Published online Aug 28, 2020. doi: 10.13105/wjma.v8.i4.285
Peer-review started: March 11, 2020
First decision: June 18, 2020
Revised: July 30, 2020
Accepted: August 22, 2020
Article in press: August 22, 2020
Published online: August 28, 2020
Processing time: 183 Days and 1.1 Hours
Small cell neuroendocrine carcinoma (SNEC) is an extremely aggressive tumor and mainly occurs in the lung. Primary extra-pulmonary SNEC is rare. To date, only 11 primary SNECs occurring in the oral cavity have been reported in the English literature. We describe a case of primary SNEC of the right posterior tongue in a 46-year-old man. The patient had stage IVA disease and received adjuvant chemotherapy, followed by radical surgery and radiotherapy. He remained tumor-free for 20 mo before death due to gastrointestinal metastasis. The relevant literature on the 11 previously reported patients was reviewed, and the clinical features, histopathological characteristics, differential diagnosis and therapeutic strategies of this rare tumor were analyzed.
Core Tip: Primary extra-pulmonary small cell neuroendocrine carcinoma (SNEC) is extremely rare. There are only 11 primary SNECs occurring in the oral cavity reported in the English literature. This time, we describe a case of primary SNEC of the right posterior tongue in a 46-year-old man. The treatment of this patient is described in detail. The relevant literature of the 11 previously reported patients was reviewed, and the clinical features, histopathological characteristics, differential diagnosis and therapeutic strategies of this rare tumor were analyzed.
- Citation: Zhou Y, Zhou HC, Peng H, Zhang ZH. Primary small cell neuroendocrine carcinoma of the right posterior tongue. World J Meta-Anal 2020; 8(4): 285-291
- URL: https://www.wjgnet.com/2308-3840/full/v8/i4/285.htm
- DOI: https://dx.doi.org/10.13105/wjma.v8.i4.285
Small cell neuroendocrine carcinoma (SNEC) is the most common type of pulmonary neoplasm and is an aggressive malignant tumor with a high tendency for regional and distant metastasis. Extra-pulmonary SNECs account for 2.5%-5% of all SNECs, of which head and neck SNECs account for 10%-15%[1]. The first case of SNEC in the head and neck was reported in 1963, and the larynx is the most common site, followed by the salivary glands and the sinonasal region[2]. Primary SNEC in the oral cavity is extremely rare and to date only 11 primary SNECs occurring in the oral cavity have been reported in the English literature[3-13].
The management of extra-pulmonary SNECs has not been standardized, but is extrapolated from pulmonary SNEC due to their similar clinicopathologic features[14,15]. In addition to chemotherapy as an effective therapeutic modality, radical surgery and radiation therapy may also play an important role depending on the primary site and clinical stage of the tumor[16]. The current report presents a rare case of primary SNEC arising in the right posterior tongue. The clinicopathologic characteristics of this rare tumor are discussed and the relevant literature is reviewed.
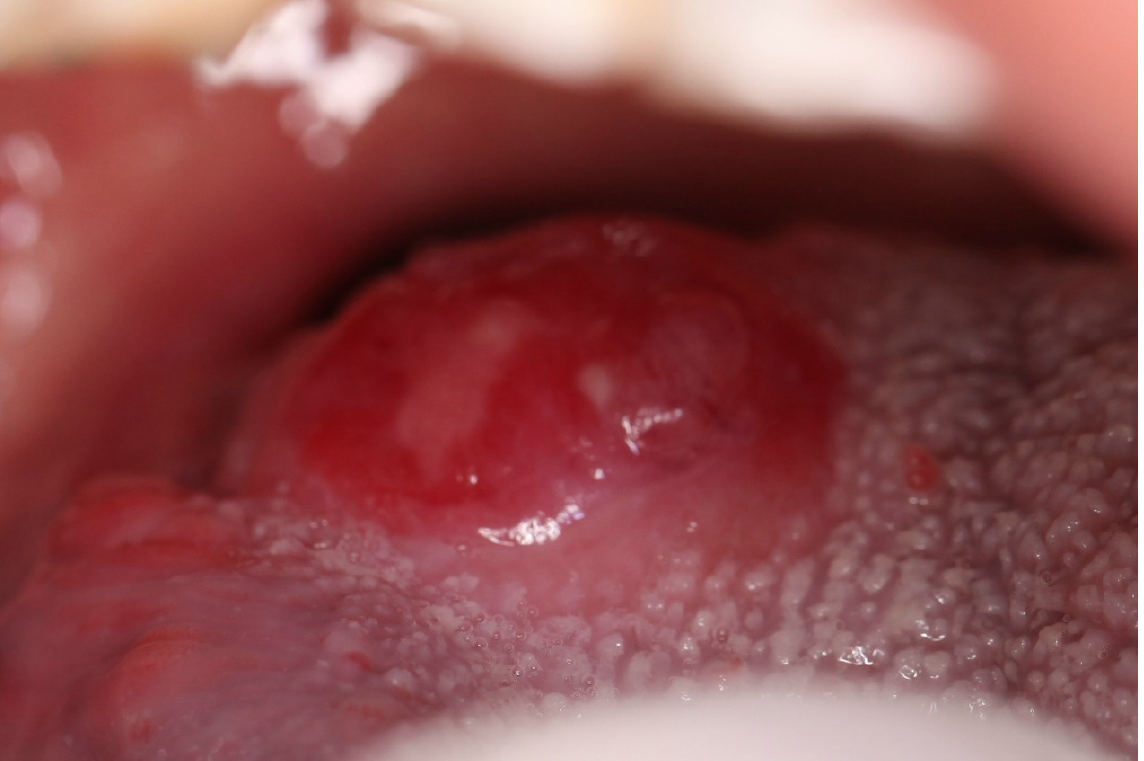
A 46-year-old man, who had a 30-year history of smoking and alcohol consumption, presented to the oral and maxillofacial department in September 2013 with a painful mass which grew rapidly of the right posterior tongue during the previous 3 mo and a painful mass in the contralateral upper jugular area for 1 mo. Physical examination identified a hard polypoid mass of the right posterior tongue, measuring 2.5 cm × 2.5 cm, with obvious tenderness and low mobility (Figure 1). A painful mass with low mobility, measuring 2.0 cm × 3.0 cm, in the contralateral upper jugular area was also identified.
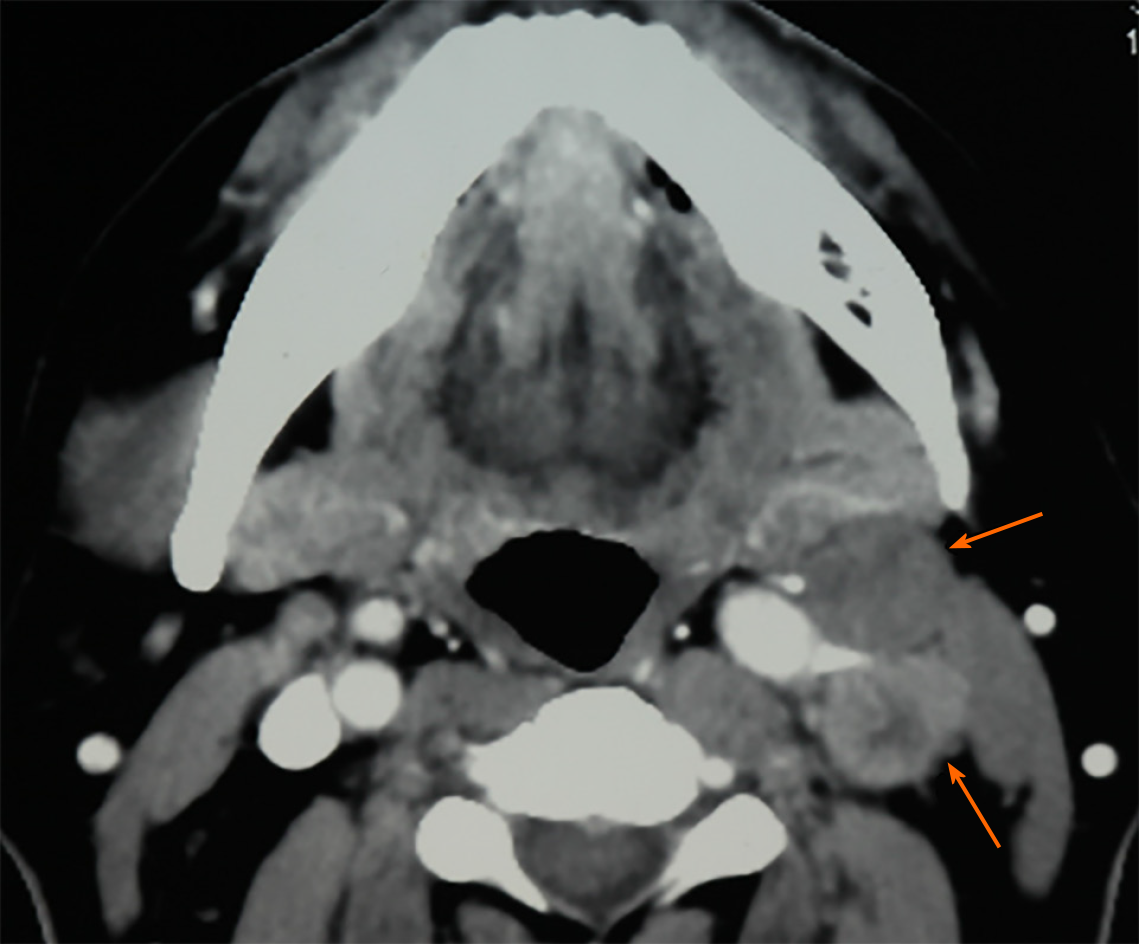
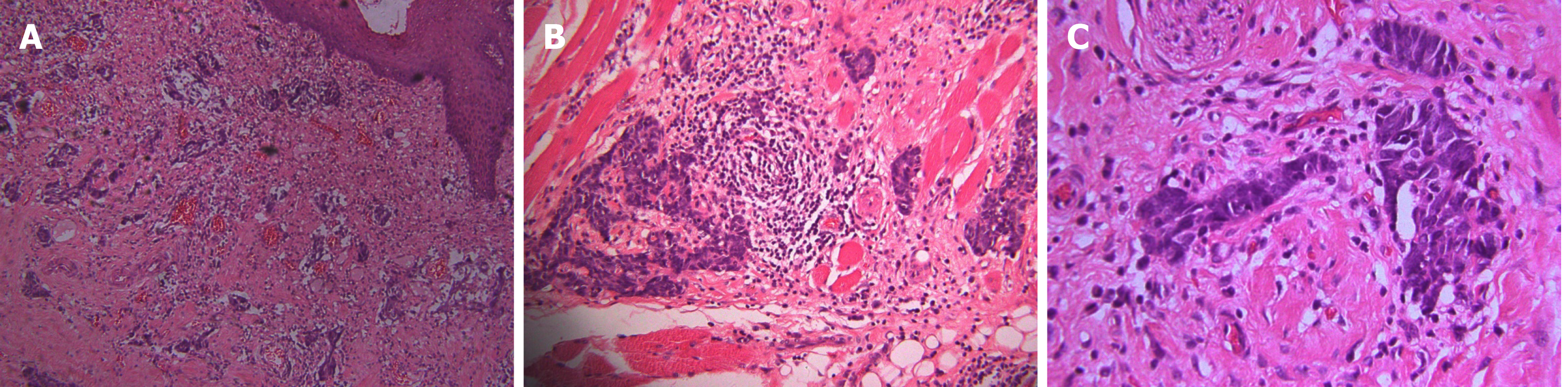
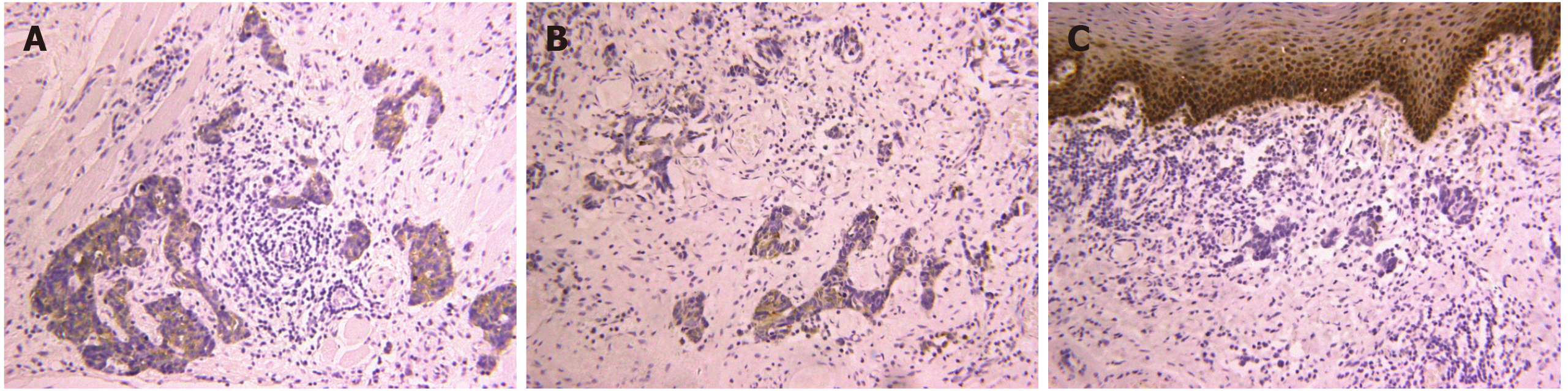
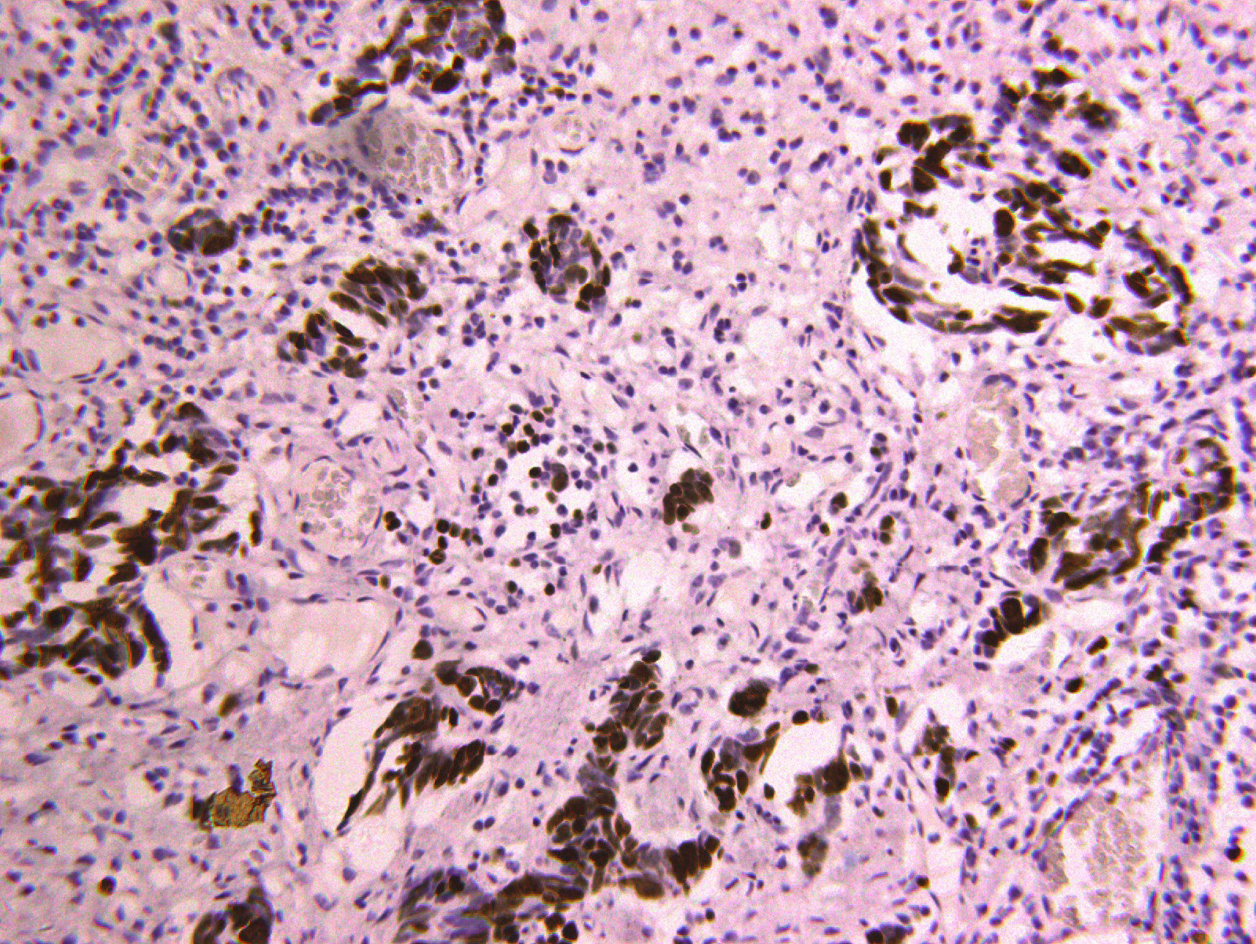
Computerized tomography (CT) scanning showed an ill-defined mass with heterogeneous enhancement in the right posterior tongue and contralateral cervical lymph node enlargement (Figure 2). A CT scan of the chest and abdomen and a positron emission tomography-CT scan revealed no abnormalities. Findings from laboratory examinations, including routine blood, blood biochemistry and urine analysis, were within normal limits. An incision biopsy under local anesthesia was performed. Microscopically, round and spindly small cells presented with ovoid or spindle shaped nuclei, fine granular chromatin, inconspicuous nucleoli and scant cytoplasm, forming broad nests, sheets or cord shapes (Figure 3). Immunohisto-chemically, these cells were positive for synaptophysin (referred to as syn), N-CAM (CD56), chromogranin A and cytokeratin AE1/AE3 (Figure 4). The proliferation index evaluated by Ki-67 labeling was 90% (Figure 5). The cells were negative for human melanoma black45 (referred to as HMB-45), cytokeratin 20 (referred to as CK20), vimentin, S100 protein, leucocyte common antigen, CD99, smooth muscle actin and thyroid transcription factor1.
A combined modality therapy was approved by multidisciplinary discussion. After two cycles of chemotherapy (cisplatin 75 mg/m2, day 1 and etoposide 100 mg/m2, day 1-3, 3 wk per cycle), the primary tumor and cervical lymph nodes partially decreased according to the Response Evaluation Criteria in Solid Tumors. Radical resection, including extensive resection of the primary tumor with contralateral radical and homolateral functional neck dissection and reconstruction of the tongue with a left free forearm flap was performed. The diagnosis of SCNC was confirmed by postoperative pathology. A 60 Gy dose of radiotherapy was administered after surgery. The patient remained tumor-free for 20 mo before his death due to gastrointestinal metastasis.
According to the World Health Organization Blue Book 2017, neuroendocrine carcinomas (NECs) are now classified into three categories: Well-differentiated, moderately-differentiated and poorly-differentiated, which is additionally divided into two subtypes: SNEC and large cell NEC[17]. Primary NECs in the oral cavity have been subclassified into typical carcinoid, atypical carcinoid, large cell NEC and SNEC[18]. The synonyms for SNEC include “small cell carcinoma”, “oat cell carcinoma” and “anaplastic small cell carcinoma”[19]. Primary SNEC occurring in the oral cavity is extremely rare and has a poor prognosis. Positron emission tomography-CT and CT images confirmed that the current patient had a rare primary SNEC of the oral cavity, and not a metastatic SNEC arising from the lung.
The clinical characteristics of these rare cases reported in the English literature are shown in Table 1. Most of the patients were men (81.8%). The median age of the patients was 67.5 years (range: 40-83 years). Smoking and alcohol abuse, as the major risk factors, were also common among these patients. These results are similar to those of NEC in the head and neck[10]. The tongue was the most common subsite (63.6%). Minor salivary glands may be the prevailing origin of primary SNEC in the oral cavity[11]. Most of the patients had lymph node and distant metastasis with stage III, IVA, IVB and IVC tumors (72.7%), which are also similar to the results of previous studies on extra-pulmonary SNEC in the head and neck. In patients with positive cervical lymph nodes, the recurrence rate is high and distant metastasis frequently occurs. In addition, these patients have a poor median survival of 10.0 mo and a 2-year survival rate of 15.7%[16]. Therefore, a comprehensive examination is mandatory to exclude distant metastasis. The outcomes of the previously reported 11 patients were as follows: 3 died of tumor, 1 died from other causes, 6 showed no evidence of disease, and the status of 1 patient was unknown.
| Publication year | Age in yr | Sex | Smoking | Alcohol | Primary subsite | Disease stage | Therapy | RT dose in Gy | CM | Follow-up time in mo | RE | DM | Patient status1 |
| 1984 | 62 | M | + | + | Tongue | IVA | S + RT + CM | Un | C+A+O | 10 | - | + | Died of DM |
| 1995 | 76 | M | + | + | Tongue | IVA | RT | 72 | - | 2 | - | - | Died of debility |
| 2001 | 62 | M | + | + | Mandible | IVA | - | - | - | 3 | - | + | Died of DM |
| 2013 | 75 | M | Un | Un | Tongue | III | S + RT | Un | - | Un | Un | Un | Un |
| 2013 | 85 | M | Un | Un | Cheek | III | CRT + S | 40 | CA | 2.5 | - | + | Died of DM |
| 2013 | 59 | M | Un | Un | Cheek | IVC | CM | Un | CI | 16 | - | + | Alive |
| 2014 | 55 | F | + | - | Tongue | IVA | S + CM | Un | Un | Un | - | - | Alive |
| 2015 | 73 | M | + | + | Lower gingiva | II | S + CM | - | CI + E | 14 | - | - | Alive |
| 2015 | 54 | M | Un | Un | Tongue | I | S + CM + RT | 66 | CI + E | Un | - | - | Alive |
| 2015 | 40 | F | Un | Un | Tongue | I | S + CM + CRT + CM | 60 | CI + E | 52 | - | - | Alive |
| 2017 | 74 | M | Un | Un | Tongue | IVC | CM | - | CA + E | 9 | - | - | Alive |
The definite diagnosis of SNEC depends on histopathology and immunohisto-chemistry analyses. Morphological examination showed extensive proliferation of round and spindly small cells with ovoid-to-spindle shaped nuclei, fine granular chromatin, inconspicuous nucleoli and scant cytoplasm, which could help in the differential diagnosis of typical and atypical carcinoids and large cell NEC[8]. These small cells formed broad nests, sheets or cord shapes. Necrosis is typically extensive and the mitotic count is high. The presence of electron-dense and neurosecretory granules is distinctive in SNEC[20]. The cells are distinctively positive for neuroendocrine markers including syn, N-CAM (CD56) and chromogranin A[17]. High Ki-67 labeling (90%) indicates high proliferative activity of tumor cells[8].
Immunohistochemical staining is crucial for the differential diagnosis of SNEC from other malignancies. Extensive mitotic index and necrosis can distinguish SNEC from typical and atypical carcinoids. In contrast to SNEC, large cell NEC contains cells with a relatively large cytoplasm and vesicular chromatin and prominent nucleoli. Primary cutaneous high-grade NEC, which mainly develops due to ultraviolet light exposure, is termed Merkel cell carcinoma. Primary mucosal high-grade NEC, which typically develops because of heavy smoking and alcohol abuse, is called SNEC. In addition, CK20 is commonly positive in Merkel cell carcinoma, and is negative in SNEC[21]. HMB45 and S100 are special markers for malignant melanoma and malignant lymphoma. They can be used for detecting metastatic squamous cell carcinoma of the lung when thyroid transcription factor1 is positive[11]. Cytokeratin AE1/AE3 and P63 staining is helpful in identifying a basal cell carcinoma or a squamous cell-originated tumor[6,10,11].
Due to the paucity of primary SNECs in the oral cavity, the lack of definitive treatment recommendations is a challenge for clinicians. The appropriate treatment strategies for extra-pulmonary SNECs are extrapolated from their pulmonary counterparts[14]. A variety of therapeutic modalities, including surgery, chemotherapy, radiotherapy and chemoradiotherapy, are thought to be appropriate treatments[16,22]. Surgery could be curative when SNECs are limited within the primary sites. In addition, surgery is the main treatment of NECs at different body sites and has been reported to significantly improve overall survival, over other single-modality treatments. To decrease local recurrence of SNECs, a wide excision is mandatory (up to 3 cm)[7]. In our case, the patient underwent extensive surgical excision of the primary tumor.
As SNEC is an extremely aggressive malignancy with high rates of local recurrence and distant metastasis, multimodal therapy is required. For patients without distant metastasis, chemotherapy can reduce tumor size and decrease the risk of distant metastasis. In early and locally advanced SNECs of the head and neck, chemoradiotherapy is also an effective treatment[16]. In patients with extensive SNEC, chemotherapy is recommended to prolong survival and improve prognosis[6,12]. The combination of cisplatin and etoposide is the first-line chemotherapy regimen for SNEC[23]. In recent research, chemotherapy in combination with first-line atezolizumab prolonged survival rate compared with chemotherapy alone in the treatment of extra-pulmonary SNECs with distant metastases[2].
Furthermore, a few novel therapeutic strategies for small cell carcinoma of the lung which are unsuccessful, including mammalian target of rapamycin inhibitors, breakpoint cluster region–Abelson tyrosine kinase inhibitors, epidermal growth factor receptor tyrosine kinase inhibitors, vascular endothelial growth factor inhibitors, deoxyribonucleic acid repair inhibitors, immunotherapies, and anti-delta-like protein 3 antibody-drug conjugates, have been introduced[24,25]. Of the 11 patients with primary SNEC in the oral cavity, 1 patient received radiotherapy only, due to his poor physical condition. Chemotherapy was the most common treatment in the majority of patients (75%). In the current case, the patient showed partial remission after two cycles of adjuvant chemotherapy. However, the patient died when gastrointestinal metastasis occurred 20 mo after treatment.
Primary SNEC occurring in the oral cavity represents a rare clinical entity, and is aggressive with a poor prognosis. The diagnosis of SNEC requires morphology and immunohistochemistry analyses. Due to its highly metastatic characteristics, a comprehensive clinical examination of the neck, chest, abdomen and bone is mandatory in SNEC patients. Multimodal therapy may be an effective treatment strategy for SNEC of the oral cavity. However, more effective treatments to improve the survival rate of patients with SNEC in the oral cavity are required.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Yoshimoto S S-Editor: Zhang L L-Editor: Filipodia P-Editor: Li JH
| 1. | Galanis E, Frytak S, Lloyd RV. Extrapulmonary small cell carcinoma. Cancer. 1997;79:1729-1736. [PubMed] |
| 2. | Wakasaki T, Yasumatsu R, Masuda M, Matsuo M, Tamae A, Kubo K, Kogo R, Uchi R, Taura M, Nakagawa T. Small Cell Carcinoma in the Head and Neck. Ann Otol Rhinol Laryngol. 2019;128:1006-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 3. | Hull MT, Eble JN, Warfel KA. Extrapulmonary oat-cell carcinoma of the tongue: an electron-microscopic study. J Oral Pathol. 1984;13:489-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Yoshida H, Onizawa K, Hirohata H. Neuroendocrine carcinoma of the tongue: report of a case. J Oral Maxillofac Surg. 1995;53:823-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Kim SG, Jang HS. Small cell carcinoma of the oral cavity: report of a case. J Oral Maxillofac Surg. 2001;59:680-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Nishihara K, Nozoe E, Hirayama Y, Miyawaki A, Semba I, Nakamura N. A case of small cell carcinoma in the buccal region. Int J Oral Maxillofac Surg. 2009;38:1000-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Cymerman JA, Kulkarni R, Gouldesbrough D, McCaul J. Small cell neuroendocrine tumour of the anterior tongue: A case report. Int J Surg Case Rep. 2013;4:753-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Terada T. Small cell carcinoma of the oral cavity (cheek mucosa): a case report with an immunohistochemical and molecular genetic analysis. Int J Clin Exp Pathol. 2013;6:780-787. [PubMed] |
| 9. | Singla A, Singla A, Gallagher R. A rare case and literature review of primary neuroendocrine carcinoma of the tongue†. J Surg Case Rep. 2014;2014. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Zeng M, Yang SD, Zhang JL, Chen XM. Primary small cell neuroendocrine carcinoma of the oral cavity: A case report and review of the literature. Oncol Lett. 2015;10:887-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Gumusay O, Yilmaz G, Aydil U, Ozet A, Tufan G, Erdem O, Kizil Y, Benekli M. Small cell neuroendocrine carcinoma of the posterior tongue. J Cancer Res Ther. 2015;11:651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Esmati E, Babaei M, Matini A, Ashtiani MS, Hamed EA, Nosrati H, Razi F, Ganjalikhani M. Neuroendocrine carcinoma of the tongue. J Cancer Res Ther. 2015;11:659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Xue LW, Chen X, Yin DD, Wang XX. Report: Small cell neuroendocrine carcinoma (SCNEC) of the tongue: A case report. Pak J Pharm Sci. 2017;30:1191-1194. [PubMed] |
| 14. | Yasumatsu R, Nakashima T, Yamauchi M, Toh S, Komune S. Extrapulmonary small cell carcinoma in head and neck. J Laryngol Otol. 2015;129 Suppl 2:S83-S85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Walenkamp AM, Sonke GS, Sleijfer DT. Clinical and therapeutic aspects of extrapulmonary small cell carcinoma. Cancer Treat Rev. 2009;35:228-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 147] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 16. | Pointer KB, Ko HC, Brower JV, Witek ME, Kimple RJ, Lloyd RV, Harari PM, Baschnagel AM. Small cell carcinoma of the head and neck: An analysis of the National Cancer Database. Oral Oncol. 2017;69:92-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 17. | Gale N, Poljak M, Zidar N. Update from the 4th Edition of the World Health Organization Classification of Head and Neck Tumours: What is New in the 2017 WHO Blue Book for Tumours of the Hypopharynx, Larynx, Trachea and Parapharyngeal Space. Head Neck Pathol. 2017;11:23-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 98] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 18. | Mahomed F. Neuroendocrine cells and associated malignancies of the oral mucosa: a review. J Oral Pathol Med. 2010;39:121-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Renner G. Small cell carcinoma of the head and neck: a review. Semin Oncol. 2007;34:3-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 99] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 20. | Roy S, Das I, Nandi A, Roy R. Primary Merkel cell carcinoma of the oral mucosa in a young adult male: report of a rare case. Indian J Pathol Microbiol. 2015;58:214-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Lewis JS, Duncavage E, Klonowski PW. Oral cavity neuroendocrine carcinoma: a comparison study with cutaneous Merkel cell carcinoma and other mucosal head and neck neuroendocrine carcinomas. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110:209-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | van der Laan TP, Plaat BE, van der Laan BF, Halmos GB. Clinical recommendations on the treatment of neuroendocrine carcinoma of the larynx: A meta-analysis of 436 reported cases. Head Neck. 2015;37:707-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 97] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 23. | Barker JL, Glisson BS, Garden AS, El-Naggar AK, Morrison WH, Ang KK, Chao KS, Clayman G, Rosenthal DI. Management of nonsinonasal neuroendocrine carcinomas of the head and neck. Cancer. 2003;98:2322-2328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ, Huemer F, Losonczy G, Johnson ML, Nishio M, Reck M, Mok T, Lam S, Shames DS, Liu J, Ding B, Lopez-Chavez A, Kabbinavar F, Lin W, Sandler A, Liu SV; IMpower133 Study Group. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med. 2018;379:2220-2229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1567] [Cited by in RCA: 2372] [Article Influence: 338.9] [Reference Citation Analysis (0)] |
| 25. | Srivastava R, Lebowicz Y, Jamil MO. Targeted agents in the management of small cell lung cancer - present and future. Drugs Today (Barc). 2018;54:479-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |