Published online Nov 28, 2019. doi: 10.13105/wjma.v7.i9.406
Peer-review started: August 9, 2019
First decision: September 19, 2019
Revised: October 3, 2019
Accepted: October 20, 2019
Article in press: October 20, 2019
Published online: November 28, 2019
Processing time: 114 Days and 16 Hours
Esophagectomy is considered the primary form of management for esophageal adenocarcinoma (EAC); however, the surgery is associated with high rates of morbidity and mortality. For patients with early-stage EAC, endoscopic resection (ER) presents a potential curative treatment option that is less invasive and carries fewer risks procedure related risks, but it is associated with higher rates of cancer recurrence following the procedure. For some patients, age and comorbidities may prevent them from having esophagectomy as a treatment option, while other patients may be operative candidates but do not wish to undergo esophagectomy for a variety of reasons related to their values and preferences. Furthermore, while anxiety of cancer recurrence following ER may significantly diminish a patient’s quality of life (QOL), so might the morbidity surrounding esophagectomy. In addition to considering health status, patient preferences, and impacts on QOL, physicians and patients must also consider what treatments would be both beneficial and available to the patient, considering esophagectomy methods-minimally invasive vs open-or the use of chemoradiotherapy in addition to ER. Our article reviews and summarizes available treatment options for patients with early EAC and their potential effects on the health and wellbeing of patients based on the current data. We conclude with a request for more research of available options for early EAC patients, the conditions that determine when each option should be employed, and their effects not only on patient health but also QOL.
Core tip: This paper is an important source of information for patients and clinicians faced with a diagnosis of T1b esophageal adenocarcinoma (T1b EAC). This paper explores and then outlines the potential benefits and risks of the numerous treatment options for T1b EAC, highlighting the integral role a patient’s individual wishes and values play into making a treatment decision that achieves the greatest outcome for that patient. The review advocates for further research regarding the effects of T1b EAC treatment options on a patient’s quality of life.
- Citation: Kumble LD, Silver E, Oh A, Abrams JA, Sonett JR, Hur C. Treatment of early stage (T1) esophageal adenocarcinoma: Personalizing the best therapy choice. World J Meta-Anal 2019; 7(9): 406-417
- URL: https://www.wjgnet.com/2308-3840/full/v7/i9/406.htm
- DOI: https://dx.doi.org/10.13105/wjma.v7.i9.406
Esophageal carcinoma is the eighth most common cancer and the sixth most deadly cancer worldwide[1]. While esophageal squamous-cell carcinoma (SCC) is the most prevalent type of esophageal carcinoma globally, esophageal adenocarcinoma (EAC) has a higher incidence than SCC within the United States and much of the Western world[2]. The incidence rate of EAC has increased rapidly since the 1970s, which has corresponded with a rise in the incidence of risk factors for EAC such as obesity, a high-fat diet, chronic gastroesophageal reflux, and Barrett’s esophagus (BE)[3-5]. For the purposes of this review, we focus primarily on treatments for early (T1) EAC.
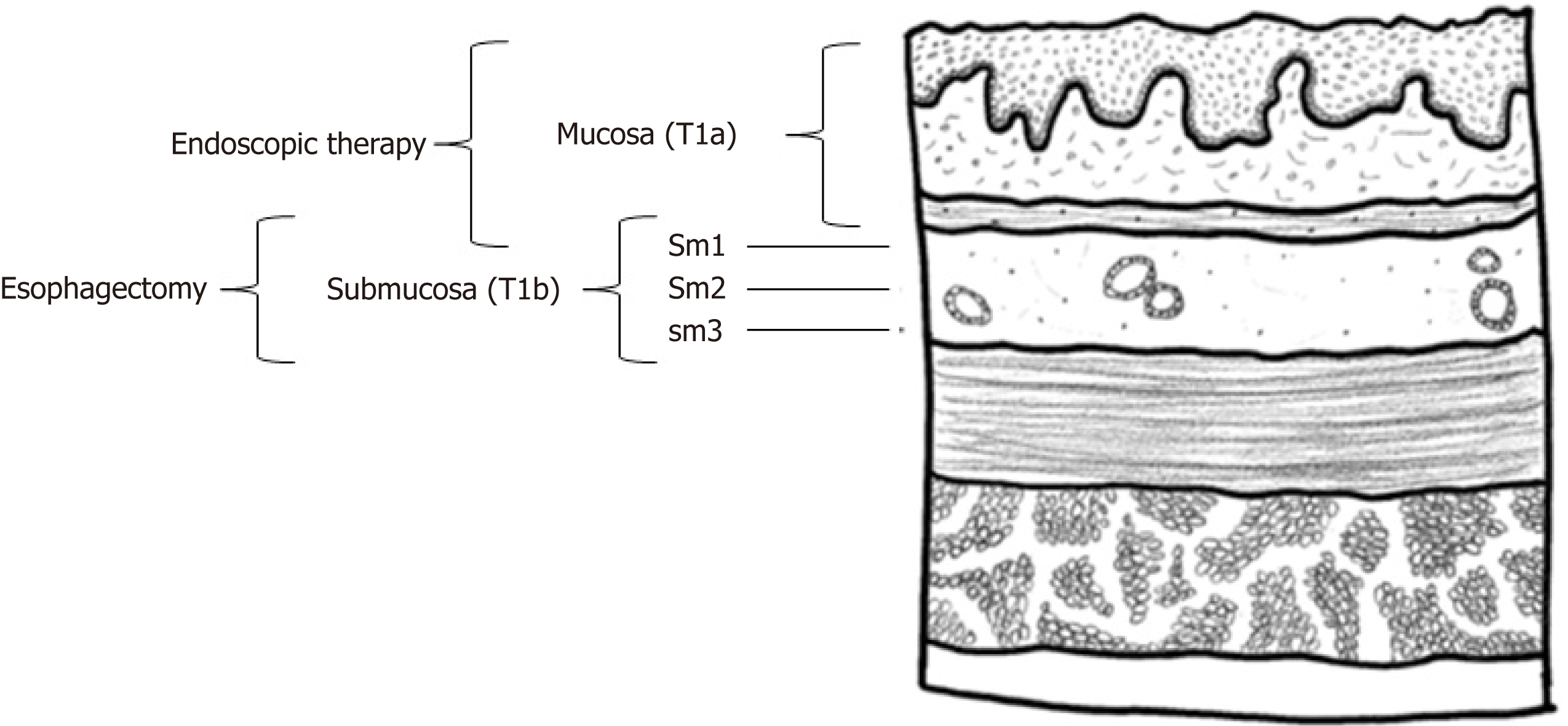
Esophageal cancer is staged by tumor-node-metastasis based on depth of invasion of the primary tumor, lymph node involvement, and extent of metastatic disease (see Figure 1)[6,7]. Tumor staging in T1 EAC is further subdivided and reflects the increasing likelihood of lymph node involvement with increasing lesion depth[7,8]. Specifically, T1 EAC is divided into Tis (carcinoma in situ; also known as high-grade dysplasia), T1a (confined to the mucosa, without lympho-vascular involvement), and T1b (involvement of submucosa)[9-11]. T1b can be substaged further into T1bsm1, T1bsm2, and T1bsm3, each of which represent further invasion into the submucosa, although the reliability of correctly substaging T1b tumors is limited by the precision and accuracy of staging techniques[12]. Despite the rising incidence of EAC in recent years, 5-year survival rates have improved for a sub-set of patients, specifically those diagnosed with early-stage EAC, for whom the cancer is confined only to the esophagus[13]. This suggests the importance of examining which treatment modalities for T1 EAC confer the most benefit relative to the trade-off between quality of life (QOL) and risk of recurrence.
Until recently, esophagectomy was recommended for all stages of EAC tumors; however, given high rates of morbidity (30%-50%) and mortality (1%-10%) associated with esophagectomy, less aggressive treatment such as endoscopic resection (ER) are increasingly utilized, especially for T1a tumors[7,8,10,14]. For patients considering esophagectomy, minimally invasive esophagectomy (MIE) is becoming a more frequently used alternative to the traditional and morbid open esophagectomy (OE). MIE is associated with fewer respiratory complications, intensive care unit stays, faster recovery time, and improved patient satisfaction compared to OE; however, the procedure requires an experienced surgeon due to its steep learning curve, and the clinical relevance of MIE’s benefits relative to OE has been contested[15-17]. By contrast, endoscopic therapies offer a promising alternative for patients with stage T1a and, potentially, T1bsm1 lesions[7,8,10,18]. In fact, according to National Comprehensive Cancer Network 2018 guidelines, endoscopic resection (ER) is the preferred treatment for patients with T1a and T1bsm1 adenocarcinoma without signs of lymph node involvement[19]. ER may also offer an alternative for patients ineligible for surgery due to advanced age and/or high comorbidity, or those who wish to pursue a less invasive treatment[10,18,20,21]. Aligned with this view, a recent decision analysis found that for T1b patients older than 70 or patients with high comorbidity, ER, rather than esophagectomy, is the most cost-effective treatment[22]. However, ER therapies cannot address nodal involvement and therefore carry greater risk of incomplete resection for patients with T1bsm2, T1bsm3, or later tumors, which are more likely to involve lymph nodes[7]. Additionally, it is challenging to assess submucosal substaging using ER. A full thickness resection of the submucosa is necessary to accurately identify the depth of submucosal invasion; ER resections often do not include the full submucosa, and when they do, these specimens may be compromised by saline injection and resection[23].
Several factors complicate treatment decision-making. Age and age-related comorbidities must be considered as 30% of EAC patients are 75 and older at the time of diagnosis[24]. Concerns about treatment morbidity and negative side effects are important factors for patients regardless of age[25]. Imaging techniques such as computerized tomography, positron-emission tomography scan, and endoscopic ultrasound (EUS), run the risk of understaging T1 and T2 lesions, which should be considered when weighing treatment options with varying levels of risk of recurrence[6,11,25,26]. The extant literature, reviewed in detail below, suggests that the choice of treatment for early-stage (particularly T1b) EAC patients is highly complex; it depends not only on stage and sub-stage, but also on patient preferences for treatment aggressiveness versus cancer risk, and patient characteristics such as age and comorbidity. Table 1[16,22,25,27-29] summarizes the different treatment options for patients with T1 EAC.
| Treatment option | Potential benefits | Potential disadvantages |
| Endoscopic resection with or without radiofrequency ablation | Organ preserving; very low mortality; low morbidity; small, transient effect on quality of life | Does not address potential lymph node metastases; higher risk of recurrence and lower rate of complete response (particularly in T1b patients) |
| Esophagectomy | High curative rate; higher disease related survival rate; lower recurrence rates; addresses lymph node metastases | High rates of early and long-term morbidity; considerable rates of surgical mortality; large decrement in quality of life; high post-operative pain; complicated surgical procedure with high operative times and financial costs |
Due to the variability in outcomes associated with different treatment modalities, decisions in this realm are preference-sensitive; that is, decision-making is contingent on the intersection of patients’ values and understandings of treatment options[30]. As such, the primary aim of this review is to overview the current landscape of early-stage EAC treatment options, including their associated risks of cancer recurrence and morbidity.
As a diagnostic technique, ER is more accurate and precise at staging early EAC than imaging methods such as EUS, reducing the risk of under-or over-staging the disease[31]. ER is a crucial component of treatment decisions for early EAC, as treatment plans are highly dependent on tumor staging and appearance, a topic more thoroughly addressed below.
Curative ER is an option for patients at low risk of lymph node involvement. These patients are characterized by having no or minimal submucosal invasion, no lympho-vascular involvement, negative deep margins, and well- to moderately-differentiated tumor biology; the majority of patients with these characteristics are staged as T1a[32,33]. ER offers these patients a potentially curative treatment while preserving the esophagus. For later-stage patients, ER serves primarily as a staging procedure, more accurate than available imaging methods[34]. In T1a patients, the risk of lymph node metastases is comparable to the mortality rate associated with esophagectomy, suggesting that esophagectomy is not appropriate for this population[9]. Additionally, ER has been shown to yield local control rates exceeding 95% in T1a patients, with survival times comparable to age and sex-matched adults without cancer[7]. A decision analysis found that ER resulted in more quality-adjusted life years than esophagectomy for T1a patients regardless of age or comorbidity, but for T1b patients, ER was only cost-effective relative to esophagectomy for older patients or patients with high comorbidity[22]. Thus, evidence suggests that endoscopic therapy is an acceptable first-line treatment with curative intent for T1a patients.
The low (0%-3.9%) risk of nodal metastases for T1a tumors is sufficient to justify using ER as a primary curative treatment, which is up to 98% effective for patients with Barrett’s Esophagus (BE) and early neoplasia[35-37]. T1b tumors, by contrast, carry a 20% risk of lymph node metastasis, indicating that ER alone leaves a patient at considerable risk of unaddressed lymph node metastasis and cancer progression[36,37].
The mortality and morbidity associated with esophagectomy may reach unacceptable levels even for those with riskier T1b lesions, especially older patients with comorbidities. Consequently, T1b patients unwilling or unable to undergo esophagectomy may elect to undergo ER. While ER is ineffective for T1b patients with lymph node metastases, it can potentially be curative for T1b patients without nodal involvement.
Lymph node metastasis is a robust predictor of recurrence and disease-specific survival, and tumor depth is a predictor of lymph node metastasis[14,38]. A study (n = 69) of T1b patients found that deeper submucosal invasion was associated with poorer outcomes from ER[39]. Of those at low risk of nodal involvement (T1bsm1) managed with radical ER, there was only one local recurrence; this recurrence was treated with repeat ER, suggesting that ER is a viable option for T1b patients with minimal submucosal invasion. However, of the 30 high-risk patients with deeper submucosal invasion who opted for ER, six developed metastatic disease during follow-up, and five died of cancer. Among higher-risk patients who did undergo esophagectomy, two of 25 patients went on to develop metastases and die of cancer. Because a greater portion of high-risk patients developed metastatic cancer under ER than under surgical management, the authors concluded that esophagectomy remains the optimal treatment for T1b tumors with deep invasion of the submucosa[39].
Another study (n = 107) also demonstrated the curative potential of ER for EAC patients with only mucosal or superficial submucosal involvement and found a 5-year recurrence-free rate of 97% and only one case of lymph node metastasis. However, 18 out of 41 patients with T1bsm2-3 tumors showed lymph node metastases and only 57% were recurrence-free after 5 years[40]. These data indicate that, on one hand, ER is a valuable curative treatment for T1bsm1 patients with lower morbidity and mortality rates than esophagectomy, but, on the other hand, ER potentially confers higher cancer risk.
For some T1bsm1 patients, the QOL benefit of organ-preserving ER may be worth the potential risk of untreated nodal metastasis, assuming initial staging and substaging were correct. However, once tumor depth reaches T1bsm2-3, rates of lymph node metastases and cancer recurrence become much higher, and esophagectomy should be the primary form of curative treatment[40]. It is important to reiterate that accurate, precise EAC staging and substaging of T1a and T1b is difficult[6,11,25,26]. Consequently, the risk of potentially understaging patients should be communicated by the clinician and considered when deciding between conservative ER and esophagectomy, as patients may desire a more aggressive treatment when presented with the possibility that their cancer may be understaged.
As previously mentioned, tumor depth is not the only predictor of lymph node involvement; there are other factors to consider when determining risk of lymph node metastases, including tumor morphology, histologic grade, and lymphovascular invasion[41,42]. In one study (n = 85), T1b tumors were grouped according to depth of submucosal invasion in conjunction with histological grade and lymphovascular invasion. Patients with tumors that were well- or moderately-differentiated and had no lymphovascular invasion had rates of overall and disease-specific survival closer to T1a tumors than to T1b tumors with poor differentiation and T1b tumors with lymphovascular invasion[43].
Another study (n = 66), examined the effects of ER on T1bsm1 patients defined as low risk, characterized by having macroscopically polypoid or flat lesions and good to moderate tumor differentiation (G1-G2), and found that out of the 61 patients whose remissions were assessed, 53 achieved complete endoluminal remission (CER) and fifty-one achieved long-term remission[44]. When focal lesions were smaller than 2 cm, the CER rate jumped from 87% to 97%. There were no associated tumor deaths. The rate of major complications from ER was 1.5% with a 0% mortality rate, and biopsy and imaging results showed that only one patient had lymph node metastases[44]. Ishihara et al.[45] found no metastasis in any of the 32 T1b lesions smaller than 30 mm without lymphovascular involvement and a poorly differentiated component in their study of the risk of metastasis of EAC, concluding such lesions as good candidates for ER.
These studies highlight that T1b tumors have similar prognostic outcomes to T1a tumors if their histology and morphology do not indicate nodal involvement. Furthermore, they identify the importance of considering multiple tumor characteristics to determine the risk of lymph node metastasis. As previously stated, ER techniques may not always accurately stage submucosal involvement; therefore, examining multiple tumor characteristics to inform tumor classification and treatment decisions is important[23].
Despite the benefits associated with organ preservation, the impact of fear of cancer recurrence on QOL is a factor to consider for T1b patients eligible for ER. One study (n = 91) examined QOL and fear of cancer recurrence following endoscopic versus surgical treatment for early-stage EAC (defined as BE with high-grade dysplasia, T1, T1sm, and T2N0M0 tumors) and found no differences in health-related or cancer-specific QOL between treatment groups[46]. While the surgical group reported significantly more reflux and eating problems, the ER group reported greater fear of cancer recurrence and anxiety[46]. The authors of the study noted that leaving the esophagus intact may prompt cancer anxiety among patients treated with ER and especially among T1b patients, for whom the chance of incomplete eradication and nodal metastases are higher[27]. Additionally, a study (n = 20) eliciting health state utility values associated with dysplastic BE-related health states from non-dysplastic BE patients reported that the utility of states associated with potential cancer recurrence were comparable to the utility associated with esophagectomy[47]. Along these lines, a decision analysis comparing ER and esophagostomy outcomes for T1 EAC patients found that the optimal treatment strategy depended most heavily on the post-treatment health statue utility values, indicating that for patients with T1b EAC, treatment decisions should be centered around patient preferences[22]. Together, these studies suggest that patient perceptions of and preferences for cancer risk are important to consider when making treatment decisions for T1b EAC.
Patients who have early-stage EAC that has developed within a large segment of BE have the option of ER followed by radiofrequency ablation (RFA) or other ablative therapies to eliminate the remaining BE[34,48-50]. Ablative techniques are important as they reduce the risk of metachronous neoplasia developing in residual BE[51].
Research with BE patients suggests that RFA is an effective method for eradicating areas with dysplasia or early intramucosal adenocarcinoma[52]. Although RFA carries a 7.8%-11.3% risk of adverse events (predominantly strictures and bleeding) these events are often low grade, with a low rate (0.6%) of severe adverse events (e.g. perforation)[53,54]. The impact of RFA on QOL in EAC patients has not been specifically studied. However, one study reported the effects of RFA on patients with dysplastic BE and found that performing RFA improved QOL by reducing anxiety about developing cancer, worry about needing an esophagectomy, stress, impact on daily QOL, dissatisfaction with the condition of their esophagus, and impact on work and family life[55]. These findings indicate a potential benefit of RFA for patients with T1a or T1bsm1 EAC and for patients with T1bsm2 or sm3 EAC who are unable or unwilling to undergo esophagectomy.
Research suggests cryotherapy is also an effective method of eradication of dysplasia[50,56]. Cryotherapy is also utilized as treatment for patients with EAC who are unwilling or unable to undergo more aggressive treatments[57-60]. A 2017 analysis of the safety and efficacy of liquid nitrogen spray for EAC patients who were not candidates for conventional therapy demonstrated complete response rates for 76.3% of T1a patients, 45.8% of T1b patients, and 66.2% for all T1 patients, with a low rate of low-grade strictures (13.6%)[57]. A 2013 assessment of patients ineligible for conventional EAC therapy found that 75% of patients with T1a EAC and 60% of patients with T1b EAC showed complete endoscopic response, with benign strictures occurring in 13% of patients[58]. These data suggest that cryotherapy is tolerable and effective for T1 EAC patients who cannot undergo conventional treatments; however, data regarding long-term outcomes are lacking[59,60].
Esophagectomy is the mainstay of treatment for resectable esophageal cancer and is indicated for T1b tumors with more than minimal submucosal invasion or later-stage tumors due to increased risk of nodal involvement[9,10]. The pain and discomfort experienced by patients after surgery has a significant negative effect on a patient’s QOL[61]. Specifically, a 2014 meta-analysis found significant and lasting detrimental effects of surgery on QOL: Social functioning, fatigue, pain, reflux, dyspnea, and coughing problems were significantly worse than pre-operation for at least nine to twelve months after surgery, at times extending beyond one year[62].
Regardless of the modality for esophagectomy (e.g., open or minimally-invasive), optimal surgical outcomes occur in high-volume centers with highly-skilled and well-practiced surgeons. Esophagectomy in high-volume surgical centers reduces the morbidity, mortality, length of hospital stay, and cost of the procedure[63]. Additionally, the long-term prognosis of a patient following the surgery is associated with the case-volume of the surgical center, with a higher volume predicting a better prognosis[64,65]. These data indicate that, when possible, patients may benefit from assuming the additional burden of seeking high-volume hospitals with highly experienced staff and the necessary resources to prevent and manage potential complications.
Treatment esophagectomy consists of two primary surgical techniques: OE and MIE. MIE is performed laparoscopically or thoracoscopically, where access to the abdominal and thoracic cavity is granted via small abdominal incisions. OE, on the other hand, requires a right thoracotomy and laparotomy, which involves large incisions where the ribs and abdominal wall are opened widely. Both OE and MIE carry significant risk of complications; one meta-analysis found complication rates of 48.2% and 41.5% in patients undergoing OE and MIE, respectively[66]. Although each of these rates are high, the difference between the two was significant and favored MIE. This meta-analysis also found a post-operative mortality risk that again significantly favored MIE, with an average mortality risk of 3.8% and 4.5% for MIE and OE respectively[66]. MIE has also been shown to result in superior short-term outcomes relative to OE, with reduced blood loss, fewer pulmonary and respiratory complications, lower total morbidity rates, and shorter post-operative hospital stays[66-71]. Owing to the complexity of the procedure, the operative time for MIE is, however, longer than that of OE[16]. MIE and OE have not been shown to differ in oncologic outcomes, with comparable lymph node retrieval and overall survival[72].
Regarding the impact of each procedure on QOL, a systematic review comparing MIE and OE found that, while overall health and social and emotional function more frequently improved following MIE relative to OE, other QOL outcomes were comparably and negatively associated with both surgery types[73]. Another meta-analysis found that MIE patients reported higher QOL than OE patients immediately after surgery, but evidence for this disparity was less robust 1-year post-operation[74]. Thus, while MIE may not have as large of an immediate decrement on QOL, MIE, like OE, remains an aggressive procedure that carries risks and can produce complications.
Important to note is the varying accessibility of MIE versus OE. Although there is evidence to suggest superiority of MIE over OE, the choice between surgical techniques may not be available to some patients because of geographic and logistical barriers such as the steep learning curve of MIE and the low availability of surgical tools necessary for MIE[16,75,76]. Therefore, OE may be the only option for patients who do not have access to centers equipped for MIE or do not have the resources to seek such centers.
As mentioned above, ER is primarily recommended for T1a patients due to concerns regarding the increased rate of lymph node metastases following submucosal invasion[28]. Lymph node metastases can be addressed through lymphadenectomy during esophagectomy and/or chemoradiotherapy (CRT). CRT alone is sometimes used as a non-operative treatment in place of surgery for older patients with locally advanced EAC who are unable, unwilling, or not referred to undergo esophagectomy despite the risk of nodal involvement. However, current data show that, compared with esophagectomy, definitive CRT for stage I-III EAC patients is ineffective[77-83]. Additionally, CRT’s impact on QOL and the differences in severe adverse events relative to esophagectomy have not been well documented. With this in mind, ER in combination with chemotherapy or CRT warrants further exploration as a potential organ-preserving alternative to esophagectomy that can address lymph node metastases, particularly in patients who are older and/or have comorbid conditions who are poor operative candidates[84].
Existing research regarding CRT + ER is limited for early-stage EAC patients. Minashi et al[85] found that ER and selective CRT provided to patients with T1b (sm1-2) resulted in a 3-year survival rate of 90.7%, which is comparable to that of surgery. A review of six studies (n = 168) in which all patients had superficial esophageal SCC treated with CRT + ER found promising rates of control of local recurrence following treatment, ranging from 0%-9%, and 3-year overall survival rates ranging from 87%-100%[86]. Patients who developed metachronous esophageal lesions after ER and adjuvant CRT were all successfully treated with salvage ER[86]. The major limitation of these findings is that the patients in this review had SCC, which tends to have a better response to CRT than EAC; therefore, these results alone cannot be used to justify the use of ER and CRT for T1 EAC[82]. Another study (n = 32) compared outcomes of ER alone, CRT + ER, and esophagectomy in patients with T1b EAC[87]. This study found an EAC recurrence rate of 11% with CRT + ER, compared to a 38% EAC recurrence rate with ER alone, and a 29% EAC recurrence rate with esophagectomy. Although there was a trend toward better outcomes for CRT + ER, differences in EAC recurrence rates were not statistically significant, potentially due to a lack of statistical power; however, these findings suggest that CRT + ER could be a viable treatment option for T1b EAC patients unable or unwilling to undergo esophagectomy[87]. Another report assessed the efficacy of salvage ER following CRT in two patients with T2N0M0 EAC who were unfit for esophagectomy[88]. Both patients achieved complete endoscopic and histological remission after removing residual lesions with ER following treatment with CRT regimen. While this sample size is too small to generalize the results to the early EAC patient population, these results suggest the utility of future work examining the efficacy of CRT + ER in early-stage EAC patients[88].
Another avenue of research with promising therapeutic potential is the identification of prognostic and predictive biomarkers of EAC and tailored treatment plans that target these biomarkers. These targeted therapies work by acting on molecular characteristics of a patient’s tumor, rather than applying a systemic conventional chemotherapy. Prognostic biomarkers of overall survival for patients with EAC have been identified, and include SPARC, SPP1, and MET gene expression, COX-2 angiogenic factor expression, and HER2 positivity[89-92]. Some molecular profiles are more common in EACs than others; EGFR (16% of EACs), HER2 (19% of EACs), and MET (6% of EACs), some of the more common biomarkers in EAC, have available targeted therapies, although they have mostly been explored in adenocarcinoma of the gastroesophageal junction[93]. HER2 positivity and EGFR overexpression are prognostic biomarkers, the presence of which indicate a poorer EAC prognosis as they promote cancer growth[94,95]. Patients with HER2 positivity are considered for treatment with trastuzumab, which acts on HER2 cells to inhibit tumor cell growth[96]. Those with EGFR overexpression may be candidates for treatment with cetuximab with chemotherapy, which has shown a trend toward improved survival relative to chemotherapy alone[93]. Because targeted therapies work only on cells that express a given biomarker, therapies such as trastuzumab and cetuximab are most likely to yield positive outcomes only in the subset of EAC patients with HER2 positivity and EGFR overexpression, respectively[93,97]. The efficacy of these targeted treatments, which offer the benefits of superior outcomes for a subset of patients and lower toxicity than conventional CRT, merit further study as a potential definitive and/or neoadjuvant treatment for EAC patients.
A thorough understanding of available treatment options for early-stage EAC and their effects on survival, health, and QOL is paramount for informing treatment-related decisions and improving patient outcomes. Many patients diagnosed with early-stage EAC are older, have comorbid conditions, or are eligible to undergo esophagectomy but choose not to have it. Future research concerning these patients’ preferences and effects of different treatments on QOL are warranted. Additional exploration of T1b tumor characteristics that can accurately predict whether the tumors are at high or low risk of lymph node metastasis would also aid in treatment choice optimization for T1b patients. The potential for the combination of ER and CRT to provide an effective, organ-preserving treatment for some early-stage EAC patients is important and requires further investigation.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Hu B, Nishida T, Tsuji Y S-Editor: Zhang L L-Editor:A E-Editor: Xing YX
| 1. | Liu S, Dai JY, Yao L, Li X, Reid B, Self S, Ma J, Chang Y, Feng S, Tapsoba Jde D, Sun X, Sun X. Esophageal Adenocarcinoma and Its Rare Association with Barrett's Esophagus in Henan, China. PLoS One. 2014;9:e110348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 2. | Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381:400-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1956] [Cited by in RCA: 1961] [Article Influence: 163.4] [Reference Citation Analysis (5)] |
| 3. | Lagergren J, Bergström R, Nyrén O. Association between body mass and adenocarcinoma of the esophagus and gastric cardia. Ann Intern Med. 1999;130:883-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 480] [Cited by in RCA: 441] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 4. | Mayne ST, Navarro SA. Diet, obesity and reflux in the etiology of adenocarcinomas of the esophagus and gastric cardia in humans. J Nutr. 2002;132:3467S-3470S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 96] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 5. | Veugelers PJ, Porter GA, Guernsey DL, Casson AG. Obesity and lifestyle risk factors for gastroesophageal reflux disease, Barrett esophagus and esophageal adenocarcinoma. Dis Esophagus. 2006;19:321-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 129] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 6. | Berry MF. Esophageal cancer: staging system and guidelines for staging and treatment. J Thorac Dis. 2014;6 Suppl 3:S289-S297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 77] [Reference Citation Analysis (0)] |
| 7. | Rubenstein JH, Shaheen NJ. Epidemiology, Diagnosis, and Management of Esophageal Adenocarcinoma. Gastroenterology. 2015;149:302-17.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 262] [Article Influence: 26.2] [Reference Citation Analysis (1)] |
| 8. | Rice TW, Blackstone EH, Rybicki LA, Adelstein DJ, Murthy SC, DeCamp MM, Goldblum JR. Refining esophageal cancer staging. J Thorac Cardiovasc Surg. 2003;125:1103-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 127] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 9. | Gamboa AM, Kim S, Force SD, Staley CA, Woods KE, Kooby DA, Maithel SK, Luke JA, Shaffer KM, Dacha S, Saba NF, Keilin SA, Cai Q, El-Rayes BF, Chen Z, Willingham FF. Treatment allocation in patients with early-stage esophageal adenocarcinoma: Prevalence and predictors of lymph node involvement. Cancer. 2016;122:2150-2157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Merkow RP, Bilimoria KY, Keswani RN, Chung J, Sherman KL, Knab LM, Posner MC, Bentrem DJ. Treatment trends, risk of lymph node metastasis, and outcomes for localized esophageal cancer. J Natl Cancer Inst. 2014;106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 111] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 11. | Worrell SG, Alicuben ET, Oh DS, Hagen JA, DeMeester SR. Accuracy of Clinical Staging and Outcome With Primary Resection for Local-Regionally Limited Esophageal Adenocarcinoma. Ann Surg. 2018;267:484-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Mocanu A, Bârla R, Hoara P, Constantinoiu S. Current endoscopic methods of radical therapy in early esophageal cancer. J Med Life. 2015;8:150-156. [PubMed] |
| 13. | Hur C, Miller M, Kong CY, Dowling EC, Nattinger KJ, Dunn M, Feuer EJ. Trends in esophageal adenocarcinoma incidence and mortality. Cancer. 2013;119:1149-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 397] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 14. | Rice TW, Zuccaro G, Adelstein DJ, Rybicki LA, Blackstone EH, Goldblum JR. Esophageal Carcinoma: Depth of Tumor Invasion Is Predictive of Regional Lymph Node Status. The Annals of thoracic surgery. 1998;65:787-792. [RCA] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 260] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 15. | Luketich JD, Alvelo-Rivera M, Buenaventura PO, Christie NA, McCaughan JS, Litle VR, Schauer PR, Close JM, Fernando HC. Minimally invasive esophagectomy: outcomes in 222 patients. Ann Surg. 2003;238:486-94; discussion 494-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 707] [Cited by in RCA: 640] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 16. | Sihag S, Kosinski AS, Gaissert HA, Wright CD, Schipper PH. Minimally Invasive Versus Open Esophagectomy for Esophageal Cancer: A Comparison of Early Surgical Outcomes From The Society of Thoracic Surgeons National Database. Ann Thorac Surg. 2016;101:1281-8; discussion 1288-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 162] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 17. | Yerokun BA, Sun Z, Yang CJ, Gulack BC, Speicher PJ, Adam MA, D'Amico TA, Onaitis MW, Harpole DH, Berry MF, Hartwig MG. Minimally Invasive Versus Open Esophagectomy for Esophageal Cancer: A Population-Based Analysis. Ann Thorac Surg. 2016;102:416-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 123] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 18. | Prasad GA, Wu TT, Wigle DA, Buttar NS, Wongkeesong LM, Dunagan KT, Lutzke LS, Borkenhagen LS, Wang KK. Endoscopic and surgical treatment of mucosal (T1a) esophageal adenocarcinoma in Barrett's esophagus. Gastroenterology. 2009;137:815-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 255] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 19. | Barret M, Prat F. Diagnosis and treatment of superficial esophageal cancer. Ann Gastroenterol. 2018;31:256-265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Ginsberg GG. Endoscopic approaches to Barrett's oesophagus with high-grade dysplasia/early mucosal cancer. Best Pract Res Clin Gastroenterol. 2008;22:751-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Kothari S, Kaul V. Endoscopic Mucosal Resection and Endoscopic Submucosal Dissection for Endoscopic Therapy of Barrett's Esophagus-related Neoplasia. Gastroenterol Clin North Am. 2015;44:317-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Chu JN, Choi J, Tramontano A, Morse C, Forcione D, Nishioka NS, Abrams JA, Rubenstein JH, Kong CY, Inadomi JM, Hur C. Surgical vs Endoscopic Management of T1 Esophageal Adenocarcinoma: A Modeling Decision Analysis. Clin Gastroenterol Hepatol. 2018;16:392-400.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Namasivayam V, Wang KK, Prasad GA. Endoscopic mucosal resection in the management of esophageal neoplasia: current status and future directions. Clin Gastroenterol Hepatol. 2010;8:743-54; quiz e96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Zeng Y, Liang W, Liu J, He J, Ng CSH, Liu CC, Petersen RH, Rocco G, D'Amico T, Brunelli A, Chen H, Zhi X, Dong X, Wang W, Cui F, Xiao D, Wang W, Yang W, Pan H, He J; written on behalf of the AME Thoracic Surgery Collaborative Group. Esophageal cancer in elderly patients: a population-based study. J Thorac Dis. 2018;10:448-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 25. | Puts MT, Tapscott B, Fitch M, Howell D, Monette J, Wan-Chow-Wah D, Krzyzanowska M, Leighl NB, Springall E, Alibhai SM. A systematic review of factors influencing older adults' decision to accept or decline cancer treatment. Cancer Treat Rev. 2015;41:197-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 188] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 26. | Napier KJ, Scheerer M, Misra S. Esophageal cancer: A Review of epidemiology, pathogenesis, staging workup and treatment modalities. World J Gastrointest Oncol. 2014;6:112-120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 499] [Cited by in RCA: 596] [Article Influence: 54.2] [Reference Citation Analysis (4)] |
| 27. | Ell C, May A, Pech O, Gossner L, Guenter E, Behrens A, Nachbar L, Huijsmans J, Vieth M, Stolte M. Curative endoscopic resection of early esophageal adenocarcinomas (Barrett's cancer). Gastrointest Endosc. 2007;65:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 342] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 28. | Sohda M, Kuwano H. Current Status and Future Prospects for Esophageal Cancer Treatment. Ann Thorac Cardiovasc Surg. 2017;23:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 148] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 29. | Zehetner J, DeMeester SR, Hagen JA, Ayazi S, Augustin F, Lipham JC, DeMeester TR. Endoscopic resection and ablation versus esophagectomy for high-grade dysplasia and intramucosal adenocarcinoma. J Thorac Cardiovasc Surg. 2011;141:39-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 30. | Stiggelbout AM, Pieterse AH, De Haes JC. Shared decision making: Concepts, evidence, and practice. Patient Educ Couns. 2015;98:1172-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 567] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 31. | Lin JL. T1 esophageal cancer, request an endoscopic mucosal resection (EMR) for in-depth review. J Thorac Dis. 2013;5:353-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 32. | Cho JW, Choi SC, Jang JY, Shin SK, Choi KD, Lee JH, Kim SG, Sung JK, Jeon SW, Choi IJ, Kim GH, Jee SR, Lee WS, Jung HY; Korean ESD Study Group. Lymph Node Metastases in Esophageal Carcinoma: An Endoscopist's View. Clin Endosc. 2014;47:523-529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 33. | Dubecz A, Kern M, Solymosi N, Schweigert M, Stein HJ. Predictors of Lymph Node Metastasis in Surgically Resected T1 Esophageal Cancer. Ann Thorac Surg. 2015;99:1879-85; discussion 1886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 34. | Moss A, Bourke MJ, Hourigan LF, Gupta S, Williams SJ, Tran K, Swan MP, Hopper AD, Kwan V, Bailey AA. Endoscopic resection for Barrett's high-grade dysplasia and early esophageal adenocarcinoma: an essential staging procedure with long-term therapeutic benefit. Am J Gastroenterol. 2010;105:1276-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 144] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 35. | Pouw RE, Gondrie JJ, Sondermeijer CM, ten Kate FJ, van Gulik TM, Krishnadath KK, Fockens P, Weusten BL, Bergman JJ. Eradication of Barrett esophagus with early neoplasia by radiofrequency ablation, with or without endoscopic resection. J Gastrointest Surg. 2008;12:1627-36; discussion 1636-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 36. | Leers JM, DeMeester SR, Oezcelik A, Klipfel N, Ayazi S, Abate E, Zehetner J, Lipham JC, Chan L, Hagen JA, DeMeester TR. The prevalence of lymph node metastases in patients with T1 esophageal adenocarcinoma a retrospective review of esophagectomy specimens. Ann Surg. 2011;253:271-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 187] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 37. | Dunbar KB, Spechler SJ. The risk of lymph-node metastases in patients with high-grade dysplasia or intramucosal carcinoma in Barrett's esophagus: a systematic review. Am J Gastroenterol. 2012;107:850-62; quiz 863. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 161] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 38. | Nigro JJ, DeMeester SR, Hagen JA, DeMeester TR, Peters JH, Kiyabu M, Campos GM, Oberg S, Gastal O, Crookes PF, Bremner CG. Node status in transmural esophageal adenocarcinoma and outcome after en bloc esophagectomy. J Thorac Cardiovasc Surg. 1999;117:960-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 111] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 39. | Schölvinck D, Künzli H, Meijer S, Seldenrijk K, van Berge Henegouwen M, Bergman J, Weusten B. Management of patients with T1b esophageal adenocarcinoma: a retrospective cohort study on patient management and risk of metastatic disease. Surg Endosc. 2016;30:4102-4113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 40. | Westerterp M, Koppert LB, Buskens CJ, Tilanus HW, ten Kate FJ, Bergman JJ, Siersema PD, van Dekken H, van Lanschot JJ. Outcome of surgical treatment for early adenocarcinoma of the esophagus or gastro-esophageal junction. Virchows Arch. 2005;446:497-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 221] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 41. | Eloubeidi MA, Desmond R, Arguedas MR, Reed CE, Wilcox CM. Prognostic factors for the survival of patients with esophageal carcinoma in the U.S.: the importance of tumor length and lymph node status. Cancer. 2002;95:1434-1443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 293] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 42. | Yamada A. Radiologic assessment of resectability and prognosis in esophageal carcinoma. Gastrointest Radiol. 1979;4:213-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 43. | Barbour AP, Jones M, Brown I, Gotley DC, Martin I, Thomas J, Clouston A, Smithers BM. Risk stratification for early esophageal adenocarcinoma: analysis of lymphatic spread and prognostic factors. Ann Surg Oncol. 2010;17:2494-2502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 44. | Manner H, Pech O, Heldmann Y, May A, Pohl J, Behrens A, Gossner L, Stolte M, Vieth M, Ell C. Efficacy, safety, and long-term results of endoscopic treatment for early stage adenocarcinoma of the esophagus with low-risk sm1 invasion. Clin Gastroenterol Hepatol. 2013;11:630-5; quiz e45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 155] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 45. | Ishihara R, Oyama T, Abe S, Takahashi H, Ono H, Fujisaki J, Kaise M, Goda K, Kawada K, Koike T, Takeuchi M, Matsuda R, Hirasawa D, Yamada M, Kodaira J, Tanaka M, Omae M, Matsui A, Kanesaka T, Takahashi A, Hirooka S, Saito M, Tsuji Y, Maeda Y, Yamashita H, Oda I, Tomita Y, Matsunaga T, Terai S, Ozawa S, Kawano T, Seto Y. Risk of metastasis in adenocarcinoma of the esophagus: a multicenter retrospective study in a Japanese population. J Gastroenterol. 2017;52:800-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 46. | Rosmolen WD, Boer KR, de Leeuw RJ, Gamel CJ, van Berge Henegouwen MI, Bergman JJ, Sprangers MA. Quality of life and fear of cancer recurrence after endoscopic and surgical treatment for early neoplasia in Barrett's esophagus. Endoscopy. 2010;42:525-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 47. | Hur C, Wittenberg E, Nishioka NS, Gazelle GS. Quality of life in patients with various Barrett's esophagus associated health states. Health Qual Life Outcomes. 2006;4:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 48. | Hvid-Jensen F, Pedersen L, Drewes AM, Sørensen HT, Funch-Jensen P. Incidence of adenocarcinoma among patients with Barrett's esophagus. N Engl J Med. 2011;365:1375-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 985] [Cited by in RCA: 979] [Article Influence: 69.9] [Reference Citation Analysis (1)] |
| 49. | Perry KA, Walker JP, Salazar M, Suzo A, Hazey JW, Melvin WS. Endoscopic management of high-grade dysplasia and intramucosal carcinoma: experience in a large academic medical center. Surg Endosc. 2014;28:777-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 50. | Shaheen NJ, Greenwald BD, Peery AF, Dumot JA, Nishioka NS, Wolfsen HC, Burdick JS, Abrams JA, Wang KK, Mallat D, Johnston MH, Zfass AM, Smith JO, Barthel JS, Lightdale CJ. Safety and efficacy of endoscopic spray cryotherapy for Barrett's esophagus with high-grade dysplasia. Gastrointest Endosc. 2010;71:680-685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 215] [Cited by in RCA: 185] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 51. | Sampliner RE. Endoscopic ablative therapy for Barrett's esophagus: current status. Gastrointest Endosc. 2004;59:66-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 52. | Luigiano C, Iabichino G, Eusebi LH, Arena M, Consolo P, Morace C, Opocher E, Mangiavillano B. Outcomes of Radiofrequency Ablation for Dysplastic Barrett's Esophagus: A Comprehensive Review. Gastroenterol Res Pract. 2016;2016:4249510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 53. | Belghazi K, Bergman J, Pouw RE. Endoscopic Resection and Radiofrequency Ablation for Early Esophageal Neoplasia. Dig Dis. 2016;34:469-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 54. | Qumseya BJ, Wani S, Desai M, Qumseya A, Bain P, Sharma P, Wolfsen H. Adverse Events After Radiofrequency Ablation in Patients With Barrett's Esophagus: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2016;14:1086-1095.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 108] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 55. | Shaheen NJ, Peery AF, Hawes RH, Rothstein RI, Spechler SJ, Galanko JA, Campbell M, Carr C, Fowler B, Walsh J, Siddiqui AA, Infantolino A, Wolfsen HC; AIM Dysplasia Trial Investigators. Quality of life following radiofrequency ablation of dysplastic Barrett's esophagus. Endoscopy. 2010;42:790-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 56. | Gosain S, Mercer K, Twaddell WS, Uradomo L, Greenwald BD. Liquid nitrogen spray cryotherapy in Barrett's esophagus with high-grade dysplasia: long-term results. Gastrointest Endosc. 2013;78:260-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 57. | Ghorbani S, Tsai FC, Greenwald BD, Jang S, Dumot JA, McKinley MJ, Shaheen NJ, Habr F, Coyle WJ. Safety and efficacy of endoscopic spray cryotherapy for Barrett's dysplasia: results of the National Cryospray Registry. Dis Esophagus. 2016;29:241-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 58. | Greenwald BD, Dumot JA, Abrams JA, Lightdale CJ, David DS, Nishioka NS, Yachimski P, Johnston MH, Shaheen NJ, Zfass AM, Smith JO, Gill KR, Burdick JS, Mallat D, Wolfsen HC. Endoscopic spray cryotherapy for esophageal cancer: safety and efficacy. Gastrointest Endosc. 2010;71:686-693. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 59. | Canto MI, Shin EJ, Khashab MA, Molena D, Okolo P, Montgomery E, Pasricha P. Safety and efficacy of carbon dioxide cryotherapy for treatment of neoplastic Barrett's esophagus. Endoscopy. 2015;47:582-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 60. | Dumot JA, Vargo JJ 2nd, Falk GW, Frey L, Lopez R, Rice TW. An open-label, prospective trial of cryospray ablation for Barrett's esophagus high-grade dysplasia and early esophageal cancer in high-risk patients. Gastrointest Endosc. 2009;70:635-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 113] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 61. | Derogar M, Orsini N, Sadr-Azodi O, Lagergren P. Influence of major postoperative complications on health-related quality of life among long-term survivors of esophageal cancer surgery. J Clin Oncol. 2012;30:1615-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 164] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 62. | Jacobs M, Macefield RC, Elbers RG, Sitnikova K, Korfage IJ, Smets EM, Henselmans I, van Berge Henegouwen MI, de Haes JC, Blazeby JM, Sprangers MA. Meta-analysis shows clinically relevant and long-lasting deterioration in health-related quality of life after esophageal cancer surgery. Qual Life Res. 2014;23:1155-1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 63. | Giwa F, Salami A, Abioye AI. Hospital esophagectomy volume and postoperative length of stay: A systematic review and meta-analysis. The American Journal of Surgery. 2018;215:155-162. [RCA] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 64. | Metzger R, Bollschweiler E, Vallböhmer D, Maish M, DeMeester TR, Hölscher AH. High volume centers for esophagectomy: what is the number needed to achieve low postoperative mortality? Dis Esophagus. 2004;17:310-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 158] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 65. | Speicher PJ, Englum BR, Ganapathi AM, Wang X, Hartwig MG, D'Amico TA, Berry MF. Traveling to a High-volume Center is Associated With Improved Survival for Patients With Esophageal Cancer. Ann Surg. 2017;265:743-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 66. | Yibulayin W, Abulizi S, Lv H, Sun W. Minimally invasive oesophagectomy versus open esophagectomy for resectable esophageal cancer: a meta-analysis. World J Surg Oncol. 2016;14:304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 177] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 67. | Wallner G, Zgodziński W, Masiak-Segit W, Skoczylas T, Dąbrowski A. Minimally invasive surgery for esophageal cancer - benefits and controversies. Kardiochir Torakochirurgia Pol. 2014;11:151-155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 68. | Yanasoot A, Yolsuriyanwong K, Ruangsin S, Laohawiriyakamol S, Sunpaweravong S. Costs and benefits of different methods of esophagectomy for esophageal cancer. Asian Cardiovasc Thorac Ann. 2017;25:513-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 69. | Biere SS, van Berge Henegouwen MI, Maas KW, Bonavina L, Rosman C, Garcia JR, Gisbertz SS, Klinkenbijl JH, Hollmann MW, de Lange ES, Bonjer HJ, van der Peet DL, Cuesta MA. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet. 2012;379:1887-1892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1067] [Cited by in RCA: 1206] [Article Influence: 92.8] [Reference Citation Analysis (0)] |
| 70. | Nagpal K, Ahmed K, Vats A, Yakoub D, James D, Ashrafian H, Darzi A, Moorthy K, Athanasiou T. Is minimally invasive surgery beneficial in the management of esophageal cancer? A meta-analysis. Surg Endosc. 2010;24:1621-1629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 239] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 71. | Mariette C, Markar SR, Dabakuyo-Yonli TS, Meunier B, Pezet D, Collet D, D'Journo XB, Brigand C, Perniceni T, Carrère N, Mabrut JY, Msika S, Peschaud F, Prudhomme M, Bonnetain F, Piessen G; Fédération de Recherche en Chirurgie (FRENCH) and French Eso-Gastric Tumors (FREGAT) Working Group. Hybrid Minimally Invasive Esophagectomy for Esophageal Cancer. N Engl J Med. 2019;380:152-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 474] [Article Influence: 79.0] [Reference Citation Analysis (0)] |
| 72. | Smithers BM, Gotley DC, Martin I, Thomas JM. Comparison of the outcomes between open and minimally invasive esophagectomy. Ann Surg. 2007;245:232-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 287] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 73. | Taioli E, Schwartz RM, Lieberman-Cribbin W, Moskowitz G, van Gerwen M, Flores R. Quality of Life after Open or Minimally Invasive Esophagectomy in Patients With Esophageal Cancer-A Systematic Review. Semin Thorac Cardiovasc Surg. 2017;29:377-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 74. | Giugliano DN, Berger AC, Rosato EL, Palazzo F. Total minimally invasive esophagectomy for esophageal cancer: approaches and outcomes. Langenbecks Arch Surg. 2016;401:747-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 75. | Song SY, Na KJ, Oh SG, Ahn BH. Learning curves of minimally invasive esophageal cancer surgery. Eur J Cardiothorac Surg. 2009;35:689-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 76. | Tapias LF, Morse CR. Minimally invasive Ivor Lewis esophagectomy: description of a learning curve. J Am Coll Surg. 2014;218:1130-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 110] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 77. | Ohba A, Kato K, Ito Y, Katada C, Ishiyama H, Yamamoto S, Ura T, Kodaira T, Kudo S, Tamaki Y. Chemoradiation therapy with docetaxel in elderly patients with stage II/III esophageal cancer: A phase 2 trial. Adv Radiat Oncol. 2016;1:230-236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 78. | Herskovic A, Martz K, al-Sarraf M, Leichman L, Brindle J, Vaitkevicius V, Cooper J, Byhardt R, Davis L, Emami B. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med. 1992;326:1593-1598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1514] [Cited by in RCA: 1445] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 79. | Anderson SE, Minsky BD, Bains M, Hummer A, Kelsen D, Ilson DH. Combined modality chemoradiation in elderly oesophageal cancer patients. Br J Cancer. 2007;96:1823-1827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 80. | Abrams JA, Buono DL, Strauss J, McBride RB, Hershman DL, Neugut AI. Esophagectomy compared with chemoradiation for early stage esophageal cancer in the elderly. Cancer. 2009;115:4924-4933. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 81. | Duan XF, Tang P, Yu ZT. Neoadjuvant chemoradiotherapy for resectable esophageal cancer: an in-depth study of randomized controlled trials and literature review. Cancer Biol Med. 2014;11:191-201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 82. | Jin HL, Zhu H, Ling TS, Zhang HJ, Shi RH. Neoadjuvant chemoradiotherapy for resectable esophageal carcinoma: a meta-analysis. World J Gastroenterol. 2009;15:5983-5991. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 78] [Cited by in RCA: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 83. | van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, Richel DJ, Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ, Cuesta MA, Blaisse RJ, Busch OR, ten Kate FJ, Creemers GJ, Punt CJ, Plukker JT, Verheul HM, Spillenaar Bilgen EJ, van Dekken H, van der Sangen MJ, Rozema T, Biermann K, Beukema JC, Piet AH, van Rij CM, Reinders JG, Tilanus HW, van der Gaast A; CROSS Group. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074-2084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3288] [Cited by in RCA: 4077] [Article Influence: 313.6] [Reference Citation Analysis (0)] |
| 84. | Pech O, May A, Rabenstein T, Ell C. Endoscopic resection of early oesophageal cancer. Gut. 2007;56:1625-1634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 67] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 85. | Minashi K, Nihei K, Mizusawa J, Takizawa K, Yano T, Ezoe Y, Tsuchida T, Ono H, Iizuka T, Hanaoka N, Oda I, Morita Y, Tajika M, Fujiwara J, Yamamoto Y, Katada C, Hori S, Doyama H, Oyama T, Nebiki H, Amagai K, Kubota Y, Nishimura K, Kobayashi N, Suzuki T, Hirasawa K, Takeuchi T, Fukuda H, Muto M. Efficacy of Endoscopic Resection and Selective Chemoradiotherapy for Stage I Esophageal Squamous Cell Carcinoma. Gastroenterology. 2019;157:382-390.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 139] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 86. | Kam TY, Kountouri M, Roth A, Frossard JL, Huber O, Mönig S, Zilli T. Endoscopic resection with adjuvant chemo-radiotherapy for superficial esophageal squamous cell carcinoma: A critical review. Crit Rev Oncol Hematol. 2018;124:61-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 87. | Ballard DD, Choksi N, Lin J, Choi EY, Elmunzer BJ, Appelman H, Rex DK, Fatima H, Kessler W, DeWitt JM. Outcomes of submucosal (T1b) esophageal adenocarcinomas removed by endoscopic mucosal resection. World J Gastrointest Endosc. 2016;8:763-769. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 88. | Noordzij IC, Curvers WL, Huysentruyt CJ, Nieuwenhuijzen GAP, Creemers GJ, van der Sangen MJC, Schoon EJ. Salvage endoscopic resection in patients with esophageal adenocarcinoma after chemoradiotherapy. Endosc Int Open. 2018;6:E1126-E1129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 89. | Gowryshankar A, Nagaraja V, Eslick GD. HER2 status in Barrett's esophagus & esophageal cancer: a meta analysis. J Gastrointest Oncol. 2014;5:25-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 90. | Kim SM, Park YY, Park ES, Cho JY, Izzo JG, Zhang D, Kim SB, Lee JH, Bhutani MS, Swisher SG, Wu X, Coombes KR, Maru D, Wang KK, Buttar NS, Ajani JA, Lee JS. Prognostic biomarkers for esophageal adenocarcinoma identified by analysis of tumor transcriptome. PLoS One. 2010;5:e15074. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 116] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 91. | McManus DT, Olaru A, Meltzer SJ. Biomarkers of esophageal adenocarcinoma and Barrett's esophagus. Cancer Res. 2004;64:1561-1569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 88] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 92. | Nagaraja V, Eslick GD. Forthcoming prognostic markers for esophageal cancer: a systematic review and meta-analysis. J Gastrointest Oncol. 2014;5:67-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 93. | Pectasides E. Genomic Alterations and Targeted Therapy in Gastric and Esophageal Adenocarcinoma. Clin Ther. 2016;38:1589-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 94. | Davidson M, Starling N. Trastuzumab in the management of gastroesophageal cancer: patient selection and perspectives. Onco Targets Ther. 2016;9:7235-7245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 95. | Guo YM, Yu WW, Zhu M, Guo CY. Clinicopathological and prognostic significance of epidermal growth factor receptor overexpression in patients with esophageal adenocarcinoma: a meta-analysis. Dis Esophagus. 2015;28:750-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 96. | Sliwkowski MX, Lofgren JA, Lewis GD, Hotaling TE, Fendly BM, Fox JA. Nonclinical studies addressing the mechanism of action of trastuzumab (Herceptin). Semin Oncol. 1999;26:60-70. [PubMed] |
| 97. | Van Cutsem E, Bang YJ, Feng-Yi F, Xu JM, Lee KW, Jiao SC, Chong JL, López-Sanchez RI, Price T, Gladkov O, Stoss O, Hill J, Ng V, Lehle M, Thomas M, Kiermaier A, Rüschoff J. HER2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer. 2015;18:476-484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 452] [Cited by in RCA: 426] [Article Influence: 42.6] [Reference Citation Analysis (0)] |