Published online Oct 28, 2021. doi: 10.13105/wjma.v9.i5.411
Peer-review started: March 31, 2021
First decision: July 28, 2021
Revised: August 13, 2021
Accepted: September 10, 2021
Article in press: September 10, 2021
Published online: October 28, 2021
Processing time: 211 Days and 3 Hours
In recent years, anterior cruciate ligament (ACL) reconstruction has generally yielded favorable outcomes. However, ACL reconstruction has not provided satisfactory results in terms of the rate of returning to sports and prevention of osteoarthritis (OA) progression. In this paper, we outline current techniques for ACL reconstruction such as graft materials, double-bundle or single-bundle reconstruction, femoral tunnel drilling, all-inside technique, graft fixation, preservation of remnant, anterolateral ligament reconstruction, ACL repair, revision surgery, treatment for ACL injury with OA and problems, and discuss expected future trends. To enable many more orthopedic surgeons to achieve excellent ACL reconstruction outcomes with less invasive surgery, further studies aimed at improving surgical techniques are warranted. Further development of biological augmentation and robotic surgery technologies for ACL reconstruction is also required.
Core Tip: Although anterior cruciate ligament (ACL) reconstruction has offered great benefits, particularly to athletes and physical laborers, there is a great deal of room for improvement through technology development aimed at achieving more excellent outcomes and restoring performance to a level equal to or higher than before the injury. The all-inside ACL reconstruction technique is a relatively new, minimally invasive method in which both femoral and tibial tunnels are drilled from inside the joint, and its advantages include less postoperative pain and less bleeding. A new computer-aided ACL reconstruction system with high efficacy needs to be developed.
- Citation: Takahashi T, Watanabe S, Ito T. Current and future of anterior cruciate ligament reconstruction techniques. World J Meta-Anal 2021; 9(5): 411-437
- URL: https://www.wjgnet.com/2308-3840/full/v9/i5/411.htm
- DOI: https://dx.doi.org/10.13105/wjma.v9.i5.411
The anterior cruciate ligament (ACL), located in the middle of the knee joint, is significantly associated with the stability of the knee. Numerous studies have contributed to the advancement of knowledge and treatment of the ACL, as well as knee surgery. Given the anatomical location and roles of the ACL, the strategies for treating it, and the approaches to researching it, one could say that “all roads of the knee lead to the ACL”. This article discusses current and future trends of surgical treatment for ACL.
Based on the challenges that our predecessors faced, ACL repair is rarely indicated for ACL injury. Instead, ACL reconstruction is usually performed.
In recent years, ACL reconstruction has generally yielded favorable outcomes. This procedure is a minimally invasive arthroscopic surgery involving small incisions, and rarely causes significant complications[1,2]. Therefore, ACL reconstruction has been actively performed for in more and more patients, including young professional athletes as well as middle-aged and older amateur athletes[3-5]. Currently, the number of ACL reconstruction procedures performed in the United States is approximately 200000 per year and is expected to increase further[6,7]. Considering the increasing level of orthopedic treatment in the world, as well as an increase in the number of people who desire a high quality of life that permits a high level of activity, there is no doubt that the number of ACL reconstruction procedures will increase.
Nevertheless, ACL reconstruction requires about 6 mo to return to sport after surgery. Consequently, high school and college athletes, as well as professional athletes, often end up stepping down as a player without a complete comeback after undergoing ACL reconstruction because their time as a player is limited. Even after returning to play, some athletes who have undergone the surgery cannot perform as they did previously because a sense of knee instability remains and the muscle strength does not sufficiently recover. The causes of these unsatisfactory outcomes include the complication of meniscal injury[8], time from injury to reconstruction surgery, progression of osteoarthritis (OA), weakness of the quadriceps, joint laxity such as hyperextended knee, and anatomical characteristics such as excessive posterior tibial slope together with narrow intercondylar fossa[9].
The common causes of ACL reconstruction failure include new traumatic events (38%), technical failure (22%), and combined causes (19%)[10]. Anatomical ACL reconstruction can reduce the risk of post-traumatic OA[11]. Factors associated with postoperative outcome include drill hole position, method of fixation, mechanical strength of the tendon graft, drilling method, whether the ACL remnant is preserved, pediatric patients before epiphyseal closure and treatment of the meniscus[12].
Because ACL reconstruction enables athletes to return to sports and also people of working age (15–65 years old) to return to work[13], it can contribute to maintaining and activating industries in their communities. Although ACL reconstruction has offered great benefits, particularly to athletes and physical laborers, there is a great deal of room for improvement through technology development aimed at achieving more excellent outcomes and restoring performance to a level equal to or higher than before the injury.
In this paper, we outline current techniques for ACL reconstruction, describe their features and problems, and discuss expected future trends.
Preparation: Hamstrings: The hamstring tendon is harvested by making a 3–4 cm skin incision 2 cm distal to the medial tibial articular surface. First, the sartorius tendon is divided along the length of the tendon. The underlying gracilis tendon is confirmed and pulled proximally. The semitendinosus tendon is confirmed distally. The distal branch of the semitendinosus tendon is dissected, bluntly detached with forceps and a gauze ball, and collected using a tendon stripper. If the semitendinosus tendon is short or thin, the gracilis tendon is also harvested.
Bone–patellar tendon–bone: The bone–patellar tendon–bone (BTB) graft is harvested by making a longitudinal skin incision of approximately 5 cm along the medial edge of the patellar tendon. An incision is made in the central 9–10 mm of the width of the patellar tendon with a scalpel. Trapezoidal bone fragments with a width of 8–10 mm and a length of 15 mm are collected from the patella and tibial tuberosity. In a BTB graft, when the length of the tibial tunnel is short and the patellar tendon is long, the bone fragment on the tibial side is exposed outside of the tibial bone tunnel. Thus, it is necessary to prepare a fixture such as a post screw.
Quadriceps: The surface layer of the quadriceps is the rectus femoris tendon. The middle layer consists of the tendons of the vastus medialis and vastus lateralis muscles. The deep layer is the vastus intermedius tendon. The width is narrowest approximately 5 cm proximal to the patellar attachment[14]. To harvest the quadriceps tendon, a scalpel is used to make a full-thickness incision 5–6 mm from the patellar attachment to the proximal end of harvested tendon. Next, an incision is extended up to the patellar attachment along the length of the tendon. The width of adherent portion is 8–10 mm. Next, a tendon with a thickness of approximately 10 mm is harvested. A Krackow suture is performed with two sets of No. 2 sutures. The length of the grafted tendon can be easily adjusted.
On the patellar side, a trapezoidal bone fragment with a length of 15 mm and a width of 8–10 mm is harvested. Even if the width of the quadriceps tendon is 5–6 mm, the thickness (approximately 10 mm) is sufficient in combination with the rectus femoris, vastus medialis, vastus lateralis, and vastus intermedius. The cross-sectional area of the quadriceps tendon is almost twice that of the BTB graft. The quadriceps tendon has higher load to failure and stiffness than the BTB graft[15]. The quadriceps tendons may be easier to use than the BTB graft in patients with anterior knee pain and pain during kneeling[16]. To fix the grafted ligament, an interference screw or a cortical button such as the CL-BTB is used as a patellar fragment on the femoral side. The thread is tied tightly to the cortical button on the tibial side.
Hamstring and BTB is commonly used as autografts[17]. Several studies reported no significant difference between these materials in postoperative clinical outcome; knee stability evaluated by KT-1000 (MED metric, San Diego, CA) test, Lachman test, and/or pivot shift test; International Knee Documentation Committee (IKDC) score; knee injury and osteoarthritis outcome (KOOS) score; limitation in range of motion; or the rate of return to sports[18-21]. However, there are some reported that the rate of graft rupture was slightly higher in patients who used hamstring autograft than in those who used BTB autograft[22-24] (Table 1).
| Hamstrings | BTB | Quadriceps | |
| Cross-sectional area | Good to excellent | Fair to good | Good to excellent |
| Mechanical strength | Good | Good to excellent | Excellent |
| Adjustment of graft length | Possible | Difficult | Easy |
| All-inside technology | Easy | Sometimes difficult | Easy |
| Preservation of remnant | Possible | Sometimes difficult | Sometimes difficult |
| Double bundle | Easy | Difficult | Possible |
| Graft fixation | |||
| Femoral | Cortical button | Interference screw | Interference screw |
| (metalic > bioabsorbable) | (metalic > bioabsorbable) | ||
| Cortical button | Cortical button | ||
| Tibial | Cortical button | Interference screw | Cortical button |
| Interference screw | (metallic ≥ bioabsorbable) | Interference screw | |
| (metalic < bioabsorbable) | |||
| Post fixation | |||
| Complication | Nerve injury | Patellar fracture | Patellar fracture |
| (infra-patellar branches of the saphenous nerve) | |||
| Decrease of Flexor muscle strength | Kneeling pain | ||
| Anterior knee pain | |||
| Decrease of extensor muscle strength | Decrease of extensor muscle strength | ||
| Indication | |||
| Recommend | Amateur athlete | Amateur and professional athlete | Amateur and professional athlete |
| (high-intensity sports) | (high-intensity sports) | ||
| Revision surgery | |||
| Not recommend | Ballet dancer | Wrestler, Judo, Karate |
Characteristics: Hamstrings: Among the hamstrings, the semitendinosus tendon and gracilis tendon are most commonly used for ACL reconstruction in patients, including amateur athletes[25]. If possible, it is preferable to harvest only the semitendinosus tendon, in order to prevent postoperative muscle weakness[26]. For single-bundle ACL reconstruction, the semitendinosus tendon is folded into four layers to obtain a diameter ≥ 8 mm. If sufficient length or diameter of autograft cannot be obtained, there is no choice but to use the gracilis tendon. A possible solution when performing ACL reconstruction using only the semitendinosus tendon autograft is to fill the socket-like drill tunnels with graft and use a technique with fewer bungee cord and windshield wiper effects. Harvesting the hamstring may result in reduced mobility of the knees at a high flexion position in some cases such as ballet dancer. An advantage of ACL reconstruction with hamstring autograft is that it makes it easy to perform double-bundle ACL reconstruction (Table 1).
BTB: ACL reconstruction with BTB autograft has commonly been used around the world because the patellar tendon provides high mechanical strength and interference screws provide strong fixation. However, because the autograft is harvested with bone, this technique causes postoperative tenderness of the anterior knee region where harvest was performed. This pain can persist for several years. Accordingly, the use of BTB autograft might be avoided for people in some Asian countries where it is common to sit on the Japanese sitting or kneeling and for athletes that requires often kneeling, such as wrestler judo or karate. Even in these countries, however, BTB autograft is often considered to be the first choice in male patients who do high-intensity sports because it can provide better postoperative stability relative to ACL reconstruction with hamstring autograft[21,27,28] (Table 1).
Quadriceps: Reports on the use of quadriceps tendon autograft with or without a bone block have increased since 2015, although the method had been used previously in clinical practice. Some studies reported that the clinical outcomes of ACL reconstruction with quadriceps tendon autograft were comparable or superior to those of reconstruction with BTB or hamstring autograft[28-31], whereas other studies reported that the rate of graft rupture was higher in ACL reconstruction with quadriceps tendon autograft than in reconstruction with BTB or hamstring autograft[32]. ACL reconstruction with quadriceps tendon autograft has several advantages: it causes less pain in the anterior knee region because there is a bone plug on one side only[29,33], less risk of injury to the infrasaphenous branch and the procedure requires only a small skin incision[34]. By contrast, ACL reconstruction with BTB autograft damages the tibial tuberosity. Although several studies reported ACL reconstruction with quadriceps tendon autograft, most were based on short-term follow-up. Hence, further studies based on long-term follow-up are needed to explore the possibility of reduced muscle strength for knee extension associated with the procedure (Table 1).
ACL reconstruction with BTB or quadriceps tendon autograft temporary decreases muscle strength for knee extension because it damages the knee extensor mechanism. In addition, reduced quadriceps strength may be exacerbate knee OA. Therefore, sufficient training for these muscles is required after surgery.
Allograft: ACL reconstruction with allograft is an alternative technique because it does not damage the patient’s own tissues. Therefore, it has been performed widely in the United States and Europe, and its outcomes are comparable to those of ACL reconstruction with autograft. However, after a study reported that the rate of graft rupture in patients who underwent ACL reconstruction with allograft was higher than that in those who received autograft[35,36], the use of allograft for primary ACL reconstruction has gradually decreased, although ACL reconstruction with fresh frozen and non-irradiated allograft is sometimes performed for revision ACL reconstruction[37,38] and multiligament reconstruction. The use of allograft is generally not recommended for primary ACL reconstruction in elite athletes.
The most common technical error in ACL reconstruction procedure is femoral tunnel malposition (63%), which causes poor clinical outcome due to residual instability or graft rupture[39].
Independent drilling technique is a method for drilling femoral and tibial tunnels separately (Table 2). There are two types of independent drilling: 1) the anteromedial (transportal) technique, in which a femoral tunnel is drilled from the inside to the outside; and 2) the outside-in technique, in which a femoral tunnel is drilled from the outside to the inside on a footprint identified with a dedicated drill guide (Table 1).
| Independent technique | Dependent technique | ||||
| Anteromedial | Outside-in | TT | Modified TT | TT with modified devices | |
| Femoral tunnel position | Anatomical | Anatomical | Somewhat unanatomical | Anatomical | Anatomical |
| Complexity of technique | Relatively simple | Somewhat complicated | Simple | Simple | Simple |
Anteromedial technique: The arthroscope is inserted from the anterolateral portal with the knee bent to 120–130 degrees. The drill guide pin is inserted from the anteromedial portal. The pin tip is placed in the center of the femoral footprint of the ACL. After drilling, it penetrates from the lateral cortex to the skin surface. First, a PL bundle bone tunnel is made, followed by an AM bundle bone tunnel in the same manner. If the knee flexion angle is 120 degrees or less, blowout of the posterolateral cortex would occur[40-42], making it impossible to fix the femoral side; this increases the risk of peroneal nerve palsy.
Outside-in technique: The arthroscope is inserted from the anteromedial portal with the knee bent to approximately 90 degrees. The femoral drill guide is inserted into the joint from the anterolateral portal and held firmly in place. Next, the guide pin is inserted so that it does not shift from the center of the femoral footprint of the ACL. The drill-guided trocar should be placed on the femur. A skin incision should be made in the lateral thigh to locate the lateral cortex.
With the anteromedial and outside-in approaches, after creating the femoral bone tunnel, a tibial drill guide is used to create a tibial bone tunnel from the anterior surface of the tibia based on the bone tunnel diameter. Both techniques are useful in that they enable surgeons to accurately create a femoral tunnel on the target footprint. Although both techniques allow for accurate femoral tunnel positioning, some studies have reported that the outside-in technique is more effective because it results in a more oblique tunnel and a longer femoral tunnel relative to the anteromedial technique[43-45]. Disadvantages of the anteromedial technique include that it is associated with the risk of short femoral tunnel, posterior-wall blowout, and iatrogenic damage to the cartilage of the medial femoral condyle by a more horizontal direction of the femoral tunnel in Three-dimensional (3D) plane[41]. However, the anteromedial technique can also produce a long femoral tunnel by using a flexible reamer, and the risk of peroneal nerve paralysis associated with this technique can be avoided using a specific procedure[46,47]. Disadvantages of the outside-in technique include the need for small incision in the femur[42]. At present, the anteromedial technique is most commonly used in the world[48,49].
Transtibial technique: This is a classical drilling method for ACL reconstruction, a tibial tunnel is initially created using a tibial drill guide. An arthroscope is inserted from the anterolateral portal. A tibial drill guide is inserted into the joint from the anteromedial portal. The tip of the guide is applied to the footprint of the tibia and then a guide pin is inserted through the tibial tunnel to create a femoral tunnel (Table 1). The position of the femoral tunnel in this technique depends on the orientation and position of the tibial tunnel. Therefore, this technique is called dependent drilling. Some studies reported that the anteromedial technique was associated with less femoral tunnel positioning errors and provided better stability and clinical outcomes than the transtibial technique[10,50,51], whereas other studies reported no difference between these techniques in clinical outcomes, patient satisfaction, or rate of revision reconstruction surgery in recreational athletes[52,53]. The independent drilling techniques (anteromedial and outside-in) allow for the creation of femoral tunnels at more anatomical positions than the transtibial technique[54]. To decrease the rate of erroneous femoral tunnel positioning with the transtibial technique, several technologies assist in determining the center of the femoral tunnel, e.g., the Wire-navigator® device (a guidewire navigation device; Smith & Nephew Japan Inc., Tokyo, Japan), which is composed of a Navi-tip consisting of tibial and femoral indicators[55] and a laser-beam guided drill guide[56]. These devices can indicate the center of femoral tunnel.
In the modified transtibial technique, the patient’s leg is placed in a figure-of-4 position (the knee is in 90° flexion, varus and internal rotation of the tibia, and the hip is abducted) when the guide pin is inserted[57,58]. This technique is easy to perform for many surgeons who are accustomed to the transtibial technique[59]. Some studies reported that this technique resulted in better femoral tunnel positioning than the transtibial technique, as well as femoral positioning and clinical outcomes comparable to those of the independent techniques; in addition, the technique is easy to perform[58,60-62].
In our opinion, it is not ideal to perform the transtibial technique in the classical manner, relying on the surgeon’s experience or gut feeling, because in some cases it results in poor femoral tunnel positioning. Therefore, if the transtibial technique is performed, assistive devices such as a dedicated drill guide should be used, and the patient’s leg should be placed in a position suitable for this technique. To confirm the femoral tunnel position, some studies have recommended using arthroscopic views through the anteromedial portal as well as intraoperative fluoroscopic views[39].
There are knacks and pitfalls in any of the techniques mentioned above. Therefore, it is important for surgeons to master techniques that they are good at, taking their learning curve into account.
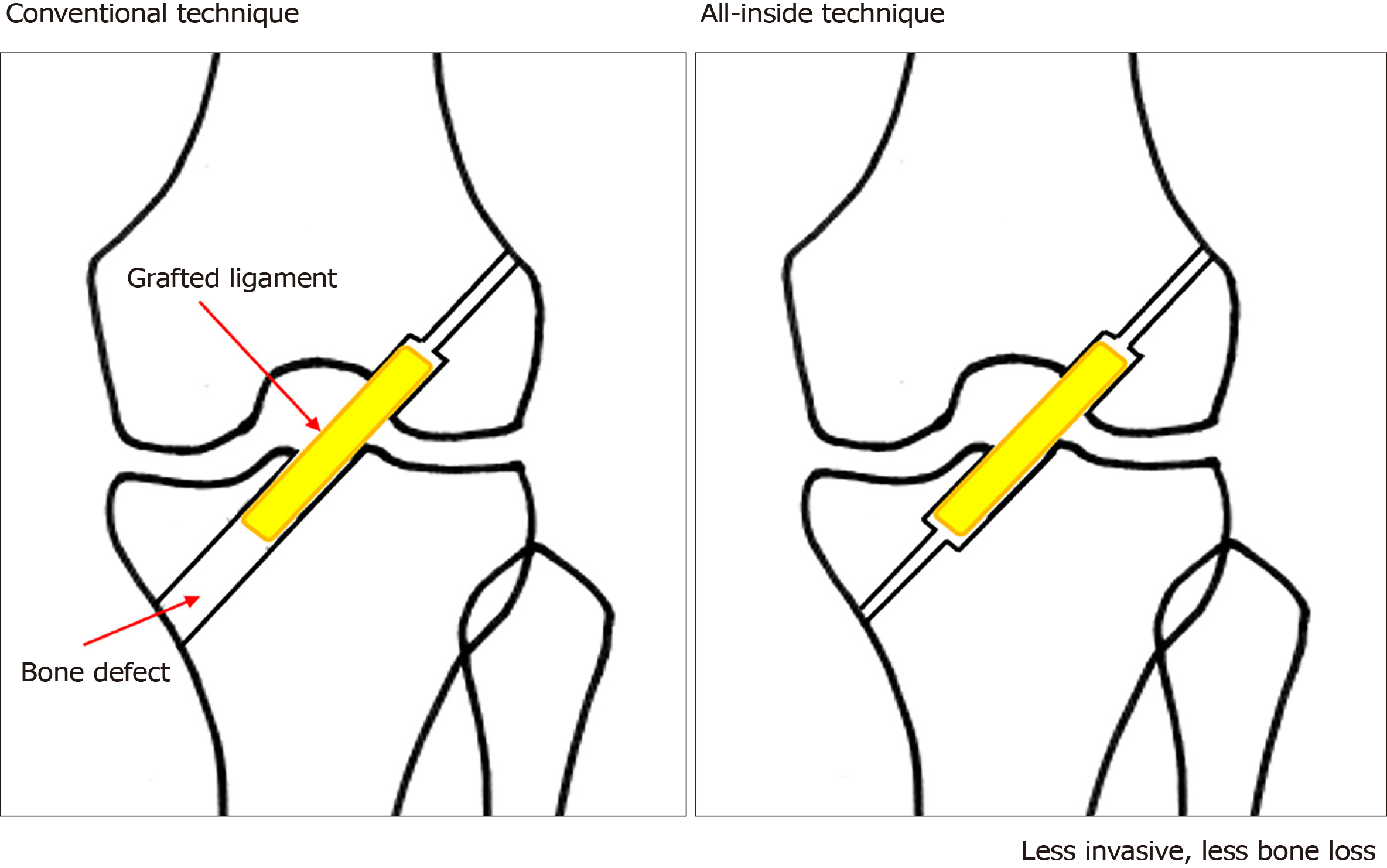
The all-inside ACL reconstruction technique (Figure 1) is a relatively new, minimally invasive method in which both femoral and tibial tunnels are drilled from inside the joint[63-65]. It has recently become more common in clinical practice, and the number of its case studies has been increasing[66]. The all-inside technique may be a reasonable option. When drilling femoral and tibial tunnels using this technique, special care should be taken not to damage the trabecular bone structure adjacent to the graft and the sutures used to pull the graft. This technique can alleviate postoperative pain because it causes less damage to the tibial bone and the periosteum[67-69]. Moreover, it allows for creation of an autograft using only the semitendinosus tendon by the hamstrings tendons. All-inside ACL reconstruction with a semitendinosus tendon autograft has achieved good postoperative stability of the knee relative to ACL reconstruction with an autograft using both the semitendinosus tendon and the gracilis tendon[70] and ACL reconstruction with BTB[71].
For the all-inside technique, independent drilling with a retrograde drill is commonly performed, and devices that have been modified for this technique are available[68,72,73]. In our hospital, we assemble a dedicated guide pin and reamer in the joint, fix them, and drill the tunnels from inside the joint using the dependent technique[74]. We have achieved good postoperative outcomes in transtibial ACL reconstruction procedures, in which the center of the femoral footprint is irradiated with a laser beam to determine the tunnel position[75]. To date, we also have achieved good postoperative stability in patients who underwent transfemoral all-inside ACL reconstruction.
The advantages of the all-inside technique include less postoperative pain[68] and less bleeding, which may decrease the risk of postoperative infection. If this technique becomes less complicated, more surgeons may choose it (Table 3).
| Conventional technique | All-inside technique | |
| Invasiveness in the tibial tunnel | ||
| Bone damage | Moderate | Minor |
| Bleeding | Moderate | Minor |
| Invasiveness during autograft harvesting | Minor or moderate | Minor |
| Postoperative pain | Moderate | Minor |
| Complexity of surgery | Minor | Moderate |
In most all-inside ACL reconstruction procedures in which both ends of an autograft are fixed with cortical buttons, only the semitendinosus tendon is used to create the autograft. In such cases, it is possible to create a large-diameter autograft and to obtain a better knee flexion strength in comparison with ACL reconstruction using both the semitendinosus tendon and gracilis tendon[76]. Several studies reported that all-inside ACL reconstruction using adjustable-length loop cortical button fixation resulted in less tibial tunnel widening than ACL reconstruction using bioabsorbable interference screw fixation[77,78], suggesting this approach may avoid two stage revision in revision ACL reconstruction surgery. However, there is no difference between these techniques in postoperative knee stability, clinical outcome, or rate of graft rupture[79]. It is expected that discussion of the benefits and reliability of the all-inside technique will become more active, as at present there have been few reports describing its long-term outcomes (≥ 5 years).
Since the double-bundle ACL reconstruction technique was reported by Muneta et al[80] in 1999, it has been used around the world.
A double bundle consists of two routes: AM and PL bundles. Two bone tunnels are created for the femur and tibia, respectively. The graft material consists of hamstring autograft or allograft. In most cases, a hamstring autograft is used. The anteromedial approach is often used to create bone tunnels, but the transtibial or outside-in approach may be used.
An arthroscopic ruler can be used to measure the insertion site of the patient’s native ACL. This measurement can help decide whether to perform double- or single-bundle reconstruction[81]. When a patient has femoral and tibial insertion sites that are larger than 14 mm, double-bundle reconstruction is indicated. When a patient has a notch width of less than 12 mm, double-bundle reconstruction often cannot be performed because the AM guide pin cannot be placed in the native femoral insertion site.
Comparing with single-bundle ACL reconstruction, double-bundle ACL reconstruction can better reproduce the natural anatomical structure of the ACL and provide better ACL function. In addition, several studies reported that double-bundle ACL reconstruction had a lower positive rate in the pivot shift test than that of one-bundle ACL reconstruction[82-84]. Moreover, double-bundle ACL reconstruction is more effective for improving rotatory instability. The rate of revision ACL reconstruction was lower in patients who received double-bundle ACL reconstruction in their primary surgery[85]. In addition, patients who received double-bundle ACL reconstruction exhibited less widening of the bone tunnel diameter, which leads to joint instability of the knee, than those who received single-bundle ACL reconstruction[86].
However, there was no difference between these techniques in the incidence of side-to-side difference evaluated by KT–1000 testing, Lysholm score, KOOS score, or in the rate of graft rupture[87,88]. Specifically, there was no difference in 5-year or longer outcomes between them.
Although the double-bundle ACL reconstruction technique is commonly used in Japan, the single-bundle ACL reconstruction technique is very common in other countries, including the United States. This may be because single route by BTB autograft is performed in many cases in those countries.
The advantages of single-bundle ACL reconstruction with hamstrings autograft include low costs of material fixation: because the autograft is fixed only at two positions, the number of devices required for fixation is half of that required for the double-bundle technique. In addition, surgical time is shorter because fewer bone tunnels need to be created. There is ongoing discussion regarding which technique is better. Therefore, further studies may be necessary to compare the progression of OA, the rate of graft rupture, and knee stability between single- and double-bundle techniques based on long-term follow-up.
Ten to sixteen percent of patients who undergo ACL reconstruction need revision ACL reconstruction due to new traumatic events or poor postoperative outcomes[89]. Therefore, the fixation technique for the primary ACL reconstruction should be selected to avoid challenging or highly-invasive procedures in possible revision ACL reconstruction surgery.
Currently available fixation techniques for ACL reconstruction include one method in which the ligament substance and bone parts are fixed with interference screws, and another method in which an ACL graft with a suture or artificial ligament is fixed with cortical buttons, post screws, or staples and so on.
A literature review study comparing suspensory fixation with interference screw fixation reported that suspensory fixation resulted in less side-to-side difference in KT-1000 measurements, whereas the interference screw exhibited a higher incidence of ligament rupture; however, there was no difference in IKDC scores between the two approaches[72,90].
Suspensory cortical button: The EndoButton is most commonly used for femoral fixation because it allows for easy and strong fixation and achieves favorable long-term outcomes[25,91]. The CL-EndoButton and CL-BTB EndoButton are available as fixed-loop devices. Recently, adjustable-loop devices have been increasingly used in clinical practice. Such devices are believed to be useful for filling gaps in bone tunnels. Some studies that performed comparisons of mechanical strength between fixed-loop and adjustable-loop devices reported that fixed-type loop devices have higher maximum tensile strength with less displacement[77,92-96], whereas other studies reported no difference between them[97-99]. In a clinical study, no difference in KT-1000 arthrometer measurements was observed between the two types of devices[100]. A modified suspension device with higher tensile strength and stiffness that was recently developed makes graft fixation easier under tension[101], and is expected to be used in clinical practice.
Interference screw: Interference screw fixation is most commonly used in ACL reconstruction with BTB because it provides strong fixation[102]. Although many interference screws are made of titanium, which has high biocompatibility, bioabsorbable interference screws are also used in clinical practice. It should be noted that, in some cases, titanium screws implanted into bones may be difficult to remove in revision ACL reconstruction. Interference screw fixation is commonly used for metallic one in femoral side, for absorbable one in tibial side.
Staple: Staples are commonly used to fix an artificial ligament to the bone when both ends of the graft (i.e., the fixation parts inside the bone tunnels) are reinforced with an artificial ligament. Although they provide strong fixation, care should be taken to prevent bone damage that may occur if the cortex of the tibia is vulnerable.
Post-screw: The ACL graft is anchored to the tibia by inserting a post screw with a washer at the distal part of the tibial tunnel. This technique is easy to perform.
Cross-pin: Fixation of an ACL graft with a cross-pin on the femoral side is associated with lower rate of graft rupture[103]. This technique has been used mainly in Europe and the United States, but its frequency has been decreasing.
Double spike plate: The plate is fixed to the tibia by hammering its spikes into the bone under the index tension. Finally, the fixation is completed by inserting a screw[104].
There are two options for preserving the ACL remnant: (1) Selective augmentation of the anteromedial or posterolateral bundle that is partially damaged[105]; and (2) Double-bundle ACL reconstruction while preserving the ACL remnant[106,107].
One advantage of ACL remnant preservation is that mechanoreceptors preserved in ACL remnant and promote angiogenesis. In addition, this approach is associated with less anterior tibial translation and a lower rate of positive pivot-shift test. Despite these advantages, this technique may cause cyclops lesion. However, one study reported no difference in Lachman test, pivot shift test and IKDC score between ACL reconstruction techniques with or without remnant preservation[108]. In general, small scarred bundles of the anteromedial or posterolateral bundle are augmented in ACL reconstruction with remnant preservation. If preservation of the ACL remnant makes it difficult to create bone tunnels at appropriate positions, it is important to remove the remnant.
Claes et al[109] named the ligament-like tissue on the lateral margin of the tibial plateau the anterolateral ligament (ALL). ALL reconstruction is an extra-articular procedure that has recently been performed in combination with standard ACL reconstruction[110]. This combination technique achieves favorable outcomes with a low rate of graft failure[111]. The anterolateral complex of the knee contributes to anterolateral rotatory stability as a secondary stabilizer to the ACL[112], although surgical reconstruction of the anterolateral complex may cause constraint of internal rotation of the tibia[113]. Combined ACL and ALL reconstruction are performed mainly in Europe, but its indication is limited to patients who have severe knee instability due to injury of ACL and other combined ligaments, or who have severe knee instability after ACL reconstruction because it is a highly invasive procedure.
A study on the addition of a lateral extra-articular tenodesis (LET) to ACL reconstruction with BTB graft reported no significant differences in long-term outcomes after ACL reconstruction with or without an LET, but LET may increase the risk of lateral compartment OA[114]. Another study reported that ACL reconstruction in combination with LET was associated with a higher risk of tunnel convergence[115].
Current graft options for ALL reconstruction include iliotibial band, gracilis tendon autograft or allograft, and semitendinosus tendon autograft or allograft; fixation angle varies from 0° to 90°[116]. Further prospective studies, such as a randomized control trial, are needed to compare clinical outcomes, indications and fixation techniques between ACL reconstruction with and without ALL reconstruction[116].
ACL repair with suture anchor for patients with avulsion ACL tears[117], as well as ACL repair combined with biologic healing augmentation for patients with incomplete tears[118], achieves successful outcomes.
An ACL repair technique with additional internal bracing was introduced in a recent study[119-122]. A study of this technique based on a short-term follow-up with small sample size reported that its outcomes were comparable to those of ACL reconstruction[123]. ACL repair may be a good treatment option for partial proximal ACL tears and pediatric ACL tears[124,125]. Although a systematic review of contemporary studies revealed no differences between ACL repair and reconstruction with respect to knee stability and the rate of graft rupture, further studies are needed because these studies were based on short-term follow-up with small sample size[123].
Infection and its prevention: The incidence of knee joint infection after ACL reconstruction is low because it is performed arthroscopically with saline irrigation[126]. However, infection has been reported to occur in 0.14% to 2.6 % of patients who undergo ACL reconstruction[7,127,128]. The most common pathogen of infection after ACL reconstruction is Staphylococcus aureus. Acute infection can be caused by pathogen contamination of the tibial tunnel or the skin incisions made for arthroscopy[126]. Pre-soaking hamstring autografts in gentamicin reduce intra-articular infection rates[129]. Bleeding and subcutaneous hematoma of these sites after surgery can also be a cause of infection. Therefore, it is important to decrease the amount of bleeding by icing and to treat wounds carefully. On the other hand, chronic infection can be caused by screws and tendon suture materials[126]. Special care should be taken for patients with atopic dermatitis, as these patients have a higher infection risk.
Deep vein thrombosis: The incidence of deep vein thrombosis after ACL reconstruction ranges from 0.3%[130] to 0.4%[131]. The incidence of pulmonary embolism is 0.18%[130] to 0.046%[130]. The only significant risk factor is age. Therefore, thromboprophylaxis should be considered in older patients.
Hemarthrosis: Hemarthrosis after ACL reconstruction can delay rehabilitation. The use of intravenous tranexamic acid in ACL reconstruction results in reduced joint drain output and hemarthrosis as well as less pain and greater range of motion during the early postoperative period[132]. Tranexamic acid does not increase the risk of deep vein thrombosis after surgery[133].
Joint stiffness: The incidence of joint stiffness after ACL reconstruction is overall 3%[134] to 8.8%[135]. There was no significant difference between BTB graft and hamstring tendon with respect to the frequency[135] and the interval between trauma to surgery[134].
Cyclops syndrome after ACL reconstruction is due to a fibrous nodule in the anterior part of the intercondylar notch. It restricts the full extension of the knee[136]. The incidence of symptomatic cyclops syndrome ranges from 1.9% to 10.9%[136].
Arthrofibrosis is a rare but potentially devastating complication after ACL reconstruction[7]. Approximately 2% of patients have postoperative stiffness that requires intervention[137]. However, arthrofibrosis remains poorly defined and there are no clear treatment guidelines[138].
Nerve injury: Tendon harvesting for ACL reconstruction often injures sensory branches of the saphenous nerve[139]. Injuries to the sartorial branch of the saphenous nerve associated with medial incisions for hamstring tendon harvesting are more common than injuries to the infrapatellar branch associated with midline incisions for patellar tendon harvesting[139]. Numbness of the skin surface supplied by the infrapatellar branches of the saphenous nerve after ACL reconstruction are less common with the quadriceps tendon compared with the hamstring tendon[140]. Regarding hamstring tendon harvesting for ACL reconstruction, vertical incisions increase the risk of iatrogenic injury to the IPBNS compared with oblique incisions[141-144].
Patellar fracture: The incidence of patellar fracture during BTB harvesting ranges from 0.3%[135] to 1.3%[145]. It is a rare but serious complication[146]. To eliminate the risk of perioperative patellar fracture, the bone-tendon-autograft technique, which does not harvest the inferior patellar bone, might be an alternative graft option[147].
The incidence of intraoperative patellar fracture after harvest of a quadriceps tendon autograft is reported to be 3.5%. It is necessary to use care when harvesting the bone block from a central position[148] and to limit the depth of bone harvesting to less than 50% of the depth of the patella with a shorter bone plug length. Longitudinal cuts can be angled centrally to produce a trapezoidal bone block with shallower bone removal[148].
We usually use only the semitendinosus tendon for primary ACL reconstruction. We produce the bone tunnel with the all-inside method using a single quadruped semitendinosus graft. We fix the grafted ligament with a cortical suspension button on both sides. In the all-inside method, the knee is flexed to approximately 90 degrees, the lower leg is internally rotated, and varus stress is applied to the knee using the dependent method.
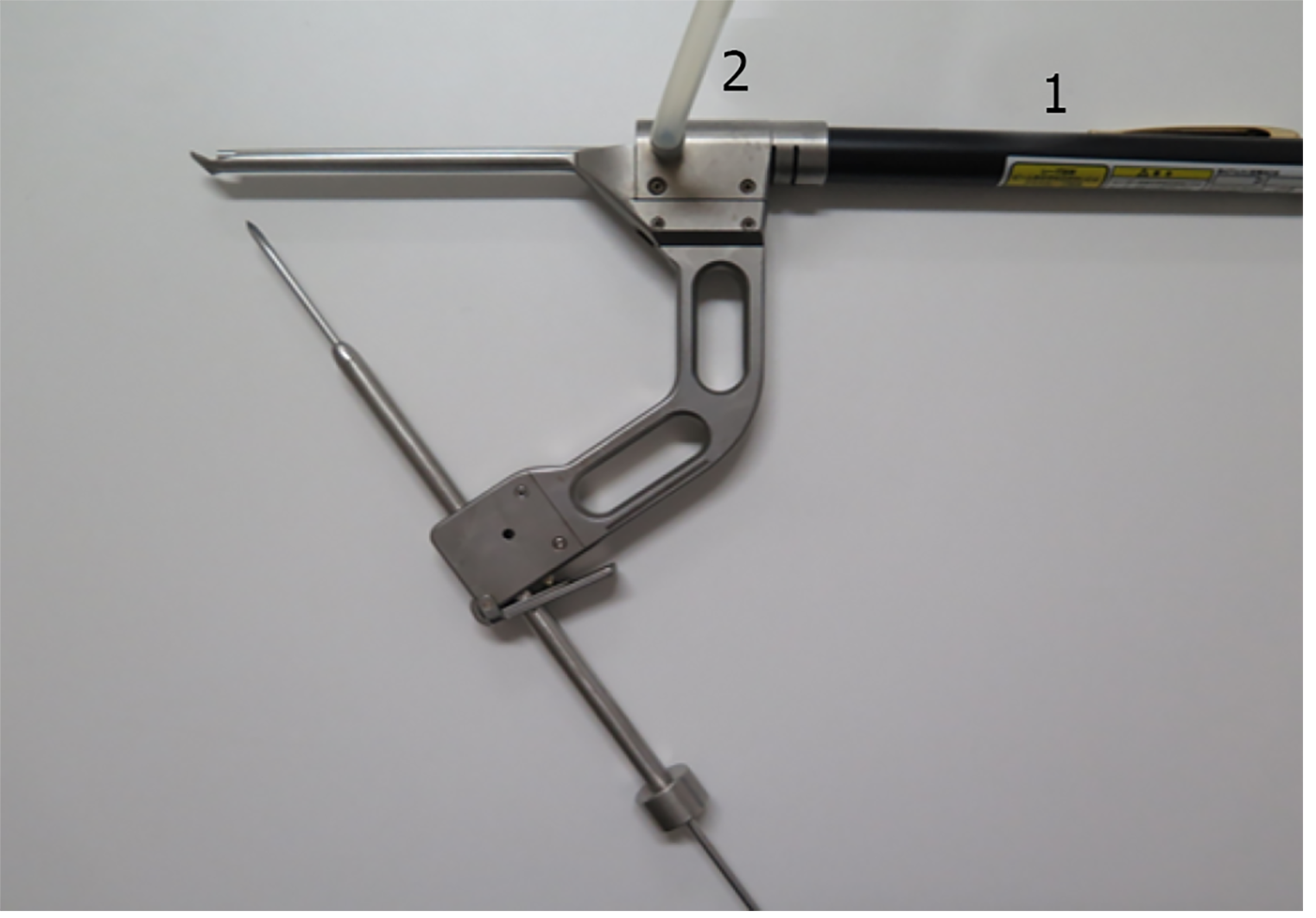
We developed a tibial drill guide with a laser beam that can identify the optimal location for the femoral tunnel during creation of tibial tunnel in a modified transtibial method. We used it in a clinical application during ACL reconstruction.
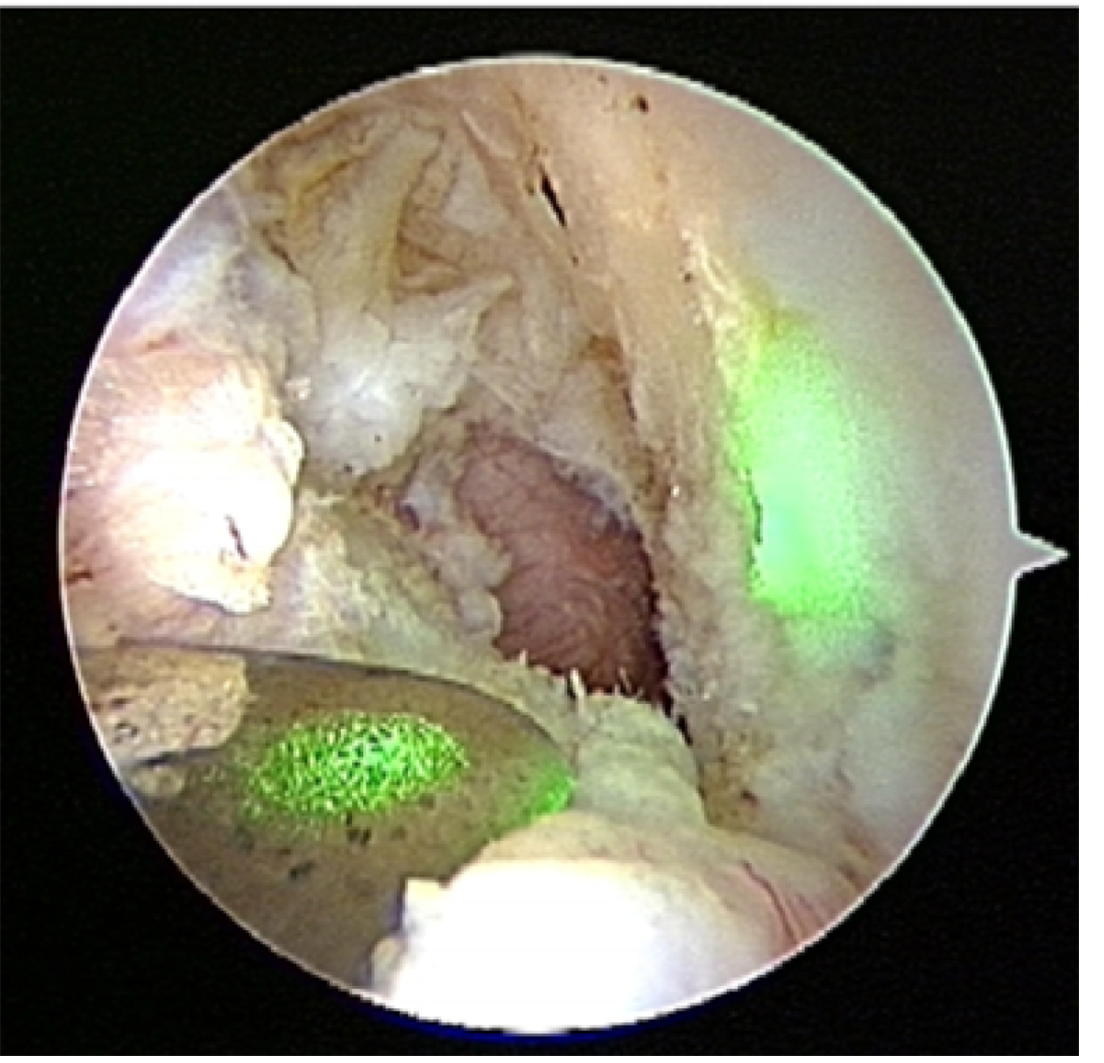
The new drill guide system: The structure of the tibial drill guide with a laser beam is shown in Figure 2. This laser beam-guided technique with a special tibial drill guide produces both tibial and femoral tunnels. The laser pointer was visible light semiconductor laser, maximum output energy of 1mW. The guide contains a metal tube for passage of laser beam (Figure 3), which can be filled with saline for irrigation. The reflected beam indicates appropriate position on the extension of the pin (Figure 4). Figure 5 shows an arthroscopic photography with the laser beam.
Transtibial guide pin placement and tunnel placement: The special drill guide is inserted through the anteromedial portal, and placed at the anatomical tibial foot print. A laser beam is reflected by reflecting plate of tip of the guide. The laser pointer illuminates the tunnel which is where femoral bundle should be made appropriately (Figure 4). A transtibial guide pin of 2.4 mm in diameter is inserted into the intra-articular portion of proximal tibia. The diameter of tibial tunnel is similar to that of grafted tendon. The guide pin is set at appropriate location of femoral tunnel. Method of making femoral tunnel and graft fixation was performed according to our previously described[56,75]. Our method is a useful way to select an appropriate anatomical site for the bone tunnels accurately and obtain excellent clinical results with ACL reconstruction.
Recently, we have produced and used a drill guide for the all-inside transfemoral method. The grafted ligaments are fixed with a CL-BTB endobutton on the femoral side and a cortical button on the tibial side with knee flexion of approximately 20 degrees. We often use a BTB graft to obtain strong fixation for young men who are active athletes needing to withstand strong collisions, such as in rugby and football. However, the quadriceps tendon is also useful. Residual ligaments are often preserved if they are thin but relatively tense. At this time, double-bundle reconstruction with residual ligaments is not performed because it is difficult to make two bone tunnels at appropriate positions. We reconstruct the AM or PL bundle based on preoperative MRI evaluation and intraoperative arthroscopic findings. ACL reinforcement is often performed to reconstruct the PL bundle, which can lead to definite symptoms of rotatory instability.
In revision reconstructive surgery, we use the ipsilateral semitendinosus tendon, BTB graft, or quadriceps tendon with a patellar fragment, unless the tendon has already been used. Quadriceps tendon with a patellar fragment has excellent mechanical strength. Reconstruction can be performed with the all-inside method, which reduces trabecular damage in the bone tunnel. Thus, we plan to increase its use in the future. At this time, the grafted ligament is fixed with a patellar fragment on the femoral side using an interference screw. On the tibial side, the grafted ligament is fixed with a cortical button, which is sutured using the Krackow method with two sets of no. 2 sutures.
Some patients may need to receive revision ACL reconstruction due to graft rupture or residual knee instability.
When an ACL graft cannot be fixed at an appropriate position because the bone is severely damaged, bone tunnel grafting with an Iliac bone autograft is performed in two-stage revision ACL reconstruction[149]. In most cases, however, one-stage revision ACL reconstruction is selected because the two-stage procedure requires time for healing after bone grafting.
Prior to revision ACL reconstruction, computed tomography scanning is performed to three-dimensionally assess the positions and sizes of bone tunnels created in the primary surgery and to determine the positions of bone tunnels for revision ACL reconstruction. During the assessment, it is important to confirm the type of fixture used in the primary surgery. It should be noted that, if titanium interference screws were used in the primary surgery, it may become more difficult to remove them over time: due to the high biocompatibility of titanium, the screws become surrounded by bone on both femoral and tibial sides. Therefore, appropriate screw drivers should be prepared to remove them. Besides BTB and hamstring grafts, a quadriceps tendon graft is often used in revision ACL reconstruction because it has advantages in terms of strength and diameter[150].
Several studies reported that revision ACL reconstruction is inferior to primary reconstruction in postoperative outcomes and the rate of returning to sport[151,152]. However, the data regarding long-term clinical outcomes from large-scale cohort studies are limited; accordingly, further studies are needed[150].
ACL insufficiency persisting after ACL injury often accelerates age-related OA change, worsens wear of cartilages of the medial knee joint, and results in varus deformity. In this case, ACL reconstruction in combination with high tibial osteotomy (HTO) is indicated for individuals younger than 70 years old who want to engage in a high level of physical activity such as sports or heavy physical labor and have less OA change in the patellofemoral joint[153,154]. Slope-reducing tibial osteotomy with this combination procedure can further improve knee stability in patients with varus deformity and excessive posterior tibial slope[155,156]. There are surgical techniques for HTO include opening-wedge[157], closed-wedge[158], and dome-shaped[159,160] osteotomy. HTO should be performed with one-stage ACL reconstruction simultaneously, postoperative rehabilitation after HTO combined with ACL reconstruction can be performed in the same manner as rehabilitation after HTO alone. Long-term outcome regarding one-stage HTO and ACL reconstruction suggested that it is an effective and safe procedure[161]. A systematic review of studies on one-stage HTO and ACL reconstruction reported that the percentages of patients who received opening-wedge and closed-wedge HTO were 57.4% and 42.6%, respectively, and the percentages of patients who received hamstring and BTB autograft for ACL reconstruction were 85.6% and 12.8%, respectively[162]. Although currently available data indicates high patient satisfaction and high rate of returning sport after combined with HTO and ACL reconstruction[163], further studies are needed to compare clinical outcomes between combined with HTO and ACL reconstruction and HTO alone.
In most studies, ACL repair resulted in failure or unfavorable results. Recently, however, experimental and clinical studies on biological augmentation of mesenchymal stem cells, platelet-rich plasma (PRP), or other biologic agents with scaffold are being conducted to assess the effects of such biotherapies on ACL repair and reconstruction[164,165].
The four main components of tissue engineering such as cells, growth factors, scaffolds, and mechanical stimuli, are combined using various methods of bioaugmentation. They have been increasingly explored to improve outcomes after surgical treatment of ACL injury[164,166-169].
Scaffolds: Stem cell-based tissue regeneration combined with scaffolds represent a novel treatment for torn ligaments[170-172]. 3D scaffolds seeded with mesenchymal stem cells yielded excellent results in osteointegration enhancement between the tendon and bone tunnel in ACL reconstruction with a rabbit model[173]. PRP combined with a gelatin sponge to prolong PRP bioactivity promotes mesenchymal stem cell proliferation in vitro[174].
Cell sources: The main cell sources are mesenchymal stem cells and ACL fibroblasts[175]. Mesenchymal stem cells have higher proliferation and collagen production rates than ligament fibroblasts[176]. ACL-derived human-induced pluripotent stem cells might be a promising cell source for ligaments and related tissue engineering applications[177].
Growth factors: PRP is obtained by plasma separation. PRP contains platelets, blood proteins such as fibrin, and a mixture of growth factors such as platelet derived growth factor, insulin-like growth factor, vascular endothelial growth factor, and transforming growth factor-beta, which are involved in general healing processes.
PRP has been used to treat knee OA and to promote ligament healing. Recently, it has been used experimentally in ACL reconstruction to promote graft maturation and osteointegration[178]. However, no clinical efficacy data have been reported yet[179,180].
Mechanical stimulation: Mechanical stimuli and dynamic loading are necessary for ligaments to enhance matrix synthesis and maintain their strength[181]. Electrospinning has been effective for cell proliferation and extracellular matrix production of scaffolds for ligament tissue engineering[182]. However, whether any mechanical stimulation is required to implant tissue-engineered ACL constructs is controversial[175].
In recent studies, bioenhanced ACL repair had similar results as ACL recons
In other clinical departments, robotic surgery with the da Vinci™ Surgical System (Intuitive Surgical, Sunnyvale, CA, United States) has become more widespread, mainly in large-scale hospitals. In orthopedic surgery, computer-assisted navigation has come to be used for spine surgery[183-186], total hip arthroplasty, and total knee arthroplasty[187,188].
There are four main types of applications for navigation systems in ACL reconstruction[189,190]: (1) Technical assistance of tunnel placement for tibial or femoral tunnel drilling; (2) Kinematic evaluation to analyze the biomechanical behavior of the ACL and surrounding structures during reconstructive surgery[191]; (3) Comparison of the effectiveness of different surgical techniques for making laxity measurements[192]; and (4) Navigation to improve clinical outcomes and cost-effectiveness of ACL reconstruction.
3D fluoroscopy-based navigation system: It is essential to perform preoperative planning using 3D computed tomography (CT) images before operation. A reference frame is rigidly attached to the femur with two half-pins at the beginning of surgery. An intraoperative 3D image of the distal femur is obtained with the C-arm of the image intensifier, which is equipped with a wireless tracker. The image is reconstructed into a 3D image on the computer screen. A navigation computer helps the surgeon visualize the entire area for bone tunnel creation. However, this system requires fixation with two half-pins in the lateral femur, which necessitates an additional skin incision and more drill holes[193,194].
CT-based navigation without intraoperative fluoroscopy: This system uses a preoperatively generated 3D model from CT images or intraoperative 3D bone morphing with an optical tracking system. The optical tracking system captures reference markers that are rigidly attached to the patient and surgical tools. After fixing the tracking markers, approximately 20 Landmark points are collected on the surface of the bone with probes[195-197].
Anatomical reconstruction using the anteromedial technique is associated with more risks including: (1) A short femoral tunnel; (2) Posterior wall blowout; and (3) Iatrogenic damage to the cartilage of the medial femoral condyle due to the more horizontal direction of the femoral tunnel in the 3D plane[41]. Navigation systems with enhanced registration accuracy can reduce surgical failures such as short femoral tunnels and posterior wall breakage of the distal femur[195,198].
Image free navigation system: This method does not require preoperative CT or intraoperative fluoroscopy. The transmitters for the femur and tibia are fixed with pins to register intra- and extra-articular landmarks intraoperatively. Next, the transmitter is attached to the tibial drill guide to determine the location of the tibial bone tunnel. The same maneuver is used for the femoral bone tunnel[199].
There is considerable variability in intra-articular landmark identification with image- free navigation. There is a potential risk of miscalculating tunnel positions[200].
Guided drilling of the tunnel leads to errors as small as 2.5 mm in the footprint and in the orientation of the intra-operative video for guiding the drilling of the tunnel with a set of contours which is reconstructed by touching the bone surface with an instrumented tool[201].
There are some studies on the use of computer-assisted navigation for bone tunnel positioning and evaluating joint instability in ACL reconstruction[202-204]. Clinical, radiological, and functional comparisons between computer-assisted and conventional ACL reconstruction have found increased accuracy in femoral tunnel placement with the use of navigation systems compared with traditional techniques alone[196,204-207]. Some studies reported that computer-assisted navigation improved the accuracy of tunnel positioning[208-210]. For inexperienced surgeons, navigation systems could be useful in ACL surgery to avoid malpositioning of bone tunnels[211,212]. However, another study showed that experienced surgeons could achieve more accurate tunnel positioning than computer-assisted positioning[211]. Consequently, computer-assisted navigation has not become common in clinical practice.
Although currently available navigation systems can enable more accurate femoral tunnel positioning and assist less experienced surgeons[201], they are not cost-efficient and require extra time for registration of operative positioning data[190,213]. Moreover, there is no difference in clinical outcomes between ACL reconstruction with and without computer-assisted navigation[214]. Therefore, a completely new system with high efficacy needs to be developed.
3D fluoroscopy-based navigation systems might be useful for confirming the native ACL footprint in remnant-preserving ACL reconstruction[215,216]. Several studies have described the use of navigation-assisted surgery to increase the possibility of achieving adequate tunnel position in revision ACL reconstruction[194,217].
Kinematic assessment of knee laxity among different ACL surgical procedures have been evaluated[218,219].
With the advancement of robotic surgery, remote surgical assistance will be available. At present, most ACL reconstructions are performed in urban hospitals by arthroscopic surgery specialists. It is not recommended that ACL reconstruction would be performed by a surgical team with no training in the procedure. However, if remote surgery assistance becomes available, and ACL reconstruction can be performed in rural areas where advanced medical care is unavailable, it will be helpful for residents in these areas. The da Vinci™ Surgical System has already been used for remote surgery assistance in some hospitals. In laparoscopic surgery assisted by the da Vinci™ Surgical System, the robot arms in a local hospital are remotely controlled by an advising surgeon in an operating room of an advanced medical facility. Such remote assistance enhances the skills of surgeons in local hospitals. In the future, robot-assisted ACL reconstruction surgery could also be achieved by remote instruction or remote control of robot by an arthroscopic surgery specialist. We hope that these technologies make advanced ACL surgery available to people living in countries and regions where advanced ACL treatment is unavailable.
There is no question that ACL reconstruction is necessary in order for patients with ACL injury to maintain a high level of activities, including sports, and that the number of patients receiving ACL reconstruction will increase around the world. However, ACL reconstruction has not provided satisfactory results in terms of the rate of returning to sports and prevention of OA progression. To enable many more orthopedic surgeons to achieve excellent ACL reconstruction outcomes with less invasive surgery, further studies aimed at improving surgical techniques are warranted. Further development of robotic surgery technologies for ACL reconstruc
Manuscript source: Invited manuscript
Specialty type: Orthopedics
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Liu L S-Editor: Liu M L-Editor: A P-Editor: Liu M
| 1. | Chambat P, Guier C, Sonnery-Cottet B, Fayard JM, Thaunat M. The evolution of ACL reconstruction over the last fifty years. Int Orthop. 2013;37:181-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 169] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 2. | Fu FH, van Eck CF, Tashman S, Irrgang JJ, Moreland MS. Anatomic anterior cruciate ligament reconstruction: a changing paradigm. Knee Surg Sports Traumatol Arthrosc. 2015;23:640-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 130] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 3. | Ovigue J, Bouguennec N, Graveleau N. Arthroscopic anterior cruciate ligament reconstruction is a reliable option to treat knee instability in patients over 50 years old. Knee Surg Sports Traumatol Arthrosc. 2020;28:3686-3693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Tan CW, Hsu WH, Yu PA, Chen CL, Kuo LT, Chi CC, Kim D, Park G. Anterior Cruciate Ligament Reconstruction in Patients Older Than 50 Years: A Systematic Review and Meta-analysis. Orthop J Sports Med. 2020;8:2325967120915698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Toanen C, Demey G, Ntagiopoulos PG, Ferrua P, Dejour D. Is There Any Benefit in Anterior Cruciate Ligament Reconstruction in Patients Older Than 60 Years? Am J Sports Med. 2017;45:832-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 6. | Buller LT, Best MJ, Baraga MG, Kaplan LD. Trends in Anterior Cruciate Ligament Reconstruction in the United States. Orthop J Sports Med. 2015;3:2325967114563664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 222] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 7. | Eckenrode BJ, Carey JL, Sennett BJ, Zgonis MH. Prevention and Management of Post-operative Complications Following ACL Reconstruction. Curr Rev Musculoskelet Med. 2017;10:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 8. |
|
| 9. | Diermeier T, Rothrauff BB, Engebretsen L, Lynch AD, Ayeni OR, Paterno MV, Xerogeanes JW, Fu FH, Karlsson J, Musahl V, Svantesson E, Hamrin Senorski E, Rauer T, Meredith SJ; Panther Symposium ACL Treatment Consensus Group. Treatment after anterior cruciate ligament injury: Panther Symposium ACL Treatment Consensus Group. Knee Surg Sports Traumatol Arthrosc. 2020;28:2390-2402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 85] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 10. | Vermeijden HD, Yang XA, van der List JP, DiFelice GS, Rademakers MV, Kerkhoffs GMMJ. Trauma and femoral tunnel position are the most common failure modes of anterior cruciate ligament reconstruction: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2020;28:3666-3675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 11. | Rothrauff BB, Jorge A, de Sa D, Kay J, Fu FH, Musahl V. Anatomic ACL reconstruction reduces risk of post-traumatic osteoarthritis: a systematic review with minimum 10-year follow-up. Knee Surg Sports Traumatol Arthrosc. 2020;28:1072-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 12. | Ishibashi Y, Adachi N, Koga H, Kondo E, Kuroda R, Mae T, Uchio Y. Japanese Orthopaedic Association (JOA) clinical practice guidelines on the management of anterior cruciate ligament injury - Secondary publication. J Orthop Sci. 2020;25:6-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 13. | Groot JA, Jonkers FJ, Kievit AJ, Kuijer PP, Hoozemans MJ. Beneficial and limiting factors for return to work following anterior cruciate ligament reconstruction: a retrospective cohort study. Arch Orthop Trauma Surg. 2017;137:155-166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Iriuchishima T, Shirakura K, Yorifuji H, Fu FH. Anatomical evaluation of the rectus femoris tendon and its related structures. Arch Orthop Trauma Surg. 2012;132:1665-1668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Shani RH, Umpierez E, Nasert M, Hiza EA, Xerogeanes J. Biomechanical Comparison of Quadriceps and Patellar Tendon Grafts in Anterior Cruciate Ligament Reconstruction. Arthroscopy. 2016;32:71-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 122] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 16. | Slone HS, Romine SE, Premkumar A, Xerogeanes JW. Quadriceps tendon autograft for anterior cruciate ligament reconstruction: a comprehensive review of current literature and systematic review of clinical results. Arthroscopy. 2015;31:541-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 216] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 17. | Middleton KK, Hamilton T, Irrgang JJ, Karlsson J, Harner CD, Fu FH. Anatomic anterior cruciate ligament (ACL) reconstruction: a global perspective. Part 1. Knee Surg Sports Traumatol Arthrosc. 2014;22:1467-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 147] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 18. | Poehling-Monaghan KL, Salem H, Ross KE, Secrist E, Ciccotti MC, Tjoumakaris F, Ciccotti MG, Freedman KB. Long-Term Outcomes in Anterior Cruciate Ligament Reconstruction: A Systematic Review of Patellar Tendon Versus Hamstring Autografts. Orthop J Sports Med. 2017;5:2325967117709735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 96] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 19. | Samuelsen BT, Webster KE, Johnson NR, Hewett TE, Krych AJ. Hamstring Autograft versus Patellar Tendon Autograft for ACL Reconstruction: Is There a Difference in Graft Failure Rate? Clin Orthop Relat Res. 2017;475:2459-2468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 290] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 20. | Sasaki S, Tsuda E, Hiraga Y, Yamamoto Y, Maeda S, Sasaki E, Ishibashi Y. Prospective Randomized Study of Objective and Subjective Clinical Results Between Double-Bundle and Single-Bundle Anterior Cruciate Ligament Reconstruction. Am J Sports Med. 2016;44:855-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 21. | Xie X, Liu X, Chen Z, Yu Y, Peng S, Li Q. A meta-analysis of bone-patellar tendon-bone autograft versus four-strand hamstring tendon autograft for anterior cruciate ligament reconstruction. Knee. 2015;22:100-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 238] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 22. | Gifstad T, Foss OA, Engebretsen L, Lind M, Forssblad M, Albrektsen G, Drogset JO. Lower risk of revision with patellar tendon autografts compared with hamstring autografts: a registry study based on 45,998 primary ACL reconstructions in Scandinavia. Am J Sports Med. 2014;42:2319-2328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 238] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 23. | Rahardja R, Zhu M, Love H, Clatworthy MG, Monk AP, Young SW. Factors associated with revision following anterior cruciate ligament reconstruction: A systematic review of registry data. Knee. 2020;27:287-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 24. | Widner M, Dunleavy M, Lynch S. Outcomes Following ACL Reconstruction Based on Graft Type: Are all Grafts Equivalent? Curr Rev Musculoskelet Med. 2019;12:460-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 96] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 25. | Grassi A, Carulli C, Innocenti M, Mosca M, Zaffagnini S, Bait C; SIGASCOT Arthroscopy Committee. New Trends in Anterior Cruciate Ligament Reconstruction: A Systematic Review of National Surveys of the Last 5 Years. Joints. 2018;6:177-187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 26. | Roger J, Bertani A, Vigouroux F, Mottier F, Gaillard R, Have L, Rongièras F. ACL reconstruction using a quadruple semitendinosus graft with cortical fixations gives suitable isokinetic and clinical outcomes after 2 years. Knee Surg Sports Traumatol Arthrosc. 2020;28:2468-2477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 27. | Shino K, Mae T, Tachibana Y. Anatomic ACL reconstruction: rectangular tunnel/bone-patellar tendon-bone or triple-bundle/semitendinosus tendon grafting. J Orthop Sci. 2015;20:457-468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 28. | Yang XG, Wang F, He X, Feng JT, Hu YC, Zhang H, Yang L, Hua K. Network meta-analysis of knee outcomes following anterior cruciate ligament reconstruction with various types of tendon grafts. Int Orthop. 2020;44:365-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 29. | Mouarbes D, Menetrey J, Marot V, Courtot L, Berard E, Cavaignac E. Anterior Cruciate Ligament Reconstruction: A Systematic Review and Meta-analysis of Outcomes for Quadriceps Tendon Autograft Versus Bone-Patellar Tendon-Bone and Hamstring-Tendon Autografts. Am J Sports Med. 2019;47:3531-3540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 282] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 30. | Nyland J, Collis P, Huffstutler A, Sachdeva S, Spears JR, Greene J, Caborn DNM. Quadriceps tendon autograft ACL reconstruction has less pivot shift laxity and lower failure rates than hamstring tendon autografts. Knee Surg Sports Traumatol Arthrosc. 2020;28:509-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (1)] |
| 31. | Runer A, Csapo R, Hepperger C, Herbort M, Hoser C, Fink C. Anterior Cruciate Ligament Reconstructions With Quadriceps Tendon Autograft Result in Lower Graft Rupture Rates but Similar Patient-Reported Outcomes as Compared With Hamstring Tendon Autograft: A Comparison of 875 Patients. Am J Sports Med. 2020;48:2195-2204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 32. | Lind M, Strauss MJ, Nielsen T, Engebretsen L. Quadriceps tendon autograft for anterior cruciate ligament reconstruction is associated with high revision rates: results from the Danish Knee Ligament Registry. Knee Surg Sports Traumatol Arthrosc. 2020;28:2163-2169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 33. | Cruz CA, Goldberg D, Wake J, Sy J, Mannino BJ, Min KS, Bottoni CR. Comparing Bone-Tendon Autograft With Bone-Tendon-Bone Autograft for ACL Reconstruction: A Matched-Cohort Analysis. Orthop J Sports Med. 2020;8:2325967120970224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 34. | Mouarbes D, Dagneaux L, Olivier M, Lavoue V, Peque E, Berard E, Cavaignac E. Lower donor-site morbidity using QT autografts for ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2020;28:2558-2566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 35. | Engelman GH, Carry PM, Hitt KG, Polousky JD, Vidal AF. Comparison of allograft versus autograft anterior cruciate ligament reconstruction graft survival in an active adolescent cohort. Am J Sports Med. 2014;42:2311-2318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 36. | Kaeding CC, Pedroza AD, Reinke EK, Huston LJ, Hewett TE, Flanigan DC; MOON Knee Group, Spindler KP. Change in Anterior Cruciate Ligament Graft Choice and Outcomes Over Time. Arthroscopy. 2017;33:2007-2014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 37. | Condello V, Zdanowicz U, Di Matteo B, Spalding T, Gelber PE, Adravanti P, Heuberer P, Dimmen S, Sonnery-Cottet B, Hulet C, Bonomo M, Kon E. Allograft tendons are a safe and effective option for revision ACL reconstruction: a clinical review. Knee Surg Sports Traumatol Arthrosc. 2019;27:1771-1781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 38. | Hulet C, Sonnery-Cottet B, Stevenson C, Samuelsson K, Laver L, Zdanowicz U, Stufkens S, Curado J, Verdonk P, Spalding T. The use of allograft tendons in primary ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2019;27:1754-1770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 39. | Robinson J, Inderhaug E, Harlem T, Spalding T, Brown CH Jr. Anterior Cruciate Ligament Femoral Tunnel Placement: An Analysis of the Intended Versus Achieved Position for 221 International High-Volume ACL Surgeons. Am J Sports Med. 2020;48:1088-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 40. | Bedi A, Raphael B, Maderazo A, Pavlov H, Williams RJ 3rd. Transtibial versus anteromedial portal drilling for anterior cruciate ligament reconstruction: a cadaveric study of femoral tunnel length and obliquity. Arthroscopy. 2010;26:342-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 170] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 41. | Lubowitz JH. Anteromedial portal technique for the anterior cruciate ligament femoral socket: pitfalls and solutions. Arthroscopy. 2009;25:95-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 195] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 42. | Robin BN, Jani SS, Marvil SC, Reid JB, Schillhammer CK, Lubowitz JH. Advantages and Disadvantages of Transtibial, Anteromedial Portal, and Outside-In Femoral Tunnel Drilling in Single-Bundle Anterior Cruciate Ligament Reconstruction: A Systematic Review. Arthroscopy. 2015;31:1412-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 131] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 43. | Osti M, Krawinkel A, Ostermann M, Hoffelner T, Benedetto KP. Femoral and tibial graft tunnel parameters after transtibial, anteromedial portal, and outside-in single-bundle anterior cruciate ligament reconstruction. Am J Sports Med. 2015;43:2250-2258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 44. | Jamsher M, Ballarati C, Viganò M, Hofbauer M, Togninalli D, Lafranchi S, de Girolamo L, Denti M. Graft Inclination Angles in Anterior Cruciate Ligament Reconstruction Vary Depending on Femoral Tunnel Reaming Method: Comparison Among Transtibial, Anteromedial Portal, and Outside-In Retrograde Drilling Techniques. Arthroscopy. 2020;36:1095-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 45. | Sim JA, Kim JM, Lee S, Bae JY, Seon JK. Comparison of tunnel variability between trans-portal and outside-in techniques in ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2017;25:1227-1233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 46. | Fitzgerald J, Saluan P, Richter DL, Huff N, Schenck RC. Anterior Cruciate Ligament Reconstruction Using a Flexible Reamer System: Technique and Pitfalls. Orthop J Sports Med. 2015;3:2325967115592875. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 47. | Silver AG, Kaar SG, Grisell MK, Reagan JM, Farrow LD. Comparison between rigid and flexible systems for drilling the femoral tunnel through an anteromedial portal in anterior cruciate ligament reconstruction. Arthroscopy. 2010;26:790-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 48. | Tibor L, Chan PH, Funahashi TT, Wyatt R, Maletis GB, Inacio MC. Surgical Technique Trends in Primary ACL Reconstruction from 2007 to 2014. J Bone Joint Surg Am. 2016;98:1079-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 93] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 49. | Vascellari A, Grassi A, Canata GL, Zaffagnini S, Gokeler A, Jones H. Hamstrings substitution via anteromedial portal with optional anterolateral ligament reconstruction is the preferred surgical technique for anterior cruciate ligament reconstruction: a survey among ESSKA members. Knee Surg Sports Traumatol Arthrosc. 2021;29:1120-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 50. | Keller TC, Tompkins M, Economopoulos K, Milewski MD, Gaskin C, Brockmeier S, Hart J, Miller MD. Tibial tunnel placement accuracy during anterior cruciate ligament reconstruction: independent femoral versus transtibial femoral tunnel drilling techniques. Arthroscopy. 2014;30:1116-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 51. | Moorthy V, Sayampanathan AA, Tan AHC. Superior Postoperative Stability and Functional Outcomes With Anteromedial Versus Transtibial Technique of Single-Bundle Autologous Hamstring Anterior Cruciate Ligament Reconstruction: A Meta-analysis of Prospective Randomized Controlled Trials. Arthroscopy. 2021;37:328-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 52. | Rahardja R, Zhu M, Love H, Clatworthy MG, Monk AP, Young SW. No difference in revision rates between anteromedial portal and transtibial drilling of the femoral graft tunnel in primary anterior cruciate ligament reconstruction: early results from the New Zealand ACL Registry. Knee Surg Sports Traumatol Arthrosc. 2020;28:3631-3638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 53. | Ruiz-Lozano M, Miralles-Muñoz FA, Rubio-Morales M, Martin-Grandes R, Lizaur-Utrilla A, Vizcaya-Moreno MF. Similar outcomes and satisfaction after transtibial versus transportal femoral drilling for anterior cruciate ligament reconstruction in young adult recreational athletes. Knee Surg Sports Traumatol Arthrosc. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 54. | Gadikota HR, Sim JA, Hosseini A, Gill TJ, Li G. The relationship between femoral tunnels created by the transtibial, anteromedial portal, and outside-in techniques and the anterior cruciate ligament footprint. Am J Sports Med. 2012;40:882-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 55. | Kondo E, Yasuda K, Onodera J, Kawaguchi Y, Kitamura N. Effects of Remnant Tissue Preservation on Clinical and Arthroscopic Results After Anatomic Double-Bundle Anterior Cruciate Ligament Reconstruction. Am J Sports Med. 2015;43:1882-1892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 56. | Takahashi T, Takeda H, Watanabe S, Yamamoto H. Laser-guided placement of the tibial guide in the transtibial technique for anterior cruciate ligament reconstruction. Arthroscopy. 2009;25:212-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 57. | Youm YS, Cho SD, Eo J, Lee KJ, Jung KH, Cha JR. 3D CT analysis of femoral and tibial tunnel positions after modified transtibial single bundle ACL reconstruction with varus and internal rotation of the tibia. Knee. 2013;20:272-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 58. | Youm YS, Cho SD, Lee SH, Youn CH. Modified transtibial versus anteromedial portal technique in anatomic single-bundle anterior cruciate ligament reconstruction: comparison of femoral tunnel position and clinical results. Am J Sports Med. 2014;42:2941-2947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 59. | Zhang Q, Kou Y, Yuan Z. A meta-analysis on anterior cruciate ligament reconstruction: Is modified transtibial technique inferior to independent drilling techniques? Exp Ther Med. 2018;16:1790-1799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 60. | Han JK, Chun KC, Lee SI, Kim S, Chun CH. Comparison of Modified Transtibial and Anteromedial Portal Techniques in Anatomic Single-Bundle ACL Reconstruction. Orthopedics. 2019;42:83-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 61. | Lee DW, Kim JG, Lee JH, Park JH, Kim DH. Comparison of Modified Transtibial and Outside-In Techniques in Anatomic Single-Bundle Anterior Cruciate Ligament Reconstruction. Arthroscopy. 2018;34:2857-2870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 62. | Lee JK, Lee S, Seong SC, Lee MC. Anatomic single-bundle ACL reconstruction is possible with use of the modified transtibial technique: a comparison with the anteromedial transportal technique. J Bone Joint Surg Am. 2014;96:664-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 63. | Abdul W, Guro R, Jawad Z, Kotwal R, Chandratreya A. Clinical outcomes of primary anatomic all-inside Anterior Cruciate Ligament reconstruction using the TransLateral technique: A minimum one-year follow-up study. Knee. 2020;27:1753-1763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 64. | Lubowitz JH, Ahmad CS, Anderson K. All-inside anterior cruciate ligament graft-link technique: second-generation, no-incision anterior cruciate ligament reconstruction. Arthroscopy. 2011;27:717-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 181] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 65. | Schurz M, Tiefenboeck TM, Winnisch M, Syre S, Plachel F, Steiner G, Hajdu S, Hofbauer M. Clinical and Functional Outcome of All-Inside Anterior Cruciate Ligament Reconstruction at a Minimum of 2 Years' Follow-up. Arthroscopy. 2016;32:332-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 66. | Ashraf Y, Senevirathna SR, Ashraf T. Conventional versus 'all-inside' anterior cruciate ligament reconstruction: a randomized controlled trial comparing hamstring strength and functional outcome. Bone Jt Open. 2020;1:706-708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 67. | Benea H, d'Astorg H, Klouche S, Bauer T, Tomoaia G, Hardy P. Pain evaluation after all-inside anterior cruciate ligament reconstruction and short term functional results of a prospective randomized study. Knee. 2014;21:102-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 68. | Lubowitz JH, Schwartzberg R, Smith P. Randomized controlled trial comparing all-inside anterior cruciate ligament reconstruction technique with anterior cruciate ligament reconstruction with a full tibial tunnel. Arthroscopy. 2013;29:1195-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 69. | Osti M, Krawinkel A, Hoffelner T, Benedetto KP. Quantification of tibial bone loss in antegrade versus retrograde tunnel placement for anterior cruciate ligament reconstruction. Int Orthop. 2015;39:1611-1614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 70. | Yasen SK, Borton ZM, Eyre-Brook AI, Palmer HC, Cotterill ST, Risebury MJ, Wilson AJ. Clinical outcomes of anatomic, all-inside, anterior cruciate ligament (ACL) reconstruction. Knee. 2017;24:55-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 71. | Smith PA, Cook CS, Bley JA. All-Inside Quadrupled Semitendinosus Autograft Shows Stability Equivalent to Patellar Tendon Autograft Anterior Cruciate Ligament Reconstruction: Randomized Controlled Trial in Athletes 24 Years or Younger. Arthroscopy. 2020;36:1629-1646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 72. | Lubowitz JH, Schwartzberg R, Smith P. Cortical Suspensory Button Versus Aperture Interference Screw Fixation for Knee Anterior Cruciate Ligament Soft-Tissue Allograft: A Prospective, Randomized Controlled Trial. Arthroscopy. 2015;31:1733-1739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 73. | Geeslin AG, Jansson KS, Wijdicks CA, Chapman MA, Fok AS, LaPrade RF. Tibial tunnel aperture irregularity after drilling with 5 reamer designs: a qualitative micro-computed tomography analysis. Am J Sports Med. 2011;39:825-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 74. | Takahashi T, Watanabe S, Miura H. All-Inside Double-Bundle Anterior Cruciate Ligament Reconstruction via the Transtibial Approach With a Laser-Tip Guide System for Drilling. Arthrosc Tech. 2019;8:e755-e762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 75. | Watanabe S, Takahashi T, Hino K, Kutsuna T, Ohnishi Y, Ishimaru M, Miura H. Short-Term Study of the Outcome of a New Instrument for All-Inside Double-Bundle Anterior Cruciate Ligament Reconstruction. Arthroscopy. 2015;31:1893-1902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 76. | Kouloumentas P, Kavroudakis E, Charalampidis E, Kavroudakis D, Triantafyllopoulos GK. Superior knee flexor strength at 2 years with all-inside short-graft anterior cruciate ligament reconstruction vs a conventional hamstring technique. Knee Surg Sports Traumatol Arthrosc. 2019;27:3592-3598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 77. | Mayr R, Smekal V, Koidl C, Coppola C, Eichinger M, Rudisch A, Kranewitter C, Attal R. ACL reconstruction with adjustable-length loop cortical button fixation results in less tibial tunnel widening compared with interference screw fixation. Knee Surg Sports Traumatol Arthrosc. 2020;28:1036-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 78. | Monaco E, Fabbri M, Redler A, Gaj E, De Carli A, Argento G, Saithna A, Ferretti A. Anterior cruciate ligament reconstruction is associated with greater tibial tunnel widening when using a bioabsorbable screw compared to an all-inside technique with suspensory fixation. Knee Surg Sports Traumatol Arthrosc. 2019;27:2577-2584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 79. | Fu CW, Chen WC, Lu YC. Is all-inside with suspensory cortical button fixation a superior technique for anterior cruciate ligament reconstruction surgery? BMC Musculoskelet Disord. 2020;21:445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 80. | Muneta T, Sekiya I, Yagishita K, Ogiuchi T, Yamamoto H, Shinomiya K. Two-bundle reconstruction of the anterior cruciate ligament using semitendinosus tendon with endobuttons: operative technique and preliminary results. Arthroscopy. 1999;15:618-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 245] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 81. | van Eck CF, Lesniak BP, Schreiber VM, Fu FH. Anatomic single- and double-bundle anterior cruciate ligament reconstruction flowchart. Arthroscopy. 2010;26:258-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 217] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 82. | Kondo E, Yasuda K, Azuma H, Tanabe Y, Yagi T. Prospective clinical comparisons of anatomic double-bundle versus single-bundle anterior cruciate ligament reconstruction procedures in 328 consecutive patients. Am J Sports Med. 2008;36:1675-1687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 224] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 83. | Muneta T, Koga H, Mochizuki T, Ju YJ, Hara K, Nimura A, Yagishita K, Sekiya I. A prospective randomized study of 4-strand semitendinosus tendon anterior cruciate ligament reconstruction comparing single-bundle and double-bundle techniques. Arthroscopy. 2007;23:618-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 307] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 84. | Yagi M, Kuroda R, Nagamune K, Yoshiya S, Kurosaka M. Double-bundle ACL reconstruction can improve rotational stability. Clin Orthop Relat Res. 2007;454:100-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 297] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 85. | Svantesson E, Cristiani R, Hamrin Senorski E, Forssblad M, Samuelsson K, Stålman A. Meniscal repair results in inferior short-term outcomes compared with meniscal resection: a cohort study of 6398 patients with primary anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2018;26:2251-2258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 86. | Aga C, Wilson KJ, Johansen S, Dornan G, La Prade RF, Engebretsen L. Tunnel widening in single- versus double-bundle anterior cruciate ligament reconstructed knees. Knee Surg Sports Traumatol Arthrosc. 2017;25:1316-1327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 87. | Aga C, Risberg MA, Fagerland MW, Johansen S, Trøan I, Heir S, Engebretsen L. No Difference in the KOOS Quality of Life Subscore Between Anatomic Double-Bundle and Anatomic Single-Bundle Anterior Cruciate Ligament Reconstruction of the Knee: A Prospective Randomized Controlled Trial With 2 Years' Follow-up. Am J Sports Med. 2018;46:2341-2354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 88. | Chen K, Zhu W, Zheng Y, Zhang F, Ouyang K, Peng L, Liu H, Feng W, Huang Y, Zhang G, Deng Z, Lu W. A retrospective study to compare the clinical effects of individualized anatomic single- and double-bundle anterior cruciate ligament reconstruction surgery. Sci Rep. 2020;10:14712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 89. | Webster KE, Feller JA, Kimp AJ, Whitehead TS. Revision Anterior Cruciate Ligament Reconstruction Outcomes in Younger Patients: Medial Meniscal Pathology and High Rates of Return to Sport Are Associated With Third ACL Injuries. Am J Sports Med. 2018;46:1137-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 90. | Browning WM 3rd, Kluczynski MA, Curatolo C, Marzo JM. Suspensory Versus Aperture Fixation of a Quadrupled Hamstring Tendon Autograft in Anterior Cruciate Ligament Reconstruction: A Meta-analysis. Am J Sports Med. 2017;45:2418-2427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 91. | Hagemans FJA, Jonkers FJ, van Dam MJJ, von Gerhardt AL, van der List JP. Clinical and Radiographic Outcomes of Anterior Cruciate Ligament Reconstruction With Hamstring Tendon Graft and Femoral Cortical Button Fixation at Minimum 20-Year Follow-up. Am J Sports Med. 2020;48:2962-2969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 92. | Ahmad SS, Hirschmann MT, Voumard B, Kohl S, Zysset P, Mukabeta T, Evangelopoulos DS, Ateschrang A. Adjustable loop ACL suspension devices demonstrate less reliability in terms of reproducibility and irreversible displacement. Knee Surg Sports Traumatol Arthrosc. 2018;26:1392-1398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 93. | Barrow AE, Pilia M, Guda T, Kadrmas WR, Burns TC. Femoral suspension devices for anterior cruciate ligament reconstruction: do adjustable loops lengthen? Am J Sports Med. 2014;42:343-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 126] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 94. | Jin C, Paluvadi SV, Lee S, Yoo S, Song EK, Seon JK. Biomechanical comparisons of current suspensory fixation devices for anterior cruciate ligament reconstruction. Int Orthop. 2018;42:1291-1296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 95. | Nye DD, Mitchell WR, Liu W, Ostrander RV. Biomechanical Comparison of Fixed-Loop and Adjustable-Loop Cortical Suspensory Devices for Metaphyseal Femoral-Sided Soft Tissue Graft Fixation in Anatomic Anterior Cruciate Ligament Reconstruction Using a Porcine Model. Arthroscopy. 2017;33:1225-1232.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 96. | Petre BM, Smith SD, Jansson KS, de Meijer PP, Hackett TR, LaPrade RF, Wijdicks CA. Femoral cortical suspension devices for soft tissue anterior cruciate ligament reconstruction: a comparative biomechanical study. Am J Sports Med. 2013;41:416-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 141] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 97. | Born TR, Biercevicz AM, Koruprolu SC, Paller D, Spenciner D, Fadale PD. Biomechanical and Computed Tomography Analysis of Adjustable Femoral Cortical Fixation Devices for Anterior Cruciate Ligament Reconstruction in a Cadaveric Human Knee Model. Arthroscopy. 2016;32:253-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 98. | Johnson JS, Smith SD, LaPrade CM, Turnbull TL, LaPrade RF, Wijdicks CA. A biomechanical comparison of femoral cortical suspension devices for soft tissue anterior cruciate ligament reconstruction under high loads. Am J Sports Med. 2015;43:154-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 99. | Rylander L, Brunelli J, Taylor M, Baldini T, Ellis B, Hawkins M, McCarty E. A biomechanical comparison of anterior cruciate ligament suspensory fixation devices in a porcine cadaver model. Clin Biomech (Bristol, Avon). 2014;29:230-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 100. | Boyle MJ, Vovos TJ, Walker CG, Stabile KJ, Roth JM, Garrett WE Jr. Does adjustable-loop femoral cortical suspension loosen after anterior cruciate ligament reconstruction? Knee. 2015;22:304-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 101. | Takahashi T, Takahashi M. The improved cortical button shows better breaking strength of sutures compared with 10 original cortical button after cyclic loading. J Exp Orthop. 2020;7:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 102. | Kurosaka M, Yoshiya S, Andrish JT. A biomechanical comparison of different surgical techniques of graft fixation in anterior cruciate ligament reconstruction. Am J Sports Med. 1987;15:225-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 571] [Cited by in RCA: 475] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 103. | Eysturoy NH, Nissen KA, Nielsen T, Lind M. The Influence of Graft Fixation Methods on Revision Rates After Primary Anterior Cruciate Ligament Reconstruction. Am J Sports Med. 2018;46:524-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 104. | Shino K, Mae T, Maeda A, Miyama T, Shinjo H, Kawakami H. Graft fixation with predetermined tension using a new device, the double spike plate. Arthroscopy. 2002;18:908-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 105. | Ochi M, Adachi N, Deie M, Kanaya A. Anterior cruciate ligament augmentation procedure with a 1-incision technique: anteromedial bundle or posterolateral bundle reconstruction. Arthroscopy. 2006;22:463.e1-463.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 127] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 106. | Muneta T, Koga H, Nakamura T, Horie M, Watanabe T, Yagishita K, Sekiya I. A new behind-remnant approach for remnant-preserving double-bundle anterior cruciate ligament reconstruction compared with a standard approach. Knee Surg Sports Traumatol Arthrosc. 2015;23:3743-3749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 107. | Yasuda K, Kondo E, Kitamura N, Kawaguchi Y, Kai S, Tanabe Y. A pilot study of anatomic double-bundle anterior cruciate ligament reconstruction with ligament remnant tissue preservation. Arthroscopy. 2012;28:343-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 108. | Wang H, Liu Z, Li Y, Peng Y, Xu W, Hu N, Huang W. Is Remnant Preservation in Anterior Cruciate Ligament Reconstruction Superior to the Standard Technique? Biomed Res Int. 2019;2019:1652901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 109. | Claes S, Vereecke E, Maes M, Victor J, Verdonk P, Bellemans J. Anatomy of the anterolateral ligament of the knee. J Anat. 2013;223:321-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 546] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 110. | Jesani S, Getgood A. Modified Lemaire Lateral Extra-Articular Tenodesis Augmentation of Anterior Cruciate Ligament Reconstruction. JBJS Essent Surg Tech. 2019;9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 111. | Littlefield CP, Belk JW, Houck DA, Kraeutler MJ, LaPrade RF, Chahla J, McCarty EC. The Anterolateral Ligament of the Knee: An Updated Systematic Review of Anatomy, Biomechanics, and Clinical Outcomes. Arthroscopy. 2021;37:1654-1666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 112. | Delaloye JR, Hartog C, Blatter S, Schläppi M, Müller D, Denzler D, Murar J, Koch PP. Anterolateral Ligament Reconstruction and Modified Lemaire Lateral Extra-Articular Tenodesis Similarly Improve Knee Stability After Anterior Cruciate Ligament Reconstruction: A Biomechanical Study. Arthroscopy. 2020;36:1942-1950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 81] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 113. | Getgood A, Brown C, Lording T, Amis A, Claes S, Geeslin A, Musahl V; ALC Consensus Group. The anterolateral complex of the knee: results from the International ALC Consensus Group Meeting. Knee Surg Sports Traumatol Arthrosc. 2019;27:166-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 259] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 114. | Castoldi M, Magnussen RA, Gunst S, Batailler C, Neyret P, Lustig S, Servien E. A Randomized Controlled Trial of Bone-Patellar Tendon-Bone Anterior Cruciate Ligament Reconstruction With and Without Lateral Extra-articular Tenodesis: 19-Year Clinical and Radiological Follow-up. Am J Sports Med. 2020;48:1665-1672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 115. | Jaecker V, Ibe P, Endler CH, Pfeiffer TR, Herbort M, Shafizadeh S. High Risk of Tunnel Convergence in Combined Anterior Cruciate Ligament Reconstruction and Lateral Extra-articular Tenodesis. Am J Sports Med. 2019;47:2110-2115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 116. | Kraeutler MJ, Welton KL, Chahla J, LaPrade RF, McCarty EC. Current Concepts of the Anterolateral Ligament of the Knee: Anatomy, Biomechanics, and Reconstruction. Am J Sports Med. 2018;46:1235-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 117. | DiFelice GS, Villegas C, Taylor S. Anterior Cruciate Ligament Preservation: Early Results of a Novel Arthroscopic Technique for Suture Anchor Primary Anterior Cruciate Ligament Repair. Arthroscopy. 2015;31:2162-2171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 150] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 118. | Gobbi A, Whyte GP. Long-term Outcomes of Primary Repair of the Anterior Cruciate Ligament Combined With Biologic Healing Augmentation to Treat Incomplete Tears. Am J Sports Med. 2018;46:3368-3377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 119. | Jonkergouw A, van der List JP, DiFelice GS. Arthroscopic primary repair of proximal anterior cruciate ligament tears: outcomes of the first 56 consecutive patients and the role of additional internal bracing. Knee Surg Sports Traumatol Arthrosc. 2019;27:21-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 105] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 120. | Massey P, Parker D, McClary K, Robinson J, Barton RS, Solitro GF. Biomechanical comparison of anterior cruciate ligament repair with internal brace augmentation versus anterior cruciate ligament repair without augmentation. Clin Biomech (Bristol, Avon). 2020;77:105065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 121. | Smith JO, Yasen SK, Palmer HC, Lord BR, Britton EM, Wilson AJ. Paediatric ACL repair reinforced with temporary internal bracing. Knee Surg Sports Traumatol Arthrosc. 2016;24:1845-1851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 122. | Smith PA, Cook CS. Knotless Primary Anterior Cruciate Ligament Repair with Adjustable Loop Device and Internal Brace Augmentation. Arthrosc Tech. 2020;9:e1967-e1975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 123. | Kandhari V, Vieira TD, Ouanezar H, Praz C, Rosenstiel N, Pioger C, Franck F, Saithna A, Sonnery-Cottet B. Clinical Outcomes of Arthroscopic Primary Anterior Cruciate Ligament Repair: A Systematic Review from the Scientific Anterior Cruciate Ligament Network International Study Group. Arthroscopy. 2020;36:594-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 124. | Liao W, Zhang Q. Is Primary Arthroscopic Repair Using the Pulley Technique an Effective Treatment for Partial Proximal ACL Tears? Clin Orthop Relat Res. 2020;478:1031-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 125. | Dabis J, Yasen SK, Foster AJ, Pace JL, Wilson AJ. Paediatric proximal ACL tears managed with direct ACL repair is safe, effective and has excellent short-term outcomes. Knee Surg Sports Traumatol Arthrosc. 2020;28:2551-2556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 126. | Mouzopoulos G, Fotopoulos VC, Tzurbakis M. Septic knee arthritis following ACL reconstruction: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2009;17:1033-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 87] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 127. | Gobbi A, Karnatzikos G, Chaurasia S, Abhishek M, Bulgherhoni E, Lane J. Postoperative Infection After Anterior Cruciate Ligament Reconstruction. Sports Health. 2016;8:187-189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 128. | Munch DRK, Hansen TI, Mikkelsen KL, Krogsgaard MR. Complications and technical failures are rare in knee ligament reconstruction: analyses based on 31,326 reconstructions during 10 years in Denmark. Knee Surg Sports Traumatol Arthrosc. 2019;27:2672-2679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 129. | Moriarty P, Kayani B, Wallace C, Chang J, Plastow R, Haddad FS. Gentamicin pre-soaking of hamstring autografts decreases infection rates in anterior cruciate ligament reconstruction. Bone Jt Open. 2021;2:66-71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 130. | Jameson SS, Dowen D, James P, Serrano-Pedraza I, Reed MR, Deehan D. Complications following anterior cruciate ligament reconstruction in the English NHS. Knee. 2012;19:14-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 131. | Kraus Schmitz J, Lindgren V, Janarv PM, Forssblad M, Stålman A. Deep venous thrombosis and pulmonary embolism after anterior cruciate ligament reconstruction: incidence, outcome, and risk factors. Bone Joint J. 2019;101-B:34-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 132. | Johns WL, Walley KC, Hammoud S, Gonzalez TA, Ciccotti MG, Patel NK. Tranexamic Acid in Anterior Cruciate Ligament Reconstruction: A Systematic Review and Meta-analysis. Am J Sports Med. 2021;363546521988943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 133. | Reale D, Andriolo L, Gursoy S, Bozkurt M, Filardo G, Zaffagnini S. Complications of Tranexamic Acid in Orthopedic Lower Limb Surgery: A Meta-Analysis of Randomized Controlled Trials. Biomed Res Int. 2021;2021:6961540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 134. | Wang B, Zhong JL, Xu XH, Shang J, Lin N, Lu HD. Incidence and risk factors of joint stiffness after Anterior Cruciate Ligament reconstruction. J Orthop Surg Res. 2020;15:175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 135. | Rousseau R, Labruyere C, Kajetanek C, Deschamps O, Makridis KG, Djian P. Complications After Anterior Cruciate Ligament Reconstruction and Their Relation to the Type of Graft: A Prospective Study of 958 Cases. Am J Sports Med. 2019;47:2543-2549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 86] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 136. | Noailles T, Chalopin A, Boissard M, Lopes R, Bouguennec N, Hardy A. Incidence and risk factors for cyclops syndrome after anterior cruciate ligament reconstruction: A systematic literature review. Orthop Traumatol Surg Res. 2019;105:1401-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 137. | Sanders TL, Kremers HM, Bryan AJ, Kremers WK, Stuart MJ, Krych AJ. Procedural intervention for arthrofibrosis after ACL reconstruction: trends over two decades. Knee Surg Sports Traumatol Arthrosc. 2017;25:532-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 91] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 138. | Ekhtiari S, Horner NS, de Sa D, Simunovic N, Hirschmann MT, Ogilvie R, Berardelli RL, Whelan DB, Ayeni OR. Arthrofibrosis after ACL reconstruction is best treated in a step-wise approach with early recognition and intervention: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2017;25:3929-3937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 139. | Haviv B, Bronak S, Rath E, Yassin M. Nerve injury during anterior cruciate ligament reconstruction: A comparison between patellar and hamstring tendon grafts harvest. Knee. 2017;24:564-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 140. | Horteur C, Cavalié G, Gaulin B, Cohen Bacry M, Morin V, Cavaignac E, Pailhé R. Saphenous nerve injury after anterior cruciate ligament reconstruction: Reduced numbness area after ligamentoplasty using quadriceps tendon compared with hamstring tendon. Knee. 2020;27:1151-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 141. | Grassi A, Perdisa F, Samuelsson K, Svantesson E, Romagnoli M, Raggi F, Gaziano T, Mosca M, Ayeni O, Zaffagnini S. Association between incision technique for hamstring tendon harvest in anterior cruciate ligament reconstruction and the risk of injury to the infra-patellar branch of the saphenous nerve: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2018;26:2410-2423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 142. | Hardy A, Casabianca L, Andrieu K, Baverel L, Noailles T; Junior French Arthroscopy Society. Complications following harvesting of patellar tendon or hamstring tendon grafts for anterior cruciate ligament reconstruction: Systematic review of literature. Orthop Traumatol Surg Res. 2017;103:S245-S248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 143. | Pękala PA, Tomaszewski KA, Henry BM, Ramakrishnan PK, Roy J, Mizia E, Walocha JA. Risk of iatrogenic injury to the infrapatellar branch of the saphenous nerve during hamstring tendon harvesting: A meta-analysis. Muscle Nerve. 2017;56:930-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 144. | Ruffilli A, De Fine M, Traina F, Pilla F, Fenga D, Faldini C. Saphenous nerve injury during hamstring tendons harvest: Does the incision matter? Knee Surg Sports Traumatol Arthrosc. 2017;25:3140-3145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 145. | Stein DA, Hunt SA, Rosen JE, Sherman OH. The incidence and outcome of patella fractures after anterior cruciate ligament reconstruction. Arthroscopy. 2002;18:578-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 68] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 146. | Tjoumakaris FP, Herz-Brown AL, Bowers AL, Sennett BJ, Bernstein J. Complications in brief: Anterior cruciate ligament reconstruction. Clin Orthop Relat Res. 2012;470:630-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 147. | Wilding CSR, Cruz CCA, Mannino LBJ, Deal CJB, Wake CJ, Bottoni CR. Bone-Tendon-Autograft Anterior Cruciate Ligament Reconstruction: A New Anterior Cruciate Ligament Graft Option. Arthrosc Tech. 2020;9:e1525-e1530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 148. | Fu FH, Rabuck SJ, West RV, Tashman S, Irrgang JJ. Patellar Fractures After the Harvest of a Quadriceps Tendon Autograft With a Bone Block: A Case Series. Orthop J Sports Med. 2019;7:2325967119829051. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 149. | Salem HS, Axibal DP, Wolcott ML, Vidal AF, McCarty EC, Bravman JT, Frank RM. Two-Stage Revision Anterior Cruciate Ligament Reconstruction: A Systematic Review of Bone Graft Options for Tunnel Augmentation. Am J Sports Med. 2020;48:767-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 150. | Horvath A, Senorski EH, Westin O, Karlsson J, Samuelsson K, Svantesson E. Outcome After Anterior Cruciate Ligament Revision. Curr Rev Musculoskelet Med. 2019;397-405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 151. | Kievit AJ, Jonkers FJ, Barentsz JH, Blankevoort L. A cross-sectional study comparing the rates of osteoarthritis, laxity, and quality of life in primary and revision anterior cruciate ligament reconstructions. Arthroscopy. 2013;29:898-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 152. | Gifstad T, Drogset JO, Viset A, Grøntvedt T, Hortemo GS. Inferior results after revision ACL reconstructions: a comparison with primary ACL reconstructions. Knee Surg Sports Traumatol Arthrosc. 2013;21:2011-2018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 153. | Dean CS, Liechti DJ, Chahla J, Moatshe G, LaPrade RF. Clinical Outcomes of High Tibial Osteotomy for Knee Instability: A Systematic Review. Orthop J Sports Med. 2016;4:2325967116633419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 154. | Herman BV, Giffin JR. High tibial osteotomy in the ACL-deficient knee with medial compartment osteoarthritis. J Orthop Traumatol. 2016;17:277-285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 155. | Fu FH, Byrne KJ, Godshaw BM. Editorial Commentary: Remember the Risk Factors During Individualized, Anatomic, Value-Based Anterior Cruciate Ligament Reconstruction. Arthroscopy. 2021;37:206-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 156. | Song GY, Ni QK, Zheng T, Zhang ZJ, Feng H, Zhang H. Slope-Reducing Tibial Osteotomy Combined With Primary Anterior Cruciate Ligament Reconstruction Produces Improved Knee Stability in Patients With Steep Posterior Tibial Slope, Excessive Anterior Tibial Subluxation in Extension, and Chronic Meniscal Posterior Horn Tears. Am J Sports Med. 2020;48:3486-3494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 157. | Hernigou P, Medevielle D, Debeyre J, Goutallier D. Proximal tibial osteotomy for osteoarthritis with varus deformity. A ten to thirteen-year follow-up study. J Bone Joint Surg Am. 1987;69:332-354. [PubMed] |
| 158. | Coventry MB. Upper tibial osteotomy for osteoarthritis. J Bone Joint Surg Am. 1985;67:1136-1140. [PubMed] |
| 159. | Maquet P. The treatment of choice in osteoarthritis of the knee. Clin Orthop Relat Res. 1985;108-112. [PubMed] |
| 160. | Takahashi T, Wada Y, Tanaka M, Iwagawa M, Ikeuchi M, Hirose D, Yamamoto H. Dome-shaped proximal tibial osteotomy using percutaneous drilling for osteoarthritis of the knee. Arch Orthop Trauma Surg. 2000;120:32-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 161. | Schuster P, Schlumberger M, Mayer P, Eichinger M, Geßlein M, Schulz-Jahrsdörfer M, Richter J. Excellent long-term results in combined high tibial osteotomy, anterior cruciate ligament reconstruction and chondral resurfacing in patients with severe osteoarthritis and varus alignment. Knee Surg Sports Traumatol Arthrosc. 2020;28:1085-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 162. | Malahias MA, Shahpari O, Kaseta MK. The clinical Outcome of One-stage High Tibial Osteotomy and Anterior Cruciate Ligament Reconstruction. A Current Concept Systematic and Comprehensive Review. Arch Bone Jt Surg. 2018;6:161-168. [PubMed] |
| 163. | Li Y, Zhang H, Zhang J, Li X, Song G, Feng H. Clinical outcome of simultaneous high tibial osteotomy and anterior cruciate ligament reconstruction for medial compartment osteoarthritis in young patients with anterior cruciate ligament-deficient knees: a systematic review. Arthroscopy. 2015;31:507-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 164. | Looney AM, Leider JD, Horn AR, Bodendorfer BM. Bioaugmentation in the surgical treatment of anterior cruciate ligament injuries: A review of current concepts and emerging techniques. SAGE Open Med. 2020;8:2050312120921057. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 165. | Uchida R, Jacob G, Shimomura K, Horibe S, Nakamura N. Biological Augmentation of ACL Repair and Reconstruction: Current Status and Future Perspective. Sports Med Arthrosc Rev. 2020;28:49-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 166. | Leong NL, Petrigliano FA, McAllister DR. Current tissue engineering strategies in anterior cruciate ligament reconstruction. J Biomed Mater Res A. 2014;102:1614-1624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 167. | Mengsteab PY, Otsuka T, McClinton A, Shemshaki NS, Shah S, Kan HM, Obopilwe E, Vella AT, Nair LS, Laurencin CT. Mechanically superior matrices promote osteointegration and regeneration of anterior cruciate ligament tissue in rabbits. Proc Natl Acad Sci U S A. 2020;117:28655-28666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 168. | Patel S, Caldwell JM, Doty SB, Levine WN, Rodeo S, Soslowsky LJ, Thomopoulos S, Lu HH. Integrating soft and hard tissues via interface tissue engineering. J Orthop Res. 2018;36:1069-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 93] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 169. | Proffen BL, Perrone GS, Roberts G, Murray MM. Bridge-enhanced ACL repair: A review of the science and the pathway through FDA investigational device approval. Ann Biomed Eng. 2015;43:805-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 170. | Archer DE, Mafi R, Mafi P, Khan WS. Preclinical Studies on Biomaterial Scaffold use in Knee Ligament Regeneration: A Systematic Review. Curr Stem Cell Res Ther. 2018;13:691-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 171. | Parry JA, Wagner ER, Kok PL, Dadsetan M, Yaszemski MJ, van Wijnen AJ, Kakar S. A Combination of a Polycaprolactone Fumarate Scaffold with Polyethylene Terephthalate Sutures for Intra-Articular Ligament Regeneration. Tissue Eng Part A. 2018;24:245-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 172. | Vaquette C, Sudheesh Kumar PT, Petcu EB, Ivanovski S. Combining electrospinning and cell sheet technology for the development of a multiscale tissue engineered ligament construct (TELC). J Biomed Mater Res B Appl Biomater. 2018;106:399-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 173. | Park SH, Choi YJ, Moon SW, Lee BH, Shim JH, Cho DW, Wang JH. Three-Dimensional Bio-Printed Scaffold Sleeves With Mesenchymal Stem Cells for Enhancement of Tendon-to-Bone Healing in Anterior Cruciate Ligament Reconstruction Using Soft-Tissue Tendon Graft. Arthroscopy. 2018;34:166-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 174. | Zhang M, Zhen J, Zhang X, Yang Z, Zhang L, Hao D, Ren B. Effect of Autologous Platelet-Rich Plasma and Gelatin Sponge for Tendon-to-Bone Healing After Rabbit Anterior Cruciate Ligament Reconstruction. Arthroscopy. 2019;35:1486-1497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 175. | Nau T, Teuschl A. Regeneration of the anterior cruciate ligament: Current strategies in tissue engineering. World J Orthop. 2015;6:127-136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 60] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 176. | Ge Z, Goh JC, Lee EH. Selection of cell source for ligament tissue engineering. Cell Transplant. 2005;14:573-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 177. | Woods S, Bates N, Dunn SL, Serracino-Inglott F, Hardingham TE, Kimber SJ. Generation of Human-Induced Pluripotent Stem Cells From Anterior Cruciate Ligament. J Orthop Res. 2020;38:92-104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 178. | Perrone GS, Proffen BL, Kiapour AM, Sieker JT, Fleming BC, Murray MM. Bench-to-bedside: Bridge-enhanced anterior cruciate ligament repair. J Orthop Res. 2017;35:2606-2612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 179. | Riediger MD, Stride D, Coke SE, Kurz AZ, Duong A, Ayeni OR. ACL Reconstruction with Augmentation: a Scoping Review. Curr Rev Musculoskelet Med. 2019;12:166-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 180. | Davey MS, Hurley ET, Withers D, Moran R, Moran CJ. Anterior Cruciate Ligament Reconstruction with Platelet-Rich Plasma: A Systematic Review of Randomized Control Trials. Arthroscopy. 2020;36:1204-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 181. | Lee KI, Lee JS, Kang KT, Shim YB, Kim YS, Jang JW, Moon SH, D'Lima DD. In Vitro and In Vivo Performance of Tissue-Engineered Tendons for Anterior Cruciate Ligament Reconstruction. Am J Sports Med. 2018;46:1641-1649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 182. | Cardwell RD, Dahlgren LA, Goldstein AS. Electrospun fibre diameter, not alignment, affects mesenchymal stem cell differentiation into the tendon/ligament lineage. J Tissue Eng Regen Med. 2014;8:937-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 183. | Burström G, Buerger C, Hoppenbrouwers J, Nachabe R, Lorenz C, Babic D, Homan R, Racadio JM, Grass M, Persson O, Edström E, Elmi Terander A. Machine learning for automated 3-dimensional segmentation of the spine and suggested placement of pedicle screws based on intraoperative cone-beam computer tomography. J Neurosurg Spine. 2019;31:147-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 184. | Cho JY, Chan CK, Lee SH, Lee HY. The accuracy of 3D image navigation with a cutaneously fixed dynamic reference frame in minimally invasive transforaminal lumbar interbody fusion. Comput Aided Surg. 2012;17:300-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 185. | Edström E, Burström G, Nachabe R, Gerdhem P, Elmi Terander A. A Novel Augmented-Reality-Based Surgical Navigation System for Spine Surgery in a Hybrid Operating Room: Design, Workflow, and Clinical Applications. Oper Neurosurg (Hagerstown). 2020;18:496-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 186. | Elmi-Terander A, Nachabe R, Skulason H, Pedersen K, Söderman M, Racadio J, Babic D, Gerdhem P, Edström E. Feasibility and Accuracy of Thoracolumbar Minimally Invasive Pedicle Screw Placement With Augmented Reality Navigation Technology. Spine (Phila Pa 1976). 2018;43:1018-1023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 91] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 187. | Goradia VK. Computer-assisted and robotic surgery in orthopedics: where we are in 2014. Sports Med Arthrosc Rev. 2014;22:202-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 188. | Karkenny AJ, Mendelis JR, Geller DS, Gomez JA. The Role of Intraoperative Navigation in Orthopaedic Surgery. J Am Acad Orthop Surg. 2019;27:e849-e858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 189. | Klos TV. Computer-assisted anterior cruciate ligament reconstruction. Four generations of development and usage. Sports Med Arthrosc Rev. 2014;22:229-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 190. | Zaffagnini S, Urrizola F, Signorelli C, Grassi A, Di Sarsina TR, Lucidi GA, Marcheggiani Muccioli GM, Bonanzinga T, Marcacci M. Current use of navigation system in ACL surgery: a historical review. Knee Surg Sports Traumatol Arthrosc. 2016;24:3396-3409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 191. | Harms SP, Noyes FR, Grood ES, Jetter AW, Huser LE, Levy MS, Gardner EJ. Anatomic Single-Graft Anterior Cruciate Ligament Reconstruction Restores Rotational Stability: A Robotic Study in Cadaveric Knees. Arthroscopy. 2015;31:1981-1990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 192. | Hofbauer M, Valentin P, Kdolsky R, Ostermann RC, Graf A, Figl M, Aldrian S. Rotational and translational laxity after computer-navigated single- and double-bundle anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2010;18:1201-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 193. | Meuffels DE, Reijman M, Verhaar JA. Computer-assisted surgery is not more accurate or precise than conventional arthroscopic ACL reconstruction: a prospective randomized clinical trial. J Bone Joint Surg Am. 2012;94:1538-1545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 194. | Taketomi S, Inui H, Nakamura K, Hirota J, Takei S, Takeda H, Tanaka S, Nakagawa T. Three-dimensional fluoroscopic navigation guidance for femoral tunnel creation in revision anterior cruciate ligament reconstruction. Arthrosc Tech. 2012;1:e95-e99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 195. | Lee BH, Kum DH, Rhyu IJ, Kim Y, Cho H, Wang JH. Clinical advantages of image-free navigation system using surface-based registration in anatomical anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2016;24:3556-3564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 196. | Luites JW, Wymenga AB, Blankevoort L, Eygendaal D, Verdonschot N. Accuracy of a computer-assisted planning and placement system for anatomical femoral tunnel positioning in anterior cruciate ligament reconstruction. Int J Med Robot. 2014;10:438-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 197. | Park SH, Moon SW, Lee BH, Park S, Kim Y, Lee D, Lim S, Wang JH. Arthroscopically blind anatomical anterior cruciate ligament reconstruction using only navigation guidance: a cadaveric study. Knee. 2016;23:813-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 198. | Kim Y, Lee BH, Mekuria K, Cho H, Park S, Wang JH, Lee D. Registration accuracy enhancement of a surgical navigation system for anterior cruciate ligament reconstruction: A phantom and cadaveric study. Knee. 2017;24:329-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 199. | Ishibashi Y, Tsuda E, Fukuda A, Tsukada H, Toh S. Intraoperative biomechanical evaluation of anatomic anterior cruciate ligament reconstruction using a navigation system: comparison of hamstring tendon and bone-patellar tendon-bone graft. Am J Sports Med. 2008;36:1903-1912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 200. | Shafizadeh S, Balke M, Hagn U, Grote S, Bouillon B, Banerjee M. Variability of landmark acquisition affects tunnel calculation in image-free ACL navigation. Knee Surg Sports Traumatol Arthrosc. 2015;23:1917-1924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 201. | Raposo C, Barreto JP, Sousa C, Ribeiro L, Melo R, Oliveira JP, Marques P, Fonseca F, Barrett D. Video-based computer navigation in knee arthroscopy for patient-specific ACL reconstruction. Int J Comput Assist Radiol Surg. 2019;14:1529-1539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 202. | Burkart A, Debski RE, McMahon PJ, Rudy T, Fu FH, Musahl V, van Scyoc A, Woo SL. Precision of ACL tunnel placement using traditional and robotic techniques. Comput Aided Surg. 2001;6:270-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 203. | Musahl V, Burkart A, Debski RE, Van Scyoc A, Fu FH, Woo SL. Anterior cruciate ligament tunnel placement: Comparison of insertion site anatomy with the guidelines of a computer-assisted surgical system. Arthroscopy. 2003;19:154-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 67] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 204. | Zaffagnini S, Klos TV, Bignozzi S. Computer-assisted anterior cruciate ligament reconstruction: an evidence-based approach of the first 15 years. Arthroscopy. 2010;26:546-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 205. | Hart R, Krejzla J, Sváb P, Kocis J, Stipcák V. Outcomes after conventional versus computer-navigated anterior cruciate ligament reconstruction. Arthroscopy. 2008;24:569-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 206. | Luites JW, Wymenga AB, Blankevoort L, Kooloos JM, Verdonschot N. Development of a femoral template for computer-assisted tunnel placement in anatomical double-bundle ACL reconstruction. Comput Aided Surg. 2011;16:11-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 207. | Shafizadeh S, Balke M, Wegener S, Tjardes T, Bouillon B, Hoeher J, Baethis H. Precision of tunnel positioning in navigated anterior cruciate ligament reconstruction. Arthroscopy. 2011;27:1268-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 208. | Endele D, Jung C, Becker U, Bauer G, Mauch F. Anterior cruciate ligament reconstruction with and without computer navigation: a clinical and magnetic resonance imaging evaluation 2 years after surgery. Arthroscopy. 2009;25:1067-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 209. | Plaweski S, Cazal J, Rosell P, Merloz P. Anterior cruciate ligament reconstruction using navigation: a comparative study on 60 patients. Am J Sports Med. 2006;34:542-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 82] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 210. | Taketomi S, Inui H, Nakamura K, Hirota J, Sanada T, Masuda H, Takeda H, Tanaka S, Nakagawa T. Clinical outcome of anatomic double-bundle ACL reconstruction and 3D CT model-based validation of femoral socket aperture position. Knee Surg Sports Traumatol Arthrosc. 2014;22:2194-2201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 211. | Anthony CA, Duchman K, McCunniff P, McDermott S, Bollier M, Thedens DR, Wolf BR, Albright JP. Double-bundle ACL reconstruction: novice surgeons utilizing computer-assisted navigation versus experienced surgeons. Comput Aided Surg. 2013;18:172-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 212. | Zhu W, Lu W, Han Y, Hui S, Ou Y, Peng L, Fen W, Wang D, Zhang L, Zeng Y. Application of a computerised navigation technique to assist arthroscopic anterior cruciate ligament reconstruction. Int Orthop. 2013;37:233-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 213. | Margier J, Tchouda SD, Banihachemi JJ, Bosson JL, Plaweski S. Computer-assisted navigation in ACL reconstruction is attractive but not yet cost efficient. Knee Surg Sports Traumatol Arthrosc. 2015;23:1026-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 214. | Eggerding V, Reijman M, Scholten RJ, Meuffels DE. Computer-assisted surgery for knee ligament reconstruction. Cochrane Database Syst Rev. 2014;CD007601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 215. | Taketomi S, Inui H, Sanada T, Nakamura K, Yamagami R, Masuda H, Tanaka S, Nakagawa T. Remnant-preserving anterior cruciate ligament reconstruction using a three-dimensional fluoroscopic navigation system. Knee Surg Relat Res. 2014;26:168-176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 216. | Tsukada H, Ishibashi Y, Tsuda E, Fukuda A, Toh S. Anatomical analysis of the anterior cruciate ligament femoral and tibial footprints. J Orthop Sci. 2008;13:122-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 153] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 217. | Plaweski S, Schlatterer B, Saragaglia D; Computer Assisted Orthopedic Surgery - France (CAOS - France). The role of computer assisted navigation in revision surgery for failed anterior cruciate ligament reconstruction of the knee: A continuous series of 52 cases. Orthop Traumatol Surg Res. 2015;101:S227-S231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 218. | Musahl V, Voos JE, O'Loughlin PF, Choi D, Stueber V, Kendoff D, Pearle AD. Comparing stability of different single- and double-bundle anterior cruciate ligament reconstruction techniques: a cadaveric study using navigation. Arthroscopy. 2010;26:S41-S48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 219. | Verhelst L, Van Der Bracht H, Oosterlinck D, Bellemans J. ACL repair with a single or double tunnel: a comparative laboratory study of knee stability using computer navigation. Acta Orthop Belg. 2012;78:771-778. [PubMed] |