Published online Feb 28, 2021. doi: 10.13105/wjma.v9.i1.1
Peer-review started: December 23, 2020
First decision: December 30, 2020
Revised: January 2, 2021
Accepted: February 25, 2021
Article in press: February 25, 2021
Published online: February 28, 2021
Processing time: 68 Days and 12.9 Hours
Inflammatory pseudotumor (IPT)-like follicular dendritic cell (FDC) sarcoma is rare. The 2017 World Health Organization classification of tumors of hematopoietic and lymphoid tissues noted that data on its clinical outcome are limited, but that the tumor appears to be indolent. The aim of this study was to summarize the clinical characteristics, treatment outcomes, and prognostic factors for IPT-like FDC sarcoma. A literature review was conducted on retrospective analyses of clinical data and prognostic information on IPT-like FDC sarcoma reported between 2001 and 2020. A total of 67 cases of IPT-like FDC sarcoma were retrieved from the literature, documenting that it occurs predominantly in middle-aged adults, with a marked female predilection. Six patients had a separate malignancy and five had an autoimmune disease. Typically involving the spleen and/or liver, it may also selectively involve the abdomen, gastrointes-tinal tract, pancreas, retroperitoneum, and mesentery. Necrosis, hemorrhage, noncaseating epithelioid granulomas, and fibrinoid deposits in blood vessel walls are often present. The neoplastic cells are predominantly positive for follicular dendritic cell markers such as cluster of differentiation 21 (CD21), CD23, CD35 and CNA.42 and are consistently Epstein-Barr virus (EBV)-positive. Mitoses were very rare in most cases. Most patients were treated by surgery alone. Disease status at the time of last follow-up was known for 57 patients with follow-up time ranging from 2 to 144 mo. Local and/or distant recurrence after initial treatment was seen in 15.8% of the patients. The 1- and 5-year progression-free survival for the entire group was 91.5% and 56.1%, respectively. Kaplan-Meier and multivariate analyses showed that age, sex, tumor size, and pathological features were not risk factors for disease progression. IPT-like FDC sarcoma appears to be mildly aggressive and requires annual surveillance. Surgery is the most effective treatment modality, and the role of adjuvant chemotherapy for postoperative management is unclear. EBV is likely to play an important role in the etiology of IPT-like FDC sarcoma.
Core Tip: We show that inflammatory pseudotumor-like follicular dendritic cell (FDC) sarcoma appears to be mildly aggressive and does require annual surveillance. Surgery is the most effective treatment modality, and the role of adjuvant chemotherapy for postoperative management is unclear. Epstein-Barr virus is likely to play an important role in the etiology of inflammatory pseudotumor-like FDC sarcoma.
- Citation: Wu H, Liu P, Xie XR, Chi JS, Li H, Xu CX. Inflammatory pseudotumor-like follicular dendritic cell sarcoma: Literature review of 67 cases. World J Meta-Anal 2021; 9(1): 1-11
- URL: https://www.wjgnet.com/2308-3840/full/v9/i1/1.htm
- DOI: https://dx.doi.org/10.13105/wjma.v9.i1.1
Follicular dendritic cells (FDCs) develop from perivascular precursors of stromal cell origin that are seeded throughout the body. They are centrally located within B cell follicles and act as a bridge between innate and adaptive responses[1]. Monda et al[2] first reported 4 cases of tumors derived from FDC in 1986. FDC sarcoma is an uncommon neoplasm that can involve lymph nodes or extranodal sites. It usually has an aggressive course in the abdomen[3]. Cheuk et al[4] first characterized inflammatory pseudotumor(IPT)-like FDC sarcoma as an EBV-associated tumor of FDC origin in 2001. The most recent World Health Organization (WHO) classification noted that IPT-like FDC sarcoma appears to be indolent and that data on clinical outcome are limited[5].
In this study, we detail the features of 67 cases with IPT-like FDC sarcoma, providing an overview of the current knowledge about the clinical, pathological, and prognostic characteristics of this disease entity.
We systematically searched PubMed, EMBASE, and MEDLINE databases using the search terms “inflammatory pseudotumor-like” combined with “follicular dendritic cell sarcoma” or “follicular dendritic cell tumor” or “fibroblastic dendritic cell sarcoma” or “fibroblastic dendritic cell tumor” and with the restrictions “English” language and “original article.” We collated demographic, clinicopathological and follow-up information.
All statistical analyses were performed using SPSS Statistics 25.0 software (IBM Corporation, Armonk, NY, United States). Survival curves were replotted with Graphpad prism 8.0 software (Graphpad Inc., La Jolla, CA, United States). P < 0.05 was considered statistically significant, and variables pertaining to accuracy were calculated with 95% confidence intervals.
Data on a total of 67 cases of IPT-like FDC sarcoma were retrieved from the literature. Patients and disease characteristics are summarized in Table 1. The median age at initial presentation was 53 years (range: 19-79). Females were more commonly affected than males (n = 42 vs 25). Patients were asymptomatic or presented with abdominal distension or pain, sometimes accompanied by systemic symptoms such as fever, fatigue, and weight loss. Other cancers were observed in 6 patients before their diagnosis with IPT-like FDC sarcoma including gastric cancer (n = 3), breast cancer (n = 1), pituitary adenoma, meningioma, and acoustic neuroma (n = 1) and diffuse large B-cell lymphoma (n = 1). Five patients (7.5%) had pre-existing or subsequent autoimmune disease including pemphigus and bronchiolitis obliterans (n = 1), IgA nephropathy (n = 1), nephrotic syndrome (n = 1), bronchial asthma (n = 1), and idiopathic thrombocytopenic purpura (n = 1).
| Case No. | Ref. | Sex/age | Site | Maximum diameter in cm | Symptom | Treatment | Time to recurrence in mo | Last follow-up in mo | Outcome |
| 1 | Rao et al[22] | 39/M | Spleen | 7.2 | Asymptomatic | Surgery | NA | NA | NA |
| 2 | Zhao[23] | 28/F | Abdomen | 9 | Skin rash, cough | Surgery | NA | NA | NA |
| 3 | Kazemimood et al[12] | 53/F | Colon | 3 | Abdominal discomfort | Surgery | NA | NA | NA |
| 4 | Hommel et al[24] | 77/F | Spleen | 12 | Left upper quadrant abdominal pain | Surgery | - | 4 | NED |
| 5 | Hang et al[25] | 57/M | Spleen | 2.7 | Asymptomatic | Surgery | - | 9 | NED |
| 6 | Granados et al[26] | 57/F | Liver | 13 | Abdominal pain, vomiting | Surgery | - | 24 | NED |
| 7 | Kiryu et al[27] | 56/F | Spleen | 4 | Asymptomatic | Surgery | - | 24 | NED |
| 8 | Li et al[28] | 32/F | Liver | 3 | - | Surgery | - | 8 | NED |
| 9 | Pan et al[29] | 78/F | Colon | 3.9 | Abdominal discomfort, bloody stool | Surgery | - | 5 | NED |
| 10 | Ang et al[30] | 63/F | Liver | 13.4 | Fever and lethargy | Surgery | - | 48 | NED |
| 11 | Wu et al[31] | 45/M | Liver | 8 | Abdominal pain and weight loss | Surgery | - | 9 | NED |
| 12 | Zhang et al[32] | 19/F | Liver | 6 | Abdominal discomfort | Surgery | - | 12 | NED |
| 13 | Deng et al[33] | 67/F | Liver | 4 | Cough | Surgery | NA | NA | NA |
| 14 | Horiguchi et al[34] | 77/F | Spleen | 8.5 | Epigastralgia | Surgery | - | 36 | NED |
| 15 | Kitamura et al[35] | 74/F | Spleen | 3.6 | Asymptomatic | Surgery | - | 24 | NED |
| 16 | Kim et al[36] | 76/M | Spleen | 3.2 | Asymptomatic | Surgery | - | 7 | NED |
| 17 | Kwon[37] | 58/F | Spleen | 5 | Asymptomatic | Surgery | - | 24 | NED |
| 18 | Nishiyama et al[38] | 73/F | Spleen | 8 | Asymptomatic | Surgery | - | 144 | NED |
| 19 | Bui et al[39] | 50/F | Spleen | 8.5 | Abdominal pain | Surgery | NA | NA | NA |
| 20 | Mograbi et al[40] | 70/F | Spleen, Pancreas | Spleen: 3, Pancreas: 7 | Asymptomatic | Surgery | NA | NA | NA |
| 21 | Hu et al[41] | 49/F | Retroperitoneum | 5 | Asymptomatic | Surgery | Tail of pancreas (60) | 60 | NED |
| 22 | Vardas et al[42] | 61/M | Spleen | 10 | Abdominal pain | Surgery | - | 12 | NED |
| 23 | Agaimy et al[43] | 52/M | Ileum and mesentery | 6 | Abdominal pain | Surgery | NA | NA | NA |
| 24 | Ge et al[44] | 54/F | Spleen | 3.5 | Left upper quadrant abdominal pain | Surgery | - | 10 | NED |
| 25 | Ge et al[44] | 79/M | Spleen | 6 | Asymptomatic | Surgery | - | 18 | NED |
| 26 | Zhang et al[45] | 31/F | Liver | 2 masses: 3.5, 2.5 | Anorexia | Surgery | - | 10 | NED |
| 27 | Zhang et al[45] | 48/M | Liver and hepatoduodenal ligament lymph node | Liver: 10, Lymph: 3.5 | Asymptomatic | Surgery | - | 2 | NED |
| 28 | Ke et al[46] | 53/M | Colon | 1 | Chest and back pain | ESD | - | 11 | NED |
| 29 | Ke et al[46] | 48/M | Colon | 4.5 | Left lower quadrant pain | Surgery | - | 7 | NED |
| 30 | Li et al[47] | 64/F | Spleen | 7.2 | Upper abdominal pain | Surgery | - | 8 | NED |
| 31 | Li et al[47] | 61/M | Spleen | 6.2 | Asymptomatic | Surgery | - | 16 | NED |
| 32 | Li et al[47] | 42/F | Spleen | 4 | Left-sided flank pain | Surgery | - | 9 | NED |
| 33 | Li et al[47] | 57/F | Spleen | 13.3 | Upper abdominal pain | Surgery | Pulmonary (4) | 4 | LWD |
| 34 | Li et al[47] | 52/M | Spleen | 2.9, 3.7 | Back pain | Surgery, chemotherapy | Multiple bone (5) | 5 | LWD |
| 35 | Li et al[48] | 49/F | Spleen | 4.7 | Asymptomatic | Surgery | - | NA | NA |
| 36 | Li et al[48] | 56/F | Spleen | 8 | Abdominal pain | Surgery | - | 17 | NED |
| 37 | Li et al[48] | 38/M | Liver | 8.5 | Fatigue, anorexia | Surgery | - | 11 | NED |
| 38 | Li et al[48] | 42/F | Liver | 2, 1.7 | Abdominal pain | Surgery | - | 36 | NED |
| 39 | Li et al[48] | 50/M | Spleen and liver | Spleen: 10, Liver: 3, 1.5, 1 | Abdominal bloating, fatigue | Surgery | - | 17 | NED |
| 40 | Li et al[48] | 39/F | Liver | 9 | Asymptomatic | Surgery, chemotherapy | Liver (12) | 84 | NED |
| 41 | Choe et al[19] | 64/F | Spleen | 5.5 | Asymptomatic | Surgery | - | 78 | NED |
| 42 | Choe et al[19] | 72/F | Spleen | 7.2 | Asymptomatic | Surgery | - | 18 | NED |
| 43 | Choe et al[19] | 53/F | Spleen | 3.2 | Asymptomatic | Surgery | - | 13 | NED |
| 44 | Choe et al[19] | 76/M | Spleen | 3.2 | Asymptomatic | Surgery | - | 8 | NED |
| 45 | Choe et al[19] | 72/M | Spleen | 6 | Asymptomatic | Surgery | - | 18 | NED |
| 46 | Choe et al[19] | 75/M | Spleen | 3.5 | Abdominal pain | Surgery | - | 30 | NED |
| 47 | Chen et al[49] | 28/F | Liver | 6 | Abdominal pain, fatigue, anorexia | Surgery | Liver (48) | 48 | LWD |
| 48 | Chen et al[49] | 39/M | Spleen | 7.4 | Asymptomatic | Surgery | - | 40 | NED |
| 49 | Chen et al[49] | 48/M | Liver | 23.3 | Abdominal pain, fever, fatigue | Surgery | - | 23 | NED |
| 50 | Chen et al[49] | 65/M | Spleen and liver | Spleen: 22.3, Liver: Multiple masses (largest: 5.8) | Abdominal pain, fever, fatigue, anorexia, weight loss | Surgery | - | 2 | DOD |
| 51 | Chen et al[49] | 51/M | Spleen | 8.5 | Weight loss | Surgery | - | 19 | NED |
| 52 | Chen et al[49] | 68/M | Spleen | 2.3 | Asymptomatic | Surgery | - | 6 | NED |
| 53 | Chen et al[49] | 51/F | Spleen | 5.3 | Epigastric discomfort | Surgery | - | 5 | NED |
| 54 | Chen et al[49] | 67/M | Spleen | 7.5 | Asymptomatic | Surgery | - | 5 | NED |
| 55 | Chen et al[49] | 60/M | Liver | 3 | Asymptomatic | Surgery | - | 3 | NED |
| 56 | Chen et al[49] | 52/F | Spleen | 0.9 | Asymptomatic | Surgery | - | 12 | NED |
| 57 | Cheuk et al[4] | 19/F | Liver | 12 | Right upper quadrant pain, weight loss | Surgery | - | 40 | NED |
| 58 | Cheuk et al[4] | 56/F | Liver | 15 | Abdominal discomfort | Surgery | Liver (15, 27 ,35) | 56 | LWD |
| 59 | Cheuk et al[4] | 40/F | Liver | 12.5 | Abdominal pain, weight loss | Surgery | Intraabdominal (108) | 108 | LWD |
| 60 | Cheuk et al[4] | 49/F | Liver | 4.2 | Asymptomatic | Surgery | - | 9 | NED |
| 61 | Cheuk et al[4] | 37/M | Liver | 15 | Weight loss | Surgery | - | 42 | NED |
| 62 | Cheuk et al[4] | 35/F | Liver | 20 | Abdominal discomfort, fever, weight loss | Surgery | Liver (30, 40), peritoneum and ascending colon (60) | 95 | DOD |
| 63 | Cheuk et al[4] | 31/F | Liver | 15 | Abdominal distension, weight loss | Surgery | - | 60 | NED |
| 64 | Cheuk et al[4] | 58/F | Spleen | 22 | Abdominal distension | Surgery | - | 4 | NED |
| 65 | Cheuk et al[4] | 39/F | Spleen | 7.5 | Weight loss, fever | Surgery | - | 2 | NED |
| 66 | Cheuk et al[4] | 61/F | Spleen | 3.5 | Asymptomatic | Surgery | NA | NA | NA |
| 67 | Cheuk et al[4] | 49/F | Peri-pancreas | 9.5 | Abdominal distension | Surgery | NA | NA | NA |
Thirty-five patients (52.2%) presented solely with spleen involvement and twenty (29.9%) only with liver. For the remaining patients, lesion sites included the abdomen (n = 1), colon (n = 4), spleen and pancreas (n = 1), retroperitoneum (n = 1), ileum and mesentery (n = 1), liver and hepatoduodenal ligament lymph node (n = 1), spleen and liver (n = 2) and peri-pancreas (n = 1). The average size of tumors at all sites was 7.6 cm, ranging from 0.9 to 23.3 cm (median, 6.2 cm). Bulky tumor (≥ 5 cm) was noted in 43 patients (64.2%). Seven patients presented with more than one lesion. The radiologic findings of IPT-like FDC sarcoma have been described in several cases. The abdominal computed tomography scan showed a well-circumscribed or ill-defined, hypodense mass with weak delayed heterogeneous enhancement after contrast enhancement in the spleen or liver. Some of these lesions reveal irregular areas of nonenhancement related to foci of tumoral necrosis and hemorrhage.
The pathological features are summarized in Table 2. In all, 40 cases (59.7%) presented with necrosis, 22 (32.8%) with hemorrhage, 23 (34.3%) with noncaseating epithelioid granulomas and 22 (32.8%) with fibrinoid deposits in the blood vessel wall. The neoplastic cells were predominantly positive for the follicular dendritic cell markers CD21(54/65), CD23 (34/47), CD35 (50/55) and CNA.42 (13/13). Tumor cells were usually positive for clusterin (8/11), vimentin (6/6), fascin (9/9), and smooth muscle actin (32/39), and variably positive for S100 (6/23), D2-40 (6/10), epidermal growth factor receptor (3/8), muscle-specific actin (5/9), epithelial membrane antigen (2/8), CD68 (8/16), tubulin beta 3 class III (3/6) andγ-synuclein (2/6). There was no staining for CD117, cytokeratin, caldesmon, activin-like kinase 1 (ALK1), desmin or human herpes virus 8 (HHV8). In 4 cases, the tumor cells resembled Reed-Sternberg cells. The neoplastic cells were consistently associated with EBV, and EBV-encoded small RNA (EBER) was positive in 60 cases (95.2%) by in situ hybridization. Immunoreactivity for EBV-encoded latent membrane protein 1 (LMP1) was present in 28 cases (82.4%). One case present with LMP1 gene fragment deletions and point mutations. Increased IgG4-postive plasma cells were found in 6 cases. Mitoses were very rare in most cases and the Ki-67 proliferation index ranged from 3% to 30%.
| Markers | Number of cases positive/number of cases tested | Cases positive, % |
| Necrosis | 40/67 | 59.7 |
| Hemorrhage | 22/67 | 32.8 |
| Granuloma | 23/67 | 34.3 |
| Fibrinoid deposits in blood vessel wall | 22/67 | 32.8 |
| CD1a | 0/6 | 0 |
| CD3 | 0/7 | 0 |
| CD20 | 1/9 | 11.1 |
| CD21 | 54/65 | 83.1 |
| CD23 | 34/47 | 72.3 |
| CD30 | 1/27 | 3.7 |
| CD31 | 1/8 | 12.5 |
| CD34 | 5/15 | 33.3 |
| CD35 | 50/55 | 90.9 |
| CD68 | 8/16 | 50 |
| CD117 | 0/9 | 0 |
| LMP1 | 28/34 | 82.4 |
| EBER | 60/63 | 95.2 |
| D2-40 | 6/10 | 60 |
| CNA.42 | 13/13 | 100 |
| Clusterin | 8/11 | 72.7 |
| EGFR | 3/8 | 37.5 |
| Vimentin | 6/6 | 100 |
| CK-pan | 0/9 | 0 |
| Caldesmon | 0/7 | 0 |
| ALK1 | 0/42 | 0 |
| Desmin | 0/30 | 0 |
| Fascin | 9/9 | 100 |
| SMA | 32/39 | 82.1 |
| MSA | 5/9 | 55.5 |
| EMA | 2/8 | 25 |
| S100 | 6/23 | 26.1 |
| p53 | 1/6 | 16.7 |
| HHV-8 | 0/7 | 0 |
| TUBB3 | 3/6 | 50 |
| γ-synuclein | 2/6 | 33.3 |
Information on treatment was available for all 67 patients, most of whom (65/67) underwent surgery alone. Only 2 patients received surgery and chemotherapy (one with two cycles of cyclophosphamide, doxorubicin, vincristine and prednisone, and the other with CHOP-based chemotherapy).
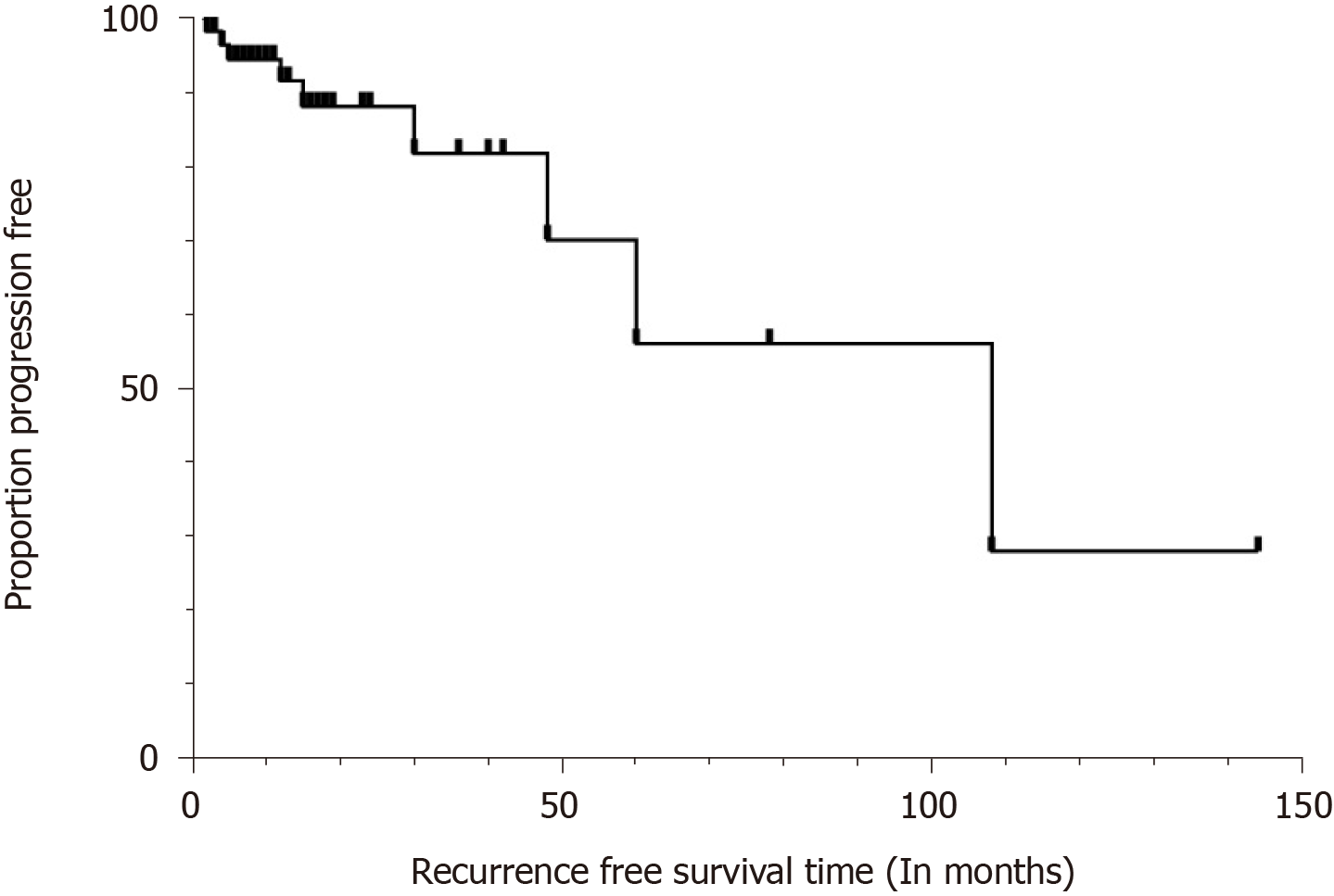
Disease status at the time of last follow-up was known for 57 of the 67 patients; these patients were eligible for progression-free survival (PFS) analyses. The follow-up time ranged from 2 to 144 mo (mean, 22 mo; median, 12 mo). Overall, 9 of the 57 (15.8%) had local and/or distant recurrence after initial treatment. Two patients died due to disease progression, but two others had no evidence of disease after undergoing a second surgery. The 1- and 5-year PFS for the entire group was 91.5% and 56.1%, respectively (Figure 1). Young age, male sex, and large tumor size may contribute to less disease progression. However, Kaplan-Meier and multivariate analyses failed to confirm that age, sex, tumor size or and pathological features are risk factors for disease progression (Table 3).
| Variable | Univariate analysis | Multivariate analysis | ||
| P value | HR (95%CI) | P value | HR (95%CI) | |
| Age ≤ 40 yr | 0.50 | 1.55 (0.39-6.16) | 0.48 | 1.78 (0.36-8.77) |
| Gender, male/female | 0.97 | 0.97 (0.21-4.64) | 0.67 | 1.51 (0.23-10.04) |
| Tumor size < 5 cm | 0.59 | 0.59 (0.11-3.32) | 0.43 | 0.38 (0.04-4.10) |
| Necrosis | 0.31 | 0.51 (0.10-2.59) | 0.24 | 0.25 (0.03-2.49) |
| Hemorrhage | 0.99 | 0.99 (0.27-3.70) | 0.84 | 1.25 (0.13-11.91) |
| Granuloma | 0.58 | 0.66 (0.16-2.71) | 0.85 | 0.84 (0.14-5.15) |
In 1986 Monda et al[2] first reported 4 cases of tumors derived from FDC and in 2001 Cheuk et al[4] reported that IPT-like FDC sarcoma were EBV-associated tumors of FDC origin. Currently, FDC sarcomas are classified into conventional and IPT-like FDC sarcoma[5]. Our study indicated that IPT-like FDC sarcoma occurs predominantly in middle-aged adults, with a marked female predilection unlike the equal distribution between the sexes of conventional FDC sarcoma. IPT-like FDC sarcoma typically involves the spleen and/or liver. Genetic lineage-tracing approaches show that FDC develop from splenopancreatic embryonic mesenchymal cells of the Nkx2-5(+) Islet1(+) lineage[6]. FDC emerge from perivascular precursors, engaging B cells in germinal centers of secondary lymphoid organs[1,7]. Our analysis indicated that 7.7% patients had pre-existing or subsequent autoimmune disease. Conventional FDC sarcoma also showed a trend for occurring coincidentally with autoimmune disease[3]. FDC can retain antigens to establish contact with B cells, and secrete milk-fat globule epidermal growth factor 8, which controls the engulfment of apoptotic B cells by macrophages. Excessive accumulation of apoptotic B cells may result in lupus-like autoimmunity[8,9]. Furthermore, FDC was shown to be important for the retention of self-antigen-containing immune complexes in a spontaneous arthritis model[10].
We found that the average size of tumors was 7.6 cm, ranging from 0.9 to 23.3 cm (median, 6.2 cm). Bulky tumor (≥ 5 cm) was noted in 43 patients. Necrosis, hemorrhage, noncaseating epithelioid granulomas, and fibrinoid deposits in blood vessel walls are often present in IPT-like FDC sarcoma. A pooled analysis demonstrated that young age, large tumor size and coagulative necrosis may predict poor prognosis in conventional FDC sarcoma[11], but our data failed to demonstrate that age, large tumor size, and necrosis are risk factors for disease progression in IPT-like FDC sarcoma. The neoplastic spindle-shaped cells of the latter are present within a prominent lymphoplasmacytic infiltrate and may even resemble Reed-Sternberg cells[4,12]. IPTs can also be found in the spleen and liver, and tend to appear as plump spindle cells in a polymorphic inflammatory cell infiltrate[13,14]. IPT-like FDC sarcomas are predominantly positive for the FDC markers CD21, CD23, CD35 and CNA.42, thus distinguishing them from Hodgkin lymphoma and IPTs. Moreover, IPT-like FDC sarcomas are negative for cytokeratin, CD117, caldesmon, ALK1, desmin and HHV8, which can help in the differential diagnosis of epithelial carcinoma, gastrointestinal stromal tumor, inflammatory myofibroblastic tumor, anaplastic large cell lymphoma, rhabdomyosarcoma and Kaposi sarcoma. IPT-like FDC sarcomas are usually positive for clusterin, vimentin and fascin. Strong clusterin staining appears to be a highly sensitive marker of FDC sarcoma. Vimentin staining may confirm mesenchymal origin, but is relatively nonspecific. Fascin staining was not specific for spindle cell tumors and thus does not imply a dendritic cell lineage[15,16].
Our study concluded that 95.2% of patients possessed tumor cells positive for EBER by in situ hybridization, and 82.4% were immunoreactive for EBV-encoded LMP1. EBV has a long history of coevolution with humans. The result is a finely balanced relationship that usually allows the virus to be carried as a lifelong asymptomatic infection. However, the pathogenic potential of EBV has been confirmed in several autoimmune disorders, particularly multiple sclerosis, and a wide range of human tumors[17]. Approximately 2.2 million cases of cancers in 2018 were attributable to infectious agents. It is estimated that EBV accounts for more than 150000 of these, mainly nasopharyngeal carcinomas, Hodgkin lymphomas and Burkitt lymphomas[18]. IPT-like FDC sarcoma cells were consistently positive for EBER and LMP1. Interestingly, increased IgG4-postive plasma cells were found in 6 cases[19]. There is evidence for the presence of increased numbers of EBV-infected cells in IgG4-related lymphadenopathy[20]. EBV lytic reactivation induces IgG4 production in Graves’ disease[21]. The etiology of EBV in IPT-like FDC sarcoma needs further research.
Surgery is the most effective therapy for IPT-like FDC sarcoma, and the number of cases treated by adjuvant chemotherapy is limited. Nevertheless, here we saw that 15.8% patients had local and/or distant recurrence after initial treatment. The WHO has noted that data on clinical outcomes are limited, but that the tumor appears to be indolent[5]. Our research revealed that 1- and 5-year PFS for the entire group was 91.5% and 56.1%, respectively.
There is a certain risk of relapse after the initial therapy of IPT-like FDC sarcoma, which therefore needs annual surveillance. Surgery is the most effective treatment modality, and the role of adjuvant chemotherapy for the management of postoperative is not clear. EBV plays an important role in the etiology of IPT-like FDC sarcoma.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Ho CM S-Editor: Wang JL L-Editor: Filipodia P-Editor: Li JH
| 1. | Krautler NJ, Kana V, Kranich J, Tian Y, Perera D, Lemm D, Schwarz P, Armulik A, Browning JL, Tallquist M, Buch T, Oliveira-Martins JB, Zhu C, Hermann M, Wagner U, Brink R, Heikenwalder M, Aguzzi A. Follicular dendritic cells emerge from ubiquitous perivascular precursors. Cell. 2012;150:194-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 306] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 2. | Monda L, Warnke R, Rosai J. A primary lymph node malignancy with features suggestive of dendritic reticulum cell differentiation. A report of 4 cases. Am J Pathol. 1986;122:562-572. [PubMed] |
| 3. | Jain P, Milgrom SA, Patel KP, Nastoupil L, Fayad L, Wang M, Pinnix CC, Dabaja BS, Smith GL, Yu J, Hu S, Bueso Ramos CE, Kanagal-Shamanna R, Medeiros LJ, Oki Y, Fowler N. Characteristics, management, and outcomes of patients with follicular dendritic cell sarcoma. Br J Haematol. 2017;178:403-412. |
| 4. | Cheuk W, Chan JK, Shek TW, Chang JH, Tsou MH, Yuen NW, Ng WF, Chan AC, Prat J. Inflammatory pseudotumor-like follicular dendritic cell tumor: a distinctive low-grade malignant intra-abdominal neoplasm with consistent Epstein-Barr virus association. Am J Surg Pathol. 2001;25:721-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 219] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 5. | Steven HS, Elias C, Nancy LH, Elaine SJ, Stefano AP, Harald S. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon: IARC press; 2017: 476-479. |
| 6. | Castagnaro L, Lenti E, Maruzzelli S, Spinardi L, Migliori E, Farinello D, Sitia G, Harrelson Z, Evans SM, Guidotti LG, Harvey RP, Brendolan A. Nkx2-5(+)islet1(+) mesenchymal precursors generate distinct spleen stromal cell subsets and participate in restoring stromal network integrity. Immunity. 2013;38:782-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 7. | Aguzzi A, Kranich J, Krautler NJ. Follicular dendritic cells: origin, phenotype, and function in health and disease. Trends Immunol. 2014;35:105-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 117] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 8. | Hanayama R, Tanaka M, Miyasaka K, Aozasa K, Koike M, Uchiyama Y, Nagata S. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science. 2004;304:1147-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 737] [Cited by in RCA: 760] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 9. | Kranich J, Krautler NJ, Heinen E, Polymenidou M, Bridel C, Schildknecht A, Huber C, Kosco-Vilbois MH, Zinkernagel R, Miele G, Aguzzi A. Follicular dendritic cells control engulfment of apoptotic bodies by secreting Mfge8. J Exp Med. 2008;205:1293-1302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 147] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 10. | Victoratos P, Kollias G. Induction of autoantibody-mediated spontaneous arthritis critically depends on follicular dendritic cells. Immunity. 2009;30:130-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Saygin C, Uzunaslan D, Ozguroglu M, Senocak M, Tuzuner N. Dendritic cell sarcoma: a pooled analysis including 462 cases with presentation of our case series. Crit Rev Oncol Hematol. 2013;88:253-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 170] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 12. | Kazemimood R, Saei HF, Sharif A, Gaitonde S, Wiley E, Giulianotti PC. A Rare Case of Epstein-Barr Virus Negative Inflammatory Pseudotumor-like Follicular Dendritic Cell Sarcoma Presenting as a Solitary Colonic Mass in a 53-Year-Old Woman; Case Report and Review of Literature. Appl Immunohistochem Mol Morphol. 2017;25:e30-e33. |
| 13. | Van BC, Van DJ. Splenic Epstein-Barr Virus-Associated Inflammatory Pseudotumor. Arch Pathol Lab Med. 2017;141:722-727. |
| 14. | You Y, Shao H, Bui K, Bui M, Klapman J, Cui Q, Coppola D. Epstein-Barr virus positive inflammatory pseudotumor of the liver: report of a challenging case and review of the literature. Ann Clin Lab Sci. 2014;44:489-498. [PubMed] |
| 15. | Grogg KL, Lae ME, Kurtin PJ, Macon WR. Clusterin expression distinguishes follicular dendritic cell tumors from other dendritic cell neoplasms: report of a novel follicular dendritic cell marker and clinicopathologic data on 12 additional follicular dendritic cell tumors and 6 additional interdigitating dendritic cell tumors. Am J Surg Pathol. 2004;28:988-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 92] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 16. | Grogg KL, Macon WR, Kurtin PJ, Nascimento AG. A survey of clusterin and fascin expression in sarcomas and spindle cell neoplasms: strong clusterin immunostaining is highly specific for follicular dendritic cell tumor. Mod Pathol. 2005;18:260-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 62] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Taylor GS, Long HM, Brooks JM, Rickinson AB, Hislop AD. The immunology of Epstein-Barr virus-induced disease. Annu Rev Immunol. 2015;33:787-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 433] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 18. | de Martel C, Georges D, Bray F, Ferlay J, Clifford GM. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob Health. 2020;8:e180-e190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1058] [Cited by in RCA: 1318] [Article Influence: 263.6] [Reference Citation Analysis (0)] |
| 19. | Choe JY, Go H, Jeon YK, Yun JY, Kim YA, Kim HJ, Huh J, Lee H, Shin DH, Kim JE. Inflammatory pseudotumor-like follicular dendritic cell sarcoma of the spleen: a report of six cases with increased IgG4-positive plasma cells. Pathol Int. 2013;63:245-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (1)] |
| 20. | Takeuchi M, Sato Y, Yasui H, Ozawa H, Ohno K, Takata K, Gion Y, Orita Y, Tachibana T, Itoh T, Asano N, Nakamura S, Swerdlow SH, Yoshino T. Epstein-Barr virus-infected cells in IgG4-related lymphadenopathy with comparison with extranodal IgG4-related disease. Am J Surg Pathol. 2014;38:946-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Nagata K, Hara S, Nakayama Y, Higaki K, Sugihara H, Kuwamoto S. Epstein-Barr Virus Lytic Reactivation Induces IgG4 Production by Host B Lymphocytes in Graves' Disease Patients and Controls: A Subset of Graves' Disease Is an IgG4-Related Disease-Like Condition. Viral Immunol. 2018;31:540-547. |
| 22. | Rao L, Yang Z, Wang X, Zhang X, Shen B. Imaging findings of inflammatory pseudotumor-like follicular dendritic cell tumor of spleen. Clin Nucl Med. 2014;39:e286-e289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Zhao C. A case in which paraneoplastic pemphigus and bronchiolitis obliterans are the main manifestations of inflammatory pseudotumour-like follicular dendritic cell sarcoma. Australas J Dermatol. 2020;61:e376-e377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Hommel C, Dargent JL, Druez P. A Rare Cause of Left Upper Quadrant Abdominal Pain. Gastroenterology. 2018;154:e7-e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 25. | Hang JF, Wang LC, Lai CR. Cytological features of inflammatory pseudotumor-like follicular dendritic cell sarcoma of spleen: A case report. Diagn Cytopathol. 2017;45:230-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Granados R, Aramburu JA, Rodríguez JM, Nieto MA. Cytopathology of a primary follicular dendritic cell sarcoma of the liver of the inflammatory pseudotumor-like type. Diagn Cytopathol. 2008;36:42-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Kiryu S, Takeuchi K, Shibahara J, Uozaki H, Fukayama M, Tanaka H, Maeda E, Akahane M, Ohtomo K. Epstein-Barr virus-positive inflammatory pseudotumour and inflammatory pseudotumour-like follicular dendritic cell tumour. Br J Radiol. 2009;82:e67-e71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Li Y, Zeng KN, Ruan DY, Yao J, Yang Y, Chen GH, Wang GS. Feasibility of laparoscopic isolated caudate lobe resection for rare hepatic mesenchymal neoplasms. World J Clin Cases. 2019;7:3194-3201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Pan ST, Cheng CY, Lee NS, Liang PI, Chuang SS. Follicular Dendritic Cell Sarcoma of the Inflammatory Pseudotumor-like Variant Presenting as a Colonic Polyp. Korean J Pathol. 2014;48:140-145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | Ang WW, Bundele MM, Shelat VG. Follicular dendritic cell sarcoma: Rare presentation of incidental large hepatic mass. Ann Hepatobiliary Pancreat Surg. 2019;23:74-76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | Wu CY, Wang RC, Chen BJ, Chen WY, Jhuang JY, Chang MC. Granuloma With an Underlying Lymphoma: A Diagnostic Challenge and a Wider Histologic Spectrum Including Adult T-Cell Leukemia/Lymphoma. Appl Immunohistochem Mol Morphol. 2020;28:316-624. |
| 32. | Zhang X, Zhu C, Hu Y, Qin X. Hepatic inflammatory pseudotumour-like follicular dendritic cell tumor: A case report. Mol Clin Oncol. 2017;6:547-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Deng S, Gao J. Inflammatory pseudotumor-like follicular dendritic cell sarcoma: a rare presentation of a hepatic mass. Int J Clin Exp Pathol. 2019;12:3149-3155. [PubMed] |
| 34. | Horiguchi H, Matsui-Horiguchi M, Sakata H, Ichinose M, Yamamoto T, Fujiwara M, Ohse H. Inflammatory pseudotumor-like follicular dendritic cell tumor of the spleen. Pathol Int. 2004;54:124-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 35. | Kitamura Y, Takayama Y, Nishie A, Asayama Y, Ushijima Y, Fujita N, Morita K, Baba S, Kubo Y, Shirabe K, Honda H. Inflammatory Pseudotumor-like Follicular Dendritic Cell Tumor of the Spleen: Case Report and Review of the Literature. Magn Reson Med Sci. 2015;14:347-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 36. | Kim HJ, Kim JE, Kang GH, Kim JY, Park K. Inflammatory Pseudotumor-like Follicular Dendritic Cell Tumor of the Spleen with Extensive Histiocytic Granulomas and Necrosis: A Case Report and Literature Review. Korean J Pathol. 2013;47:599-602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 37. | Kwon H. Inflammatory pseudotumor-like follicular dendritic cell tumor of the spleen. Turk J Gastroenterol. 2018;29:128-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 38. | Nishiyama R, Baba S, Watahiki Y, Maruo H. Inflammatory pseudotumour-like follicular dendritic cell tumour of the spleen. BMJ Case Rep. 2015;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 39. | Bui PL, Vicens RA, Westin JR, Jensen CT. Multimodality imaging of Epstein-Barr virus-associated inflammatory pseudotumor-like follicular dendritic cell tumor of the spleen: case report and literature review. Clin Imaging. 2015;39:525-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 40. | Mograbi M, Stump MS, Luyimbazi DT, Shakhatreh MH, Grider DJ. Pancreatic Inflammatory Pseudotumor-Like Follicular Dendritic Cell Tumor. Case Rep Pathol. 2019;2019:2648123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 41. | Hu J, Chen LL, Ding BW, Jin DY, Xu XF. Resection is an effective treatment for recurrent follicular dendritic cell sarcoma from retroperitoneum: unusual presentation of a rare tumor. Int J Clin Exp Med. 2015;8:8218-8221. [PubMed] |
| 42. | Vardas K, Manganas D, Papadimitriou G, Kalatzis V, Kyriakopoulos G, Chantziara M, Exarhos D, Drakopoulos S. Splenic inflammatory pseudotumor-like follicular dendritic cell tumor. Case Rep Oncol. 2014;7:410-416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 43. | Agaimy A, Wünsch PH. Follicular dendritic cell tumor of the gastrointestinal tract: Report of a rare neoplasm and literature review. Pathol Res Pract. 2006;202:541-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 44. | Ge R, Liu C, Yin X, Chen J, Zhou X, Huang C, Yu W, Shen X. Clinicopathologic characteristics of inflammatory pseudotumor-like follicular dendritic cell sarcoma. Int J Clin Exp Pathol. 2014;7:2421-2429. [PubMed] |
| 45. | Zhang BX, Chen ZH, Liu Y, Zeng YJ, Li YC. Inflammatory pseudotumor-like follicular dendritic cell sarcoma: A brief report of two cases. World J Gastrointest Oncol. 2019;11:1231-1239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 46. | Ke X, He H, Zhang Q, Yuan J, Ao Q. Epstein-Barr virus-positive inflammatory follicular dendritic cell sarcoma presenting as a solitary colonic mass: two rare cases and a literature review. Histopathology. 2020;77:832-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 47. | Li X, Shi Z, You R, Li Y, Cao D, Lin R, Huang X. Inflammatory Pseudotumor-Like Follicular Dendritic Cell Sarcoma of the Spleen: Computed Tomography Imaging Characteristics in 5 Patients. J Comput Assist Tomogr. 2018;42:399-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 48. | Li XQ, Cheuk W, Lam PW, Wang Z, Loong F, Yeong ML, Browett P, McCall J, Chan JK. Inflammatory pseudotumor-like follicular dendritic cell tumor of liver and spleen: granulomatous and eosinophil-rich variants mimicking inflammatory or infective lesions. Am J Surg Pathol. 2014;38:646-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 49. | Chen Y, Shi H, Li H, Zhen T, Han A. Clinicopathological features of inflammatory pseudotumour-like follicular dendritic cell tumour of the abdomen. Histopathology. 2016;68:858-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |