cytotoxic T cells, which is an effective means to fight tumor cells[17-20]. So far, the United States FDA has only approved one DC vaccine sipuleucel-T for the treatment of metastatic CRPC; Switzerland and Brazil approved two DC vaccines- DCVax®-Brain for the treatment of brain tumors and HybriCell for the treatment of kidney cancer and melanoma[6].
Stapuldencel-T (DCVAC/PCa) is a vaccine which a Czech biotech company (Sotio a.s.) uses autologous leukocytes obtained from prostate cancer patients during the leukapheresis process as raw material to grow immature DCs in vitro. The high hydrostatic pressure kills the immunogenic tumor cells which sensitize the immature DCs and make them mature. The loaded mature DCs are then be inoculated into prostate cancer patients. Podrazil et al[21] conducted a phase I/II clinical trial (EudraCT 2009-017295-24) of combining DCVAC/PCa and docetaxel to treat 25 patients with metastatic CRPC, the median OS (mOS) of the subjects was 19 mo, which is obviously longer than the mOS of 11.8 and 13 mo predicted by Halabi nomogram and MSKCC nomogram, respectively. There were no DCVAC/PCa-related adverse reactions. Long-term vaccination with DCVAC/PCa can induce and maintain the growth of prostate-specific antigen (PSA)-specific T cells. Fucikova et al[22] conducted a phase I/II trial (EudraCT 2009-017259-91) involving 27 patients with rising PSA levels. The median PSADT (PSA doubling time) in all treated patients increased from 5.67 mo prior to immunotherapy to 18.85 mo after 12 doses (P < 0.0018). Moreover, specific PSA-reacting T lymphocytes were increased significantly already after the 4th dose.
Sotio has accomplished 5 earlier trials of DCVAC/PCa in prostate cancer at varying stages namely SP001 (NCT02105675), SP002 (NCT02107391), SP003 (NCT02107404), SP004 (NCT02107430), SP010 (NCT02137746). Based on previous trials, it launched an extensive global multi-center phase III clinical trial studying DCVAC/PCa in prostate cancer (SP005:NCT02111577) to determine whether DCVAC/PCa added onto standard of care (SOC) therapy can improve survival rate. The VIABLE study (actiVe ImmunotherApy using DC-Based treatment for late stage prostatE cancer) enrolled 1182 prostate cancer patients across 21 European countries and the United States. As of January 21, 2021, results of VIABLE study were submitted to United States trial registry but have not yet been announced. However, SOTIO terminates the phase I/II SP015 trial (NCT03514836; EudraCT2015-004314-15) in prostate cancer in Czech Republic owing to insufficient patient accrual.
Rocapuldencel-T (AGS-003) is a mature monocyte-derived DC vaccine developed by Argos Therapeutics, Inc. using patients’ own amplified tumor RNA plus synthetic CD40L RNA for electroporation, which induces the activation and expansion of new T cells (including persistent memory cells and killer cells) based on Arcelis technology platform, specifically attacking the unique antigens of each patient’s tumor. Amin et al[23] carried out a phase II clinical trial that combined AGS-003 and sunitinib in 21 patients with advanced renal cell carcinoma (RCC). The results showed that 13 patients (62%) were effective in this therapy (9 patients responded and 4 patients were in stable condition), but none of the patients achieved complete remission. The median progression-free survival (PFS) of all patients was 11.2 mo (95%CI: 6.0-19.4), and the mOS was 30.2 mo (95%CI: 9.4-57.1); 7 patients (33%) survived at least 4.5 years, 5 cases (24%) survived for more than 5 years, including 2 cases in the continuous response period without disease progression at the completion of the report; the patients tolerated AGS-003 well, and only mild adverse reactions occurred at the vaccination site.
The ADAPT trial recruited 462 patients that were randomized 2:1, 307 to the combination group and 155 to the SOC group between 2013 and 2016. mOS in the combination group was 27.7 mo (95%CI: 23.0-35.9) and 32.4 mo (95%CI: 22.5-not reached) in the SOC group HR of 1.10 (95%CI: 0.83-1.40). PFS was 6.0 mo and 7.83 mo for the combination and SOC groups, respectively [HR = 1.15 (95%CI: 0.92-1.44)]. The ORR was 42.7% (95%CI: 37.1-48.4) for the combination group and 39.4% (95%CI: 31.6-47.5) for the SOC group. Median follow up was 29 mo (0.4-47.7 mo). On account of the lack of clinical efficacy, the ADAPT trial was terminated on February 17, 2017. Immune responses were detected in 70% of patients treated with Rocapuldencel-T, and the magnitude of the immune response positively correlated with OS. Figlin et al[24] has conducted the phase III trial to investigate the safety and efficacy of a combination therapy dosing regimen of Rocapuldencel-T plus sunitinib in patients with metastatic RCC. The results indicated that the combination therapy did not improve the patient's OS. Nevertheless, the phase III trial identified two potential survival-predictive biomarkers namely interleukin (IL)-12 produced by the DC vaccine and higher numbers of T regulatory cells present in the peripheral blood of advanced RCC patients.
DCVax® was developed and is being commercialized by Northwest Biotherapeutics, Inc. (MD, United States), serving as a platform technology that uses activated autologous DCs to reinvigorate and educate the immune system to attack cancers. DCVax®-L) is designed to cover all solid tumor cancers in which the tumors can be surgically removed. Theoretically, DCVax®-L induces the differentiation and maturation of peripheral blood mononuclear cells into DCs, which are activated and loaded with biomarkers (specific antigens) obtained from the patient's own tumor tissue. Antigens can be derived from autologous tumor lysates as in DCVax®-L for glioblastoma multiforme (GBM) or specific recombinant antigenic epitopes[25,26]. The loading of biomarkers into the DCs “educates” them about what the immune system needs to attack. The activated, educated DCs are then isolated with very high purity and comprise the DCVax®-L personalized vaccine[26].
A 348-patient double blind, randomized, placebo-controlled phase III clinical trial (NCT00045968) with DCVax®-L for newly diagnosed GBM is being implemented, whose enrollment completed in 2015. The primary endpoint of the trial is PFS, and secondary endpoints include OS and other measures. The trial is under way at 51 sites (medical centers) across the United States. Liau et al[27] posted its first results on survival indicating that addition of DCVax®-L to standard therapy is feasible and safe in glioblastoma patients and may extend survival. mOS was 23.1 mo from surgery without DCVax®-L. As of this analysis involving 331 patients in 2018, 223 patients are ≥ 30 mo past their surgery date; 67 of these (30.0%) have lived ≥ 30 mo and have a Kaplan-Meier-derived mOS of 46.5 mo. 182 patients are ≥ 36 mo past surgery; 44 of these (24.2%) have lived ≥ 36 mo and have a KM-derived mOS of 88.2 mo[27].
Peptide vaccines that initially targeted tumor enriched antigens can be classified into two distinct categories: Tumor-associated antigens (TAA) and tumor-specific neoantigens antigens[28,29]. Tumor neoantigen is a specific peptide epitope of tumor cells that can be recognized by T cells due to gene mutations in tumor cells, which can activate T cells and exert anti-tumor immune responses. Currently, Peptide vaccines are mainly used in patients with advanced tumors, and clinical trials have been carried out for patients with CRPC, lung cancer, gastrointestinal tumors, cholangiocarcinoma, pancreatic cancer and GBM. Most of the peptide vaccine research is currently in phase I and phase II clinical trials.
Seviprotimut-L (POL-103A) is currently in orphan drug status and developed by Polynoma Lewis Lung Carcinoma (LLC), which is a combination of shed antigens produced by three proprietary melanoma cell lines. Polynoma LLC announced the start of Melanoma Antigen Vaccine Immunotherapy Study (MAVIS), the company’s phase III trial of POL-103A vaccine for melanoma in June 2012. MAVIS (NCT01546571), a global, multi-center, double-blind, placebo-controlled study, is expected to recruit 1224 participants with resected stage IIb, IIc or III melanoma and a high risk of recurrence. The trial is expected to be initially completed on January 1, 2025[30].
Tedopi® is a synthetic peptide vaccine developed by the French company OSE Immunotherapeutics, which is a specific treatment for HLA-A2+ patients, a key receptor for the cytotoxic T-immune response, through its proprietary combination of 9 optimized neo-epitopes plus one epitope giving universal helper T cell response targeting T cell activation. Currently, Tedopi® is being investigated in two major cancer indications: Non-small cell lung cancer (NSCLC) with an ongoing phase III trial and pancreatic cancer with an ongoing phase II trial[31].
In February 2016, OSE Immunotherapeutics launched the phase III clinical trial (NCT02654587) named Atalante 1 that compared OSE-2101 as a second and third-line drug with docetaxel or pemetrexed for HLA A2+ IIIB or IV NSCLC patients after immune checkpoint inhibitor (CPI)s [programmed death 1 (PD1)/programmed death-ligand 1] failure. The trial included 99 HLA-A2-positive patients with stage IIIB or metastatic stage IV. They were randomly divided into Tedopi® vaccine treatment group or chemotherapy group (pemetrexed or docetaxel) at a ratio of 2:1. The trial is expected to be completed in December 2021 and was initially completed in February 2020. According to the positive step-1 phase III results announced at the European Society for Medical Oncology Virtual Congress 2020, among the 63 patients in the Tedopi® group, 29 patients survived at least 12 mo and the 12-mo survival rate was 46% higher than expected 25%. In the chemotherapy control group, 13 of the 36 patients survived at least 12 mo, which is equivalent to a 12-mo survival rate of 36%[32].
In previous phase II clinical trials of IDM-2101, this vaccine also achieved promising data.
IDM-2101 (previously EP-2101) was administered for a total of 63 patients positive for HLA-A2 every 3 wk for the first 15 wk, then every 2 mo through year 1, then quarterly through year 2, for a total of 13 doses. Results showed that one-year survival in the treated patients was 60%, and median survival was 17.3 mo[33-35].
Nucleic acids have been well acknowledged as potent adjuvants[36,37]. Nucleic acid vaccines include plasmid DNA vaccines, RNA vaccines and viral vector vaccines. Both RNA and DNA have been utilized as adjuvants, meanwhile they take the responsibility to code for TAA[38]. RNA is transcribed in vitro (IVT) by a DNA template encoding the antigen and bacteriophage RNA polymerase; RNA vaccines can release a large number of tumor-derived specific antigens and induce humoral and cellular immune responses, provide costimulatory signals, and are well tolerated without carcinogenic potential[39,40].
VGX-3100 is a DNA vaccine developed by INOVIO Pharmaceuticals, Inc. in the United States. The vaccine contains two DNA plasmids targeting E6 and E7 oncogenes associated with HPV-16 as well as HPV-18, which are responsible for transforming HPV-infected cells into precancerous lesions or cancer cells. Therefore, the vaccine is designed to increase the T cell immune response to eliminate infections caused by HPV-16 and HPV-18 and to destroy precancerous cells or lesions, without the associated risk of losing the patient’s reproductive function[41,42].
Trimble et al[43] conducted a randomized, double-blind, placebo-controlled phase IIb clinical trial in patients with high-grade cervical squamous intraepithelial lesions (HSIL) related to HPV types 16 and 18, and 125 patients were divided into the VGX-3100 group; 42 patients were assigned to the placebo group. Results showed that 55 out of 114 patients in the VGX-3100 group (48.2%) and 12 out of 40 patients in the placebo group (30.0%) had histopathological regression [percentage difference between the two groups was 18.2% (95%CI: 1.3%-34.4%), P = 0.034)]. Patients in the treatment group were well tolerated, and the most common adverse reaction was erythema at the vaccination site, and no serious adverse events were reported.
The company launched the VGX-3100 critical phase III trial (REVEAL 1: NCT03185013) in June 2017 and completed the initial goal of recruiting 198 participants in June 2019. On March 1, 2021, INOVIO announced that the REVEAL 1 study has reached the primary and secondary clinical endpoints, thus being the first DNA medicine to achieve efficacy endpoints in a phase III trial. The REVEAL 1 study enrolled 201 patients with HPV-16/18-related HSIL. Among the 193 patients with evaluable efficacy, 23.7% (31/131) of the these in the treatment group reached the common primary endpoint of achieving histopathological regression of HSIL combined with virologic clearance of HPV-16 and/or HPV-18 at week 36, while the placebo group was 11.3% (7/62) and results were statistically significant (P = 0.022; 95%CI: 0.4-22.5). The study reached all secondary endpoints as well.
ProstAtak® is an adenovirus vector tumor vaccine developed by Advantagene, Inc. in the United States to prevent and treat recurrence of prostate cancer. It utilizes a gene transfer method to directly deliver a vaccine containing the herpes simplex virus thymidine kinase gene (aglatimagene besadenovec, AdV-tk) followed by an anti-herpetic prodrug valacyclovir into the prostate tumor via trans-rectal ultrasound guided injection, and then the patient continuously takes valacyclovir for 14 d. Theoretically, the initial local cytotoxicity is mediated by nucleoside analogues produced by valacyclovir phosphorylation, which activates the immune system by stimulating T-cell proliferation and IL-2 production therefore generates a systemic anti-tumor immune response. Advantagene Biotech launched a randomized, completely blind, placebo-controlled phase III clinical trial of ProstAtak® (PrTK03; NCT01436968) combined with radiotherapy in 711 patients with moderate to high-risk localized prostate cancer in September 2011. The subjects were randomly divided into treatment group and control group at a ratio of 2:1. The trial is expected to be initially completed in September 2023. Additionally, the company's phase II clinical trial of ProstAtak® (ULYSSES; NCT02768363) for patients with localized prostate cancer was also launched in May 2016. The trial has recruited 187 participants and its primary completion time was estimated to be March 2021.
It has been well-acknowledged that mRNA has the potential to be promoted as an important character in therapeutic regimens since over 20 years ago. Since the successful development and current massive use of mRNA vaccines for coronavirus disease 2019 (COVID-19) immunization, more mRNA-based tumor immunotherapies have been under-developed. Some typical mRNA-based tumor vaccines and COVID-19 vaccines are listed in Tables 2 and 3. FixVac (BNT111) is an intravenously administered liposomal RNA (RNA-LPX) vaccine developed by Biopharmaceutical New Technologies (BioNTech), which comprises RNA-LPX encoding 4 TAAs—NY-ESO-1, melanoma-associated antigen A3, tyrosinase, and trans-membrane phospha
| Vaccine | mRNA-encoded antigen | Formulation type | Disease | NCT ID | Phases | Status | Sponsor/collaborator | Results |
| mRNA-2416 | OX40L | LNP | Relapsed/Refractory Solid Tumor Malignancies or Lymphoma Ovarian Cancer | NCT03323398 | Phase I/II | Recruiting | ModernaTX, Inc. | Any dose of intratumoral injection is tolerable when mRNA-2416 is administered alone. Results indicate increased OX40L protein expression, elevated PD-L1 levels and pro-inflammatory activity after mRNA-2416 injection |
| mRNA-2572 | OX40L, IL-23, IL-36γ | LNP | Dose Escalation: Relapsed/Refractory Solid Tumor Malignancies or Lymphoma Dose Expansion: Triple Negative Breast Cancer, Head and Neck Squamous Cell Carcinoma, Non-Hodgkin Lymphoma, and Urothelial Cancer | NCT03739931 | Phase I | Recruiting | ModernaTX, Inc., AstraZeneca | Any dose of intratumoral injection is tolerable when mRNA-2572 is administered alone or in combination with PD-L1 inhibitor. IFN-γ, TNF-α, and PD-L1 levels increased |
| mRNA-4157 KEYNOTE-603 | Neo-Ag | LNP | Solid Tumors | NCT03313778 | Phase I | Recruiting | ModernaTX, Inc., Merck Sharp & Dohme Corp. | All tested doses is tolerated, and clinical responses were observed when mRNA-4157 is combined with Pembrolizumab |
| KEYNOTE-942 | Neo-Ag | LNP | Melanoma | NCT03897881 | Phase II | Recruiting | ModernaTX, Inc., Merck Sharp & Dohme Corp. | Not available |
| mRNA-5671/Merck V941 | KRAS mutations: G12D, G12V, G13D, G12C | LNP | NSCLC, Pancreatic cancer, Colorectal cancer | NCT03948763 | Phase I | Recruiting | Merck Sharp & Dohme Corp. | Not available |
| FixVac (BNT111); Lipo-MERIT | NY-ESO-1, MAGEC3, tyrosinase, TPTE | Lipo-MERIT, LNP | Melanoma | NCT02410733 | Phase I | Active, not recruiting | BioNTech SE | BNT111 alone or in combination with PD1, mediates durable objective responses in CPI-experienced patients with unresectable melanoma. Durable clinical responses in both monotherapy and combination with CPI are accompanied by the induction of strong CD4+ and CD8+ T cell immunity. BNT111 vaccination was safe and well tolerated with no dose limiting toxicity |
| RO7198457 (BNT122) | Neo-Ag | Lipo-MERIT, LNP | Melanoma, NSCLC, Bladder Cancer, CRC, Breast Cancer etc. | NCT03289962 | Phase I | Recruiting | BioNTech, Genentech | The combination of RO7198457 and atezolizumab is generally well tolerated. RO7198457 combined with atezolizumab can induce pro-inflammatory cytokine release and peripheral T cell response in most patients |
| Neo-Ag | Lipo-MERIT, LNP | Advanced Melanoma | NCT03815058 | Phase II | Recruiting | Genentech, Inc., BioNTech SE | Not available | |
| Neo-Ag | Lipo-MERIT, LNP | Stage II and III CRC (surgically resected) | NCT04486378 | Phase II | Recruiting | BioNTech SE | Not available | |
| Neo-Ag | Lipo-MERIT, LNP | Pancreatic Cancer (surgically resected) | NCT04161755 | Phase I | Recruiting | Memorial Sloan Kettering Cancer Center, Genentech, Inc. | Not available | |
| Neo-Ag | Lipo-MERIT, LNP | NSCLC | NCT04267237 | Phase II | Withdrawn | Hoffmann-La Roche | Not available | |
| SAR441000 (BNT131) | IL-12sc, IL-15sushi, IFNα and GM-CSF | Various formulations | advanced melanoma | NCT03871348 | Phase I | Recruiting | Sanofi, BioNTech RNA Pharmaceuticals GmbH | Not available |
| RiboMab (BNT141) | mRNA encoding secreted IgG antibodies that target multiple epithelial solid tumors | Various liver-targeting LNP formulations | CLDN18.2-positive Solid Tumors | NCT04683939 | Phase I/II | Not yet recruiting | BioNTech SE | Not available |
| IVAC MUTANOME, RBL001/RBL002 | Neo-Ag/TAA | naked mRNA | Advanced Melanoma | NCT02035956 | Phase I | Completed | BioNTech RNA Pharmaceuticals GmbH, BioNTech SE | |
| CV8102 | TLR7/8/RIG-1 agonist based on noncoding single stranded RNA | RNActive, (Protamine) | Melanoma (Skin), Squamous Cell Carcinoma of the Skin Carcinoma, Squamous Cell of Head and Neck Carcinoma, Adenoid Cystic | NCT03291002 | Phase I | Recruiting | CureVac AG, Syneos Health | Not available |
| Peptide vaccine and mRNA | IMA970A plus CV8102 and Cyclophosphamide | Hepatocellular carcinoma | NCT03203005 | Phase I/II | Completed | National Cancer Institute, Naples, immatics Biotechnologies GmbH, CureVac AG, European Commission-FP7-Health-2013- Innovation-1 | Not available | |
| BI-1361849 (CV9202) | NY-ESO-1, MAGE-C2, MAGE-C1, survivin, 5 T4, MUC1 | RNActive, Protamine | Metastatic NSCLC | NCT03164772 | Phase I/II | Active, not recruiting | Ludwig Institute for Cancer Research, Cancer Research Institute, New York City; Boehringer Ingelheim, MedImmune LLC, CureVac AG, PharmaJet, Inc. | CV9202 was well-tolerated, and antigen specific immune responses were detected in majority of patients (84%) |
| CV9201 | MAGE-C1, MAGE-C2, NY-SEO-1, survivin,5 T4 | RNActive, Protamine | Stage IIIB/IV NSCLC | NCT00923312 | Phase I/II | Completed | CureVac AG | CV9201 was well-tolerated and results indicated immune responses after vaccination. Median PFS and OS were 5 and 10.8 mo, respectively |
| CV9103 | PSA, PSCA, PSMA, STEAP1 | RNActive, Protamine | Prostate cancer | NCT00831467 | Phase I/II | Completed | CureVac AG | CV9103 is well tolerated and immunogenic |
| CV9104 | PSA, PSCA, PSMA, STEAP1, PAP, MUC1 | RNActive, Protamine | Prostate cancer | NCT01817738 | Phase I/II | Terminated | CureVac AG | Terminated due to insufficient activities |
| Vaccine | NCT ID | Title | Phase | Status | Estimated number of participants | Sponsor/collaborator |
| BNT162b2 | NCT04816669 | Study to Evaluate the Safety, Tolerability, and Immunogenicity of a Lyophilized Formulation of BNT162b2 Against COVID-19 in Healthy Adults | Phase III | Recruiting | 550 | BioNTech SE, Pfizer |
| NCT04713553 | A Phase 3 Study to Evaluate the Safety, Tolerability, and Immunogenicity of Multiple Production Lots and Dose Levels of BNT162b2 Against COVID-19 in Healthy Participants | Phase III | Recruiting | 1530 | BioNTech SE, Pfizer | |
| NCT04754594 | Study to Evaluate the Safety, Tolerability, and Immunogenicity of SARS-CoV-2 RNA Vaccine Candidate (BNT162b2) Against COVID-19 in Healthy Pregnant Women 18 Years of Age and Older | Phase II/III | Recruiting | 4000 | BioNTech SE, Pfizer | |
| NCT04775069 | Antibody Response to COVID-19 Vaccines in Liver Disease Patients | Phase IV | Not yet recruiting | 900 | Humanity & Health Medical Group Limited | |
| mRNA-1273 | NCT04860297 | A Study to Evaluate Safety and Immunogenicity of mRNA-1273 Vaccine to Prevent COVID-19 in Adult Organ Transplant Recipients and in Healthy Adult Participants | Phase III | Recruiting | 240 | ModernaTX, Inc. |
| NCT04796896 | A Study to Evaluate Safety and Effectiveness of mRNA-1273 Vaccine in Healthy Children Between 6 Months of Age and Less Than 12 Years of Age | Phase II/III | Recruiting | 6750 | ModernaTX, Inc. | |
| NCT04470427 | A Study to Evaluate Efficacy, Safety, and Immunogenicity of mRNA-1273 Vaccine in Adults Aged 18 Years and Older to Prevent COVID-19 | Phase III | Active, not recruiting | 30420 | ModernaTX, Inc., Biomedical Advanced Research and Development Authority, National Institute of Allergy and Infectious Diseases (NIAID) | |
| NCT04649151 | A Study to Evaluate the Safety, Reactogenicity, and Effectiveness of mRNA-1273 Vaccine in Adolescents 12 to <18 Years Old to Prevent COVID-19 | Phase II/III | Active, not recruiting | 3000 | ModernaTX, Inc., Biomedical Advanced Research and Development Authority | |
| CV-NCOV-011 | NCT04848467 | A Trial Studying the SARS-CoV-2 mRNA Vaccine CVnCoV to Learn About the Immune Response, the Safety, and the Degree of Typical Vaccination Reactions When CVnCoV is Given at the Same Time as a Flu Vaccine Compared to When the Vaccines Are Separately Given in Adults 60 Years of Age and Older (CV-NCOV-011) | Phase III | Not yet recruiting | 1000 | Bayer, CureVac AG |
| CVnCoV | NCT04860258 | A Study to Evaluate Safety, Reactogenicity and Immunogenicity of the SARS-CoV-2 mRNA Vaccine CVnCoV in Adults With Co-morbidities for COVID-19 | Phase III | Not yet recruiting | 1200 | CureVac AG |
| NCT04838847 | A Study to Evaluate the Immunogenicity and Safety of the SARS-CoV-2 mRNA Vaccine CVnCoV in Elderly Adults Compared to Younger Adults for COVID-19 | Phase III | Not yet recruiting | 180 | CureVac AG | |
| NCT04652102 | A Study to Determine the Safety and Efficacy of SARS-CoV-2 mRNA Vaccine CVnCoV in Adults for COVID-19 | Phase II/III | Recruiting | 36500 | CureVac AG | |
| NCT04674189 | A Study to Evaluate the Safety and Immunogenicity of Vaccine CVnCoV in Healthy Adults in Germany for COVID-19 | Phase III | Recruiting | 2520 | CureVac AG | |
| SARS-CoV-2 mRNA Vaccine | NCT04847102 | A Phase III Clinical Study of a SARS-CoV-2 Messenger Ribonucleic Acid (mRNA) Vaccine Candidate Against COVID-19 in Population Aged 18 Years and Above | Phase III | Not yet recruiting | 28000 | Walvax Biotechnology Co., Ltd., Abogen Biosciences Co. Ltd., Yuxi Walvax Biotechnology Co., Ltd., |
| CoVPN 3006 | NCT04811664 | A Study of SARS CoV-2 Infection and Potential Transmission in University Students Immunized With Moderna COVID-19 Vaccine (CoVPN 3006) | Phase III | Recruiting | 37500 | National Institute of Allergy and Infectious Diseases (NIAID) |
| KYRIOS | NCT04869358 | Exploring the Immune Response to SARS-CoV-2/COVID-19 Vaccines in Patients With Relapsing Multiple Sclerosis (RMS) Treated With Ofatumumab (KYRIOS) | Phase IV | Not yet recruiting | 40 | |
| ENFORCE | NCT04760132 | National Cohort Study of Effectiveness and Safety of SARS-CoV-2/COVID-19 Vaccines (ENFORCE) (ENFORCE) | Phase IV | Recruiting | 10000 | Jens D Lundgren, MD, Ministry of the Interior and Health, Denmark; Rigshospitalet, Denmark |
| AMA-VACC | NCT04792567 | Exploring the Immune Response to SARS-CoV-2 modRNA Vaccines in Patients With Secondary Progressive Multiple Sclerosis (AMA-VACC) (AMA-VACC) | Phase IV | Recruiting | 60 | |
| COVAXID | NCT04780659 | COVID-19 Vaccination of Immunodeficient Persons (COVAXID) (COVAXID) | Phase IV | Recruiting | 540 | Karolinska University Hospital, Karolinska Institutet |
| DemiVac | NCT04852861 | Safety and Immunogenicity of Demi-dose of Two Covid-19 mRNA Vaccines in Healthy Population (DemiVac) | Phase IV | Not yet recruiting | 200 | Sciensano, Mensura EDPB, Institute of Tropical Medicine, Belgium; Erasme University Hospital |
Sahin et al[47] has conducted the clinical trial named Lipo-MERIT (NCT02410733), which is a multicenter, open-label, dose-escalation phase 1 trial to evaluate the safety and tolerability of vaccinated patients with stage IIIB-C and stage IV melanoma. According to the interim analysis as of July 29, 2019 of 89 patients who was intravenously administered BNT111 ranging from 7.2 μg to 400 μg, BNT111 alone or in combination with blockade of the CPI PD1, mediates durable objective responses in CPI-experienced patients with unresectable melanoma. Durable clinical responses in both monotherapy and combinatory therapy were accompanied by the induction of strong CD4+ and CD8+ T cell immunity. BNT111 vaccination was safe and well tolerated with no dose limiting toxicity. Most common adverse events were mild to moderate, transient flu-like symptoms, such as pyrexia and chills. Mostly they are early-onset, transient and manageable with antipyretics, and could be resolved within 24 h.
Based on the promising results of Lipo-MERIT, BioNTech launched the randomized, multi-site, phase II trial (NCT04526899) designed to evaluate the efficacy, tolerability, and safety of BNT111 combined with cemiplimab (Libtayo®) in anti-PD1-refractory/ relapsed patients with unresectable Stage III or IV melanoma. The trial was scheduled to recruit 120 participants and estimated to start in May 2021[48]. In addition, iNeST is another typical platform in BioNTech and represents the pioneer in developing fully individualized cancer immunotherapies, which utilizes optimized mRNA encoding neoantigens identified on particular patients and features proprietary size- and charge-based RNA-LPX targeting DCs formulation[44]. There are four ongoing clinical trials based on its product candidate RO7198457 (BNT122), two of which has entered phase 2.
The pursuit of tumor vaccines has been for more than a century. In the field of immunotherapy, the past decade has witnessed tremendous progress in the usage of immune checkpoint blockades and the adoptive cell therapy, although still many patients fail to benefit from the immune therapies alone. Such effectiveness of novel immune therapies has greatly motivated people to revisit the concept of tumor vaccines. At present, one of the main restricting factors of tumor vaccines is the weak immunogenicity of the tumor antigens, which poses tumor immune tolerance or immune escape. Moreover, since the tumors in patients are highly heterogeneous, the development of tumor vaccines is undergoing a transition from universality to individualization, so that the treatment is more tailored to individual patient. Different types of vaccines have their own distinct advantages and disadvantages. Tumor cell vaccine contains the full spectrum of tumor antigens and it is simple to prepare. However, it requires a large amount of autologous tumor tissues or allogeneic tumor cell lines, and their immunogenicity is usually weak. DC vaccine can stimulate a wide range of immune responses and can be loaded with antigens in diverse ways, but DC cell culture in vitro is challenging, and the vaccine preparation process may generate immature DCs which may induce immune tolerance. Peptide vaccine has strong specificity and high safety, and is not restricted by MHC haplotype and easy to modify, but it tends to provoke a weak immune response and is prone to tumor antigen modulation. With regard to the nucleic acid vaccine, it is easy to produce, economical and safe, and can elicit a wide range of immune responses, but it requires to be used in a large amount so that it can be taken up by cells in sufficient amount to stimulate effective immunity. It is also worth noting that storage, stability and delivery techniques of nucleic acid vaccine are also issues to be overcome.
The past 20 years have witnessed the application of mRNA technology in multiple indications and its transition from theory to vaccine products and clinical treatments. Before the global health pandemic COVID-19, mRNA technology had already been regarded as the most advanced in the area of cancer immunotherapy but its full potential remains latent. The efforts made to the recent fast approval of two mRNA-based COVID-19 vaccines, mRNA-1273 (Moderna) and BNT162b2 (Pfizer/BioNTech), definitely promotes the mRNA vaccine development in every aspect, such as its modification strategy to stabilize and to control its immunogenicity, cell delivery strategy and transportation and maintenance strategy. Undoubtedly, this will be a huge push to apply mRNA technology in additional infectious disease prevention and in the area of cancer treatment. We envision mRNA technology is poised to be the next generation cancer immunotherapy in the near future.
In summary, we are experiencing an outbreak of different types of tumor vaccines, and we are making every effort to transform the idea of therapeutic tumor vaccines into a standard clinical application. Many pending questions remain to be addressed. However, with the advancement of new technologies and deepened understanding of tumor immunology, the joint efforts of scientific researchers from all over the world will certainly make the development of therapeutic tumor vaccines a good prospect.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Korbelik M S-Editor: Fan JR L-Editor: Filipodia P-Editor: Li JH
| 1. | Slaney CY, Kershaw MH. Challenges and Opportunities for Effective Cancer Immunotherapies. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (1)] |
| 2. | Guo C, Manjili MH, Subjeck JR, Sarkar D, Fisher PB, Wang XY. Therapeutic cancer vaccines: past, present, and future. Adv Cancer Res. 2013;119:421-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 386] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 3. | Wong KK, Li WA, Mooney DJ, Dranoff G. Advances in Therapeutic Cancer Vaccines. Adv Immunol. 2016;130:191-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 4. | Dores GM, Bryant-Genevier M, Perez-Vilar S. Adverse Events Associated With the Use of Sipuleucel-T Reported to the US Food and Drug Administration's Adverse Event Reporting System, 2010-2017. JAMA Netw Open. 2019;2:e199249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 5. | Andtbacka RH, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J, Delman KA, Spitler LE, Puzanov I, Agarwala SS, Milhem M, Cranmer L, Curti B, Lewis K, Ross M, Guthrie T, Linette GP, Daniels GA, Harrington K, Middleton MR, Miller WH Jr, Zager JS, Ye Y, Yao B, Li A, Doleman S, VanderWalde A, Gansert J, Coffin RS. Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma. J Clin Oncol. 2015;33:2780-2788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1504] [Cited by in RCA: 1591] [Article Influence: 159.1] [Reference Citation Analysis (0)] |
| 6. | Ogi C, Aruga A. Clinical evaluation of therapeutic cancer vaccines. Hum Vaccin Immunother. 2013;9:1049-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 7. | Sprooten J, Ceusters J, Coosemans A, Agostinis P, De Vleeschouwer S, Zitvogel L, Kroemer G, Galluzzi L, Garg AD. Trial watch: dendritic cell vaccination for cancer immunotherapy. Oncoimmunology. 2019;8:e1638212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 116] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 8. | Roy S, Sethi TK, Taylor D, Kim YJ, Johnson DB. Breakthrough concepts in immune-oncology: Cancer vaccines at the bedside. J Leukoc Biol. 2020;108:1455-1489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 9. | Vermorken JB, Claessen AM, van Tinteren H, Gall HE, Ezinga R, Meijer S, Scheper RJ, Meijer CJ, Bloemena E, Ransom JH, Hanna MG Jr, Pinedo HM. Active specific immunotherapy for stage II and stage III human colon cancer: a randomised trial. Lancet. 1999;353:345-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 340] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 10. | OncoVAX® Phase IIIa Study (8701) Results in Stage II Colon Cancer. OncoVAX® Cancer Vaccine Clinical Results - Vaccinogen (vaccinogen-oncovax.com). [cited 5 May 2021]. Available from: https://vaccinogen-oncovax.com/oncovax/clinical-results/. |
| 11. | Michael G. Vaccine Therapy in Treating Patients With Stage II or Stage III Colon Cancer That Has Been Removed During Surgery. In: ClinicalTrials.gov [Internet]. National Cancer Institute (NCI): U.S. National Library of Medicine. [cited 5 May 2021]. Available from: https://www.clinicaltrials.gov/ct2/show/NCT00016133. |
| 12. | Senzer N, Barve M, Kuhn J, Melnyk A, Beitsch P, Lazar M, Lifshitz S, Magee M, Oh J, Mill SW, Bedell C, Higgs C, Kumar P, Yu Y, Norvell F, Phalon C, Taquet N, Rao DD, Wang Z, Jay CM, Pappen BO, Wallraven G, Brunicardi FC, Shanahan DM, Maples PB, Nemunaitis J. Phase I trial of "bi-shRNAi(furin)/GMCSF DNA/autologous tumor cell" vaccine (FANG) in advanced cancer. Mol Ther. 2012;20:679-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 145] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 13. | Nemunaitis J, Barve M, Orr D, Kuhn J, Magee M, Lamont J, Bedell C, Wallraven G, Pappen BO, Roth A, Horvath S, Nemunaitis D, Kumar P, Maples PB, Senzer N. Summary of bi-shRNA/GM-CSF augmented autologous tumor cell immunotherapy (FANG™) in advanced cancer of the liver. Oncology. 2014;87:21-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Oh J, Barve M, Matthews CM, Koon EC, Heffernan TP, Fine B, Grosen E, Bergman MK, Fleming EL, DeMars LR, West L, Spitz DL, Goodman H, Hancock KC, Wallraven G, Kumar P, Bognar E, Manning L, Pappen BO, Adams N, Senzer N, Nemunaitis J. Phase II study of Vigil® DNA engineered immunotherapy as maintenance in advanced stage ovarian cancer. Gynecol Oncol. 2016;143:504-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 15. | Rocconi RP, Grosen EA, Ghamande SA, Chan JK, Barve MA, Oh J, Tewari D, Morris PC, Stevens EE, Bottsford-Miller JN, Tang M, Aaron P, Stanbery L, Horvath S, Wallraven G, Bognar E, Manning L, Nemunaitis J, Shanahan D, Slomovitz BM, Herzog TJ, Monk BJ, Coleman RL. Gemogenovatucel-T (Vigil) immunotherapy as maintenance in frontline stage III/IV ovarian cancer (VITAL): a randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Oncol. 2020;21:1661-1672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 85] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 16. | Rocconi RP, Monk BJ, Walter A, Herzog TJ, Galanis E, Manning L, Bognar E, Wallraven G, Stanbery L, Aaron P, Senzer N, Coleman RL, Nemunaitis J. Gemogenovatucel-T (Vigil) immunotherapy demonstrates clinical benefit in homologous recombination proficient (HRP) ovarian cancer. Gynecol Oncol. 2021;161:676-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 17. | Kalinski P, Okada H. Polarized dendritic cells as cancer vaccines: directing effector-type T cells to tumors. Semin Immunol. 2010;22:173-182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | Steinman RM. Decisions about dendritic cells: past, present, and future. Annu Rev Immunol. 2012;30:1-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 809] [Cited by in RCA: 966] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 19. | Barth RJ Jr, Fisher DA, Wallace PK, Channon JY, Noelle RJ, Gui J, Ernstoff MS. A randomized trial of ex vivo CD40L activation of a dendritic cell vaccine in colorectal cancer patients: tumor-specific immune responses are associated with improved survival. Clin Cancer Res. 2010;16:5548-5556. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 20. | Lesterhuis WJ, de Vries IJ, Schreibelt G, Lambeck AJ, Aarntzen EH, Jacobs JF, Scharenborg NM, van de Rakt MW, de Boer AJ, Croockewit S, van Rossum MM, Mus R, Oyen WJ, Boerman OC, Lucas S, Adema GJ, Punt CJ, Figdor CG. Route of administration modulates the induction of dendritic cell vaccine-induced antigen-specific T cells in advanced melanoma patients. Clin Cancer Res. 2011;17:5725-5735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 137] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 21. | Podrazil M, Horvath R, Becht E, Rozkova D, Bilkova P, Sochorova K, Hromadkova H, Kayserova J, Vavrova K, Lastovicka J, Vrabcova P, Kubackova K, Gasova Z, Jarolim L, Babjuk M, Spisek R, Bartunkova J, Fucikova J. Phase I/II clinical trial of dendritic-cell based immunotherapy (DCVAC/PCa) combined with chemotherapy in patients with metastatic, castration-resistant prostate cancer. Oncotarget. 2015;6:18192-18205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 113] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 22. | Fucikova J, Podrazil M, Jarolim L, Bilkova P, Hensler M, Becht E, Gasova Z, Klouckova J, Kayserova J, Horvath R, Fialova A, Vavrova K, Sochorova K, Rozkova D, Spisek R, Bartunkova J. Phase I/II trial of dendritic cell-based active cellular immunotherapy with DCVAC/PCa in patients with rising PSA after primary prostatectomy or salvage radiotherapy for the treatment of prostate cancer. Cancer Immunol Immunother. 2018;67:89-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 23. | Amin A, Dudek AZ, Logan TF, Lance RS, Holzbeierlein JM, Knox JJ, Master VA, Pal SK, Miller WH Jr, Karsh LI, Tcherepanova IY, DeBenedette MA, Williams WL, Plessinger DC, Nicolette CA, Figlin RA. Survival with AGS-003, an autologous dendritic cell-based immunotherapy, in combination with sunitinib in unfavorable risk patients with advanced renal cell carcinoma (RCC): Phase 2 study results. J Immunother Cancer. 2015;3:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 155] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 24. | Figlin RA, Tannir NM, Uzzo RG, Tykodi SS, Chen DYT, Master V, Kapoor A, Vaena D, Lowrance W, Bratslavsky G, DeBenedette M, Gamble A, Plachco A, Norris MS, Horvatinovich J, Tcherepanova IY, Nicolette CA, Wood CG; ADAPT study group. Results of the ADAPT Phase 3 Study of Rocapuldencel-T in Combination with Sunitinib as First-Line Therapy in Patients with Metastatic Renal Cell Carcinoma. Clin Cancer Res. 2020;26:2327-2336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 25. | DCVax ® Technology. Northwest Biotherapeutics. [cited 5 May 2021]. Available from: https://nwbio.com/dcvax-technology/. |
| 26. | Hdeib A, Sloan AE. Dendritic cell immunotherapy for solid tumors: evaluation of the DCVax® platform in the treatment of glioblastoma multiforme. CNS Oncol. 2015;4:63-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Liau LM, Ashkan K, Tran DD, Campian JL, Trusheim JE, Cobbs CS, Heth JA, Salacz M, Taylor S, D'Andre SD, Iwamoto FM, Dropcho EJ, Moshel YA, Walter KA, Pillainayagam CP, Aiken R, Chaudhary R, Goldlust SA, Bota DA, Duic P, Grewal J, Elinzano H, Toms SA, Lillehei KO, Mikkelsen T, Walbert T, Abram SR, Brenner AJ, Brem S, Ewend MG, Khagi S, Portnow J, Kim LJ, Loudon WG, Thompson RC, Avigan DE, Fink KL, Geoffroy FJ, Lindhorst S, Lutzky J, Sloan AE, Schackert G, Krex D, Meisel HJ, Wu J, Davis RP, Duma C, Etame AB, Mathieu D, Kesari S, Piccioni D, Westphal M, Baskin DS, New PZ, Lacroix M, May SA, Pluard TJ, Tse V, Green RM, Villano JL, Pearlman M, Petrecca K, Schulder M, Taylor LP, Maida AE, Prins RM, Cloughesy TF, Mulholland P, Bosch ML. First results on survival from a large Phase 3 clinical trial of an autologous dendritic cell vaccine in newly diagnosed glioblastoma. J Transl Med. 2018;16:142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 223] [Cited by in RCA: 378] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 28. | van der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, Van den Eynde B, Knuth A, Boon T. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643-1647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2388] [Cited by in RCA: 2369] [Article Influence: 69.7] [Reference Citation Analysis (0)] |
| 29. | Kallen KJ, Gnad-Vogt U, Scheel B, Rippin G, Stenzl A. A phase I/IIa study of the mRNA based cancer vaccine CV9103 prepared with the RNActive technology results in distinctly longer survival than predicted by the Halabi Nomogram which correlates with the induction of antigen-specific immune responses. J Immunother Cancer. 2013;1:P219. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Craig S. Study of a Melanoma Vaccine in Stage IIb, IIc, and III Melanoma Patients (MAVIS). In: ClinicalTrials.gov [Internet]. Polynoma LLC: U.S. National Library of Medicine. [cited 6 May 2021]. Available from: https://clinicaltrials.gov/ct2/show/NCT01546571. |
| 31. | Tedopi®. OSE Immunotherapeutics. [cited 6 May 2021]. Available from: https://ose-immuno.com/en/ose-product/tedopi/. |
| 32. | Santiago V. Early signs of activity of Tedopi (OSE2101), a multiple neoepitope vaccine, in a phase 3 trial in advanced lung cancer patients after failure to previous immune checkpoint inhibitors (ICI) Atalante -1 study trial. AACR 2019. [cited 6 May 2021]. Available from: https://ose-immuno.com/wp-content/uploads/2019/04/Viteri-Early-sings-Tedopi-Final-1.pdf. |
| 33. | Beebe M, Qin M, Moi M, Wu S, Heiati H, Walker L, Newman M, Fikes J, Ishioka GY. Formulation and characterization of a ten-peptide single-vial vaccine, EP-2101, designed to induce cytotoxic T-lymphocyte responses for cancer immunotherapy. Hum Vaccin. 2008;4:210-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 34. | Nemunaitis J, Cunningham C, Bender J, Ishioka G, Maples P, Pappen B, Stephenson J, Morse M, Mills B, Greco A, McCune D, Steis R, Nugent F, Khong HT, Richards D. Phase II trial of a 10-epitope CTL vaccine, IDM-2101, in metastatic NSCLC patients: Induction of immune responses and clinical efficacy. J Clin Oncol. 2007;25:3068-3068. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 35. | Barve M, Bender J, Senzer N, Cunningham C, Greco FA, McCune D, Steis R, Khong H, Richards D, Stephenson J, Ganesa P, Nemunaitis J, Ishioka G, Pappen B, Nemunaitis M, Morse M, Mills B, Maples PB, Sherman J, Nemunaitis JJ. Induction of immune responses and clinical efficacy in a phase II trial of IDM-2101, a 10-epitope cytotoxic T-lymphocyte vaccine, in metastatic non-small-cell lung cancer. J Clin Oncol. 2008;26:4418-4425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 36. | Restifo NP, Ying H, Hwang L, Leitner WW. The promise of nucleic acid vaccines. Gene Ther. 2000;7:89-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 95] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 37. | Hobernik D, Bros M. DNA Vaccines-How Far From Clinical Use? Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 227] [Cited by in RCA: 329] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 38. | Ulmer JB, Mason PW, Geall A, Mandl CW. RNA-based vaccines. Vaccine. 2012;30:4414-4418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 133] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 39. | Pardi N, Muramatsu H, Weissman D, Karikó K. In vitro transcription of long RNA containing modified nucleosides. Methods Mol Biol. 2013;969:29-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 134] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 40. | McNamara MA, Nair SK, Holl EK. RNA-Based Vaccines in Cancer Immunotherapy. J Immunol Res. 2015;2015:794528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 169] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 41. | Morrow MP, Kraynyak KA, Sylvester AJ, Dallas M, Knoblock D, Boyer JD, Yan J, Vang R, Khan AS, Humeau L, Sardesai NY, Kim JJ, Plotkin S, Weiner DB, Trimble CL, Bagarazzi ML. Clinical and Immunologic Biomarkers for Histologic Regression of High-Grade Cervical Dysplasia and Clearance of HPV16 and HPV18 after Immunotherapy. Clin Cancer Res. 2018;24:276-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 42. | Bagarazzi ML, Yan J, Morrow MP, Shen X, Parker RL, Lee JC, Giffear M, Pankhong P, Khan AS, Broderick KE, Knott C, Lin F, Boyer JD, Draghia-Akli R, White CJ, Kim JJ, Weiner DB, Sardesai NY. Immunotherapy against HPV16/18 generates potent TH1 and cytotoxic cellular immune responses. Sci Transl Med. 2012;4:155ra138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 243] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 43. | Trimble CL, Morrow MP, Kraynyak KA, Shen X, Dallas M, Yan J, Edwards L, Parker RL, Denny L, Giffear M, Brown AS, Marcozzi-Pierce K, Shah D, Slager AM, Sylvester AJ, Khan A, Broderick KE, Juba RJ, Herring TA, Boyer J, Lee J, Sardesai NY, Weiner DB, Bagarazzi ML. Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: a randomised, double-blind, placebo-controlled phase 2b trial. Lancet. 2015;386:2078-2088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 515] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 44. | BIONTECH. BioNTech Publishes Data from mRNA-based BNT111 FixVac Melanoma Trial in Nature. [cited 6 May 2021]. Available from: https://investors.biontech.de/news-releases/news-release-details/biontech-publishes-data-mrna-based-bnt111-fixvac-melanoma-trial/. |
| 45. | Miao L, Zhang Y, Huang L. mRNA vaccine for cancer immunotherapy. Mol Cancer. 2021;20:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 381] [Cited by in RCA: 576] [Article Influence: 144.0] [Reference Citation Analysis (0)] |
| 46. | Hollingsworth RE, Jansen K. Turning the corner on therapeutic cancer vaccines. NPJ Vaccines. 2019;4:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 420] [Cited by in RCA: 484] [Article Influence: 80.7] [Reference Citation Analysis (0)] |
| 47. | Sahin U, Oehm P, Derhovanessian E, Jabulowsky RA, Vormehr M, Gold M, Maurus D, Schwarck-Kokarakis D, Kuhn AN, Omokoko T, Kranz LM, Diken M, Kreiter S, Haas H, Attig S, Rae R, Cuk K, Kemmer-Brück A, Breitkreuz A, Tolliver C, Caspar J, Quinkhardt J, Hebich L, Stein M, Hohberger A, Vogler I, Liebig I, Renken S, Sikorski J, Leierer M, Müller V, Mitzel-Rink H, Miederer M, Huber C, Grabbe S, Utikal J, Pinter A, Kaufmann R, Hassel JC, Loquai C, Türeci Ö. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. Nature. 2020;585:107-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 631] [Article Influence: 126.2] [Reference Citation Analysis (0)] |
| 48. | Agents in Patients With Anti-PD1-refractory/Relapsed. Unresectable Stage III or IV Melanoma. In: ClinicalTrials.gov [Internet]. BioNTech SE: U.S. National Library of Medicine. [cited 25 April 2015]. Available from: https://clinicaltrials.gov/ct2/show/NCT04526899?cond=NCT04526899&draw=2&rank=1. |