Published online Apr 28, 2020. doi: 10.13105/wjma.v8.i2.98
Peer-review started: December 26, 2019
First decision: January 19, 2020
Revised: March 17, 2020
Accepted: March 19, 2020
Article in press: March 19, 2020
Published online: April 28, 2020
Processing time: 124 Days and 8.7 Hours
The infection and drug resistance rates of Helicobacter pylori (H. pylori) are high and must be prevented and treated by better strategies. Based on recent research advances in this field as well as the results from our team and those on traditional Chinese medicine, we review the causes of drug resistance, and prevention and treatment strategies for drug-resistant H. pylori infection, with an aim to make suggestions for the development of new drugs, such as establishment of new target identification and screening systems, modification of existing drug structures, use of new technologies, application of natural products, and using a commercial compound library. This article may provide reference for eradication of drug-resistant H. pylori.
Core tip: The resistance rate of Helicobacter pylori is increasing and there is an urgent need to develop better treatment strategies. We review the current progress in the field with regard to prevention of drug-resistant bacterial infection, effective diagnosis and standardized treatment, rational application of antibacterial drugs, and prevention of drug-resistant bacterial transmission. The factors causing drug resistance are suggested to be eliminated so as to ensure the success of the first-line treatment of patients with drug resistance, whose accurate treatment is based on individual drug history and drug-sensitivity testing results. Traditional Chinese medicine has good effects in the treatment of drug-resistant bacterial infection and should be more widely applied.
- Citation: Li RJ, Dai YY, Qin C, Li XH, Qin YC, Pan Y, Huang YY, Huang ZS, Huang YQ. Treatment strategies and preventive methods for drug-resistant Helicobacter pylori infection. World J Meta-Anal 2020; 8(2): 98-108
- URL: https://www.wjgnet.com/2308-3840/full/v8/i2/98.htm
- DOI: https://dx.doi.org/10.13105/wjma.v8.i2.98
Helicobacter pylori (H. pylori) is an important cause of chronic gastritis, peptic ulcers, gastric cancer, and other diseases[1-3]. In addition, H. pylori is associated with a variety of extra-intestinal diseases, such as periodontitis and secondary thrombocytopenic purpura[4]. H. pylori now infects more than half of the world’s population, and the infection rate is higher in developing countries compared to developed countries, with more than 80% of cases diagnosed in underdeveloped areas[5-7]. Due to the high infection rate of H. pylori and the widespread use of antibiotics for treatment, the drug resistance rate of H. pylori is increasing along with a simultaneous decrease in eradication rate, which poses a serious threat to public health. There is an urgent need for better strategies to prevent and treat H. pylori infection. In this paper, we review the current advances in methods of prevention and treatment of drug-resistant H. pylori infection, aiming to provide reference for eradication of drug-resistant H. pylori.
The main methods of the international H. pylori eradication program include standard triple, non-bismuth quadruple, bismuth quadruple (a proton pump inhibitor + bismuth + two antimicrobial agents) treatments. The non-bismuth quadruple regimens consist of sequential, concomitant, and mixed therapies. Currently, bismuth quadruple treatment is preferentially recommended. The Kyoto consensus emphasizes that eradication is the first-line treatment for patients with H. pylori infection with dyspepsia[8]. The Toronto consensus provides recommendations on H. pylori eradication methods for adult patients[9]. The Maastricht V/Florence Consensus points out that when H. pylori is sensitive to clarithromycin (CLA), the eradication rate of the international standard triple protocol is 97.3%[10]. In areas with high resistance to CLA or double resistance, the eradication rate of quadruple therapy containing bismuth is up to 86%[11]. In the Fifth National Consensus Conference on the Management of H. pylori Infection held in China, the eradication rates of the seven regimens are all around 90%[12].
Although the efficacy of first-line treatment containing various antibiotics to which H. pylori is sensitive, the eradication rate is less than 100%, and with the failure of the first eradication, the rate of drug resistance increases and so radical treatment becomes more difficult. The causes of eradication failure and drug resistance mainly include history of antimicrobial use, improper use of antibiotics, course of digestive diseases, and certain drug characteristics[13], which are listed in Table 1. Although amoxicillin is not prone to resistance, the rate of resistance to amoxicillin has gradually increased in recent years[13], which highlights the severity of drug resistance of H. pylori. Consequently, in 2017, the World Health Organization listed CLA-resistant H. pylori as one of the 12 pathogens in urgent need of new antibiotics[14].
| Level | Causes |
| Main factors | History of antimicrobial use |
| Antibiotics are not properly used | |
| Course of disease | |
| Drugs tend to develop resistance | |
| Transmission of drug-resistant plasmids | |
| Secondary factors | Age |
| Gender | |
| Race | |
| Other factors | Territory |
| Planting site and density |
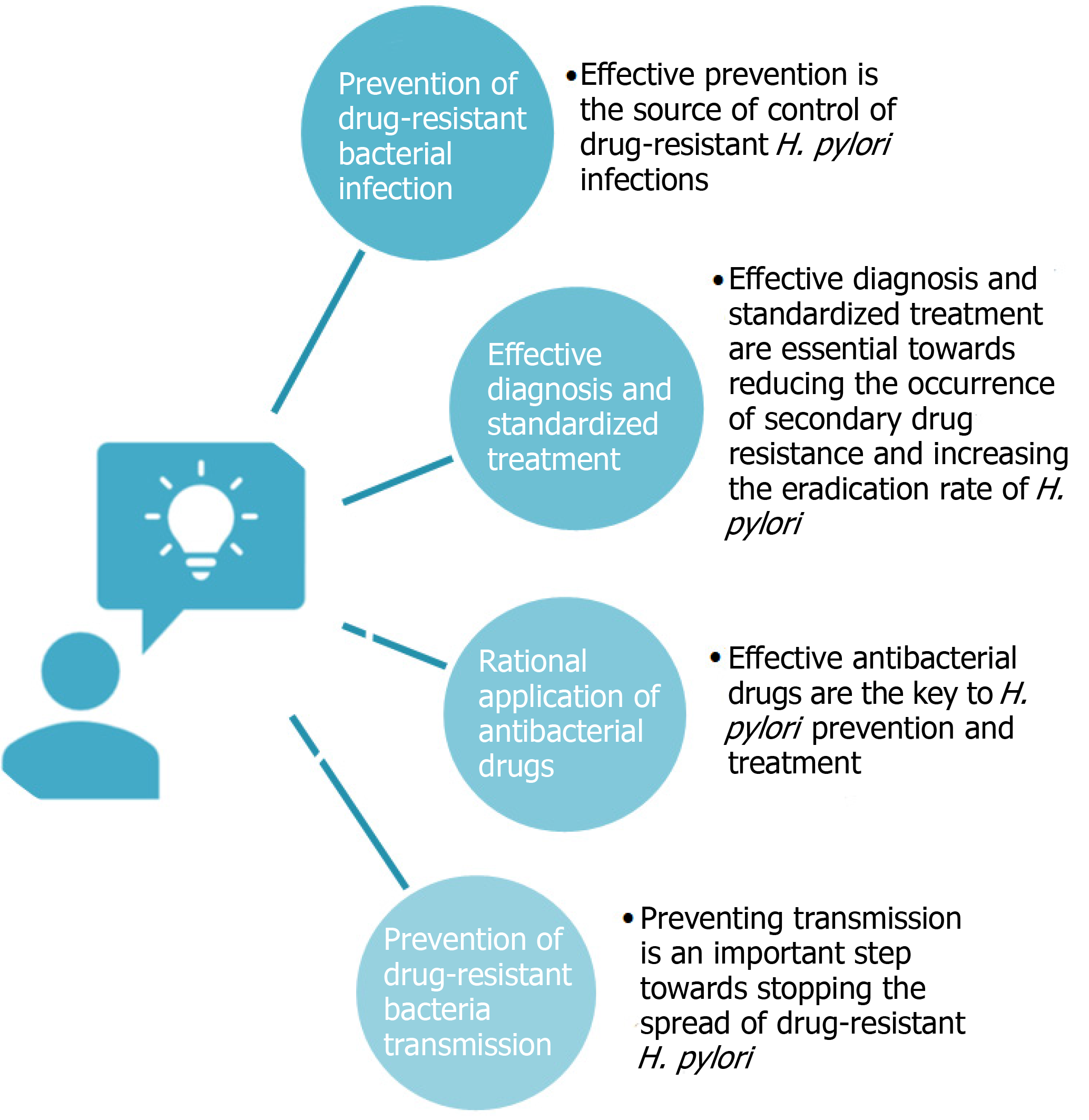
At present, strategies targeting drug-resistant H. pylori include prevention of drug-resistant bacterial infection, effective diagnosis and standardized treatment, rational application of antibacterial drugs, and prevention of drug-resistant bacteria transmission (Figure 1). Effective prevention is the source of control of drug-resistant H. pylori infections. Only with reasonable prevention can the incidence of infections be effectively decreased. When drug-resistant infections occur, effective diagnosis and standardized treatment are essential towards reducing the occurrence of secondary drug resistance and increasing the eradication rate of H. pylori. Invasive or non-invasive testing is the key to rapid and effective diagnosis. Selection of antibiotics based on the sensitivity to antibacterial drugs and formulation of rational, standardized, and accurate treatment plans based on patient condition are also critical[12].
Effective antibacterial drugs are the key to H. pylori prevention and treatment. The government, health administration departments, drug regulatory departments, and hospital medical staff must proactively perform their respective duties to enhance antibacterial drug management. Other important aspects include forming a management system for rational drug use, formulating medication guidelines, establishing antibacterial drug guidelines and drug resistance monitoring networks, and supporting the development of new types of drugs. Furthermore, preventing transmission is an important step towards stopping the spread of drug-resistant H. pylori. Security control in laboratories and hospitals should be strengthened to prevent the spread of drug-resistant strains.
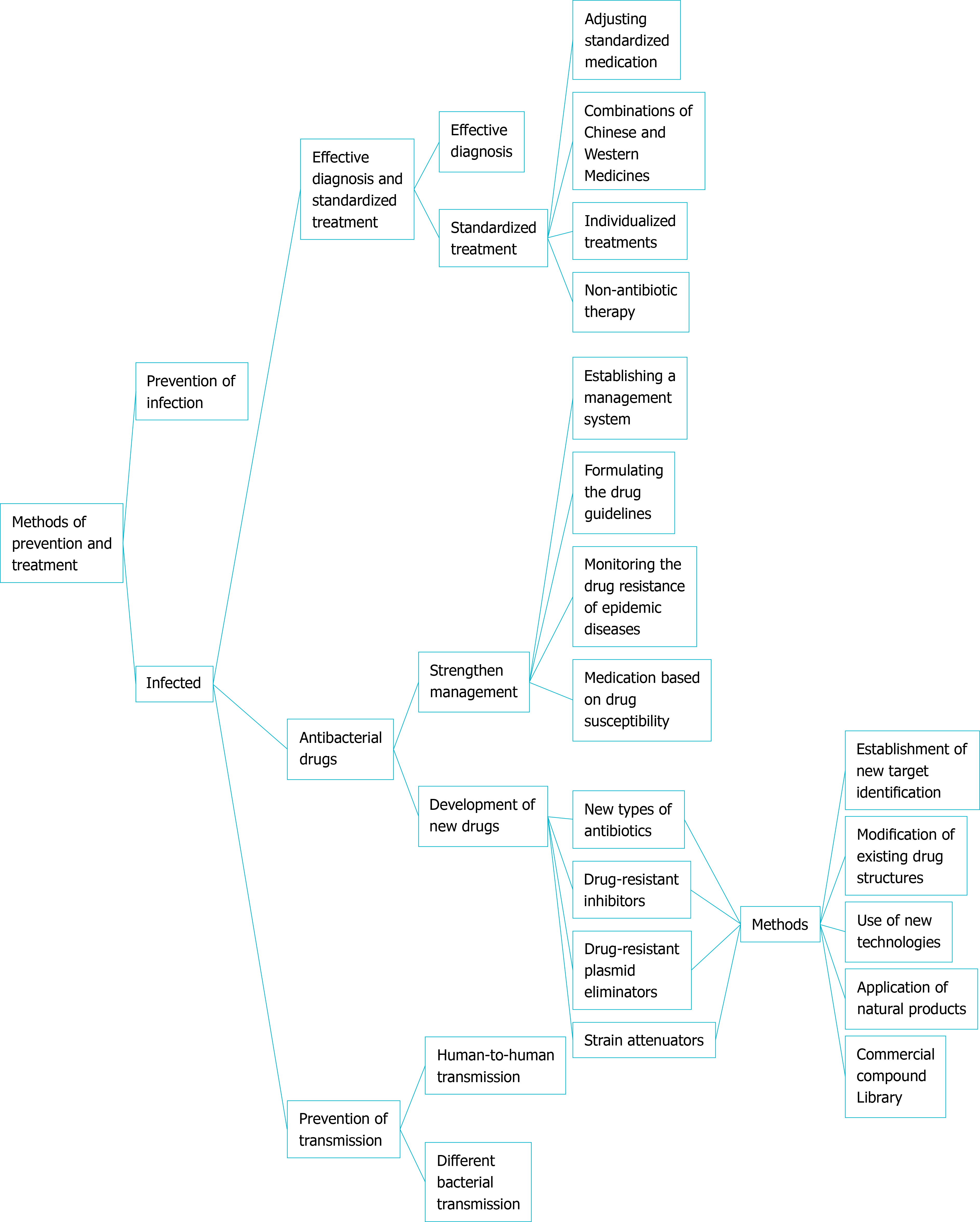
Based on the above-mentioned four strategies for the control of drug-resistant H. pylori and combined with research from our research group, we will conduct a detailed analysis of the prevention methods (Figure 2).
Prevention in adolescents: One third of children worldwide have been infected with H. pylori, highlighting the importance of prevention in adolescents[15]. Screening for the disease in adolescents can decrease the lifetime risk of gastric cancer. As H. pylori is transmitted mainly through fecal-oral and oral-oral routes, parents should pay attention to the diet of adolescents, keep oral hygiene, avoid mouth-to-mouth feeding, promote meal sharing, and avoid mixing water cups, toothbrushes, and mouthwash cups amongst family members.
Prevention in adults < 50 years old with a low gastric cancer risk: For this population, an H. pylori infection test and a gastric atrophy test should be combined to effectively prevent transmission to the next generation. In addition, tableware and toilets should be disinfected frequently, and people should develop good eating habits and pay attention to the hygiene of drinking water. Medical workers in hospitals should pay special attention to the transmission of H. pylori in hospitals.
Prevention in adults ≥ 50 years old with a high gastric cancer risk: H. pylori infection increases with age. One of the main reasons is cross-infection in the home or in the population. In addition to paying attention to health problems in the family, people with a high gastric cancer risk should also undergo screening for the disease. The combined detection of serum pepsinogen and H. pylori antibodies can increase the level of prevention for people with a high gastric cancer risk[16].
Diagnosis of H. pylori infection is extremely important. Invasive or non-invasive detection methods are usually used in the individual diagnosis of H. pylori infection. Invasive detection methods include endoscopy[17], rapid urease test[18], histological method, and bacterial culture[19]. Non-invasive detection methods include 13C urea breath test[20], serum antibody test[21], stool antigen test[22], and other molecular biology techniques. Each test method has its own advantages and disadvantages, which should be selected from person to person. Considering the accuracy and safety of diagnosis, non-invasive detection methods are generally recommended. There are also some other approaches used to assist diagnosis such as serum pepsinogen measurement[23] and gastric X-ray[24].
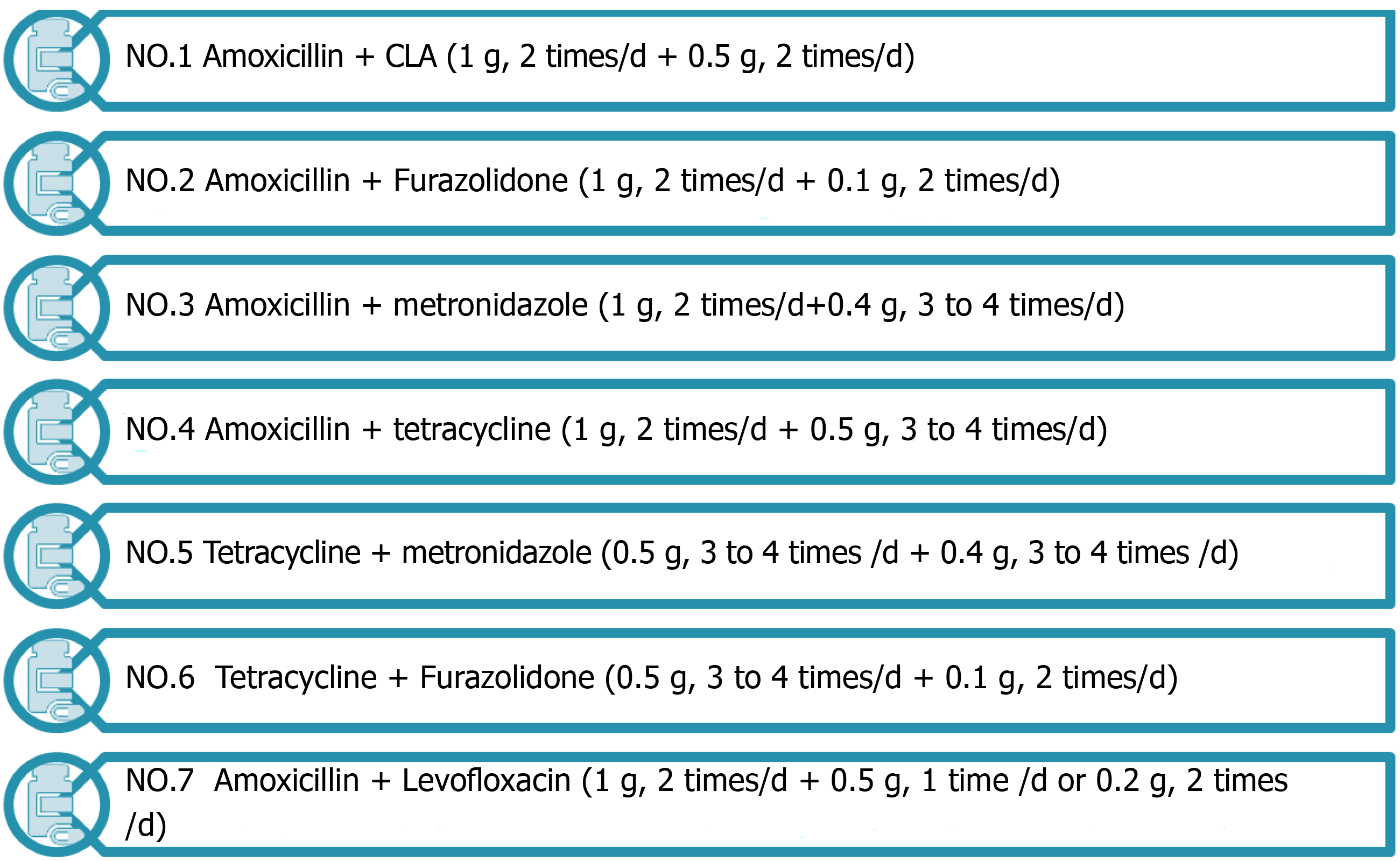
Adjusting standardized medication: Drug-resistant H. pylori is a strain that is not easily eradicated after the first standardized treatment. To treat this refractory disease caused by drug resistance, antibiotics should be adjusted in time for remedial treatment based on the results of drug sensitivity testing or medication history. Seven common antibiotic combinations[25], as shown in Figure 3, are used in the remedy in the treatment of antimicrobial drugs with low drug resistance. In the protocol, antibacterial drugs such as tetracycline, metronidazole, and amoxicillin have a high eradication rate[26]. H. pylori has high resistance to CLA, with a primary resistance rate of 20% to 50%. In areas with high rates of CLA, or high rates of metronidazole-CLA dual resistance, quadruple therapy is recommended in preference to CLA and metronidazole as first-line therapy[27]. Non-bismuth quadruple concomitant and sequential therapies can also be used as an eradication protocol. Patients were less well tolerated and had poor compliance to these two treatment regimens[28]. The 7-d concomitant therapy has been shown to be better than 7-d or 10-d triple therapy[29]. With the same treatment course, concomitant therapy is superior to sequential therapy[30]. Moreover, antimicrobial treatment should be controlled[31]. The actual trend is towards the use of quadruple instead of triple therapy and prolongation of the duration of each eradication regimen (10-14 d).
New quadruple therapy has a modification to the traditional triple and quadruple therapy that should be used as the first treatment option. Recent studies indicated that minocycline, cephalosporins[32], and rabeprazole can be used as alternatives to antibacterial agents in quadruple therapy[33]. However, new quadruple therapy is in its early stage, and the dosage and course of treatment should be further optimized.
Combination of Chinese and Western medicines: Antibiotics are broad-spectrum agents and prone to drug resistance, while traditional Chinese medicines are less prone to drug resistance, less toxic, and have complex mechanisms. Combining the two can effectively treat H. pylori infection. In a previous study, 162 patients with H. pylori infection were randomly divided into two groups. The treatment group received traditional Chinese medicine combined with Western medicine for anti-H. pylori therapy. After three courses, the effective rate was as high as 100%, which was significantly higher than that of the control group treated with Western medicine alone[34]. This result showed that the combination of traditional Chinese and Western medicines is significantly better than Western medicine alone, which may improve the prognosis of patients.
Individualized treatments in special pathological conditions: As amoxicillin is not prone to drug resistance and has few adverse reactions, it is often the first choice for H. pylori eradication therapy. However, when patients are allergic to penicillin, other antibiotics with low resistance should be selected instead of amoxicillin, such as tetracycline[35]. When choosing a regimen containing antibiotics to which H. pylori is highly-resistant, treatment time can be appropriately extended[28]. Patients with H. pylori infection should have their blood glucose well-controlled during treatment[36]. In the treatment of such patients with H. pylori infection accompanied by special pathological conditions, individualized treatment plans should be formulated based on the patient’s condition.
Non-antibiotic therapy: Currently, triple or quadruple therapies are widely accepted as first-line H. pylori eradication treatment regimens; however, these regimens often lead to some adverse outcomes, such as intestinal flora imbalance and drug resistance. Probiotics are an important factor in the human body to maintain the micro-ecological balance. Combining probiotics with other therapies to treat H. pylori infection is safe, feasible, and beneficial. For example, Lactobacillus can influence the colonization of H. pylori[37], and the triple treatment combined with Blaird's yeast had a significantly higher eradication rate than the traditional triple treatment[38]. However, the adjuvant role of probiotics in the eradication of H. pylori remains controversial and more research is warranted[39].
Traditional Chinese medicine treatments are methods with Chinese characteristics. Some monomer components containing Chinese medicine mucosal-protective agents exhibit the characteristics of high eradication rate, low drug resistance, few adverse reactions, and low toxicity. These medicines even kill drug-resistant H. pylori and may therefore provide new tools for the eradication of H. pylori[40]. For example, some quinolone alkaloids in Fructus Evodia inhibit the growth of H. pylori and achieve eradication without affecting the intestinal flora[41]. According to epidemiological statistics, the total effective rate of traditional Chinese medicine treatment can reach 95.45%[42].
The bacteriostatic effect of berberine is strongest amongst the monomer components of Chinese medicine, followed by rhubarb, and scutellaria. Cortex, Radix Ginseng, Forsythia, and Hedyotis diffusa also exert a certain antibacterial effect[43]. The mechanism of action of traditional Chinese medicines may be related to the inhibition of functional protein synthesis[44], biofilm synthesis[45], inflammatory factor release[46], and virulence factor release[44] and the reduction of adhesion[47]. The mechanism of action for these medicines is complex. Also, the extraction and analysis of active ingredients have not fully completed, and the course of treatment is difficult to control.
Strengthening the management of antibiotics: According to the regulations covering the use of antibiotics in the "Administrative Measures for the Classification of Prescription Drugs and Over-the-Counter Drugs"[48], the management system for antibiotics should be strictly enforced. Measures include enhancing the management of antibiotics in hospitals, formulating a reasonable medication management system, and preventing antibiotic abuse. In particular, medical workers should ensure that patients use antibiotics safely, reasonably, and effectively, to ensure their health and well-being.
Following the principles of antibiotic use: The principles of antibiotic use should be strictly followed: (1) Clear medication indications and corresponding antibiotics need to be used for H. pylori infection; (2) For targeted use, triple therapy is preferred; (3) The rational dosage of drugs and the sufficient course of treatment can not only ensure efficacy but also prevent the development of drug resistance; (4) The patients should be asked about the history of drug allergy in detail before the medication; (5) The appropriate method of administration needs to be selected so that the general H. pylori medication is orally administered; (6) The drug should be carefully changed along with the treatment plan after confirming failure of the triple or quadruple therapy; and (7) Patients with impaired liver and kidney function should be cautious in medicine taking.
Biological standards for rational drug use: Rational drug use refers to the selection of the best drug and the formulation of a dosing plan to effectively, safely, and economically prevent and cure diseases. The World Health Organization has established biological standards for rational drug use as follows: (1) Proper use of drugs needs to be ensured; (2) The drug information is appropriate; (3) The efficacy, safety, use, and price are appropriate for patients; (4) Dosage, usage, and course of treatment should be appropriate; (5) The subject is appropriate, without contraindications or significant adverse reactions; (6) The drug resource allocation is correct; and (7) Patients have good drug compliance.
Indications for antibiotic application: Antibiotics can be classified into first, second, and third-line drugs according to the antibiotics management classification. H. pylori infections are usually treated with first- and second-line drugs. First-line drugs refer to antibiotic drugs that are non-restricted, narrow-spectrum, and positive in effect, have slight adverse reactions and low prices, and are available in sufficient supply. Second-line drugs are the drugs that are restricted in use, have a broad antibacterial spectrum and good curative effects, but have obvious adverse reactions or are more expensive, or are drug varieties that may develop rapid resistance and have controlled use. Third-line drugs are generally used in a unique way as they exert curative effects but are relatively toxic and expensive. They are a class of drugs that will have serious consequences once drug resistance occurs. In the treatment of drug-resistant H. pylori infection, the most suitable antibiotic should be selected and used according to the best course of treatment. Narrow-spectrum, "low-grade" antibiotics should be used as much as possible.
Drug susceptibility testing for accurate treatment: Differences in the rates of drug resistance are closely associated with region[49], medical standards, economic development level, and quality of life[50]. The resistance rate of H. pylori to antibacterial drugs can determine the eradication rate of treatment options. The epidemic of drug resistance differ among different regions. Based on local drug resistance monitoring data, specific drug resistance conditions should be combined with drug sensitivity tests to make a reasonable plan to achieve the purpose of a precise treatment.
H. pylori has serious drug resistance, particularly multiple drug resistance, for which there are not many drug candidates available. Therefore, new types of antibiotics, drug-resistance inhibitors, drug-resistance plasmid eliminators, and strain attenuators urgently need to be researched and developed based on the following methods:
Establishment of new target identification and screening systems: Screening new targets provides new avenues for the development of new antibacterial drugs. Some enzymes are involved in the biosynthesis of unsaturated fatty acids, such as H. PYLORI0773 (FabX), a decapeptide from the decanoyl-acyl carrier protein (ACP) in a parallel reaction with the first enzyme of acyl-CoA dehydrogenase of the fatty acid β-oxidation cycle. Also it isomerizes trans-2-decenol-ACP to form a key UFA synthesis intermediate, cis-3-decenoyl-ACP, which reverses the normal fatty acid synthesis cycle of H. pylori in the c10 phase[51]. However, there remains a certain distance from the screening of targets to drugs entering clinical trials.
Modification of existing drug structures: Modification, semi-synthesis, and synthesis of existing drugs are currently the recommended methods. Amoxicillin-UCS-2/tripolyphosphate (TPP) nanoparticles constructed with urea-modified chitosan derivatives UCS-2 and sodium tripolyphosphate (STPP) have more effective and specific effects in eliminating H. pylori in vitro. Amoxicillin UCCS-2/TPP nanoparticles reduced the levels of pro-inflammatory cytokines and decreased inflammatory damage caused by H. pylori infection[52]. Modification of drugs can ensure their activity and shorten the time of preparation and mechanistic exploration; however, the toxicity of newly modified drugs should also be tested.
Use of new technologies: MicroRNAs (miRNAs) are a class of small non-coding RNAs widely found in intergenic or intron regions. They play a role in suppressing cancer mainly by regulating the expression of tumor suppressor genes[53]. MiRNA210 is a candidate molecule that is often highly expressed in gastric cancer and mediates epithelial-mesenchymal transition. It is considered a viable molecular target in the treatment of gastric cancer and can inhibit the invasion and metastasis of gastric cancer[54].
Application of natural products: Natural products include plants, microbial secondary metabolites, and marine life. Both live cells and the supernatant of Lactobacillus plantarum ZDY201 can inhibit the growth and urease activity of H. pylori. Owing to its good lactic acid production and anti-inflammatory effects against H. pylori SS1 infection, it is expected to become a candidate strain of probiotics[55]. Traditional Chinese medicine has an H. pylori killing effect, low drug resistance, and low toxicity, and can be used as non-antibiotic drugs to treat H. pylori infection[56]. Some natural products and agents have been shown to affect the TLR4 and MAPK signaling pathway activation by H. pylori[57]. Screening active ingredients from plants is a fast and effective method for treating drug-resistant H. pylori infection.
Commercial compound library: Some old drugs that are used in clinical trials can be used. For example, furazolidone has been used as a replacement for CLA or metronidazole[58]. However, due to the limited types of old medicines, it is difficult to purchase such medicines, which limits their usage.
Although drug-resistant H. pylori is weakly infectious, in terms of preventing transmission, in addition to preventing human-to-human transmission, the most important thing is to prevent transmission of drug-resistant plasmids. The spread of drug-resistant plasmids can occur between different bacteria, as well as between different individuals, strains, and animals, making humans susceptible to drug free H. pylori infection[59]. Livestock management[60], drug resistance testing[61], and management of antibiotics for other infectious diseases should also be implemented.
Through the unremitting efforts of scientific researchers and people from all works of life, some results in the prevention and treatment of drug-resistant H. pylori have been acquired. For example, zinc linolenate can specifically act on H. pylori and does not easily result in drug resistance[62]. However, there is still a long way to deal with the key problem of low H. pylori eradication rate. Drug resistance monitoring, application of drug susceptibility testing, and research and development of new drugs warrant further exploration. H. pylori associated gastritis is an infectious disease. Vaccines are the most effective method for H. pylori infection prevention, however, no vaccine is currently available. A vaccine that is expected to prevent H. pylori infection will be introduced to the market in the near future.
Currently, there is no effective way to prevent and treat drug resistance in H. pylori infection. To cope with this situation, we suggest comprehensive prevention and treatment measures. First, the factors of causing drug resistance are suggested to be eliminated to ensure the success of the first triple or quadruple treatment for patients with drug resistance, whose accurate treatment is based on individual drug history and drug-sensitivity testing results. Traditional Chinese medicine plays a unique role in the treatment of drug-resistant bacteria with relatively few side effects, which is worthy of further exploration.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Romo-Gonzalez C, Savarino V, Slomiany BL S-Editor: Ma YJ L-Editor: Wang TQ E-Editor: Liu MY
| 1. | Sonnenberg A, Lash RH, Genta RM. A national study of Helicobactor pylori infection in gastric biopsy specimens. Gastroenterology. 2010;139:1894-1901.e2; quiz e12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 158] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 2. | Yang YS, Ji LM. Harm and prevention of Helicobacter pylori. Shijie Zuixin Yixue Xinxi Wenzhai. 2019;19:315. [DOI] [Full Text] |
| 3. | Plummer M, de Martel C, Vignat J, Ferlay J, Bray F, Franceschi S. Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Glob Health. 2016;4:e609-e616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1068] [Cited by in RCA: 1015] [Article Influence: 112.8] [Reference Citation Analysis (0)] |
| 4. | Sultan S, Ahmed SI, Murad S, Irfan SM. Primary versus secondary immune thrombocytopenia in adults; a comparative analysis of clinical and laboratory attributes in newly diagnosed patients in Southern Pakistan. Med J Malaysia. 2016;71:269-274. [PubMed] |
| 5. | Nagy P, Johansson S, Molloy-Bland M. Systematic review of time trends in the prevalence of Helicobacter pylori infection in China and the USA. Gut Pathog. 2016;8:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 144] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 6. | Chi ZC, Tong YQ, Dong QJ. Diagnosis and treatment of Helicobacter pylori infection and related diseases. Beijing: Military Medical Science Press, 2008. |
| 7. | Knorr J, Ricci V, Hatakeyama M, Backert S. Classification of Helicobacter pylori Virulence Factors: Is CagA a Toxin or Not? Trends Microbiol. 2019;27:731-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Sugano K, Tack J, Kuipers EJ, Graham DY, El-Omar EM, Miura S, Haruma K, Asaka M, Uemura N, Malfertheiner P; faculty members of Kyoto Global Consensus Conference. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64:1353-1367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1322] [Cited by in RCA: 1173] [Article Influence: 117.3] [Reference Citation Analysis (0)] |
| 9. | Fallone CA, Chiba N, van Zanten SV, Fischbach L, Gisbert JP, Hunt RH, Jones NL, Render C, Leontiadis GI, Moayyedi P, Marshall JK. The Toronto Consensus for the Treatment of Helicobacter pylori Infection in Adults. Gastroenterology. 2016;151:51-69.e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 695] [Cited by in RCA: 629] [Article Influence: 69.9] [Reference Citation Analysis (0)] |
| 10. | Malfertheiner P, Megraud F, O'Morain CA, Gisbert JP, Kuipers EJ, Axon AT, Bazzoli F, Gasbarrini A, Atherton J, Graham DY, Hunt R, Moayyedi P, Rokkas T, Rugge M, Selgrad M, Suerbaum S, Sugano K, El-Omar EM; European Helicobacter and Microbiota Study Group and Consensus panel. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut. 2017;66:6-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2220] [Cited by in RCA: 1973] [Article Influence: 246.6] [Reference Citation Analysis (1)] |
| 11. | Suzuki H, Mori H. World trends for H. pylori eradication therapy and gastric cancer prevention strategy by H. pylori test-and-treat. J Gastroenterol. 2018;53:354-361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 102] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 12. | Liu WZ, Xie Y, Lu H, Cheng H, Zeng ZR, Zhou LY, Chen Y, Wang JB, Du YQ, Lu NH; Chinese Society of Gastroenterology, Chinese Study Group on Helicobacter pylori and Peptic Ulcer. Fifth Chinese National Consensus Report on the management of Helicobacter pylori infection. Helicobacter. 2018;23:e12475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 327] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 13. | Morilla AM, Álvarez-Argüelles ME, Duque JM, Armesto E, Villar H, Melón S. Primary antimicrobial resistance rates and prevalence of Helicobacter pylori infection in the north of Spain. A 13-year retrospective study. Gastroenterol Hepatol. 2019;42:476-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Hashemi SJ, Sheikh AF, Goodarzi H, Yadyad MJ, Seyedian SS, Aslani S, Assarzadegan MA. Genetic basis for metronidazole and clarithromycin resistance in Helicobacter pylori strains isolated from patients with gastroduodenal disorders. Infect Drug Resist. 2019;12:535-543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 15. | Zabala Torrres B, Lucero Y, Lagomarcino AJ, Orellana-Manzano A, George S, Torres JP, O'Ryan M. Review: Prevalence and dynamics of Helicobacter pylori infection during childhood. Helicobacter. 2017;22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 16. | Agréus L, Kuipers EJ, Kupcinskas L, Malfertheiner P, Di Mario F, Leja M, Mahachai V, Yaron N, van Oijen M, Perez Perez G, Rugge M, Ronkainen J, Salaspuro M, Sipponen P, Sugano K, Sung J. Rationale in diagnosis and screening of atrophic gastritis with stomach-specific plasma biomarkers. Scand J Gastroenterol. 2012;47:136-147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 112] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 17. | Tahara T, Horiguchi N, Yamada H, Yoshida D, Terada T, Okubo M, Funasaka K, Nakagawa Y, Shibata T, Ohmiya N. Comparative study of magnifying narrow-band imaging and conventional white light endoscopy in the diagnosis of Helicobacter pylori status after eradication therapy. Medicine (Baltimore). 2019;98:e17697. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Murata H, Kawano S, Tsuji S, Tsujii M, Sawaoka H, Iijima H, Kawai N, Hori M. Evaluation of the PyloriTek test for detection of Helicobacter pylori infection in cases with and without eradication therapy. Am J Gastroenterol. 1998;93:2102-2105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 19. | Laine L, Lewin DN, Naritoku W, Cohen H. Prospective comparison of H&E, Giemsa, and Genta stains for the diagnosis of Helicobacter pylori. Gastrointest Endosc. 1997;45:463-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 91] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Ohara S, Kato M, Saito M, Fukuda S, Kato C, Hamada S, Nagashima R, Obara K, Suzuki M, Honda H, Asaka M, Toyota T. Comparison between a new 13C-urea breath test, using a film-coated tablet, and the conventional 13C-urea breath test for the detection of Helicobacter pylori infection. J Gastroenterol. 2004;39:621-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Nurgalieva ZZ, Graham DY. Pearls and pitfalls of assessing Helicobacter pylori status. Dig Liver Dis. 2003;35:375-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Sato M, Shimoyama T, Takahashi R, Kajiyama H, Sano Y, Sakaedani N, Kato A, Hirata H, Fukuda Y. Characterization and usefulness of stool antigen tests using a monoclonal antibody to Helicobacter pylori catalase. J Gastroenterol Hepatol. 2012;27 Suppl 3:23-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Ohkusa T, Miwa H, Nomura T, Asaoka D, Kurosawa A, Sakamoto N, Abe S, Hojo M, Terai T, Ogihara T, Sato N. Improvement in serum pepsinogens and gastrin in long-term monitoring after eradication of Helicobacter pylori: comparison with H. pylori-negative patients. Aliment Pharmacol Ther. 2004;20 Suppl 1:25-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Itoh T, Saito M, Marugami N, Hirai T, Marugami A, Takahama J, Tanaka T, Kichikawa K. Correlation between the ABC classification and radiological findings for assessing gastric cancer risk. Jpn J Radiol. 2015;33:636-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Chinese Society of Gastroenterology, Chinese Study Group on Helicobacter pylori., Liu WZ, Xie Y, Cheng H, Lu NH, Hu FL, Zhang WD, Zhou LY, Chen Y, Zeng ZR, Wang CW, Xiao SD, Pan GZ, Hu PJ; . Fourth Chinese National Consensus Report on the management of Helicobacter pylori infection. J Dig Dis. 2013;14:211-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 26. | Hu Y, Zhu Y, Lu NH. Primary Antibiotic Resistance of Helicobacter pylori in China. Dig Dis Sci. 2017;62:1146-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (2)] |
| 27. | Farzi N, Yadegar A, Sadeghi A, Asadzadeh Aghdaei H, Marian Smith S, Raymond J, Suzuki H, Zali MR. High Prevalence of Antibiotic Resistance in Iranian Helicobacter pylori Isolates: Importance of Functional and Mutational Analysis of Resistance Genes and Virulence Genotyping. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 28. | Lee HJ, Kim JI, Lee JS, Jun EJ, Oh JH, Cheung DY, Chung WC, Kim BW, Kim SS. Concomitant therapy achieved the best eradication rate for Helicobacter pylori among various treatment strategies. World J Gastroenterol. 2015;21:351-359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 59] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 29. | Gisbert JP, Calvet X. Update on non-bismuth quadruple (concomitant) therapy for eradication of Helicobacter pylori. Clin Exp Gastroenterol. 2012;5:23-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 100] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 30. | Lim JH, Lee DH, Choi C, Lee ST, Kim N, Jeong SH, Kim JW, Hwang JH, Park YS, Lee SH, Shin CM, Jo HJ, Jang ES, Song Is, Jung HC. Clinical outcomes of two-week sequential and concomitant therapies for Helicobacter pylori eradication: a randomized pilot study. Helicobacter. 2013;18:180-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 31. | Fischbach L, Evans EL. Meta-analysis: the effect of antibiotic resistance status on the efficacy of triple and quadruple first-line therapies for Helicobacter pylori. Aliment Pharmacol Ther. 2007;26:343-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 282] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 32. | Bai P, Zhou LY, Xiao XM, Luo Y, Ding Y. Susceptibility of Helicobacter pylori to antibiotics in Chinese patients. J Dig Dis. 2015;16:464-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 33. | Wang M. Analysis of the effect of rabeprazole combined with berberine new quadruple therapy on peptic ulcer. Yixue Lunli Yu Shijian. 2018;31:996-997. [DOI] [Full Text] |
| 34. | Wang SY, Wang SL. Progress in the study of Helicobacter pylori in traditional Chinese medicine. Zhongguo Minjian Liaofa. 2020;96-98. [DOI] [Full Text] |
| 35. | Lee JY, Kim N, Nam RH, In Choi S, Lee JW, Lee DH. Primary and secondary antibiotic resistance of Helicobacter pylori in Korea from 2003 to 2018. Helicobacter. 2019;24:e12660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 36. | Cheng KP, Yang YJ, Hung HC, Lin CH, Wu CT, Hung MH, Sheu BS, Ou HY. Helicobacter pylori eradication improves glycemic control in type 2 diabetes patients with asymptomatic active Helicobacter pylori infection. J Diabetes Investig. 2019;10:1092-1101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 37. | Kabir AM, Aiba Y, Takagi A, Kamiya S, Miwa T, Koga Y. Prevention of Helicobacter pylori infection by lactobacilli in a gnotobiotic murine model. Gut. 1997;41:49-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 204] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 38. | Szajewska H, Horvath A, Piwowarczyk A. Meta-analysis: the effects of Saccharomyces boulardii supplementation on Helicobacter pylori eradication rates and side effects during treatment. Aliment Pharmacol Ther. 2010;32:1069-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 151] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 39. | Zhang MM, Qian W, Qin YY, He J, Zhou YH. Probiotics in Helicobacter pylori eradication therapy: a systematic review and meta-analysis. World J Gastroenterol. 2015;21:4345-4357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 108] [Cited by in RCA: 114] [Article Influence: 11.4] [Reference Citation Analysis (1)] |
| 40. | Li L, Meng F, Zhu S, Guo S, Wang Y, Zhao X, Sun Y, Zhang Y, Wang Q, Xu H, Zhang S. Efficacy and Safety of Wei Bi Mei, a Chinese Herb Compound, as an Alternative to Bismuth for Eradication of Helicobacter pylori. Evid Based Complement Alternat Med. 2018;2018:4320219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 41. | Hamasaki N, Ishii E, Tominaga K, Tezuka Y, Nagaoka T, Kadota S, Kuroki T, Yano I. Highly selective antibacterial activity of novel alkyl quinolone alkaloids from a Chinese herbal medicine, Gosyuyu (Wu-Chu-Yu), against Helicobacter pylori in vitro. Microbiol Immunol. 2000;44:9-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 64] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 42. | Ning WH. Observation of curative effect of traditional Chinese medicine on Helicobacter pylori infectious gastric disease. Quanke Kouqiang Yixue Zazhi. 2019;6:14-24. [DOI] [Full Text] |
| 43. | Shi B, Liu NY, Bi HY, Tang XD, Li ZH. Research progress of Chinese medicine in treating Helicobacter pylori infection. Zhonguo Zhongxiyi Jiehe Zazhi. 2017;507-511. [DOI] [Full Text] |
| 44. | Liu S, Sun Y, Li W, Yu H, Li X, Liu Z, Zeng J, Zhou Y, Chen C, Jia J. The antibacterial mode of action of allitridi for its potential use as a therapeutic agent against Helicobacter pylori infection. FEMS Microbiol Lett. 2010;303:183-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 45. | Huang YQ, Huang QR, Li XH, Huang XF, Wei LD, Wei HY, Chen YYH, Tang HY, Yang S, Qin YC. Effects of Chinese herbal extracts on the biofilm formation of resistant Helicobacter pylori. Yiyao Daobao. 2013;32:1407-1409. [DOI] [Full Text] |
| 46. | Yan X, Kita M, Minami M, Yamamoto T, Kuriyama H, Ohno T, Iwakura Y, Imanishi J. Antibacterial effect of Kampo herbal formulation Hochu-ekki-to (Bu-Zhong-Yi-Qi-Tang) on Helicobacter pylori infection in mice. Microbiol Immunol. 2002;46:475-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 47. | O'Mahony R, Al-Khtheeri H, Weerasekera D, Fernando N, Vaira D, Holton J, Basset C. Bactericidal and anti-adhesive properties of culinary and medicinal plants against Helicobacter pylori. World J Gastroenterol. 2005;11:7499-7507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 106] [Cited by in RCA: 89] [Article Influence: 4.7] [Reference Citation Analysis (2)] |
| 48. | Drug Administration. Administrative Measures for Classification of Prescription Drugs and OTC Drugs. 1999. Aviable from: http://www.nmpa.gov.cn/WS04/CL2077/300625.html. |
| 49. | Xia Y, Meng G, Zhang Q, Liu L, Wu H, Shi H, Bao X, Su Q, Gu Y, Fang L, Yu F, Yang H, Yu B, Sun S, Wang X, Zhou M, Jia Q, Zhao H, Song K, Niu K. Dietary Patterns are Associated with Helicobacter Pylori Infection in Chinese Adults: A Cross-Sectional Study. Sci Rep. 2016;6:32334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 50. | Bi H, Zhu L, Jia J, Zeng L, Cronan JE. Unsaturated Fatty Acid Synthesis in the Gastric Pathogen Helicobacter pylori Proceeds via a Backtracking Mechanism. Cell Chem Biol. 2016;23:1480-1489. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 51. | Jing ZW, Luo M, Jia YY, Li C, Zhou SY, Mei QB, Zhang BL. Anti-Helicobacterpylori effectiveness and targeted delivery performance of amoxicillin-UCCs-2/TPP nanoparticles based on ureido-modified chitosan derivative. Int J Biol Macromol. 2018;115:367-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 52. | Yu P, Fan S, Huang L, Yang L, Du Y. MIR210 as a potential molecular target to block invasion and metastasis of gastric cancer. Med Hypotheses. 2015;84:209-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 53. | Suzuki H, Maruyama R, Yamamoto E, Kai M. Epigenetic alteration and microRNA dysregulation in cancer. Front Genet. 2013;4:258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 133] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 54. | Zhao K, Xie Q, Xu D, Guo Y, Tao X, Wei H, Wan C. Antagonistics of Lactobacillus plantarum ZDY2013 against Helicobacter pylori SS1 and its infection in vitro in human gastric epithelial AGS cells. J Biosci Bioeng. 2018;126:458-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 55. | Hosseini V, Mokhtare M, Gholami M, Taghvaei T, Maleki I, Valizadeh M, Bari Z, Fakheri H. A Comparison between Moderate- and High-dose Furazolidone in Triple Regimens for Helicobacterpylori Eradication in Iran. Middle East J Dig Dis. 2014;6:195-202. [PubMed] |
| 56. | Hu FL, Zhang SS. National Consensus Expert Group on Integrated Helicobacter Pylori Treatment with Traditional Chinese and Western Medicine. Weichangbing Xue And Ganzangbing Xue. 2018;98:2066-2072. [DOI] [Full Text] |
| 57. | Lin ZQ, Wang DX, Hong SS, Fu XY. [Effects of Xiangsha Liujunzi decoction on TLR signal pathway in gastric mucosa tissues of rats with Helicobacter pylori-induced chronic atrophic gastritis]. Zhongguo Zhong Yao Za Zhi. 2016;41:3078-3083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 58. | Yang YJ, Sheu BS. Probiotics-containing yogurts suppress Helicobacter pylori load and modify immune response and intestinal microbiota in the Helicobacter pylori-infected children. Helicobacter. 2012;17:297-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 59. | Çiftçiler R, Koluman A, Haznedaroğlu İC, Akar N. Effects of Ankaferd Hemostat on Helicobacter pylori strains and antibiotic resistance. Turk J Med Sci. 2019;49:347-355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 60. | Päivärinta M, Latvio S, Fredriksson-Ahomaa M, Heikinheimo A. Whole genome sequence analysis of antimicrobial resistance genes, multilocus sequence types and plasmid sequences in ESBL/AmpC Escherichia coli isolated from broiler caecum and meat. Int J Food Microbiol. 2020;315:108361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 61. | French NP, Zhang J, Carter GP, Midwinter AC, Biggs PJ, Dyet K, Gilpin BJ, Ingle DJ, Mulqueen K, Rogers LE, Wilkinson DA, Greening SS, Muellner P, Fayaz A, Williamson DA. Genomic Analysis of Fluoroquinolone- and Tetracycline-Resistant Campylobacter jejuni Sequence Type 6964 in Humans and Poultry, New Zealand, 2014-2016. Emerg Infect Dis. 2019;25:2226-2234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 62. | Huang Y, Hang X, Jiang X, Zeng L, Jia J, Xie Y, Li F, Bi H. In Vitro and In Vivo Activities of Zinc Linolenate, a Selective Antibacterial Agent against Helicobacter pylori. Antimicrob Agents Chemother. 2019;63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |