Published online Oct 28, 2020. doi: 10.13105/wjma.v8.i5.375
Peer-review started: October 7, 2020
First decision: October 22, 2020
Revised: October 26, 2020
Accepted: October 27, 2020
Article in press: October 27, 2020
Published online: October 28, 2020
Processing time: 20 Days and 20.1 Hours
Pheochromocytomas are tumors arising from the chromaffin cell of the adrenal gland and paragangliomas as tumors from extra-adrenal sympathetic chromaffin cells. The combined yearly incidence of pheochromocytoma and paraganglioma (PPGL) is approximately 0.8 per 100000 person/year. Malignant pheochro-mocytoma is defined only by the presence of metastasis, as there is no confirmatory histology or biomarkers. The most common metastatic sites of these chromaffin tumors are the lymph node, bone, lungs, and liver. This review focuses on relevant clinical and immunohistological factors that are predictive of malignant PPGL or metastasis and determinants of prognosis. Findings showed that the risk of malignant PPGL, along with disease survival, is closely associated with age, primary tumor size, gender, synchronous metastasis, and absence of surgical excision. Other essential biomarkers or immunohistology investigated were galectin-3, COX-2, nm-23, microRNA-210, ERBB-2 overexpression and succinate dehydrogenase subunit mutation, which were predictive of malignancy as well as disease prognosis. Curative resection is possible but most metastatic diseases are amenable to radiopharmaceuticals and chemotherapy due to late presentation. Other therapeutic options, like molecular-targeted therapy, are still undergoing clinical trials.
Core Tip: The diagnosis of malignant pheochromocytoma/paraganglioma (PPGL) is challenging. To date, confirmatory histology and biomarkers are still lacking. Data from recent studies have shown various biomarkers and genetic mutations that are predictive of malignancy, metastasis and disease prognosis of PPGL, but primary tumor size, male sex and synchronous metastasis have revealed consistent association with malignant PPGL and its prognostication.
- Citation: Cassell III AK, Bague AH. Current trend in the diagnosis and management of malignant pheochromocytoma: Clinical and prognostic factors. World J Meta-Anal 2020; 8(5): 375-382
- URL: https://www.wjgnet.com/2308-3840/full/v8/i5/375.htm
- DOI: https://dx.doi.org/10.13105/wjma.v8.i5.375
The World Health Organization (WHO) in 2004 defined pheochromocytomas as tumors arising from the chromaffin cell of the adrenal gland and paragangliomas as tumors from extra-adrenal sympathetic chromaffin cells or parasympathetic nonchromaffin cells of the head and neck[1]. Pheochromocytoma and paraganglioma (PPGL) are histologically similar, with both secreting catecholamine (norepinephrine), but pheochromocytoma may also secrete epinephrine[2]. The combined yearly incidence of PPGL is approximately 0.8 per 100000 person/year[3]. Although the majority of PPGL are benign, about 10% to 31% are metastatic[3,4]. According to the WHO, malignant pheochromocytoma is defined only by the presence of metastasis[4].
Despite PPGL being mostly sporadic, nearly 40% are linked to somatic and/or germline mutations in at least 20 known susceptibility genes associated with the pathogenesis of these tumors[5]. PPGL can also occur with hereditary syndromes and genetic testing in patients may be required[5].
Genetically, PPGL with associated succinate dehydrogenase subunit B (SDHB) germline mutations may require a stringent follow-up, as the risk of malignancy is almost 40% and these tumors display aggressive behaviors[6]. Biochemical studies using plasma-free metanephrines or 24-h urine collection for fractionated metanephrines can rule-out PPGL in most instances.
Currently, no diagnostic molecular or histological marker exists for malignancy and most patients are diagnosed with malignant PPGL after they have developed unresectable disease and metastatic disease[7]. The most common metastatic sites of these chromaffin tumors are the lymph node, bone, lungs, and liver[8]. The 5-year survival without metastasis has been reported to be approximately 89.3%[8]. When metastasis is detected, the prognosis is usually poor, with a 5-year survival rate of only 60%[9]. Most of these cases then resort to systemic chemotherapy or radiopharm-aceutical agents, such as metaiodobenzylguanidine. These therapies lead mostly to partial radiographic response, disease stabilization, and symptomatic control in about 40% of cases[7,9].
This review has focused on relevant clinical and immuno-histological factors that are predictive of malignant PPGL or metastasis. The review also highlights essential biomarkers that are relevant to prognostication of malignant PPGL. A succinct overview of the current standard of care is provided in the Discussion.
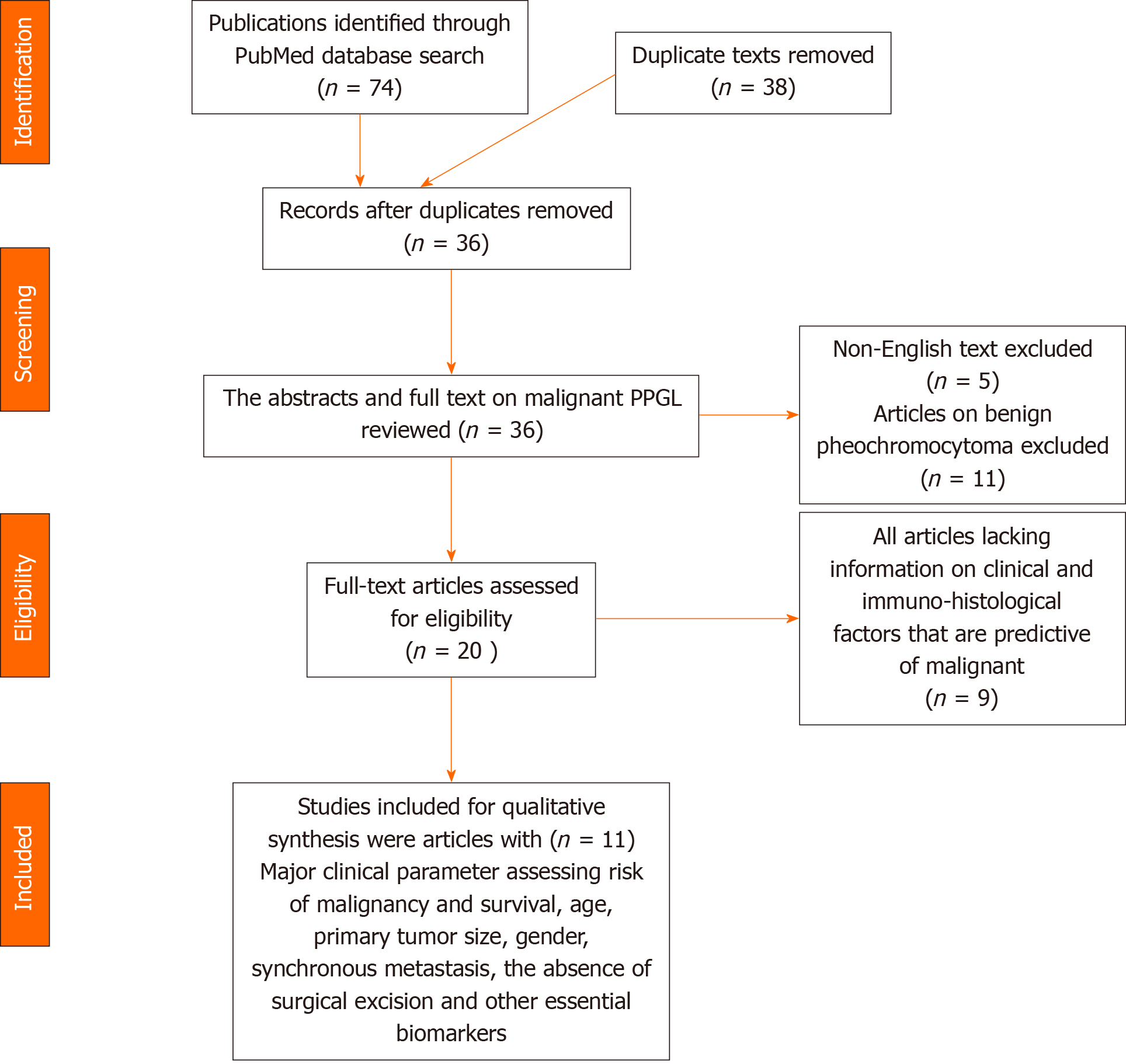
An extensive literature search was conducted from 2000 to 2020 using the PubMed database and search engine. The keyword utilized was “clinical and prognostic factors of malignant pheochromocytoma”. There were a total of 74 articles. The abstracts and full texts of 36 publications on malignant PPGL were reviewed after extraction of duplicate text.
After perusal of abstracts and full texts, 11 publications investigating the clinical parameters and biomarkers on the risk of malignancy, metastasis and prognosis were selected for qualitative and quantitative synthesis as projected in Table 1. All articles on benign pheochromocytoma exclusively were excluded from the study. All case reports and case series with less than 10 subjects were not eligible for inclusion.
| Ref. | Study duration and type | Study population | Study protocol/objective | Study outcome |
| Choi et al[10], 2015 | 1997-2013, retrospective | 345 pts | Prognostic factors associated with survival for PPGL | Poor survival: Older age and synchronous metastasis |
| De Wailly et al[11], 2012 | 1993-2009, retrospective | 53 pts | New immunohistologic elements correlating to malignant PPGL | Risk of malignancy/recurrence: Tumor necrosis, Ki-67 index > 4% and pS100 |
| Hamidi et al[12], 2017 | 1960-2016, retrospective | 272 pts | Epidemiology and survival outcome of malignant PPGL | MOS: 24.6 years, DSS: 33.7 years; poor survival: Older age at diagnosis, male sex, synchronous metastasis, larger tumor size, elevated dopamine |
| Mei et al[13], 2019 | 1973-2013, retrospective | 1014 pts | Survival pattern of malignant PPGL | Survival advantage: Younger age, female sex, surgical resection and origin from of aortic/carotid bodies |
| Ruff et al[14], 2019 | Prospective | 35 pts | Serum miR-210 levels as a biomarker of malignancy | Lower serum miR-210 expression level and larger primary tumor size strongly correlated with malignant disease |
| Zelinka et al[15], 2011 | Retrospective | 41 pts | Biochemical and clinical outcome of malignant PPGL | Metastatic pheochromocytoma presented at a significantly younger age, with larger tumor, tumor necrosis and more frequent secreted norepinephrine |
| Hamidi et al[16], 2017 | Systemic review and meta-analysis of 20 studies | 1338 pts | Baseline characteristics and outcome of patients with malignant PPGL | 5-year and 10-year mortality rates of 37% (95%CI: 24%-51%) and 29% (95%CI: 17%-42%), respectively; male sex and synchronous metastases were associated with higher mortality |
| Saffar et al[17], 2011 | 1986-2006, retrospective | 51 pts | Galectin-3, COX-2, and nm-23 to discriminate benign disease from malignancy | Negative nm-23, along with positive galectin-3 or COX-2 were predictive of malignancy, with a sensitivity of 100% |
| Suh et al[18], 2017 | 1988-2013, retrospective | 176 pts | Variations in genomic expressions and mutations of malignant PPGL | mRNA expression of malignant PPGL was up-regulated in five pathways; disease-free survival rates were closely related the presence or absence of mutations |
| Szalat et al[19], 2011 | 1980-2008, retrospective | 16 pts | Predictive features of malignancy in PPGL | High levels of chromogranin A at the time of diagnosis were associated with malignancy; 10-year survival rate after initial diagnosis was 50% and 25% after metastasis |
| Zhong et al[20], 2017 | 2002-2014, retrospective | 414 pts | Clinical significance of biomarker nomogram in detecting malignancy and rate of metastasis | Tumor size, tumor location, vascular invasion, ERBB-2 overexpression and SDHB mutation were independent predictors of malignancy and metastasis |
A total of 11 articles met the desired objective for further qualitative analysis. All studies with research methodology emphasizing clinical and immuno-histological factors that are predictive of malignant PPGL or metastasis were selected (Figure 1).
The selected articles were reviewed for study type and duration, study population, study protocol/objectives, clinical parameters, biomarker, and study outcome. The retrieved information was analyzed and reflected in the main text of the Results and Discussion.
The term “pheochromocytoma and paraganglioma” was used concurrently and abbreviated as PPGL.
A total of 11 studies[10-20] were synthesized (1 systematic review, 1 meta-analysis, 1 prospective study, and 9 retrospective studies) to include 3755 patients with malignant PPGL. The various objectives outlined essential clinical factors and immuno-histological factors that are predictive of malignant PPGL or metastasis. The major clinical parameters assessed for risk of malignancy and survival were age, primary tumor size, gender, synchronous metastasis, and absence of surgical excision. Other essential biomarkers/immunohistology features investigated were galectin-3, COX-2, nm-23, microRNA-210 (miR-210), and ERBB-2 overexpression, and SDHB mutation.
A retrospective study by Choi et al[10] of 345 patients treated for PPGL evaluating prognostic factors associated with survival for PPGL showed that the median survival was 7.2 years, with a 5-year survival rate of 75.4%. Univariate analysis revealed that older age (> 45 years), larger tumor size (> 6 cm), synchronous metastasis and lack of surgical excision correlated with poor survival. Further multivariate analysis showed that older age and synchronous metastasis were associated with poor overall survival.
De Wailly et al[11], in a review of 53 patients operated for PPGL, assessed for new immunohistological elements correlating to malignant pheochromocytoma and the findings indicated that the size and weight of pheochromocytoma were directly related to the Pheochromocytoma of Adrenal gland Scaled Score and malignancy[11]. The presence of tumor necrosis, Ki-67 index > 4%, and absence of pS100 indicated the need for close follow-up, as there was a high risk of malignancy or recurrence.
A retrospective review of 272 patients with malignant PPGL by Hamidi et al[12] assessing survivor outcome and predictors of shorter survivors displayed that the median overall survival was 24.6 years and disease-specific survival was 33.7 years. Older age at diagnosis, male sex, synchronous metastasis, larger tumor size, elevated dopamine, and not undergoing primary tumor resection were associated with shorter survival.
Review of the Surveillance, Epidemiology, and End Results program and Cancer Genome Atlas (commonly known as TCGA) database (data from 1973 to 2013) with 1014 patients to analyze epidemiology and survival pattern of malignant PPGL found that younger age, female sex, surgical resection, and origin from of aortic/carotid bodies conferred remarkable survival advantage[13]. Opposingly, distant metastasis was associated with a worse prognosis. ATRX was the most common oncogenic mutation in the metastatic group, while SHDB was higher among males.
A prospective study by Ruff et al[14] of 35 patients investigating serum miR-210 levels as a biomarker of malignancy revealed that the most common germline mutation involved SDHB[14]. Univariate analysis showed that lower serum miR-210 expression level and larger primary tumor size strongly correlated with malignant disease.
Zelinka et al[15] reported from a retrospective review investigating biochemical and clinical outcome of 41 cases of malignant pheochromocytoma to 108 cases of benign disease that patients with metastatic pheochromocytoma presented at a significantly younger age, with larger tumor, and more often secreted norepinephrine compared to benign pheochromocytomas.
The histological marker of potential malignancy of tumor necrosis was more consistent with metastatic pheochromocytoma. The median period to the development of metastasis from initial diagnosis was 3.6 years.
Results from a systematic review and meta-analysis of 20 retrospective studies of 1338 patients with malignant PPGL by Hamidi et al[16] showed that 40.4% of patients had synchronous metastasis. The 5-year and 10-year mortality rates were 37% (95% confidence interval (CI): 24%-51%) and 29% (95%CI: 17%-42%), respectively. Male sex and synchronous metastases were associated with higher mortality.
Retrospective data from Saffar et al[17] involving 55 cases of pheochromocytoma determining the concurrent expression of galectin-3, COX-2, and nm-23 to discriminate benign disease from malignancy showed that negativity for nm-23 along with positivity for galectin-3 or COX-2 were predictive of malignancy, with a sensitivity of 100%. Meanwhile, positivity of nm-23 along with dual negativity of COX-2 and galectin-3 showed a benign disease, with a 100% sensitivity.
A multigenomic analysis of data collected from 1988 to 2013 involving 176 patients by Suh et al[18] assessing the variations in genomic expressions and mutations of malignant PPGL with TCGA found that mRNA expression of malignant PPGL was up-regulated in five pathways, namely cell cycle (BUB1, BUB1B, CCNB2, CDC2, ESPL1), gap junction (CDC2, PRKCB1), calcium signaling (CCNB2, CDC2, PRKCB1), regulation of actin cytoskeleton (DIAPH3, FGF18, IQGAP3), and phosphatidylinositol (PRKCB1, TTK). Disease-free survival rates were closely related to the presence or absence of mutations, such as RP11-798G7.5, HERC2, SETD2, TGDS, TRHDE, FKBP9, and BMS1.
Data from Szalat et al[19] in a retrospective study of 16 patients from 1980 to 2008 identifying the predictive features of malignancy in PPGL displayed that high levels of chromogranin A at the time of diagnosis was associated with malignancy, metastasis and poor prognosis. The 10-year survival rate after initial diagnosis was 50% and 25% after metastasis is found.
A retrospective study by Zhong et al[20] randomly assigned 414 patients with PPGL to a training or validation group investigating the clinical significance of biomarker nomogram in detecting malignancy and rate of metastasis. The overall rate of metastasis was 10.6%. Univariate analysis revealed that primary tumor size, tumor location, vascular invasion, capsular invasion, ERBB-2 overexpression, and SDHB mutation were remarkably associated with malignancy[20]. Multivariate logistic regression analysis showed that tumor size, tumor location, vascular invasion, ERBB-2 overexpression, and SDHB mutation were independent predictors of malignancy and metastasis. Further analysis revealed that biomarker-based nomogram was useful for assessing the risk of metastasis compared to nomogram without biomarkers (ERBB-2 overexpression and SDHB mutation).
It has been proven that measurement of metanephrine is superior to other routine catecholamines and metabolites, like vanillylmandelic acid; therefore, the measurement of 24-h plasma metanephrine or fractionated urinary metanephrine is acceptable[21].
The role of imaging is to evaluate primary tumor, assess multifocality, and identify metastasis. Computed tomography scan and magnetic resonance imaging (commonly known as MRI) are ideal for primary tumors, with a sensitivity of 98%-100% for adrenal lesions[21]. MRI is more sensitive for extra-adrenal paraganglioma, with sensitivities of 93% vs 90% respectively.
Metaiodobenzylguanidine scintigraphy (I131/I123 MIBG) has been used for decades to image neuroendocrine tumors because the uptake represents adrenergic innervation and catecholamine excretion. In patients with large tumors, inherited tumors or multifocal tumors, conventional imaging along with I123 MIBG or 18F-fluorodeoxyglucose positron emission tomography can be used to detect metastases[22].
The American Joint Committee on Cancer has recently developed a staging criterion for malignant PPGL using the tumor-node-metastasis classification. The classification was based on certain parameters that allow the size of the primary tumor to reliably predict the presence of malignancy and shorter survival[23]. Patients with primary PPGL lager than 5 cm have a shorter overall survival than patients with smaller tumors[23]. Therefore, a pheochromocytoma lesser than 5 cm is stage as T1. Tumors that are larger than 5 cm are staged T2. Pheochromocytomas that invade surrounding structures, like the liver or kidney, requiring extensive surgery are staged T3.
Treatment for malignant PPGL is palliative; therefore, a shared decision-making is required for the patient’s quality-of-life. Concerns are focused on the tumor growth, tumor hypersecretion, or the intervention itself. Bony metastasis to the spine is usually painful, with additional risk of spinal cord compression. Therefore, treatment of pain with nonsteroidal anti-inflammatory, analgesics, bisphosphonates, and radiotherapy are all viable options.
Patients with malignant PPGL may already present with cardiovascular complications from excess catecholamine, like acute adrenergic cardiomyopathy, arrhythmia, myocardial infarction, stroke, and sudden death[22,24]. For patients who may require surgery, preoperative control of blood pressure and adrenergic outflow is crucial to prevent adverse events. The first pharmaceutical agent of choice is an alpha-blocker preferably, either noncompetitive (phenoxybenzamine) or competitive (doxazosin, prazosin, and terazosin)[25,26]. Beta-blockers may be needed to prevent or ameliorate reflex tachycardia or orthostatic hypotension associated with the alpha-blocker. If the pressure is not controlled by an alpha- or beta-blocker, calcium channel inhibitor or metyrosine are alternatives[26]. Liberal salt and fluid intake are encouraged to promote intravascular expansion.
The only definitive treatment for PPGL is surgical resection. Though surgery may not be curative in most scenarios, it offers long-term disease remission in some patients. Patients with malignant PPGL and isolated metastasis can have a better result with surgery. In patients with incurable disease, resection can reduce the catecholamine hypersecretion and avoid complication of tumoral compression on vital organs[21,22]. Accurate preoperative imaging is crucial to assess the vasculature of the tumor. The tumor should be dissected without capsular infraction. It is advised that the veins be ligated first, to prevent excess catecholamine release into circulation. If regional lymph nodes are assessed preoperative or intraoperatively, a regional lymphadenectomy should be performed[21,22].
I123 MIBG is frequently being investigated as a treatment option for metastatic PPGL. It is recommended as the first-line treatment in patients with slow-growing I123 MIBG-positive metastasis. Treatment can be administered as both fractionated low dose and high dose; both are shown to have considerable efficacy[27]. A systematic review and meta-analysis of 17 studies involving 243 patients with malignant PPGL evaluating the effect of I131 MIBG therapy on tumor volume in patients with malignant PPGL showed that the complete response was 3%, partial response was 27% and stable disease was 52%[28]. Separate analysis showed better hormonal response among paraganglioma than pheochromocytoma.
Chemotherapy may have a role in the management of malignant PPGL. The best recommended chemotherapeutic protocol includes cyclophosphamide, vincristine, and dacarbazine (CVD) administered as (cyclophosphamide 750 mg/m2, vincristine 1.4 mg/m2, and dacarbazine 600 mg/m2 on day 1 and dacarbazine 600 mg/m2 on day 2). A systematic review and meta-analysis of four studies, by Niemeijer et al[29], using chemotherapy with CVD involving a pool of 50 patients with PPGL revealed varying response of malignant PPGL to CVD[29]. The pooled percentages were 4% for complete response (95%CI: 1%-15%), 37% for partial response (95%CI: 25%-51%) and 14% for stable disease (95%CI: 7%-27%). Sub analysis of two studies also showed varying response of catecholamine excess, with complete response in 14% (95%CI: 6%-30%), partial response in 40% (95%CI: 25%-57%), and stable hormonal response in 20% (95%CI: 10%-36%)[29].
Molecular-targeted therapies, like receptor tyrosine kinase inhibitors, mTORC1 inhibitors, HIF-2α antagonist and SSTR2 analogues, are still investigational[3,7]. Receptor tyrosine kinase inhibitors like unitinib, cabozantinib, axitinib and pazopanib are considered to have potential therapeutic benefits and still part of ongoing clinical trials[7,9,30].
The diagnosis of malignant PPGL is challenging. To date, confirmatory histology and biomarkers are still lacking. The presence of metastasis is still being considered as a reference for malignancy. Data from recent studies have shown various biomarkers and genetic mutations that are predictive of malignancy, metastasis and disease prognosis of PPGL. Biomarkers like galectin-3, COX-2, nm-23, miR-210, ERBB-2 overexpression and SDHB mutation have shown correlation to malignant PPGL and disease prognosis. However, primary tumor size, male sex and synchronous metastasis have revealed consistent association with malignancy PPGL and prognostication.
Most malignant PPGL may not be amenable or curable with surgical excision. As such, radiopharmaceuticals and chemotherapy are alternative treatment options. Other therapeutic options, like molecular-targeted therapy, are still undergoing clinical trials.
Special thanks to my wife Lydia Olasupo Cassell.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: The International College of Surgeons (Fellow), No. C21329; The American Urological Association (Member), No. 01034597; The American College of Surgeon (Associate Fellow), No. 03338579; and The Faculty of Surgical Trainer-The Royal College of Surgeon of Edinburgh (Member), No. 191761.
Specialty type: Oncology
Country/Territory of origin: Senegal
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): E
P-Reviewer: Liu Y, Ullah M S-Editor: Wang JL L-Editor: Filipodia P-Editor: Li JH
| 1. | Angelousi A, Kassi E, Zografos G, Kaltsas G. Metastatic pheochromocytoma and paraganglioma. Eur J Clin Invest. 2015;45:986-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 2. | Guo Z, Lloyd RV. Pheochromocytomas and Paragangliomas: An Update on Recent Molecular Genetic Advances and Criteria for Malignancy. Adv Anat Pathol. 2015;22:283-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Corssmit EPM, Snel M, Kapiteijn E. Malignant pheochromocytoma and paraganglioma: management options. Curr Opin Oncol. 2020;32:20-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 4. | Grogan RH, Mitmaker EJ, Duh QY. Changing paradigms in the treatment of malignant pheochromocytoma. Cancer Control. 2011;18:104-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (1)] |
| 5. | Hamidi O. Metastatic pheochromocytoma and paraganglioma: recent advances in prognosis and management. Curr Opin Endocrinol Diabetes Obes. 2019;26:146-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | Henry JF, Peix JL, Kraimps JL. Positional statement of the European Society of Endocrine Surgeons (ESES) on malignant adrenal tumors. Langenbecks Arch Surg. 2012;397:145-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Jimenez C, Rohren E, Habra MA, Rich T, Jimenez P, Ayala-Ramirez M, Baudin E. Current and future treatments for malignant pheochromocytoma and sympathetic paraganglioma. Curr Oncol Rep. 2013;15:356-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 125] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 8. | Korevaar TI, Grossman AB. Pheochromocytomas and paragangliomas: assessment of malignant potential. Endocrine. 2011;40:354-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 9. | Jimenez P, Tatsui C, Jessop A, Thosani S, Jimenez C. Treatment for Malignant Pheochromocytomas and Paragangliomas: 5 Years of Progress. Curr Oncol Rep. 2017;19:83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 10. | Choi YM, Sung TY, Kim WG, Lee JJ, Ryu JS, Kim TY, Kim WB, Hong SJ, Song DE, Shong YK. Clinical course and prognostic factors in patients with malignant pheochromocytoma and paraganglioma: A single institution experience. J Surg Oncol. 2015;112:815-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | de Wailly P, Oragano L, Radé F, Beaulieu A, Arnault V, Levillain P, Kraimps JL. Malignant pheochromocytoma: new malignancy criteria. Langenbecks Arch Surg. 2012;397:239-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 12. | Hamidi O, Young WF Jr, Iñiguez-Ariza NM, Kittah NE, Gruber L, Bancos C, Tamhane S, Bancos I. Malignant Pheochromocytoma and Paraganglioma: 272 Patients Over 55 Years. J Clin Endocrinol Metab. 2017;102:3296-3305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 235] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 13. | Mei L, Khurana A, Al-Juhaishi T, Faber A, Celi F, Smith S, Boikos S. Prognostic Factors of Malignant Pheochromocytoma and Paraganglioma: A Combined SEER and TCGA Databases Review. Horm Metab Res. 2019;51:451-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 14. | Ruff SM, Ayabe RI, Malekzadeh P, Good ML, Wach MM, Gonzales MK, Tirosh A, Nilubol N, Pacak K, Kebebew E, Patel D. MicroRNA-210 May Be a Preoperative Biomarker of Malignant Pheochromocytomas and Paragangliomas. J Surg Res. 2019;243:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Zelinka T, Musil Z, Dušková J, Burton D, Merino MJ, Milosevic D, Widimský J Jr, Pacak K. Metastatic pheochromocytoma: does the size and age matter? Eur J Clin Invest. 2011;41:1121-1128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Hamidi O, Young WF Jr, Gruber L, Smestad J, Yan Q, Ponce OJ, Prokop L, Murad MH, Bancos I. Outcomes of patients with metastatic phaeochromocytoma and paraganglioma: A systematic review and meta-analysis. Clin Endocrinol (Oxf). 2017;87:440-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 84] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 17. | Saffar H, Sanii S, Heshmat R, Haghpanah V, Larijani B, Rajabiani A, Azimi S, Tavangar SM. Expression of galectin-3, nm-23, and cyclooxygenase-2 could potentially discriminate between benign and malignant pheochromocytoma. Am J Clin Pathol. 2011;135:454-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Suh YJ, Choe JY, Park HJ. Malignancy in Pheochromocytoma or Paraganglioma: Integrative Analysis of 176 Cases in TCGA. Endocr Pathol. 2017;28:159-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Szalat A, Fraenkel M, Doviner V, Salmon A, Gross DJ. Malignant pheochromocytoma: predictive factors of malignancy and clinical course in 16 patients at a single tertiary medical center. Endocrine. 2011;39:160-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Zhong X, Ye L, Su T, Xie J, Zhou W, Jiang Y, Jiang L, Ning G, Wang W. Establishment and evaluation of a novel biomarker-based nomogram for malignant phaeochromocytomas and paragangliomas. Clin Endocrinol (Oxf). 2017;87:127-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Lowery AJ, Walsh S, McDermott EW, Prichard RS. Molecular and therapeutic advances in the diagnosis and management of malignant pheochromocytomas and paragangliomas. Oncologist. 2013;18:391-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Plouin PF, Fitzgerald P, Rich T, Ayala-Ramirez M, Perrier ND, Baudin E, Jimenez C. Metastatic pheochromocytoma and paraganglioma: focus on therapeutics. Horm Metab Res. 2012;44:390-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 23. | Roman-Gonzalez A, Jimenez C. Malignant pheochromocytoma-paraganglioma: pathogenesis, TNM staging, and current clinical trials. Curr Opin Endocrinol Diabetes Obes. 2017;24:174-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 24. | Almeida MQ, Bezerra-Neto JE, Mendonça BB, Latronico AC, Fragoso MCBV. Primary malignant tumors of the adrenal glands. Clinics (Sao Paulo). 2018;73:e756s. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Harari A, Inabnet WB 3rd. Malignant pheochromocytoma: a review. Am J Surg. 2011;201:700-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 26. | Naranjo J, Dodd S, Martin YN. Perioperative Management of Pheochromocytoma. J Cardiothorac Vasc Anesth. 2017;31:1427-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 121] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 27. | Nölting S, Grossman A, Pacak K. Metastatic Phaeochromocytoma: Spinning Towards More Promising Treatment Options. Exp Clin Endocrinol Diabetes. 2019;127:117-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 28. | van Hulsteijn LT, Niemeijer ND, Dekkers OM, Corssmit EP. (131)I-MIBG therapy for malignant paraganglioma and phaeochromocytoma: systematic review and meta-analysis. Clin Endocrinol (Oxf). 2014;80:487-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 133] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 29. | Niemeijer ND, Alblas G, van Hulsteijn LT, Dekkers OM, Corssmit EP. Chemotherapy with cyclophosphamide, vincristine and dacarbazine for malignant paraganglioma and pheochromocytoma: systematic review and meta-analysis. Clin Endocrinol (Oxf). 2014;81:642-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 149] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 30. | Khatami F, Mohammadamoli M, Tavangar SM. Genetic and epigenetic differences of benign and malignant pheochromocytomas and paragangliomas (PPGLs). Endocr Regul. 2018;52:41-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |