Published online Oct 28, 2020. doi: 10.13105/wjma.v8.i5.348
Peer-review started: July 30, 2020
First decision: September 13, 2020
Revised: September 26, 2020
Accepted: October 26, 2020
Article in press: October 26, 2020
Published online: October 28, 2020
Processing time: 89 Days and 22.7 Hours
Originating from Wuhan in China, coronavirus disease 2019 (COVID-19) spread globally within months and was declared a pandemic by World Health Organization in March 2020, making it one of the biggest healthcare calamities of our time. As more data on COVID-19 infection became available, what was initially thought to be a simple respiratory illness was found to be more complex. Many extra-pulmonary manifestations are now frequently reported for COVID-19 in available literature, most commonly gastrointestinal and hepatopancreato-biliary manifestations. Due to early scarcity of data, extra pulmonary manifestations were initially overlooked and may have contributed to nosocomial spread of the infection. Practitioners, especially gastroenterologists, who frequently encounter patients with these symptoms, need to be aware of them. This can not only help minimize the nosocomial spread, ensure safety of provider but also help conserve already stretched-thin healthcare resources. A tremendous amount of COVID-19 related literature is getting added to the growing pool every day, making it difficult for providers to follow. The aim of our review is to summarize the available evidence for gastrointestinal and hepatopancreatobiliary manifestations of COVID-19. We here briefly discussed the possible pathophysiologic mechanism for these manifestations and summarized the recommendations put forward by multiple gastrointestinal societies regarding safe and effective clinical practice during the ongoing pandemic.
Core Tip: This review is to summarize the available evidence for extrapulmonary manifestations of coronavirus disease 2019 infection, particularly emphasizing on gastrointestinal and hepatopancreatobiliary manifestations. Here we briefly discussed the possible pathophysiologic mechanism for the disease, and summarized the recommendations of multiple gastrointestinal societies regarding safe and effective clinical practice during the ongoing pandemic. Lastly, we've touched briefly upon management of chronic liver disease and transplant patients.
- Citation: Pasha SB, Swi A, Hammoud GM. Gastrointestinal and hepatic manifestations of COVID-19 infection: Lessons for practitioners. World J Meta-Anal 2020; 8(5): 348-374
- URL: https://www.wjgnet.com/2308-3840/full/v8/i5/348.htm
- DOI: https://dx.doi.org/10.13105/wjma.v8.i5.348
The pathogen responsible for a mysterious cause of pneumonia, linked to a seafood market in Wuhan, China, first reported in early December 2019, was later identified as a novel coronavirus by the Chinese Center for Disease Control (CDC)[1,2]. Based on phylogenetics, the Coronavirus Study Group of the International Committee on Taxonomy of Viruses named it the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) and the disease was later named coronavirus disease 2019 (COVID-19) by the World Health Organization (WHO)[3-5]. Within months, the disease spread across the globe like wildfire infecting many and was declared a pandemic by the WHO on March 11, 2020[6].
This is not the first time that a CoV has caused a global health crisis. Just within the last two decades, the SARS- and MERS-CoVs had been responsible for two major viral outbreaks, causing the death of 774 and 838, respectively[7-9]. This number in comparison to the ongoing pandemic appears to be miniscule. Within a period of nine months, the SARS-CoV-2 has managed to infect more than 28 million people and has nearly claimed a million lives[10]. The ongoing pandemic has left the global economies shattered and health care systems exhausted. The current pandemic has reached a level not seen since the Spanish flu of 1918 and will go down the history books as the worst healthcare crisis experienced by mankind in the 21st century.
Early in the pandemic, COVID-19 was thought be an exclusively respiratory illness. However, as the number of cases grew and reports of numerous extra pulmonary and atypical presentations emerged, it became apparent that COVID-19, is much more than a simple case of viral pneumonia.
Typical symptoms of COVID-19 include fever, cough, dyspnea, fatigue which are accompanied by radiographic evidence of pneumonia[11]. Additionally, a wide range of extra-pulmonary symptoms, including gastrointestinal (GI) and hepatic dysfunction are frequently reported. The presence of viral RNA in fecal samples from COVID-19 patients also raise concern for fecal-oral transmission in addition to the already established droplet and airborne routes[11].
GI symptoms of COVID-19 may not only precede the typical respiratory manifestations but can sometimes be the only presenting symptom[12]. Interestingly, the first reported case of COVID-19 in the US developed GI symptoms such as vomiting and diarrhea prior to the onset of respiratory symptoms[11]. The predominant focus on the respiratory aspect of the disease can allow these atypical presenters to slip through the cracks. This can potentially result in significant exposure and increased risk of transmission in unsuspecting healthcare workers (HCWs). Wang et al[13]’s study had particularly highlighted this concern. They reported isolated GI symptoms in around 10% of cases and mentioned of one patient who was overlooked and admitted under a surgical specialty. This single patient was inadvertently responsible for infecting more than 10 HCWs[13].
Thus, it is important for clinicians, especially gastroenterologists to be aware of these atypical and often missed presentations of COVID-19. This will not only serve to mitigate the spread but also help reduce clinician’s anxiety during this pandemic.
Before moving on to these extra-pulmonary manifestations of COVID-19, a brief overview of SARS-CoV-2 would better serve the ensuing discussion. Ranging from 60 to 140 nm in diameter, coronaviruses are one of the largest, single-stranded RNA viruses known. Around 39 different types of coronaviruses have been identified since they were first discovered[11,14-16].
Most of them are zoonotic and their natural hosts include mammals and birds[17-20]. They are responsible for a multitude of diseases involving respiratory, enteric and other organ systems in their hosts[21,22]. Majority of coronaviruses that are capable of infecting humans have been thought to originate from an inter-specie crossover event between animals and humans[2].
SARS-CoV-2 is the seventh and the newest member of human coronaviruses, which also include OC43-, 229E-, NL63-, HKU1-, SARS- and MERS -CoVs[23,24]. The former four are endemic and have been responsible for common seasonal upper respiratory tract illnesses, while the latter two and the SARS-CoV-2, cause severe pneumonia outbreaks which can lead to multi organ failure and death[25].
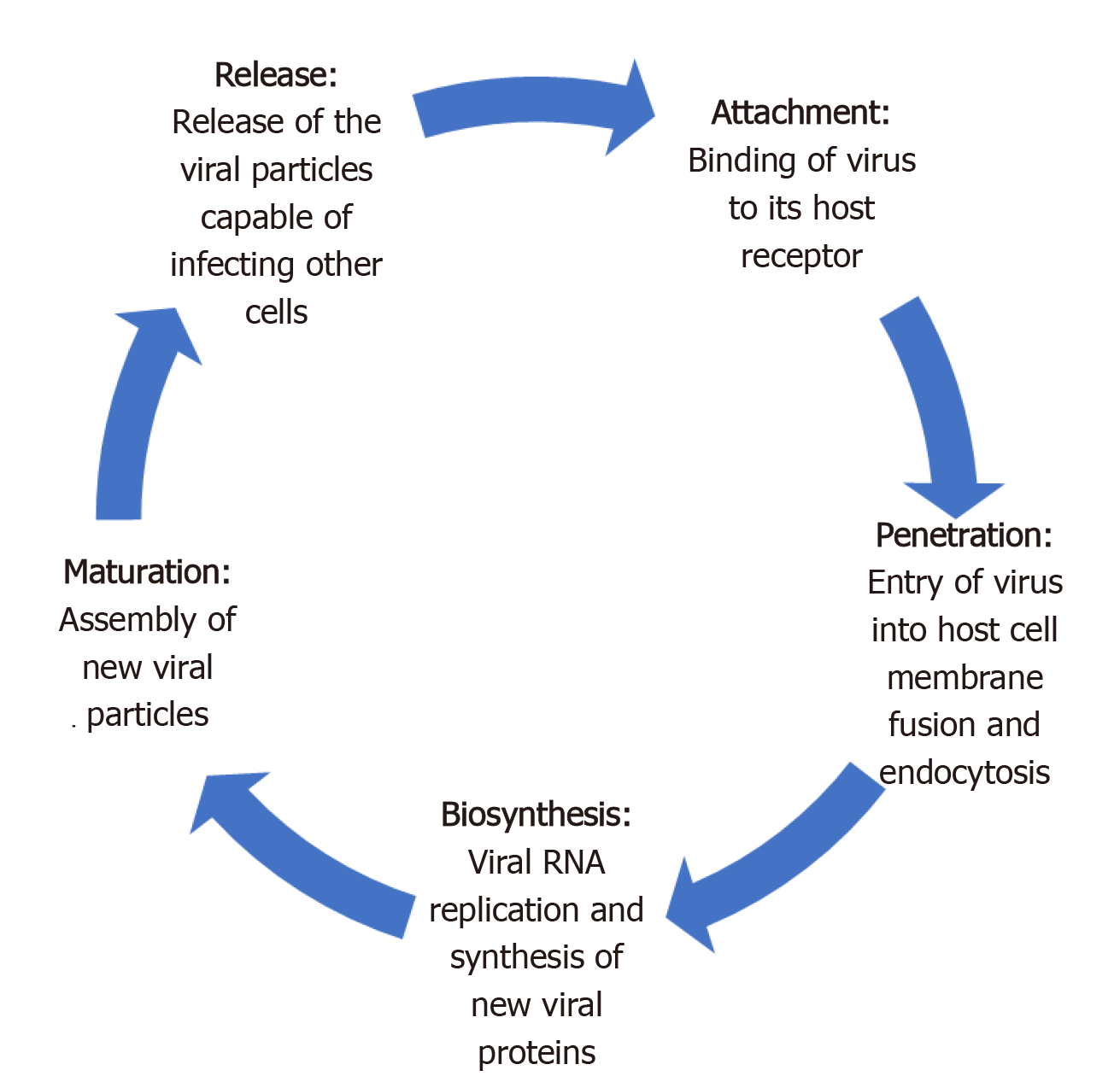
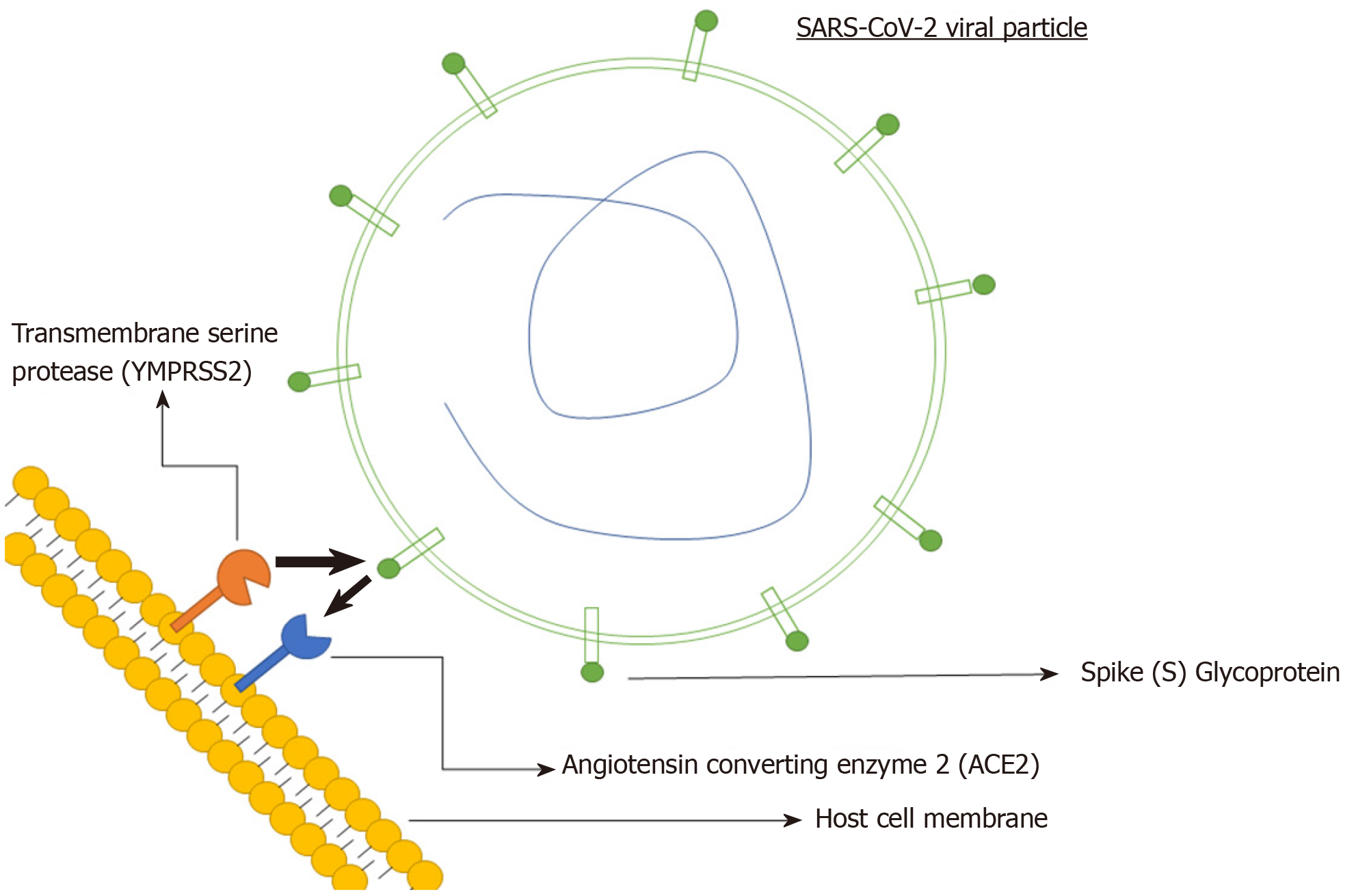
Like most viruses, there are five key steps in the life cycle of coronaviruses. These include attachment, penetration, biosynthesis, maturation and release (Figure 1)[26]. Attachment and penetration are crucial steps which rely on receptor recognition and subsequent downstream processes allowing virus to gain entry into host cell (Figure 2)[27]. The structural transmembrane glycoprotein called Spike (S) is important for this process. Functionally it consists of two subunits, S1 and S2, which after a host protease dependent activation, allows the virus to bind to its specific receptor and the host-virus membrane fusion to take place[26]. In the case of SARS-CoV-2, the target receptor is the Angiotensin- converting enzyme 2 (ACE2) receptor and the host protease required for S glycoprotein activation is identified as a transmembrane serine protease called TMPRSS2[28-33].
The degree to which the virus specific receptor is expressed on a host cell determines viral tropism[34]. The high expression of SARS-CoV-2’s target receptor, the ACE2 in lungs and distal airway makes them an easy target for SARS-CoV-2 and explain the predominant respiratory nature of the disease[35]. However, this ACE2 receptor is not exclusive to pulmonary tissues and is widely expressed across a broad range of human tissues. They are abundant throughout the glandular epithelial cells of the GI tract, cholangiocytes, proximal renal tubules among many others[12,36-38]. A lot of these cell types also have high co-expression of TMPRSS2, which makes them a target for SARS-CoV-2[39,40].
Another important concept is the affinity of a virus for its specific receptor. This not only is an important determinant of its successful replication, but also demonstrates a correlation with transmissibility and disease severity. The evidence for this comes from an observation made during the 2002-03 SARS pandemic. Compared to the earlier 2002 phase, a lower pathogenicity and transmissibility was seen with the 2003 re-emergence phase which also co-related with a lower viral affinity for its receptor[27]. The role of host proteases such as the TMPRSS2 is also important in this regard. Not only can they facilitate viral penetrance but can also enhance it. This can allow the virus to infect cells with relatively low ACE2 expression, which would otherwise not be possible[30]. The binding affinity of SAR-CoV-2 is 10 to 20-fold greater than that of SARS-CoV[41]. This can not only explain its higher transmissibility but also the involvement of multiple organ systems in COVID-19.
The innate immune system consists of epithelial cells, dendritic cells and macrophages[35]. These cells not only serve as the first line of defense, but by the process of antigen presentation also activate the adaptive arm of immune system. Effectors cells of adaptive immunity are CD4 and the CD8 T-Cells. By activating B-Cells, the CD4 T-cells also activate humoral immunity, while CD8 T-cells directly kill the virus infected cell[26]. This is body’s natural immune response to any foreign pathogen. However, in a subset of COVID-19 patients, overactivation of this normal immune response can occur. This results in severe manifestations of the disease including acute respiratory distress syndrome and multi-organ failure. Higher levels of various pro-inflammatory cytokine, including IL-6, IL-10, granulocyte-colony stimulating factor, monocyte chemoattractant protein 1, macrophage inflammatory protein 1 alpha and tumor necrosis factor (TNF) - alpha is often seen in such patients. This phenomenon is also called ‘cytokine storm’[42-44]. The levels of these pro-inflammatory cytokines correlates with disease severity[26]. Thus an aberrant immune response is the underlying mechanism for severe COVID-19.
Case fatality rate (CFR), which is a crude way to assess mortality has demonstrated tremendous regional and demographic variability for COVID-19[45]. While a mild disease phenotype is reported for the majority (80%), epidemiological studies have indicated a higher mortality in the elderly (> 60 years)[46].
Initial studies from China estimated a CFR between 1.36% and 33% and reported majority (80%) of deaths in the elderly[42,47-50]. The official numbers later released by the Chinese government reported it at 3.17%[51]. The US CDC not only reported variable CFRs across different age groups but also found a positive correlation between older age and rates of hospitalization[46]. CFRs ranged from 0 in < 19 years to up to 10%-27% in > 85 years[46]. Similarly, the rates of hospitalization also ranged between 2%-3% in <19 years to greater than 31% in > 85 years[46]. The worldwide CFR for COVID-19 for all age groups currently stands at approximately 3% but can range from as high as 12% in Italy to < 0.1% in Singapore[45].
Some authors have attributed the observed regional differences in mortality to their respective demographic characteristics. For example, the abnormally high CFR reported from Italy was thought to be related to its relatively older population[26]. While the morbidity and mortality of COVID-19 is almost certainly higher in older age groups, it is important to be aware that severe disease can occur in any age group[46].
Compared to its predecessors, the SARS-CoV (approximately 10%) and MERS-CoV (34%-37%), the mortality rate for COVID-19 appears to be substantially low[45,52,53]. But longer incubation period and sustained viral shedding during pre-symptomatic phase have enabled a much more efficient spread for SARS-CoV-2[53,54]. The estimated reproduction number for SARS-CoV-2 ranges from 2.24 to 3.58, which is higher than that of its predecessor[55,56]. The mean incubation period for SARS-CoV-2 is 5.2 days (95%CI: 4.1-7.0)[57], but the incubation period can generally range from 1 to 14 d[16,56,58].
A direct person-to-person transmission by respiratory droplets is the predominant route of transmission for COVID-19[59]. This can occur during coughing, sneezing, or normal breathing. Droplets generated via these routes can travel up to six feet and can remain suspended in air for up to three hours[60]. Aerosol transmission during aerosol generating procedures, such as endotracheal intubation and nebulization can also occur[61]. Indirect transmission via fomites is another potential mode of transmission. Viral particles can remain viable on different surfaces (plastic, steel etc.) for up to a few days[62]. Viral particles have also been detected in samples from toilet bowl and sink used by COVID-19 patients[63]. A cluster of COVID-19 cases associated with a shopping mall in Wenzhou, China was thought to be linked to a common restroom[64].
During the SARS outbreak of 2003, the SARS-CoV particles were detected in the GI biopsy and stool specimens, indicating potential for fecal-oral transmission and GI troposim[65]. Based on shared homology, the same was speculated for SARS-CoV-2, which was afterwards confirmed by the presence of fecal viral RNA and nucleocapsid proteins in the cytoplasm of GI epithelial cells on autopsy[66,67]. Around 36% to 53% of stool samples can test positive for SARS-CoV-2 viral RNA[38]. The stool sample from the first US case of COVID-19 also tested positive for the viral RNA[68].
Patients with GI symptoms can have a 70% higher chance of testing positive on the nasopharyngeal swabs, suggesting a higher overall viral burden[69]. Stool samples can turn positive around 2-5 d after a positive respiratory specimen and can remain positive despite a negative respiratory specimen in up to 23% to 82% of cases[38]. The reason for this can be a longer survival or persistent viral replication in the GI environment[38,68].
A study from China reported positive stool samples in more than half (53%) of the cases, which can remain positive up to 12 d. Around 23% of these samples can continue to test positive despite a negative respiratory test[67]. Another study reported 53% of stool samples testing positive on RT-PCR. But reported lower fecal viral load compared to respiratory samples[70]. Zhang et al[71] also demonstrated presence of viral particles in oral and anal swabs. While it can be argued that positive RT-PCR does not necessarily mean a live virus capable of causing infections, Wang et al[72] actually found live SARS-CoV-2 virus in stool samples.
In summary, droplet and aerosol routes are still responsible for bulk of transmission but fecal-oral transmission can be another possibility. Gastroenterologists who can encounter patients with GI symptoms in their practice should be aware of the risk of such transmissions and adopt necessary precautions.
GI symptoms such as diarrhea, anorexia, nausea, vomiting and abdominal pain are commonly reported in COVID-19[25,73]. The retrospective study by Guan et al[48] which included 1099 COVID-19 cases from 552 centers across China, reported nausea, vomiting in 5% and diarrhea in 3.8% cases. Another study reported at least one GI symptom in 32.5% of cases. These included anorexia (56.7%), diarrhea (37.8%), nausea (16.5%), abdominal pain (10.4%) and vomiting (7.9%)[39]. A cross-sectional study found GI symptoms in more than half (50.5%) of the cases on initial presentation. The severity of these symptoms correlated with the overall disease severity and significant liver function abnormalities[74]. The retrospective study by Luo et al[75] which included 1141 cases found at least one GI symptom in 16% of cases on initial presentation. These included anorexia (98%), nausea, vomiting (66%), diarrhea (37%) and abdominal pain (25%). Jin et al[76]’s study which was the first COVID-19 study outside Wuhan reported the prevalence of GI symptoms at 11.4%. Diarrhea, at 8.14% was the most common of these symptoms[76].
A single-center case series from Flushing, New York, including 892 cases, reported GI symptoms in a quarter. Diarrhea (19.8%) was the most common, followed by nausea (16.6%), anorexia (11.8%), vomiting (10.2%) and abdominal pain (7.8%)[77]. Another retrospective study from New York which included 1059 patients, reported diarrhea in 22% of cases. Nausea, vomiting and abdominal pain were reported in 16%, 9% and 7%, respectively[78]. A multicenter, retrospective cohort study, from Massachusetts reported at least one GI symptom in up to two-thirds (61.3%) of cases. These symptoms included anorexia (34.8%), diarrhea (33.7%) and nausea (26.4%). Around 20% experienced isolated GI symptoms, while 14% developed these symptoms prior to the onset of other disease manifestations[79]. An outpatient questionnaire based study by Sierpiński et al[80] which included 1942 cases with mild to moderate disease reported at least one GI symptom in 53.6%. These included Anorexia (47%) and diarrhea (24%).
A meta-analysis of 60 studies including 4243 cases from 6 countries, found pooled prevalence of GI symptoms at 17.6%. Anorexia (26.8%) was most common, followed by diarrhea (12.5%), nausea/vomiting (10.2%) and abdominal pain/discomfort
GI symptoms are also seen in pediatric cases. A retrospective study including 20 pediatric cases from Wuhan, reported GI symptoms, such as diarrhea and vomiting in 15% and 10% of cases, respectively[81].
In addition to GI symptoms, gross and microscopic changes of the GI tract are also seen on autopsy of COVID-19 patients. Segmental dilation and stenosis of small intestine, along with mucosal shedding and necrosis is reported on autopsy, while inflammatory infiltrates and interstitial edema, consistent with a colitis/enteritis like picture is seen on histology[38,67]. Macroscopic abnormalities of GI tract can also be evident on imaging. Lui et al[82] reported bowel wall thickening involving any portion of the bowel, as well as ileus, ascites, pneumatosis intestinalis and pneumoperitoneum on abdominal imaging.
We have summarized the most frequently reported GI symptoms across different studies in Tables 1-4.
| Ref. | Number of patients | Diarrhea | Nausea | Vomiting | Anorexia | Abdominal pain |
| Huang et al[42] | 41 | 1 (3) | NA | NA | NA | NA |
| Yang et al[58] | 52 | NA | NA | 2 (4) | NA | NA |
| Chen et al[1] | 99 | 2 (2) | 1(1) | NA | NA | |
| Ping et al[83] | 9 | 1 (11.1) | 1(11.1) | 1(11.1) | 6 (66.7) | NA |
| Wang et al[13] | 138 | 14 (10.1) | 14 (10.1) | 55 (40) | 3 (2.2) | |
| Luo et al[75] | 1141 | 68 (6) | 134 (11.7) | 119 (10.4) | NA | 45 (3.9) |
| Zhou et al[84] | 191 | 9 (4.7) | 7 (3.7) | NA | NA | |
| Zhang et al[85] | 140 | 18 (12.9) | 24 (17.3) | 7 (5) | NA | 8 (5.8) |
| Chen et al[86] | 274 | 77 (28.1) | 24 (8.8) | 16 (5.8) | NA | 19 (6.9) |
| Xu et al[87] | 1324 | 28 (2.1) | NA | NA | 56 (4.2) | NA |
| Shi et al[88] | 645 | 29 (4.5) | NA | NA | NA | NA |
| Han et al[89] | 108 | 15 (14) | NA | NA | NA | NA |
| Fang et al[90] | 305 | 146 (49.5) | 59 (29.4) | 3 (2) | 101 (50.2) | 12 (6) |
| Xu et al[91] | 355 | 130 (36.6) | NA | NA | NA | NA |
| Ma et al[92] | 81 | 6 (7.41) | NA | NA | NA | NA |
| Liu et al[93] | 238 | 14 (9.2) | 2 (1.3) | 3 (2) | 14 (9.2) | 1 (0.7) |
| Huang et al[94] | 36 | 3 (8.3) | NA | NA | NA | NA |
| Mao et al[95] | 214 | 41 (19.2) | NA | NA | NA | 10 (4.7) |
| Liu et al[96] | 109 | 12 (11) | NA | NA | NA | NA |
| Shu et al[97] | 545 | 49 (8.9) | 0 (0) | NA | NA | |
| Ref. | Number of patients | Diarrhea | Nausea | Vomiting | Anorexia | Abdominal pain |
| Ai et al[98] | 102 | 15 (14.3) | 9 (8.8) | 2 (2) | NA | 3 (2.9) |
| Chen et al[99] | 9 | 2 (22) | 0 (0) | NA | 0 (0) | |
| Zhao et al[100] | 77 | 1 (1.3) | 6 (7.8) | NA | NA | NA |
| Chang et al[101] | 13 | 1 (7.7) | NA | NA | NA | NA |
| Zhao et al[102] | 75 | 7 (9.3) | NA | NA | NA | 1 (1.3) |
| Yang et al[103] | 55 | 2 (3.6) | NA | NA | NA | NA |
| Li et al[104] | 83 | 7 (8.4) | NA | NA | NA | 7 (8.4) |
| Qi et al[105] | 267 | 10 (3.7) | 6 (2.2) | 46 (17.2) | NA | |
| Xu et al[106] | 90 | 5(5.6) | 2 (2.2) | 5 (5.6) | NA | NA |
| Xiao et al[67] | 73 | 26 (35.6) | NA | NA | NA | NA |
| Lin et al[107] | 95 | 23 (24.2) | 17 (17.9) | 4 (4.2) | 17 (17.8) | 2 (2.1) |
| Wen et al[108] | 417 | 29 (7) | NA | NA | NA | NA |
| Xu et al[109] | 45 | 0 (0) | NA | NA | NA | NA |
| Yan et al[110] | 168 | 12 (7.1) | 9 (5.4) | 7 (4.2) | 14 (8.3) | 7 (4.2) |
| Wang et al[111] | 18 | 3 (16.7) | 1 (5.6) | NA | NA | |
| Chen et al[112] | 291 | 25 (8.6) | 17 (5.8) | NA | 1 (0.3) | |
| Liu et al[113] | 620 | 53 (8.5) | NA | NA | NA | NA |
| Fan et al[114] | 55 | 6 (10.9) | NA | 4 (7.3) | NA | NA |
| Yao et al[115] | 40 | 3 (7.5) | 3 (7.5) | NA | NA | NA |
| Tian et al[116] | 37 | 8 (25.8) | NA | NA | NA | NA |
| Song et al[117] | 51 | 5 (10) | 3 (6) | 9 (18) | NA | NA |
| Lu et al[118] | 265 | 17 (6.4) | 6 (2.3) | NA | NA | |
| Fu et al[119] | 52 | 7 (13.5) | 1 (1.9) | NA | NA | NA |
| Fu et al[120] | 36 | 3 (8.3) | NA | NA | NA | NA |
| Xu et al[121] | 62 | 3 (4.8) | NA | NA | NA | NA |
| Jin et al[76] | 651 | 56 (8.6) | 28 (4.3) | NA | NA | |
| Qian et al[122] | 91 | 21 (23.1) | 11 (12.1) | 6 (6.6) | 23 (25.3) | NA |
| Chen et al[123] | 175 | 35 (20) | NA | 7 (4) | NA | 5 (3) |
| Kuang et al[124] | 944 | 21 (2.7) | NA | NA | NA | NA |
| Hu et al[125] | 24 | 2 (8.3) | NA | NA | NA | NA |
| Guan et al[48] | 1099 | 42 (3.8) | 55 (5) | NA | NA | |
| Ref. | Number of patients | Diarrhea | Nausea | Vomiting | Anorexia | Abdominal pain |
| Cholankeril et al[126] | 207 | 22 (10.8) | 22 (10.8) | NA | 14 (7.1) | |
| Hajifathalian et al[78] | 1059 | 234 (22.1) | 168 (15.3) | 91 (8.6) | 240 (22.7) | 72 (6.8) |
| Kujawski et al[127] | 12 | 4 (33.3) | 3 (25) | NA | NA | 2 (16.7) |
| Ferm et al[77] | 892 | 177 (19.8) | 148 (16.6) | 91 (10.2) | 105 (11.8) | 70 (7.8) |
| Redd et al[79] | 318 | 107 (33.7) | 84 (26.4) | 49 (15.4) | 110 (34.8) | 46 (14.5) |
| Ref. | Location | Number of patients | Diarrhea | Nausea | Vomiting | Anorexia | Abdominal pain |
| COVID-19 National Emergency response Center[128] | South Korea | 28 | 2 (7) | NA | NA | NA | 1 (4) |
| Young et al[129] | Singapore | 18 | 3 (17) | NA | NA | NA | NA |
| Pung et al[130] | Singapore | 17 | 4 (23.5) | 1 (5.9) | NA | NA | |
| Tabata et al[131] | Diamond Princess Cruise Ship | 104 | 8 (9.6) | NA | NA | NA | NA |
| Kluytmans et al[132] | Netherlands | 86 | 16 (18.6) | NA | NA | NA | 5 (5.8) |
| Wölfel et al[133] | Germany | 9 | 2 (22) | NA | NA | NA | NA |
| Dreher et al[134] | Germany | 50 | 8 (16) | 1 (2) | 2 (4) | NA | NA |
| Gritti et al[135] | Italy | 21 | 5 (23.8) | NA | NA | NA | NA |
| Spiteri et al[136] | Europe | 38 | 1 (3.2) | NA | NA | NA | NA |
| COVID-19 National Incident Room Surveillance Team[137] | Australia | 295 | 48 (16.3) | 34 (11.5) | NA | 6 (1) | |
| Sierpiński et al[80] | Poland | 1942 | 470 (24.2) | NA | NA | NA | NA |
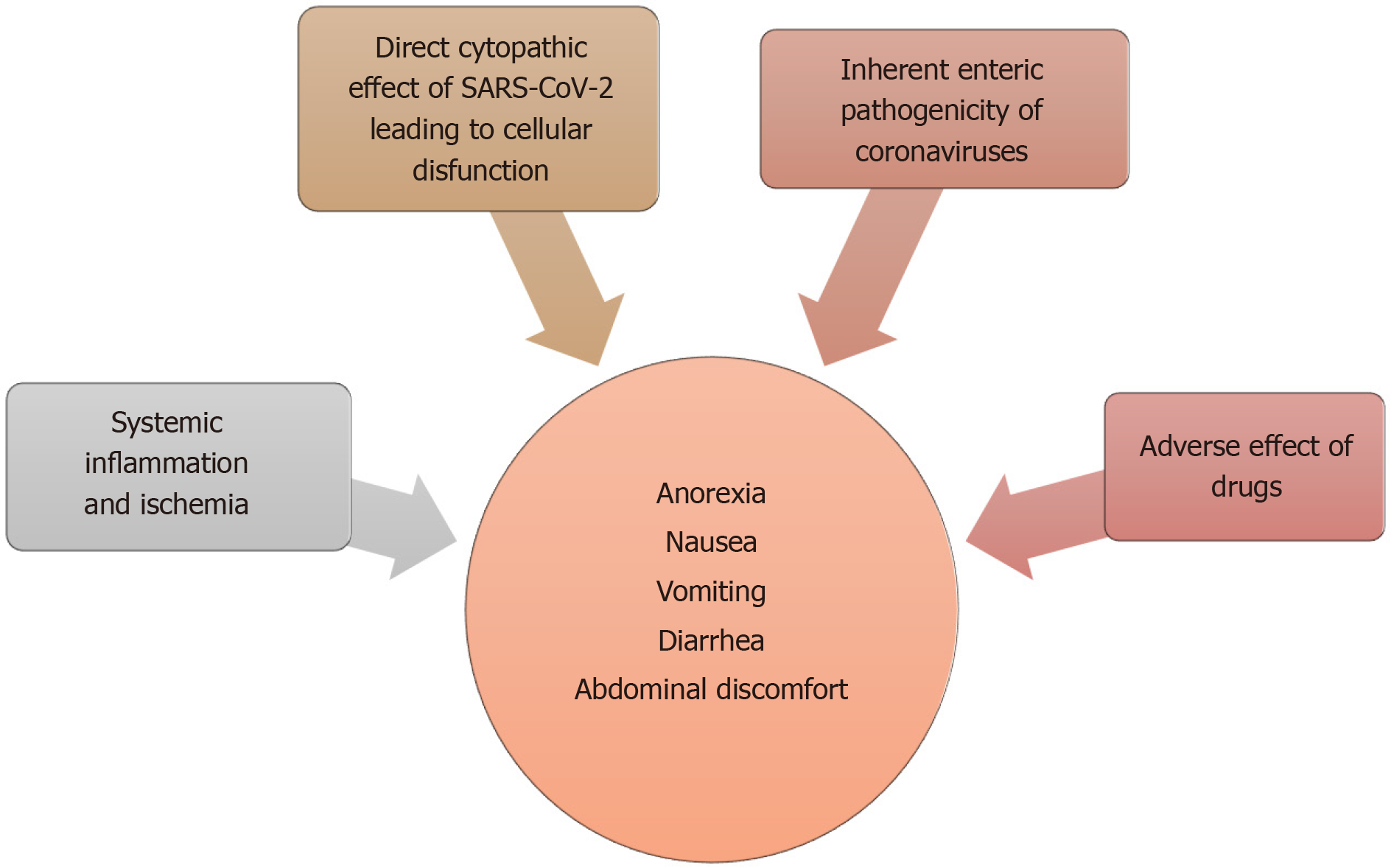
There are several proposed mechanisms for the GI manifestations of COVID-19 (Figure 3). Subjective and nonspecific symptoms such as anorexia and nausea are difficult to accurately characterize and can be over-reported. Objective symptoms, such as diarrhea and vomiting are speculated to be either a result of direct viral attack or due to an adverse drug reaction. An overwhelming systemic inflammatory response commonly seen in severe cases can also produce these symptoms.
The structural similarities and inter-specie immunologic cross-reactivity between animal and human coronaviruses have indicated a zoonotic origin for most human coronaviruses[54]. Some of these inherently have the ability to cause gastroenteritis in their animal hosts[18,20,54]. Some of the endemic human coronaviruses, such as OC43 and 229E have antigenic similarities to these animal coronaviruses[54,138,141]. It is possible that the recombination events resulting in these inter-specie barrier crossover can allow some of them to retain their enteric infectivity[54]. This can be a possible scenario in SARS-CoV-2, which via the ACE2 receptor can infect and exert a direct cytopathic effect on enterocytes. Fecal leukocytes and occult blood which can be seen in other cases of viral diarrhea is reported in 1.7% and 5.2% of fecal samples, respectively[38]. Increased inflammatory infiltrates and interstitial edema of the GI tract indicating active inflammation is also observed on histology[67]. The presence of an infectious SARS-CoV-2 virion in stool, and persistent fecal viral RNA shedding which in some cases, can outlast respiratory shedding points towards viral replication in GI tract[67,72]. Together, these findings can support a direct viral attack on enterocytes resulting in cellular dysfunction and increased permeability which in turn can lead to malabsorption, culminating in diarrhea[73]. It is also a possibility that viral involvement of upper GI glandular epithelium produces nausea and vomiting in a similar way.
Adverse drug reactions can be another possible explanation. Some of the antivirals, antibiotics and immunomodulators, commonly prescribed to COVID-19 patients are known to cause diarrhea. Compared to earlier studies, a higher prevalence of GI symptoms was reported in later studies. This temporal variation also happens to coincide with a more widespread use of these medications[38]. Hypoxia induced necrosis and cellular injury resulting in enterocyte dysfunction is another possibility[38].
Hepatic dysfunction, as evidenced by abnormal laboratory tests is frequently seen in COVID-19. Histological changes including lobular and portal inflammation have also been reported[142]. Most cases of COVID-19 associated hepatic dysfunction are mild and transient, but in a small subset of patients a higher degree of dysfunction and hepatic enzyme derangements can be seen[143].
One of the earliest studies from Wuhan, reported aspartate transaminase (AST) elevation in 37% of cases[42]. This number was even higher in critically ill [62% vs 25% intensive care unit (ICU) vs non-ICU cases, respectively][42]. Another study from Wuhan, reported liver function test (LFT) abnormalities in 43% of cases. AST elevations were more common than alanine aminotransferase (ALT) (35% vs 28%), while total bilirubin was elevated in only 18%[1]. Though most cases had only mild to moderate degree of LFT derangements, a case of severe liver injury with profound ALT and AST elevation was also reported[1]. Guan et al[48]’s analysis of data reported to the Chinese National Health Commission found AST, ALT and total bilirubin elevation in 22.2%, 21.3% and 10.5% of cases, respectively. LFT derangements were more commonly seen in severe cases (AST 39.4% vs 18.2%: ALT 28.1% vs 19.8% and T. Bili 13.3% vs 9.9%; Severe vs non-severe, respectively)[48]. Data from 56 COVID-19 patients admitted to the People’s Liberation Army Hospital in Beijing reported LFT abnormalities in 28.6% of cases[144].
A study from New York, reported mild (2-5 × ULN) AST and ALT elevation in 13.8% and 11.5% cases on admission. Severe (> 5 × ULN) AST and ALT elevations were seen in 2.8% and 1.9%. 4.3% had abnormal bilirubin (> 1.2 mg/dL), 11.9% had abnormal alkaline phosphatase and 24% had an INR > 1.13[77]. LFT abnormalities, especially AST elevations were associated with severe disease and higher mortality[77]. The strength of this study was that it looked at the prevalence of LFT derangements on admission. This can help mitigate the effects of confounders such as medications and other factors.
Like adult cases, pediatric cases have also demonstrated hepatic dysfunction. A study from Wuhan reported ALT elevation in a quarter of pediatric cases[81].
In summary, liver dysfunction seen in COVID-19 is mostly transient and mild with a hepatocellular, rather than cholestatic pattern[145]. AST elevations are also more common than ALT, which is in contrasts to the typical pattern of viral induced liver injury[77]. Liver injury is more commonly seen in severe cases. Except for a few, most studies have not described prevalence of pre-existing liver diseases which can be a potential confounder[145].
We have summarized studies which have reported hepatic dysfunction in Table 5.
| Ref. | Location | Number of patients | AST elevation | ALT elevation | T. Bili elevation |
| Huang et al[42] | Wuhan, China | 41 | 15 (37) | NA | NA |
| Chen et al[1] | Wuhan, China | 99 | 35 (35) | 28 (28) | 18 (18) |
| Zhou et al[84] | Wuhan, China | 191 | NA | 59 (31) | NA |
| Chen et al[86] | Wuhan, China | 274 | 84 (31) | 60 (27) | NA |
| Xu et al[91] | Wuhan, China | 355 | 102 (28.7) | 91 (25.6) | 66 (18.6) |
| Huang et al[94] | Wuhan, China | 36 | 18 (58) | 4 (13.3) | 4 (12.9) |
| Shu et al[97] | Wuhan, China | 545 | 35 (10.1) | 41 (7.5) | 189 (34.7) |
| Cai et al[146] | Shenzhen City, Guangdong, China | 298 | 25 (8.4) | 39 (13.1) | 24 (8.1) |
| Ai et al[98] | Xiangyang, China | 102 | 26 (25.5) | 20 (19.6) | NA |
| Zhao et al[102] | Hefei, Anhui, China | 75 | 14 (18.7) | 15 (20) | 12 (16) |
| Zhao et al[100] | Beijing, China | 77 | 20 (26) | 26 (33.8) | NA |
| Qi et al[105] | Chongqing, China | 267 | 19 (7.1) | 20 (7.5) | 6 (2.2) |
| Lin et al[107] | Zhuhai, Guangdong, China | 95 | 4 (4.2) | 5 (5.3) | 22 (23.2) |
| Xu et al[109] | Multiple locations in China (All outside Hubei province) | 45 | NA | NA | NA |
| Yan et al[110] | Hainan Province, China | 168 | 18 (17.3) | 9 (8) | 7 (6.4) |
| Wang et al[111] | Zhengzhou, Henan, China | 18 | Abnormal LFTs 4 (25) | NA | |
| Chen et al[112] | Changsha and Loudi, Hunan, China | 291 | 44 (15.1) | 30 (10.3) | 27(9.3) |
| Yao et al[115] | Xi’an, Shaanxi, China | 40 | 16 (40) | 21 (52.5) | 10 (25) |
| Tian et al[116] | Liaocheng, Shandong, China | 37 | 4 (10.8) | 2 (5.4) | 13 (35.1) |
| Fu et al[120] | Kunming, Yunnan, China | 36 | 4 (11.1) | 4 (11.1) | 11 (30.56) |
| Xu et al[121] | Zhejiang, China | 62 | 10 (16.1) | NA | NA |
| Qian et al[122] | Multiple sites, Zhejiang, China | 91 | 9 (9.9) | 7 (7.7) | NA |
| Chen et al[123] | Multiple sites, Wenzhou, Zhejiang, China | 175 | 19 (11) | 23 (13) | NA |
| Guan et al[48] | Multiple sites across China | 1099 | 168 (22.2) | 158 (21.3) | 76 (10.5) |
| Ferm et al[77] | New York, United States | 892 | Borderline elevation (1-2 × ULN) 40% | Borderline elevation (1-2 × ULN) 26.5% | 4.3% |
| mild elevation (2-5 × ULN) 13.8% | mild elevation (2-5 × ULN) 11.5% | ||||
| moderate to severe elevation (> 5 × ULN) 2.8% | moderate to severe (> 5 × ULN) 1.9% | ||||
| Tabata et al[131] | Diamond Princess Cruise, Japan | 104 | 18 (17.3) | 17 (16.3) | NA |
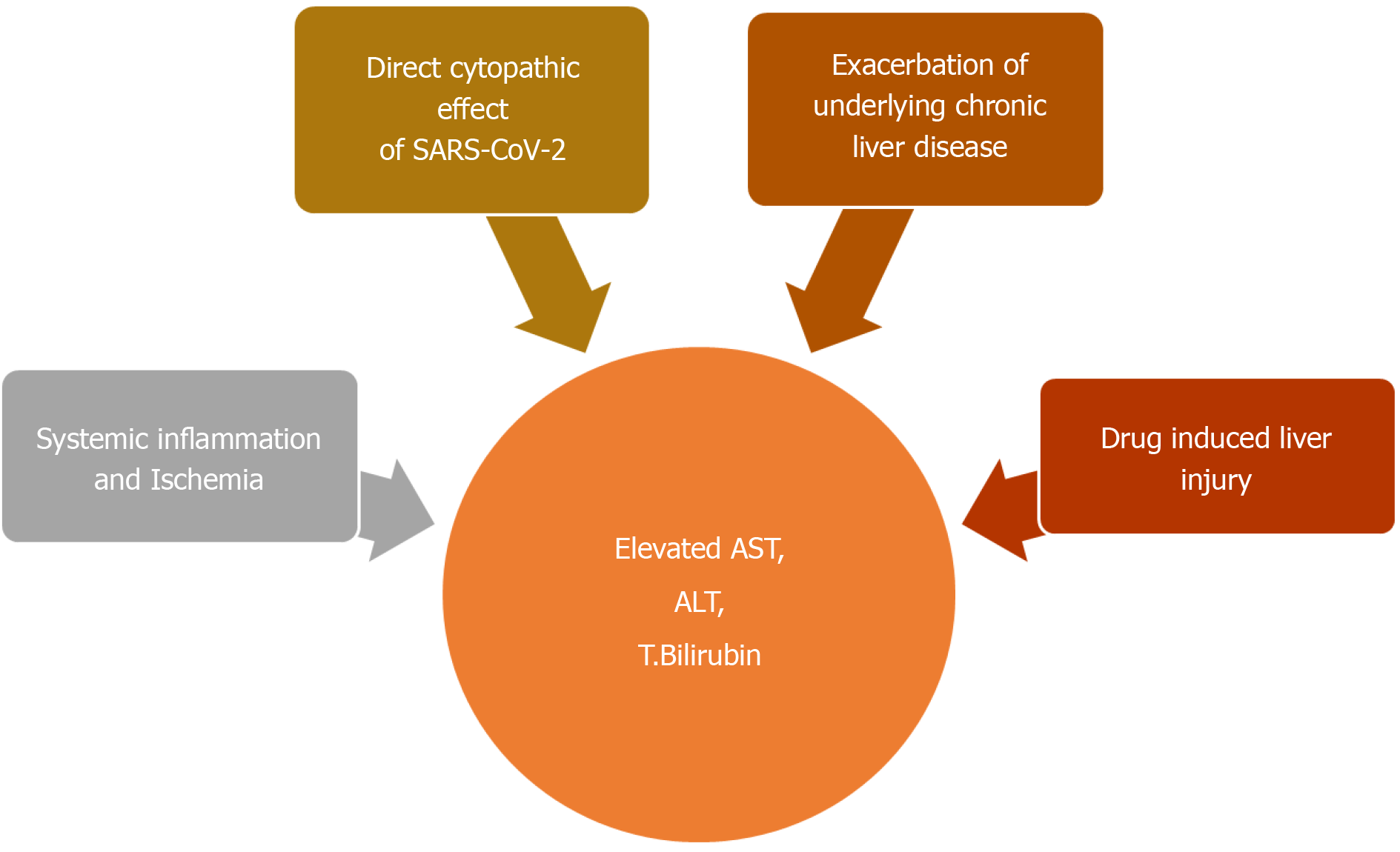
There are many possible explanations for COVID-19 associated hepatic dysfunction. SARS-CoV-2 can directly infect hepatocytes and cholangiocytes, which also harbor the virus specific ACE2 receptors (Figure 4)[145]. Expression profiling have indicated a 20-fold higher expression of ACE2 in cholangiocytes, compared to hepatocytes[145]. In addition to maintaining normal function of biliary system, cholangiocytes also play an important role in liver regeneration and immune response. Direct involvement of these cells can cause liver injury as manifested by impaired hepatic transaminases[145]. Liver biopsy on autopsy of COVID-19 patients show micro vesicular steatosis as well as lobular and portal inflammation[142]. Although non-specific, these findings can represent direct hepatic injury from SARS-CoV-2 infection. However, no viral inclusions have been detected to date in hepatic or biliary tissue and the pattern of LFT derangement seen is mostly hepatocellular rather than cholestatic, which is expected in biliary and cholangiocyte involvement[145]. This indicates that the liver involvement in COVID-19 is not a likely consequence of direct liver injury.
Indirect liver injury from overwhelming release of inflammatory cytokines (cytokine storm) or ischemia is a more likely explanation[144]. AST elevation is more commonly seen than ALT which is in contrast to the pattern of liver injury typically seen in viral hepatitis[147]. Preferential AST elevations are more commonly seen with toxic and ischemic liver injury which involves zone 3 hepatocytes[147]. A higher prevalence of LFT abnormalities in severe cases of COVID-19 also supports this finding[77]. Overwhelming release of inflammatory cytokines such TNF-α, interferon-λ and various interleukins such IL-2, IL-6, IL-7, IL-8 can lead to global tissue damage and multiorgan dysfunction including the liver[145].
Liver injury seen in COVID-19 can also be drug induced. A wide range of medications can cause hepatotoxicity. These can range from commonly taken OTC acetaminophen to antiviral drugs currently under investigation for COVID-19. A summary of these drugs will be mentioned in Table 6.
| Drug | Proposed mechanism in COVID-19 | GI side effects | Hepatic side effects | Pancreatic side effects |
| Remdesivir | Inhibitor of viral RNA-dependent RNA polymerase. | Nausea, Vomiting, gastroparesis, GI bleeding | Increased LFTs | NA |
| Chloroquine and Hydroxychloroquine | Disruption of endosome-mediated viral entry and transport. | Nausea, vomiting, abdominal cramping | NA | NA |
| Lopinavir/Ritonavir | Protease inhibitor. Disrupts viral replication. | Diarrhea, nausea, vomiting | LFT elevation, hepatitis, worsening of underlying CLD | Pancreatitis |
| Ribavirin | Nucleoside analog inhibitor of viral RNA synthesis. | Nausea | NA | NA |
| Nitazoxanide | Increase production of type I IFNs enhancing antiviral activity. | Abdominal pain, nausea, diarrhea, acid reflux | NA | NA |
| Nelfinavir | HIV-1 protease inhibitor. Action against SARS-CoV-2 unclear. | Diarrhea, nausea, increased flatulence | LFT elevation | NA |
| INF-α | Enhances cell mediated immune response against the virus. | NA | Auto-immune hepatitis | NA |
| Barcitinib | JAK inhibitor. Downregulation of hyper-inflammatory state seen in severe disease. | Nausea | LFT elevation | NA |
| Tocilizumab | IL-6 inhibitor. Downregulation of hyper-inflammatory state seen in severe disease. | Gastrointestinal perforation in patients on high dose steroids or history of diverticulitis | LFT elevation | NA |
Due to high worldwide prevalence of chronic liver disease (CLD), it is possible that hepatic dysfunction seen with COVID-19 is a consequence of pre-existing liver disease exacerbation[143]. Patients with CLD can have interruptions in their ongoing treatment resulting in exacerbations. Corticosteroids and immunomodulators which are increasingly being used to treat COVID-19 can also exacerbate underlying viral hepatitis. Singh et al[148] did a comparative study of patients with and without pre-existing CLD. Their study, which included 2780 patients, reported similar elevations in ALT, AST, GGT, Alkaline Phosphatase and total bilirubin in both groups, suggesting COVID-19 associated hepatic injury as a more likely reason for LFT derangements[148].
Data on the effects of COVID-19 on pre-existing liver disease, at present is limited. Most studies have not specified the prevalence of chronic liver disease in their COVID-19 cases. The few studies that have, report it between 1.25% to 11%[145]. 2.1% of 1099 COVID-19 cases included in Guan et al[48]’s study had pre-existing chronic hepatitis B which was associated with worse outcomes. In Singh et al[148]’s study, 9% of COVID-19 patients also had pre-existing liver disease. At 42%, nonalcoholic steatohepatitis was the most common liver condition, while cirrhosis was present in 1.8% of cases. The authors also compared laboratory and clinical characteristics of patients with pre-existing liver disease to those without. The results demonstrated a significantly higher mortality rate with chronic liver disease (RR 3.0, 95%CI: 1.5-6.0; P = 0.001) and cirrhosis (RR 4.6, 95%CI: 2.6-8.3, P < 0.001), irrespective of the cause[148]. However, liver function derangements from baseline were seen in both groups and the overall prevalence of hepatic dysfunction during the disease course did not appear to be different either[148].
Another study found a significantly higher risk of disease progression, LFT derangement and prolonger viral shedding in patients with co-existing nonalcoholic fatty liver disease[149]. Impaired hepatic immune system in these patients was speculated to be a reason[149]. A case series from Northern Italy did not find any COVID-19 related complications in the majority of Autoimmune liver disease patients[150]. Another Italian study did not find any difference in disease course between patients with autoimmune hepatitis (AIH) on immunosuppressive therapy and who are those not[151].
To address the current scarcity of data for CLD patients, two international registries have been created. These are the Surveillance Epidemiology of Coronavirus (COVID-19) Under Research Exclusion (SECURE-Cirrhosis) and European association for the study of liver (EASL) supported COVID-HEP registries. According to the combined weekly update (released on August 25th, 2020) a 31% mortality rate is seen in CLD patients with cirrhosis compared to 7% in those without. In liver transplant recipients, the mortality rate is 18%[152].
To summarize, an overall higher COVID-19 related mortality is seen with pre-existing liver diseases, especially cirrhosis. However, autoimmune hepatitis appears to be an exception to this rule.
Although not as common as the other extra-pulmonary organs, pancreatic involvement can also be seen in COVID-19. A few cases of possible COVID-19 induced pancreatitis have been reported[153,154]. A Chinese and an Italian study also found some form of pancreatic injury in 17% and 8.5% of cases, respectively[155,156].
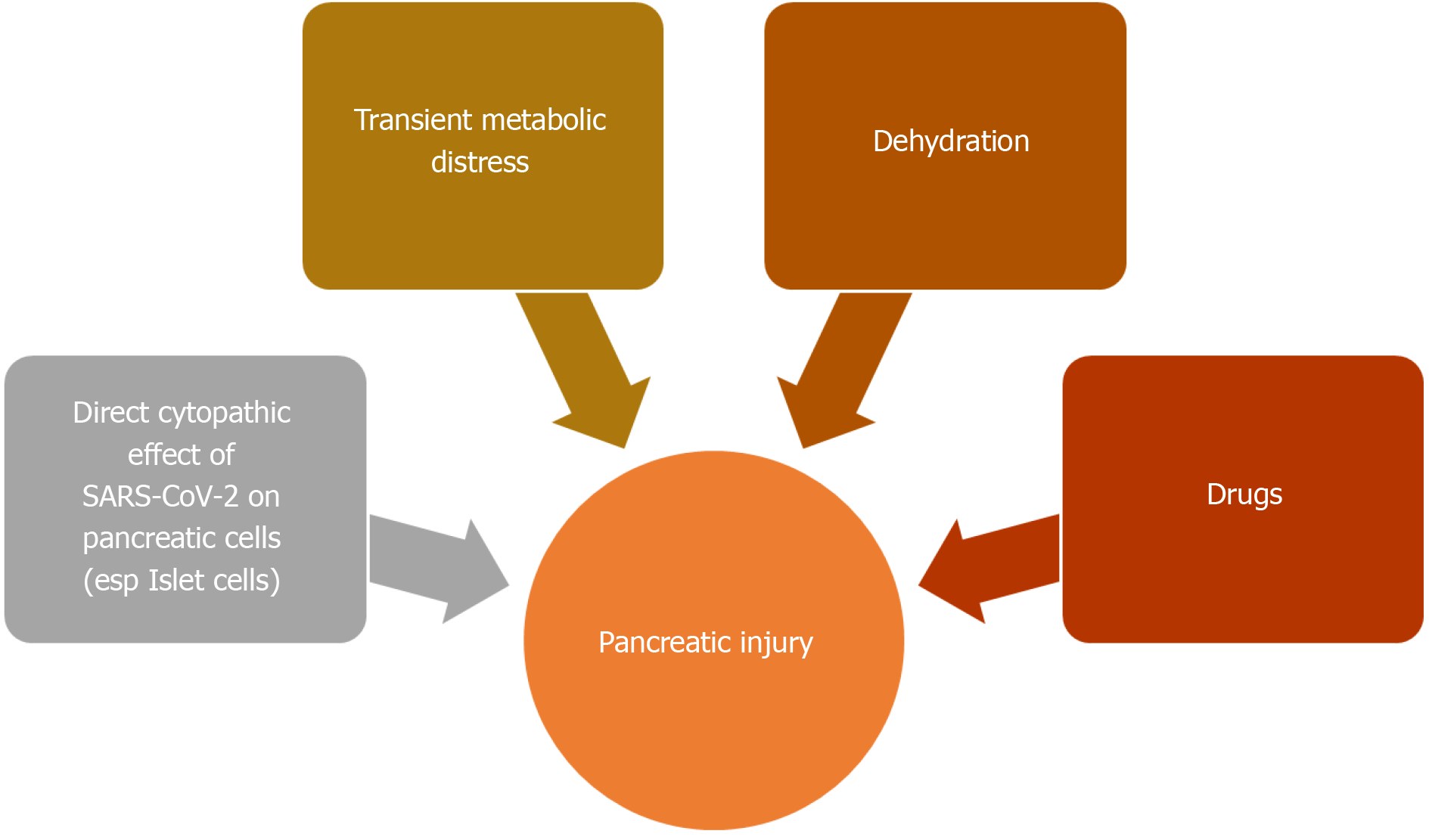
ACE2 receptor is also found in pancreas, especially its islet cells[157]. Pancreatic islet cell injury and ensuing, acute diabetes was previously seen with SARS infection[157]. The SARS-CoV virus was detected in pancreatic cells, indicating direct viral attack[158]. Like its predecessor, SARS-CoV-2 can also involve the pancreas[159]. The two-thirds of cases reported by Wang et al[155] developed hyperglycemia, which can be a consequence of endocrine pancreas involvement. Pancreatic injury can also be a part of the multi organ failure seen in severe COVID-19[155,156,160]. An English study from Liverpool reported 5 COVID-19 related cases of metabolic-pancreatitis. All these patients were overweight or obese and had hypertriglyceridemia and glucose intolerance. Transient metabolic distress induced by COVID-19 was hypothesized to cause pancreatitis in these cases[161]. Dehydration and drug induced pancreatic injury are other possibilities (Figure 5)[156].
Many of the commonly encountered extra-pulmonary manifestations of COVID-19 can be an adverse reaction to frequently prescribed drugs. We will summarize some of the well-known GI and hepatic side effects of these commonly administered drugs (Table 6)[162].
GI symptoms associated with COVID-19 are mostly mild to moderate in severity. Diarrhea seen in these patients is typically low volume and does not cause severe dehydration[74]. Management of these patients should involve adequate hydration and correction of electrolyte imbalances. Anti-emetics can also be administered for nausea and vomiting, provided there are no contraindications. Anorexia is a nonspecific symptom and is likely due to severe inflammation. Enteral nutrition should be encouraged whenever possible. A dietician consultation can also be beneficial for these patients.
COVID-19 associated liver injury is mostly transient and is likely indirect, which could be related to hypoxia, systemic inflammatory response, and drugs. Therefore, management of liver injury in COVID-19 should revolve around correction or removal of underlying etiology. Correction of hypoxia via oxygen supplementation or mechanical ventilation, renal replacement therapy for cytokine storm and restoration of effective intravascular volume in cases of shock can often correct liver injury[145]. Prompt identification and discontinuation or dose reduction of drugs responsible for drug induced liver injury is also important. Hepato-protective anti-inflammatory drugs such as L-ornithine-L-aspartate can also be used as adjuvant treatment in severe cases[145].
Significantly higher COVID-19 associated mortality is seen in cirrhotics[163]. Data from international registries have also pointed towards a higher mortality in these patients[152]. Therefore cirrhotics and CLD patients warrant special attention and for this reason three liver associations have provided recommendations for management of CLD during the pandemic (Table 7)[164].
| Selected recommendations | |
| To limit nosocomial spread | (1) Limit in-person visits by effective triaging; (2) Use of virtual platforms such as telehealth to provide alternatives for in-person visits; (3) Symptom and exposure screening prior to entering the healthcare facility to identify at-risk individuals; (4) Minimize staffing to essential staff only; (5) Decrease frequency of laboratory and imaging monitoring; (6) Ensure adherence to recommended PPE by HCW and patients; (7) Ensure adequate social distancing at the healthcare facility (Remodel if necessary); (8) Postpone and delay nonurgent or elective procedures (refer to Table 12); and (9) Minimize research activities including clinical trials wherever possible. |
| Management of CLD patients with COVID-19 | (1) Early hospital admission is recommended for these patients; (2) Prioritization of COVID-19 testing for cirrhotics, CLD patients on immunosuppressive therapy and those with acute decompensation; (3) Frequent LFT monitoring is recommended; (4) Consider early enrollment in clinical trial when possible; (5) Include non-COVID-19 etiologies in differentials for liver dysfunction; (6) Pay special attention to COVID-19 patients with NAFLD, which is often associated with severe COVID-19; (7) Consider hepatitis B surface antigen screening; (8) Monitor for drug induced liver injury; (9) 2-3 g/d of acetaminophen is generally safe and can be used in these patients. NSAIDs can also be used as needed but limit their use whenever possible; (10) Consider HBV prophylaxis prior to initiating immunosuppressive medications, especially IL-6; and (11) Hold Remdesivir in patients with decompensated liver disease and ALT > 5 × ULN. |
| Management of chronic viral hepatitis (HCV and HBV) | (1) Continuing treatment for chronic HCV and HBV despite COVID-19 status is recommended; (2) Can hold initiating treatment for HBV in the absence of flare; and (3) Treatment for HCV and HBV in the uninfected should continue according to established guidelines. |
| Management of HCC | (1) Based on the risk and benefit, a delay in surveillance of up to 2 mo is acceptable for high risk individuals; (2) Continuation of HCC treatment per guidelines is recommended, however can be postponed if necessary; (3) For COVID-19 patients, can consider postponing elective transplant and resection surgery and withholding immunotherapy; and (4) Early inpatient admission is advised for HCC patients. |
| Management of pre- and post- transplant recipients | (1) Screening of both, the donor and recipient for COVID-19 is recommended; (2) Donors testing positive for COVID-19 should be deferred; (3) CMS has classified transplant surgeries as Tier 3b. As such, these procedures should not be postponed or delayed; (4) Patients with poor short-term prognosis should be prioritized; (5) Low threshold for admitting transplant listed COVID-19 patients to the hospital is recommended; (6) For post-transplant patients, immunosuppressive dose reductions can be considered in moderate COVID-19 cases. For mild COVID-19 cases no immunosuppressive dose reduction is advised; and (7) Vaccination against pneumonia and influenza is recommended in post-transplant recipients. |
The EASL[165], the American Association for the Study of Liver Diseases (AASLD)[166] and three European referral centers have also made recommendation for the management of AIH which are summarized in Table 8[167].
| Selected recommendations from EASL, AASLD for management of auto-immune hepatitis | |
| (1) In-person visits should be minimized as much as possible. Switch to virtual platforms whenever possible; and (2) COVID-19 testing is recommended in cases of acute decompensation and liver failure. | |
| Low risk of complications (stable patient on chronic immunosuppressive treatment) | (1) Frequent patient-provider communications for close monitoring; (2) Use of virtual platforms should be preferred whenever possible; and (3) Ensure adequate drug supply and refills to reduce running out of medications. |
| Moderate risk of complications (symptomatic disease in the absence of cirrhosis, compensated cirrhosis) | (1) Can empirically treat via virtual healthcare platform whenever possible; and (2) Avoiding liver biopsy whenever possible is also recommended. |
| High risk of complications (acute flare, decompensated cirrhosis) | (1) Minimize invasive procedures wherever possible; (2) In COVID-19 patients, dose reduction of antimetabolites is recommended if lymphopenia develops; and (3) In case of infection corticosteroids should be tapered as quickly as possible. |
At present, the data on the safety of corticosteroids or other immunosuppressive medications is limited. Given the incomplete understanding of the effect of immune-modulating therapy on COVID-19, as well as the effect of COVID‐19 on immunosuppressed patients, the AASLD and EASL have provided guidance on the use of these drugs which is summarized in Table 9[168].
| Selected recommendations from AASLD and EASL on use of immunosuppressive therapies in CLD patients | |
| (1) Initiating corticosteroids and immunosuppressive therapy should be preceded; and (2) by careful risk vs benefit assessment. | |
| Patients uninfected by COVID-19 on immunosuppressive therapy | Dosage reductions or adjustment is not advised. |
| Patients infected with COVID-19 on immunosuppressive therapy | (1) Corticosteroid dose reductions after specialist consultation (consider tapering to avoid adrenal insufficiency); and (2) Dose reductions in azathioprine, cyclosporine, mycophenolate is recommended in severe COVID-19 (especially with accompanying lymphopenia). |
| Patients requiring initiation of immunosuppressive therapy | Initiating treatment is recommended in these patients regardless of COVID-19 status. |
Management of chronic GI disorders such as inflammatory bowel diseases (IBD) which require close follow ups and often require immunosuppressive medications is also challenging. Despite its limitations, virtual platforms, such as telehealth offer a safe and convenient alternative to in-person visits and can be an effective platform for triaging. This can minimize potential exposure to COVID-19 and can determine need for in-person visits and urgent interventions. In situations where no alternative to in-person evaluation exists, precautions including social distancing, proper hand hygiene, wearing of facemasks at all times and symptoms and exposure screening prior to entry in the healthcare facility is recommended[169].
Another problem gastroenterologists face during the pandemic is to determine whether to continue chronic immunosuppressive treatments that are often required for these conditions. Many of these medications carry an increased risk of infections which range between 0.5% to 30%[169]. Societies like American Gastroenterological Association (AGA), European Crohn’s and Colitis Organization and International Organization for the Study of IBD have provided recommendations regarding their use during the pandemic which will be summarized in Table 10[169].
| Asymptomatic infection | Mild to moderate infection | Severe or Critical infection | |
| Corticosteroids | (1) Dose reduction of Prednisone to < 20 mg/d; and (2) Consider switching to budesonide. | (1) Discontinue; and (2) Taper in chronic users. | |
| Immunomodulators (thiopurines, methotrexate) | (1) Consider risks vs benefits; and (2) Can resume after 14 d in asymptomatic patients or when test negative. | ||
| Biologicals | (1) Consider risks vs benefits; (2) Can resume after 14 d in asymptomatic patients or when test negative; (3) Can decrease infusion frequencies for infliximab and vedolizumab; and (4) Tofacitinib and JAK inhibitors should be discontinued. | ||
The ongoing pandemic has put an immense strain on healthcare systems and created and overwhelming demand for critical supplies. Judicious use of available resources and ensuring safety of HCWs who are constantly exposed, with consequently higher rates of infections is essential for optimum functioning of healthcare machinery during the pandemic[170,171]. The US CDC has issued interim guidelines for infection prevention and control which is summarized in Table 11[172].
| Interim Infection Prevention and Control guidelines by CDC |
| Encourage and promote Telehealth visits over in-person visits whenever possible. |
| COVID-19 symptom screening for everyone entering a healthcare facility. |
| Limiting entry points to a healthcare facility, ensuring effective screening for all. |
| Encourage hand hygiene and source control measures for everyone, especially HCWs. |
| Encourage physical distancing and limiting the number of visitors and personnel at any given time. |
| Implementing use of PPEs (e.g. N95/PAPR) for HCWs in areas with moderate to substantial community spread. |
| To mitigate spread from asymptomatic carriers; depending on the availability, a targeted SARS-CoV-2 testing for all patients can be considered. |
| To reduce exposures for HCWs the use of Engineering controls should be optimized. |
To mitigate the spread and to conserve limited resources, Joint Gastroenterology Societies, the Centers for Medicare and Medicaid Services, the US surgeon General and American College of Surgeons have all proposed delaying non-urgent and elective procedures[173-176]. AGA recommends a review of any planned procedures by trained medical personnel to determine urgency of a procedure[171]. AGA and AASLD have also provided an outline for triaging[166,171]. Though this approach is quite reasonable; the ensuing diagnostic and therapeutic delays is another challenge that needs to be addressed. Therefore, gradual, and stepwise resumption of procedures, while, of course ensuring patient and provider safety is the need of the day. Canadian Association of Gastroenterology has recently laid a framework for resumption of nonurgent endoscopic procedures[177]. Stepwise increment and expansion of endoscopic services with a minimum of 2 wk’ time in between is recommended. Factors related to local risk of transmission, infection dynamics and success of mitigation measures in the community must considered before this[177]. Adequate availability of healthcare resources including personal protective equipment (PPE), staff and equipment, as well as the ability to implement recommended social distancing must be taken into consideration as well[177]. Lastly, triaging which is a powerful tool for efficient healthcare delivery should be vigilantly implemented at every level. Evidence based approach to triaging endoscopic procedures are summarized in Table 12.
| Procedures that can be delayed | Procedures that cannot be delayed |
| Diagnostic procedures for mild/stable dysphagia[177]. | Endoscopies for upper GI bleeding (Blatchford score > 1)[166,171,177]. |
| Diagnostic procedures for suspected GI malignancy can be delayed for up to a few months[171]. | Upper endoscopies for foreign body with severe/progressive dysphagia[177]. |
| Routine surveillance and screening colonoscopies are non-urgent and can be postponed[171]. | Lower endoscopies for acute obstruction requiring decompression[177]. |
| Procedures for asymptomatic (with normal LFTs) gallstones and biliary strictures can be postponed[177]. | ERCP for acute cholangitis or symptomatic common bile duct stone[166,177]. |
| Follow up Colonoscopies for positive FIT test can be delayed for up to 7 to 9 mo without any adverse impact on outcomes[178]. | Follow up band ligation for recent variceal bleeding[166,177]. |
| Placement of percutaneous endoscopic gastrostomy or jejunostomy tubes[177]. | |
| Procedures that can significantly impact medical decisions should be performed. These can include endoscopies for evaluation of high likelihood GI malignancies, resection of high-grade dysplasia or histologically proven neoplasia and investigation for IBD flares[171,177]. | |
| Colonoscopies for lower GI bleeding can be delayed up to 72 h without any change in outcome[178]. | |
| Liver biopsies to diagnose autoimmune hepatitis and transplant rejection in liver transplant patients[166]. | |
| Transplant surgeries are categorized Tier 3b by CMS and should be performed[175]. | |
| Endoscopic drainage of infected pancreatic fluid collections and necrosectomies[177]. |
When a procedure needs to be performed, proper personal protection equipment must be worn by the providers. Recommendations for PPEs during GI procedures are summarized in Table 13[171].
| Recommendations for PPEs during GI procedures | |
| Masks | Regardless of patient’s COVID-19 status, NIOSH approved N95/PAPR should be used by staff for any GI procedure. |
| Gloves | Regardless of patient’s COVID-19 status, gloves should be worn by providers during any GI procedure. Double gloves are preferred over single. |
| Negative Pressure Rooms | GI procedures for confirmed COVID-19 patients should be performed in negative pressure rooms when available. |
| Disinfection | Standard endoscope disinfectants can reduce microorganisms by 99.99% and should be sufficient for SARS-CoV-2. |
The disease caused by the novel coronavirus is more of a systemic condition than an isolated respiratory illness. GI and hepatic manifestations of COVID-19 are frequently reported and anorexia, nausea, vomiting, diarrhea are few of the commonly seen GI symptoms in COVID-19. Hepatic function derangement is also common, mostly mild, and transient and pancreatic injury is also reported. These symptoms can be a result of a direct infection or an indirect consequence of associated systemic inflammation or hypoxia from respiratory failure. Side effects of drugs currently being used to treat COVID-19 could also be a possible explanation for these manifestations. Fecal oral transmission in the setting of these symptoms poses a considerable risk of exposure for gastroenterologists who deal with these patients. The limited availability of PPE only makes matters worse. Effective and safe delivery of care while conserving these critical supplies is of utmost importance. For this purpose, various gastroenterology societies have also put forward their recommendations. With no end to the pandemic in sight, it is important for HCWs and gastroenterologists to be aware of and adherent to these guidelines.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Gupta V, Hu B, M Joseph P, Trkulja V S-Editor: Wang JL L-Editor: A P-Editor: Li JH
| 1. | Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14869] [Cited by in RCA: 12979] [Article Influence: 2595.8] [Reference Citation Analysis (1)] |
| 2. | Hussain A, Yadav S, Hadda V, Suri TM, Tiwari P, Mittal S, Madan K, Mohan A. Covid-19: a comprehensive review of a formidable foe and the road ahead. Expert Rev Respir Med. 2020;14:869-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Cheung KS, Hung IFN, Chan PPY, Lung KC, Tso E, Liu R, Ng YY, Chu MY, Chung TWH, Tam AR, Yip CCY, Leung KH, Fung AY, Zhang RR, Lin Y, Cheng HM, Zhang AJX, To KKW, Chan KH, Yuen KY, Leung WK. Gastrointestinal Manifestations of SARS-CoV-2 Infection and Virus Load in Fecal Samples From a Hong Kong Cohort: Systematic Review and Meta-analysis. Gastroenterology. 2020;159:81-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1113] [Cited by in RCA: 1133] [Article Influence: 226.6] [Reference Citation Analysis (1)] |
| 4. | He X, Lau EHY, Wu P, Deng X, Wang J, Hao X, Lau YC, Wong JY, Guan Y, Tan X, Mo X, Chen Y, Liao B, Chen W, Hu F, Zhang Q, Zhong M, Wu Y, Zhao L, Zhang F, Cowling BJ, Li F, Leung GM. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26:672-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2588] [Cited by in RCA: 2857] [Article Influence: 571.4] [Reference Citation Analysis (0)] |
| 5. | Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270-273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15248] [Cited by in RCA: 14133] [Article Influence: 2826.6] [Reference Citation Analysis (1)] |
| 6. | World Health Organization. Virtual Press Conference on COVID-19 2020. Available from: https://www.who.int/docs/default-source/coronaviruse/transcripts/who-audio-emergencies-coronavirus-press-conference-full-and-final-11mar2020.pdf?sfvrsn=cb432bb3_2. |
| 7. | Al-Tawfiq JA, Memish ZA. Middle East Respiratory Syndrome Coronavirus and Severe Acute Respiratory Syndrome Coronavirus. Semin Respir Crit Care Med. 2020;41:568-578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | de Wit E, van Doremalen N, Falzarano D, Munster VJ. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14:523-534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2157] [Cited by in RCA: 2281] [Article Influence: 253.4] [Reference Citation Analysis (0)] |
| 9. | Peiris JS, Yuen KY, Osterhaus AD, Stöhr K. The severe acute respiratory syndrome. N Engl J Med. 2003;349:2431-2441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 936] [Cited by in RCA: 900] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 10. | World Health Organization. Weekly Epidemiological Update 14 September 2020. Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200914-weekly-epi-update-5.pdf?sfvrsn=cf929d04_2. |
| 11. | Galanopoulos M, Gkeros F, Doukatas A, Karianakis G, Pontas C, Tsoukalas N, Viazis N, Liatsos C, Mantzaris GJ. COVID-19 pandemic: Pathophysiology and manifestations from the gastrointestinal tract. World J Gastroenterol. 2020;26:4579-4588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 98] [Cited by in RCA: 105] [Article Influence: 21.0] [Reference Citation Analysis (5)] |
| 12. | Beattie RM, Ashton JJ, Penman ID. COVID-19 and the gastrointestinal tract: recent data. Frontline Gastroenterol. 2020;11:371-374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14113] [Cited by in RCA: 14767] [Article Influence: 2953.4] [Reference Citation Analysis (0)] |
| 14. | Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536-544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5202] [Cited by in RCA: 4633] [Article Influence: 926.6] [Reference Citation Analysis (0)] |
| 15. | Brian DA, Baric RS. Coronavirus genome structure and replication. Curr Top Microbiol Immunol. 2005;287:1-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 416] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 16. | Huang X, Wei F, Hu L, Wen L, Chen K. Epidemiology and Clinical Characteristics of COVID-19. Arch Iran Med. 2020;23:268-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 130] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 17. | Chakraborty C, Sharma A, Bhattacharya M, Sharma G, Lee SS. The 2019 novel coronavirus disease (COVID-19) pandemic: A zoonotic prospective. Asian Pac J Trop Med. 2020;13:242-246. [RCA] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 18. | Chen Y, Liu Q, Guo D. Emerging coronaviruses: Genome structure, replication, and pathogenesis. J Med Virol. 2020;92:418-423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1873] [Cited by in RCA: 1874] [Article Influence: 374.8] [Reference Citation Analysis (1)] |
| 19. | Cui J, Li F, Shi ZL. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17:181-192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3747] [Cited by in RCA: 3309] [Article Influence: 551.5] [Reference Citation Analysis (0)] |
| 20. | Kapikian AZ. The coronaviruses. Dev Biol Stand. 1975;28:42-64. [PubMed] |
| 21. | Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Di Napoli R. Features, Evaluation, and Treatment of Coronavirus (COVID-19). In: StatPearls. Treasure Island: StatPearls Publishing, 2020. [PubMed] |
| 22. | Ashour HM, Elkhatib WF, Rahman MM, Elshabrawy HA. Insights into the Recent 2019 Novel Coronavirus (SARS-CoV-2) in Light of Past Human Coronavirus Outbreaks. Pathogens. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 389] [Cited by in RCA: 356] [Article Influence: 71.2] [Reference Citation Analysis (1)] |
| 23. | Corman VM, Muth D, Niemeyer D, Drosten C. Hosts and Sources of Endemic Human Coronaviruses. Adv Virus Res. 2018;100:163-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 557] [Cited by in RCA: 628] [Article Influence: 89.7] [Reference Citation Analysis (0)] |
| 24. | Liu L, Wang T, Lu J. The prevalence, origin, and prevention of six human coronaviruses. Virol Sin. 2016;31:94-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Sultan S, Altayar O, Siddique SM, Davitkov P, Feuerstein JD, Lim JK, Falck-Ytter Y, El-Serag HB; AGA Institute. AGA Institute Rapid Review of the Gastrointestinal and Liver Manifestations of COVID-19, Meta-Analysis of International Data, and Recommendations for the Consultative Management of Patients with COVID-19. Gastroenterology 2020; 159: 320-334. e27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 309] [Cited by in RCA: 293] [Article Influence: 58.6] [Reference Citation Analysis (1)] |
| 26. | Yuki K, Fujiogi M, Koutsogiannaki S. COVID-19 pathophysiology: A review. Clin Immunol. 2020;215:108427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1218] [Cited by in RCA: 1192] [Article Influence: 238.4] [Reference Citation Analysis (0)] |
| 27. | Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020; 181: 281-292. e6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4743] [Cited by in RCA: 6163] [Article Influence: 1232.6] [Reference Citation Analysis (0)] |
| 28. | Gallagher TM, Buchmeier MJ. Coronavirus spike proteins in viral entry and pathogenesis. Virology. 2001;279:371-374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 487] [Cited by in RCA: 491] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 29. | Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020; 181: 271-280. e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11946] [Cited by in RCA: 14275] [Article Influence: 2855.0] [Reference Citation Analysis (0)] |
| 30. | Letko M, Marzi A, Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol. 2020;5:562-569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1933] [Cited by in RCA: 2217] [Article Influence: 443.4] [Reference Citation Analysis (0)] |
| 31. | Li F. Structure, Function, and Evolution of Coronavirus Spike Proteins. Annu Rev Virol. 2016;3:237-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2002] [Cited by in RCA: 1840] [Article Influence: 204.4] [Reference Citation Analysis (0)] |
| 32. | Simmons G, Zmora P, Gierer S, Heurich A, Pöhlmann S. Proteolytic activation of the SARS-coronavirus spike protein: cutting enzymes at the cutting edge of antiviral research. Antiviral Res. 2013;100:605-614. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 292] [Cited by in RCA: 304] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 33. | Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor Recognition by the Novel Coronavirus from Wuhan: an Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. J Virol. 2020;94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3085] [Cited by in RCA: 2915] [Article Influence: 583.0] [Reference Citation Analysis (0)] |
| 34. | Lukassen S, Chua RL, Trefzer T, Kahn NC, Schneider MA, Muley T, Winter H, Meister M, Veith C, Boots AW, Hennig BP, Kreuter M, Conrad C, Eils R. SARS-CoV-2 receptor ACE2 and TMPRSS2 are primarily expressed in bronchial transient secretory cells. EMBO J. 2020;39:e105114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 465] [Cited by in RCA: 675] [Article Influence: 135.0] [Reference Citation Analysis (0)] |
| 35. | Yoshikawa T, Hill T, Li K, Peters CJ, Tseng CT. Severe acute respiratory syndrome (SARS) coronavirus-induced lung epithelial cytokines exacerbate SARS pathogenesis by modulating intrinsic functions of monocyte-derived macrophages and dendritic cells. J Virol. 2009;83:3039-3048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 214] [Cited by in RCA: 198] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 36. | Liang W, Feng Z, Rao S, Xiao C, Xue X, Lin Z, Zhang Q, Qi W. Diarrhoea may be underestimated: a missing link in 2019 novel coronavirus. Gut. 2020;69:1141-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 269] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 37. | Qi F, Qian S, Zhang S, Zhang Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem Biophys Res Commun. 2020;526:135-140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 523] [Cited by in RCA: 742] [Article Influence: 148.4] [Reference Citation Analysis (0)] |
| 38. | Tian Y, Rong L, Nian W, He Y. Review article: gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment Pharmacol Ther. 2020;51:843-851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 594] [Cited by in RCA: 583] [Article Influence: 116.6] [Reference Citation Analysis (0)] |
| 39. | Zhang H, Liao YS, Gong J, Liu J, Xia X, Zhang H. Clinical characteristics of coronavirus disease (COVID-19) patients with gastrointestinal symptoms: A report of 164 cases. Dig Liver Dis. 2020;52:1076-1079. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (3)] |
| 40. | Sungnak W, Huang N, Bécavin C, Berg M, Queen R, Litvinukova M, Talavera-López C, Maatz H, Reichart D, Sampaziotis F, Worlock KB, Yoshida M, Barnes JL; HCA Lung Biological Network. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26:681-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1985] [Cited by in RCA: 1956] [Article Influence: 391.2] [Reference Citation Analysis (0)] |
| 41. | Bourgonje AR, Abdulle AE, Timens W, Hillebrands JL, Navis GJ, Gordijn SJ, Bolling MC, Dijkstra G, Voors AA, Osterhaus AD, van der Voort PH, Mulder DJ, van Goor H. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J Pathol. 2020;251:228-248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 661] [Cited by in RCA: 740] [Article Influence: 148.0] [Reference Citation Analysis (0)] |
| 42. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30124] [Article Influence: 6024.8] [Reference Citation Analysis (3)] |
| 43. | Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, Xie C, Ma K, Shang K, Wang W, Tian DS. Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71:762-768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2495] [Cited by in RCA: 3341] [Article Influence: 668.2] [Reference Citation Analysis (0)] |
| 44. | Zhou Y, Fu B, Zheng X, Wang D, Zhao C, Qi Y, Sun R, Tian Z, Xu X, Wei H. Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. Natl Sci Rev. 2020;7:998-1002. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 727] [Cited by in RCA: 715] [Article Influence: 143.0] [Reference Citation Analysis (0)] |
| 45. | Max Roser HR, Ortiz-Ospina E, Hasell J. Coronavirus Pandemic (COVID-19) 2020. Available from: https://ourworldindata.org/coronavirus. |
| 46. | CDC COVID-19 Response Team. Severe Outcomes Among Patients with Coronavirus Disease 2019 (COVID-19) - United States, February 12-March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:343-346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1690] [Cited by in RCA: 1513] [Article Influence: 302.6] [Reference Citation Analysis (0)] |
| 47. | Epidemiology Working Group for NCIP Epidemic Response, Chinese Center for Disease Control and Prevention. [The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China]. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41:145-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1341] [Reference Citation Analysis (0)] |
| 48. | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708-1720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19202] [Cited by in RCA: 18881] [Article Influence: 3776.2] [Reference Citation Analysis (7)] |
| 49. | Wu P, Hao X, Lau EHY, Wong JY, Leung KSM, Wu JT, Cowling BJ, Leung GM. Real-time tentative assessment of the epidemiological characteristics of novel coronavirus infections in Wuhan, China, as at 22 January 2020. Euro Surveill. 2020;25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 255] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 50. | Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W; China Novel Coronavirus Investigating and Research Team. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382:727-733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18987] [Cited by in RCA: 17651] [Article Influence: 3530.2] [Reference Citation Analysis (0)] |
| 51. | Sun P, Lu X, Xu C, Sun W, Pan B. Understanding of COVID-19 based on current evidence. J Med Virol. 2020;92:548-551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 514] [Cited by in RCA: 559] [Article Influence: 111.8] [Reference Citation Analysis (0)] |
| 52. | Ahn DG, Shin HJ, Kim MH, Lee S, Kim HS, Myoung J, Kim BT, Kim SJ. Current Status of Epidemiology, Diagnosis, Therapeutics, and Vaccines for Novel Coronavirus Disease 2019 (COVID-19). J Microbiol Biotechnol. 2020;30:313-324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 549] [Cited by in RCA: 565] [Article Influence: 113.0] [Reference Citation Analysis (0)] |
| 53. | Munster VJ, Koopmans M, van Doremalen N, van Riel D, de Wit E. A Novel Coronavirus Emerging in China - Key Questions for Impact Assessment. N Engl J Med. 2020;382:692-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 922] [Cited by in RCA: 835] [Article Influence: 167.0] [Reference Citation Analysis (0)] |
| 54. | Cimolai N. Features of enteric disease from human coronaviruses: Implications for COVID-19. J Med Virol. 2020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 55. | Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int J Antimicrob Agents. 2020;55:105924. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3736] [Cited by in RCA: 3197] [Article Influence: 639.4] [Reference Citation Analysis (0)] |
| 56. | Zhai P, Ding Y, Wu X, Long J, Zhong Y, Li Y. The epidemiology, diagnosis and treatment of COVID-19. Int J Antimicrob Agents. 2020;55:105955. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 633] [Cited by in RCA: 509] [Article Influence: 101.8] [Reference Citation Analysis (0)] |
| 57. | Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, Ren R, Leung KSM, Lau EHY, Wong JY, Xing X, Xiang N, Wu Y, Li C, Chen Q, Li D, Liu T, Zhao J, Liu M, Tu W, Chen C, Jin L, Yang R, Wang Q, Zhou S, Wang R, Liu H, Luo Y, Liu Y, Shao G, Li H, Tao Z, Yang Y, Deng Z, Liu B, Ma Z, Zhang Y, Shi G, Lam TTY, Wu JT, Gao GF, Cowling BJ, Yang B, Leung GM, Feng Z. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med. 2020;382:1199-1207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11224] [Cited by in RCA: 9319] [Article Influence: 1863.8] [Reference Citation Analysis (0)] |
| 58. | Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475-481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6231] [Cited by in RCA: 6660] [Article Influence: 1332.0] [Reference Citation Analysis (0)] |
| 59. | Smyk W, Janik MK, Portincasa P, Milkiewicz P, Lammert F, Krawczyk M. COVID-19: Focus on the lungs but do not forget the gastrointestinal tract. Eur J Clin Invest. 2020;50:e13276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (1)] |
| 60. | Lotfi M, Hamblin MR, Rezaei N. COVID-19: Transmission, prevention, and potential therapeutic opportunities. Clin Chim Acta. 2020;508:254-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 525] [Cited by in RCA: 461] [Article Influence: 92.2] [Reference Citation Analysis (0)] |
| 61. | Wei WE, Li Z, Chiew CJ, Yong SE, Toh MP, Lee VJ. Presymptomatic Transmission of SARS-CoV-2 - Singapore, January 23-March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:411-415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 653] [Cited by in RCA: 621] [Article Influence: 124.2] [Reference Citation Analysis (0)] |
| 62. | van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, Tamin A, Harcourt JL, Thornburg NJ, Gerber SI, Lloyd-Smith JO, de Wit E, Munster VJ. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N Engl J Med. 2020;382:1564-1567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5894] [Cited by in RCA: 5667] [Article Influence: 1133.4] [Reference Citation Analysis (0)] |
| 63. | Ong SWX, Tan YK, Chia PY, Lee TH, Ng OT, Wong MSY, Marimuthu K. Air, Surface Environmental, and Personal Protective Equipment Contamination by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) From a Symptomatic Patient. JAMA. 2020;323:1610-1612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1461] [Cited by in RCA: 1392] [Article Influence: 278.4] [Reference Citation Analysis (0)] |
| 64. | Cai J, Sun W, Huang J, Gamber M, Wu J, He G. Indirect Virus Transmission in Cluster of COVID-19 Cases, Wenzhou, China, 2020. Emerg Infect Dis. 2020;26:1343-1345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 318] [Cited by in RCA: 335] [Article Influence: 67.0] [Reference Citation Analysis (0)] |
| 65. | Leung WK, To KF, Chan PK, Chan HL, Wu AK, Lee N, Yuen KY, Sung JJ. Enteric involvement of severe acute respiratory syndrome-associated coronavirus infection. Gastroenterology. 2003;125:1011-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 295] [Article Influence: 13.4] [Reference Citation Analysis (1)] |
| 66. | Chen L, Lou J, Bai Y, Wang M. COVID-19 Disease With Positive Fecal and Negative Pharyngeal and Sputum Viral Tests. Am J Gastroenterol. 2020;115:790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 105] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 67. | Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for Gastrointestinal Infection of SARS-CoV-2. Gastroenterology 2020; 158: 1831-1833. e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1985] [Cited by in RCA: 1995] [Article Influence: 399.0] [Reference Citation Analysis (1)] |
| 68. | Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, Spitters C, Ericson K, Wilkerson S, Tural A, Diaz G, Cohn A, Fox L, Patel A, Gerber SI, Kim L, Tong S, Lu X, Lindstrom S, Pallansch MA, Weldon WC, Biggs HM, Uyeki TM, Pillai SK; Washington State 2019-nCoV Case Investigation Team. First Case of 2019 Novel Coronavirus in the United States. N Engl J Med. 2020;382:929-936. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4155] [Cited by in RCA: 3822] [Article Influence: 764.4] [Reference Citation Analysis (1)] |
| 69. | Nobel YR, Phipps M, Zucker J, Lebwohl B, Wang TC, Sobieszczyk ME, Freedberg DE. Gastrointestinal Symptoms and Coronavirus Disease 2019: A Case-Control Study From the United States. Gastroenterology 2020; 159: 373-375. e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 265] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 70. | Pan Y, Zhang D, Yang P, Poon LLM, Wang Q. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect Dis. 2020;20:411-412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1120] [Cited by in RCA: 1127] [Article Influence: 225.4] [Reference Citation Analysis (0)] |
| 71. | Zhang W, Du RH, Li B, Zheng XS, Yang XL, Hu B, Wang YY, Xiao GF, Yan B, Shi ZL, Zhou P. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect. 2020;9:386-389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1192] [Cited by in RCA: 1219] [Article Influence: 243.8] [Reference Citation Analysis (0)] |
| 72. | Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, Tan W. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA. 2020;323:1843-1844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1894] [Cited by in RCA: 2658] [Article Influence: 531.6] [Reference Citation Analysis (0)] |
| 73. | Gu J, Han B, Wang J. COVID-19: Gastrointestinal Manifestations and Potential Fecal-Oral Transmission. Gastroenterology. 2020;158:1518-1519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 954] [Cited by in RCA: 949] [Article Influence: 189.8] [Reference Citation Analysis (1)] |
| 74. | Pan L, Mu M, Yang P, Sun Y, Wang R, Yan J, Li P, Hu B, Wang J, Hu C, Jin Y, Niu X, Ping R, Du Y, Li T, Xu G, Hu Q, Tu L. Clinical Characteristics of COVID-19 Patients With Digestive Symptoms in Hubei, China: A Descriptive, Cross-Sectional, Multicenter Study. Am J Gastroenterol. 2020;115:766-773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1160] [Cited by in RCA: 1206] [Article Influence: 241.2] [Reference Citation Analysis (0)] |
| 75. | Luo S, Zhang X, Xu H. Don't Overlook Digestive Symptoms in Patients With 2019 Novel Coronavirus Disease (COVID-19). Clin Gastroenterol Hepatol. 2020;18:1636-1637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 234] [Cited by in RCA: 233] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 76. | Jin X, Lian JS, Hu JH, Gao J, Zheng L, Zhang YM, Hao SR, Jia HY, Cai H, Zhang XL, Yu GD, Xu KJ, Wang XY, Gu JQ, Zhang SY, Ye CY, Jin CL, Lu YF, Yu X, Yu XP, Huang JR, Xu KL, Ni Q, Yu CB, Zhu B, Li YT, Liu J, Zhao H, Zhang X, Yu L, Guo YZ, Su JW, Tao JJ, Lang GJ, Wu XX, Wu WR, Qv TT, Xiang DR, Yi P, Shi D, Chen Y, Ren Y, Qiu YQ, Li LJ, Sheng J, Yang Y. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69:1002-1009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 944] [Cited by in RCA: 871] [Article Influence: 174.2] [Reference Citation Analysis (0)] |
| 77. | Ferm S, Fisher C, Pakala T, Tong M, Shah D, Schwarzbaum D, Cooley V, Hussain S, Kim SH. Analysis of Gastrointestinal and Hepatic Manifestations of SARS-CoV-2 Infection in 892 Patients in Queens, NY. Clin Gastroenterol Hepatol 2020; 18: 2378-2379. e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 78. | Hajifathalian K, Krisko T, Mehta A, Kumar S, Schwartz R, Fortune B, Sharaiha RZ; WCM-GI research group*. Gastrointestinal and Hepatic Manifestations of 2019 Novel Coronavirus Disease in a Large Cohort of Infected Patients From New York: Clinical Implications. Gastroenterology 2020; 159: 1137-1140. e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 107] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 79. | Redd WD, Zhou JC, Hathorn KE, McCarty TR, Bazarbashi AN, Thompson CC, Shen L, Chan WW. Prevalence and Characteristics of Gastrointestinal Symptoms in Patients With Severe Acute Respiratory Syndrome Coronavirus 2 Infection in the United States: A Multicenter Cohort Study. Gastroenterology 2020; 159: 765-767. e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 241] [Cited by in RCA: 283] [Article Influence: 56.6] [Reference Citation Analysis (0)] |
| 80. | Sierpiński R, Pinkas J, Jankowski M, Zgliczyński WS, Wierzba W, Gujski M, Szumowski Ł. Sex differences in the frequency of gastrointestinal symptoms and olfactory or taste disorders in 1942 nonhospitalized patients with coronavirus disease 2019 (COVID-19). Pol Arch Intern Med. 2020;130:501-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 81. | Xia W, Shao J, Guo Y, Peng X, Li Z, Hu D. Clinical and CT features in pediatric patients with COVID-19 infection: Different points from adults. Pediatr Pulmonol. 2020;55:1169-1174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 683] [Cited by in RCA: 636] [Article Influence: 127.2] [Reference Citation Analysis (0)] |
| 82. | Lui K, Wilson MP, Low G. Abdominal imaging findings in patients with SARS-CoV-2 infection: a scoping review. Abdom Radiol (NY). 2020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 83. | An P, Chen H, Jiang X, Su J, Xiao Y, Ding Y, Ren H, Ji M, Chen Y, Chen W. Clinical features of 2019 novel coronavirus pneumonia presented gastrointestinal symptoms but without fever onset. SSRN. 2020. [RCA] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Reference Citation Analysis (2)] |
| 84. | Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054-1062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17476] [Cited by in RCA: 18204] [Article Influence: 3640.8] [Reference Citation Analysis (0)] |
| 85. | Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, Akdis CA, Gao YD. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75:1730-1741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2139] [Cited by in RCA: 2342] [Article Influence: 468.4] [Reference Citation Analysis (0)] |
| 86. | Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, Ma K, Xu D, Yu H, Wang H, Wang T, Guo W, Chen J, Ding C, Zhang X, Huang J, Han M, Li S, Luo X, Zhao J, Ning Q. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2289] [Cited by in RCA: 2550] [Article Influence: 510.0] [Reference Citation Analysis (2)] |
| 87. | Xu H, Yan L, Qiu C, Jiao B, Chen Y, Tan X, Chen Z, Ai L, Xiao Y, Luo A, Li S. Analysis and Prediction of False Negative Results for SARS-CoV-2 Detection with Pharyngeal Swab Specimen in COVID-19 Patients: A Retrospective Study. 2020 Preprint. Available from: medRxiv:2020.03.26.20043042. [DOI] [Full Text] |
| 88. | Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, Huang H, Yang B, Huang C. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2428] [Cited by in RCA: 3009] [Article Influence: 601.8] [Reference Citation Analysis (1)] |
| 89. | Han R, Huang L, Jiang H, Dong J, Peng H, Zhang D. Early Clinical and CT Manifestations of Coronavirus Disease 2019 (COVID-19) Pneumonia. AJR Am J Roentgenol. 2020;215:338-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 219] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 90. | Fang D, Ma J, Guan J, Wang M, Song Y, Tian D, Li P. Manifestations of digestive system in hospitalized patients with novel coronavirus pneumonia in Wuhan, China: a single-center, descriptive study. Zhonghua Xiaohua Zazhi. 2020;40. [DOI] [Full Text] |
| 91. | Xu S, Fu L, Fei J, Xiang HX, Xiang Y, Tan ZX, Li MD, Liu FF, Li Y, Han MF, Li XY, Yu DX, Zhao H, Xu DX. Acute kidney injury at early stage as a negative prognostic indicator of patients with COVID-19: a hospital-based retrospective analysis. 2020 Preprint. Available from: medRxiv:2020.03.24.20042408. [DOI] [Full Text] |
| 92. | Ma L, Xie W, Li D, Shi L, Mao Y, Xiong Y, Zhang Y, Zhang M. Effect of SARS-CoV-2 infection upon male gonadal function: A single center-based study. 2020 Preprint. Available from: medRxiv:2020.03.21.20037267. [DOI] [Full Text] |
| 93. | Liu L, Liu W, Zheng Y, Jiang X, Kou G, Ding J, Wang Q, Huang Q, Ding Y, Ni W, Wu W, Tang S, Tan L, Hu Z, Xu W, Zhang Y, Zhang B, Tang Z, Zhang X, Li H, Rao Z, Jiang H, Ren X, Wang S, Zheng S. A preliminary study on serological assay for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in 238 admitted hospital patients. Microbes Infect. 2020;22:206-211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 109] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 94. | Huang Y, Yang R, Xu Y, Gong P. Clinical characteristics of 36 non-survivors with COVID-19 in Wuhan, China. 2020 Preprint. Available from: medRxiv:2020.02.27.20029009. [DOI] [Full Text] |
| 95. | Mao L, Wang M, Chen S, He Q, Chang J, Hong C, Zhou Y, Wang D, Li Y, Jin H, Hu B. Neurological Manifestations of Hospitalized Patients with COVID-19 in Wuhan, China: a retrospective case series study. 2020 Preprint. Available from: medRxiv:2020.02.22.20026500. [RCA] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 167] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 96. | Liu Y, Sun W, Li J, Chen L, Wang Y, Zhang L, Yu L. Clinical features and progression of acute respiratory distress syndrome in coronavirus disease 2019. 2020 Preprint. Available from: medRxiv:2020.02.17.20024166. [DOI] [Full Text] |
| 97. | Shu L, Wang X, Li M, Chen X, Ji N, Shi L, Wu M, Deng K, Wei J, Wang X, Cao Y, Yan J, Feng G. Clinical Characteristics of Moderate COVID-19 Patients Aggravation in Wuhan Stadium Cabin Hospital: A 571 Cases of Retrospective Cohort Study. J Med Virol. 2020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 98. | Ai J, Chen J, Wang Y, Liu X, Fan W, Qu G, Zhang M, Pei SP, Tang B, Yuan S, Li Y, Wang L, Huang G, Pei B. The cross-sectional study of hospitalized coronavirus disease 2019 patients in Xiangyang, Hubei province. 2020 Preprint. Available from: medRxiv:2020.02.19.20025023. [DOI] [Full Text] |
| 99. | Chen Q, Quan B, Li X, Gao G, Zheng W, Zhang J, Zhang Z, Liu C, Li L, Wang C, Zhang G, Li J, Dai Y, Yang J, Han W. A report of clinical diagnosis and treatment of nine cases of coronavirus disease 2019. J Med Virol. 2020;92:683-687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 100. | Zhao W, Yu S, Zha X, Wang N, Pang Q, Li T, Li A. Clinical characteristics and durations of hospitalized patients with COVID-19 in Beijing: a retrospective cohort study. 2020 Preprint. Available from: medRxiv:2020.03.13.20035436. [DOI] [Full Text] |
| 101. | Chang, Lin M, Wei L, Xie L, Zhu G, Dela Cruz CS, Sharma L. Epidemiologic and Clinical Characteristics of Novel Coronavirus Infections Involving 13 Patients Outside Wuhan, China. JAMA. 2020;323:1092-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 439] [Article Influence: 87.8] [Reference Citation Analysis (0)] |
| 102. | Zhao Z, Xie J, Yin M, Yang Y, He H, Jin T, Li W, Zhu X, Xu J, Zhao C, Li L, Li Y, Mengist HM, Zahid A, Yao Z, Ding C, Qi Y, Gao Y, Ma X. Clinical and Laboratory Profiles of 75 Hospitalized Patients with Novel Coronavirus Disease 2019 in Hefei, China. 2020 Preprint. Available from: medRxiv:2020.03.01.20029785. [DOI] [Full Text] |
| 103. | Yang P, Ding Y, Xu Z, Pu R, Li P, Yan J, Liu J, Meng F, Huang L, Shi L, Jiang T, Qin E, Zhao M, Zhang D, Zhao P, Yu L, Wang Z, Hong Z, Xiao Z, et al Epidemiological and clinical features of COVID-19 patients with and without pneumonia in Beijing, China. 2020 Preprint. Available from: medRxiv:2020.02.28.20028068. [DOI] [Full Text] |
| 104. | Li K, Wu J, Wu F, Guo D, Chen L, Fang Z, Li C. The Clinical and Chest CT Features Associated With Severe and Critical COVID-19 Pneumonia. Invest Radiol. 2020;55:327-331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 882] [Cited by in RCA: 797] [Article Influence: 159.4] [Reference Citation Analysis (0)] |
| 105. | Qi D, Yan X, Tang X, Peng J, Yu Q, Feng L, Yuan G, Zhang A, Chen Y, Yuan J, Huang X, Zhang X, Hu P, Song Y, Qian C, Sun Q, Wang D, Tong J, Xiang J. Epidemiological and clinical features of 2019-nCoV acute respiratory disease cases in Chongqing municipality, China: a retrospective, descriptive, multiple-center study. 2020 Preprint. Available from: medRxiv:2020.03.01.20029397. [DOI] [Full Text] |
| 106. | Xu X, Yu C, Qu J, Zhang L, Jiang S, Huang D, Chen B, Zhang Z, Guan W, Ling Z, Jiang R, Hu T, Ding Y, Lin L, Gan Q, Luo L, Tang X, Liu J. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2. Eur J Nucl Med Mol Imaging. 2020;47:1275-1280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 457] [Cited by in RCA: 491] [Article Influence: 98.2] [Reference Citation Analysis (0)] |
| 107. | Lin L, Jiang X, Zhang Z, Huang S, Zhang Z, Fang Z, Gu Z, Gao L, Shi H, Mai L, Liu Y, Lin X, Lai R, Yan Z, Li X, Shan H. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut. 2020;69:997-1001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 630] [Cited by in RCA: 658] [Article Influence: 131.6] [Reference Citation Analysis (0)] |
| 108. | Wen Y, Wei L, Li Y, Tang X, Feng S, Leung K, Wu X, Pan XF, Chen C, Xia J, Zou X, Feng T, Mei S. Epidemiological and clinical characteristics of COVID-19 in Shenzhen, the largest migrant city of China. 2020 Preprint. Available from: medRxiv:2020.03.22.20035246. [DOI] [Full Text] |
| 109. | Xu Y, Xu Z, Liu X, Cai L, Zheng H, Huang Y, Zhou L, Huang L, Lin Y, Deng L, Li J, Chen S, Liu D, Lin Z, Zhou L, He W, Liu X, Li Y. Clinical findings in critical ill patients infected with SARS-Cov-2 in Guangdong Province, China: a multi-center, retrospective, observational study. 2020 Preprint. Available from: medRxiv:2020.03.03.20030668. [DOI] [Full Text] |
| 110. | Yan S, Song X, Lin F, Zhu H, Wang X, Li M, Ruan J, Lin C, Liu X, Wu Q, Luo Z, Fu W, Chen S, Yuan Y, Liu S, Yao J, Lv C. Clinical Characteristics of Coronavirus Disease 2019 in Hainan, China. 2020 Preprint. Available from: medRxiv:2020.03.19.20038539. [DOI] [Full Text] |
| 111. | Wang L, Gao YH, Lou LL, Zhang GJ. The clinical dynamics of 18 cases of COVID-19 outside of Wuhan, China. Eur Respir J. 2020;55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 81] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 112. | Chen X, Zheng F, Qing Y, Ding S, Yang D, Lei C, Yin Z, Zhou X, Jiang D, Zuo Q, He J, Lv J, Chen P, Chen Y, Peng H, Li H, Xie Y, Liu J, Zhou Z. Epidemiological and clinical features of 291 cases with coronavirus disease 2019 in areas adjacent to Hubei, China: a double-center observational study. 2020 Preprint. Available from: medRxiv:2020.03.03.20030353. [DOI] [Full Text] |
| 113. | Liu S, Luo H, Wang Y, Wang D, Ju S, Yang YJC. Characteristics and associations with severity in COVID-19 patients: a multicentre cohort study from Jiangsu Province, China. SSRN. 2020;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 114. | Fan L, Liu C, Li N, Liu H, Gu Y, Liu Y, Chen Y. Medical treatment of 55 patients with COVID-19 from seven cities in northeast China who fully recovered: a single-center, retrospective, observational study. 2020 Preprint. Available from: medRxiv:2020.03.28.20045955. [DOI] [Full Text] |
| 115. | Yao N, Wang SN, Lian JQ, Sun YT, Zhang GF, Kang WZ, Kang W. [Clinical characteristics and influencing factors of patients with novel coronavirus pneumonia combined with liver injury in Shaanxi region]. Zhonghua Gan Zang Bing Za Zhi. 2020;28:234-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 39] [Reference Citation Analysis (0)] |
| 116. | Tian S, Chang Z, Wang Y, Wu M, Zhang W, Zhou G, Zou X, Tian H, Xiao T, Xing J, Chen J, Han J, Ning K, Wu T. Clinical characteristics and reasons of different duration from onset to release from quarantine for patients with COVID-19 Outside Hubei province, China. 2020 Preprint. Available from: medRxiv:2020.03.21.20038778. [DOI] [Full Text] |
| 117. | Song F, Shi N, Shan F, Zhang Z, Shen J, Lu H, Ling Y, Jiang Y, Shi Y. Emerging 2019 Novel Coronavirus (2019-nCoV) Pneumonia. Radiology. 2020;295:210-217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 903] [Cited by in RCA: 784] [Article Influence: 156.8] [Reference Citation Analysis (0)] |
| 118. | Lu H, Ai J, Shen Y, Li Y, Li T, Zhou X, Zhang H, Zhang Q, Ling Y, Wang S, Qu H, Gao Y, Li Y, Yu K, Zhu D, Zhu H, Tian R, Zeng M, Li Q, et al A descriptive study of the impact of diseases control and prevention on the epidemics dynamics and clinical features of SARS-CoV-2 outbreak in Shanghai, lessons learned for metropolis epidemics prevention. 2020 Preprint. Available from: medRxiv:2020.02.19.20025031. [DOI] [Full Text] |
| 119. | Fu H, Xu H, Zhang N, Xu H, Li Z, Chen H, Xu R, Sun R, Wen L, Xie L, Liu H, Zhang K, Fu C, Hou K, Yang Z, Yang M, Guo Y. Association between Clinical, Laboratory and CT Characteristics and RT-PCR Results in the Follow-up of COVID-19 patients. 2020 Preprint. Available from: medRxiv:2020.03.19.20038315. [DOI] [Full Text] |
| 120. | Fu H, Li H, Tang X, Li X, Shen J, Zhou Y, Xu B, Luo Y. Analysis on the Clinical Characteristics of 36 Cases of Novel Coronavirus Pneumonia in Kunming. 2020 Preprint. Available from: medRxiv:2020.02.28.20029173. [DOI] [Full Text] |
| 121. | Xu XW, Wu XX, Jiang XG, Xu KJ, Ying LJ, Ma CL, Li SB, Wang HY, Zhang S, Gao HN, Sheng JF, Cai HL, Qiu YQ, Li LJ. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1298] [Cited by in RCA: 1263] [Article Influence: 252.6] [Reference Citation Analysis (0)] |
| 122. | Qian GQ, Yang NB, Ding F, Ma AHY, Wang ZY, Shen YF, Shi CW, Lian X, Chu JG, Chen L, Wang ZY, Ren DW, Li GX, Chen XQ, Shen HJ, Chen XM. Epidemiologic and clinical characteristics of 91 hospitalized patients with COVID-19 in Zhejiang, China: a retrospective, multi-centre case series. QJM. 2020;113:474-481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 249] [Cited by in RCA: 262] [Article Influence: 52.4] [Reference Citation Analysis (0)] |
| 123. | Chen D, Li X, Song Q, Hu C, Su F, Dai J. Hypokalemia and Clinical Implications in Patients with Coronavirus Disease 2019 (COVID-19). 2020 Preprint. Available from: medRxiv:2020.02.27.20028530. [DOI] [Full Text] |
| 124. | Kuang Y, Zhang H, Zhou R, Lin S, Lin M, Wang J, Pang P, Ma L, Ji WJZ, China. Epidemiological and clinical characteristics of 944 cases of 2019 novel Coronavirus infection of non-COVID-19 exporting city, Zhejiang, China. SSRN. 2020. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 125. | Hu Z, Song C, Xu C, Jin G, Chen Y, Xu X, Ma H, Chen W, Lin Y, Zheng Y, Wang J, Hu Z, Yi Y, Shen H. Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China. Sci China Life Sci. 2020;63:706-711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 802] [Cited by in RCA: 858] [Article Influence: 171.6] [Reference Citation Analysis (0)] |
| 126. | Cholankeril G, Podboy A, Aivaliotas V, Pham EA, Tarlow B, Spencer S, Kim D, Hsing A, Ahmed A. Association of Digestive Symptoms and Hospitalization in Patients with SARS-CoV-2 Infection. 2020 Preprint. Available from: medRxiv:2020.04.23.20076935. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 127. | Kujawski SA, Wong KK, Collins JP, Epstein L, Killerby ME, Midgley CM, Abedi GR, Ahmed NS, Almendares O, Alvarez FN, Anderson KN, Balter S, Barry V, Bartlett K, Beer K, Ben-Aderet MA, Benowitz I, Biggs H, Binder AM, et al First 12 patients with coronavirus disease 2019 (COVID-19) in the United States. 2020 Preprint. Available from: medRxiv:2020.03.09.20032896. [RCA] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 128. | COVID-19 National Emergency Response Center, Epidemiology and Case Management Team, Korea Centers for Disease Control and Prevention. Early Epidemiological and Clinical Characteristics of 28 Cases of Coronavirus Disease in South Korea. Osong Public Health Res Perspect. 2020;11:8-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 93] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 129. | Young BE, Ong SWX, Kalimuddin S, Low JG, Tan SY, Loh J, Ng OT, Marimuthu K, Ang LW, Mak TM, Lau SK, Anderson DE, Chan KS, Tan TY, Ng TY, Cui L, Said Z, Kurupatham L, Chen MI, Chan M, Vasoo S, Wang LF, Tan BH, Lin RTP, Lee VJM, Leo YS, Lye DC; Singapore 2019 Novel Coronavirus Outbreak Research Team. Epidemiologic Features and Clinical Course of Patients Infected With SARS-CoV-2 in Singapore. JAMA. 2020;323:1488-1494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1346] [Cited by in RCA: 1366] [Article Influence: 273.2] [Reference Citation Analysis (0)] |
| 130. | Pung R, Chiew CJ, Young BE, Chin S, Chen MI, Clapham HE, Cook AR, Maurer-Stroh S, Toh MPHS, Poh C, Low M, Lum J, Koh VTJ, Mak TM, Cui L, Lin RVTP, Heng D, Leo YS, Lye DC, Lee VJM; Singapore 2019 Novel Coronavirus Outbreak Research Team. Investigation of three clusters of COVID-19 in Singapore: implications for surveillance and response measures. Lancet. 2020;395:1039-1046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 471] [Cited by in RCA: 437] [Article Influence: 87.4] [Reference Citation Analysis (0)] |
| 131. | Tabata S, Imai K, Kawano S, Ikeda M, Kodama T, Miyoshi K, Obinata H, Mimura S, Kodera T, Kitagaki M, Sato M, Suzuki S, Ito T, Uwabe Y, Tamura K. The clinical characteristics of COVID-19: a retrospective analysis of 104 patients from the outbreak on board the Diamond Princess cruise ship in Japan. 2020 Preprint. Available from: medRxiv:2020.03.18.20038125. [DOI] [Full Text] |
| 132. | Kluytmans M, Buiting A, Pas S, Bentvelsen R, van den Bijllaardt W, van Oudheusden A, van Rijen M, Verweij J, Koopmans M, Kluytmans J. SARS-CoV-2 infection in 86 healthcare workers in two Dutch hospitals in March 2020. 2020 Preprint. Available from: medRxiv:2020.03.23.20041913. [DOI] [Full Text] |
| 133. | Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, Niemeyer D, Jones TC, Vollmar P, Rothe C, Hoelscher M, Bleicker T, Brünink S, Schneider J, Ehmann R, Zwirglmaier K, Drosten C, Wendtner C. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4682] [Cited by in RCA: 4811] [Article Influence: 962.2] [Reference Citation Analysis (0)] |
| 134. | Dreher M, Kersten A, Bickenbach J, Balfanz P, Hartmann B, Cornelissen C, Daher A, Stöhr R, Kleines M, Lemmen SW, Brokmann JC, Müller T, Müller-Wieland D, Marx G, Marx N. The Characteristics of 50 Hospitalized COVID-19 Patients With and Without ARDS. Dtsch Arztebl Int. 2020;117:271-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 134] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 135. | Gritti G, Raimondi F, Ripamonti D, Riva I, Landi F, Alborghetti L, Frigeni M, Damiani M, Micò C, Fagiuoli S, Cosentini R, Luca Lorini F, Gandini L, Novelli L, Morgan JP, Owens BMJ, Kanhai K, Tonkovic Reljanovic G, Rizzi M. IL-6 signalling pathway inactivation with siltuximab in patients with COVID-19 respiratory failure: an observational cohort study. 2020 Preprint. Available from: medRxiv:2020.04.01.20048561. [DOI] [Full Text] |
| 136. | Spiteri G, Fielding J, Diercke M, Campese C, Enouf V, Gaymard A, Bella A, Sognamiglio P, Sierra Moros MJ, Riutort AN, Demina YV, Mahieu R, Broas M, Bengnér M, Buda S, Schilling J, Filleul L, Lepoutre A, Saura C, Mailles A, Levy-Bruhl D, Coignard B, Bernard-Stoecklin S, Behillil S, van der Werf S, Valette M, Lina B, Riccardo F, Nicastri E, Casas I, Larrauri A, Salom Castell M, Pozo F, Maksyutov RA, Martin C, Van Ranst M, Bossuyt N, Siira L, Sane J, Tegmark-Wisell K, Palmérus M, Broberg EK, Beauté J, Jorgensen P, Bundle N, Pereyaslov D, Adlhoch C, Pukkila J, Pebody R, Olsen S, Ciancio BC. First cases of coronavirus disease 2019 (COVID-19) in the WHO European Region, 24 January to 21 February 2020. Euro Surveill. 2020;25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 360] [Cited by in RCA: 365] [Article Influence: 73.0] [Reference Citation Analysis (0)] |
| 137. | COVID-19 National Incident Room Surveillance Team. COVID-19, Australia: Epidemiology Report 7 (Reporting week ending 19:00 AEDT 14 March 2020). Commun Dis Intell (2018). 2020;44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 138. | Cereda PM, Pagani L, Romero E. Prevalence of antibody to human coronaviruses 229E, OC43 and neonatal calf diarrhea coronavirus (NCDCV) in patients of Northern Italy. Eur J Epidemiol. 1986;2:112-117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 139. | Gerna G, Cereda PM, Revello MG, Cattaneo E, Battaglia M, Gerna MT. Antigenic and biological relationships between human coronavirus OC43 and neonatal calf diarrhoea coronavirus. J Gen Virol. 1981;54:91-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 140. | Gerna G, Passarani N, Cereda PM, Battaglia M. Antigenic relatedness of human enteric coronavirus strains to human coronavirus OC43: a preliminary report. J Infect Dis. 1984;150:618-619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 141. | Small JD, Woods RD. Relatedness of rabbit coronavirus to other coronaviruses. Adv Exp Med Biol. 1987;218:521-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 142. | Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5228] [Cited by in RCA: 5786] [Article Influence: 1157.2] [Reference Citation Analysis (2)] |
| 143. | Li Y, Xiao SY. Hepatic involvement in COVID-19 patients: Pathology, pathogenesis, and clinical implications. J Med Virol. 2020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 144. | Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428-430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1348] [Cited by in RCA: 1295] [Article Influence: 259.0] [Reference Citation Analysis (4)] |
| 145. | Yang RX, Zheng RD, Fan JG. Etiology and management of liver injury in patients with COVID-19. World J Gastroenterol. 2020;26:4753-4762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 58] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (2)] |
| 146. | Cai Q, Huang D, Ou P, Yu H, Zhu Z, Xia Z, Su Y, Ma Z, Zhang Y, Li Z, He Q, Liu L, Fu Y, Chen J. COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy. 2020;75:1742-1752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 332] [Article Influence: 66.4] [Reference Citation Analysis (0)] |
| 147. | Kasarala G, Tillmann HL. Standard liver tests. Clin Liver Dis (Hoboken). 2016;8:13-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 148. | Singh S, Khan A. Clinical Characteristics and Outcomes of Coronavirus Disease 2019 Among Patients With Preexisting Liver Disease in the United States: A Multicenter Research Network Study. Gastroenterology 2020; 159: 768-771. e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 270] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 149. | Ji D, Qin E, Xu J, Zhang D, Cheng G, Wang Y, Lau G. Non-alcoholic fatty liver diseases in patients with COVID-19: A retrospective study. J Hepatol. 2020;73:451-453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 336] [Cited by in RCA: 408] [Article Influence: 81.6] [Reference Citation Analysis (2)] |
| 150. | Di Giorgio A, Nicastro E, Speziani C, De Giorgio M, Pasulo L, Magro B, Fagiuoli S, D' Antiga L. Health status of patients with autoimmune liver disease during SARS-CoV-2 outbreak in northern Italy. J Hepatol. 2020;73:702-705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 151. | Gerussi A, Rigamonti C, Elia C, Cazzagon N, Floreani A, Pozzi R, Pozzoni P, Claar E, Pasulo L, Fagiuoli S, Cristoferi L, Carbone M, Invernizzi P. Coronavirus Disease 2019 (COVID-19) in autoimmune hepatitis: a lesson from immunosuppressed patients. Hepatol Commun. 2020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 152. | SECURE-Cirrhosis and EASL supported COVID-Hep. Combined update - 25 Aug 2020. Available from: https://covid-hep.net/img/update_20200825.pdf. |
| 153. | Hadi A, Werge M, Kristiansen KT, Pedersen UG, Karstensen JG, Novovic S, Gluud LL. Coronavirus Disease-19 (COVID-19) associated with severe acute pancreatitis: Case report on three family members. Pancreatology. 2020;20:665-667. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 169] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 154. | Kumaran NK, Karmakar BK, Taylor OM. Coronavirus disease-19 (COVID-19) associated with acute necrotising pancreatitis (ANP). BMJ Case Rep. 2020;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 155. | Wang F, Wang H, Fan J, Zhang Y, Wang H, Zhao Q. Pancreatic Injury Patterns in Patients With Coronavirus Disease 19 Pneumonia. Gastroenterology. 2020;159:367-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 276] [Cited by in RCA: 325] [Article Influence: 65.0] [Reference Citation Analysis (0)] |
| 156. | Bruno G, Fabrizio C, Santoro CR, Buccoliero GB. Pancreatic injury in the course of coronavirus disease 2019: A not-so-rare occurrence. J Med Virol. 2020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 157. | Yang JK, Lin SS, Ji XJ, Guo LM. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47:193-199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 686] [Cited by in RCA: 781] [Article Influence: 52.1] [Reference Citation Analysis (0)] |
| 158. | Ding Y, He L, Zhang Q, Huang Z, Che X, Hou J, Wang H, Shen H, Qiu L, Li Z, Geng J, Cai J, Han H, Li X, Kang W, Weng D, Liang P, Jiang S. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J Pathol. 2004;203:622-630. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 729] [Cited by in RCA: 789] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 159. | Liu F, Long X, Zhang B, Zhang W, Chen X, Zhang Z. ACE2 Expression in Pancreas May Cause Pancreatic Damage After SARS-CoV-2 Infection. Clin Gastroenterol Hepatol 2020; 18: 2128-2130. e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 343] [Cited by in RCA: 473] [Article Influence: 94.6] [Reference Citation Analysis (0)] |
| 160. | Li H, Liu L, Zhang D, Xu J, Dai H, Tang N, Su X, Cao B. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 2020;395:1517-1520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 772] [Cited by in RCA: 887] [Article Influence: 177.4] [Reference Citation Analysis (0)] |
| 161. | Szatmary P, Arora A, Thomas Raraty MG, Joseph Dunne DF, Baron RD, Halloran CM. Emerging Phenotype of Severe Acute Respiratory Syndrome-Coronavirus 2-associated Pancreatitis. Gastroenterology. 2020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 162. | Barlow A, Landolf KM, Barlow B, Yeung SYA, Heavner JJ, Claassen CW, Heavner MS. Review of Emerging Pharmacotherapy for the Treatment of Coronavirus Disease 2019. Pharmacotherapy. 2020;40:416-437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 139] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 163. | Iavarone M, D'Ambrosio R, Soria A, Triolo M, Pugliese N, Del Poggio P, Perricone G, Massironi S, Spinetti A, Buscarini E, Viganò M, Carriero C, Fagiuoli S, Aghemo A, Belli LS, Lucà M, Pedaci M, Rimondi A, Rumi MG, Invernizzi P, Bonfanti P, Lampertico P. High rates of 30-day mortality in patients with cirrhosis and COVID-19. J Hepatol. 2020;73:1063-1071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 279] [Cited by in RCA: 276] [Article Influence: 55.2] [Reference Citation Analysis (2)] |
| 164. | Lau G, Ward JW. Synthesis of Liver Associations Recommendations for Hepatology and Liver Transplant Care During the COVID-19 Pandemic. Clin Liver Dis (Hoboken). 2020;15:204-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 165. | Boettler T, Newsome PN, Mondelli MU, Maticic M, Cordero E, Cornberg M, Berg T. Care of patients with liver disease during the COVID-19 pandemic: EASL-ESCMID position paper. JHEP Rep. 2020;2:100113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 312] [Cited by in RCA: 334] [Article Influence: 66.8] [Reference Citation Analysis (1)] |
| 166. | Fix OK, Hameed B, Fontana RJ, Kwok RM, McGuire BM, Mulligan DC, Pratt DS, Russo MW, Schilsky ML, Verna EC, Loomba R, Cohen DE, Bezerra JA, Reddy KR, Chung RT. Clinical Best Practice Advice for Hepatology and Liver Transplant Providers During the COVID-19 Pandemic: AASLD Expert Panel Consensus Statement. Hepatology. 2020;72:287-304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 280] [Cited by in RCA: 422] [Article Influence: 84.4] [Reference Citation Analysis (0)] |
| 167. | Lleo A, Invernizzi P, Lohse AW, Aghemo A, Carbone M. Management of patients with autoimmune liver disease during COVID-19 pandemic. J Hepatol. 2020;73:453-455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 168. | Kushner T, Cafardi J. Chronic Liver Disease and COVID-19: Alcohol Use Disorder/Alcohol-Associated Liver Disease, Nonalcoholic Fatty Liver Disease/Nonalcoholic Steatohepatitis, Autoimmune Liver Disease, and Compensated Cirrhosis. Clin Liver Dis (Hoboken). 2020;15:195-199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 169. | Chela H, Pasha SB, Wan XF, Ghouri YA. A review on medical management of inflammatory bowel disease during the coronavirus disease 2019 pandemic. J Gastroenterol Hepatol. 2020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 170. | World Health Organization. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19) 16-24 February 2020. Available from: https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf. |
| 171. | Sultan S, Lim JK, Altayar O, Davitkov P, Feuerstein JD, Siddique SM, Falck-Ytter Y, El-Serag HB, AGA Institute. AGA Rapid Recommendations for Gastrointestinal Procedures During the COVID-19 Pandemic. Gastroenterology. 2020;159:739-758.e4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 244] [Cited by in RCA: 278] [Article Influence: 55.6] [Reference Citation Analysis (0)] |
| 172. | Centers for Disease Control and Prevention. Infection Control Guidance 2020. Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html. |
| 173. | Joint Gastroenterology Societies. Joint GI Society message: COVID-19 clinical insights for our community of gastroenterologists and gastroenterology care providers. Available from: https://gi.org/2020/03/15/joint-gi-society-message-on-covid-19/. |
| 174. | American College of Surgeons. COVID-19: Recommendations for management of elective surgical procedures. Available from: https://www.facs.org/covid-19/clinical-guidance/elective-surgery. |
| 175. | Centers for Medicare & Medicaid Services. Non-Emergent, Elective Medical Services, and Treatment Recommendations 2020. Available from: https://www.cms.gov/files/document/cms-non-emergent-elective-medical-recommendations.pdf. |
| 176. | Politico. Surgeon General advises hospitals to cancel elective surgeries. Available from: https://www.politico.com/news/2020/03/14/surgeon-general-elective-surgeries-coronavirus-129405. |
| 177. | Ménard C, Waschke K, Tse F, Borgaonkar M, Forbes N, Barkun A, Martel M. COVID-19: Framework for the Resumption of Endoscopic Activities From the Canadian Association of Gastroenterology. J Can Assoc Gastroenterol. 2020;3:243-245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 178. | Tsay C, Shung D, Stemmer Frumento K, Laine L. Early Colonoscopy Does Not Improve Outcomes of Patients With Lower Gastrointestinal Bleeding: Systematic Review of Randomized Trials. Clin Gastroenterol Hepatol. 2020;18:1696-1703.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |