Published online Aug 28, 2021. doi: 10.13105/wjma.v9.i4.342
Peer-review started: March 28, 2021
First decision: April 28, 2021
Revised: July 3, 2021
Accepted: August 24, 2021
Article in press: August 24, 2021
Published online: August 28, 2021
Processing time: 159 Days and 2.9 Hours
The increase in circulating Troponin-I in the blood of patients suffering coronavirus disease 2019 (COVID-19) can be a strong prognostic factor for predicting disease poorer outcome.
To review the literatures to approve this claim systematically.
Two blinded reviewers independently screened the titles and abstracts of the manuscripts using the keywords and deeply searching the databanks including PubMed, Scopus, Google Scholar, and Web of knowledge, followed by profo
The manuscripts entered into our final assessment were categorized as the two groups including 10 manuscripts describing and comparing death and disease-related complications between the subgroups of patients with raised serum troponin level and those with normal ranges of this biomarker and 7 manuscripts comparing the mean level of serum troponin concentration across the survived and non-survived groups. Comparing outcome of COVID-19 disease in the groups with raised troponin level and normal level of this markers showed increased the likelihood of death [hazard ratio (HR) = 4.967, P < 0.001], acute respiratory distress syndrome (HR = 5.914, P < 0.001), acute kidney injury (HR = 3.849, P < 0.001), and intensive care unit (ICU) admission (HR = 3.780, P < 0.001) following raise of troponin. The pooled analysis showed significantly higher concentration of this marker in the survived group compared to non-survived group (weighted mean differences of 22.278, 95%CI: 15.647 to 28.927, P < 0.001).
Raising troponin-I on admission can be linked to the increase risk for in-hospital death, acute respiratory distress syndrome, kidney injury, and ICU admission by 4.9, 5.9, 3.8, and 3.7 times as compared to those with initial normal troponin-I concentration. Thus, raising baseline value of troponin-I can be used as a prognostic factor for poor outcome of COVID-19.
Core Tip: We systematically reviewed the literatures to assess this claim that an increase in troponin-I levels could be a prognostic factor in predicting disease severity and mortality in patients with coronavirus disease 2019. According to our findings, regardless of the history of myocardial injuries or the presence of cardiovascular risk profile, the value of troponin I should be accurately assessed on admission. Raising troponin-I on admission can be linked to the increase risk for in-hospital death, acute respiratory distress syndrome, kidney injury, and intensive care unit admission by 4.9, 5.9, 3.8, and 3.7 times as compared to those with initial normal troponin I concentration.
- Citation: Ashraf H, Soleimani A, Kazemi saeid A, Sadat Naseri A, Majidi F, Peirovi N, Karbalai Saleh S. Troponin I biomarker as a strong prognostic factor for predicting COVID-19 mortality: A systematic review. World J Meta-Anal 2021; 9(4): 342-352
- URL: https://www.wjgnet.com/2308-3840/full/v9/i4/342.htm
- DOI: https://dx.doi.org/10.13105/wjma.v9.i4.342
The new coronavirus known as coronavirus disease 2019 (COVID-19) has so far (15 February 15) infected more than 108 million people, leading to more than 2 million deaths. About 15 to 20 percent of patients experience a severe illness resulting in 2 to 3 percent mortality. The disease presents with acute respiratory syndrome with fever, dry cough, dyspnea and myalgia[1-4]. To date, there is no confirmed treatment for this disease. Therefore, accurate and early diagnosis and determination of its severity can prevent its further progression.
Along with acute respiratory failure as a prominent and debilitating manifestation of COVID-19, multidimensional organ defects have been also detected in the affected patients certainly resulting from the virus triggering role on pro-inflammatory cytokines activation and secretion, as well as coagulation abnormalities[5,6]. A complex of such events can predispose the patients to cardiovascular ischemic and thromboembolic events[7,8]. Some clinical data have supported the strong link of COVID-19 infection to cardiac and cerebrovascular ischemic events leading to high mortality and disabilities[9]. This infection is now suggested to promote myocardial injuries leading to cardiac arrhythmias, myocardial hypertrophy, acute coronary syndrome and even acute heart failure. We obvious a bidirectional interaction between the cardiovascular system and infection to COVID-19, however, the exact mechanisms responsible for such cardiovascular defects remains elusive. Some molecular-based studies emphasize the central role of pro-inflammatory cascades such as the activation of interleukin-6, interleukin-1beta, and tumor necrosis factor-α as the main flaring factors for such events[10]. Also, the presence of some specific receptors for the virus such as ACE2 as a gateway for the virus to enter tissues such as the myocardium seems necessary for the mentioned injuries[9]. There is also strong evidence on a close association between the effects of the virus and underlying cardiovascular risk factors such as hypertension and diabetes so that the presence of any of these risk factors increases the risk of ischemic events many times over. The body of evidence highlights the high risk of ischemic heart disease such as myocardial infarction in patients with COVID-19[11,12].
Therefore, one of the relatively prevalent adversity in patients with COVID-19, particularly in the elderly and patients with prior predisposing factors like hypertension or diabetes mellitus is cardiovascular lesions in the form of ischemic heart attacks, arrhythmias, and vascular disorders. In this regard, considering that the increase in the level of cardiac biomarkers such as troponin-I as a marker of myocardial damage has been fully proven[13], it seems that in COVID-19 patients with myocardial and arrhythmic lesions, we see an increase in this marker. The increase in circulating troponin-I in the blood of patients may be also a prognostic factor for predicting the severity of the disease and its mortality[14]. In this study, we systematically reviewed the literature to assess this claim that an increase in troponin-I levels could be a prognostic factor in predicting disease severity and mortality in patients with COVID-19.
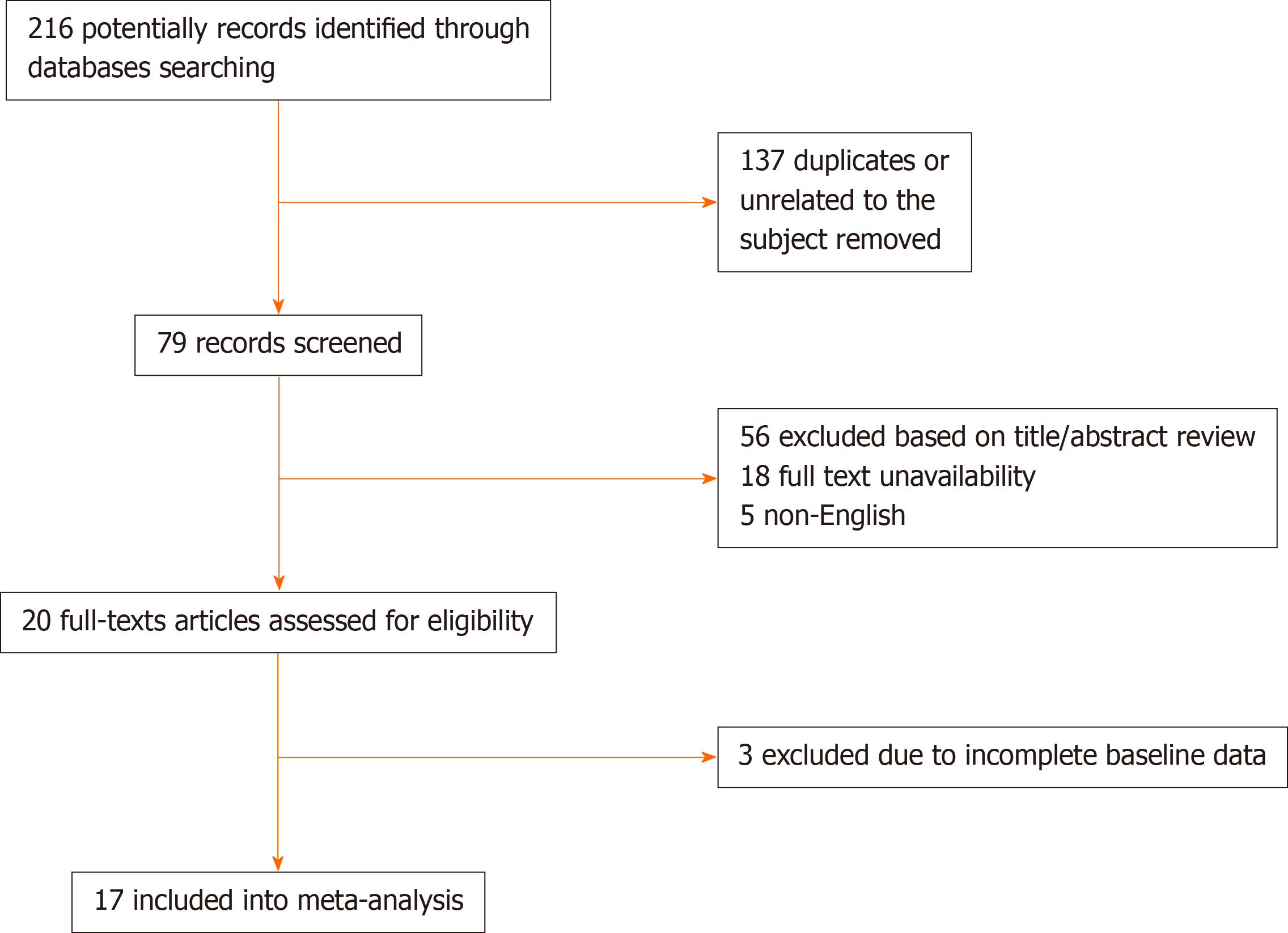
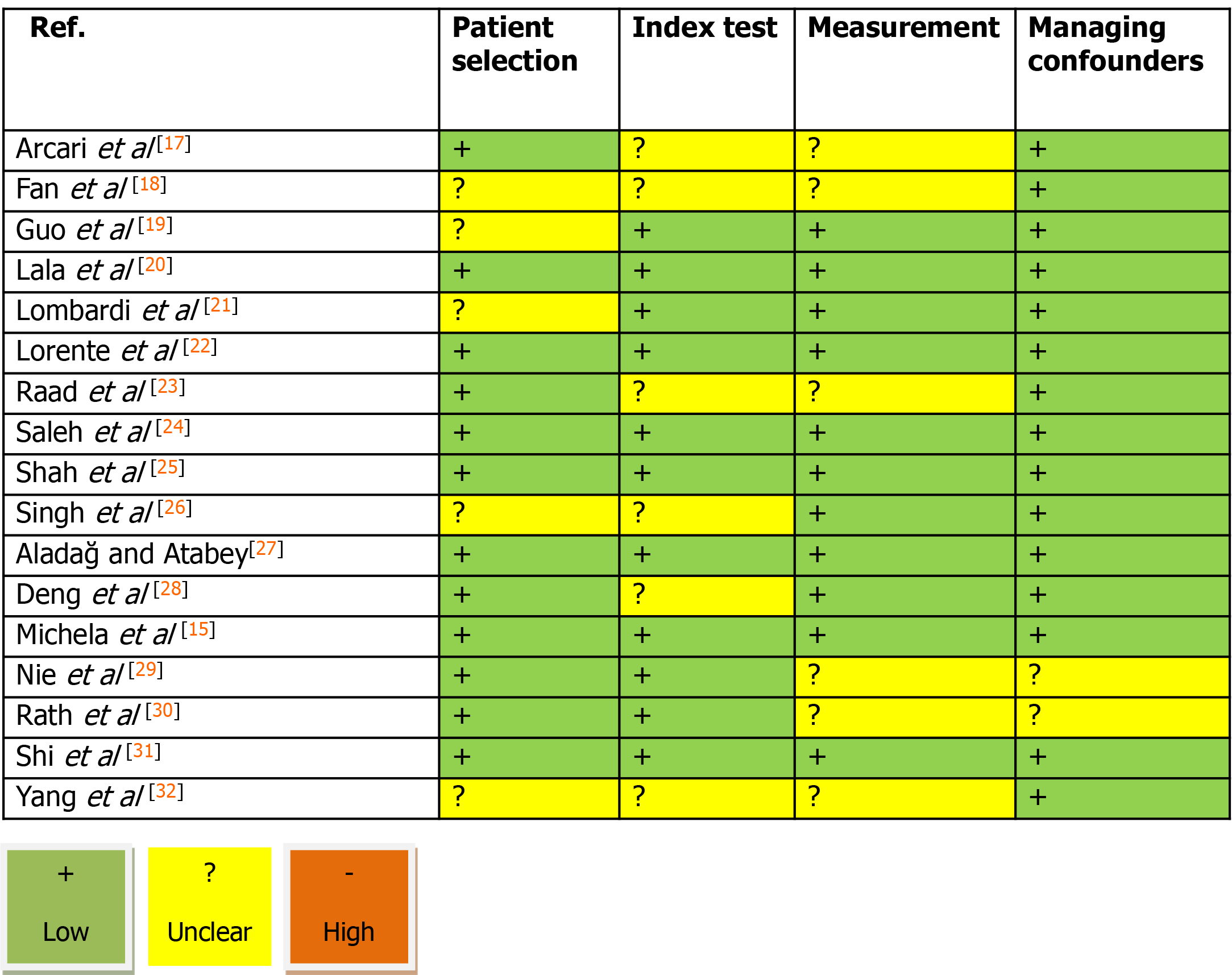
For performing the present systematic review and meta-analysis, the full guideline of the “Preferred Reporting Items for Systematic Reviews and Meta-Analyses” was followed[15]. In the first step and after explaining the study’s main question and specific goals, all prospective and retrospective comparative studies that evaluated the link between the serum level of troponin-I and two COVID-19 related parameters including disease severity and mortality were considered to be eligible for primary assessment. In this regard, deeply searching the manuscript databanks including PubMed, Scopus, Google Scholar, and Web of knowledge was planned from inception to October 2020; The main keywords were: ‘covid-19’ OR ‘sars-cov-2’ OR ‘2019-ncov’ AND ‘troponin’ AND ‘mortality’ OR ‘death’. In the searches, the review papers, case presentations, letter to editors, and abstracts without full text access were all excluded. Non-English studies were excluded from the meta-analysis. Also, in cases of lack of access to the full text of the articles, correspondence was made with the author in charge of the articles to obtain the full article, and in case of lack of access to the original article; it was removed from the study. The manuscript reviewing was done by two blinded reviewers, screening the titles and abstracts followed by profound appraisement of the full texts independently to assess the inclusion appropriateness. The presence of any disagreement between them was judged and checked again by another reviewer as the last arbiter. The eligibility and reasons for not including the papers are schematically presented in Figure 1. The bias hazard was blindly assessed by two authors using the Cochrane risk of bias tool, afore finalizing the meta-analysis. The level of bias was qualitatively classified into high, uncertain, or low bias[16]. Accordingly, the following domains are typically used to specify the level of bias: How to select the participants (selection bias), how to perform the measurement of troponin-I along the management of disease-related outcomes including disease severity and in-hospital death, and how to manage confounders and missing data. The permanent effects or random-effects (in case of significant heterogeneity across the data) models were used to obtained pooled relative risk (with 95% confidence interval and corresponding P-values) for disease severity and death due to troponin-I raising as well as to obtained pooled dichotomous data using the mean difference (MD) for the level of troponin-I. The incongruity between the studies was evaluated by determining I2values. A sensitivity analysis was also performed, in which observational studies at critical risk of bias were excluded from the analysis. Publication bias was also appraised by the rank correlation test and also affirmed by the funnel plot analysis. Reported values were two-tailed, and hypothesis testing results were assumed statistically significant at P = 0.05. We used the Comprehensive Meta-Analysis Software (CMA, version 3.0) for statistical analysis.
The study selection process is shown in Figure 1. On the field, 216 articles were prepared by the database searching at the beginning. We removed 137 articles as they were duplicated or unrelated to the subject of the systematic review. At first, 79 articles were initially under-screened. Fifty-nine articles were excluded based on the titles and abstracts. The extant 20 articles were specified for subsequent eligibility. We also excluded 3 more articles because the data and contents were not completed. Eventually, 17 articles were qualified for the final analysis[17-32]. Table 1 describes the baseline characteristics of the included studies. Evaluation of the publication and systematic bias demonstrated that approximately all articles were supposed as low risk or with unclear biases; hence, the obtained results could be considered valid and none of the articles was supposed to have a high risk of bias (Figure 2).
| Ref. | Country | Population | Mean age | Male/female | Cutoff for TnI raising |
| Arcari et al[17] | Italy | Normal TnI: 39 | 66 ± 17 | 28/11 | 14 pg/mL |
| Raised TnI: 64 | 79 ± 13 | 19/45 | |||
| Fan et al[18] | China | Normal TnI: 67 | 58 ± 15 | 38/29 | 0.01 μg/L |
| Raised TnI: 22 | 71 ± 12 | 11/11 | |||
| Guo et al[19] | China | Normal TnI: 135 | 53 ± 13 | 57/78 | 99th percentile |
| Raised TnI: 52 | 71 ± 9 | 34/18 | |||
| Lala et al[20] | United States | Normal TnI: 2206 | 66 ± 13 | 1312/894318/212 | 0.09 ng/mL |
| Raised TnI: 530 | 68 ± 15 | ||||
| Lombardi et al[21] | Italy | Normal TnI: 336 | 64 ± 13 | 234/102201/77 | 99th percentile |
| Raised TnI: 278 | 71 ± 12 | ||||
| Lorente-Ros et al[22] | Spain | Normal TnI: 560 | 63 ± 12 | 367/193 | 14 ng/L |
| Raised TnI: 147 | 78 ± 14 | 77/70 | |||
| Raad et al[23] | United States | Normal TnI: 630 | 59 ± 11 | 280/350 | 18 ng/L |
| Raised TnI: 390 | 70 ± 13 | 229/161 | |||
| Karbalai Saleh et al[24] | Iran | Normal TnI: 271 | 57 ± 15 | 167/104 | 99th percentile |
| Raised TnI: 115 | 65 ± 15 | 69/46 | |||
| Shah et al[25] | United States | Raised TnI: 116 | 59 ± 14 | 70/123 | 0.05 ng/ml |
| Normal TnI: 193 | 68 ± 14 | 62/54 | |||
| Singh et al[26] | United States | Normal TnI: 129 | 53 ± 11 | 54/75 | 17 ng/L |
| Raised TnI: 132 | 71 ± 12 | 70/62 |
The manuscripts entered into our final assessment were categorized as the two groups including 10 manuscripts describing and comparing death and disease-related complications between the subgroups of patients with raised serum troponin level and those with normal ranges of this biomarker[17-26] and 7 manuscripts comparing the mean level of serum troponin concentration across the survived and non-survived groups[18-33]. The main point concerning the present meta-analysis was to first present the value of troponin with different laboratory units that of course could be matched through unit conversion. However, the different techniques employed for troponin value assessment, the type of study as prospective or retrospective, the different cutoff values considered for defining troponin abnormal raise as well as the time considering for patients’ follow-up might lead to high heterogeneity across the two groups that were measured in our meta-analysis.
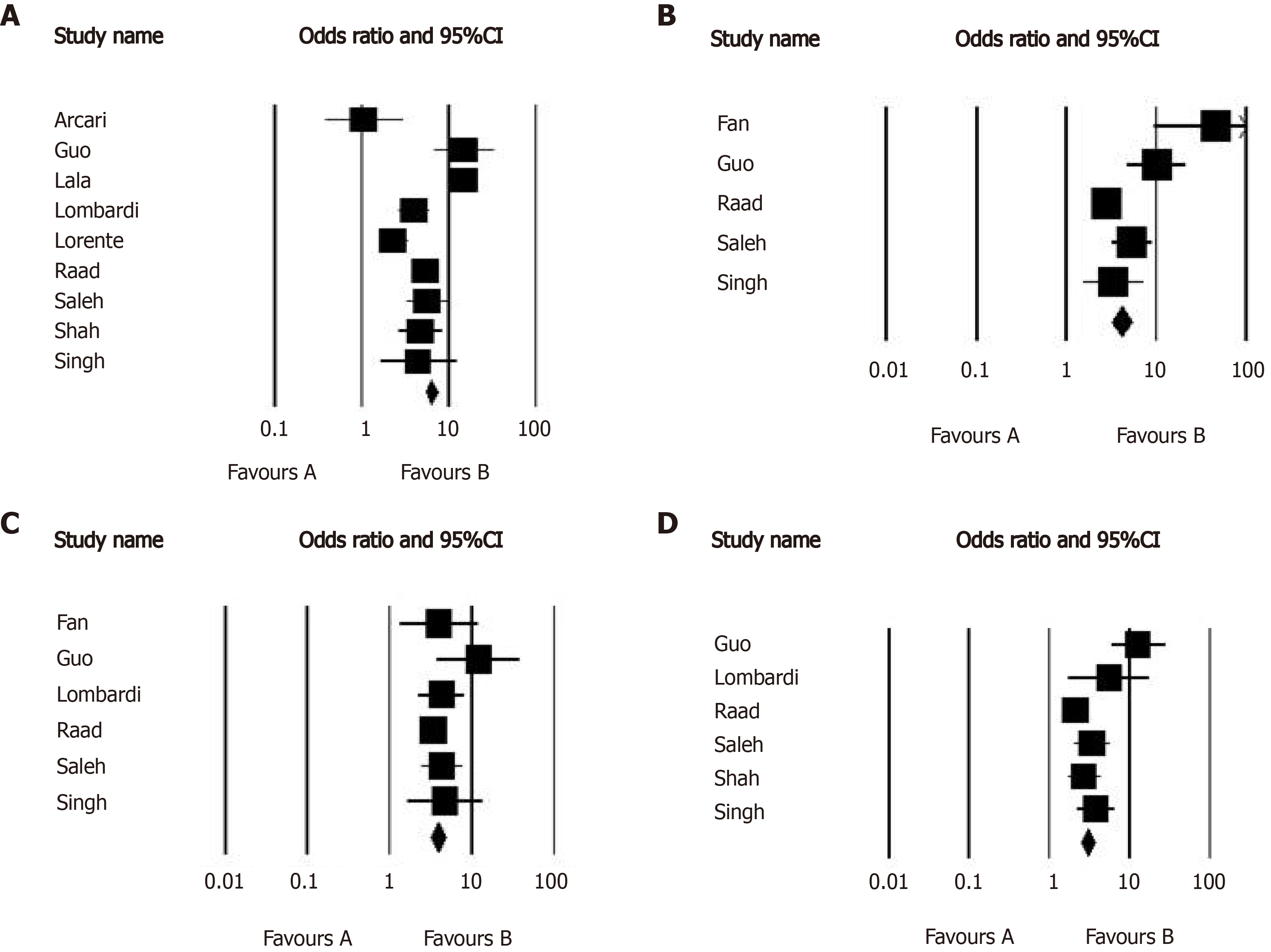
Overall, 10 studies assessed the early mortality and morbidity in the groups with and without raised serum troponin level (Table 1). In this regard, the outcome of COVID-19 was compared between the group with a normal troponin range (n = 4566) and the group with raised troponin level (n = 1846). The average age of participants in the two groups was 59.8 years and 71.2 years respectively. First, the overall rate of raising serum troponin level was estimated to be 32.2% (95%CI: 25.0% to 40.5%) in COVID-19 patients admitted to the hospitals. Comparing the outcome of COVID-19 disease in the groups with raised troponin level and normal level of this marker (Table 2) showed an increased likelihood of death [hazard ratio (HR) = 4.967, 95%CI: 2.883 to 8.557, P < 0.001], acute respiratory distress syndrome (ARDS) (HR = 5.914, 95%CI: 3.027 to 11.555, P < 0.001), acute kidney injury (HR = 3.849, 95%CI: 3.112 to 4.760, P < 0.001), and also intensive care unit (ICU) admission (HR = 3.780, 95%CI: 2.405 to 5.943, P < 0.001) following raise of troponin and thus the abnormal value of troponin on admission could effectively predict poor outcome in COVID-19 patients (Figure 3). The heterogeneity across the studies in the assessment of disease outcome was relevant with the I2values ranged 74.877 to 91.317.
| Ref. | Raised TnI rate | Death rate | ARDS | Kidney injury | ICU admission |
| Arcari et al[17] | 39/103 | Raised TnI: 12 | --- | --- | --- |
| Normal TnI: 7 | |||||
| Fan et al[18] | 22/89 | --- | Raised TnI: 20 | Raised TnI: 9 | --- |
| Normal TnI: 12 | Normal TnI: 10 | ||||
| Guo et al[19] | 52/187 | Raised TnI:3 1 | Raised TnI: 30 | Raised TnI: 14 | Raised TnI: 31 |
| Normal TnI: 12 | Normal TnI: 16 | Normal TnI: 4 | Normal TnI: 14 | ||
| Lala et al[20] | 530/2736 | Raised TnI: 223 | --- | --- | --- |
| Normal TnI: 102 | |||||
| Lombardi et al[21] | 278/614 | Raised TnI: 102 | --- | Raised TnI: 41 | --- |
| Normal TnI: 43 | Normal TnI: 13 | ||||
| Lorente-Ros et al[22] | 147/707 | Raised TnI: 60; Normal TnI: 130 | --- | --- | Raised TnI: 7Normal TnI: 5 |
| Raad et al[23] | 390/1020 | Raised TnI: 128 | Raised TnI: 93 | Raised TnI: 224 | Raised TnI: 105 |
| Normal TnI: 52 | Normal TnI: 64 | Normal TnI:178 | Normal TnI:93 | ||
| Karbalai Saleh et al[24] | 115/386 | Raised TnI: 47 | Raised TnI: 46 | Raised TnI: 35 | Raised TnI: 41 |
| Normal TnI: 30 | Normal TnI: 30 | Normal TnI: 25 | Normal TnI: 38 | ||
| Shah et al[25] | 116/309 | Raised TnI: 44 | --- | Raised TnI: 13 | Raised TnI: 58 |
| Normal TnI: 22 | Normal TnI: 5 | Normal TnI: 52 | |||
| Singh et al[26] | 132/276 | Raised TnI: 20 | Raised TnI: 29 | --- | Raised TnI: 63 |
| Normal TnI: 5 | Normal TnI: 10 | Normal TnI: 25 |
In the second assessment concerning the difference in the value of troponin-I between survived and non-survived patients suffering COVID-19 (Table 3), The pooled analysis showed significantly higher concentration of troponin-I in the survived group compared to non-survived group (weighted MD of 22.278, 95%CI: 15.647 to 28.927, P < 0.001). The statistical heterogeneity was significant with an I2of 99.123. We showed a significant publication bias as evidenced by either funnel plot asymmetry or the Egger test for all comparative analyses.
| Ref. | Country | Population | Mean age | Male/female | Mean TnI |
| Aladağ and Atabey[27] | Turkey | Survived: 35 | 68 ± 14 | 22/13 | Survived: 0.001 ng/mL |
| Non-survived: 15 | 68 ± 15 | 6/9 | Non-survived: 0.010 ng/mL | ||
| Deng et al[28] | China | Survived: 212 | 62 ± 11 | 97/115 | Survived: 0.006 ng/mL |
| Non-survived: 52 | 74 ± 15 | 33/19 | Non-survived: 0.051 ng/mL | ||
| Liberati et al[15] | Italy | Survived: 425 | 61 ± 13 | 287/138 | Survived: 0.006 ng/mL |
| Non-survived: 98 | 76 ± 10 | 68/30 | Non-survived: 0.036 ng/mL | ||
| Nie et al[29] | China | Survived: 200 | 60 ± 11 | 180/20 | Survived: 0.002 ng/mL |
| Non-survived: 111 | 72 ± 12 | 88/23 | Non-survived: 0.032 ng/mL | ||
| Rath et al[30] | Germany | Survived: 107 | 67 ± 15 | 65/42 | Survived: 0.014 ng/mL |
| Non-survived: 16 | 73 ± 16 | 12/4 | Non-survived: 0.024 ng/mL | ||
| Shi et al[31] | China | Survived: 609 | 61 ± 13 | 287/322 | Survived: 0.006 ng/mL |
| Non-survived: 62 | 74 ± 15 | 35/27 | Non-survived: 0.023 ng/mL | ||
| Yang et al[32] | China | Survived: 145 | 57 ± 13 | 77/68 | Survived: 0.004 ng/mL |
| Non-survived: 58 | 67 ± 15 | 38/20 | Non-survived: 0.020 ng/mL |
High-sensitive troponin-I has been well known as a strong and even specific cardiac biomarker for diagnosing ischemic cardiac injury as well as predicting its poorer outcome. The observations among critically ill patients who are suffering from COVID-19 especially those who require intensive care also suggested higher levels of this marker on admission as well as within hospitalization. In this regard, recent studies have focused this point that by initially measuring troponin-I in patients hospitalized due to definitive diagnosis of COVID-19, predicting outcomes of disease especially occurring in-hospital death and disease severity may be certainly possible. As shown in our systematic review, assessing the baseline level of troponin-I not only can strongly predict death in COVID-19 patients, but also it can be used as a valuable factor to predict disease sequels including ARDS, kidney injury, and requiring ICU admission. Due to this fact, the appearance of each of these complications are indicators for disease severity (particularly ICU admission). Therefore, raising in troponin-I is a valuable indicator for disease severity. Overall, as well indicated in our meta-analysis, raising troponin-I on admission can be linked to the increased risk for in-hospital death, ARDS, kidney injury, and ICU admission by 4.9, 5.9, 3.8, and 3.7 times as compared to those with initial normal troponin-I concentration. It should be noted that the use of this parameter along with other known predictive parameters can be more valuable to predict non-survived patients or those with disease-related adverse events. Despite demonstrating the value of troponin-I as a strong predictor for COVID-19 poorer outcomes, our results exposed to a high heterogeneity across the findings. Such heterogeneity can be explained by first the difference insignificant divergent in the cutoff points defined for troponin-I raising, also by the difference in the baseline characteristics of study populations especially with respect to the presence of cardiovascular risk profiles, the sample size of the studies, the time for patients’ following–up, as well as the techniques for measuring troponin-I concentration. However, despite such heterogeneities across the studies, this marker should also be considered as an important and strong predictor of disease consequences. In a meta-analysis by Lippi et al[33] demonstrated a significant association between COVID-19 severity and elevated troponin-I level, despite their high heterogeneity.
Regarding the association between the raise of troponin-I and COVID-19 outcome, some probable mechanisms have been delivered. Firstly, it has been revealed that the patients with raised troponin-I level had higher rates of cardiovascular risk factors that are now shown to be closely linked to the increased risk for death and severity of COVID-19[19]. Also, any severe respiratory viral infections especially when lead to sepsis are also associated with increasing the level of troponin-I. Recently, a quadrilateral mediator loop is revealed describing the association between raising troponin levels and disease outcome[34,35]. These four loops include: (1) Secretion of pro-inflammatory cytokines that raised favored by ACE2 receptor suppression and induced oxidative stress and endothelial dysfunction; (2) Microangiopathy and prothrombotic states usually stimulated by both oxidative stress and endothelial dysfunction; (3) Myocardial infarction induced directly by viral invasion or by inflammatory cascades activation; and (4) Myocarditis by inflammatory reactions[36].
In conclusion, according to our findings, regardless of the history of myocardial injuries or the presence of cardiovascular risk profile, the value of troponin-I should be accurately assessed on admission because of its high predicting value for COVID-19 related mortality and morbidity.
Troponin-I on admission has a high predicting value for coronavirus disease 2019 (COVID-19) related mortality. Troponin-I on admission has a high predicting value for COVID-19 related morbidity. Troponin-I can strongly predict disease sequels including acute respiratory distress syndrome (ARDS), kidney injury, and Intensive care units (ICU) admission requirement.
Accurate and early diagnosis and determination of COVID-19 severity can prevent its further progression. The increase in circulating troponin-I in the blood of patients suffering COVID-19 can be a strong prognostic factor for predicting disease poorer outcome. We systematically reviewed the literatures to approve this claim.
The increase in circulating troponin-I in the blood of patients suffering COVID-19 can be a strong prognostic factor for predicting disease poorer outcome.
Deeply searching the manuscript databanks was planned. All studies that evaluated the link between the serum level of troponin-I and two COVID-19 related parameters including disease severity and mortality were considered to be eligible for primary assessment. The review papers, case presentations, letter to editors, non-English studies, and abstracts without full text access were all excluded. The manuscript reviewing was done by two blinded reviewers, screening the titles and abstracts followed by profound appraisement of the full texts independently to assess the inclusion appropriateness. The presence of any disagreement between them was judged and checked again by another reviewer as the last arbiter.
Comparing outcome of COVID-19 disease in the groups with raised troponin level and normal level of this markers showed increased the likelihood of death [hazard ratio (HR) = 4.967, P < 0.001], acute respiratory distress syndrome (HR = 5.914, P < 0.001), acute kidney injury (HR = 3.849, P < 0.001), and ICU admission (HR = 3.780, P < 0.001) following raise of troponin. The pooled analysis showed significantly higher concentration of this marker in the survived group compared to non-survived group (weighted mean differences of 22.278, 95%CI: 15.647 to 28.927, P < 0.001).
In conclusion, according to our findings, regardless of the history of myocardial injuries or the presence of cardiovascular risk profile, the value of troponin-I should be accurately assessed on admission because of its high predicting value for COVID-19 related mortality and morbidity.
The value of troponin-I should be accurately assessed on admission because of its high predicting value for COVID-19 related mortality and morbidity.
We are indebted to Research Development Center of Sina Hospital for their technical help.
Manuscript source: Unsolicited Manuscript
Specialty type: Cardiac and Cardiovascular Systems
Country/Territory of origin: Iran
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Azarbakhsh H, Peng D, Poggio PD, Wu HHL S-Editor: Liu M L-Editor: A P-Editor: Li JH
| 1. | World Health Organization. Coronavirus disease (COVID-19) Weekly Epidemiological Update and Weekly Operational Update. [cited 16 February 2021]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports. |
| 2. | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708-1720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19202] [Cited by in RCA: 18877] [Article Influence: 3775.4] [Reference Citation Analysis (7)] |
| 3. | Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475-481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6231] [Cited by in RCA: 6660] [Article Influence: 1332.0] [Reference Citation Analysis (0)] |
| 4. | Ai T, Yang Z, Hou H, Zhan C, Chen C, Lv W, Tao Q, Sun Z, Xia L. Correlation of Chest CT and RT-PCR Testing for Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases. Radiology. 2020;296:E32-E40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3614] [Cited by in RCA: 3285] [Article Influence: 657.0] [Reference Citation Analysis (0)] |
| 5. | Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20:363-374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3174] [Cited by in RCA: 2918] [Article Influence: 583.6] [Reference Citation Analysis (0)] |
| 6. | Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, Nigoghossian C, Ageno W, Madjid M, Guo Y, Tang LV, Hu Y, Giri J, Cushman M, Quéré I, Dimakakos EP, Gibson CM, Lippi G, Favaloro EJ, Fareed J, Caprini JA, Tafur AJ, Burton JR, Francese DP, Wang EY, Falanga A, McLintock C, Hunt BJ, Spyropoulos AC, Barnes GD, Eikelboom JW, Weinberg I, Schulman S, Carrier M, Piazza G, Beckman JA, Steg PG, Stone GW, Rosenkranz S, Goldhaber SZ, Parikh SA, Monreal M, Krumholz HM, Konstantinides SV, Weitz JI, Lip GYH; Global COVID-19 Thrombosis Collaborative Group; Endorsed by the ISTH, NATF, ESVM, and the IUA; Supported by the ESC Working Group on Pulmonary Circulation and Right Ventricular Function. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;75:2950-2973. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2178] [Cited by in RCA: 2191] [Article Influence: 438.2] [Reference Citation Analysis (0)] |
| 7. | Connors JM, Levy JH. Thromboinflammation and the hypercoagulability of COVID-19. J Thromb Haemost. 2020;18:1559-1561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 454] [Cited by in RCA: 470] [Article Influence: 94.0] [Reference Citation Analysis (0)] |
| 8. | Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential Effects of Coronaviruses on the Cardiovascular System: A Review. JAMA Cardiol. 2020;5:831-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1095] [Cited by in RCA: 1249] [Article Influence: 249.8] [Reference Citation Analysis (2)] |
| 9. | Clerkin KJ, Fried JA, Raikhelkar J, Sayer G, Griffin JM, Masoumi A, Jain SS, Burkhoff D, Kumaraiah D, Rabbani L, Schwartz A, Uriel N. COVID-19 and Cardiovascular Disease. Circulation. 2020;141:1648-1655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1022] [Cited by in RCA: 1220] [Article Influence: 244.0] [Reference Citation Analysis (0)] |
| 10. | Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607-1608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1565] [Cited by in RCA: 1766] [Article Influence: 353.2] [Reference Citation Analysis (0)] |
| 11. | Centers for Disease Control and Prevention. Underlying Medical Conditions. [cited 16 February 2021]. Available from: https://cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html. |
| 12. | Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11409] [Cited by in RCA: 11507] [Article Influence: 2301.4] [Reference Citation Analysis (0)] |
| 13. | Babapoor-Farrokhran S, Gill D, Walker J, Rasekhi RT, Bozorgnia B, Amanullah A. Myocardial injury and COVID-19: Possible mechanisms. Life Sci. 2020;253:117723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 228] [Cited by in RCA: 308] [Article Influence: 61.6] [Reference Citation Analysis (0)] |
| 14. | Salvatici M, Barbieri B, Cioffi SMG, Morenghi E, Leone FP, Maura F, Moriello G, Sandri MT. Association between cardiac troponin I and mortality in patients with COVID-19. Biomarkers. 2020;25:634-640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 15. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13930] [Cited by in RCA: 13355] [Article Influence: 834.7] [Reference Citation Analysis (0)] |
| 16. | Higgins J, Altman D, Sterne J. Chapter 8: Assessing risk of bias in included studies In: Higgins JPT. Cochrane Handbook for systematic reviews of interventions version 6.2. The Cochrane Collaboration, 2017: 520. |
| 17. | Arcari L, Luciani M, Cacciotti L, Musumeci MB, Spuntarelli V, Pistella E, Martolini D, Manzo D, Pucci M, Marone C, Melandri S, Ansalone G, Santini C, Martelletti P, Volpe M, De Biase L. Incidence and determinants of high-sensitivity troponin and natriuretic peptides elevation at admission in hospitalized COVID-19 pneumonia patients. Intern Emerg Med. 2020;15:1467-1476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 18. | Fan ZX, Yang J, Zhang J, He C, Wu H, Yang CJ, Zheng T, Ma C, Xiang ZJ, Zhai YH, Jiang J, Qiu SQ. Analysis of influencing factors related to elevated serum troponin I level for COVID-19 patients in Yichang, China. Cardiovasc Diagn Ther. 2020;10:678-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020;5:811-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2516] [Cited by in RCA: 2842] [Article Influence: 568.4] [Reference Citation Analysis (0)] |
| 20. | Lala A, Johnson KW, Januzzi JL, Russak AJ, Paranjpe I, Richter F, Zhao S, Somani S, Van Vleck T, Vaid A, Chaudhry F, De Freitas JK, Fayad ZA, Pinney SP, Levin M, Charney A, Bagiella E, Narula J, Glicksberg BS, Nadkarni G, Mancini DM, Fuster V; Mount Sinai COVID Informatics Center. Prevalence and Impact of Myocardial Injury in Patients Hospitalized With COVID-19 Infection. J Am Coll Cardiol. 2020;76:533-546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 515] [Cited by in RCA: 557] [Article Influence: 111.4] [Reference Citation Analysis (0)] |
| 21. | Lombardi CM, Carubelli V, Iorio A, Inciardi RM, Bellasi A, Canale C, Camporotondo R, Catagnano F, Dalla Vecchia LA, Giovinazzo S, Maccagni G, Mapelli M, Margonato D, Monzo L, Nuzzi V, Oriecuia C, Peveri G, Pozzi A, Provenzale G, Sarullo F, Tomasoni D, Ameri P, Gnecchi M, Leonardi S, Merlo M, Agostoni P, Carugo S, Danzi GB, Guazzi M, La Rovere MT, Mortara A, Piepoli M, Porto I, Sinagra G, Volterrani M, Specchia C, Metra M, Senni M. Association of Troponin Levels With Mortality in Italian Patients Hospitalized With Coronavirus Disease 2019: Results of a Multicenter Study. JAMA Cardiol. 2020;5:1274-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 147] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 22. | Lorente-Ros A, Monteagudo Ruiz JM, Rincón LM, Ortega Pérez R, Rivas S, Martínez-Moya R, Sanromán MA, Manzano L, Alonso GL, Ibáñez B, Zamorano JL. Myocardial injury determination improves risk stratification and predicts mortality in COVID-19 patients. Cardiol J. 2020;27:489-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 23. | Raad M, Dabbagh M, Gorgis S, Yan J, Chehab O, Dagher C, Jamoor K, Hussein IH, Cook B, Van Harn M, Singh G, McCord J, Parikh S. Cardiac Injury Patterns and Inpatient Outcomes Among Patients Admitted With COVID-19. Am J Cardiol. 2020;133:154-161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 24. | Karbalai Saleh S, Oraii A, Soleimani A, Hadadi A, Shajari Z, Montazeri M, Moradi H, Talebpour M, Sadat Naseri A, Balali P, Akhbari M, Ashraf H. The association between cardiac injury and outcomes in hospitalized patients with COVID-19. Intern Emerg Med. 2020;15:1415-1424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 25. | Shah P, Doshi R, Chenna A, Owens R, Cobb A, Ivey H, Newton S, Mccarley K. Prognostic Value of Elevated Cardiac Troponin I in Hospitalized Covid-19 Patients. Am J Cardiol. 2020;135:150-153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 26. | Singh N, Anchan RK, Besser SA, Belkin MN, Cruz MD, Lee L, Yu D, Mehta N, Nguyen AB, Alenghat FJ. High sensitivity Troponin-T for prediction of adverse events in patients with COVID-19. Biomarkers. 2020;25:626-633. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 27. | Aladağ N, Atabey RD. The role of concomitant cardiovascular diseases and cardiac biomarkers for predicting mortality in critical COVID-19 patients. Acta Cardiol. 2021;76:132-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 28. | Deng Q, Hu B, Zhang Y, Wang H, Zhou X, Hu W, Cheng Y, Yan J, Ping H, Zhou Q. Suspected myocardial injury in patients with COVID-19: Evidence from front-line clinical observation in Wuhan, China. Int J Cardiol. 2020;311:116-121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 274] [Cited by in RCA: 301] [Article Influence: 60.2] [Reference Citation Analysis (0)] |
| 29. | Nie SF, Yu M, Xie T, Yang F, Wang HB, Wang ZH, Li M, Gao XL, Lv BJ, Wang SJ, Zhang XB, He SL, Qiu ZH, Liao YH, Zhou ZH, Cheng X. Cardiac Troponin I Is an Independent Predictor for Mortality in Hospitalized Patients With COVID-19. Circulation. 2020;142:608-610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 30. | Rath D, Petersen-Uribe Á, Avdiu A, Witzel K, Jaeger P, Zdanyte M, Heinzmann D, Tavlaki E, Müller K, Gawaz MP. Impaired cardiac function is associated with mortality in patients with acute COVID-19 infection. Clin Res Cardiol. 2020;109:1491-1499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 100] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 31. | Shi S, Qin M, Cai Y, Liu T, Shen B, Yang F, Cao S, Liu X, Xiang Y, Zhao Q, Huang H, Yang B, Huang C. Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019. Eur Heart J. 2020;41:2070-2079. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 349] [Cited by in RCA: 351] [Article Influence: 70.2] [Reference Citation Analysis (0)] |
| 32. | Yang C, Liu F, Liu W, Cao G, Liu J, Huang S, Zhu M, Tu C, Wang J, Xiong B. Myocardial injury and risk factors for mortality in patients with COVID-19 pneumonia. Int J Cardiol. 2021;326:230-236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 33. | Lippi G, Lavie CJ, Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): Evidence from a meta-analysis. Prog Cardiovasc Dis. 2020;63:390-391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 406] [Cited by in RCA: 477] [Article Influence: 95.4] [Reference Citation Analysis (0)] |
| 34. | Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, Huang H, Yang B, Huang C. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2428] [Cited by in RCA: 3009] [Article Influence: 601.8] [Reference Citation Analysis (1)] |
| 35. | Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054-1062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17476] [Cited by in RCA: 18201] [Article Influence: 3640.2] [Reference Citation Analysis (0)] |
| 36. | Tersalvi G, Vicenzi M, Calabretta D, Biasco L, Pedrazzini G, Winterton D. Elevated Troponin in Patients With Coronavirus Disease 2019: Possible Mechanisms. J Card Fail. 2020;26:470-475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 217] [Cited by in RCA: 220] [Article Influence: 44.0] [Reference Citation Analysis (0)] |