Published online Apr 28, 2020. doi: 10.13105/wjma.v8.i2.119
Peer-review started: February 5, 2020
First decision: March 21, 2020
Revised: April 8, 2020
Accepted: April 21, 2020
Article in press: April 21, 2020
Published online: April 28, 2020
Processing time: 82 Days and 22.8 Hours
Evidence relating tobacco smoking to type 2 diabetes has accumulated rapidly in the last few years, rendering earlier reviews considerably incomplete.
To review and meta-analyse evidence from prospective studies of the relationship between smoking and the onset of type 2 diabetes.
Prospective studies were selected if the population was free of type 2 diabetes at baseline and evidence was available relating smoking to onset of the disease. Papers were identified from previous reviews, searches on Medline and Embase and reference lists. Data were extracted on a range of study characteristics and relative risks (RRs) were extracted comparing current, ever or former smokers with never smokers, and current smokers with non-current smokers, as well as by amount currently smoked and duration of quitting. Fixed- and random-effects estimates summarized RRs for each index of smoking overall and by various subdivisions of the data: Sex; continent; publication year; method of diagnosis; nature of the baseline population (inclusion/exclusion of pre-diabetes); number of adjustment factors; cohort size; number of type 2 diabetes cases; age; length of follow-up; definition of smoking; and whether or not various factors were adjusted for. Tests of heterogeneity and publication bias were also conducted.
The literature searches identified 157 relevant publications providing results from 145 studies. Fifty-three studies were conducted in Asia and 53 in Europe, with 32 in North America, and seven elsewhere. Twenty-four were in males, 10 in females and the rest in both sexes. Fifteen diagnosed type 2 diabetes from self-report by the individuals, 79 on medical records, and 51 on both. Studies varied widely in size of the cohort, number of cases, length of follow-up, and age. Overall, random-effects estimates of the RR were 1.33 [95% confidence interval (CI): 1.28-1.38] for current vs never smoking, 1.28 (95%CI: 1.24-1.32) for current vs non-smoking, 1.13 (95%CI: 1.11-1.16) for former vs never smoking, and 1.25 (95%CI: 1.21-1.28) for ever vs never smoking based on, respectively, 99, 156, 100 and 100 individual risk estimates. Risk estimates were generally elevated in each subdivision of the data by the various factors considered (exceptions being where numbers of estimates in the subsets were very low), though there was significant (P < 0.05) evidence of variation by level for some factors. Dose-response analysis showed a clear trend of increasing risk with increasing amount smoked by current smokers and of decreasing risk with increasing time quit. There was limited evidence of publication bias.
The analyses confirmed earlier reports of a modest dose-related association of current smoking and a weaker dose-related association of former smoking with type 2 diabetes risk.
Core tip: Based on data from 145 follow-up studies of individuals free of type 2 diabetes at baseline, we confirm evidence of a modest association of smoking with subsequent onset of the disease. Meta-analysis showed relative risks of 1.33 [95% confidence interval (CI): 1.28-1.38] for current vs never smoking, 1.28 (95%CI: 1.24-1.32) for current vs non-smoking, and 1.13 (95%CI: 1.11-1.16) for former smoking. Risks increased with amount smoked and decreased with time quit. Elevated risks were consistently seen when the data were subdivided by various factors, suggesting that the associations are not a result of uncontrolled confounding.
- Citation: Lee PN, Coombs KJ. Systematic review with meta-analysis of the epidemiological evidence relating smoking to type 2 diabetes. World J Meta-Anal 2020; 8(2): 119-152
- URL: https://www.wjgnet.com/2308-3840/full/v8/i2/119.htm
- DOI: https://dx.doi.org/10.13105/wjma.v8.i2.119
Pan et al[1], 2015 published a meta-analysis and systematic review of the relationship of active, passive and quitting smoking with incident type 2 diabetes. Based on 88 prospective studies, they reported pooled relative risks (RRs) and 95% confidence intervals (CIs) compared to never smoking of 1.37 (95%CI: 1.33-1.42) for current smoking, 1.14 (95%CI: 1.10-1.18) for former smoking and 1.22 (95%CI: 1.10-1.25) for passive smoking, and evidence of a dose-relationship with amount smoked and years quit. This was an update of a previous review by the US Surgeon General, 2014[2], which based on 46 studies, had argued for a causal relationship. As evidence on tobacco smoking and type 2 diabetes has accumulated rapidly in the last few years, we wanted to investigate more extensively how this relationship may vary based on characteristics of the study or of the RR. We conducted our own updated review and meta-analysis, based solely on active smoking of cigarettes, with or without use of pipes, cigars or smokeless tobacco.
Epidemiological prospective studies of populations without type 2 diabetes at baseline in which smoking was related to subsequent incidence of the disease.
The studies had to provide RR estimates for one or more defined major or dose-related smoking indices. The defined “major indices” compare ever, current or ex-smokers with never smokers, or current smokers with non-current smokers, and refer to smoking of any product (cigarettes, pipes, cigars and combinations) or to smoking of cigarettes. The defined “dose related indices” concern the amount currently smoked and the duration of quitting.
Studies were excluded where the participants were restricted to those with diseases related to type 2 diabetes.
This was carried out in five steps.
Step 1 identified relevant papers from four previously published reviews of evidence from relevant prospective studies. The review in the 2014 United States Surgeon-General Report[2], presented an analysis based on 46 prospective studies, taking into account studies reported in an earlier review by Willi et al[3], 2007 and adding additional studies. Since that Report, which included studies published up to 2010, two further meta-analyses have been published. That by Pan et al[1], 2015 included 88 studies, all but five of those considered by the United States Surgeon-General, along with many other studies published up to May 3, 2015. Another review by Akter et al[4], 2017 was limited to studies in Japan, and also considered studies up to 2015.
Step 2, carried out on January 31, 2019, repeated the Medline searches described by Pan et al[1], 2015, but with the search date restricted to January 1, 2015 onwards.
Step 3 was based on a search on our in-house reference system for papers with keywords DIABETES.
Step 4, carried out on March 1, 2019, repeated the Embase searches described by Pan et al[1], 2015, with the search restricted to papers not on Medline.
Finally, Step 5 was based on reference lists of papers identified in Steps 2, 3 and 4, looking for additional potentially relevant papers published from 2015.
In Steps 2 and 4, abstracts were examined first, with full texts obtained only for papers which appeared likely to be relevant. This step was initially carried out by Coombs KJ, with a 20% check made by Lee PN.
At each step, papers (or abstracts) examined for potential relevance were only those not previously considered.
At the end of this process, a set of potentially relevant papers was obtained. Subsequently, more detailed examination of the full texts at the data entry stage revealed that some papers did not actually meet the inclusion criteria, leading to a reduction in the list of relevant papers.
Relevant information was entered onto a publication database and a linked RR database.
The publication database contains a record for each publication describing the following aspects: In-house reference ID of the publication; first author; publication year; location (continent/country); study name; study title; population studied; beginning and end year of baseline; end year of follow-up; length of follow-up; definition of type 2 diabetes (for both baseline exclusion and subsequent incidence) and source of diagnosis; cohort size; number of type 2 diabetes cases; age at baseline; sexes considered; races considered; definition of smoking; results available (current, former, ever, amount smoked, and years quit); details of results available for specific subsets [sex, age, body mass index (BMI), physical activity, alcohol, family history of type 2 diabetes, education, diet, and others]; and details of factors adjusted for in analyses (sex, age, BMI, physical activity, alcohol, family history of type 2 diabetes, education, diet, blood pressure, cholesterol, glucose, triglycerides, waist size, and others).
The RR database holds the detailed results, typically containing multiple records for each publication. Each record is linked to the relevant publication and refers to a specific comparison. The record includes details of the publication reference ID, study name, sex, age range at baseline, length of follow-up, BMI range, definition of smoking, and smoking status of the numerator (current, former or ever), and of the denominator (never or non). Where the smoking status is former, the range of years quit is entered. The range of amount smoked is also entered. For unadjusted RR estimates, the numbers of cases and at risk (or person years) are entered for both the numerator and denominator.
For adjusted RR estimates, the RR and 95%CI are entered, taken directly from the publication, or estimated using standard methods[5], with details also entered of the factors adjusted for.
Numbers of cases and at risk, or RRs and 95%CIs, are only entered for the whole population or for subgroups defined by sex, age group or BMI group. As noted above, the availability of results by other factors is recorded in the publication database, but the detailed results have not so far been entered. Results are also only entered unadjusted for potential confounding variables and adjusted for the most confounding variables for which results were available.
All data were entered by Coombs KJ and checked by Lee PN, with any disagreements discussed and resolved.
Once the data were entered, the list of publications was sorted into studies. Where the RRs from only one publication needed to be used in analysis, with the others providing no useful extra data (e.g., providing similar data for a shorter follow-up), these “other” publications were rejected, with the reasons for rejection noted. Where more than one publication from the same study provided useful data (e.g., for different aspects of smoking), one publication was nominated as the main reference for the study (typically, the publication providing the most detailed results) and others were nominated as subsidiary references. Thus, it was possible to have main, subsidiary and rejected references from the same study. Another possibility is that a publication may give a pooled analysis of several individual studies, including useful data for aspects not covered in the main publications of the separate studies. These pooled publications are also nominated as subsidiary references.
Fixed-effect and random-effects meta-analyses were conducted using the method of Fleiss and Gross, 1991[6], with heterogeneity quantified by H, the ratio of the heterogeneity chisquared to its degrees of freedom. H is directly related to the statistic I2[7] by the formula I2 = 100 (H−1)/H. For all meta-analyses, Egger’s test of publication bias[8] was included.
Meta-analyses were conducted using the available data for current vs never, current vs non, ever vs never, and former vs never smoking. Where there was a choice of estimates for a study, preference was given to results that were for the full range of amount smoked, the longest follow-up, the most adjusted, the widest age range, and the preferred product, with preference being given, in order to results for: Cigarettes; smoking excluding exclusive pipe/cigar; smoking; and tobacco; but not exclusive cigar, pipe or smokeless tobacco. For a study of both sexes, preference was also given to separate estimates for the two sexes, if available. While in most studies, the choice of estimates was straightforward, in others it was not (e.g., between an unadjusted RR for a longer follow-up from one publication and an adjusted RRs for a shorter follow-up from another). Here Coombs KJ and Lee PN agreed and recorded the most relevant RR to choose (disregarding its magnitude). For a particular exposure (e.g., current vs never) each study could provide only the estimate or two sex-specific estimates for inclusion in the meta-analysis.
Effect estimates were derived based on all the selected RRs as well as for those subdivided by various categorical variables: Sex (male, female, and sexes combined); continent (Asia, Europe, Americas, and Oceania); publication year (before 2005, 2005-14, 2015 or later); diagnosis of type 2 diabetes (self-reported, medical data only, both); population (general, pre-diabetics only, excludes pre-diabetics); total number of adjustment factors (0, 1-5, 6-10, 11+); cohort size (< 5000, 5000-20000, > 20000); number of type 2 diabetes cases (< 500, 500-999, 1000-2000, 2001+); highest baseline age (< 60, 60-74, 75+ years); length of follow-up (< 5, 5-10, > 10 years); definition of smoking [cigarettes, smoking (whether or not excluding exclusive pipe/cigar), tobacco]; and whether each of a range of different variables were adjusted for.
When comparing RRs by amount currently smoked (with a reference group of never smokers) or non-smokers, or by years quit (with a reference group of never smokers), a study typically provides a set of non-independent RRs for each dose-category, expressed relative to a common base. To avoid double-counting, it is necessary to include only one in any one meta-analysis.
For amount smoked, three methods were used. One method used only for studies that reported results for two levels of amount smoked, was to compare results for 1-19 and 20+ cigs/d, the most common subdivision used. The second, used only for studies that reported results for three levels of amount smoked was to compare results for low, medium and high cigs/d regardless of the levels selected. The third involved defining a set of key values (10, 20 and 40 cigs/d) and carrying out a separate meta-analysis for each key value. For an RR to be allocated to a key value its dose category had to include that key value and no other. This method was only applied for studies reporting results by three or more levels, with all three key value results available. These methods were used for data on current vs never smoking, and for current vs non-smoking.
For years quit, two methods were used. One simply used the shortest and longest categories. The other used the key values approach with values of 3, 7 and 12 years quit.
For each of the studies that reported independent RR estimates separately for different subdivisions of the population by level of BMI, estimates were made, for each smoking index for which data were available, of the ratio of the RR for highest vs lowest BMI group, these ratios then also being meta-analysed.
When conducting meta-analyses care was taken to minimize overlap of cases. Thus, results from subsidiary papers were used only when the main paper did not provide the result required for the particular meta-analysis. Also, if an RR was available from three separate studies, and also from a combined analysis from the three studies, the individual results were preferred, only using the combined RR for a smoking index for which results were not reported in all the different studies.
As summarized in Table 1[9-15], 221 publications were originally identified as likely to be relevant, with 42 later rejected during data entry, the reasons for rejection being given in Supplementary File 1. As seven of the publications provided results for two independent data sets (either presenting separate results for two studies or for two non-overlapping follow-up periods), data entry was carried out initially for 186 publication records. On investigation of studies with multiple records, 29 records were rejected as providing no useful information extra to those provided in other records) and 12 were classified as subsidiary, providing some limited extra information for records classified as main. This meant that there were 145 studies, 144 separate studies plus the combined analysis of three studies (HPFUS, NHS and NHSII). Table 2[9-14,16-161] summarizes some characteristics of these studies, while Supplementary file 1 also gives information on why some publications were rejected or only provided subsidiary information.
| Step | Papers originally selected as probably relevant1 | Papers rejected during data entry2 | Papers providing separate results for multiple studies3 | |
| 1 | Previous reviews | 98 | 10 | 3[9-11] |
| 2 | Medline search | 74 (from 3365 hits) | 23 | 4[12-15] |
| 3 | In-house database | 1 | 0 | 0 |
| 4 | Embase search | 33 (from 5433 hits) | 7 | 0 |
| 5 | Secondary references4 | 15 (of 30 identified) | 2 | 0 |
| Total | 221 | 42 | 7 | |
| Study Ref. | Main/ Other Ref. | Continent | Country, location2 | Study Population3 | Sex | Baseline | Follow-up (yr)4 | Diagnosis code5 | Cohort size | Diabetes cases | Age |
| 3 studies6 | [16] | North America | United States | Medical professionals | M+F | 1984-1991 | 19.6 | 3 | 162807 | 12384 | 25-75 |
| AICHI | [17]/[18] | Asia | Japan, Aichi | Civil servants | M+F | 2002 | 9.0 | 3 | 3338 | 225 | 35-66 |
| AIZAWA | [19] | Asia | Japan, Matsumoto | Participants from hospital (not otherwise defined) | M+F | 2005 | 4.9 | 2 | 4159 | 279 | Any |
| ALEIN | [20] | Asia | Taiwan (China), A-Lein | Persons undergoing community wide screening for hepatitis | M+F | 1996-1997 | 8.0 | 2 | 3539 | 423 | 40-69 |
| ALSWH | [21] | Oceania | Australia | General population | F | 1998 | 12.0 | 1 | 12367 | 871 | 47-52 |
| ANSAN | [22]/[23,24] | Asia | South Korea, Ansun and Ansan | Community based | M+F | 2001-2002 | 4.0 | 2 | 4041 | 329 | 40-69 |
| ARIC | [25]/[26] | North America | United States, North Carolina, Mississippi, Maryland | Probability sample from 4 US communities with exclusive sampling of African Americans in one of the four sites, Black or White | M+F | 1987-1989 | 9.0 | 3 | 10892 | 1254 | 45-64 |
| ASAN | [27] | Asia | South Korea, Asan | Attending voluntary medical check-ups | M+F | 2000 | 5.0 | 2 | 5372 | 234 | 20-79 |
| ATTICA | [28] | Europe | Greece, Athens | General population | M+F | 2001-2002 | 10.0 | 2 | 1485 | 191 | 18-89 |
| Ausdiab | [29] | Oceania | Australia | General population | M+F | 1990-2000 | 5.0 | 2 | 5842 | 244 | 25+ |
| BEDFORD | [30] | Europe | England, Bedford | Borderline diabetics with a 2h fasting glucose of 6.7-11.1 mmol/L | M+F | 1962-1964 | 10.0 | 2 | 241 | 36 | 18+ |
| BIP | [31] | Asia | Israel | Subjects with impaired functional capacity (New York Heart Association class II and III) | M+F | 1990-1993 | 6.2 | 2 | 630 | 98 | 45-74 |
| BMES | [32] | Oceania | Australia, West of Sydney | Non institutionalised residents | M+F | 1992-1994 | 10.0 | 3 | 2123 | 165 | 49+ |
| BOGALUSA | [33] | North America | United States, Bogalusa | General population | M+F | 1973-2010 | 9.1 | 2 | 7725 | 176 | <18 |
| BOTNIA | [9]/[34] | Europe | Finland, Botnia | Family members of diabetics | M+F | 1990 | 7.6 | 2 | 2770 | 138 | Any |
| BRHS | [35] | Europe | Britain | General population | M | 1978-1980 | 16.8 | 3 | 7124 | 290 | 40-59 |
| BRUNECK | [36] | Europe | Italy, Bruneck | General population, White | M+F | 1990 | 10.0 | 2 | 837 | 64 | 40-79 |
| BURKE | [37] | Oceania | Australia Kimberley | General population, Aboriginal | M+F | 1988-1989 | 12.9 | 2 | 493 | 104 | 15-88 |
| BWHS | [38] | North America | United States | African American subscribers to magazine targeted at black women | F | 1995 | 16.0 | 3 | 43003 | 4387 | 21-69 |
| CASSAN | [39] | North America | United States | Majority were veterans, 98% Caucasian | M | 1963 | 18.0 | 2 | 1972 | 226 | 20-80 |
| CCHS | [40] | North America | United States, Cleveland | General population | M+F | 2008 | 5.0 | 2 | 5084 | 872 | 18+ |
| CDCdeC | [41] | Europe | Spain, Canaries | General population | M+F | 2000-2005 | 3.5 | 3 | 5521 | 146 | 18-75 |
| CEHSC | [42] | Asia | Hong Kong (China) | General population volunteers | M+F | 1998-2001 | 9.8 | 2 | 53905 | 806 | 65+ |
| CKB | [43] | Asia | China | General population | M+F | 2004-2008 | 7.2 | 2 | 461211 | 8784 | 30-79 |
| CoLaus | [44] | Europe | Switzerland, Lausanne | General population | M+F | 2003-2006 | 5.5 | 2 | 3166 | 47 | 35-75 |
| CPSI | [45] | North America | United States | General Population | M+F | 1959-1960 | 12.0 | 3 | 709827 | 25397 | 30+ |
| CRISPS | [46] | Asia | Hong Kong (China) | General population, Chinese | M+F | 2000-2004 | 9.0 | 2 | 1380 | 123 | Any |
| CURES | [47] | Asia | India, Chennai | General population | M+F | 2001-2003 | 9.1 | 2 | 1376 | 385 | 20+ |
| DAQING | [48] | Asia | China | Care clinic patients with pre-diabetes, part of diabetes prevention intervention | M+F | 1986 | 23.0 | 3 | 568 | 436 | Any |
| DEHGHA | [49] | Europe | Netherlands, Ommoord | General population | M+F | 1990-1993 | 10.8 | 2 | 6935 | 645 | 55+ |
| DE-PLAN | [11] | Europe | Spain, Navarra, Reus and Barcelona | Participants in clinical trial on Mediterranean diet, Caucasian | M+F | 2006 | 4.2 | 2 | 552 | 124 | 45-75 |
| DESIR | [50] | Europe | France, Western | Volunteers for periodic health checks | M+F | 1998 | 9.0 | 2 | 3817 | 203 | 30-64 |
| DLCS | [51] | Europe | Netherlands, Northern | General population, Western Europe | M+F | 2007-2013 | 4.0 | 3 | 72880 | 1056 | 18-90 |
| DNC | [52] | Europe | Denmark | Nurses | F | 1993-1999 | 15.3 | 2 | 24174 | 1137 | 44+ |
| DONGFENG | [53] | Asia | China, Da Qing | Retired employees | M+F | 2008-2010 | 4.0 | 3 | 17690 | 1390 | Any |
| DWECS | [54] | Europe | Denmark | Workers | M+F | 1995-2005 | 5.0 | 2 | 6823 | NA | 30-59 |
| EPIC-IN | [55] | Europe | 8 countries7 | Subset of participants in EPIC-InterAct cohort | M+F | 1991 | 11.7 | 3 | 23501 | 10327 | Any |
| ESTHER | [56] | Europe | Germany, Saarland | General population | M+F | 2000-2002 | 8.0 | 3 | 7462 | 718 | 50-75 |
| FAGERB | [57] | Europe | Sweden, Göteborg | General population, Caucasian | F | 2001-2004 | 5.5 | 2 | 341 | 69 | 64 |
| FINNMARK | [58] | Europe | Norway, Finnmark | General population | M+F | 1997-1978 | 12.0 | 2 | 11654 | 162 | 35-52 |
| GLOSTRUP | [59] | Europe | Denmark, Glostrup | General population | M | 1982-2001 | 18.9 | 2 | 5350 | 211 | 30-70 |
| GNHIES | [60] | Europe | Germany | General population (non institutionalized) | M+F | 1997-1999 | 5.0 | 2 | 3625 | 82 | 18-79 |
| HDNNCDS | [12] | Asia | China, Harbin | General population, Chinese | M+F | 2010 | 4.2 | 3 | 7133 | 578 | 20-74 |
| HEALTH2000 | [10] | Europe | Finland | General population | M+F | 2000-2001 | 7.0 | 2 | 4110 | 81 | 40-79 |
| HEINZN | [61]/[62] | Europe | Germany, Western | General population | M+F | 2000-2003 | 5.1 | 3 | 3547 | 319 | 45-75 |
| HENAN | [63] | Asia | China, Henan | General population, N Chinese ancestry | M+F | 2007-2008 | 6.0 | 3 | 12272 | 775 | 18+ |
| HIPOP-OHP | [64] | Asia | Japan | Employees | M+F | 1999 | 3.4 | 3 | 6498 | 229 | Any |
| HIPPIS1 | [65] | Europe | England and Wales | Primary care patients | M+F | 1993-2008 | 8.0 | 2 | 2540753 | 78081 | 25-79 |
| HIPPIS2 | [66] | Europe | England | Primary care patients | M+F | 2005-2016 | 3.9 | 2 | 8186705 | 178314 | 25-84 |
| HISAYAMA | [67] | Asia | Japan, Hisayama | General population | M+F | 1988 | 11.8 | 2 | 1935 | 286 | 40-79 |
| HPFUS | [68] | North America | United States | Health professionals | M | 1986 | 6.0 | 3 | 41810 | 509 | 40-75 |
| HPHS | [12] | Asia | China, Harbin | General population, Chinese | M+F | 2008 | 4.2 | 3 | 3350 | 244 | 20-74 |
| HUNT | [69] | Europe | Norway, Nord-Trøndelag | General population | M+F | 1984-1997 | 11.0 | 3 | 90819 | 1860 | 20+ |
| ICARIA | [70] | Europe | Spain | Spanish workers | M+F | 2004-2007 | 4.1 | 3 | 380366 | 9960 | 18-65 |
| ICS | [71] | Asia | Iran, Isfahan, Arak and Najafabad | General population | M+F | 2001 | 7.0 | 2 | 2980 | 389 | 35+ |
| IPC | [72] | Europe | France, Paris | Workers and those seeking employment who had undergone 2 health checks | M+F | 1998-2010 | 5.3 | 2 | 22567 | 527 | 18+ |
| IRAS | [73] | North America | United States, 4 areas8 | General population | M+F | 1992-1993 | 5.0 | 2 | 906 | 148 | 40-69 |
| IWHS | [74] | North America | United States, Iowa | Community based | F | 1986 | 13.2 | 1 | 36839 | 3281 | 55-69 |
| JACC | [75] | Asia | Japan | Community based | M+F | 1988-1990 | 5.0 | 1 | 16160 | 396 | 40-79 |
| J-ECOH | [76]/[77] | Asia | Japan | Employees | M+F | 2008-2010 | 3.9 | 2 | 53930 | 2441 | 15-83 |
| JHS | [78] | North America | United States, Mississippi | General population, Black | M+F | 2000-2004 | 8.0 | 2 | 2991 | 479 | 21-84 |
| JPHC | [79] | Asia | Japan | General population | M+F | 1990 | 10.0 | 1 | 28893 | 1183 | 40-59 |
| JPHC2 | [80] | Asia | Japan | General population | M+F | 1995-1998 | 5.0 | 1 | 59834 | 1100 | 45-74 |
| KANGBUK | [81] | Asia | South Korea, Seoul | Individuals undergoing health screening | M+F | 2002 | 5.6 | 3 | 174314 | 5544 | 18+ |
| KAWAHA | [82] | Asia | Japan, Kitakyushu City | City workers | M+F | 2008 | 3.7 | 2 | 52781 | 4369 | 20-89 |
| KAWAKA | [83] | Asia | Japan, electrical company | Employees of large electrical company | M | 1984 | 8.0 | 2 | 2312 | 41 | 18-53 |
| KMIC | [84] | Asia | South Korea | Government and school employees | M | 1990-1986 | 8.0 | 2 | 27635 | 1170 | 35-44 |
| KoGES-K | [85]/[86] | Asia | South Korea, Kangwha | Community based | M+F | 2006-2011 | 4.0 | 2 | 2079 | 142 | 40+ |
| KORA F4/FF4 | [87] | Europe | Germany, Augsburg | General population | M+F | 2006-2008 | 7.0 | 2 | 504 | 76 | 62-81 |
| KORA S4/F4 | [88] | Europe | Germany, Augsburg | General population | M+F | 1999-2001 | 7.0 | 2 | 887 | 93 | 55-74 |
| KPNW | [89] | North America | United States, Portland | Health care members | M+F | 1997-2000 | 6.8 | 2 | 46578 | 1854 | 40+ |
| LEICESTER | [90] | Europe | England, Leicester | With clinical diagnosis of polycystic ovary syndrome | F | 1988-2009 | 5.2 | 2 | 2164 | 138 | 16-79 |
| LIETO | [91] | Europe | Finland, Leito | General population | M | 1998-1999 | 9.0 | 2 | 430 | 30 | 64+ |
| LINDBE | [92] | Europe | Denmark, Copenhagen | General population | M+F | 2001-2003 | 8.5 | 2 | 5349 | 136 | 20-94 |
| LLP | [93] | Europe | England, Liverpool | General population | M+F | 1998-2008 | 10.0 | 2 | 8753 | 763 | 45-79 |
| MAILES | [94] | Oceania | Australia, Adelaide | General population | M | 2002-2006 | 4.9 | 3 | 1597 | 232 | 35-80 |
| MANSON | [95] | North America | United States | Physicians in randomized trial | M | 1982 | 12.0 | 1 | 21068 | 770 | 40-84 |
| MECC | [96] | North America | United States, Hawaii and California | General population, African American and Latino | M+F | 1993-1996 | 14.0 | 3 | 48995 | 15833 | 50-75 |
| MECH | [97] | North America | United States, Hawaii | General population, Caucasian, Hawaiian, Japanese, American | M+F | 1993-1996 | 12.1 | 3 | 74970 | 8559 | 45-75 |
| MESA | [98]/[99] | North America | United States, 6 states9 | General population, White, Black, Hispanic or Chinese | M+F | 2000-2002 | 10.2 | 2 | 5931 | 359 | 45-84 |
| MFH | [10] | Europe | Finland | General population | M+F | 1978-1980 | 10.0 | 2 | 4517 | 145 | 40-79 |
| MJH | [100] | Asia | Taiwan (China) | Paid members of private health screening program, Chinese | M+F | 2001-2014 | 6.7 | 3 | 147908 | 4781 | 18+ |
| MONICAG | [101] | Europe | Germany, Augsburg | General population | M+F | 1984-1995 | 12.5 | 3 | 10892 | 672 | 25-74 |
| MONICAS | [102] | Europe | Sweden, Northern | General population | M | 1986-1994 | 8.7 | 3 | 1275 | 27 | 25-74 |
| MORIMO | [103]/[104] | Asia | Japan, Nagano prefecture | Volunteers in Nagano Prefecture | M+F | 1990-1992 | 10.1 | 3 | 5872 | 595 | 40-69 |
| MOZAFF | [105] | North America | United States, 4 states10 | Ambulatory, noninstitutionalized subjects | M+F | 1989-1992 | 10.0 | 2 | 4883 | 337 | 65+ |
| MPBB | [106] | North America | United States, Michigan | Subjects who had injected food contaminated with polybrominated biphenyls, 99.8% White | M+F | 1976 | 25.0 | 3 | 1384 | 180 | 20+ |
| MPP | [9] | Europe | Sweden, Malmo | General population | M+F | 1974-1992 | 24.8 | 2 | 16061 | 2063 | Any |
| MUTUAL | [107] | Asia | Japan | Civil servants | M+F | 2000 | 6.5 | 2 | 5848 | 287 | 30-59 |
| MYHUS | [108] | Asia | Japan | Employees | M+F | 2004 | 5.0 | 3 | 13700 | 408 | 36-55 |
| NAGALA | [109]/[110] | Asia | Japan, Gifu | Subjects receiving medical check-ups | M+F | 2004-2015 | 5.1 | 3 | 17810 | 804 | Any |
| NAGAYA | [111] | Asia | Japan, Nagoya | Volunteer attendants of annual health check ups | M | 1988-1990 | 7.4 | 3 | 16829 | 869 | 30-59 |
| NAKANI | [112] | Asia | Japan, Osaka | Employees | M | 1994 | 5.0 | 2 | 1266 | 54 | 35-59 |
| NCDS | [113] | Europe | Britain | Birth cohort from March 1958 | M+F | 1974 | 17.0 | 1 | 4945 | 28 | 16 |
| NHANES | [114] | North America | United States | General population | M+F | 1971-1975 | 18.0 | 3 | 4830 | 443 | 25-74 |
| NHIC | [115] | Asia | South Korea | Recipients of biennial medical check-ups | M+F | 1992-1995 | 14.0 | 2 | 1236443 | 89422 | 30-95 |
| NHIS-HEALS | [116] | Asia | South Korea | Recipients of national health screen test | M+F | 2002-2003 | 10.8 | 2 | 359349 | 37678 | 40-79 |
| NHIS-NCS | [117] | Asia | South Korea | Nationally representative | M+F | 2002 | 6.8 | 2 | 51405 | 2749 | 20+ |
| NHS | [118] | North America | United States | Registered Nurses | F | 1976-1982 | 24.0 | 3 | 100526 | 5392 | 30-55 |
| NHSII | [13] | North America | United States | Registered Nurses | F | 1989-1991 | 23.0 | 3 | 88086 | 5441 | 25-42 |
| NIH -AARP | [119] | North America | United States, 6 states11 | General population | M+F | 1995-1996 | 11.0 | 1 | 207479 | 18000 | 50-71 |
| NOMAS | [120] | North America | United States, North Manhattan | General population, White, Black or Hispanic | M+F | 1993-2001 | 11.0 | 3 | 2430 | 449 | 40+ |
| NOVAK | [121] | Europe | Sweden, Gothenburg | General population (intervention group in ineffective trial) | M | 1970-1973 | 35.0 | 2 | 6828 | 899 | 47-56 |
| OLMSTED | [122] | North America | United States, Rochester | General population who also took at least one medication | M+F | 1999-2004 | 6.0 | 2 | 13508 | 1182 | 18+ |
| ONAT | [123] | Asia | Turkey | Participants in nationwide survey | M+F | 1997-1998 | 5.9 | 3 | 3385 | 216 | 28+ |
| OSAKA | [124] | Asia | Japan, Osaka | General population undergoing basic health check-ups | M+F | 2001 | 4.0 | 2 | 9327 | 171 | 40-74 |
| OSLO | [125] | Europe | Norway, Oslo | General population | M | 1972-1973 | 28.0 | 3 | 6382 | 584 | 40-49 |
| OSTENS | [126] | Europe | Sweden, Stockholm | General population | M | 1992-1994 | 10.0 | 2 | 2383 | 99 | 35-56 |
| PARK | [127] | Asia | South Korea, not known | Undergoing health examinations | M | 2002 | 4.0 | 2 | 1717 | 50 | Any |
| PATJA | [128] | Europe | Finland, North Karelia and Kuopio | General population | M+F | 1972-1992 | 21.0 | 2 | 41372 | 2770 | 25-64 |
| PINGLIANG | [129] | Asia | China, Ping Liang | General population pre-diabetic at baseline | M+F | 2002-2003 | 10.8 | 2 | 334 | 98 | Any |
| PMMJS | [130] | Asia | China, Jiangsu | General population | M+F | 2000-2004 | 5.0 | 2 | 3598 | 160 | 35-74 |
| PREDIMED | [11] | Europe | Spain, Navarra, Reus and Barcelona | Participants in clinical trial on Mediterranean diet, Caucasian | M+F | 2003-2009 | 4.8 | 2 | 1381 | 155 | 55-80 |
| PREDIMERC | [131] | Europe | Spain, Madrid | General population | M+F | 2007 | 6.4 | 2 | 2048 | 44 | 30-74 |
| PREVEND | [132] | Europe | Netherlands, Groningen | General population | M+F | 1997-1998 | 11.4 | 3 | 7953 | 447 | Any |
| REGARDS | [133] | North America | United States | General population, Black or White | M+F | 2003-2007 | 9.5 | 2 | 7758 | 891 | 45+ |
| SABE | [134] | South America | Brazil, Sào Paulo | General population | M+F | 2000 | 6.0 | 1 | 914 | 72 | 60+ |
| SAIREN | [135] | Asia | Japan, Ibaraki-ken | General population undergoing annual health check-ups | M+F | 1993 | 5.0 | 2 | 128141 | 7990 | 40-79 |
| SALSA | [136] | North America | United States, Sacramento | General population, Latino | M+F | 1998-1999 | 10.0 | 3 | 782 | 144 | 60+ |
| SAMSUNG | [137] | Asia | South Korea, Seoul | Undergoing health examinations, Korean | M | 2006 | 6.0 | 3 | 1774 | 180 | 20+ |
| SAPALDIA | [138] | Europe | Switzerland | General population | M+F | 2002 | 8.3 | 3 | 2631 | 110 | 18+ |
| SAWADA | [139] | Asia | Japan, Tokyo | Employees of Tokyo Gas Company | M | 1985 | 14.0 | 3 | 4745 | 280 | 20-41 |
| SAX45 | [140] | Oceania | Australia, New South Wales | General population | M+F | 2006-2008 | 3.4 | 1 | 54997 | 888 | 45+ |
| SCCS | [14] | North America | United States, Southern | General population, Black or White | M+F | 2002-2009 | 4.5 | 1 | 35892 | 3439 | 40-79 |
| SCCS2 | [14] | North America | United States, Southern | General population, Black or White | M+F | 201212 | 3.0 | 1 | 20712 | 1708 | 43-82 |
| SHFS | [141] | North America | United States, 4 states13 | Members of multiplex families, American Indians | M+F | 2001-2003 | 5.5 | 2 | 431 | 133 | 14+ |
| SHIP | [142] | Europe | Germany, Augsburg | Caucasian German citizens | M+F | 1997-2001 | 11.1 | 2 | 2034 | 206 | 20-81 |
| SMHS | [143] | Asia | China, Shanghai | General population | M | 2002-2006 | 5.4 | 3 | 51464 | 1304 | 40-74 |
| STILLW | [144] | Europe | Finland | Employees of Finnish Company | M | 1986 | 17.0 | 2 | 5827 | 313 | 18-65 |
| STRAND | [145] | Europe | Finland, Helsinki | Volunteer executives and businessmen | M | 1974-1975 | 20.0 | 3 | 1802 | 94 | 40-56 |
| STRING | [146]/[147] | Europe | England, London | Civil service employees | M+F | 1985-2002 | 23.7 | 2 | 8270 | 1286 | 50 |
| SUGIMO | [148] | Asia | Japan, Tokyo | Participants in MHTS | M+F | 1976 | 16.0 | 2 | 2573 | 296 | 18-69 |
| SULAWESI | [149] | Asia | Indonesia, South Sulawesi | Three tribes | M+F | 2013 | 3.0 | 2 | 182 | 58 | 16+ |
| SWAN | [150] | North America | United States, Michigan | Participants in study of menopause transition, Black or White | F | 1996 | 16.0 | 3 | 424 | 157 | 42-52 |
| TCS | [151] | Asia | Thailand | Students at Sukothai Thammithirat Open University | M+F | 2005 | 8.0 | 1 | 39507 | 698 | 15-88 |
| TERATA | [152] | Asia | Japan, Chiba | Steelworkers | M | 2002 | 8.0 | 2 | 8423 | 464 | Any |
| TLSA | [153] | Asia | Taiwan (China), Non-aboriginal areas | Participants in ongoing survey on aging, Taiwanese | M+F | 1999 | 4.0 | 1 | 2995 | 277 | 53+ |
| TOPICS6 | [154] | Asia | Japan, Toranomon | Government employees and some general population | M+F | 1997-2002 | 5.0 | 3 | 7654 | 289 | 40-75 |
| TROMSO | [155] | Europe | Norway, Tromsø | General population | M+F | 1994-1995 | 10.8 | 3 | 26168 | 522 | 25-98 |
| UCHIMO | [156] | Asia | Japan, Osaka | Employees of large company | M | 1981-1991 | 10.0 | 2 | 6250 | 450 | 35-60 |
| VETERAN | [157] | North America | United States | Veterans | M+F | 2002-2003 | 4.0 | 2 | 239057 | 33453 | 18-99 |
| VIP | [158] | Europe | Sweden, Västerbotten County | General population | M+F | 1990-2012 | 9.9 | 3 | 32120 | 2211 | 35-55 |
| WHI | [159] | North America | United States | Postmenopausal women in a clinical trial or an observational study | F | 1993-1998 | 11.0 | 1 | 135906 | 15076 | 50-79 |
| YOUNGF | [160] | Europe | Finland | Population based | M+F | 1980 | 24.0 | 3 | 2298 | 79 | 3-18 |
| ZUTPHEN | [161] | Europe | Netherlands, Zutphen | General population | M | 1960 | 25.0 | 2 | 841 | 58 | 40-59 |
All stages of the identification of relevant papers, classification of papers with studies, and data entry were conducted initially by Coombs KJ and checked by Lee PN. Exceptionally, Lee PN only checked 20 percent of the abstracts for the Medline and Embase searches. This 20 percent check, of a total of 8798 hits, only resulted in four extra full-text papers being examined, only one of which proved to have relevant data. Given the very limited extra information obtained, and the time spent, it was decided not to extend this to a 100 percent check.
Location: As shown in Table 2, 53 of the 145 studies were conducted in Asia (including 23 in Japan, 10 in South Korea, nine in China and 11 in other countries). Fifty-three were conducted in Europe (eight in Great Britain, eight in Finland, seven in Germany, six in Sweden, five in Spain, and 19 in other countries), with 32 in North America (all in the United States), six in Australia and one in Brazil.
Population: Ten of the studies were in females, 24 in males and 111 in both sexes. About half were of the relevant general population, with Table 2 showing further details.
Time: There was a clear increase in study frequency with time, with 17 starting before 1980, 23 starting in the 1980s, 47 in the 1990s, 42 in 2000-2005, and 16 from 2006 onwards.
Years follow up: Twenty-four studies involved less than 5 years follow-up; 62 studies involved 5-9.9 years follow-up; 36 studies involved 10-14.9 years follow-up; and 23 studies involved 15 years or more years follow-up, with the longest (NOVAK) involving 35 years.
Diagnosis: Fifteen of the studies diagnosed type 2 diabetes only on the basis of self-report of the individuals, 79 only on medical records, and 51 on both.
Size: The numbers in the cohorts studied varied from 182 to over eight million. Sixty-three were under 5000, 39 in the range 5000 to 20000 and 43 larger than this.
Type 2 diabetes cases: The number of type 2 diabetes cases varied from 27 to almost 180000. Eighty-two involved fewer than 500 cases, 21 involved 500-999 cases, 13 involved 1000-2000 cases, and 28 involved more than this. The number was not available for one study.
Age: Most of the studies included some individuals of age 75 or older at baseline. However, 24 were restricted to those aged less than 60 and 30 more were restricted to those aged less than 74.
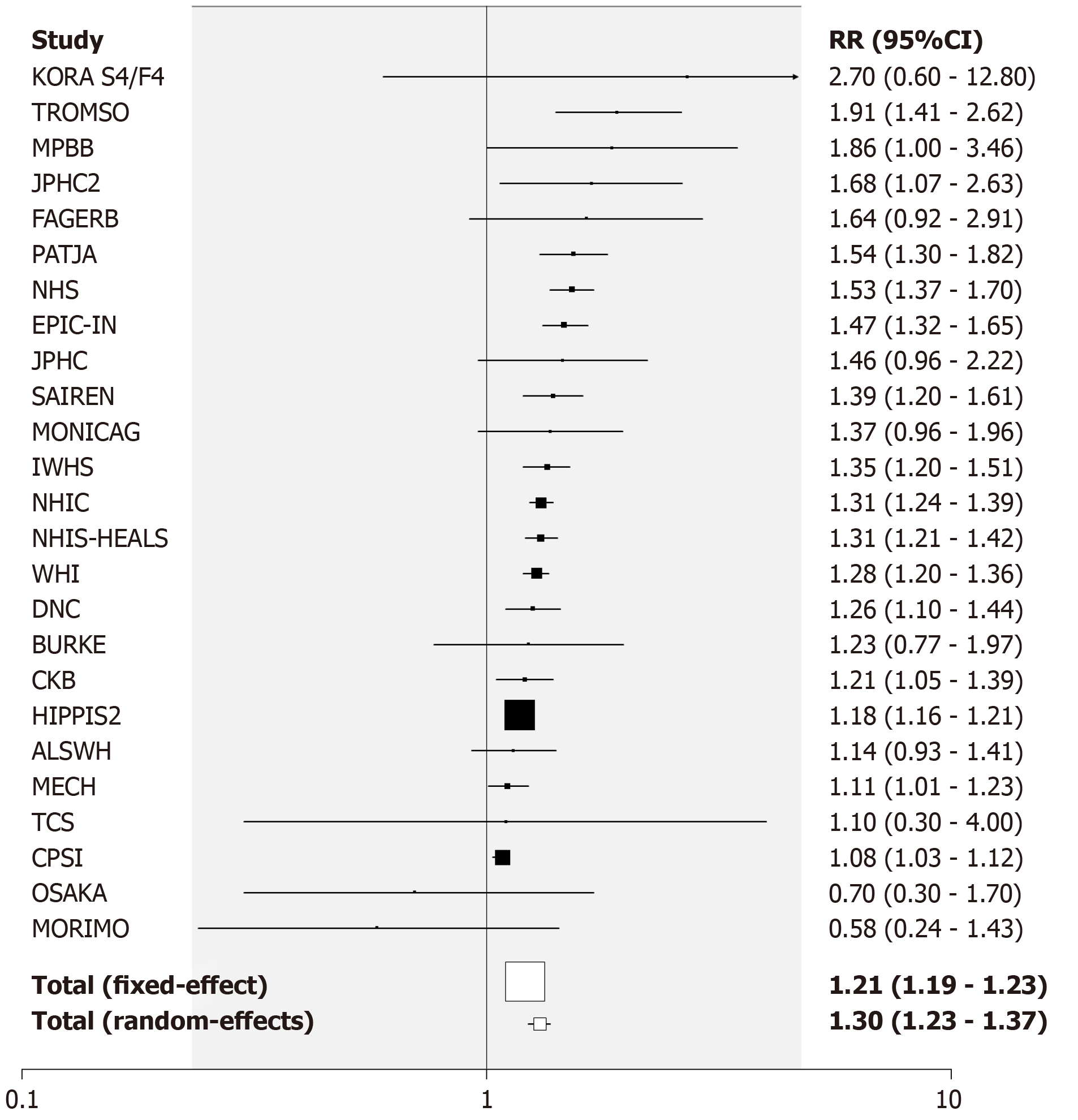
Current vs never smoking: The studies provided 99 RR estimates from 80 studies for the comparison of current vs never smoking. Nineteen studies provided estimates for both sexes, six for females only, 17 for males only and 38 only for sexes combined. Of the 99 estimates, 12 were below 1, 10 were above 2, with the remaining 77 in the range 1 to 2. The overall fixed-effect RR estimate was 1.25 (95%CI: 1.24-1.26) with highly significant heterogeneity between the estimates (Chisq. 816.8 on 98 df, P < 0.001, I2 = 88.0%). The random-effects estimate was somewhat higher at 1.33 (95%CI: 1.28-1.38). There was limited evidence of publication bias (0.01 < P < 0.05).
Table 3 presents the overall random-effects estimate, together with a breakdown of the estimates by various factors, with fuller details given in Supplementary file 2. There was evidence (P < 0.05) that the estimates varied by population type with both the estimates from studies restricted to pre-diabetics exceeding 3. There was also evidence that estimates were higher in those that were more adjusted (P < 0.05) or adjusted for various other individual factors (age, alcohol, family history of diabetes, cholesterol, triglycerides – all P < 0.05 - and glucose – P < 0.01), but were lower in those that were adjusted for education (P < 0.05). It is notable, however, that with the exception of two estimates based on less than five RRs, all the RR estimates shown in Table 3 were significantly (P < 0.05) increased.
| Grouping1 | Current vs never smoking | Current vs non-smoking | |||||
| n2 | RR (95%CI) | P | n | RR (95%CI) | P | ||
| Overall | 99 | 1.33 (1.28-1.38) | P < 0.001, P < 0.05 | 156 | 1.28 (1.24-1.32) | P < 0.001, P < 0.05 | |
| Sex | Female | 25 | 1.30 (1.23-1.37) | 31 | 1.26 (1.21-1.31) | ||
| Male | 36 | 1.40 (1.32-1.49) | 47 | 1.30 (1.24-1.36) | |||
| Combined | 38 | 1.28 (1.18-1.39) | NS3 | 78 | 1.26 (1.18-1.34) | NS | |
| Continent | Asia | 44 | 1.36 (1.30-1.43) | 57 | 1.36 (1.29-1.43) | ||
| Europe | 32 | 1.34 (1.27-1.42) | 60 | 1.25 (1.20-1.30) | |||
| North and South America | 19 | 1.27 (1.18-1.37) | 34 | 1.18 (1.12-1.25) | |||
| Oceania | 4 | 1.05 (0.68-1.62) | NS | 5 | 1.54 (1.28-1.85) | P < 0.001 | |
| Publication year | Up to 2005 | 13 | 1.41 (1.27-1.56) | 23 | 1.24 (1.16-1.33) | ||
| 2005-2014 | 47 | 1.36 (1.30-1.43) | 66 | 1.31 (1.27-1.35) | |||
| 2015 or later | 39 | 1.27 (1.20-1.35) | NS | 67 | 1.23 (1.17-1.30) | NS | |
| Basis of diagnosis | Self-report only | 12 | 1.32 (1.25-1.40) | 17 | 1.34 (1.25-1.44) | ||
| Medical records only | 49 | 1.32 (1.25-1.38) | 86 | 1.29 (1.23-1.34) | |||
| Both | 38 | 1.36 (1.27-1.46) | NS | 53 | 1.24 (1.17-1.32) | NS | |
| Population | General | 93 | 1.32 (1.28-1.37) | 147 | 1.28 (1.24-1.32) | ||
| Pre-diabetics only | 2 | 3.29 (1.51-7.21) | 3 | 1.23 (0.79-1.90) | |||
| Pre-diabetics excluded | 4 | 1.61 (1.30-1.99) | P < 0.05 | 6 | 1.38 (1.15-1.67) | NS | |
| Number of adjustment factors | 0 | 17 | 1.15 (1.00-1.33) | 33 | 1.19 (1.08-1.31) | ||
| 1 to 5 | 18 | 1.36 (1.25-1.47) | 30 | 1.38 (1.27-1.51) | |||
| 6 to 10 | 43 | 1.40 (1.32-1.48) | 64 | 1.29 (1.25-1.33) | |||
| 11 or more | 21 | 1.28 (1.20-1.37) | P < 0.05 | 29 | 1.22 (1.15-1.30) | P < 0.1 | |
| Cohort size | < 5000 | 35 | 1.36 (1.19-1.56) | 58 | 1.31 (1.20-1.42) | ||
| 5000 to 20000 | 20 | 1.38 (1.25-1.53) | 43 | 1.24 (1.17-1.32) | |||
| > 20000 | 44 | 1.32 (1.26-1.37) | NS | 55 | 1.29 (1.24-1.35) | NS | |
| Number of type 2 diabetes cases | < 500 | 44 | 1.37 (1.23-1.52) | 78 | 1.27 (1.19-1.35) | ||
| 500-999 | 18 | 1.50 (1.34-1.67) | 24 | 1.40 (1.27-1.55) | |||
| 1000-2000 | 10 | 1.26 (1.15-1.38) | 17 | 1.20 (1.11-1.30) | |||
| 2001+ | 27 | 1.29 (1.22-1.35) | P < 0.1 | 37 | 1.26 (1.20-1.33) | NS | |
| Highest age at baseline | < 60 | 13 | 1.36 (1.23-1.51) | 22 | 1.24 (1.16-1.32) | ||
| 60-74 | 27 | 1.44 (1.32-1.56) | 38 | 1.36 (1.27-1.45) | |||
| 75+ | 59 | 1.29 (1.24-1.35) | P < 0.1 | 96 | 1.26 (1.21-1.31) | NS | |
| Length of follow-up (yr) | < 5 | 14 | 1.27 (1.19-1.35) | 25 | 1.24 (1.15-1.34) | ||
| 5-10 | 55 | 1.38 (1.30-1.47) | 81 | 1.34 (1.28-1.40) | |||
| > 10 | 30 | 1.31 (1.22-1.39) | NS | 50 | 1.22 (1.17-1.28) | P < 0.05 | |
| Definition of smoking | Cigarette | 47 | 1.32 (1.27-1.38) | 63 | 1.25 (1.21-1.29) | ||
| Smoking | 50 | 1.36 (1.26-1.46) | 89 | 1.30 (1.23-1.37) | |||
| Tobacco | 2 | 1.10 (0.94-1.29) | P < 0.1 | 4 | 1.16 (1.06-1.27) | P < 0.1 | |
| Adjusted for age | No | 20 | 1.17 (1.04-1.32) | 41 | 1.22 (1.12-1.33) | ||
| Yes | 79 | 1.35 (1.31-1.41) | P < 0.05 | 115 | 1.29 (1.25-1.33) | NS | |
| Adjusted for sex | No | 72 | 1.35 (1.29-1.41) | 107 | 1.27 (1.23-1.32) | ||
| Yes | 27 | 1.29 (1.20-1.39) | NS | 49 | 1.29 (1.20-1.38) | NS | |
| Adjusted for BMI | No | 29 | 1.24 (1.11-1.38) | 55 | 1.22 (1.13-1.32) | ||
| Yes | 70 | 1.35 (1.30-1.41) | NS | 101 | 1.30 (1.26-1.34) | NS | |
| Adjusted for physical activity | No | 41 | 1.27 (1.20-1.35) | 87 | 1.27 (1.21-1.33) | ||
| Yes | 58 | 1.36 (1.30-1.43) | P < 0.1 | 69 | 1.28 (1.23-1.33) | NS | |
| Adjusted for alcohol consumption | No | 42 | 1.26 (1.19-1.34) | 87 | 1.26 (1.20-1.32) | ||
| Yes | 57 | 1.37 (1.31-1.43) | P < 0.05 | 69 | 1.29 (1.25-1.33) | NS | |
| Adjusted for family history of diabetes | No | 61 | 1.28 (1.22-1.35) | 99 | 1.23 (1.17-1.29) | ||
| Yes | 38 | 1.41 (1.33-1.49) | P < 0.05 | 57 | 1.34 (1.29-1.40) | P < 0.01 | |
| Adjusted for education | No | 63 | 1.37 (1.31-1.44) | 115 | 1.29 (1.24-1.35) | ||
| Yes | 36 | 1.28 (1.21-1.34) | P < 0.05 | 41 | 1.23 (1.18-1.28) | P < 0.1 | |
| Adjusted for diet | No | 74 | 1.35 (1.29-1.41) | 126 | 1.29 (1.24-1.34) | ||
| Yes | 25 | 1.30 (1.22-1.38) | NS | 30 | 1.23 (1.18-1.28) | P < 0.1 | |
| Adjusted for blood pressure | No | 53 | 1.31 (1.24-1.40) | 88 | 1.27 (1.21-1.34) | ||
| Yes | 46 | 1.35 (1.29-1.41) | NS | 68 | 1.28 (1.24-1.33) | NS | |
| Adjusted for cholesterol | No | 72 | 1.30 (1.25-1.35) | 115 | 1.26 (1.22-1.31) | ||
| Yes | 27 | 1.40 (1.32-1.48) | P < 0.05 | 41 | 1.32 (1.25-1.39) | NS | |
| Adjusted for glucose | No | 79 | 1.30 (1.25-1.35) | 116 | 1.26 (1.22-1.31) | ||
| Yes | 20 | 1.44 (1.35-1.54) | P < 0.01 | 40 | 1.34 (1.27-1.41) | NS | |
| Adjusted for triglycerides | No | 80 | 1.30 (1.25-1.36) | 124 | 1.27 (1.22-1.31) | ||
| Yes | 19 | 1.45 (1.33-1.58) | P < 0.05 | 32 | 1.34 (1.24-1.44) | NS | |
| Adjusted for waist circumference | No | 82 | 1.34 (1.29-1.40) | 136 | 1.28 (1.24-1.32) | ||
| Yes | 17 | 1.29 (1.19-1.41) | NS | 20 | 1.25 (1.16-1.35) | NS | |
| Adjusted for any other factors | No | 37 | 1.30 (1.19-1.42) | 62 | 1.28 (1.18-1.38) | ||
| Yes | 62 | 1.34 (1.29-1.40) | NS | 94 | 1.27 (1.23-1.30) | NS | |
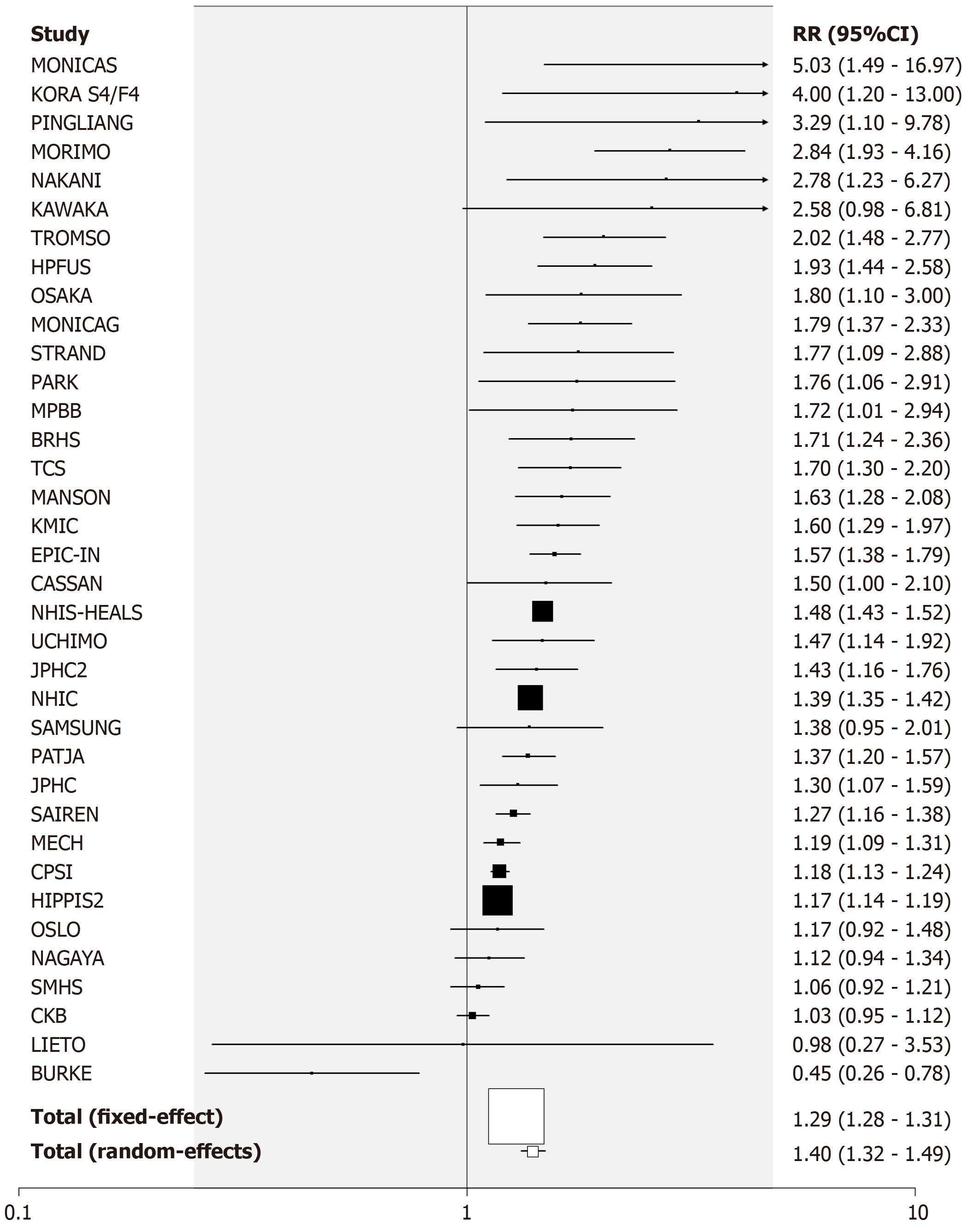
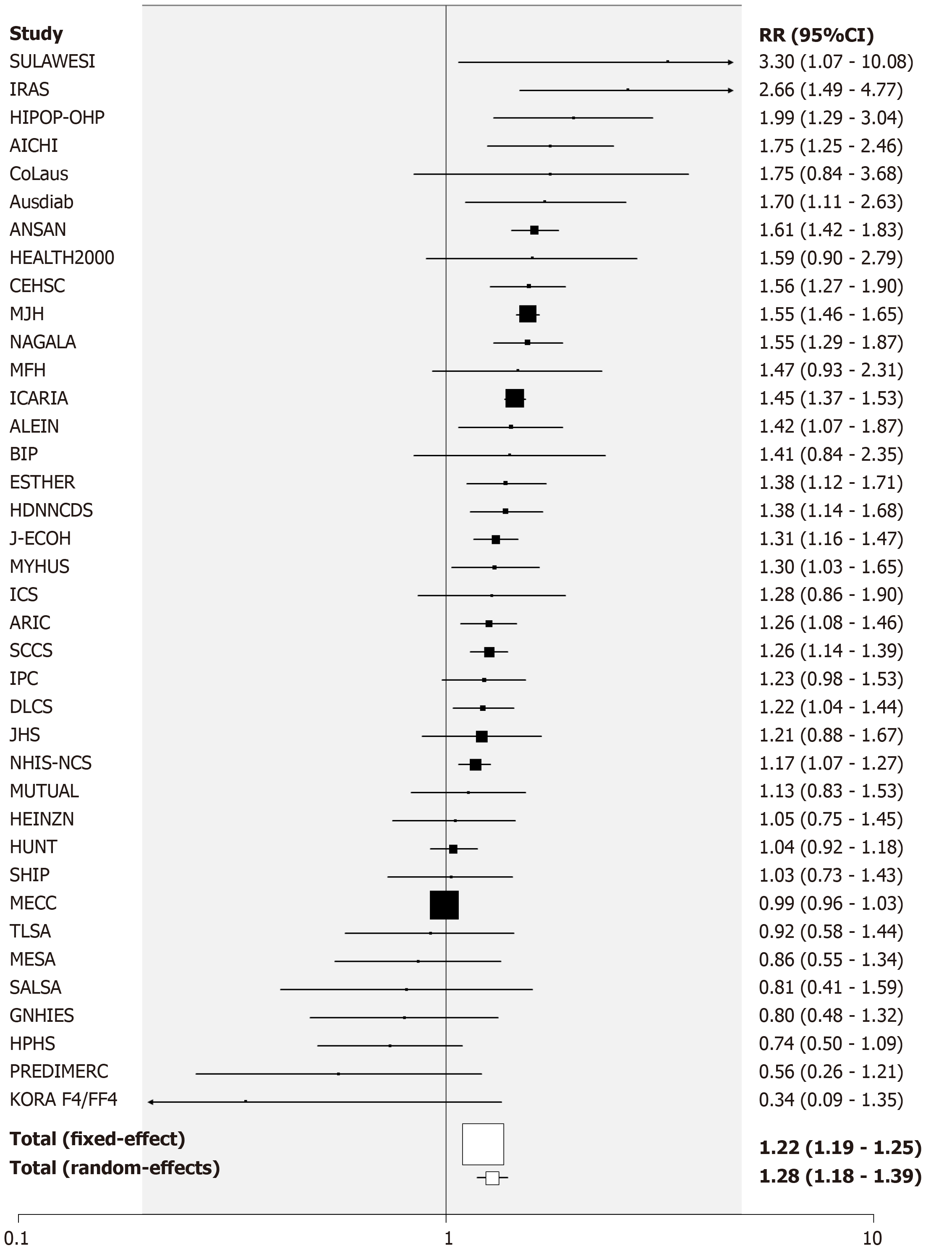
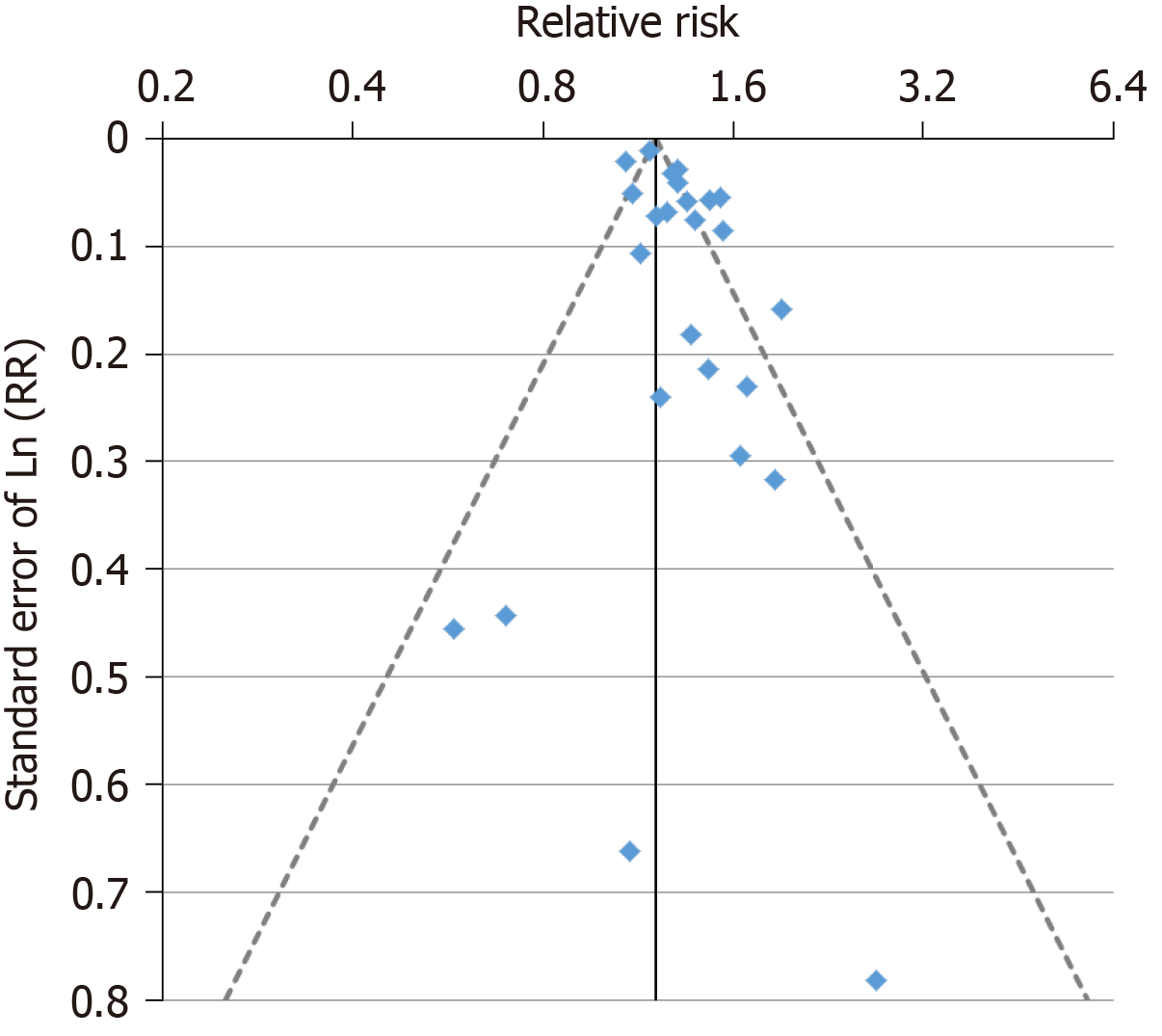
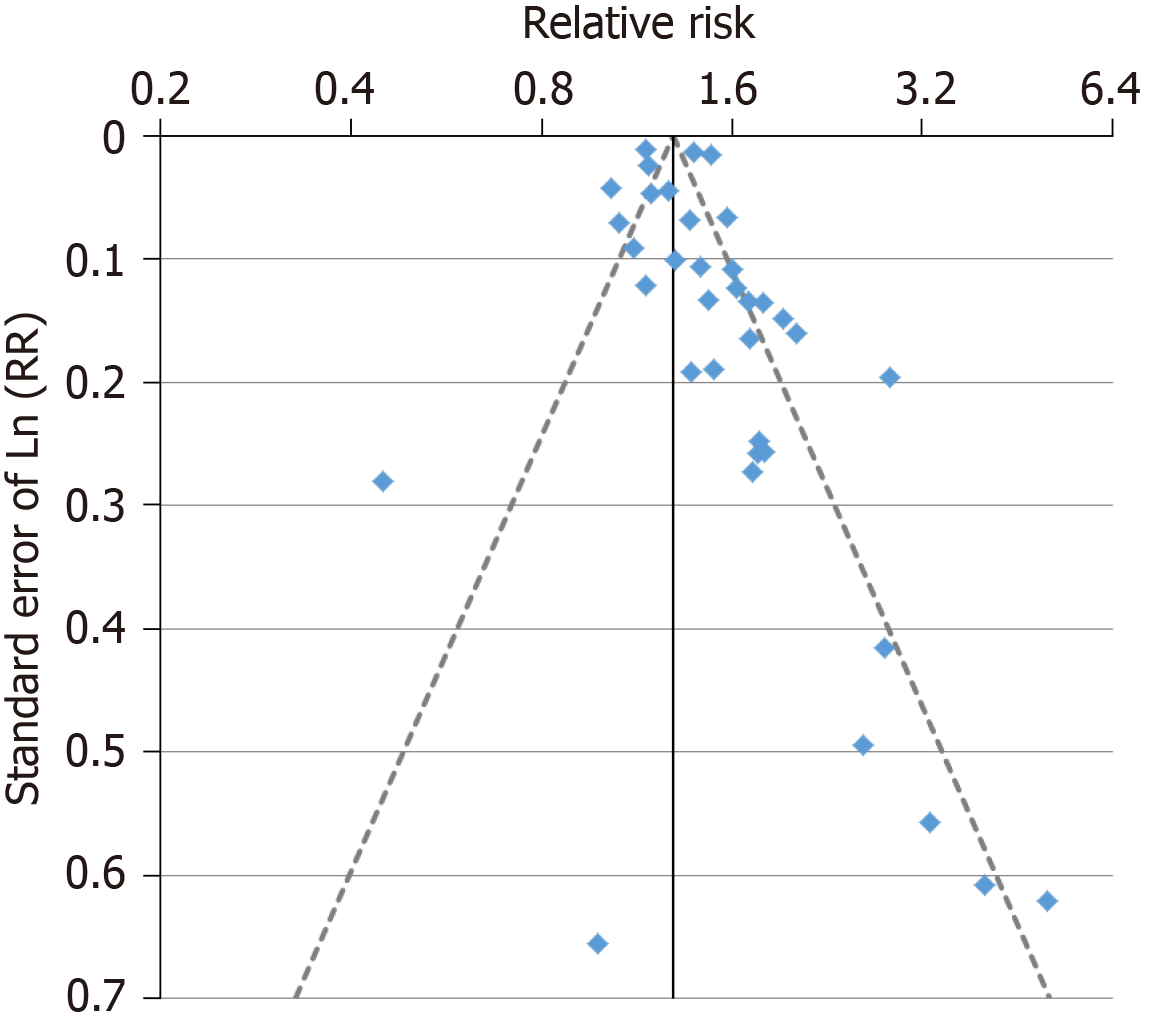
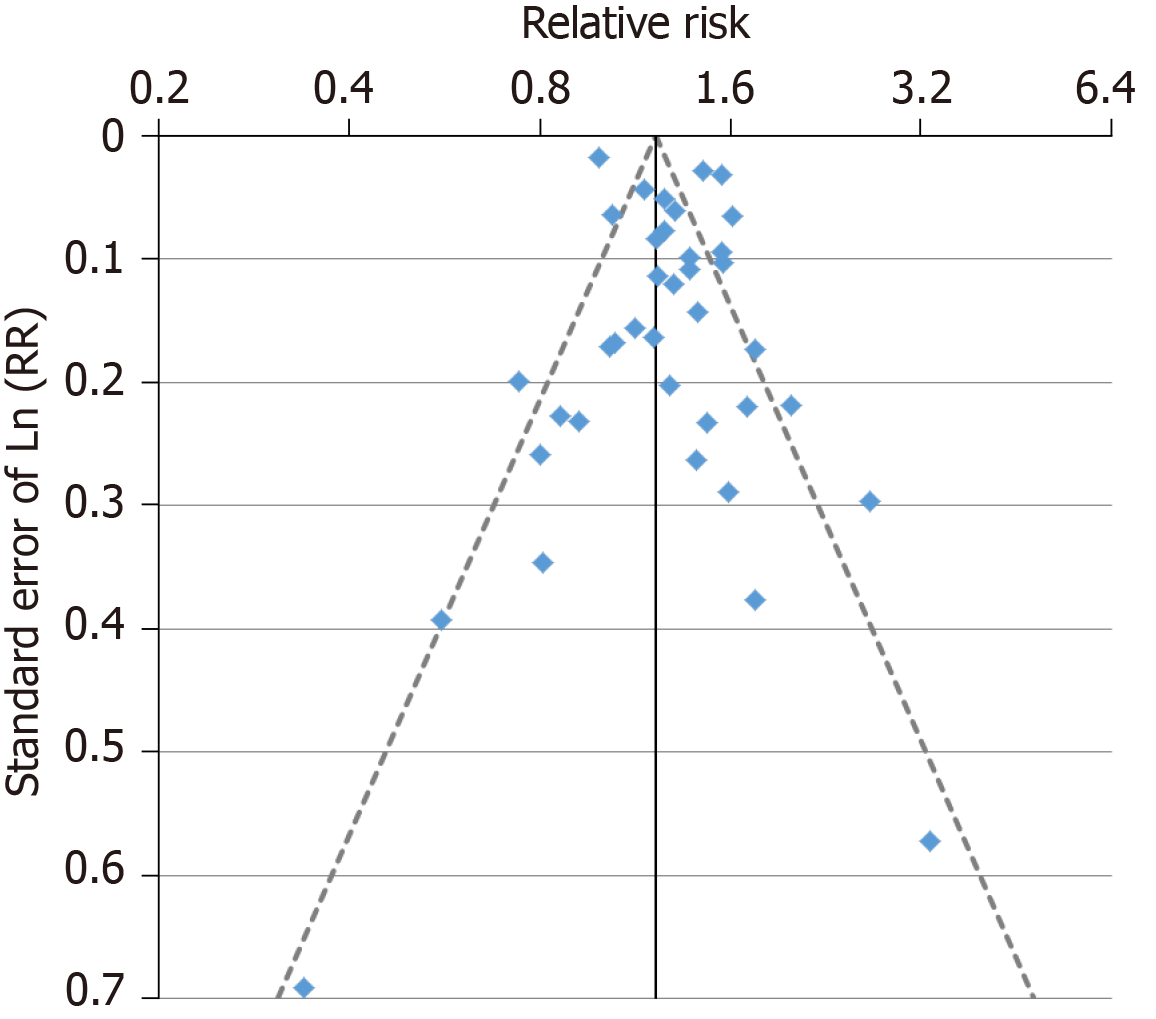
For the analysis subdivided by sex, Figure 1 (females), Figure 2 (males) and Figure 3 (sexes combined) summarize the data in forest plots, while Figure 4 (females), Figure 5 (males) and Figure 6 (sexes combined) present funnel plots to illustrate possible publication bias. No marked publication bias was evident.
Table 4 (and Supplementary file 3) summarizes the results of the dose-response analysis for current vs never smoking. Whichever of the three methods of dose-response grouping was used, the RR estimates clearly rose with increasing amount smoked, and the increase at each level remained significant (P < 0.05). Note that the sets of estimates are not independent, with all the studies providing results for the key value analysis also contributing to the low/medium/high split.
| Grouping1 | Current vs never smoking | Current vs non-smoking | ||
| n2 | RR (95%CI) | n | RR (95%CI) | |
| Using key values: | ||||
| About 10 cigs/d | 13 | 1.10 (1.03-1.18) | 13 | 1.04 (0.98-1.10) |
| About 20 cigs per d | 13 | 1.31 (1.19-1.44) | 13 | 1.27 (1.16-1.39) |
| About 40 cigs per d | 13 | 1.55 (1.39-1.72) | 13 | 1.54 (1.37-1.72) |
| Low | 23 | 1.17 (1.11-1.23) | 22 | 1.13 (1.07-1.19) |
| Medium | 23 | 1.30 (1.22-1.39) | 22 | 1.26 (1.18-1.34) |
| High | 23 | 1.53 (1.41-1.65) | 22 | 1.48 (1.37-1.60) |
| 1-19 cigs/d | 18 | 1.32 (1.20-1.45) | 17 | 1.20 (1.10-1.30) |
| 20+ | 18 | 1.58 (1.42-1.76) | 17 | 1.44 (1.31-1.59) |
Current vs non-smoking: There were 156 RR estimates from 133 studies for the comparison of current vs non- smoking. Twenty-three studies provided estimates for both sexes, eight for females only, 24 for males only and 78 for sexes combined.
Of the 156 estimates, 27 were below 1, 11 were above 2, with the remaining 118 in the range 1 to 2. The overall fixed-effect RR estimate was 1.20 (95%CI: 1.20-1.21), with highly significant heterogeneity (Chisq. 1986.7 on 155 df, P < 0.001, I2 = 92.2%), and the random-effects estimate was 1.28 (95%CI: 1.24-1.32), slightly lower than the estimate for current vs never smoking. As for current smoking, there was limited evidence of publication bias (0.01 < P < 0.05).
Table 3 also presents the overall random-effects estimate for current vs non-smoking, as well as a breakdown of the estimates by different factors (see also Supplementary file 4). As for current vs never smoking, the random-effects estimate was elevated in all subdivisions of the data, significantly so except where based on very few estimates. There was little evidence of variation in the RR in subdivisions of the data by level of the various factors studied, the most notable exceptions being the somewhat higher estimate in studies adjusted rather than unadjusted for family history of diabetes, and the variation by continent.
Table 4 (and Supplementary file 5) summarizes the results of the dose-response analysis for current vs non-smoking. As for current vs never smoking, there was clear evidence that risk rises with amount smoked, whichever dose-response grouping is used.
Forest and funnel plots for the analysis subdivided by sex are shown in Supplementary file 6.
Former vs never smoking: There were 100 RR estimates from 81 studies for the comparison of former vs never smoking. Nineteen provided estimates for both sexes, seven for females only, 17 for males only and 38 for sexes combined.
Of the 100 estimates, 18 were below 1, 7 were above 2, with the remaining 75 in the range 1 to 2. The overall fixed-effect estimate was 1.09 (95%CI: 1.08-1.10), with highly significant heterogeneity (Chisq. 263.6 on 99 df, P < 0.001, I2 = 62.4%). The random-effects estimate was 1.13 (95%CI: 1.11-1.16). Somewhat stronger evidence of publication bias (0.001 < P < 0.01) was seen than for current smoking.
Table 5 presents the overall random effects estimate, together with a breakdown of the estimates by different factors (see also Supplementary file 7). There was no strong evidence (P < 0.01) of variation in the RR by level of any factor, with estimates slightly elevated in all subgroupings except where based on very few estimates.
| Grouping1 | n2 | RR (95%CI) | P | |
| Overall | 100 | 1.13 (1.11-1.16) | P < 0.001, P < 0.01 | |
| Sex | Female | 26 | 1.13 (1.08-1.18) | |
| Male | 36 | 1.12 (1.08-1.16) | ||
| Combined | 38 | 1.16 (1.09-1.22) | NS3 | |
| Continent | Asia | 44 | 1.16 (1.10-1.22) | |
| Europe | 32 | 1.13 (1.09-1.18) | ||
| North and South America | 20 | 1.11 (1.07-1.16) | ||
| Oceania | 4 | 1.07 (0.93-1.23) | NS | |
| Publication year | Up to 2005 | 13 | 1.13 (1.06-1.21) | |
| 2005-2014 | 47 | 1.16 (1.11-1.22) | ||
| 2015 or later | 40 | 1.11 (1.08-1.15) | NS | |
| Basis of diagnosis | Self-report only | 12 | 1.17 (1.05-1.29) | |
| Medical records only | 49 | 1.11 (1.08-1.13) | ||
| Both | 39 | 1.16 (1.11-1.22) | P < 0.1 | |
| Population | General | 94 | 1.13 (1.11-1.16) | |
| Pre-diabetics only | 2 | 0.97 (0.08-12.64) | ||
| Pre-diabetics excluded | 4 | 1.11 (0.86-1.44) | NS | |
| Number of adjustment factors | 0 | 18 | 1.11 (1.01-1.23) | |
| 1 to 5 | 18 | 1.20 (1.11-1.30) | ||
| 6 to 10 | 42 | 1.12 (1.08-1.17) | ||
| 11 or more | 22 | 1.13 (1.09-1.17) | NS | |
| Cohort size | < 5000 | 35 | 1.21 (1.11-1.32) | |
| 5000 to 20000 | 20 | 1.19 (1.09-1.29) | ||
| > 20000 | 45 | 1.12 (1.09-1.15) | NS | |
| Number of type 2 diabetes cases | < 500 | 44 | 1.21 (1.12-1.30) | |
| 500 to 999 | 18 | 1.11 (1.03-1.20) | ||
| 1000 to 2000 | 10 | 1.26 (1.10-1.45) | ||
| 2001+ | 28 | 1.11 (1.08-1.14) | P < 0.1 | |
| Highest age at baseline | < 60 | 14 | 1.20 (1.10-1.30) | |
| 60-74 | 27 | 1.19 (1.10-1.29) | ||
| 75+ | 59 | 1.11 (1.09-1.14) | NS | |
| Length of follow-up (yr) | < 5 | 14 | 1.13 (1.08-1.19) | |
| 5-10 | 55 | 1.16 (1.10-1.23) | ||
| > 10 | 31 | 1.11 (1.08-1.15) | NS | |
| Definition of smoking | Cigarette | 48 | 1.12 (1.09-1.15) | |
| Smoking | 50 | 1.15 (1.10-1.21) | ||
| Tobacco | 2 | 0.95 (0.83-1.08) | P < 0.05 | |
| Adjusted for age | No | 21 | 1.13 (1.05-1.22) | |
| Yes | 79 | 1.13 (1.10-1.16) | NS | |
| Adjusted for sex | No | 75 | 1.13 (1.10-1.16) | |
| Yes | 25 | 1.13 (1.07-1.19) | NS | |
| Adjusted for BMI | No | 31 | 1.15 (1.07-1.24) | |
| Yes | 69 | 1.12 (1.10-1.15) | NS | |
| Adjusted for physical activity | No | 41 | 1.15 (1.11-1.20) | |
| Yes | 59 | 1.12 (1.09-1.16) | NS | |
| Adjusted for alcohol consumption | No | 43 | 1.15 (1.10-1.19) | |
| Yes | 57 | 1.13 (1.09-1.16) | NS | |
| Adjusted for family history of diabetes | No | 61 | 1.13 (1.10-1.17) | |
| Yes | 39 | 1.13 (1.09-1.17) | NS | |
| Adjusted for education | No | 65 | 1.16 (1.12-1.19) | |
| Yes | 35 | 1.09 (1.05-1.14) | P < 0.05 | |
| Adjusted for diet | No | 75 | 1.14 (1.11-1.17) | |
| Yes | 25 | 1.12 (1.07-1.16) | NS | |
| Adjusted for blood pressure | No | 54 | 1.14 (1.10-1.19) | |
| Yes | 46 | 1.13 (1.09-1.16) | NS | |
| Adjusted for cholesterol | No | 73 | 1.13 (1.10-1.16) | |
| Yes | 27 | 1.14 (1.08-1.20) | NS | |
| Adjusted for glucose | No | 80 | 1.13 (1.10-1.16) | |
| Yes | 20 | 1.15 (1.07-1.23) | NS | |
| Adjusted for triglycerides | No | 81 | 1.12 (1.10-1.15) | |
| Yes | 19 | 1.17 (1.08-1.27) | NS | |
| Adjusted for waist circumference | No | 83 | 1.13 (1.10-1.16) | |
| Yes | 17 | 1.14 (1.05-1.24) | NS | |
| Adjusted for other factors | No | 38 | 1.15 (1.08-1.23) | |
| Yes | 62 | 1.13 (1.10-1.15) | NS | |
Table 6 (and Supplementary file 8) summarizes the results of the dose-response analysis for former vs never smoking. These showed clear evidence that the RR declined with increasing time since quitting.
Again, forest and funnel plots are shown in Supplementary file 6.
Ever vs never smoking: One hundred RRs were available from 82 studies. The overall fixed-effect RR estimate was 1.17 (95%CI: 1.16-1.18) with evidence of considerable heterogeneity (Chisq. 897.37 on 99 df, P < 0.001, I2 = 89.0%), the random-effect estimate being 1.25 (95%CI: 1.21-1.28). There was some evidence of publication bias (0.001 < P < 0.01). RRs were generally elevated in all subgroups, the strongest evidence of variation by any factor (P < 0.001) relating to adjustment for education, unadjusted estimates (RR = 1.29, 95%CI: 1.24-1.34) being higher than adjusted ones (RR = 1.17, 95%CI: 1.12-1.21). There was also weaker evidence (P < 0.05) that RRs were somewhat higher in Asia, and somewhat lower in populations with a baseline upper age limit of 75 or more, or if the RRs were unadjusted for glucose. See Table 8 and Supplementary File 9 for fuller details.
Only one of the studies provided information on risk by amount smoked, so no dose-response meta-analyses were possible.
Again, forest and funnel plots are shown in Supplementary file 6.
Ratio of RRs for highest to lowest BMI groupings: Six studies provided results by level of BMI, three of these giving results for each sex separately. One study provided data only for current vs never and former vs never smoking, while the others also provided data for current vs non-smoking and ever vs never smoking. None of the meta-analyses provided any evidence of variation in RR by level of BMI, the random effects meta-analysis estimate of the highest to lowest ratio being 1.20 (95%CI: 0.92-1.57) for current vs never smoking, 1.06 (95%CI: 0.82-1.36) for current vs non-smoking, 1.12 (0.95-1.32) for former vs never smoking, and 1.03 (95%CI: 0.87-1.23) for ever vs never smoking, based on, respectively, 9, 7, 9 and 7 estimates. (See Supplementary file 10).
Supplementary file 1 gives further details of the literature search, including a list of the 42 publications rejected during data entry, giving the reasons for rejection, and a description of how multiple publications from a study were dealt with.
Supplementary Files 2, 4, 7 and 9 give full details of the results for the main analysis of, respectively, current vs never smoking, current vs non-smoking, former vs never smoking and ever vs never smoking. Each file is laid out similarly. Introductory pages describe the content and layout of the output, and explain the abbreviations used and the decisions made where multiple results were available for a single study. Table 1 of each Supplementary File then gives details of each candidate RR selected from the main and subsidiary publications for each study, while Table 2 of each file gives details of the RRs actually used in the analyses, and Tables 3-27 of each file give full results of the meta-analyses subdivided by each of the 25 factors considered (sex, continent, etc.).
| Grouping1 | n2 | RR (95%CI) | P | |
| Overall | 100 | 1.25 (1.21-1.28) | P < 0.001, P < 0.01 | |
| Sex | Female | 24 | 1.25 (1.18-1.31) | |
| Male | 36 | 1.25 (0.20-1.31) | ||
| Combined | 40 | 1.22 (1.14-1.31) | P < 0.05 | |
| Continent | Asia | 41 | 1.30 (1.25-1.36) | |
| Europe | 36 | 1.21 (1.17-1.26) | ||
| North and South America | 20 | 1.19 (1.13-1.26) | ||
| Oceania | 3 | 0.87 (0.48-1.57) | P < 0.05 | |
| Publication year | Up to 2005 | 13 | 1.25 (1.16-1.34) | |
| 2005-2014 | 47 | 1.26 (1.20-1.33) | ||
| 2015 or later | 40 | 1.23 (1.18-1.28) | NSc | |
| Basis of diagnosis | Self-report only | 10 | 1.35 (1.17-1.56) | |
| Medical records only | 51 | 1.22 (1.18-1.27) | ||
| Both | 39 | 1.26 (1.19-1.33) | NS | |
| Population | General | 95 | 1.24 (1.21-1.28) | |
| Pre-diabetics only | 1 | 3.30 (1.24-8.77) | ||
| Pre-diabetics excluded | 4 | 1.43 (1.17-1.76) | P < 0.1 | |
| Number of adjustment factors | 0 | 23 | 1.18 (1.06-1.32) | |
| 1 to 5 | 16 | 1.28 (1.20-1.36) | ||
| 6 to 10 | 40 | 1.24 (1.19-1.30) | ||
| 11 or more | 21 | 1.22 (1.16-1.28) | NS | |
| Cohort size | < 5000 | 39 | 1.26 (1.14-1.38) | |
| 5000 to 20000 | 17 | 1.27 (1.17-1.38) | ||
| > 20000 | 44 | 1.24 (1.20-1.28) | NS | |
| Number of type 2 diabetes cases | < 500 | 46 | 1.26 (1.16-1.36) | |
| 500 to 999 | 17 | 1.32 (1.19-1.47) | ||
| 1000 to 2000 | 9 | 1.28 (1.14-1.43) | ||
| 2001+ | 28 | 1.22 (1.17-1.26) | NS | |
| Highest age at baseline | < 60 | 13 | 1.35 (1.23-1.47) | |
| 60-74 | 27 | 1.32 (1.23-1.41) | ||
| 75+ | 60 | 1.21 (1.17-1.25) | P < 0.05 | |
| Length of follow-up (yr) | < 5 | 14 | 1.21 (1.15-1.26) | |
| 5-10 | 56 | 1.29 (1.22-1.35) | ||
| > 10 | 30 | 1.21 (1.15-1.28) | NS | |
| Definition of smoking | Cigarette | 48 | 1.22 (1.18-1.26) | |
| Smoking | 50 | 1.28 (1.20-1.36) | ||
| Tobacco | 2 | 1.09 (0.94-1.25) | P < 0.1 | |
| Adjusted for age | No | 27 | 1.19 (1.09-1.31) | |
| Yes | 73 | 1.24 (1.20-1.28) | NS | |
| Adjusted for sex | No | 77 | 1.26 (1.22-1.30) | |
| Yes | 23 | 1.20 (1.13-1.27) | NS | |
| Adjusted for BMI | No | 35 | 1.23 (1.13-1.34) | |
| Yes | 65 | 1.24 (1.20-1.28) | NS | |
| Adjusted for physical activity | No | 43 | 1.26 (1.20-1.32) | |
| Yes | 57 | 1.24 (1.19-1.29) | NS | |
| Adjusted for alcohol consumption | No | 46 | 1.24 (1.19-1.30) | |
| Yes | 54 | 1.25 (1.20-1.30) | NS | |
| Adjusted for family history of diabetes- | No | 62 | 1.22 (1.17-1.27) | |
| Yes | 38 | 1.28 (1.23-1.33) | NS | |
| Adjusted for education | No | 67 | 1.29 (1.24-1.34) | |
| Yes | 33 | 1.17 (1.12-1.21) | P < 0.001 | |
| Adjusted for diet | No | 76 | 1.26 (1.22-1.31) | |
| Yes | 24 | 1.21 (1.15-1.26) | NS | |
| Adjusted for blood pressure | No | 57 | 1.25 (1.19-1.32) | |
| Yes | 43 | 1.23 (1.19-1.27) | NS | |
| Adjusted for cholesterol | No | 75 | 1.24 (1.20-1.28) | |
| Yes | 25 | 1.27 (1.19-1.36) | NS | |
| Adjusted for glucose | No | 79 | 1.23 (1.19-1.27) | |
| Yes | 21 | 1.31 (1.25-1.37) | P < 0.05 | |
| Adjusted for triglycerides | No | 83 | 1.23 (1.20-1.27) | |
| Yes | 17 | 1.31 (1.22-1.41) | NS | |
| Adjusted for waist circumference | No | 84 | 1.25 (1.21-1.30) | |
| Yes | 16 | 1.21 (1.12-1.31) | NS | |
| Adjusted for other factors | No | 42 | 1.24 (1.15-1.33) | |
| Yes | 58 | 1.23 (1.20-1.28) | NS | |
Supplementary Files 3, 5 and 8 give full details of the dose-response analysis of respectively, current vs never smoking (by amount smoked), current vs non- smoking (by amount smoked) and former vs never smoking (by year quit). Each file includes separate blocks of description and results, similar to those for Supplementary Files 2, 4 7 and 9 , but only including Tables 1-3 of those files, with Table 3 only showing results subdivided by sex. Each block relates to a specific dose-response level (e.g., about 10 for amount smoked).
Supplementary file 6 presents forest and funnel plots for current vs non-smoking, former vs never smoking and ever vs never smoking, similar to those shown in Figures 1-6 of the paper for current vs never smoking.
Supplementary file 10 gives the results of meta-analyses of ratios of relative risks for the highest to lowest BMI groupings available.
According to the United States National Institute of Diabetes and Digestive and Kidney Diseases Health Information Center[162], risk factors for type 2 diabetes include overweight/obesity, age, a family history of diabetes, high blood pressure, low high-density lipoprotein cholesterol, high triglycerides, a history of gestational diabetes, giving birth to a baby weighing 9 pounds or more, physical inactivity, a history of heart disease or stroke, as well as being in certain ethnic groups or having certain diseases. Smoking is not mentioned as a risk factor.
The meta-analyses we conducted indicate a modest relationship of smoking to risk of type 2 diabetes. This can be seen for current smoking (whether compared with never or non-smokers), former smoking and ever smoking. While there was clear evidence of heterogeneity in the RRs, the random-effects RRs showed increased risks in males and females, in younger and older subjects, in all continents studied, regardless of the basis of diagnosis, and regardless of the definition of smoking used. Despite the evidence of heterogeneity between the individual estimates, a striking feature of the results presented in Tables 3 and 5 was the fact that the estimates were elevated in virtually every subdivision of the data, whichever factor the subdivision was based on. There was also clear evidence (see Tables 4 and 6) of an increasing risk with increasing amount smoked by current smokers and of decreasing risk with increasing time quit by former smokers. Though there was some evidence of variation in risk by level of some factors, this did not suggest that the elevation in risk was unique to some populations or could be explained by adjustment for specific confounding variables. Nor did the fact that some studies did not report an elevation affect the overall conclusion. With a relatively weak association (with RRs about 1.3 for current smoking and about 1.13 for former smoking) it might be expected that some smaller studies would not detect an elevated risk. However, this did not affect the overall conclusion. Indeed, it was notable that, of the 12 RR estimates for current vs never smoking that were below 1.0, only one was statistically significant (at P < 0.05), whereas, of the 87 estimates above 1.0, as many as 63 were.
Given the weight of evidence from this review and others, smoking may be a contributory factor to type 2 diabetes. Publication bias, for which some evidence was detected, might have led to some over-estimation of the association, due to some studies finding no relationship not presenting their results. Bias due to misclassification of smoking status would only tend to bias the observed relationship down, not produce an association that did not truly exist. Failure to control properly for diet, BMI or related factors would not seem to be an explanation of the association as elevated risks were seen in studies that adjusted for these factors. That said, it is clear from Table 3 that many of the studies did not adjust for various factors listed in the first paragraph of the discussion, so that the association seen between smoking and type 2 diabetes may have suffered from uncontrolled confounding to some extent.
This review has limitations, some unavoidable. Lack of access to individual person data limited the detail of the meta-analyses that can be carried out, but obtaining such data was not practical. Obtaining a reliable definition of outcome, exposure and adjustment variables was sometimes hindered by incomplete information in the source papers. Some studies involved relatively few type 2 diabetes cases, but associations were evident both in studies with small and large numbers. It is possible that our analyses did not make full use of all the data collected, but this is inevitable in a paper of reasonable length. We would be willing to make our database available to bona fide researchers for further analysis.
Our results are consistent with those of the earlier review by Pan et al[1] based on 88 prospective studies. Although our analyses were based on a considerably larger number of studies, 145, our estimated random-effect RRs of 1.33, 1.28 and 1.13 for current vs never, current vs non, and former vs never smoking were similar to their corresponding estimates of 1.40, 1.35 and 1.14. Like us, they also found dose-response relationships with amount smoked and years since quitting. The interested reader is referred to that paper for further discussion of limitations of the data and interpretation of the results.
That paper refers to “the high prevalence of smoking in many countries and the increasing number of diabetes worldwide” and considers that “reducing tobacco use should be prioritized as a key public health strategy to prevent and control global epidemic of diabetes”. Though reduction of smoking is clearly important to limit a range of diseases such as lung cancer, chronic obstructive pulmonary disease and cardiovascular disease, one must question this prioritization, in the light of the range of other risk factors for type 2 diabetes noted above, and the evidence that diabetes incidence is rising fast worldwide[56], while smoking is declining[2]. As a strategy, controlling diet may be much more beneficial. The work of Taylor et al[163] suggests that, in many people, type 2 diabetes can be completely reversed quite rapidly by appropriate diet and weight loss.
In conclusion, the analyses confirm earlier reports of a modest dose-related association of current smoking and a weaker dose-related association of former smoking with risk of type 2 diabetes.
A systematic review of the relationship between smoking and incident type 2 diabetes, based on 88 epidemiological prospective studies, was published in 2015. Much new evidence on this relationship has become available since then.
To obtain up-to-date evidence relating smoking to type 2 diabetes.
To systematically review available evidence from prospective studies on the relationship of type 2 diabetes onset to ever, current or former smoking of cigarettes or of any tobacco product, including dose-response data.
Attention was restricted to prospective studies of populations free of type 2 diabetes at baseline which related subsequent incidence of the disease to one or more defined major or dose-related smoking indices. The major indices compared ever, current or former smokers to never smokers and current smokers to non-current smokers. The dose-related indices concerned amount currently smoked and years quit. Literature searches identified relevant papers from previous reviews, from Medline searches and from references lists of relevant papers identified. Data were extracted on study details and on the relative risks required, estimated if required using standard methods. Care was taken to avoid overlap of data from the same study from multiple publications. Fixed-effect and random-effects meta-analyses were conducted, including tests of heterogeneity and publication bias. Where a study provided multiple estimates, a preference scheme was used involving factors such as level of adjustment for confounding factors, length of follow-up and age range considered. Sex-specific results were used, if available. Effect estimates were derived based on all the selected RRs, and also for those subdivided by various categorical variables – sex, continent, year of publication, basis of diagnosis of diabetes, initial diabetes status of the population, age, length of follow-up, definition of smoking, and whether a range of different variables were adjusted for.
The literature searches identified 157 relevant publications providing results from 145 studies. Overall random-effect RR estimates were 1.33 [95% confidence interval (CI): 1.28-1.38] for current vs never smoking, 128 (95%CI: 1.24-1.32) for current vs non-smoking, 1.13 (95%CI: 1.11-1.16) for former vs never smoking and 1.25 (95%CI: 1.21-1.28) for ever vs never smoking, each combined estimate being based on at least 99 individual estimates. Estimates were generally elevated in each subdivision of the data by the categorical variables considered, though in some cases RR estimates varied significantly (P < 0.05) by level. The dose-response analysis showed that risk increased with increasing amount smoked, and reduced with increasing time quit.
Our analyses confirmed and extended reports of a modest dose-related association of current smoking and a weaker dose-related association of former smoking with risk of type 2 diabetes. The evidence suggests smoking may contribute to the risk of type 2 diabetes, though our estimates may be affected by publication bias and some uncontrolled confounding. Although reduction of smoking is clearly important to limit risk of diseases such as lung cancer, chronic obstructive pulmonary disease and cardiovascular disease, the worldwide rise in incidence of type 2 diabetes, coupled with a decline in smoking, suggests that control of other factors, such as diet, may be much more beneficial in reducing type 2 diabetes risk.
Our analyses suggest strongly that there is a modest increased risk of type 2 diabetes associated with current smoking which is greater in heavier smokers and reduced following quitting. Further large prospective studies could characterize this more precisely by more detailed assessment of smoking history and by more fully accounting for the range of other factors known to be related to type 2 diabetes. Care should be taken to determine the accuracy of all the data used, and to assess the effect that any possible inaccuracy might have on the estimated association.
We thank Barbara Forey for assistance with classification of studies, Jan Hamling, John Hamling and John Fry for assistance in conducting the analyses described and producing the figures, and Yvonne Cooper and Diane Morris for typing various drafts of this paper.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: Royal Statistical Society (Fellow).
Specialty type: Medicine, research and experimental
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jia J, Rakhshan V S-Editor: Tang JZ L-Editor: A E-Editor: Qi LL
| 1. | Pan A, Wang Y, Talaei M, Hu FB, Wu T. Relation of active, passive, and quitting smoking with incident type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3:958-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 376] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 2. | National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention (US) 2014; 944. |
| 3. | Willi C, Bodenmann P, Ghali WA, Faris PD, Cornuz J. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2007;298:2654-2664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 849] [Cited by in RCA: 875] [Article Influence: 48.6] [Reference Citation Analysis (2)] |
| 4. | Akter S, Goto A, Mizoue T. Smoking and the risk of type 2 diabetes in Japan: A systematic review and meta-analysis. J Epidemiol. 2017;27:553-561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 106] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 5. | Lee PN, Forey BA, Coombs KJ. Systematic review with meta-analysis of the epidemiological evidence in the 1900s relating smoking to lung cancer. BMC Cancer. 2012;12:385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 191] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 6. | Fleiss JL, Gross AJ. Meta-analysis in epidemiology, with special reference to studies of the association between exposure to environmental tobacco smoke and lung cancer: a critique. J Clin Epidemiol. 1991;44:127-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 261] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 7. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 46304] [Article Influence: 2104.7] [Reference Citation Analysis (3)] |
| 8. | Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34245] [Cited by in RCA: 40337] [Article Influence: 1440.6] [Reference Citation Analysis (2)] |
| 9. | Lyssenko V, Jonsson A, Almgren P, Pulizzi N, Isomaa B, Tuomi T, Berglund G, Altshuler D, Nilsson P, Groop L. Clinical risk factors, DNA variants, and the development of type 2 diabetes. N Engl J Med. 2008;359:2220-2232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 669] [Cited by in RCA: 625] [Article Influence: 36.8] [Reference Citation Analysis (1)] |
| 10. | Laaksonen MA, Knekt P, Rissanen H, Härkänen T, Virtala E, Marniemi J, Aromaa A, Heliövaara M, Reunanen A. The relative importance of modifiable potential risk factors of type 2 diabetes: a meta-analysis of two cohorts. Eur J Epidemiol. 2010;25:115-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 11. | Guasch-Ferré M, Bulló M, Costa B, Martínez-Gonzalez MÁ, Ibarrola-Jurado N, Estruch R, Barrio F, Salas-Salvadó J; PREDI-PLAN Investigators. A risk score to predict type 2 diabetes mellitus in an elderly Spanish Mediterranean population at high cardiovascular risk. PLoS One. 2012;7:e33437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Du S, Wu X, Han T, Duan W, Liu L, Qi J, Niu Y, Na L, Sun C. Dietary manganese and type 2 diabetes mellitus: two prospective cohort studies in China. Diabetologia. 2018;61:1985-1995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 13. | Shan Z, Li Y, Zong G, Guo Y, Li J, Manson JE, Hu FB, Willett WC, Schernhammer ES, Bhupathiraju SN. Rotating night shift work and adherence to unhealthy lifestyle in predicting risk of type 2 diabetes: results from two large US cohorts of female nurses. BMJ. 2018;363:k4641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 171] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 14. | Conway BN, Han X, Munro HM, Gross AL, Shu XO, Hargreaves MK, Zheng W, Powers AC, Blot WJ. The obesity epidemic and rising diabetes incidence in a low-income racially diverse southern US cohort. PLoS One. 2018;13:e0190993. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 15. | Frisard C, Gu X, Whitcomb B, Ma Y, Pekow P, Zorn M, Sepavich D, Balasubramanian R. Marginal structural models for the estimation of the risk of Diabetes Mellitus in the presence of elevated depressive symptoms and antidepressant medication use in the Women's Health Initiative observational and clinical trial cohorts. BMC Endocr Disord. 2015;15:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Hu Y, Zong G, Liu G, Wang M, Rosner B, Pan A, Willett WC, Manson JE, Hu FB, Sun Q. Smoking Cessation, Weight Change, Type 2 Diabetes, and Mortality. N Engl J Med. 2018;379:623-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 173] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 17. | Hilawe EH, Yatsuya H, Li Y, Uemura M, Wang C, Chiang C, Toyoshima H, Tamakoshi K, Zhang Y, Kawazoe N, Aoyama A. Smoking and diabetes: is the association mediated by adiponectin, leptin, or C-reactive protein? J Epidemiol. 2015;25:99-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Yatsuya H, Li Y, Hirakawa Y, Ota A, Matsunaga M, Haregot HE, Chiang C, Zhang Y, Tamakoshi K, Toyoshima H, Aoyama A. A Point System for Predicting 10-Year Risk of Developing Type 2 Diabetes Mellitus in Japanese Men: Aichi Workers' Cohort Study. J Epidemiol. 2018;28:347-352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Miyakoshi T, Oka R, Nakasone Y, Sato Y, Yamauchi K, Hashikura R, Takayama M, Hirayama Y, Hirabayashi K, Koike H, Aizawa T. Development of new diabetes risk scores on the basis of the current definition of diabetes in Japanese subjects [Rapid Communication]. Endocr J. 2016;63:857-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Wang CS, Chang TT, Yao WJ, Wang ST, Chou P. The impact of smoking on incident type 2 diabetes in a cohort with hepatitis B but not hepatitis C infection. J Viral Hepat. 2017;24:1114-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Harris ML, Oldmeadow C, Hure A, Luu J, Loxton D, Attia J. Stress increases the risk of type 2 diabetes onset in women: A 12-year longitudinal study using causal modelling. PLoS One. 2017;12:e0172126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 22. | Cho NH, Chan JC, Jang HC, Lim S, Kim HL, Choi SH. Cigarette smoking is an independent risk factor for type 2 diabetes: a four-year community-based prospective study. Clin Endocrinol (Oxf). 2009;71:679-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 23. | Cho NH, Jang HC, Park C and Kimm KC. Evaluation of smoking effects on glucose metabolism: Community based prospective study. Proceedings of the 65th Scientific Sessions of the American Diabetes Association; 2005 Jun 10-14; San Diego, California, USA. American Diabetes Association, 2005: 987. |
| 24. | Han SJ, Kim HJ, Kim DJ, Lee KW, Cho NH. Incidence and predictors of type 2 diabetes among Koreans: A 12-year follow up of the Korean Genome and Epidemiology Study. Diabetes Res Clin Pract. 2017;123:173-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 25. | Yeh HC, Duncan BB, Schmidt MI, Wang NY, Brancati FL. Smoking, smoking cessation, and risk for type 2 diabetes mellitus: a cohort study. Ann Intern Med. 2010;152:10-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 257] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 26. | Rebholz CM, Yu B, Zheng Z, Chang P, Tin A, Köttgen A, Wagenknecht LE, Coresh J, Boerwinkle E, Selvin E. Serum metabolomic profile of incident diabetes. Diabetologia. 2018;61:1046-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 27. | Kim CH, Park JY, Lee KU, Kim JH, Kim HK. Fatty liver is an independent risk factor for the development of Type 2 diabetes in Korean adults. Diabet Med. 2008;25:476-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 94] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 28. | Koloverou E, Panagiotakos DB, Pitsavos C, Chrysohoou C, Georgousopoulou EN, Tousoulis D and Stefanadis C. The long term effect of dietary habits and physical activity on type 2 diabetes incidence: 10-year follow up of the ATTICA study (2002-2012): Diet, physical activity and diabetes. Hellenic J Atherosclerosis. 2018;9:5-16. |
| 29. | Magliano DJ, Barr EL, Zimmet PZ, Cameron AJ, Dunstan DW, Colagiuri S, Jolley D, Owen N, Phillips P, Tapp RJ, Welborn TA, Shaw JE. Glucose indices, health behaviors, and incidence of diabetes in Australia: the Australian Diabetes, Obesity and Lifestyle Study. Diabetes Care. 2008;31:267-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 163] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 30. | Keen H, Jarrett RJ, McCartney P. The ten-year follow-up of the Bedford survey (1962-1972): glucose tolerance and diabetes. Diabetologia. 1982;22:73-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 179] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 31. | Tenenbaum A, Fisman EZ, Adler Y, Motro M, Boyko V, Behar S. Smoking and development of type 2 diabetes in patients with decreased functional capacity. Int J Cardiol. 2005;104:275-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 32. | Cugati S, Wang JJ, Rochtchina E, Mitchell P. Ten-year incidence of diabetes in older Australians: the Blue Mountains Eye Study. Med J Aust. 2007;186:131-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Pollock BD, Chen W, Harville EW, Shu T, Fonseca V, Mauvais-Jarvis F, Kelly TN, Bazzano LA. Differential sex effects of systolic blood pressure and low-density lipoprotein cholesterol on type 2 diabetes: Life course data from the Bogalusa Heart Study. J Diabetes. 2018;10:449-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 34. | Lyssenko V, Almgren P, Anevski D, Perfekt R, Lahti K, Nissén M, Isomaa B, Forsen B, Homström N, Saloranta C, Taskinen MR, Groop L, Tuomi T; Botnia study group. Predictors of and longitudinal changes in insulin sensitivity and secretion preceding onset of type 2 diabetes. Diabetes. 2005;54:166-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 263] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 35. | Wannamethee SG, Shaper AG, Perry IJ; British Regional Heart Study. Smoking as a modifiable risk factor for type 2 diabetes in middle-aged men. Diabetes Care. 2001;24:1590-1595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 204] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 36. | Bonora E, Kiechl S, Willeit J, Oberhollenzer F, Egger G, Meigs JB, Bonadonna RC, Muggeo M; Bruneck study. Population-based incidence rates and risk factors for type 2 diabetes in white individuals: the Bruneck study. Diabetes. 2004;53:1782-1789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 210] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 37. | Burke V, Zhao Y, Lee AH, Hunter E, Spargo RM, Gracey M, Smith RM, Beilin LJ, Puddey IB. Predictors of type 2 diabetes and diabetes-related hospitalisation in an Australian Aboriginal cohort. Diabetes Res Clin Pract. 2007;78:360-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 38. | Coogan PF, White LF, Yu J, Burnett RT, Marshall JD, Seto E, Brook RD, Palmer JR, Rosenberg L, Jerrett M. Long term exposure to NO2 and diabetes incidence in the Black Women's Health Study. Environ Res. 2016;148:360-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 39. | Cassano PA, Rosner B, Vokonas PS, Weiss ST. Obesity and body fat distribution in relation to the incidence of non-insulin-dependent diabetes mellitus. A prospective cohort study of men in the normative aging study. Am J Epidemiol. 1992;136:1474-1486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 108] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 40. | Brateanu A, Barwacz T, Kou L, Wang S, Misra-Hebert AD, Hu B, Deshpande A, Kobaivanova N, Rothberg MB. Determining the optimal screening interval for type 2 diabetes mellitus using a risk prediction model. PLoS One. 2017;12:e0187695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 41. | de León AC, Coello SD, González DA, Díaz BB, Rodríguez JC, Hernández AG, Aguirre-Jaime A, Pérez Mdel C. Impaired fasting glucose, ancestry and waist-to-height ratio: main predictors of incident diagnosed diabetes in the Canary Islands. Diabet Med. 2012;29:399-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 42. | Qiu H, Schooling CM, Sun S, Tsang H, Yang Y, Lee RS, Wong CM, Tian L. Long-term exposure to fine particulate matter air pollution and type 2 diabetes mellitus in elderly: A cohort study in Hong Kong. Environ Int. 2018;113:350-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 43. | Lv J, Yu C, Guo Y, Bian Z, Yang L, Chen Y, Hu X, Hou W, Chen J, Chen Z, Qi L, Li L; China Kadoorie Biobank Collaborative Group. Adherence to a healthy lifestyle and the risk of type 2 diabetes in Chinese adults. Int J Epidemiol. 2017;46:1410-1420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 44. | Le Boudec J, Marques-Vidal P, Cornuz J, Clair C. Smoking cessation and the incidence of pre-diabetes and type 2 diabetes: a cohort study. J Diabetes Complications. 2016;30:43-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 45. | Will JC, Galuska DA, Ford ES, Mokdad A, Calle EE. Cigarette smoking and diabetes mellitus: evidence of a positive association from a large prospective cohort study. Int J Epidemiol. 2001;30:540-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 235] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 46. | Woo YC, Lee CH, Fong CH, Xu A, Tso AW, Cheung BM, Lam KS. Serum fibroblast growth factor 21 is a superior biomarker to other adipokines in predicting incident diabetes. Clin Endocrinol (Oxf). 2017;86:37-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 47. | Anjana RM, Shanthi Rani CS, Deepa M, Pradeepa R, Sudha V, Divya Nair H, Lakshmipriya N, Subhashini S, Binu VS, Unnikrishnan R, Mohan V. Incidence of Diabetes and Prediabetes and Predictors of Progression Among Asian Indians: 10-Year Follow-up of the Chennai Urban Rural Epidemiology Study (CURES). Diabetes Care. 2015;38:1441-1448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 180] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 48. | Li X, Wang J, Shen X, An Y, Gong Q, Li H, Zhang B, Shuai Y, Chen Y, Hu Y, Li G. Higher blood pressure predicts diabetes and enhances long-term risk of cardiovascular disease events in individuals with impaired glucose tolerance: Twenty-three-year follow-up of the Daqing diabetes prevention study. J Diabetes. 2019;11:593-598. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 49. | Dehghan A, van Hoek M, Sijbrands EJ, Stijnen T, Hofman A, Witteman JC. Risk of type 2 diabetes attributable to C-reactive protein and other risk factors. Diabetes Care. 2007;30:2695-2699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 50. | Balkau B, Lange C, Fezeu L, Tichet J, de Lauzon-Guillain B, Czernichow S, Fumeron F, Froguel P, Vaxillaire M, Cauchi S, Ducimetière P, Eschwège E. Predicting diabetes: clinical, biological, and genetic approaches: data from the Epidemiological Study on the Insulin Resistance Syndrome (DESIR). Diabetes Care. 2008;31:2056-2061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 183] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 51. | van Waateringe RP, Fokkens BT, Slagter SN, van der Klauw MM, van Vliet-Ostaptchouk JV, Graaff R, Paterson AD, Smit AJ, Lutgers HL, Wolffenbuttel BHR. Skin autofluorescence predicts incident type 2 diabetes, cardiovascular disease and mortality in the general population. Diabetologia. 2019;62:269-280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 85] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 52. | Hansen AB, Ravnskjær L, Loft S, Andersen KK, Bräuner EV, Baastrup R, Yao C, Ketzel M, Becker T, Brandt J, Hertel O, Andersen ZJ. Long-term exposure to fine particulate matter and incidence of diabetes in the Danish Nurse Cohort. Environ Int. 2016;91:243-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 111] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 53. | Han X, Wang J, Li Y, Hu H, Li X, Yuan J, Yao P, Miao X, Wei S, Wang Y, Liang Y, Zhang X, Guo H, Pan A, Yang H, Wu T, He M. Development of a new scoring system to predict 5-year incident diabetes risk in middle-aged and older Chinese. Acta Diabetol. 2018;55:13-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 54. | Poulsen K, Andersen LL. Linking data on work, health and lifestyle to explain socio-occupational inequality in Danish register-based incidence of diabetes. Scand J Public Health. 2016;44:361-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 55. | InterAct Consortium. Spijkerman AM, van der A DL, Nilsson PM, Ardanaz E, Gavrila D, Agudo A, Arriola L, Balkau B, Beulens JW, Boeing H, de Lauzon-Guillain B, Fagherazzi G, Feskens EJ, Franks PW, Grioni S, Huerta JM, Kaaks R, Key TJ, Overvad K, Palli D, Panico S, Redondo ML, Rolandsson O, Roswall N, Sacerdote C, Sánchez MJ, Schulze MB, Slimani N, Teucher B, Tjonneland A, Tumino R, van der Schouw YT, Langenberg C, Sharp SJ, Forouhi NG, Riboli E, Wareham NJ. Smoking and long-term risk of type 2 diabetes: the EPIC-InterAct study in European populations. Diabetes Care. 2014;37:3164-3171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 56. | Steele CJ, Schöttker B, Marshall AH, Kouvonen A, O'Doherty MG, Mons U, Saum KU, Boffetta P, Trichopoulou A, Brenner H, Kee F. Education achievement and type 2 diabetes-what mediates the relationship in older adults? Data from the ESTHER study: a population-based cohort study. BMJ Open. 2017;7:e013569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 57. | Fagerberg B, Kellis D, Bergström G, Behre CJ. Adiponectin in relation to insulin sensitivity and insulin secretion in the development of type 2 diabetes: a prospective study in 64-year-old women. J Intern Med. 2011;269:636-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 58. | Njølstad I, Arnesen E, Lund-Larsen PG. Sex differences in risk factors for clinical diabetes mellitus in a general population: a 12-year follow-up of the Finnmark Study. Am J Epidemiol. 1998;147:49-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 104] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 59. | Holmboe SA, Jensen TK, Linneberg A, Scheike T, Thuesen BH, Skakkebaek NE, Juul A, Andersson AM. Low Testosterone: A Risk Marker Rather Than a Risk Factor for Type 2 Diabetes. J Clin Endocrinol Metab. 2016;101:3180-3190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 60. | Paprott R, Mühlenbruch K, Mensink GB, Thiele S, Schulze MB, Scheidt-Nave C, Heidemann C. Validation of the German Diabetes Risk Score among the general adult population: findings from the German Health Interview and Examination Surveys. BMJ Open Diabetes Res Care. 2016;4:e000280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 61. | Icks A, Albers B, Haastert B, Pechlivanis S, Bokhof B, Slomiany U, Erbel R, Jöckel KH, Kruse J, Nowotny B, Herder C, Giani G, Moebus S; Heinz Nixdorf Recall Study Investigative Group; German BMBF Competence Network for Diabetes Mellitus. Diabetes incidence does not differ between subjects with and without high depressive symptoms--5-year follow-up results of the Heinz Nixdorf Recall Study. Diabet Med. 2013;30:65-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 62. | Weinmayr G, Hennig F, Fuks K, Nonnemacher M, Jakobs H, Möhlenkamp S, Erbel R, Jöckel KH, Hoffmann B, Moebus S; Heinz Nixdorf Recall Investigator Group. Long-term exposure to fine particulate matter and incidence of type 2 diabetes mellitus in a cohort study: effects of total and traffic-specific air pollution. Environ Health. 2015;14:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 142] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 63. | Zhang L, Wang B, Wang C, Li L, Ren Y, Zhang H, Yang X, Zhao Y, Han C, Zhou J, Luo X, Hu D. High pulse pressure is related to risk of type 2 diabetes mellitus in Chinese middle-aged females. Int J Cardiol. 2016;220:467-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 64. | Hayashino Y, Fukuhara S, Okamura T, Yamato H, Tanaka H, Tanaka T, Kadowaki T, Ueshima H; HIPOP-OHP Research Group. A prospective study of passive smoking and risk of diabetes in a cohort of workers: the High-Risk and Population Strategy for Occupational Health Promotion (HIPOP-OHP) study. Diabetes Care. 2008;31:732-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 65. | Hippisley-Cox J, Coupland C, Robson J, Sheikh A, Brindle P. Predicting risk of type 2 diabetes in England and Wales: prospective derivation and validation of QDScore. BMJ. 2009;338:b880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 244] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 66. | Hippisley-Cox J, Coupland C. Development and validation of QDiabetes-2018 risk prediction algorithm to estimate future risk of type 2 diabetes: cohort study. BMJ. 2017;359:j5019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 114] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 67. | Doi Y, Ninomiya T, Hata J, Hirakawa Y, Mukai N, Iwase M, Kiyohara Y. Two risk score models for predicting incident Type 2 diabetes in Japan. Diabet Med. 2012;29:107-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 68. | Rimm EB, Chan J, Stampfer MJ, Colditz GA, Willett WC. Prospective study of cigarette smoking, alcohol use, and the risk of diabetes in men. BMJ. 1995;310:555-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 361] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 69. | Rasouli B, Grill V, Midthjell K, Ahlbom A, Andersson T, Carlsson S. Smoking is associated with reduced risk of autoimmune diabetes in adults contrasting with increased risk in overweight men with type 2 diabetes: a 22-year follow-up of the HUNT study. Diabetes Care. 2013;36:604-610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 70. | Vazquez LA, Calvo-Bonacho E, Reviriego J, García-Margallo T, Caveda E, Goday A. Incidence of Diabetes in the Working Population in Spain: Results from the ICARIA Cohort. Diabetes Ther. 2019;10:57-69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 71. | Sadeghi M, Talaei M, Parvaresh Rizi E, Dianatkhah M, Oveisgharan S, Sarrafzadegan N. Determinants of incident prediabetes and type 2 diabetes in a 7-year cohort in a developing country: The Isfahan Cohort Study. J Diabetes. 2015;7:633-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 72. | Wiernik E, Nabi H, Thomas F, Pannier B, Hanon O, Simon T, Simon JM, Danchin N, Limosin F, Czernichow S, Lemogne C. Association between current perceived stress and incident diabetes is dependent on occupational status: Evidence from the IPC cohort study. Diabetes Metab. 2016;42:328-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 73. | Foy CG, Bell RA, Farmer DF, Goff DC, Wagenknecht LE. Smoking and incidence of diabetes among U.S. adults: findings from the Insulin Resistance Atherosclerosis Study. Diabetes Care. 2005;28:2501-2507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 121] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 74. | Cullen MW, Ebbert JO, Vierkant RA, Wang AH, Cerhan JR. No interaction of body mass index and smoking on diabetes mellitus risk in elderly women. Prev Med. 2009;48:74-78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 75. | Eshak ES, Iso H, Maruyama K, Muraki I, Tamakoshi A. Associations between dietary intakes of iron, copper and zinc with risk of type 2 diabetes mellitus: A large population-based prospective cohort study. Clin Nutr. 2018;37:667-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 84] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 76. | Akter S, Okazaki H, Kuwahara K, Miyamoto T, Murakami T, Shimizu C, Shimizu M, Tomita K, Nagahama S, Eguchi M, Kochi T, Imai T, Nishihara A, Sasaki N, Nakagawa T, Yamamoto S, Honda T, Uehara A, Yamamoto M, Hori A, Sakamoto N, Nishiura C, Totsuzaki T, Kato N, Fukasawa K, Pham NM, Kurotani K, Nanri A, Kabe I, Mizoue T, Sone T, Dohi S; Japan Epidemiology Collaboration on Occupational Health Study Group. Smoking, Smoking Cessation, and the Risk of Type 2 Diabetes among Japanese Adults: Japan Epidemiology Collaboration on Occupational Health Study. PLoS One. 2015;10:e0132166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 77. | Hu H, Nakagawa T, Yamamoto S, Honda T, Okazaki H, Uehara A, Yamamoto M, Miyamoto T, Kochi T, Eguchi M, Murakami T, Shimizu M, Tomita K, Nagahama S, Imai T, Nishihara A, Sasaki N, Ogasawara T, Hori A, Nanri A, Akter S, Kuwahara K, Kashino I, Kabe I, Mizoue T, Sone T, Dohi S; Japan Epidemiology Collaboration on Occupational Health Study Group. Development and validation of risk models to predict the 7-year risk of type 2 diabetes: The Japan Epidemiology Collaboration on Occupational Health Study. J Diabetes Investig. 2018;9:1052-1059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 78. | White WB, Cain LR, Benjamin EJ, DeFilippis AP, Blaha MJ, Wang W, Okhomina V, Keith RJ, Al Rifai M, Kianoush S, Winniford MD, Robertson RM, Bhatnagar A, Correa A, Hall ME. High-Intensity Cigarette Smoking Is Associated With Incident Diabetes Mellitus In Black Adults: The Jackson Heart Study. J Am Heart Assoc. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 79. | Waki K, Noda M, Sasaki S, Matsumura Y, Takahashi Y, Isogawa A, Ohashi Y, Kadowaki T, Tsugane S; JPHC Study Group. Alcohol consumption and other risk factors for self-reported diabetes among middle-aged Japanese: a population-based prospective study in the JPHC study cohort I. Diabet Med. 2005;22:323-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 131] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 80. | Oba S, Noda M, Waki K, Nanri A, Kato M, Takahashi Y, Poudel-Tandukar K, Matsushita Y, Inoue M, Mizoue T, Tsugane S; Japan Public Health Center-Based Prospective Study Group. Smoking cessation increases short-term risk of type 2 diabetes irrespective of weight gain: the Japan Public Health Center-Based Prospective Study. PLoS One. 2012;7:e17061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 81. | Lee JY, Ryu S, Sung KC. Association of baseline level of physical activity and its temporal changes with incident hypertension and diabetes mellitus. Eur J Prev Cardiol. 2018;25:1065-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 82. | Kawahara T, Imawatari R, Kawahara C, Inazu T, Suzuki G. Incidence of type 2 diabetes in pre-diabetic Japanese individuals categorized by HbA1c levels: a historical cohort study. PLoS One. 2015;10:e0122698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 83. | Kawakami N, Takatsuka N, Shimizu H, Ishibashi H. Effects of smoking on the incidence of non-insulin-dependent diabetes mellitus. Replication and extension in a Japanese cohort of male employees. Am J Epidemiol. 1997;145:103-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 67] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 84. | Hur NW, Kim HC, Nam CM, Jee SH, Lee HC, Suh I. Smoking cessation and risk of type 2 diabetes mellitus: Korea Medical Insurance Corporation Study. Eur J Cardiovasc Prev Rehabil. 2007;14:244-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 85. | Song BM, Kim HC, Lee JY, Lee JM, Kim DJ, Lee YH, Suh I. Performance of HbA1c for the prediction of diabetes in a rural community in Korea. Diabet Med. 2015;32:1602-1610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 86. | Lee SW, Kim HC, Lee JM, Yun YM, Lee JY, Suh I. Association between changes in systolic blood pressure and incident diabetes in a community-based cohort study in Korea. Hypertens Res. 2017;40:710-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 87. | Herder C, Kannenberg JM, Carstensen-Kirberg M, Huth C, Meisinger C, Koenig W, Peters A, Rathmann W, Roden M, Thorand B. Serum levels of interleukin-22, cardiometabolic risk factors and incident type 2 diabetes: KORA F4/FF4 study. Cardiovasc Diabetol. 2017;16:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 88. | Rathmann W, Strassburger K, Heier M, Holle R, Thorand B, Giani G, Meisinger C. Incidence of Type 2 diabetes in the elderly German population and the effect of clinical and lifestyle risk factors: KORA S4/F4 cohort study. Diabet Med. 2009;26:1212-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 126] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 89. | Nichols GA, Hillier TA, Brown JB. Normal fasting plasma glucose and risk of type 2 diabetes diagnosis. Am J Med. 2008;121:519-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 109] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 90. | Mani H, Levy MJ, Davies MJ, Morris DH, Gray LJ, Bankart J, Blackledge H, Khunti K, Howlett TA. Diabetes and cardiovascular events in women with polycystic ovary syndrome: a 20-year retrospective cohort study. Clin Endocrinol (Oxf). 2013;78:926-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 130] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 91. | Salminen M, Vahlberg T, Räihä I, Niskanen L, Kivelä SL, Irjala K. Sex hormones and the risk of type 2 diabetes mellitus: A 9-year follow up among elderly men in Finland. Geriatr Gerontol Int. 2015;15:559-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 92. | Lindberg S, Jensen JS, Bjerre M, Pedersen SH, Frystyk J, Flyvbjerg A, Galatius S, Jeppesen J, Mogelvang R. Adiponectin, type 2 diabetes and cardiovascular risk. Eur J Prev Cardiol. 2015;22:276-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 93. | Sherratt FC, Field JK, Marcus MW. Association between smoking and health outcomes in an economically deprived population: the Liverpool Lung Project. J Epidemiol Community Health. 2017;71:806-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 94. | Gyawali P, Martin SA, Heilbronn LK, Vincent AD, Taylor AW, Adams RJT, O'Loughlin PD, Wittert GA. The role of sex hormone-binding globulin (SHBG), testosterone, and other sex steroids, on the development of type 2 diabetes in a cohort of community-dwelling middle-aged to elderly men. Acta Diabetol. 2018;55:861-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 95. | Manson JE, Ajani UA, Liu S, Nathan DM, Hennekens CH. A prospective study of cigarette smoking and the incidence of diabetes mellitus among US male physicians. Am J Med. 2000;109:538-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 151] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 96. | Setiawan VW, Stram DO, Porcel J, Chari ST, Maskarinec G, Le Marchand L, Wilkens LR, Haiman CA, Pandol SJ, Monroe KR. Pancreatic Cancer Following Incident Diabetes in African Americans and Latinos: The Multiethnic Cohort. J Natl Cancer Inst. 2019;111:27-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 97. | Steinbrecher A, Morimoto Y, Heak S, Ollberding NJ, Geller KS, Grandinetti A, Kolonel LN, Maskarinec G. The preventable proportion of type 2 diabetes by ethnicity: the multiethnic cohort. Ann Epidemiol. 2011;21:526-535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 98. | Keith RJ, Al Rifai M, Carruba C, De Jarnett N, McEvoy JW, Bhatnagar A, Blaha MJ, Defilippis AP. Tobacco Use, Insulin Resistance, and Risk of Type 2 Diabetes: Results from the Multi-Ethnic Study of Atherosclerosis. PLoS One. 2016;11:e0157592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 99. | Joseph JJ, Echouffo-Tcheugui JB, Carnethon MR, Bertoni AG, Shay CM, Ahmed HM, Blumenthal RS, Cushman M, Golden SH. The association of ideal cardiovascular health with incident type 2 diabetes mellitus: the Multi-Ethnic Study of Atherosclerosis. Diabetologia. 2016;59:1893-1903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 100. | Lao XQ, Guo C, Chang LY, Bo Y, Zhang Z, Chuang YC, Jiang WK, Lin C, Tam T, Lau AKH, Lin CY, Chan TC. Long-term exposure to ambient fine particulate matter (PM2.5) and incident type 2 diabetes: a longitudinal cohort study. Diabetologia. 2019;62:759-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 81] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 101. | Meisinger C, Döring A, Thorand B, Löwel H. Association of cigarette smoking and tar and nicotine intake with development of type 2 diabetes mellitus in men and women from the general population: the MONICA/KORA Augsburg Cohort Study. Diabetologia. 2006;49:1770-1776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 102. | Eliasson M, Asplund K, Nasic S, Rodu B. Influence of smoking and snus on the prevalence and incidence of type 2 diabetes amongst men: the northern Sweden MONICA study. J Intern Med. 2004;256:101-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 103. | Morimoto A, Ohno Y, Tatsumi Y, Nishigaki Y, Maejima F, Mizuno S, Watanabe S. Risk of smoking and body mass index for incidence of diabetes mellitus in a rural Japanese population. Prev Med. 2012;54:341-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 104. | Morimoto A, Ohno Y, Tatsumi Y, Nishigaki Y, Maejima F, Mizuno S, Watanabe S. Impact of smoking cessation on incidence of diabetes mellitus among overweight or normal-weight Japanese men. Diabetes Res Clin Pract. 2012;96:407-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 105. | Mozaffarian D, Kamineni A, Carnethon M, Djoussé L, Mukamal KJ, Siscovick D. Lifestyle risk factors and new-onset diabetes mellitus in older adults: the cardiovascular health study. Arch Intern Med. 2009;169:798-807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 267] [Cited by in RCA: 242] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 106. | Vasiliu O, Cameron L, Gardiner J, Deguire P, Karmaus W. Polybrominated biphenyls, polychlorinated biphenyls, body weight, and incidence of adult-onset diabetes mellitus. Epidemiology. 2006;17:352-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 137] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 107. | Ide R, Hoshuyama T, Wilson D, Takahashi K, Higashi T. Periodontal disease and incident diabetes: a seven-year study. J Dent Res. 2011;90:41-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 108. | Kaneto C, Toyokawa S, Miyoshi Y, Suyama Y, Kobayashi Y. Long-term weight change in adulthood and incident diabetes mellitus: MY Health Up Study. Diabetes Res Clin Pract. 2013;102:138-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 109. | Mitsuhashi K, Hashimoto Y, Hamaguchi M, Obora A, Kojima T, Fukuda T, Fukui M. Impact of fatty liver disease and metabolic syndrome on incident type 2 diabetes; a population based cohort study. Endocr J. 2017;64:1105-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 110. | Hashimoto Y, Hamaguchi M, Nakanishi N, Ohbora A, Kojima T, Fukui M. Urinary pH is a predictor of diabetes in men; a population based large scale cohort study. Diabetes Res Clin Pract. 2017;130:9-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 111. | Nagaya T, Yoshida H, Takahashi H, Kawai M. Heavy smoking raises risk for type 2 diabetes mellitus in obese men; but, light smoking reduces the risk in lean men: a follow-up study in Japan. Ann Epidemiol. 2008;18:113-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 112. | Nakanishi N, Nakamura K, Matsuo Y, Suzuki K, Tatara K. Cigarette smoking and risk for impaired fasting glucose and type 2 diabetes in middle-aged Japanese men. Ann Intern Med. 2000;133:183-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 87] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 113. | Montgomery SM, Ekbom A. Smoking during pregnancy and diabetes mellitus in a British longitudinal birth cohort. BMJ. 2002;324:26-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 160] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 114. | Ford ES, Mannino DM; National Health and Nutrition Examination Survey Epidemiologic Follow-up Study. Prospective association between lung function and the incidence of diabetes: findings from the National Health and Nutrition Examination Survey Epidemiologic Follow-up Study. Diabetes Care. 2004;27:2966-2970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 121] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 115. | Jee SH, Foong AW, Hur NW, Samet JM. Smoking and risk for diabetes incidence and mortality in Korean men and women. Diabetes Care. 2010;33:2567-2572. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 116. | Ha KH, Lee YH, Song SO, Lee JW, Kim DW, Cho KH, Kim DJ. Development and Validation of the Korean Diabetes Risk Score: A 10-Year National Cohort Study. Diabetes Metab J. 2018;42:402-414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 117. | Kim ES, Jeong JS, Han K, Kim MK, Lee SH, Park YM, Baek KH, Moon SD, Han JH, Song KH, Kwon HS. Impact of weight changes on the incidence of diabetes mellitus: a Korean nationwide cohort study. Sci Rep. 2018;8:3735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 118. | Zhang L, Curhan GC, Hu FB, Rimm EB, Forman JP. Association between passive and active smoking and incident type 2 diabetes in women. Diabetes Care. 2011;34:892-897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 119. | Reis JP, Loria CM, Sorlie PD, Park Y, Hollenbeck A, Schatzkin A. Lifestyle factors and risk for new-onset diabetes: a population-based cohort study. Ann Intern Med. 2011;155:292-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 120. | Kulick ER, Moon YP, Cheung K, Willey JZ, Sacco RL, Elkind MS. Racial-ethnic disparities in the association between risk factors and diabetes: The Northern Manhattan Study. Prev Med. 2016;83:31-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 121. | Novak M, Björck L, Giang KW, Heden-Ståhl C, Wilhelmsen L, Rosengren A. Perceived stress and incidence of Type 2 diabetes: a 35-year follow-up study of middle-aged Swedish men. Diabet Med. 2013;30:e8-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 122. | Castro MR, Simon G, Cha SS, Yawn BP, Melton LJ, Caraballo PJ. Statin Use, Diabetes Incidence and Overall Mortality in Normoglycemic and Impaired Fasting Glucose Patients. J Gen Intern Med. 2016;31:502-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 123. | Onat A, Ozhan H, Esen AM, Albayrak S, Karabulut A, Can G, Hergenç G. Prospective epidemiologic evidence of a "protective" effect of smoking on metabolic syndrome and diabetes among Turkish women--without associated overall health benefit. Atherosclerosis. 2007;193:380-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 124. | Katsuta S. [Cigarette smoking and lifestyle-related diseases in Japan. A longitudinal study of health check-up data from urban areas]. Nihon Koshu Eisei Zasshi. 2012;59:447-456. [PubMed] [DOI] [Full Text] |
| 125. | Holme I, Tonstad S, Sogaard AJ, Larsen PG, Haheim LL. Leisure time physical activity in middle age predicts the metabolic syndrome in old age: results of a 28-year follow-up of men in the Oslo study. BMC Public Health. 2007;7:154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 126. | Östenson CG, Hilding A, Grill V, Efendic S. High consumption of smokeless tobacco ("snus") predicts increased risk of type 2 diabetes in a 10-year prospective study of middle-aged Swedish men. Scand J Public Health. 2012;40:730-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 127. | Park CH, Ga H, Leem JH, Kwak SM, Kim HC, Choi JH. [The effect of smoking status upon occurrence of impaired fasting glucose or type 2 diabetes in Korean men]. J Prev Med Public Health. 2008;41:249-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 128. | Patja K, Jousilahti P, Hu G, Valle T, Qiao Q, Tuomilehto J. Effects of smoking, obesity and physical activity on the risk of type 2 diabetes in middle-aged Finnish men and women. J Intern Med. 2005;258:356-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 129. | Song X, Qiu M, Zhang X, Wang H, Tong W, Ju L, Gu L, Sun S, Zhang H, Wang W, Tian J. Gender-related affecting factors of prediabetes on its 10-year outcome. BMJ Open Diabetes Res Care. 2016;4:e000169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 130. | Luo W, Guo Z, Wu M, Hao C, Zhou Z, Yao X. Interaction of smoking and obesity on type 2 diabetes risk in a Chinese cohort. Physiol Behav. 2015;139:240-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 131. | Gil-Montalbán E, Martín-Ríos MD, Ortiz-Marrón H, Zorrilla-Torras B, Martínez-Cortés M, Esteban-Vasallo MD, López-de-Andrés A. Incidence of type 2 diabetes and associated factors in the adult population of the Community of Madrid. PREDIMERC cohort. Rev Clin Esp. 2015;215:495-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 132. | Suthahar N, Meijers WC, Brouwers FP, Heerspink HJL, Gansevoort RT, van der Harst P, Bakker SJL, de Boer RA. Heart failure and inflammation-related biomarkers as predictors of new-onset diabetes in the general population. Int J Cardiol. 2018;250:188-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 133. | Joseph JJ, Bennett A, Echouffo Tcheugui JB, Effoe VS, Odei JB, Hidalgo B, Dulin A, Safford MM, Cummings DM, Cushman M, Carson AP. Ideal cardiovascular health, glycaemic status and incident type 2 diabetes mellitus: the REasons for Geographic and Racial Differences in Stroke (REGARDS) study. Diabetologia. 2019;62:426-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 134. | Roediger MA, Marucci MFN, Gobbo LA, Dourado DAQS, Santos JLF, Duarte YAO, Lebrão ML. Reported diabetes mellitus: incidence and determinants in cohort of community dwelling elderly people in São Paulo City, Brazil: SABE study, health, wellness and aging. Cien Saude Colet. 2018;23:3913-3922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 135. | Sairenchi T, Iso H, Nishimura A, Hosoda T, Irie F, Saito Y, Murakami A, Fukutomi H. Cigarette smoking and risk of type 2 diabetes mellitus among middle-aged and elderly Japanese men and women. Am J Epidemiol. 2004;160:158-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 136. | Jeon CY, Haan MN, Cheng C, Clayton ER, Mayeda ER, Miller JW, Aiello AE. Helicobacter pylori infection is associated with an increased rate of diabetes. Diabetes Care. 2012;35:520-525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 156] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 137. | Yu TY, Jee JH, Bae JC, Hong WJ, Jin SM, Kim JH, Lee MK. Delayed heart rate recovery after exercise as a risk factor of incident type 2 diabetes mellitus after adjusting for glycometabolic parameters in men. Int J Cardiol. 2016;221:17-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 138. | Eze IC, Foraster M, Schaffner E, Vienneau D, Héritier H, Rudzik F, Thiesse L, Pieren R, Imboden M, von Eckardstein A, Schindler C, Brink M, Cajochen C, Wunderli JM, Röösli M, Probst-Hensch N. Long-term exposure to transportation noise and air pollution in relation to incident diabetes in the SAPALDIA study. Int J Epidemiol. 2017;46:1115-1125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 106] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 139. | Sawada SS, Lee IM, Muto T, Matuszaki K, Blair SN. Cardiorespiratory fitness and the incidence of type 2 diabetes: prospective study of Japanese men. Diabetes Care. 2003;26:2918-2922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 95] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 140. | Ding D, Chong S, Jalaludin B, Comino E, Bauman AE. Risk factors of incident type 2-diabetes mellitus over a 3-year follow-up: Results from a large Australian sample. Diabetes Res Clin Pract. 2015;108:306-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 141. | Zhao J, Zhu Y, Hyun N, Zeng D, Uppal K, Tran VT, Yu T, Jones D, He J, Lee ET, Howard BV. Novel metabolic markers for the risk of diabetes development in American Indians. Diabetes Care. 2015;38:220-227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 142. | Kebede TG, Pink C, Rathmann W, Kowall B, Völzke H, Petersmann A, Meisel P, Dietrich T, Kocher T, Holtfreter B. Does periodontitis affect diabetes incidence and haemoglobin A1c change? An 11-year follow-up study. Diabetes Metab. 2018;44:243-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 143. | Shi L, Shu XO, Li H, Cai H, Liu Q, Zheng W, Xiang YB, Villegas R. Physical activity, smoking, and alcohol consumption in association with incidence of type 2 diabetes among middle-aged and elderly Chinese men. PLoS One. 2013;8:e77919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 144. | Kouvonen AM, Väänänen A, Woods SA, Heponiemi T, Koskinen A, Toppinen-Tanner S. Sense of coherence and diabetes: a prospective occupational cohort study. BMC Public Health. 2008;8:46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 145. | Strandberg TE, Salomaa V. Factors related to the development of diabetes during a 20-year follow-up. A prospective study in a homogeneous group of middle-aged men. Nutr Metab Cardiovasc Dis. 2000;10:239-246. [PubMed] |
| 146. | Singh-Manoux A, Fayosse A, Sabia S, Tabak A, Shipley M, Dugravot A, Kivimäki M. Clinical, socioeconomic, and behavioural factors at age 50 years and risk of cardiometabolic multimorbidity and mortality: A cohort study. PLoS Med. 2018;15:e1002571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 130] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 147. | Stringhini S, Tabak AG, Akbaraly TN, Sabia S, Shipley MJ, Marmot MG, Brunner EJ, Batty GD, Bovet P, Kivimäki M. Contribution of modifiable risk factors to social inequalities in type 2 diabetes: prospective Whitehall II cohort study. BMJ. 2012;345:e5452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 116] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 148. | Sugimori H, Miyakawa M, Yoshida K, Izuno T, Takahashi E, Tanaka C, Nakamura K, Hinohara S. Health risk assessment for diabetes mellitus based on longitudinal analysis of MHTS database. J Med Syst. 1998;22:27-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 149. | Waris L, Mihardja LK, Pratomo H, Lampe M, Soewondo P, Djuwita R and Ronoatmodjo S. Understanding pre-diabetic life style as a determinant factor of type-2 diabetes mellitus in south Sulawesi province, Indonesia. Indian J Public Health Res Dev. 2018;9:86-92. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 150. | Karvonen-Gutierrez CA, Peng Q, Peterson M, Duchowny K, Nan B, Harlow S. Low grip strength predicts incident diabetes among mid-life women: the Michigan Study of Women's Health Across the Nation. Age Ageing. 2018;47:685-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 151. | Papier K, Jordan S, D'Este C, Bain C, Peungson J, Banwell C, Yiengprugsawan V, Seubsman SA, Sleigh A. Incidence and risk factors for type 2 diabetes mellitus in transitional Thailand: results from the Thai cohort study. BMJ Open. 2016;6:e014102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 152. | Teratani T, Morimoto H, Sakata K, Oishi M, Tanaka K, Nakada S, Nogawa K, Suwazono Y. Dose-response relationship between tobacco or alcohol consumption and the development of diabetes mellitus in Japanese male workers. Drug Alcohol Depend. 2012;125:276-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 153. | Tsai AC, Lee SH. Determinants of new-onset diabetes in older adults—Results of a national cohort study. Clin Nutr. 2015;34:937-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 154. | Heianza Y, Arase Y, Hsieh SD, Saito K, Tsuji H, Kodama S, Tanaka S, Ohashi Y, Shimano H, Yamada N, Hara S, Sone H. Development of a new scoring system for predicting the 5 year incidence of type 2 diabetes in Japan: the Toranomon Hospital Health Management Center Study 6 (TOPICS 6). Diabetologia. 2012;55:3213-3223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 155. | Joseph J, Svartberg J, Njølstad I, Schirmer H. Incidence of and risk factors for type-2 diabetes in a general population: the Tromsø Study. Scand J Public Health. 2010;38:768-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 156. | Uchimoto S, Tsumura K, Hayashi T, Suematsu C, Endo G, Fujii S, Okada K. Impact of cigarette smoking on the incidence of Type 2 diabetes mellitus in middle-aged Japanese men: the Osaka Health Survey. Diabet Med. 1999;16:951-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 66] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 157. | Phillips LS, Ho YL, Rhee MK, Vassy JL, Gagnon DR, Wilson PWF. Levels of random plasma glucose predict the diagnosis of diabetes. Proceedings of the 70th Scientific Sessions (2010); 2010 Jun 25-29; Orlando, Florida, USA. Diabetes 2017; 66: A422. |
| 158. | Long GH, Johansson I, Rolandsson O, Wennberg P, Fhärm E, Weinehall L, Griffin SJ, Simmons RK, Norberg M. Healthy behaviours and 10-year incidence of diabetes: a population cohort study. Prev Med. 2015;71:121-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 159. | Luo J, Rossouw J, Tong E, Giovino GA, Lee CC, Chen C, Ockene JK, Qi L, Margolis KL. Smoking and diabetes: does the increased risk ever go away? Am J Epidemiol. 2013;178:937-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 160. | Pitkänen N, Juonala M, Rönnemaa T, Sabin MA, Hutri-Kähönen N, Kähönen M, Lehtimäki T, Viikari JS, Raitakari OT. Role of Conventional Childhood Risk Factors Versus Genetic Risk in the Development of Type 2 Diabetes and Impaired Fasting Glucose in Adulthood: The Cardiovascular Risk in Young Finns Study. Diabetes Care. 2016;39:1393-1399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 161. | Feskens EJ, Kromhout D. Cardiovascular risk factors and the 25-year incidence of diabetes mellitus in middle-aged men. The Zutphen Study. Am J Epidemiol. 1989;130:1101-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 136] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 162. | National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Risk factors for type 2 diabetes. 2016 Nov [Cited February 2020]. In: Diabetes Overview [Internet]. Available from: https://www.niddk.nih.gov/health-information/diabetes/overview/risk-factors-type-2-diabetes. |
| 163. | Taylor R. Life without diabetes-The definitive guide to understanding and reversing type 2 diabetes. Vol London: Short Books, 2020: 319. |