Published online Apr 28, 2021. doi: 10.13105/wjma.v9.i2.108
Peer-review started: January 23, 2021
First decision: February 28, 2021
Revised: March 12, 2021
Accepted: April 23, 2021
Article in press: April 23, 2021
Published online: April 28, 2021
Processing time: 94 Days and 20.6 Hours
Coronavirus disease 2019 is a pandemic disease caused by a novel RNA coronavirus, SARS coronavirus 2 (SARS-CoV-2), which is implicated in the respiratory system. SARS-CoV-2 also targets extrapulmonary systems, including the gastrointestinal tract, liver, central nervous system and others. SARS-CoV-2, like other RNA viruses, targets the liver and produces liver injury. This literature review showed that SARS-CoV-2-induced liver injury is different from other RNA viruses by a transient elevation of hepatic enzymes and does not progress to liver fibrosis or other unfavorable events. Moreover, SARS-CoV-2-induced liver injury usually occurs in the presence of risk factors, such as nonalcoholic liver fatty disease. This review highlights the important differences between RNA viruses inducing liver injury taking into consideration the clinical, biochemical, histopathological, postmortem findings and the chronicity of liver injury that ultimately leads to liver fibrosis and hepatocellular carcinoma.
Core Tip: Coronavirus-induced liver injury is rare, and it may be passed without a definite diagnosis. Liver function tests are simple, inexpensive and rapid and can detect acute liver injury, particularly in infected patients who have evidence of comorbidities. This review discusses the differences between RNA virus-induced liver injury focusing on the coronavirus targeting the liver as an extrapulmonary site of infection or as a part of multiple organ dysfunction
- Citation: Al-Nimer MS. Is COVID-19-induced liver injury different from other RNA viruses? World J Meta-Anal 2021; 9(2): 108-127
- URL: https://www.wjgnet.com/2308-3840/full/v9/i2/108.htm
- DOI: https://dx.doi.org/10.13105/wjma.v9.i2.108
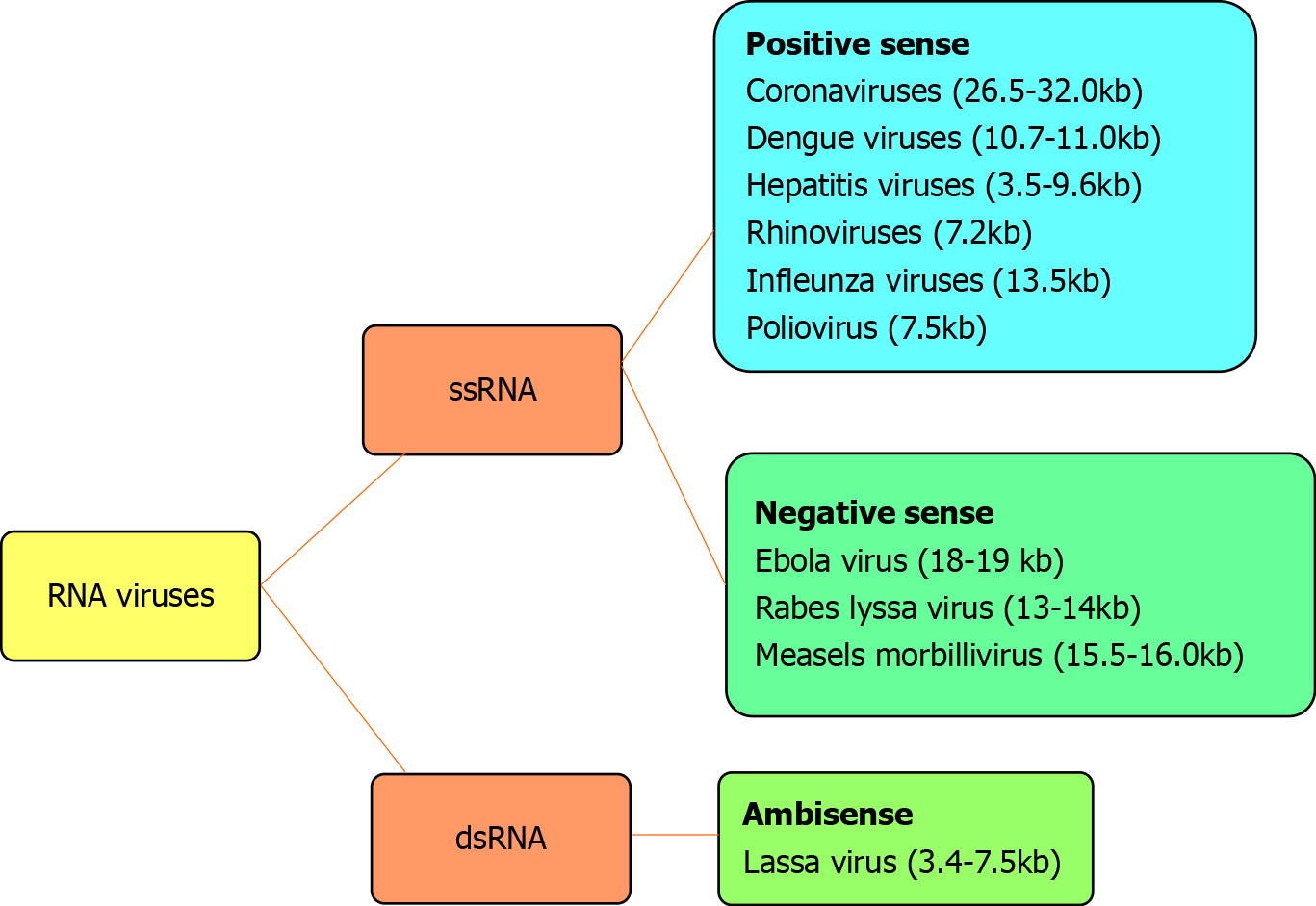
The genetic material of RNA viruses is usually single-stranded RNA and occasionally double-stranded. Examples of single-stranded RNA viruses are rhinoviruses, influenza viruses, coronaviruses, dengue viruses, hepatitis viruses, west Nile viruses, Lassa virus, Ebola virus, Rabies lyssavirus, polioviruses and measles morbillivirus. Examples of double-stranded RNA viruses are rotaviruses and picobirnaviruses. According to the polarity of the viral envelope, they are grouped into positive-sense and negative-sense (Figure 1). Coronaviruses are RNA viruses that belong to the coronaviridae family. These viruses are enveloped with a positive single-stranded RNA genome sized 26.4-31.7 kilobases[1]. The genome of coronaviruses is larger than other RNA viruses (Figure 1).
In humans, coronaviruses cause mild respiratory symptoms (e.g., rhinoviruses) or lethal respiratory stress syndrome [e.g., severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS) and coronavirus disease 2019 (COVID-19)]. In mice, coronaviruses cause hepatitis and encephalomyelitis[2,3]. Extrapulmonary organ dysfunction is also reported by coronavirus infections in humans. Dysfunction of the hepato-biliary system is an uncommon clinical presentation of coronavirus infections.
The liver is one of the extrapulmonary organs attacked by COVID-19[4-6]. Some SARS coronavirus 2 (SARS-CoV-2)-infected people showed abnormal liver function tests, and other patients showed liver injury as a late sequel of multiple organ dysfunction or failure[7]. Abnormal liver function tests, including increasing levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST) and lactic dehydrogenase are observed[8]. Serum bilirubin level is also reported to be increased in some COVID-19 patients at the time of admission into the hospital[9].
High abnormal serum levels of aminotransferases were observed in severe illnesses rather than in trivial or mild COVID-19 illnesses[10]. There are several characteristic features of high serum levels of aminotransferase enzymes. The first feature is a transient and slight increase of serum ALT and AST levels. The second feature is a dynamic pattern of liver enzymes: liver dysfunction started with an elevation of AST, slight changes of total bilirubin levels followed by increased ALT in severe patients[11], and the pattern of cholestasis is absent[8,12]. Liver failure and bile duct injuries were not pathological features of COVID-19[13]. Therefore, any elevated transaminase enzyme is considered an indirect expression of systemic inflammation, and there are no specific symptoms that have been linked to liver dysfunction[13]. The third feature is the elevation of aminotransferase enzymes is related to race as Chinese patients less frequently had high serum aminotransferases compared with United States patients infected with COVID-19[14]. These enzymes (ALT, AST, alkaline phosphatase and γ-glutamyl transpeptidase) are increased with increased disease severity. The mortality rate increased by 14.87 fold in patients with AST level (40-120 U/L), and the male gender is positively associated with elevation of AST[11].
The fourth feature is that ALT among many biochemical markers was studied as a predictor of COVID-19 severity using univariate and multivariate ordinal logistic regression models on 598 patients presenting with moderate, severe and critical illness. The results showed that patients with ALT > 50 U/L had worse severity of illness (odds ratio: 3.304, 95% confidence interval: 2.107-5.180)[15]. Serum ALT level is a stronger predictor of disease severity compared with myohemoglobin, but it is weaker than cardiac troponin I or aging and the presence of high blood pressure.
Phipps et al[16] categorized the acute liver injury in patients with coronavirus according to the serum levels of ALT into mild [ALT > the upper limit normal (ULN) < 2 times ULN], moderate (ALT 2-5 times the ULN) and severe (ALT > 5 times the ULN). Those authors found that 45% had mild, 21% moderate and 6.4% severe liver in a total number of 2273 patients with positive SARS-CoV-2 tests[16]. The category of severe acute liver injury had significantly high inflammatory markers and a higher rate of mortality that accounted for 42%[16]. In one cohort study that included 176 patients, 109 (61.9%) had evidence of liver dysfunction, and only 34 out of 109 (31.2%) had an acute liver injury (ALT and/or AST ≥ 3 ULN), which was associated with an increase in serum bilirubin, lactic dehydrogenase and C-reactive protein[17].
Another meta-analysis that included 3428 patients collected from 20 studies found that severe COVID-19 is associated with increasing levels of serum AST, ALT and bilirubin and a decreasing serum albumin level[18]. This study showed no evidence of bias of all studied variables as the calculated P value of the Egger’s test was nonsignificant (P > 0.1).
The fifth feature is that there is no clear association with adult respiratory stress syndrome. Most studies link acute liver injury with respiratory stress syndrome with hypoxemia and multiple organ failure. The sixth feature is they may be associated with an increased serum bilirubin level. In one study that included 11245 patients, the majority of patients presented with elevated serum bilirubin (9.7%), AST (23%), ALT (21.2%), gamma-glutamyl transferase (GGT) (15.5%) and alkaline phosphatase (ALP) (4%)[19]. Patients presenting with higher levels of serum bilirubin at admission indicate the progression of the disease severity[20,21].
The seventh feature is that high levels of aminotransferases are not a specific effect as other serum levels of enzymes related to the heart, kidney and muscle are also increased The eighth feature is that the high serum aminotransferases are infrequently associated with a slight increase of GGT, ALP and bilirubin[14]. During admission the serum level of ALP, an index of cholangiocyte injury, is within normal limits and several factors are associated with increased serum levels of ALP including male gender, increased neutrophil count, corticosteroid use and antifungal drugs[11]. The final feature is that SARS-CoV-2-infected children showed minimal or no increase in the hepatic enzymes[22].
The explanation of abnormal serum levels of ALT and AST are attributed to several factors. SARS-CoV-2 infected the pulmonary tissue causing hypoxemia. As a result of hypoxia-reoxygenation injury, Kupffer cells are activated leading to the generation of the free radicals that induce acute liver cell injury[23,24]. Hypoxia de novo is a form of stress that increases the activity of the sympathetic autonomic nervous system and releases adrenocorticotrophic hormone from the hypothalamus leading to acute liver injury[25]. In addition, flaring of the intestinal microflora in the critical COVID-19 cases causes septic shock, which induces hypoxia of the hepatocytes and thereby hepatocellular necrosis and cholestasis[26].
Abnormal ALT and AST serum levels are also attributed to direct transmission of SARS-CoV-2 from the bowel to the liver[13,27]. Inflammation and high levels of cytokines associated with SARS-CoV-2 infection causes multiple organ dysfunction. Biomarkers related to the inflammation are independent risk factors of developing acute liver injury in COVID-19. Interleukins (IL), including IL-1β, IL-6 and IL-8, and soluble tumor necrosis factor (TNF) receptor-1 are increased and played a role in multiple organ dysfunction[28]. Serum C-reactive protein ≥ 20 mg/L and lymphocyte count < 1.1 × 109/mm3 are significantly associated with acute live injury[7].
Chronic liver diseases also cause abnormal levels of AST and ALT. Patients with viral hepatitis are more susceptible to SARS-CoV-2 infection. Lei et al[11] reported 84 patients out of 5771 had a previous history of liver disease (4 patients had fatty liver, and 77 patients had viral hepatitis. Sarin et al[29] reported that elevation of serum bilirubin and AST/ALT ratio predicted a poor prognosis of COVID-19 (mortality rate of 43%) in patients with liver cirrhosis. On the other side, a recent meta-analysis study that included 17 studies showed that chronic liver disease had no significant effect on the poor prognosis of COVID-19[30].
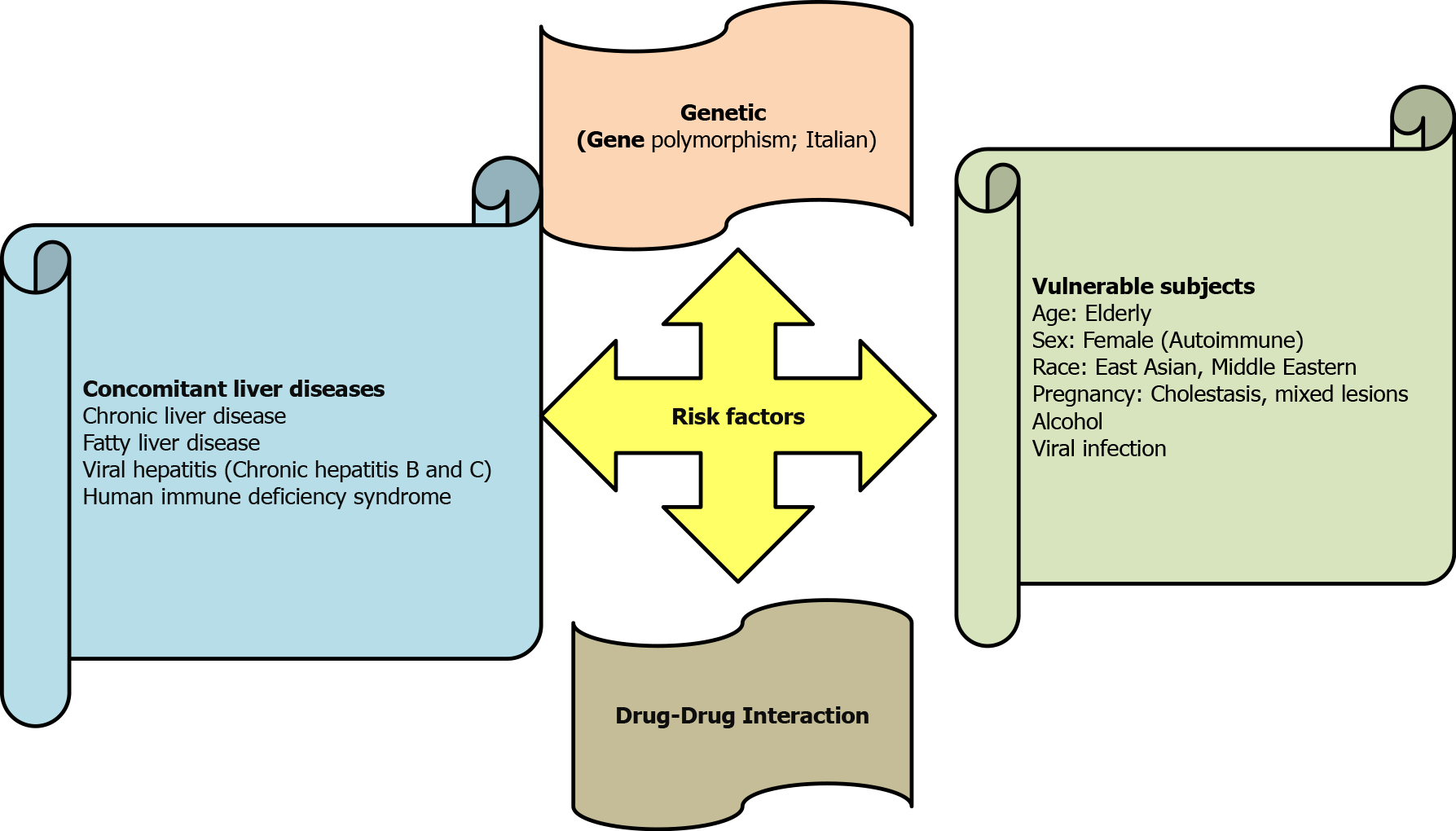
Drug-induced hepatocyte injury can also cause abnormal AST and ALT levels[31,32] (Table 1). Accordingly, drug-induced acute liver injury referred to an elevation of ALT by ≥ 5 ULN, or an elevation of ALP (in the absence of bone diseases) by ≥ 5 ULN, or the elevation of ALT by ≥ 3 ULN plus elevation of serum bilirubin level by > 2 ULN[33,34]. Drug-induced liver injury is either idiosyncratic or intrinsic in nature, and several risk factors played a role in inducing liver injury (Figure 2). Antiviral agents with potential hepatotoxic effects, e.g., remdesivir, lopinavir and tocilizumab should also be considered[35-37]. Acetaminophen, which might cause liver damage, is usually used in the management of COVID-19 to reduce body temperature[38,39]. Azithromycin therapy is a possible cause of acute liver injury in COVID-19[40].
| Drug category | Efficacy and hepatic adverse reactions |
| Protease inhibitors: lopinavir, ritonavir | They are effective against SARS and MERS but not against COVID-19. These drugs failed to improve the clinical manifestations or shorten the time of conversion to negative test of viral nucleic acids in the bronchoalveolar fluid specimens. They caused increase serum bilirubin and aminotransferases levels in COVID-19 patients. |
| Viral RNA-dependent RNA polymerase: remdesivir, favipiravir | (1) Remdesivir is an adenine nucleotide analogue used in the management of Ebola virus infection. It inhibits the activity of viral RNA-dependent RNA polymerase. It improves the clinical manifestations of COVID-19 patients presenting with hypoxia with low blood oxygen saturation. It causes a significant increase in AST/ALT in COVID-19 patients; and (2) Favipiravir is a guanosine analogue with a broad-spectrum antiviral activity similar to remdesivir. It is effective against the clinical manifestations and shortening the recovery period of moderate COVID-19 illness. Experimental studies showed that favipiravir increases the serum levels of AST, ALP, ALT and total bilirubin and increased vacuolization in hepatocytes. |
| Membrane fusion inhibitors: umifenovir | Umifenovir is an indole derivative molecule with broad-spectrum antiviral activity. It is effective in the management of COVID-19, particularly when combined with protease inhibitors. The data are very limited about the hepatotoxicity of umifenovir. Chronic users had high serum transaminases. |
| Interferon lambda | Endogenous INFλs potentiate the long-term immunity by stimulating T helper 1, cytotoxic T cell and antibody response. It prevents the development of a cytokine storm. Recombinant INFλ may be a promising therapy for COVID-19. There is no clinical evidence that INFλ induces liver injury. |
Several studies clarified that SARS-CoV-2 causes nonspecific hepatic changes according to the postmortem histopathological and immunohistochemistry studies. The histopathological changes observed in the liver autopsies are[15,41] microvascular steatosis, lobular focal necrosis, cellular neutrophil, lymphocyte and monocyte infiltration in the portal area, microthrombosis and hepatic sinuses congestion. There is no evidence suggesting liver failure or development of granuloma. Electron microscopy studies showed that the endothelial cell contained coronavirus particles[42]. The RNA sequence data findings and immunohistochemistry studies showed expression of angiotensin-converting enzyme-2 receptors (ACE-2R) in the cholangiocytes but not in Kupffer cells[43], which may not be related to COVID-19. In normal liver, immunohistochemistry showed expression of ACE-2R in the bile duct lining cell but not in the hepatocytes.
Metabolic dysfunction-associated fatty liver disease (MAFLD) is the acronym of nonalcoholic fatty liver disease (NAFLD) as NAFLD is associated with components of the metabolic syndrome, including obesity, diabetes mellitus and dyslipidemia[44]. Therefore, COVID-19 and metabolic dysfunction-associated fatty liver disease/ NAFLD can affect each other. For example, when the patients with metabolic dysfunction-associated fatty liver disease/NAFLD are infected with SARS-CoV-2, their livers are susceptible to the harmful effects of the drugs that are clinically used in the management of the COVID-19[45-47]. Also, patients with metabolic derangement are more susceptible to SARS-CoV-2 infection (e.g., diabetes mellitus)[48]. Diabetic patients already have asymptomatic or symptomatic liver dysfunction or injury.
A recent study on 202 COVID-19 patients showed NAFLD is an independent risk factor for the progression of COVID-19 with an ultimately poor prognosis[49]. The most common pattern of liver damage reported in the Ji et al[49] study is mild hepatocellular injury, while the ductular or mixed with hepatocellular injury was observed in 2.6%. Moreover, persistent abnormal liver function tests were observed in 33.2%, and NAFLD was independently associated with the progression of liver injury in 19.3% of COVID-19 patients. The percentage of liver injury induced by SARS-CoV-2 is related to the associated risk factor rather than the specificity of SARS-CoV-2 to attack the liver directly because ACE-2R is absent in Kupffer cells and available in 3% of the hepatocytes[43,50].
Acute liver injury was reported in 19 out of 187 (15.4%) patients in one study performed at the Seventh Hospital of Wuhan City, China. Five of the patients had a cardiac injury represented by a higher serum level of troponin[51]. Zhang et al[52] summarized the studies that showed an abnormally high level of ALT, AST and GGT, which is a diagnostic marker for cholangiocyte injury, was found to be elevated in 30 of 56 patients with COVID-19 during hospitalization. The other important trigger factor of the progression of COVID-19 is drug-induced liver injury. Patients with fatty liver are at a higher risk for drug-induced liver injury compared with noninfected healthy individuals or patients without fatty liver. Some drugs might cause severe liver injury in obese patients with COVID-19, while others might induce the transition of fatty liver to nonalcoholic steatohepatitis (NASH) or worsen the preexisting pathological liver lesion[53].
Hepatocellular hypoxia is the most common explanation of hepatic metabolic derangement induced by SARS-CoV-2 infection, which leads to increases of ACE-2R in liver tissue. Tissue hypoxia is a feature of COVID-19 that is followed by an insidious and paradoxical pattern. In experimental studies, ACE-2R protein was significantly increased in the liver tissue after bile duct ligation, and the activity of ACE-2R is also increased in the human cirrhotic liver compared with a healthy liver[50]. An in vitro experimental study showed that hypoxia increased the expression of ACE-2R in CD34 cells derived from mice subjected to hind-limb ischemia without alteration in the activity of ACE[54]. Hepatocellular hypoxia also increases the expression of inducible hypoxic factors. These factors play a role in the adaptation of the endothelial cell to the hypoxia by inducing the expression of genes that promote energy metabolism of the cells[55]. These factors have dual effects as HIF-1α induces inflammation by upregulating NF-kB and CD4+, CD8+ and producing proinflammatory cytokines (IL-2 and TNF-α)[56,57].
Hepatocellular hypoxia activates reactive oxygen and nitrogen species. Extensive release of cytokines (cytokines storm) play roles in recruiting the immune cells to the site of inflammation, producing vasodilation and increasing the vascular permeability and production of nitrative and oxidative free radicals, which collectively cause tissue damage[7,58-60]. A nitrative stress syndrome in COVID-19 is evoked by IL-2 and IL-6[7,61], while oxidative stress syndrome was expressed in COVID-19 by IL-6 and TNF-α, which increase the levels of superoxide anion in neutrophils and hydrogen peroxide[62-64]. Antioxidants are useful in the management of COVID-19, e.g., vitamin C, Nrf2 activators, zinc, glutathione and N-acetylcysteine[65-67]. It is important to mention that macrolides are prescribed in the management of COVID-19 because they (e.g., azithromycin and erythromycin) inhibit the generation of superoxide anion and nitric oxide[68-70].
High inflammatory response of Kupffer cells is linked to hepatocellular hypoxia. Kupffer cells are part of innate immunity that acts as scavengers and phagocytes. During COVID-19 illness, the ACE-2Rs are expressed in the circulating inflammatory macrophages and tissue macrophages, including Kupffer cells[71]. Expression of these receptors may cause Kupffer cell dysfunction and lead to acute liver injury. Yuan et al[72] reported that Kupffer cells have dual actions against viral hepatitis as they cleared the hepatitis B virus (HBV)/HCV due to their phagocytic activity and at the same time produce inflammatory mediators that eventually cause immune tolerance towards HBV/HCV.
In one study including 28 patients presenting with variable chronic liver disease of different etiological factors, it was found that patients with acute on chronic liver failure showed worse outcomes, and a poor outcome was observed in patients managed with mechanical ventilation[73]. The mortality rate was 33% in patients with decompensated liver cirrhosis, while it accounted for 8% in patients with chronic liver disease without cirrhosis[73]. The therapeutic regimen of hydroxychloroquine, remdesivir, other antivirals and plasma therapy did not improve the outcome of COVID-19 in patients with chronic liver disease. Moreover, acute liver injury was reported in 14 out of 105 (13.3%) patients with coinfections of COVID-19 and viral hepatitis due to HBV. Because 4 out of 14 (28.57%) patients progressed within a short time to acute on chronic liver failure, and the study concluded that liver injury in patients with SARS-CoV-2 and chronic HBV coinfection was associated with severity and poor prognosis[74]. Transient elevations of ALT, AST, GGT, ALP and bilirubin were observed in a small number of coinfected patients with COVID-19 and HBV[74].
Patients with liver transplantation are at risk of infection with COVID-19. Maggi et al[75] reported 16 patients managed with liver transplantation showed positive COVID-19 laboratory tests. Moreover, the mortality rate in long-term liver transplantation survivors infected with COVID-19 was 2.7% (3 out of 111 survivors), which is comparable to those without liver transplantation[76]. Drug-drug interaction should be considered in the liver transplant recipient, particularly between immunosuppressive antiviral therapeutic regimens. A combination of ritonavir and protease inhibitors should be avoided, while chloroquine and remdesivir are safe[77].
During the COVID-19 pandemic, a number of specific antivirals drugs are indicated in the management of severe COVID-19 or patients at risk of developing severe disease. These drugs are listed in Table 1.
Ritonavir (a protease inhibitor) can induce hepatotoxicity by laboratory evidence of elevation of aminotransferases[78,79] and clinically presented with jaundice and hepatomegaly. In addition, ritonavir can induce dyslipidemia characterized by increasing serum levels of triglyceride and low-density lipoprotein cholesterol, while the serum level of high-density lipoprotein cholesterol is decreased[78,80,81]. Long-term therapy can cause dyslipidemia, which is a risk factor for developing NAFLD. Therefore protease inhibitors are unlikely to cause dyslipidemia because they are used for a short period in the management of COVID-19.
Lopinavir is another protease inhibitor used for COVID-19. It caused severe liver injury in HIV-infected patients[82]. The combined therapy of ritonavir and lopinavir leads to increased toxicity of ritonavir, which presented with a higher elevation of transaminases[83]. It is important to mention that protease inhibitor-induced hepatotoxicity is not related to lopinavir plasma levels but to ritonavir, which accumulated in the liver[82]. A combination of lopinavir/ritonavir does not show significant improvement in the clinical and laboratory testing of COVID-19; indeed it may produce adverse reactions[84].
Remdesivir is an adenosine nucleotide analog with broad-spectrum antiviral activity used for RNA virus infection treatment[85]. The most common hepatotoxicity of remdesivir in COVID-19 patients is the elevation of hepatic transaminases and multiple organ failure[86]. The elevation of ALT and AST is usually reversible.
Favipiravir (6-fluoro-3-hydroxy-2-pyrazine carboxamide) is a guanosine analog with a broad-spectrum antiviral activity similar to remdesivir. It selectively inhibits the RNA-dependent RNA polymerase of the virus and has a broad-spectrum activity against influenza virus and all RNA viruses that cause hemorrhagic fever[87]. According to the evidence-based studies, favipiravir is effective against clinical manifestations and shortening the recovery period of moderate COVID-19 illness[88]. In SARS-CoV-2-infected patients, favipiravir significantly increased the ALT and AST enzymes. Experimental studies showed that favipiravir increases the serum levels of AST, ALP, ALT and total bilirubin and increased vacuolization in hepatocytes.
Chloroquine and hydroxychloroquine have antiviral activity and have been recommended as the second therapy choice in the treatment of COVID-19[89]. Limited data were available concerning the hepatotoxicity of chloroquine.
Umifenovir (Arbidol) inhibits the adsorption of the virus to the host cell and thereby prevents viral penetration of the cell. It is effective against influenza, hepatitis C and SARS-CoV-2 infections[90-92], and it has a broad therapeutic index. Chronic use of umifenovir is associated with increased transaminase levels[93]. A triple combination of lopinavir, ritonavir and umifenovir is considered in the management of COVID-19 patients, but this combination induced liver damage in 50% of patients and elevated hepatic transaminases and bilirubin[94].
Recombinant or pegylated interferon lambda (INFλ) is available endogenously in four moieties and have a potent antiviral activity as they maintain the antiviral response in the pulmonary tissue[95]. Synthetic (recombinant) INFλ has been effective against viral replication and obviates the development of a cytokine storm[96,97]. In an experimental animal model of SARS-CoV-2 infection, the production of INFλ is decreased. Therefore, administration of synthetic recombinant INFλ can restore the endogenous INFλ and improve the immune system.
MERS-CoV is a viral RNA transmitted from infected camels to humans[98,99]. Severe MERS-CoV infections were observed in immunocompromised patients, diabetes mellitus, renal failure and the elderly age group similar to COVID-19[100,101]. Acute renal failure is the most common extrapulmonary complication in MERS-CoV patients, which accounts for 75%[102,103]. Histopathological findings of the liver autopsies of patients with MERS[104,105] are similar to those observed with COVID-19, which included: (1) mild chronic lymphocytic portal inflammation; (2) mild hydropic degeneration of hepatocytes; (3) mild sinusoidal lymphocytosis; (4) no evidence of lobular or portal granuloma or fibrosis; (5) mild steatosis; and (6) occasional intralobular hemorrhage and necrosis.
Ultrastructural findings detected by electron microscopy showed MERS-CoV inside the macrophage, while SARS-CoV-2 was detected in the cytoplasm of the hepatocyte.
In SARS-CoV, the elderly age group who had the liver disease of whatever etiology showed the highest mortality rate[106]. Postmortem liver autopsy studies revealed that SARS-CoV particles were present in less than 50% of liver tissue with approximately 1.6 × 106 copies/g hepatic tissue[107]. The characteristic features of liver injury in SARS are cellular apoptosis, increased mitotic activity, balloon degeneration of the hepatocyte, lymphocytic cell infiltration and central lobular necrosis[105,108]. Infrequently, fatty degeneration was observed[109]. Real-time PCR of the liver tissue was positive (similar to SARS-CoV-2), but electron microscopy did not show virus particles[107,110].
Liver disease is the third most common cause of death in HIV patients, which is mainly due to HIV/HCV coinfections[111]. Several factors are contributed to causing death in HIV patients, including metabolic syndrome, consumption of alcohol and coinfections with hepatitis C and D viruses[111]. HIV patients are more likely to have fatty liver (alcoholic or nonalcoholic), which can present with any clinical manifesta
The characteristic features of liver diseases in patients with HIV infections are: (1) patients with HIV infection commonly have NAFLD; (2) patients with HIV and NAFLD commonly have NASH compared with those who do not have HIV infection[115,116]; (3) an HIV-infected person with NAFLD had a lower body mass index compared with patients without HIV infection despite the similarities between the two groups in the other components of metabolic syndrome[117]. Moreover, HIV-infected persons with NAFLD had a higher percentage of visceral fat compared with uninfected HIV persons[118-120]; (4) previous studies showed that antiretroviral medicines are not a cause of NAFLD, and they did not play any role in the pathogenesis of NAFLD in HIV-infected patients. HIV-infected patients managed with highly active antiretroviral therapy for 12 mo showed significant hepatotoxicity when the patients had a higher baseline serum AST level and a lower serum albumin level. Hepatotoxicity presented with elevated serum transaminases and a significantly high AST:ALT ratio[121]. One retrospective study of HIV-infected patients found that concomitant viral hepatitis, duration of antiretroviral drugs and baseline data of liver enzymes can increase the risk of liver damage in HIV-infected patients[122]; (5) cytokines, including IL-6 and TNF-α, are not responsible for inflammation that progressed to liver fibrosis and cirrhosis[123]; (6) liver fibrosis commonly occurred in patients with concomitant diseases, e.g., viral hepatitis C and HIV despite confounding factors, including using antiretroviral drugs and heavy alcohol consumption[124]; (7) in HIV-mono-infected patients, significantly high AST and ALT were found in 5.6% and 16.8%, respectively, while hepatic steatosis and fibrosis were found in 55.0% and 17.6%, respectively[125]. Middle aged males with evidence of metabolic syndrome were more vulnerable to liver fibrosis in HIV mono-infected persons; (8) noncirrhotic portal hypertension is an uncommon complication of HIV-infection[111]; (9) hepatic granuloma and hemosiderosis and chronic active hepatitis were reported in 16%, 26% and 3%, respectively. The higher percentage of hepatic granuloma is related to intravenous drug abuse in HIV patients, while the low incidence of chronic active hepatitis is attributed to T lymphocyte depletion[126]; (10) histopathological changes of the liver showed a nonspecific or pathognomonic feature that indicated HIV infection. One study including 42 liver autopsies found that histopathological changes are usually secondary to infection, inflammation, cirrhosis and cancer[127]; and (11) HIV patients with secondary specific hepatic infections have higher serum ALP and AST compared with noninfected HIV patients[126].
Influenza A virus is an enveloped, single-stranded RNA virus with eight RNA fragments. Infected patients with influenza A/Kawasaki/86 (H1N1) showed significant elevation of the serum aminotransferases indicating viral hepatitis. The significant high serum liver enzymes usually occurred after the fever had subsided, which indicates that the viral replication is not the cause but the consequent activation of the immune response[128]. Rarely, using chloroquine in the prevention of influenza can cause hepatitis (elevated serum amino transaminases)[129].
H1N1 differed from the seasonal influenza infection by inducing hepatocellular injury with increased serum AST and ALT levels[130,131], while infection with H7N9 can induce hepatic hypoxia and fatty infiltration[132,133]. In experimental studies using mice, influenza A virus types H1N1, H5N1 and H7N2 significantly increased serum hepatic enzymes on day 5 postinfection, and the immunohistochemistry showed positively infected hepatocytes with viruses[134]. In one Korean population study, the relationship between the H1N1 2009 pandemic and acute viral hepatitis A showed a significant inverse (r = -0.597) relationship, i.e. as the prevalence of hepatitis A virus increased the prevalence of H1N1 2009 decreased. This indicates that the hepatitis A virus (positive-stranded RNA virus) damped the virulence of H1N1 2009 to attack the liver and produce exacerbation of the acute liver injury by a mechanism still unknown[135].
Hepatitis A virus (HAV) produces a broad spectrum of liver injury varied from mild illness (a transient elevation of serum transaminases and bilirubin) to fulminant hepatic failure, and the HAV-RNA particle was detected in the serum of the infected patient[136]. Blood donor participants with previous HAV infection showed a high serum level of ALT and anti-HAV antibody, while the HVA-RNA was not detected in the serum[137]. Infected patients with hepatitis E virus have low serum levels of ALT compared with HAV infection, while the serum level of GTT is high, and the patients clinically are asymptomatic[138]. Infected patients with HCV may have a previous history of HBV infections, and their illness linked with liver autoimmune diseases as autoantibodies are detected in their sera[139]. Most HCV are mono-infections, and a minority have HCV and HBV coinfections at a single time point with a complex pattern of virological profiles. In patients with positive hepatitis B surface antigen, the estimated prevalence of HBV/HCV is approximately 5%-20%, while in HCV positive antibody patients is 2%-10%, considering the geographical distribution factor[140]. Patients with chronic hepatitis B surface antigen are liable to be infected with HDV[141]. Chronic hepatitis D patients showed high viral load and transaminase levels and progressed rapidly to liver cirrhosis[142].
The relationship between serum lipids and viral hepatitis are complex. There is evidence that in the acute phase of viral hepatitis, the serum triglycerides are elevated and abnormal lipoproteins are detected in the serum[143]. A low serum level of cholesterol and apolipoprotein A indicated a severe liver injury[143]. NAFLD is usually associated with HBV and HCV infection, and the reason for hepatic steatosis is related to the existence of the components of metabolic syndrome (e.g., obesity, diabetes mellitus, dyslipidemia, etc.). Hepatic steatosis is related to the HCV-genotype 3 infection, which exerts direct metabolic effects on the liver[144]. Chronic HBV and HCV infections lead to liver cirrhosis and hepatocellular carcinoma, which carries a poor liver function compared with NASH-induced hepatocellular carcinoma[145]. A case report study mentioned a 24-year-old patient presented with mild COVID-19 disease, and he experienced a cytokine storm due to coinfection with HBV leading to multiple organ dysfunction syndrome and death[146]. This case report highlights the worsening prognosis of superinfection with viral hepatitis in patients with RNA virus infection with coronaviruses and HIV.
The clinical manifestations of the dengue virus are dengue fever, dengue with plasma leakage, dengue hemorrhagic fever and dengue shock syndrome. The liver is the target of the dengue virus infection, and the severity of the infection is usually assessed by laboratory investigations, including serum aminotransferases, albumin and blood count in addition to abdominal ultrasound[147-152]. There is evidence that the dengue virus regulates hepatic lipids in order to replicate inside hepatocytes[153], leading to an increase of aminotransferases and thrombocytopenia[154-156]. Previous experimental studies showed that intracellular lipid droplets are essential for dengue virus replication, and postmortem studies confirmed that lipid vesicles in the liver autopsies are used for dengue virus replication[151,157].
NAFLD is a feature of dengue virus infection, whether related to the clinical category of dengue with or without plasma leakage[158]. A high serum ALT level is a characteristic feature of dengue virus infection during the febrile period of any clinical category[158]. Patients with dengue hemorrhagic fever complicated with multiple organ failure showed elevation of both ALT and AST, while those patients without multiple organ failure did not show elevation of the serum AST level[159]. Fatty infiltration in dengue-infected patients with plasma leakage indicating dengue severity as hemoconcentration and thrombocytopenia are commonly reported in those patients[158]. Postmortem histopathology studies of liver autopsies of dengue-infected patients showed sinusoid congestion, hepatocytes necrosis, fatty infiltration or steatosis[160].
Ebola virus disease (formerly called Ebola hemorrhagic disease) is a disease caused by the RNA virus transmitted from animals (e.g., bats) to humans and spreading through human-to-human transmission. It is a fatal disease complicated with multiple organ dysfunction like COVID-19[161]. The fatality rate of Ebola virus disease is attributed to the exaggeration of the immune response leading to extensive production of cytokines (cytokine storm) like COVID-19[162]. Hepatomegaly and hepatocellular necrosis are the characteristic features of liver lesions induced by the Ebola virus. Bradfute et al[163] demonstrated that Ebola virus induced-hepatocyte apoptosis is linked to the progression of the disease, while lymphocyte apoptosis is not involved in the progression of the disease. Patients who recovered from Ebola virus disease may complain from recurrent hepatitis in the future[164,165].
Lassa virus is an old-world arenavirus that is transmitted from small animals, e.g., rat, to humans. In 1969, the virus was first described in Nigeria, and it caused fatal hemorrhagic fever. Lassa fever can cause hepatitis, which was not the cause of death or progressed to liver failure[166]. Lassa virus-induced liver injury is acute active hepatitis without lymphocytic infiltration, which progresses to more hepatocyte necrosis or recovery. Gross and histopathological findings of liver autopsies are fatty metamorphoses but no evidence of fatty infiltration like COVID-19 and hepatocellular necrosis with eosinophilic, not lymphocytic, infiltration[167]. In an experimental hamster model, Pirital virus produced similar changes observed in human Lassa fever, including elevation of serum levels of hepatic transaminases combined with hepatocellular necrosis[168].
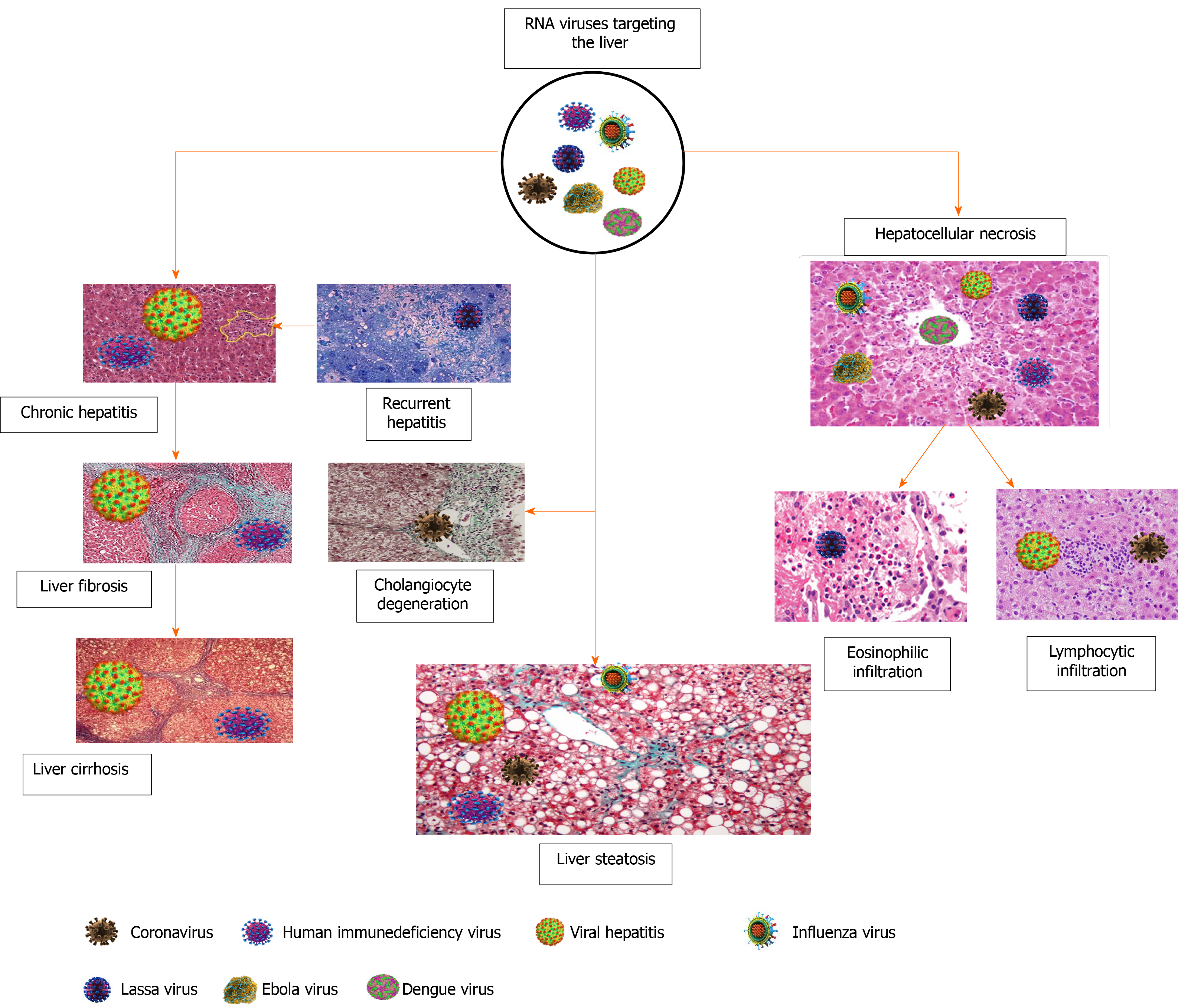
Liver fibrosis is a term for a progressive accumulation of the extracellular matrix leading to disruption of the normal liver architecture[169,170]. This condition develops in response to viral infection, metabolic disorders, chemicals, heavy alcohol consumption and autoimmune diseases[171,172]. At the molecular level, HBV through HBV X protein, which is expressed during infection, will activate the hepatic stellate cells[173] and induce fibrosis, while hepatitis delta virus is involved in the progression of existing fibrosis[174]. Hepatitis delta virus antigen (notably the large isoform) activates the HBV X protein to mediate and enhance the signals that induce liver fibrosis. On the other hand, proteins derived from HCV are de novo profibrogenic [175]. This indicates that HCV-induced liver fibrosis mechanisms differed from that mentioned with HBV or HCV. Moreover, some authors believe in the role of oxidative stress, mitochondrial dysfunction and iron accumulation in the pathogenesis of liver fibrosis[176-178]. HIV is not fibrogenic per se, but it can accelerate existing liver fibrosis induced by HBV or HCV[179]. Mono-HIV infected persons do not significantly show liver fibrosis compared with those presenting with coinfections with HBV or HCV[180]. Figure 3 summarizes the liver injury induced by RNA viruses and shows that the outcome event of liver fibrosis or cirrhosis is not a feature of COVID-19.
Acute liver injury is an asymptomatic manifestation of COVID-19 represented by a transient and reversible increase of liver enzymes. Microvascular steatosis, lobular focal necrosis, immune cell infiltration in the portal area and microthrombosis are postmortem findings of patients with multiple organ dysfunction. The liver injury produced by SARS-CoV-2 infection has completely differed from the corresponding liver damage induced by other RNA viruses. Risk factors of liver injury in COVID-19 patients are age, race, concomitant liver diseases and evidence of metabolic derangement. The chronicity of liver injury is still unknown, and further prospective studies are recommended to clarify the role of SARS-CoV-2 in inducing fibrosis and fatty liver.
The author expresses his gratitude to Professor Dr. Ismail I. Latif, the Dean of the College of Medicine, University of Diyala for his kind support, and to Assistant Lecturer Sura K. Khubala at University of Imam Ja’afar Sadiq for the technical support.
Manuscript source: Invited manuscript
Specialty type: Infectious diseases
Country/Territory of origin: Iraq
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Huang CH, Sergi C, Zhang W S-Editor: Wang JL L-Editor: Filipodia P-Editor: Ma YJ
| 1. | Woo PC, Huang Y, Lau SK, Yuen KY. Coronavirus genomics and bioinformatics analysis. Viruses. 2010;2:1804-1820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 624] [Cited by in RCA: 524] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 2. | Yin Y, Wunderink RG. MERS, SARS and other coronaviruses as causes of pneumonia. Respirology. 2018;23:130-137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 632] [Cited by in RCA: 646] [Article Influence: 80.8] [Reference Citation Analysis (0)] |
| 3. | Gretebeck LM, Subbarao K. Animal models for SARS and MERS coronaviruses. Curr Opin Virol. 2015;13:123-129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 140] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 4. | Hu LL, Wang WJ, Zhu QJ, Yang L. [Novel coronavirus pneumonia-related liver injury: etiological analysis and treatment strategy]. Zhonghua Gan Zang Bing Za Zhi. 2020;28:97-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 5. | Shen J. [Identification of essential oil from semen litchi]. Zhong Yao Tong Bao. 1988;13:31-32, 63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1106] [Cited by in RCA: 952] [Article Influence: 190.4] [Reference Citation Analysis (1)] |
| 6. | Mao R, Liang J, Shen J, Ghosh S, Zhu LR, Yang H, Wu KC, Chen MH; Chinese Society of IBD, Chinese Elite IBD Union; Chinese IBD Quality Care Evaluation Center Committee. Implications of COVID-19 for patients with pre-existing digestive diseases. Lancet Gastroenterol Hepatol. 2020;5:425-427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 218] [Cited by in RCA: 241] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 7. | Li J, Fan JG. Characteristics and Mechanism of Liver Injury in 2019 Coronavirus Disease. J Clin Transl Hepatol. 2020;8:13-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 206] [Article Influence: 41.2] [Reference Citation Analysis (2)] |
| 8. | Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14869] [Cited by in RCA: 12977] [Article Influence: 2595.4] [Reference Citation Analysis (1)] |
| 9. | Fang D, Ma J, Guan J, Wang M, Song Y, Tian D, Li P. Manifestations of digestive system in hospitalized patients with novel coronavirus pneumonia in Wuhan, China: a single-center, descriptive study. Zhonghua Xiaohua Zazhi. 2020;12:E005-E005. |
| 10. | Cai Q, Huang D, Ou P, Yu H, Zhu Z, Xia Z, Su Y, Ma Z, Zhang Y, Li Z, He Q, Liu L, Fu Y, Chen J. COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy. 2020;75:1742-1752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 332] [Article Influence: 66.4] [Reference Citation Analysis (0)] |
| 11. | Lei F, Liu YM, Zhou F, Qin JJ, Zhang P, Zhu L, Zhang XJ, Cai J, Lin L, Ouyang S, Wang X, Yang C, Cheng X, Liu W, Li H, Xie J, Wu B, Luo H, Xiao F, Chen J, Tao L, Cheng G, She ZG, Zhou J, Wang H, Lin J, Luo P, Fu S, Zhou J, Ye P, Xiao B, Mao W, Liu L, Yan Y, Liu L, Chen G, Li H, Huang X, Zhang BH, Yuan Y. Longitudinal Association Between Markers of Liver Injury and Mortality in COVID-19 in China. Hepatology. 2020;72:389-398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 318] [Cited by in RCA: 309] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 12. | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708-1720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19202] [Cited by in RCA: 18878] [Article Influence: 3775.6] [Reference Citation Analysis (7)] |
| 13. | Portincasa P, Krawczyk M, Machill A, Lammert F, Di Ciaula A. Hepatic consequences of COVID-19 infection. Lapping or biting? Eur J Intern Med. 2020;77:18-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 14. | Bertolini A, van de Peppel IP, Bodewes FAJA, Moshage H, Fantin A, Farinati F, Fiorotto R, Jonker JW, Strazzabosco M, Verkade HJ, Peserico G. Abnormal Liver Function Tests in Patients With COVID-19: Relevance and Potential Pathogenesis. Hepatology. 2020;72:1864-1872. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 219] [Cited by in RCA: 193] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 15. | Xu K, Zhou M, Yang D, Ling Y, Liu K, Bai T, Cheng Z, Li J. Application of ordinal logistic regression analysis to identify the determinants of illness severity of COVID-19 in China. Epidemiol Infect. 2020;148:e146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Phipps MM, Barraza LH, LaSota ED, Sobieszczyk ME, Pereira MR, Zheng EX, Fox AN, Zucker J, Verna EC. Acute Liver Injury in COVID-19: Prevalence and Association with Clinical Outcomes in a Large U.S. Cohort. Hepatology. 2020;72:807-817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 272] [Article Influence: 54.4] [Reference Citation Analysis (2)] |
| 17. | Da BL, Kushner T, El Halabi M, Paka P, Khalid M, Uberoi A, Lee BT, Perumalswami PV, Rutledge SM, Schiano TD, Friedman S, Saberi B. Liver Injury in Hospitalized Patients with COVID-19 Correlates with Hyper Inflammatory Response and Elevated IL-6. Hepatol Commun. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 18. | Youssef M, H Hussein M, Attia AS, M Elshazli R, Omar M, Zora G, S Farhoud A, Elnahla A, Shihabi A, Toraih EA, S Fawzy M, Kandil E. COVID-19 and liver dysfunction: A systematic review and meta-analysis of retrospective studies. J Med Virol. 2020;92:1825-1833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 19. | Wijarnpreecha K, Ungprasert P, Panjawatanan P, Harnois DM, Zaver HB, Ahmed A, Kim D. COVID-19 and liver injury: a meta-analysis. Eur J Gastroenterol Hepatol. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 20. | Feng Y, Ling Y, Bai T, Xie Y, Huang J, Li J, Xiong W, Yang D, Chen R, Lu F, Lu Y, Liu X, Chen Y, Li X, Li Y, Summah HD, Lin H, Yan J, Zhou M, Lu H, Qu J. COVID-19 with Different Severities: A Multicenter Study of Clinical Features. Am J Respir Crit Care Med. 2020;201:1380-1388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 576] [Cited by in RCA: 636] [Article Influence: 127.2] [Reference Citation Analysis (0)] |
| 21. | Liang W, Liang H, Ou L, Chen B, Chen A, Li C, Li Y, Guan W, Sang L, Lu J, Xu Y, Chen G, Guo H, Guo J, Chen Z, Zhao Y, Li S, Zhang N, Zhong N, He J, China Medical Treatment Expert Group for COVID-19. Development and Validation of a Clinical Risk Score to Predict the Occurrence of Critical Illness in Hospitalized Patients With COVID-19. JAMA Intern Med. 2020;180:1081-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 858] [Cited by in RCA: 989] [Article Influence: 197.8] [Reference Citation Analysis (0)] |
| 22. | Garrido I, Liberal R, Macedo G. Review article: COVID-19 and liver disease-what we know on 1st May 2020. Aliment Pharmacol Ther. 2020;52:267-275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 118] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 23. | Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, Bikdeli B, Ahluwalia N, Ausiello JC, Wan EY, Freedberg DE, Kirtane AJ, Parikh SA, Maurer MS, Nordvig AS, Accili D, Bathon JM, Mohan S, Bauer KA, Leon MB, Krumholz HM, Uriel N, Mehra MR, Elkind MSV, Stone GW, Schwartz A, Ho DD, Bilezikian JP, Landry DW. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26:1017-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2419] [Cited by in RCA: 2049] [Article Influence: 409.8] [Reference Citation Analysis (2)] |
| 24. | Zhong P, Xu J, Yang D, Shen Y, Wang L, Feng Y, Du C, Song Y, Wu C, Hu X, Sun Y. COVID-19-associated gastrointestinal and liver injury: clinical features and potential mechanisms. Signal Transduct Target Ther. 2020;5:256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 141] [Article Influence: 28.2] [Reference Citation Analysis (1)] |
| 25. | Porzionato A, Emmi A, Barbon S, Boscolo-Berto R, Stecco C, Stocco E, Macchi V, De Caro R. Sympathetic activation: a potential link between comorbidities and COVID-19. FEBS J. 2020;287:3681-3688. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 26. | Strnad P, Tacke F, Koch A, Trautwein C. Liver - guardian, modifier and target of sepsis. Nat Rev Gastroenterol Hepatol. 2017;14:55-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 446] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 27. | Méndez-Sánchez N, Valencia-Rodríguez A, Qi X, Yoshida EM, Romero-Gómez M, George J, Eslam M, Abenavoli L, Xie W, Teschke R, Carrion AF, Keaveny AP. What Has the COVID-19 Pandemic Taught Us so Far? Addressing the Problem from a Hepatologist's Perspective. J Clin Transl Hepatol. 2020;8:0024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 28. | McElvaney OJ, McEvoy NL, McElvaney OF, Carroll TP, Murphy MP, Dunlea DM, Ní Choileáin O, Clarke J, O'Connor E, Hogan G, Ryan D, Sulaiman I, Gunaratnam C, Branagan P, O'Brien ME, Morgan RK, Costello RW, Hurley K, Walsh S, de Barra E, McNally C, McConkey S, Boland F, Galvin S, Kiernan F, O'Rourke J, Dwyer R, Power M, Geoghegan P, Larkin C, O'Leary RA, Freeman J, Gaffney A, Marsh B, Curley GF, McElvaney NG. Characterization of the Inflammatory Response to Severe COVID-19 Illness. Am J Respir Crit Care Med. 2020;202:812-821. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 419] [Cited by in RCA: 464] [Article Influence: 92.8] [Reference Citation Analysis (0)] |
| 29. | Sarin SK, Choudhury A, Lau GK, Zheng MH, Ji D, Abd-Elsalam S, Hwang J, Qi X, Cua IH, Suh JI, Park JG, Putcharoen O, Kaewdech A, Piratvisuth T, Treeprasertsuk S, Park S, Wejnaruemarn S, Payawal DA, Baatarkhuu O, Ahn SH, Yeo CD, Alonzo UR, Chinbayar T, Loho IM, Yokosuka O, Jafri W, Tan S, Soo LI, Tanwandee T, Gani R, Anand L, Esmail ES, Khalaf M, Alam S, Lin CY, Chuang WL, Soin AS, Garg HK, Kalista K, Batsukh B, Purnomo HD, Dara VP, Rathi P, Al Mahtab M, Shukla A, Sharma MK, Omata M; APASL COVID Task Force, APASL COVID Liver Injury Spectrum Study (APCOLIS Study-NCT 04345640). Pre-existing liver disease is associated with poor outcome in patients with SARS CoV2 infection; The APCOLIS Study (APASL COVID-19 Liver Injury Spectrum Study). Hepatol Int. 2020;14:690-700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 217] [Cited by in RCA: 216] [Article Influence: 43.2] [Reference Citation Analysis (1)] |
| 30. | Sharma A, Jaiswal P, Kerakhan Y, Saravanan L, Murtaza Z, Zergham A, Honganur NS, Akbar A, Deol A, Francis B, Patel S, Mehta D, Jaiswal R, Singh J, Patel U, Malik P. Liver disease and outcomes among COVID-19 hospitalized patients - A systematic review and meta-analysis. Ann Hepatol. 2021;21:100273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 82] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 31. | Jiang F, Deng L, Zhang L, Cai Y, Cheung CW, Xia Z. Review of the Clinical Characteristics of Coronavirus Disease 2019 (COVID-19). J Gen Intern Med. 2020;35:1545-1549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 869] [Cited by in RCA: 703] [Article Influence: 140.6] [Reference Citation Analysis (0)] |
| 32. | Chen J, Ling Y, Xi X, Liu P, Li F, Li T, Shang Z, Wang M, Shen Y, Lu H. Efficacies of lopinavir/ritonavir and abidol in the treatment of novel coronavirus pneumonia. Zhonghua Chuanranbing Zazhi. 2020;38:E008. |
| 33. | Fontana RJ, Seeff LB, Andrade RJ, Björnsson E, Day CP, Serrano J, Hoofnagle JH. Standardization of nomenclature and causality assessment in drug-induced liver injury: summary of a clinical research workshop. Hepatology. 2010;52:730-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 235] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 34. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Drug-induced liver injury. J Hepatol. 2019;70:1222-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 715] [Cited by in RCA: 669] [Article Influence: 111.5] [Reference Citation Analysis (0)] |
| 35. | Li H, Liu L, Zhang D, Xu J, Dai H, Tang N, Su X, Cao B. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 2020;395:1517-1520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 772] [Cited by in RCA: 887] [Article Influence: 177.4] [Reference Citation Analysis (0)] |
| 36. | Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M, Li X, Xia J, Chen N, Xiang J, Yu T, Bai T, Xie X, Zhang L, Li C, Yuan Y, Chen H, Li H, Huang H, Tu S, Gong F, Liu Y, Wei Y, Dong C, Zhou F, Gu X, Xu J, Liu Z, Zhang Y, Li H, Shang L, Wang K, Li K, Zhou X, Dong X, Qu Z, Lu S, Hu X, Ruan S, Luo S, Wu J, Peng L, Cheng F, Pan L, Zou J, Jia C, Wang J, Liu X, Wang S, Wu X, Ge Q, He J, Zhan H, Qiu F, Guo L, Huang C, Jaki T, Hayden FG, Horby PW, Zhang D, Wang C. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. N Engl J Med. 2020;382:1787-1799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3386] [Cited by in RCA: 3627] [Article Influence: 725.4] [Reference Citation Analysis (0)] |
| 37. | Bangash MN, Patel J, Parekh D. COVID-19 and the liver: little cause for concern. Lancet Gastroenterol Hepatol. 2020;5:529-530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 324] [Cited by in RCA: 357] [Article Influence: 71.4] [Reference Citation Analysis (0)] |
| 38. | Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5228] [Cited by in RCA: 5786] [Article Influence: 1157.2] [Reference Citation Analysis (2)] |
| 39. | Deng SQ, Peng HJ. Characteristics of and Public Health Responses to the Coronavirus Disease 2019 Outbreak in China. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 40. | Martinez MA, Vuppalanchi R, Fontana RJ, Stolz A, Kleiner DE, Hayashi PH, Gu J, Hoofnagle JH, Chalasani N. Clinical and histologic features of azithromycin-induced liver injury. Clin Gastroenterol Hepatol. 2015;13:369-376.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 41. | Liu Q, Wang RS, Qu GQ, Wang YY, Liu P, Zhu YZ, Fei G, Ren L, Zhou YW, Liu L. Gross examination report of a COVID-19 death autopsy. Fa Yi Xue Za Zhi. 2020;36:21-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 129] [Reference Citation Analysis (0)] |
| 42. | Colmenero I, Santonja C, Alonso-Riaño M, Noguera-Morel L, Hernández-Martín A, Andina D, Wiesner T, Rodríguez-Peralto JL, Requena L, Torrelo A. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br J Dermatol. 2020;183:729-737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 287] [Cited by in RCA: 321] [Article Influence: 64.2] [Reference Citation Analysis (0)] |
| 43. | Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 44. | Eslam M, Sanyal AJ, George J; International Consensus Panel. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology. 2020;158:1999-2014.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2367] [Cited by in RCA: 2212] [Article Influence: 442.4] [Reference Citation Analysis (1)] |
| 45. | Schwimmer JB, Pardee PE, Lavine JE, Blumkin AK, Cook S. Cardiovascular risk factors and the metabolic syndrome in pediatric nonalcoholic fatty liver disease. Circulation. 2008;118:277-283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 307] [Cited by in RCA: 290] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 46. | Molina-Molina E, Lunardi Baccetto R, Wang DQ, de Bari O, Krawczyk M, Portincasa P. Exercising the hepatobiliary-gut axis. The impact of physical activity performance. Eur J Clin Invest. 2018;48:e12958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 47. | Molina-Molina E, Krawczyk M, Stachowska E, Lammert F, Portincasa P. Non-Alcoholic Fatty Liver Disease in Non-Obese Individuals: Prevalence, Pathogenesis and Treatment. Clin Res Hepatol Gastroenterol. 2019;43:638-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 48. | Wang B, Li R, Lu Z, Huang Y. Does comorbidity increase the risk of patients with COVID-19: evidence from meta-analysis. Aging (Albany NY). 2020;12:6049-6057. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 694] [Cited by in RCA: 800] [Article Influence: 160.0] [Reference Citation Analysis (0)] |
| 49. | Ji D, Qin E, Xu J, Zhang D, Cheng G, Wang Y, Lau G. Non-alcoholic fatty liver diseases in patients with COVID-19: A retrospective study. J Hepatol. 2020;73:451-453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 336] [Cited by in RCA: 408] [Article Influence: 81.6] [Reference Citation Analysis (2)] |
| 50. | Paizis G, Tikellis C, Cooper ME, Schembri JM, Lew RA, Smith AI, Shaw T, Warner FJ, Zuilli A, Burrell LM, Angus PW. Chronic liver injury in rats and humans upregulates the novel enzyme angiotensin converting enzyme 2. Gut. 2005;54:1790-1796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 281] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 51. | Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020;5:811-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2516] [Cited by in RCA: 2842] [Article Influence: 568.4] [Reference Citation Analysis (0)] |
| 52. | Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428-430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1348] [Cited by in RCA: 1295] [Article Influence: 259.0] [Reference Citation Analysis (4)] |
| 53. | Ferron PJ, Gicquel T, Mégarbane B, Clément B, Fromenty B. Treatments in Covid-19 patients with pre-existing metabolic dysfunction-associated fatty liver disease: A potential threat for drug-induced liver injury? Biochimie. 2020;179:266-274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (1)] |
| 54. | Joshi S, Wollenzien H, Leclerc E, Jarajapu YP. Hypoxic regulation of angiotensin-converting enzyme 2 and Mas receptor in human CD34+ cells. J Cell Physiol. 2019;234:20420-20431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 55. | Bartoszewski R, Moszyńska A, Serocki M, Cabaj A, Polten A, Ochocka R, Dell'Italia L, Bartoszewska S, Króliczewski J, Dąbrowski M, Collawn JF. Primary endothelial cell-specific regulation of hypoxia-inducible factor (HIF)-1 and HIF-2 and their target gene expression profiles during hypoxia. FASEB J. 2019;33:7929-7941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 126] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 56. | Fattahi F, Kalbitz M, Malan EA, Abe E, Jajou L, Huber-Lang MS, Bosmann M, Russell MW, Zetoune FS, Ward PA. Complement-induced activation of MAPKs and Akt during sepsis: role in cardiac dysfunction. FASEB J. 2017;31:4129-4139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 57. | Suresh MV, Balijepalli S, Zhang B, Singh VV, Swamy S, Panicker S, Dolgachev VA, Subramanian C, Ramakrishnan SK, Thomas B, Rao TC, Delano MJ, Machado-Aranda D, Shah YM, Raghavendran K. Hypoxia-Inducible Factor (HIF)-1α Promotes Inflammation and Injury Following Aspiration-Induced Lung Injury in Mice. Shock. 2019;52:612-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 58. | Akaike T, Noguchi Y, Ijiri S, Setoguchi K, Suga M, Zheng YM, Dietzschold B, Maeda H. Pathogenesis of influenza virus-induced pneumonia: involvement of both nitric oxide and oxygen radicals. Proc Natl Acad Sci USA. 1996;93:2448-2453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 358] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 59. | Sun D, Li H, Lu XX, Xiao H, Ren J, Zhang FR, Liu ZS. Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center's observational study. World J Pediatr. 2020;16:251-259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 355] [Cited by in RCA: 390] [Article Influence: 78.0] [Reference Citation Analysis (0)] |
| 60. | Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol. 2020;38:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 655] [Article Influence: 131.0] [Reference Citation Analysis (0)] |
| 61. | Hibbs JB, Westenfelder C, Taintor R, Vavrin Z, Kablitz C, Baranowski RL, Ward JH, Menlove RL, McMurry MP, Kushner JP. Evidence for cytokine-inducible nitric oxide synthesis from L-arginine in patients receiving interleukin-2 therapy. J Clin Invest. 1992;89:867-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 62. | Tsujimoto M, Yokota S, Vilcek J, Weissmann G. Tumor necrosis factor provokes superoxide anion generation from neutrophils. Biochem Biophys Res Commun. 1986;137:1094-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 202] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 63. | Kharazmi A, Nielsen H, Rechnitzer C, Bendtzen K. Interleukin 6 primes human neutrophil and monocyte oxidative burst response. Immunol Lett. 1989;21:177-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 64. | Colston JT, Chandrasekar B, Freeman GL. A novel peroxide-induced calcium transient regulates interleukin-6 expression in cardiac-derived fibroblasts. J Biol Chem. 2002;277:23477-23483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 65. | Jarosz M, Olbert M, Wyszogrodzka G, Młyniec K, Librowski T. Antioxidant and anti-inflammatory effects of zinc. Zinc-dependent NF-κB signaling. Inflammopharmacology. 2017;25:11-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 347] [Cited by in RCA: 449] [Article Influence: 56.1] [Reference Citation Analysis (0)] |
| 66. | Cai J, Chen Y, Seth S, Furukawa S, Compans RW, Jones DP. Inhibition of influenza infection by glutathione. Free Radic Biol Med. 2003;34:928-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 150] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 67. | Lai KY, Ng WY, Osburga Chan PK, Wong KF, Cheng F. High-dose N-acetylcysteine therapy for novel H1N1 influenza pneumonia. Ann Intern Med. 2010;152:687-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 68. | Levert H, Gressier B, Moutard I, Brunet C, Dine T, Luyckx M, Cazin M, Cazin JC. Azithromycin impact on neutrophil oxidative metabolism depends on exposure time. Inflammation. 1998;22:191-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 69. | Meyer M, Huaux F, Gavilanes X, van den Brûle S, Lebecque P, Lo Re S, Lison D, Scholte B, Wallemacq P, Leal T. Azithromycin reduces exaggerated cytokine production by M1 alveolar macrophages in cystic fibrosis. Am J Respir Cell Mol Biol. 2009;41:590-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 70. | Sato K, Suga M, Akaike T, Fujii S, Muranaka H, Doi T, Maeda H, Ando M. Therapeutic effect of erythromycin on influenza virus-induced lung injury in mice. Am J Respir Crit Care Med. 1998;157:853-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 76] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 71. | Song X, Hu W, Yu H, Zhao L, Zhao Y, Zhao X, Xue HH, Zhao Y. Little to no expression of angiotensin-converting enzyme-2 on most human peripheral blood immune cells but highly expressed on tissue macrophages. Cytometry A. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 83] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 72. | Yuan F, Zhang W, Mu D, Gong J. Kupffer cells in immune activation and tolerance toward HBV/HCV infection. Adv Clin Exp Med. 2017;26:739-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 73. | Shalimar, Elhence A, Vaishnav M, Kumar R, Pathak P, Soni KD, Aggarwal R, Soneja M, Jorwal P, Kumar A, Khanna P, Singh AK, Biswas A, Nischal N, Dar L, Choudhary A, Rangarajan K, Mohan A, Acharya P, Nayak B, Gunjan D, Saraya A, Mahapatra S, Makharia G, Trikha A, Garg P. Poor outcomes in patients with cirrhosis and Corona Virus Disease-19. Indian J Gastroenterol. 2020;39:285-291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 74. | Zou X, Fang M, Li S, Wu L, Gao B, Gao H, Ran X, Bian Y, Li R, ShanshanYu, Ling J, Li D, Tian D, Huang J. Characteristics of Liver Function in Patients With SARS-CoV-2 and Chronic HBV Coinfection. Clin Gastroenterol Hepatol. 2021;19:597-603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 67] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 75. | Maggi U, De Carlis L, Yiu D, Colledan M, Regalia E, Rossi G, Angrisani M, Consonni D, Fornoni G, Piccolo G, DeFeo TM. The impact of the COVID-19 outbreak on liver transplantation programs in Northern Italy. Am J Transplant. 2020;20:1840-1848. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 76. | Bhoori S, Rossi RE, Citterio D, Mazzaferro V. COVID-19 in long-term liver transplant patients: preliminary experience from an Italian transplant centre in Lombardy. Lancet Gastroenterol Hepatol. 2020;5:532-533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 205] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 77. | Alqahtani SA, Schattenberg JM. Liver injury in COVID-19: The current evidence. United European Gastroenterol J. 2020;8:509-519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 166] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 78. | Nolan D, Reiss P, Mallal S. Adverse effects of antiretroviral therapy for HIV infection: a review of selected topics. Expert Opin Drug Saf. 2005;4:201-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 79. | Sulkowski MS, Thomas DL, Chaisson RE, Moore RD. Hepatotoxicity associated with antiretroviral therapy in adults infected with human immunodeficiency virus and the role of hepatitis C or B virus infection. JAMA. 2000;283:74-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 680] [Cited by in RCA: 644] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 80. | Lv Z, Chu Y, Wang Y. HIV protease inhibitors: a review of molecular selectivity and toxicity. HIV AIDS (Auckl). 2015;7:95-104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 186] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 81. | Anđelković M, Buha A, Vukomanović P, Matović V. Bezbednost primene lekova koji se koriste u terapiji HIV infekcije. Arh Farm (Belgr.). 2016;66:161-173. |
| 82. | González-Requena D, Núñez M, Jiménez-Nacher I, González-Lahoz J, Soriano V. Short communication: liver toxicity of lopinavir-containing regimens in HIV-infected patients with or without hepatitis C coinfection. AIDS Res Hum Retroviruses. 2004;20:698-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 83. | Tu Y, Poblete RJ, Freilich BD, Zarbin MA, Bhagat N. Retinal toxicity with Ritonavir. Int J Ophthalmol. 2016;9:640-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 84. | Wen CY, Xie ZW, Li YP, Deng XL, Chen XT, Cao Y, Ou X, Lin WY, Li F, Cai WP, Li LH. [Real-world efficacy and safety of lopinavir/ritonavir and arbidol in treating with COVID-19 : an observational cohort study]. Zhonghua Nei Ke Za Zhi. 2020;59:E012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 85. | Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, Shi Z, Hu Z, Zhong W, Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269-271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4289] [Cited by in RCA: 4569] [Article Influence: 913.8] [Reference Citation Analysis (0)] |
| 86. | Grein J, Ohmagari N, Shin D, Diaz G, Asperges E, Castagna A, Feldt T, Green G, Green ML, Lescure FX, Nicastri E, Oda R, Yo K, Quiros-Roldan E, Studemeister A, Redinski J, Ahmed S, Bernett J, Chelliah D, Chen D, Chihara S, Cohen SH, Cunningham J, D'Arminio Monforte A, Ismail S, Kato H, Lapadula G, L'Her E, Maeno T, Majumder S, Massari M, Mora-Rillo M, Mutoh Y, Nguyen D, Verweij E, Zoufaly A, Osinusi AO, DeZure A, Zhao Y, Zhong L, Chokkalingam A, Elboudwarej E, Telep L, Timbs L, Henne I, Sellers S, Cao H, Tan SK, Winterbourne L, Desai P, Mera R, Gaggar A, Myers RP, Brainard DM, Childs R, Flanigan T. Compassionate Use of Remdesivir for Patients with Severe Covid-19. N Engl J Med. 2020;382:2327-2336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1926] [Cited by in RCA: 1884] [Article Influence: 376.8] [Reference Citation Analysis (0)] |
| 87. | Furuta Y, Komeno T, Nakamura T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc Jpn Acad Ser B Phys Biol Sci. 2017;93:449-463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 675] [Cited by in RCA: 692] [Article Influence: 86.5] [Reference Citation Analysis (0)] |
| 88. | Joshi S, Parkar J, Ansari A, Vora A, Talwar D, Tiwaskar M, Patil S, Barkate H. Role of favipiravir in the treatment of COVID-19. Int J Infect Dis. 2021;102:501-508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 246] [Cited by in RCA: 251] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 89. | Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, Doudier B, Courjon J, Giordanengo V, Vieira VE, Tissot Dupont H, Honoré S, Colson P, Chabrière E, La Scola B, Rolain JM, Brouqui P, Raoult D. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56:105949. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3279] [Cited by in RCA: 3272] [Article Influence: 654.4] [Reference Citation Analysis (0)] |
| 90. | Boriskin YS, Leneva IA, Pécheur EI, Polyak SJ. Arbidol: a broad-spectrum antiviral compound that blocks viral fusion. Curr Med Chem. 2008;15:997-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 276] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 91. | Zheng L, Zhang L, Huang J, Nandakumar KS, Liu S, Cheng K. Potential treatment methods targeting 2019-nCoV infection. Eur J Med Chem. 2020;205:112687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 92. | Srinivas P, Sacha GL, Koval C. Antivirals for COVID-19. Cleve Clin J Med. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 93. | Wang MZ, Cai BQ, Li LY, Lin JT, Su N, Yu HX, Gao H, Zhao JZ, Liu L. [Efficacy and safety of arbidol in treatment of naturally acquired influenza]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2004;26:289-293. [PubMed] |
| 94. | Liang T. Handbook of COVID-19 Prevention and Treatment. Zhejiang: The First Affiliated Hospital, Zhejiang University School of Medicine, 2020: 1-68. [cited 10 January 2021]. Available from: https://www.alsgbi.org/wp-content/uploads/2020/03/COVID-19-Prevention-and-Treatments-in-a-hospital.pdf. |
| 95. | Andreakos E, Zanoni I, Galani IE. Lambda interferons come to light: dual function cytokines mediating antiviral immunity and damage control. Curr Opin Immunol. 2019;56:67-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 96. | Davidson S, McCabe TM, Crotta S, Gad HH, Hessel EM, Beinke S, Hartmann R, Wack A. IFNλ is a potent anti-influenza therapeutic without the inflammatory side effects of IFNα treatment. EMBO Mol Med. 2016;8:1099-1112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 198] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 97. | Galani IE, Triantafyllia V, Eleminiadou EE, Koltsida O, Stavropoulos A, Manioudaki M, Thanos D, Doyle SE, Kotenko SV, Thanopoulou K, Andreakos E. Interferon-λ Mediates Non-redundant Front-Line Antiviral Protection against Influenza Virus Infection without Compromising Host Fitness. Immunity. 2017;46:875-890.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 368] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 98. | Azhar EI, El-Kafrawy SA, Farraj SA, Hassan AM, Al-Saeed MS, Hashem AM, Madani TA. Evidence for camel-to-human transmission of MERS coronavirus. N Engl J Med. 2014;370:2499-2505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 616] [Cited by in RCA: 630] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 99. | Memish ZA, Cotten M, Meyer B, Watson SJ, Alsahafi AJ, Al Rabeeah AA, Corman VM, Sieberg A, Makhdoom HQ, Assiri A, Al Masri M, Aldabbagh S, Bosch BJ, Beer M, Müller MA, Kellam P, Drosten C. Human infection with MERS coronavirus after exposure to infected camels, Saudi Arabia, 2013. Emerg Infect Dis. 2014;20:1012-1015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 253] [Cited by in RCA: 276] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 100. | Zumla A, Hui DS, Perlman S. Middle East respiratory syndrome. Lancet. 2015;386:995-1007. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 781] [Cited by in RCA: 787] [Article Influence: 78.7] [Reference Citation Analysis (0)] |
| 101. | Arabi YM, Balkhy HH, Hayden FG, Bouchama A, Luke T, Baillie JK, Al-Omari A, Hajeer AH, Senga M, Denison MR, Nguyen-Van-Tam JS, Shindo N, Bermingham A, Chappell JD, Van Kerkhove MD, Fowler RA. Middle East Respiratory Syndrome. N Engl J Med. 2017;376:584-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 310] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 102. | Cha RH, Joh JS, Jeong I, Lee JY, Shin HS, Kim G, Kim Y; Critical Care Team of National Medical Center. Renal Complications and Their Prognosis in Korean Patients with Middle East Respiratory Syndrome-Coronavirus from the Central MERS-CoV Designated Hospital. J Korean Med Sci. 2015;30:1807-1814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 103. | Eckerle I, Müller MA, Kallies S, Gotthardt DN, Drosten C. In-vitro renal epithelial cell infection reveals a viral kidney tropism as a potential mechanism for acute renal failure during Middle East Respiratory Syndrome (MERS) Coronavirus infection. Virol J. 2013;10:359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 104. | Alsaad KO, Hajeer AH, Al Balwi M, Al Moaiqel M, Al Oudah N, Al Ajlan A, AlJohani S, Alsolamy S, Gmati GE, Balkhy H, Al-Jahdali HH, Baharoon SA, Arabi YM. Histopathology of Middle East respiratory syndrome coronovirus (MERS-CoV) infection - clinicopathological and ultrastructural study. Histopathology. 2018;72:516-524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 204] [Cited by in RCA: 225] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 105. | Chau TN, Lee KC, Yao H, Tsang TY, Chow TC, Yeung YC, Choi KW, Tso YK, Lau T, Lai ST, Lai CL. SARS-associated viral hepatitis caused by a novel coronavirus: report of three cases. Hepatology. 2004;39:302-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 106. | Peiris JS, Chu CM, Cheng VC, Chan KS, Hung IF, Poon LL, Law KI, Tang BS, Hon TY, Chan CS, Chan KH, Ng JS, Zheng BJ, Ng WL, Lai RW, Guan Y, Yuen KY; HKU/UCH SARS Study Group. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767-1772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1737] [Cited by in RCA: 1787] [Article Influence: 81.2] [Reference Citation Analysis (0)] |
| 107. | Farcas GA, Poutanen SM, Mazzulli T, Willey BM, Butany J, Asa SL, Faure P, Akhavan P, Low DE, Kain KC. Fatal severe acute respiratory syndrome is associated with multiorgan involvement by coronavirus. J Infect Dis. 2005;191:193-197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 123] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 108. | Guo Y, Korteweg C, McNutt MA, Gu J. Pathogenetic mechanisms of severe acute respiratory syndrome. Virus Res. 2008;133:4-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 109. | Shi X, Gong E, Gao D, Zhang B, Zheng J, Gao Z, Zhong Y, Zou W, Wu B, Fang W, Liao S, Wang S, Xie Z, Lu M, Hou L, Zhong H, Shao H, Li N, Liu C, Pei F, Yang J, Wang Y, Han Z, Shi X, Zhang Q, You J, Zhu X, Gu J. Severe acute respiratory syndrome associated coronavirus is detected in intestinal tissues of fatal cases. Am J Gastroenterol. 2005;100:169-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 80] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 110. | Gu J, Gong E, Zhang B, Zheng J, Gao Z, Zhong Y, Zou W, Zhan J, Wang S, Xie Z, Zhuang H, Wu B, Zhong H, Shao H, Fang W, Gao D, Pei F, Li X, He Z, Xu D, Shi X, Anderson VM, Leong AS. Multiple organ infection and the pathogenesis of SARS. J Exp Med. 2005;202:415-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1090] [Cited by in RCA: 1104] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 111. | Puoti M, Moioli MC, Travi G, Rossotti R. The burden of liver disease in human immunodeficiency virus-infected patients. Semin Liver Dis. 2012;32:103-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 112. | Chaudhry AA, Sulkowski MS, Chander G, Moore RD. Hazardous drinking is associated with an elevated aspartate aminotransferase to platelet ratio index in an urban HIV-infected clinical cohort. HIV Med. 2009;10:133-142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 113. | Perazzo H, Cardoso SW, Yanavich C, Nunes EP, Morata M, Gorni N, da Silva PS, Cardoso C, Almeida C, Luz P, Veloso VG, Grinsztejn B. Predictive factors associated with liver fibrosis and steatosis by transient elastography in patients with HIV mono-infection under long-term combined antiretroviral therapy. J Int AIDS Soc. 2018;21:e25201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 114. | Aepfelbacher JA, Balmaceda J, Purdy J, Mattingly A, Zambell K, Hawkins K, Chairez C, Curl KA, Dee N, Hadigan C. Increased Prevalence of Hepatic Steatosis in Young Adults With Lifelong HIV. J Infect Dis. 2019;220:266-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 115. | Lui G, Wong VW, Wong GL, Chu WC, Wong CK, Yung IM, Wong RY, Yeung SL, Yeung DK, Cheung CS, Chan HY, Chan HL, Lee N. Liver fibrosis and fatty liver in Asian HIV-infected patients. Aliment Pharmacol Ther. 2016;44:411-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 116. | Vodkin I, Valasek MA, Bettencourt R, Cachay E, Loomba R. Clinical, biochemical and histological differences between HIV-associated NAFLD and primary NAFLD: a case-control study. Aliment Pharmacol Ther. 2015;41:368-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 106] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 117. | Mohammed SS, Aghdassi E, Salit IE, Avand G, Sherman M, Guindi M, Heathcote JE, Allard JP. HIV-positive patients with nonalcoholic fatty liver disease have a lower body mass index and are more physically active than HIV-negative patients. J Acquir Immune Defic Syndr. 2007;45:432-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 118. | Study of Fat Redistribution and Metabolic Change in HIV Infection (FRAM). Fat distribution in women with HIV infection. J Acquir Immune Defic Syndr. 2006;42:562-571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 108] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 119. | Brown TT, Xu X, John M, Singh J, Kingsley LA, Palella FJ, Witt MD, Margolick JB, Dobs AS. Fat distribution and longitudinal anthropometric changes in HIV-infected men with and without clinical evidence of lipodystrophy and HIV-uninfected controls: a substudy of the Multicenter AIDS Cohort Study. AIDS Res Ther. 2009;6:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 120. | Joy T, Keogh HM, Hadigan C, Dolan SE, Fitch K, Liebau J, Johnsen S, Lo J, Grinspoon SK. Relation of body composition to body mass index in HIV-infected patients with metabolic abnormalities. J Acquir Immune Defic Syndr. 2008;47:174-184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 121. | Servin-Abad L, Molina E, Baracco G, Arosemena L, Regev A, Jeffers L, Schiff E. Liver enzymes elevation after HAART in HIV-HCV co-infection. J Viral Hepat. 2005;12:429-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 122. | Qin F, Jiang J, Qin C, Huang Y, Liang B, Xu Y, Huang J, Xu Z, Ning C, Liao Y, Zang N, Lai J, Wei W, Yu J, Ye L, Qin X, Liang H. Liver damage in patients living with HIV on antiretroviral treatment with normal baseline liver function and without HBV/HCV infection: an 11-year retrospective cohort study in Guangxi, China. BMJ Open. 2019;9:e023140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 123. | Stojsavljević S, Gomerčić Palčić M, Virović Jukić L, Smirčić Duvnjak L, Duvnjak M. Adipokines and proinflammatory cytokines, the key mediators in the pathogenesis of nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20:18070-18091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 252] [Cited by in RCA: 252] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 124. | Barocas JA, So-Armah K, Cheng DM, Lioznov D, Baum M, Gallagher K, Fuster D, Gnatienko N, Krupitsky E, Freiberg MS, Samet JH. Zinc deficiency and advanced liver fibrosis among HIV and hepatitis C co-infected anti-retroviral naïve persons with alcohol use in Russia. PLoS One. 2019;14:e0218852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 125. | Lombardi R, Sambatakou H, Mariolis I, Cokkinos D, Papatheodoridis GV, Tsochatzis EA. Prevalence and predictors of liver steatosis and fibrosis in unselected patients with HIV mono-infection. Dig Liver Dis. 2016;48:1471-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 126. | Dworkin BM, Stahl RE, Giardina MA, Wormser GP, Weiss L, Jankowski R, Rosenthal WS. The liver in acquired immune deficiency syndrome: emphasis on patients with intravenous drug abuse. Am J Gastroenterol. 1987;82:231-236. [PubMed] |
| 127. | Glasgow BJ, Anders K, Layfield LJ, Steinsapir KD, Gitnick GL, Lewin KJ. Clinical and pathologic findings of the liver in the acquired immune deficiency syndrome (AIDS). Am J Clin Pathol. 1985;83:582-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 71] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 128. | Polakos NK, Cornejo JC, Murray DA, Wright KO, Treanor JJ, Crispe IN, Topham DJ, Pierce RH. Kupffer cell-dependent hepatitis occurs during influenza infection. Am J Pathol. 2006;168:1169-78; quiz 1404-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 129. | Paton NI, Lee L, Xu Y, Ooi EE, Cheung YB, Archuleta S, Wong G, Wilder-Smith A. Chloroquine for influenza prevention: a randomised, double-blind, placebo controlled trial. Lancet Infect Dis. 2011;11:677-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 141] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 130. | Cho WH, Kim YS, Jeon DS, Kim JE, Kim KI, Seol HY, Kim KU, Park HK, Lee MK, Park SK, Jeong YJ. Outcome of pandemic H1N1 pneumonia: clinical and radiological findings for severity assessment. Korean J Intern Med. 2011;26:160-167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 131. | Potapova OV, Kovner AV, Anikina AG, Cherdantseva LA, Sharkova TV, Shkurupy VA, Vasil'eva EV, Shestopalov AM. Studies of Influenza A/H1N1 A/Tomsk/13/2010 Virus Topology during Development of Infectious Process in Mammals. Bull Exp Biol Med. 2016;160:683-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 132. | Yu L, Wang Z, Chen Y, Ding W, Jia H, Chan JF, To KK, Chen H, Yang Y, Liang W, Zheng S, Yao H, Yang S, Cao H, Dai X, Zhao H, Li J, Bao Q, Chen P, Hou X, Li L, Yuen KY. Clinical, virological, and histopathological manifestations of fatal human infections by avian influenza A(H7N9) virus. Clin Infect Dis. 2013;57:1449-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 133. | Zhang Y, Liu J, Yu L, Zhou N, Ding W, Zheng S, Shi D, Li L. Prevalence and characteristics of hypoxic hepatitis in the largest single-centre cohort of avian influenza A(H7N9) virus-infected patients with severe liver impairment in the intensive care unit. Emerg Microbes Infect. 2016;5:e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 134. | Zhang S, Hu B, Xu J, Ren Q, Wang L, Wang S. Influenza A virus infection induces liver injury in mice. Microb Pathog. 2019;137:103736. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 135. | Byun JM, Kim SG, Zhang YY, Kim YS, Jeong SW, Lee SH, Jang JY, Hong SJ, Moon JH, Kim HS, Lee MS, Kim BS. Influenza A (H1N1) 2009 pandemic calm down the prevalence of acute hepatitis A in the latter half of 2009: Korean population study. Korean J Gastroenterol. 2012;59:360-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 136. | Lee HW, Chang DY, Moon HJ, Chang HY, Shin EC, Lee JS, Kim KA, Kim HJ. Clinical Factors and Viral Load Influencing Severity of Acute Hepatitis A. PLoS One. 2015;10:e0130728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 137. | Sun P, Su N, Lin FZ, Ma L, Wang HJ, Rong X, Dai YD, Li J, Jian ZW, Tang LH, Xiao W, Li CQ. Prevalence of hepatitis A viral RNA and antibodies among Chinese blood donors. Genet Mol Res. 2015;14:16431-16437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 138. | Terzić D, Mijović G, Dupanović B, Drasković N, Svirtlih N. [Comparison of clinical and laboratory characteristics of viral hepatitis A and E in Montenegro]. Med Pregl. 2010;63:175-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 139. | Lohse AW, Gerken G, Mohr H, Löhr HF, Treichel U, Dienes HP, Meyer zum Büschenfelde KH. Relation between autoimmune liver diseases and viral hepatitis: clinical and serological characteristics in 859 patients. Z Gastroenterol. 1995;33:527-533. [PubMed] |
| 140. | Caccamo G, Saffioti F, Raimondo G. Hepatitis B virus and hepatitis C virus dual infection. World J Gastroenterol. 2014;20:14559-14567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 73] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 141. | Rizzetto M. Hepatitis D Virus: Introduction and Epidemiology. Cold Spring Harb Perspect Med. 2015;5:a021576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 142. | Krivoshapkin VG, Semenov SI, Maximova SS, Tihonova NN, Sivtseva AI, Sleptsova SS. Clinical and laboratory characteristics of hepatitis d in Republic of Sakha (Yakutia). Wiad Lek. 2018;71:179-183. [PubMed] |
| 143. | Vergani C, Trovato G, Delù A, Pietrogrande M, Dioguardi N. Serum total lipids, lipoprotein cholesterol, and apolipoprotein A in acute viral hepatitis and chronic liver disease. J Clin Pathol. 1978;31:772-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 144. | Haga Y, Kanda T, Sasaki R, Nakamura M, Nakamoto S, Yokosuka O. Nonalcoholic fatty liver disease and hepatic cirrhosis: Comparison with viral hepatitis-associated steatosis. World J Gastroenterol. 2015;21:12989-12995. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 59] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 145. | Hester CA, Rich NE, Singal AG, Yopp AC. Comparative Analysis of Nonalcoholic Steatohepatitis- Versus Viral Hepatitis- and Alcohol-Related Liver Disease-Related Hepatocellular Carcinoma. J Natl Compr Canc Netw. 2019;17:322-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 146. | Ali E, Ziglam H, Kohla S, Ahmed M, Yassin M. A Case of Fulminant Liver Failure in a 24-Year-Old Man with Coinfection with Hepatitis B Virus and SARS-CoV-2. Am J Case Rep. 2020;21:e925932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 147. | Suwarto S, Nainggolan L, Sinto R, Effendi B, Ibrahim E, Suryamin M, Sasmono RT. Dengue score: a proposed diagnostic predictor for pleural effusion and/or ascites in adults with dengue infection. BMC Infect Dis. 2016;16:322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 148. | Michels M, Sumardi U, de Mast Q, Jusuf H, Puspita M, Dewi IM, Sinarta S, Alisjahbana B, van der Ven AJ. The predictive diagnostic value of serial daily bedside ultrasonography for severe dengue in Indonesian adults. PLoS Negl Trop Dis. 2013;7:e2277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 149. | Balasubramanian S, Janakiraman L, Kumar SS, Muralinath S, Shivbalan S. A reappraisal of the criteria to diagnose plasma leakage in dengue hemorrhagic fever. Indian Pediatr. 2006;43:334-339. [PubMed] |
| 150. | Huy NT, Van Giang T, Thuy DH, Kikuchi M, Hien TT, Zamora J, Hirayama K. Factors associated with dengue shock syndrome: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2013;7:e2412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 146] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 151. | Póvoa TF, Alves AM, Oliveira CA, Nuovo GJ, Chagas VL, Paes MV. The pathology of severe dengue in multiple organs of human fatal cases: histopathology, ultrastructure and virus replication. PLoS One. 2014;9:e83386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 157] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 152. | Huerre MR, Lan NT, Marianneau P, Hue NB, Khun H, Hung NT, Khen NT, Drouet MT, Huong VT, Ha DQ, Buisson Y, Deubel V. Liver histopathology and biological correlates in five cases of fatal dengue fever in Vietnamese children. Virchows Arch. 2001;438:107-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 89] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 153. | Heaton NS, Randall G. Dengue virus-induced autophagy regulates lipid metabolism. Cell Host Microbe. 2010;8:422-432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 575] [Cited by in RCA: 551] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 154. | Paes MV, Lenzi HL, Nogueira AC, Nuovo GJ, Pinhão AT, Mota EM, Basílio-de-Oliveira CA, Schatzmayr H, Barth OM, Alves AM. Hepatic damage associated with dengue-2 virus replication in liver cells of BALB/c mice. Lab Invest. 2009;89:1140-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 155. | Lin YW, Wang KJ, Lei HY, Lin YS, Yeh TM, Liu HS, Liu CC, Chen SH. Virus replication and cytokine production in dengue virus-infected human B lymphocytes. J Virol. 2002;76:12242-12249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 156. | Vaughn DW, Green S, Kalayanarooj S, Innis BL, Nimmannitya S, Suntayakorn S, Endy TP, Raengsakulrach B, Rothman AL, Ennis FA, Nisalak A. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J Infect Dis. 2000;181:2-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1124] [Cited by in RCA: 1158] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 157. | Samsa MM, Mondotte JA, Iglesias NG, Assunção-Miranda I, Barbosa-Lima G, Da Poian AT, Bozza PT, Gamarnik AV. Dengue virus capsid protein usurps lipid droplets for viral particle formation. PLoS Pathog. 2009;5:e1000632. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 409] [Cited by in RCA: 457] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 158. | Suwarto S, Diahtantri RA, Hidayat MJ, Widjaya B. Nonalcoholic fatty liver disease is associated with increased hemoconcentration, thrombocytopenia, and longer hospital stay in dengue-infected patients with plasma leakage. PLoS One. 2018;13:e0205965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 159. | Laoprasopwattana K, Jundee P, Pruekprasert P, Geater A. Outcome of Severe Dengue Viral Infection-caused Acute Liver Failure in Thai Children. J Trop Pediatr. 2016;62:200-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 160. | Aye KS, Charngkaew K, Win N, Wai KZ, Moe K, Punyadee N, Thiemmeca S, Suttitheptumrong A, Sukpanichnant S, Prida M, Halstead SB. Pathologic highlights of dengue hemorrhagic fever in 13 autopsy cases from Myanmar. Hum Pathol. 2014;45:1221-1233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 127] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 161. | Jacob ST, Crozier I, Fischer WA 2nd, Hewlett A, Kraft CS, Vega MA, Soka MJ, Wahl V, Griffiths A, Bollinger L, Kuhn JH. Ebola virus disease. Nat Rev Dis Primers. 2020;6:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 350] [Cited by in RCA: 366] [Article Influence: 73.2] [Reference Citation Analysis (0)] |
| 162. | Younan P, Iampietro M, Nishida A, Ramanathan P, Santos RI, Dutta M, Lubaki NM, Koup RA, Katze MG, Bukreyev A. Ebola Virus Binding to Tim-1 on T Lymphocytes Induces a Cytokine Storm. mBio. 2017;8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 163. | Bradfute SB, Swanson PE, Smith MA, Watanabe E, McDunn JE, Hotchkiss RS, Bavari S. Mechanisms and consequences of ebolavirus-induced lymphocyte apoptosis. J Immunol. 2010;184:327-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 164. | Kortepeter MG, Bausch DG, Bray M. Basic clinical and laboratory features of filoviral hemorrhagic fever. J Infect Dis. 2011;204 Suppl 3:S810-S816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 193] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 165. | Goeijenbier M, van Kampen JJ, Reusken CB, Koopmans MP, van Gorp EC. Ebola virus disease: a review on epidemiology, symptoms, treatment and pathogenesis. Neth J Med. 2014;72:442-448. [PubMed] |
| 166. | McCormick JB, Walker DH, King IJ, Webb PA, Elliott LH, Whitfield SG, Johnson KM. Lassa virus hepatitis: a study of fatal Lassa fever in humans. Am J Trop Med Hyg. 1986;35:401-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 71] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 167. | Winn WC, Walker DH. The pathology of human Lassa fever. Bull World Health Organ. 1975;52:535-545. [PubMed] |
| 168. | Sbrana E, Mateo RI, Xiao SY, Popov VL, Newman PC, Tesh RB. Clinical laboratory, virologic, and pathologic changes in hamsters experimentally infected with Pirital virus (Arenaviridae): a rodent model of Lassa fever. Am J Trop Med Hyg. 2006;74:1096-1102. [PubMed] |
| 169. | Iredale JP. Models of liver fibrosis: exploring the dynamic nature of inflammation and repair in a solid organ. J Clin Invest. 2007;117:539-548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 698] [Cited by in RCA: 700] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 170. | Elpek GÖ. Cellular and molecular mechanisms in the pathogenesis of liver fibrosis: An update. World J Gastroenterol. 2014;20:7260-7276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 289] [Cited by in RCA: 293] [Article Influence: 26.6] [Reference Citation Analysis (4)] |
| 171. | Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol. 2011;6:425-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1096] [Cited by in RCA: 1380] [Article Influence: 98.6] [Reference Citation Analysis (0)] |
| 172. | Puche JE, Saiman Y, Friedman SL. Hepatic stellate cells and liver fibrosis. Compr Physiol. 2013;3:1473-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 570] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 173. | Martín-Vílchez S, Sanz-Cameno P, Rodríguez-Muñoz Y, Majano PL, Molina-Jiménez F, López-Cabrera M, Moreno-Otero R, Lara-Pezzi E. The hepatitis B virus X protein induces paracrine activation of human hepatic stellate cells. Hepatology. 2008;47:1872-1883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 81] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 174. | Choi SH, Jeong SH, Hwang SB. Large hepatitis delta antigen modulates transforming growth factor-beta signaling cascades: implication of hepatitis delta virus-induced liver fibrosis. Gastroenterology. 2007;132:343-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 175. | Chouteau P, Defer N, Florimond A, Caldéraro J, Higgs M, Gaudin A, Mérour E, Dhumeaux D, Lerat H, Pawlotsky JM. Hepatitis C virus (HCV) protein expression enhances hepatic fibrosis in HCV transgenic mice exposed to a fibrogenic agent. J Hepatol. 2012;57:499-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 176. | Paracha UZ, Fatima K, Alqahtani M, Chaudhary A, Abuzenadah A, Damanhouri G, Qadri I. Oxidative stress and hepatitis C virus. Virol J. 2013;10:251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 110] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 177. | Arciello M, Gori M, Balsano C. Mitochondrial dysfunctions and altered metals homeostasis: new weapons to counteract HCV-related oxidative stress. Oxid Med Cell Longev. 2013;2013:971024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 178. | Hino K, Nishina S, Hara Y. Iron metabolic disorder in chronic hepatitis C: mechanisms and relevance to hepatocarcinogenesis. J Gastroenterol Hepatol. 2013;28 Suppl 4:93-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 179. | Saracino A, Bruno G, Scudeller L, Punzi G, Lagioia A, Ladisa N, Monno L, Angarano G. Does HIV-1 co-receptor tropism correlate with fibrosis progression in HIV/HCV co-infected patients? J Clin Virol. 2014;59:167-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 180. | Bruno R, Galastri S, Sacchi P, Cima S, Caligiuri A, DeFranco R, Milani S, Gessani S, Fantuzzi L, Liotta F, Frosali F, Antonucci G, Pinzani M, Marra F. gp120 modulates the biology of human hepatic stellate cells: a link between HIV infection and liver fibrogenesis. Gut. 2010;59:513-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 106] [Article Influence: 7.1] [Reference Citation Analysis (0)] |