Published online Nov 28, 2019. doi: 10.13105/wjma.v7.i9.418
Peer-review started: July 29, 2019
First decision: August 20, 2019
Revised: October 20, 2019
Accepted: October 30, 2019
Article in press: October 30, 2019
Published online: November 28, 2019
Processing time: 125 Days and 13.9 Hours
The plant kingdom is an important potential source of effective treatment for various diseases. Most herbs have long been used for medicinal purposes, and plant metabolites with their derivatives had been used in ethnomedicine. However, concerns exist about the quality and safety of herbal medicine products, particularly relating to safety, dosage, and mechanism of action. This mini review reveals some insights about the Hunteria umbellate seed, which is similar to that of insulin secretagogue metformin. Studies have validated its beneficial role in hyperglycemic, insulin resistance and obesity conditions, which are components of metabolic syndrome. However, none of these studies evaluated the mechanisms by which this plant extract performs its anti-hyperglycemic, insulin resistance and anti-obesity actions in metabolic syndrome. This understanding would provide considerable progress toward drug design using this plant material. Hence the need for this awareness to sensitize the researchers in this field who are passionate about drug design to consider the pathways discussed below for Hunteria umbellata seeds. Hunteria umbellata seed extract may represent a new therapeutic strategy for type-2 diabetes in place of metformin if it is well-studied.
Core tip: Herbs have been used for medicinal purposes since time is immemorial, although concerns exist about quality, safety, dosage and mechanism of action of herbal medicinal products. This mini review reveals some insights about the Hunteria umbellata seed, which is similar to metformin insulin secretagogue but with less side effects in diabetic subjects. Based on its beneficial roles that have been documented, none of the studies have evaluated the mechanisms involved. Therefore, researchers in this field who are passionate about drug design need to consider the pathways discussed below for the Hunteria umbellata seed. This may serve as a new therapeutic strategy for type-2 diabetes in place of metformin.
- Citation: Ejelonu OC. Mechanisms of action of aqueous extract from the Hunteria umbellata seed and metformin in diabetes. World J Meta-Anal 2019; 7(9): 418-422
- URL: https://www.wjgnet.com/2308-3840/full/v7/i9/418.htm
- DOI: https://dx.doi.org/10.13105/wjma.v7.i9.418
A systematic review published by Herman et al[1] revealed that eight out of ten of the world population diagnosed with type-2 diabetes are within developing and developed countries; this indicates the role of socioeconomic factors played in the demography and dynamics of type-2 diabetes. According to the International Diabetes Federation projection, one in every 10 adults will be diagnosed with diabetes by 2030[2]. A year following this press release, type-2 diabetes was described as a global epidemic requiring attention and urgent action[3]. These data clearly support that expedited actions should be directed towards these countries. Patho-physiologically, failed insulin secretion and insulin resistance drive type-2 diabetes[4]; thus, type-2 diabetes is amenable to insulinotropic drugs[5]. Due to socioeconomic constraints, low- and medium-income countries have no access to insulinotropic drugs; therefore cheaper, more accessible therapeutic options must be considered to offset current statistics and future projections.
Hunteria umbellata (K. Schum.) Hallier f. belong to the Apocynaceae family, a West African glaborous tree known as Abeere in Yoruba, Southwest Nigeria[6]. It has been widely used by different folk, which include the management of infections, diseases, treatment of pain and metabolic disorders such as diabetes mellitus and obesity[7]. Many genera in the Apocynaceae family have been well-studied, especially their chemical composition and economic importance. Studies have revealed that the aqueous seed extract of Hunteria umbellata has been tested on different experimental models of diabetes[8] for its anti-hyperglycemic effect coupled with anti-obesity and anti-hyperlipidemic potentials[9], which are well-documented. Likewise, oral toxicity studies have been conducted that authenticate its safety prior to oral administration in experimental animals[10]. Biometric analysis of the Hunteria umbellata seed conducted by Ajibola and co-researchers revealed that aqueous extract of the Hunteria umbellata seed performed well in reducing fasting blood glucose in type-2 diabetic patients in a few short weeks compared with metformin, coupled with lesser side effects. The patients placed on metformin exhibited symptoms such as abdominal pains, belching, chest pain, diarrhea, headache, nausea and vomiting, runny nose and weakness; but the only complaint recorded from patients treated with the Hunteria umbellata seed was extreme bitterness, which caused one of the patients to have an allergic reaction to the extract, causing seldom vomiting[11].
Furthermore, hypoglycemic activity of its seed extract has been documented in normal, high glucose and nicotine-induced hyperglycemic rats, mediated via intestinal uptake of glucose coupled with adrenergic inhibition, respectively[6]. Similarly, other animal models were also used and reported to ascertain the efficacy of Hunteria umbellata seed extract as an anti-diabetes and anti-obesity plant[9]. It has been documented by Boone et al[12] that the Hunteria umbellata seed possesses eburnamine, eburnamonine, hunteriamine, hunterine, vincamine and corymine as part of its phytoconstituent, with eburnamonine and eburnamine coupled with hunterine that have been indicated to possess strong and lasting hypotensive action. Cerebrovascular activity of the Hunteria umbellata seed has been traced to eburnamonine, which is reported to be more abundant[12]. This has a positive effect on general blood circulation, while anti-hypertensive and sedative properties have been attributed to vincamine[12]. Likewise, Adeneye and fellow researchers[13] suggested that anti-hyperglycemic potential posed by the Hunteria umbellata seed was attributed to an isolated, newly extracted bisindole alkaloid called erinidine, with the in vitro and in vivo anti-hyperglycemic studies conducted using erinidine confirming that the Hunteria umbellata seed can mediate its anti-hyperglycemic action via intestinal glucose uptake inhibition. Findings have revealed that none of these studies have delved into the mechanisms by which this plant extract performs its anti-hyperglycemic actions in experimental diabetes models, which would provide strong progress towards drug design using Hunteria umbellata seeds.
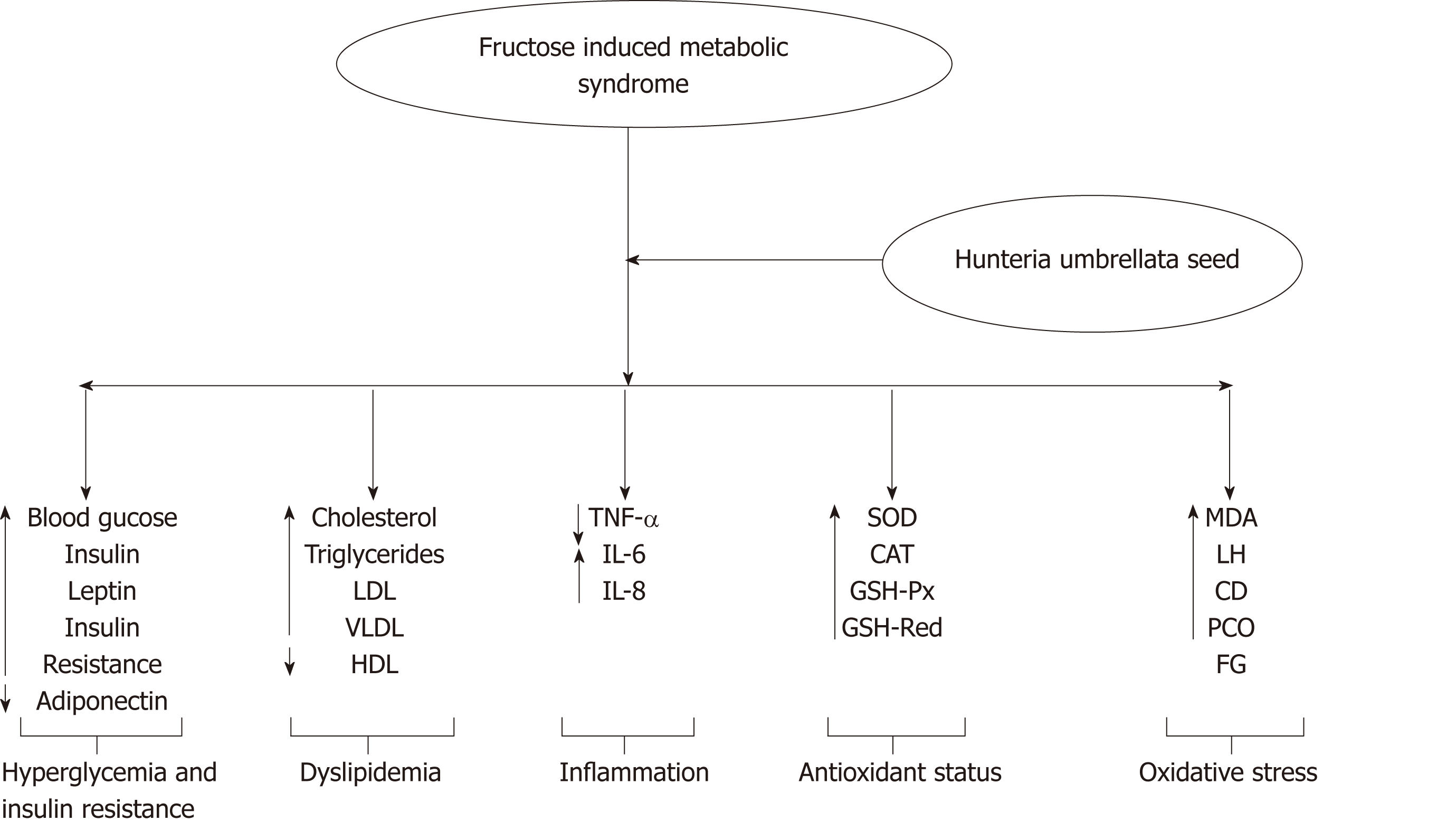
A study revealed that aqueous extract of Hunteria umbellata seeds were capable of ameliorating metabolic syndrome associated with high fructose-induced rats, (Figure 1) thereby acting as a scavenging agent for reactive oxygen species and increased enzymes activities that detoxify reactive oxygen species, which invariably revealed Hunteria umbellata seeds as a source of nutraceuticals for treating metabolic syndrome[14].
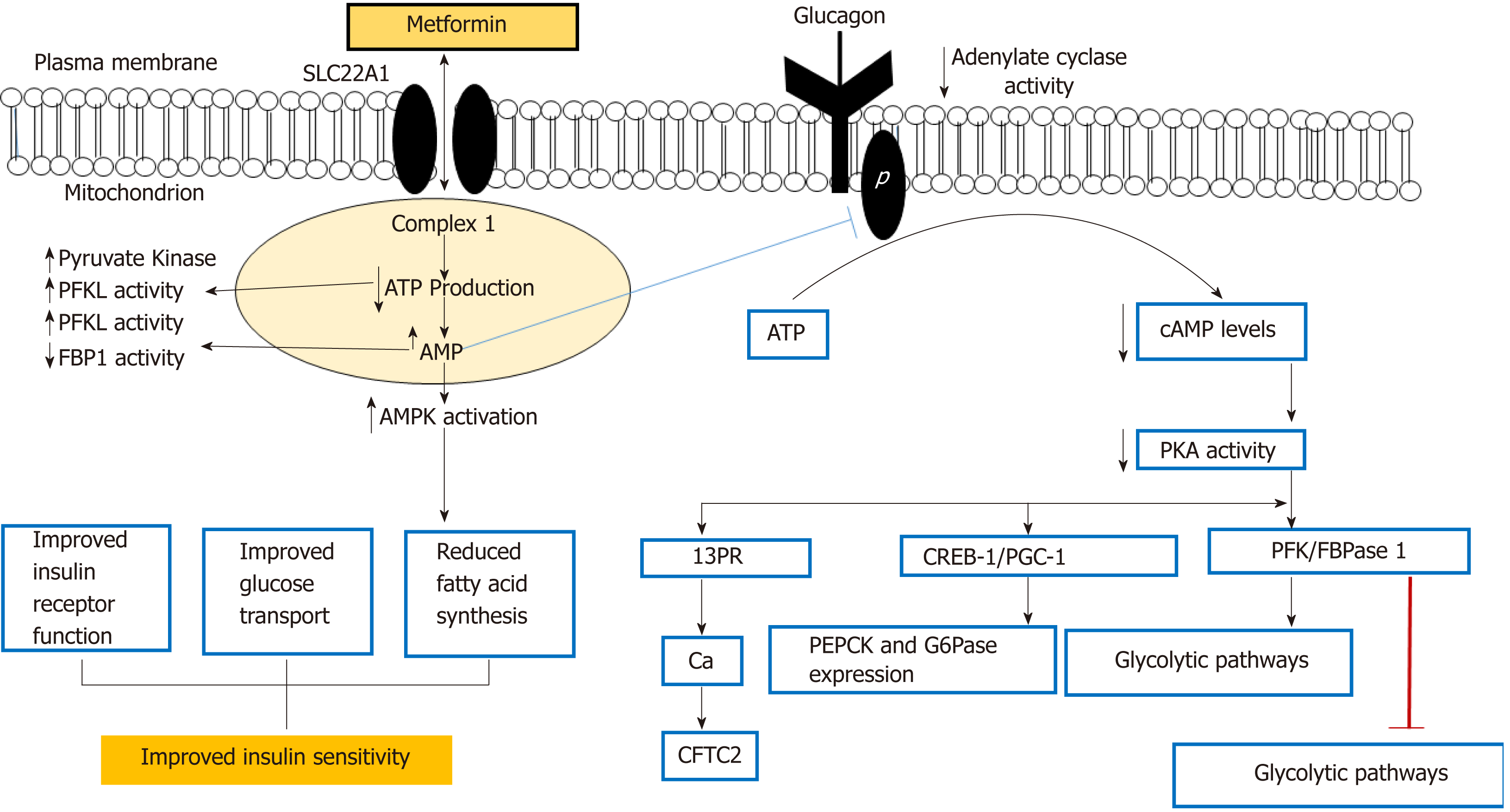
Meanwhile, metformin is known as a potent anti-hyperglycemic agent specific for type-2 diabetes, but exerts its pharmacologic actions on type-2 diabetes in routes different from any other class of drugs[15].
The apparent volume of distribution (V/F) of metformin following single oral doses of 850 mg immediate release metformin hydrochloride averaged 654 ± 358 L. Metformin is negligibly bound to plasma proteins. Metformin partitions into erythrocytes, most likely as a function of time. At usual clinical doses and dosing schedules of metformin, steady state plasma concentrations of metformin are reached within 24-48 h and are generally < 1 μg/mL during controlled clinical trials, which serve as the basis of approval for metformin. Notably, maximum metformin plasma levels did not exceed 5 μg/mL, even at maximum doses.
Intravenous single-dose studies in normal subjects demonstrate that metformin is excreted unchanged in the urine, and does not undergo hepatic metabolism or biliary excretion. Renal clearance is approximately 3.5 times greater than creatinine clearance, which indicates that tubular secretion is the major route of metformin elimination[15].
The liver is the main site of action for metformin, where it has been shown to reduce hepatic glucose output by 75%[15], and reduce hepatic glucose production mainly by inhibiting liver gluconeogenesis[16], which is the formation of glucose by the liver from non-carbohydrate sources, such as amino acids. In addition, the uptake of gluconeogenic substrates (alanine and lactate) is reduced by metformin[15]. Insulin sensitivity is increased in skeletal muscles because of an increase in the tyrosine kinase activity of the insulin receptor along with increased GLUT1 (glucose transporter) transport activity by increasing translocation to the plasma membrane[15]. Metformin altered endocrine function in the pancreas by stimulating the expression of the glucagon-like peptide 1 (GLP-1) receptor and GLP-1 protein in the pancreas. GLP-1 is responsible for increasing the secretion of insulin and lowering the secretion levels of glucagon (a hormone that raises blood glucose levels)[15]. Metformin slightly delays the absorption of glucose through the gastrointestinal (GI) tract[15].
This short communication is put together for researchers working on type-2 diabetic drug design to explore the potential of Hunteria umbellata seed extract via this pathway (Figure 2).
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: Nigeria
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): E, E
P-Reviewer: Cheng JT, Sipos F, Tan BK S-Editor: Dou Y L-Editor: Filipodia E-Editor: Xing YX
| 1. | Herman WH, Edelstein SL, Ratner RE, Montez MG, Ackermann RT, Orchard TJ, Foulkes MA, Zhang P, Saudek CD, Brown MB. The 10-year cost-effectiveness of lifestyle intervention or metformin for diabetes prevention: an intent-to-treat analysis of the DPP/DPPOS. Diabetes Care. 2012;35:723-730. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 304] [Cited by in RCA: 327] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 2. | Alemi F, Kwon E, Poole DP, Lieu T, Lyo V, Cattaruzza F, Cevikbas F, Steinhoff M, Nassini R, Materazzi S, Guerrero-Alba R, Valdez-Morales E, Cottrell GS, Schoonjans K, Geppetti P, Vanner SJ, Bunnett NW, Corvera CU. The TGR5 receptor mediates bile acid-induced itch and analgesia. J Clin Invest. 2013;123:1513-1530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 284] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 3. | Mendenhall E, Norris SA, Shidhaye R, Prabhakaran D. Depression and type 2 diabetes in low- and middle-income countries: a systematic review. Diabetes Res Clin Pract. 2014;103:276-285. [PubMed] [DOI] [Full Text] |
| 4. | Miura T, Nosaka K, Ishii H, Ishida T. Antidiabetic effect of Nitobegiku, the herb Tithonia diversifolia, in KK-Ay diabetic mice. Biol Pharm Bull. 2005;28:2152-2154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Hodge RJ, Nunez DJ. Therapeutic potential of Takeda-G-protein-receptor-5 (TGR5) agonists. Hope or hype? Diabetes Obes Metab. 2016;18:439-443. [PubMed] [DOI] [Full Text] |
| 6. | Adeneye AA, Adeyemi OO. Hypoglycaemic effects of the aqueous seed extract of Hunteria umbellata in normoglycaemic and glucose and nicotine-induced hyperglycaemic rats. Int J Natural Products Research. 2009;2:9-18. [DOI] [Full Text] |
| 7. | Falodun A, Nworgu Z, Ikponmwonsa MO. Phytochemical components of Hunteria umbellata (K. Schum) and its effect on isolated non-pregnant rat uterus in oestrus. J Pharm Sci. 2006;19:256-258. |
| 8. | Igbe I, Omogbai EKI, Ozolua RI. Hypoglycaemic activity of aqueous seed extract of Hunteria umbellata in normal and streptozotocin-induced diabetic rats. Pharm Biol. 2009;47:1011-1016. [DOI] [Full Text] |
| 9. | Adeneye AA, Adeyemi OO, Agbaje EO. Anti-obesity and antihyperlipidaemic effect of Hunteria umbellata seed extract in experimental hyperlipidaemia. J Ethnopharmacol. 2010;130:307-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Adeneye AA, Adeyemi OO, Agbaje EO, Banjo AA. Evaluation of the toxicity and reversibility profile of the aqueous seed extract of Hunteria umbellata (K. Schum.) Hallier f. in rodents. Afr J Tradit Complement Altern Med. 2010;7:350-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 11. | Ajibola OOE, Akinmegha TS, Nwaiwu O, Balogun OJ. Biometric Analysis of Hunteria umbellata (K.Schum.) Hallier f and Metformin in the treatment of diabetes. J Appl Sci Environ Manage. 2018;22:561-564. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 12. | Boone MJ, Schmelzer GH. Hunteria umbellata (K. Schum.) Hallier f. Schmelzer GH. Gurib-Fakim A. editors. Prota 11, Medicinal plants/Plantes médicinales (CD-ROM). Wageningen: PROTA; 2006; . |
| 13. | Adeneye AA, Crooks PA, Fadhel-Albayati Z, Miller AF, Zito SW, Adeyemi OO, Agbaje EO. Antihyperglycemic profile of erinidine isolated from Hunteria umbellata seed. Afr J Tradit Complement Altern Med. 2013;10:189-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Ajiboye TO, Hussaini AA, Nafiu BY, Ibitoye OB. Aqueous seed extract of Hunteria umbellata (K. Schum.) Hallier f. (Apocynaceae) palliates hyperglycemia, insulin resistance, dyslipidemia, inflammation and oxidative stress in high-fructose diet-induced metabolic syndrome in rats. J Ethnopharmacol. 2017;198:184-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 15. | Pernicova I, Korbonits M. Metformin--mode of action and clinical implications for diabetes and cancer. Nat Rev Endocrinol. 2014;10:143-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 785] [Cited by in RCA: 940] [Article Influence: 85.5] [Reference Citation Analysis (0)] |
| 16. | Foretz M, Hébrard S, Leclerc J, Zarrinpashneh E, Soty M, Mithieux G, Sakamoto K, Andreelli F, Viollet B. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J Clin Invest. 2010;120:2355-2369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 990] [Cited by in RCA: 955] [Article Influence: 63.7] [Reference Citation Analysis (0)] |