Published online Jun 30, 2019. doi: 10.13105/wjma.v7.i6.323
Peer-review started: April 8, 2019
First decision: June 4, 2019
Revised: June 11, 2019
Accepted: June 18, 2019
Article in press: June 18, 2019
Published online: June 30, 2019
Processing time: 85 Days and 17.1 Hours
A number of non-systematic reviews on the effects or mechanisms of probiotics on improving dyslipidemia, fatty liver, and obesity have been available but inconclusive to determine the independent effects of probiotics on each of the three conditions.
To perform a systematic review and meta-analysis on potential benefits of probiotics among individuals with fatty liver or obesity or hyperlipidemia.
A systematic literature search was performed using PubMed and Embase. Adult participants of any gender without major comorbidities who received probiotics were considered following these criteria: (1) Studies on a single genus of probiotics with or without prebiotics; (2) Studies specifying the probiotic dosage into colony-forming units (CFUs); and (3) Studies on food-based probiotics were excluded. The primary outcome measures for fatty liver, obesity, and dyslipidemia were fibrosis score (kPa), body mass index (BMI; kg/m2), and serum lipid profiles (mg/dL), respectively. The secondary outcome measures for fatty liver and obesity were liver enzymes (U/L) and subcutaneous fat area (cm2).
A total of 13 articles, published between 1997 and 2018, fulfilled the selection criteria. Three probiotics were included, of which Lactobacillus was the most commonly studied (10 studies), followed by Bifidobacterium (two studies) and Pediococcus (one study). Probiotics significantly reduced BMI (P = 0.013), total cholesterol (P = 0.011), and low-density lipoprotein (P = 0.006) while increased high-density lipoprotein (P = 0.028); high heterogeneities were observed. Only Lactobacillus could decrease triglyceride level (P = 0.005) with low heterogeneity. No included studies reported fibrosis score, liver functions, subcutaneous fat outcomes.
Single probiotics, especially Lactobacillus, have a potentially beneficial effect on improving obesity and dyslipidemia. Evidence on the fatty liver is limited.
Core tip: No consensus is available about the benefit of single probiotics on improving dyslipidemia, fatty liver, and obesity. This meta-analysis investigated the effect of single, non-food-based probiotics, with specified dosage and duration, on body mass index, serum lipid profiles, fibrosis score, liver functions, and subcutaneous fat.
- Citation: Pongpirul K, Janchot K, Dai Y. Single strain probiotics for dyslipidemia, fatty liver, and obesity: A systematic review and meta-analysis. World J Meta-Anal 2019; 7(6): 323-338
- URL: https://www.wjgnet.com/2308-3840/full/v7/i6/323.htm
- DOI: https://dx.doi.org/10.13105/wjma.v7.i6.323
The gut microbiota is a diverse and dynamic collection of micro-organisms that live in the human gastrointestinal tract. They are essential for maintaining the health of the human host in the ″symbiosis″ state whereas a ″dysbiosis″ could lead to a number of diseases or worsen health conditions[1]. Probiotics are live bacteria and yeasts that are presented either in ″functional food″ (i.e., fermented food such as yogurt, cheese, miso, kimchi, and kefir) or as supplements in several forms. Probiotics have been claimed to boost the digestive system, support the immune system and reduce the risks associa-ted with metabolic syndrome[2].
There are three main types of fat metabolism disorders: Dyslipidemia, fatty liver, and obesity. Identified as a major risk factor for cardiovascular disease (CVD), dyslipi-demia has been the main point of scientific interest affecting clinical practice especially pharmacological intervention[3]. Metabolic activity of the gut microbiota has been proposed as an influencer of the human serum lipid content[3]; it was estimated that 1% reduction in serum total cholesterol level could yield as high as 3% reduction in CVD risk[4]. Probiotics that exhibit a cholesterol reduction effect is of great interest because they are safer and usually cheaper than chemical drugs. Potential mechanisms for the cholesterol reduction effect of probiotics consumption have been discussed in a recent review[3].
Non-alcoholic fatty liver disease (NAFLD) has been the most common chronic liver disease along with the prevalent obesity worldwide. The alteration of gut microbiota has been shown to promote the development of NAFLD by mediating processes of inflammation, insulin resistance, bile acids, as well as choline metabolisms[5]. Probio-tics are one of the common ways to manipulate the gut microbiota as part of NAFLD management.
The number of overweight (body mass index; BMI 25-29.9 kg/m2) or obese (BMI ≥ 30 kg/m2) individuals has been rising worldwide[6]. The gut microbiota synthesizes short-chain fatty acids and amino acids, ferment otherwise indigestible carbohydrates, and contribute to the energy supplied to the animal and human host[7,8]. Evidence on the association between bacterial richness/dysbiosis and weight loss[9] suggested that modifying gut microbiota including probiotics administration is a potential target for obesity treatment[10].
Unlike other conventional interventions, the practical uses of probiotics have greatly varied. As mentioned earlier, probiotics could be in functional food or as a supplement. They could be used as live organisms with the unclear quantified amount; commonly measured in colony-forming units (CFU). Assessment of a single probiotic is scientifically difficult since more than one genus/species/strains are commonly offered simultaneously. Probiotics are usually regarded as supplements, so their therapeutic effects do not require to be supported by robust scientific evidence by the national food and drug authorities. Although conducting a randomized contro-lled trial (RCT) on this type of complex intervention is relatively more difficult than other interventions, a substantial amount of clinical experiments on probiotics have been prevalent in a variety of healthy and disease-specific study populations.
A number of non-systematic reviews on the effects or mechanisms of probiotics on improving dyslipidemia[3], fatty liver[5], and obesity[10] have been available. However, previous reviews could not determine the independent effects of probiotics on each of the three conditions. Also, many reviews could not differentiate the effects of various amounts of probiotics, especially when mixed and/or food-based probiotics were explored.
This systematic review aimed to identify clinical trials on the use of probiotics alone or in combination with prebiotics for improving fatty liver, obesity, or dyslipidemia. The selected studies must quantify the number of probiotics and explicitly describe the outcome measures. This review did not restrict to any specific kind of probiotics or any country. Probiotics in functional foods or combined in a mixture with substan-ces other than prebiotics were excluded.
This systematic review has been registered in PROSPERO (CRD42019125511) and the protocol ID=CRD42019125511.
The conducting and reporting of this systematic review and meta-analysis followed the PRISMA statement guidelines[11] whereas the inclusion criteria reporting followed the PICOS scheme. A systematic literature search was performed by two independent authors (KJ and YD) using PubMed and Embase. The search was limited to human subjects and English language. Adult individuals of any gender who received probiotics were considered as the intervention group whereas those who received placebo were considered as the comparator group. Only controlled trials with and without randomization were included. The search strategy was based on various combinations of words for both database and focused on two main concepts: probiotics and fat metabolism. The last search was conducted on March 1, 2019.
For the PubMed database the following combination was applied: ((Overweight-[Mesh] OR overweight[tiab] OR obese[tiab] OR obesity[tiab] OR "body weight"[tiab] OR "body mass index"[tiab]) OR ("Fatty Liver"[Mesh] OR "fatty liver"[tiab] OR Fibroscan[tiab] OR Ultrasound[tiab] OR "liver function tests"[Mesh] OR "liver function tests"[tiab] OR "Aspartate Aminotransferases"[tiab] OR "Alanine Transa-minase"[tiab] OR "Alkaline phosphatase"[tiab] OR "gammaglutamyl transpeptidase"[tiab] OR albumin*[tiab] OR bilirubin[tiab]) OR (Dyslipidemias[Mesh] OR dyslipidemia*[tiab] OR Hyperlipidemia[tiab] OR Hyperlipoprote-inemias[tiab] OR Hypertriglyceridemia[tiab] OR Hypercholesterolemia[tiab] OR Cholesterol[Mesh] OR cholesterol[tiab] OR plasma lipids[tiab] OR Triglycerides[Mesh] OR Triglyceride*[tiab] HDL[tiab] OR LDL[tiab] OR VLDL[tiab])) AND ((Probiotics[Mesh] OR probiotics[tiab] OR probiotic[tiab] OR (Synbiotics[Mesh] OR synbiotics[tiab] OR synbiotic[tiab]) OR (Lactobacillales[Mesh] OR Lactobacillales[tiab] OR Lactobacillus[tiab] OR Pediococcus[tiab] OR Leuconostoc [tiab] OR Oenococcus[tiab] OR Weissella[tiab] OR Lactococcus[tiab] OR Streptococcus[tiab]) OR (Bifidobacteriales[Mesh] OR Bifidobacteriales[tiab] OR Bifidobacterium[tiab] OR Aeriscardovia[tiab] OR Alloscardovia[tiab] OR Bifidobacterium[tiab] OR Bombiscardovia[tiab] OR Galliscardovia[tiab] OR Gardnerella[tiab] OR Neoscardovia[tiab] OR Parascardovia[tiab] OR Pseudoscardovia[tiab] OR Scardovia[tiab]) OR (Saccharomyces[Mesh] OR Saccharomyces[tiab])) AND (Humans[Mesh] AND English[lang]).
For the Embase database the following combination was applied: ('obesity'/exp OR 'adipositas':ti,ab OR 'adiposity':ti,ab OR 'alimentary obesity':ti,ab OR 'body weight, excess':ti,ab OR 'corpulency':ti,ab OR 'fat overload syndrome':ti,ab OR 'nutritional obesity':ti,ab OR 'obesitas':ti,ab OR 'obesity':ti,ab OR 'overweight':ti,ab OR 'body weight'/exp OR 'body weight':ti,ab OR 'total body weight':ti,ab OR 'weight, body':ti,ab OR 'body mass'/exp OR 'bmi (body mass index)':ti,ab OR 'quetelet index':ti,ab OR 'body mass':ti,ab OR 'body mass index':ti,ab OR 'fatty liver'/exp OR 'fat liver':ti,ab OR 'fatty liver':ti,ab OR 'fatty liver disease':ti,ab OR 'fatty liver infiltration':ti,ab OR 'hepatic steatosis':ti,ab OR 'hepatosteatosis':ti,ab OR 'liver fatty infiltration':ti,ab OR 'liver steatosis':ti,ab OR 'liver, fatty':ti,ab OR 'steatosis, liver':ti,ab OR 'liver function test'/exp OR 'function test, liver':ti,ab OR 'hepatic function test':ti,ab OR 'liver function test':ti,ab OR 'liver function tests':ti,ab OR 'test, liver function':ti,ab OR 'echography'/exp OR 'doptone':ti,ab OR 'duplex echography':ti,ab OR 'echogram':ti,ab OR 'echography':ti,ab OR 'echoscopy':ti,ab OR 'echosound':ti,ab OR 'high resolution echography':ti,ab OR 'scanning, ultrasonic':ti,ab OR 'sonogram':ti,ab OR 'sonography':ti,ab OR 'ultrasonic detection':ti,ab OR 'ultrasonic diagnosis':ti,ab OR 'ultrasonic echo':ti,ab OR 'ultrasonic examination':ti,ab OR 'ultrasonic scanning':ti,ab OR 'ultrasonic scintillation':ti,ab OR 'ultrasonography':ti,ab OR 'ultrasound diagnosis':ti,ab OR 'ultrasound scanning':ti,ab OR 'aspartate aminotransferase'/exp OR '1 aspartate 2 oxoglutarate aminotransferase':ti,ab OR '1 aspartate:2 oxoglutarate aminotransferase':ti,ab OR 'got':ti,ab OR 'aminotransferase, aspartate':ti,ab OR 'aspartate amino transferase':ti,ab OR 'aspartate amin-otransferase':ti,ab OR 'aspartate aminotransferases':ti,ab OR 'aspartate transaminase':ti,ab OR 'aspartic aminotransferase':ti,ab OR 'aspartic transminase':ti,ab OR 'e.c. 2.6.1.1':ti,ab OR 'glutamate aspartate transaminase':ti,ab OR 'glutamate oxalacetate transaminase':ti,ab OR 'glutamate oxalacetic transaminase':ti,ab OR 'glutamate oxalate transaminase':ti,ab OR 'glutamate oxalic transaminase':ti,ab OR 'glutamate oxaloacetate aminotransferase':ti,ab OR 'glutamate oxaloacetate transaminase':ti,ab OR 'glutamate oxaloacetic acid transaminase':ti,ab OR 'glutamatoxalacetate transaminase':ti,ab OR 'glutamic aspartic aminotransferase':ti,ab OR 'glutamic aspartic transaminase':ti,ab OR 'glutamic oxal acetatic transaminase':ti,ab OR 'glutamic oxalacetic acid transaminase':ti,ab OR 'glutamic oxalacetic transaminase':ti,ab OR 'glutamic oxalacetic transferase':ti,ab OR 'glutamic oxalic transaminase':ti,ab OR 'glutamic oxaloacetic acid transaminase':ti,ab OR 'glutamic oxaloacetic aminotransferase':ti,ab OR 'glutamic oxaloacetic transa-minase':ti,ab OR 'glutamine oxaloacetic transaminase':ti,ab OR 'glutaminic oxalacetic transaminase':ti,ab OR 'l asparate 2 oxoglutarate transaminase':ti,ab OR 'l aspartate 2 oxoglutarate aminotransferase':ti,ab OR 'l aspartate aminotransferase':ti,ab OR 'l aspartate:2 oxoglutarate aminotransferase':ti,ab OR 'l aspartate:2 oxoglutarate transaminase':ti,ab OR 'l aspatate:2 oxoglutarate aminotransferase':ti,ab OR 'levo aspartate aminotransferase':ti,ab OR 'transaminase a':ti,ab OR 'alanine aminotransferase'/exp OR 'gpt':ti,ab OR 'alanin aminotransferase':ti,ab OR 'alanine 2 oxoglutarate aminotransferase':ti,ab OR 'alanine 2 oxoisovalerate amino-transferase':ti,ab OR 'alanine alpha ketoglutarate transaminase':ti,ab OR 'alanine alpha oxoglutarate transaminase':ti,ab OR 'alanine amino transferase':ti,ab OR 'alanine aminotransferase':ti,ab OR 'alanine transaminase':ti,ab OR 'alanine transpe-ptidase':ti,ab OR 'alanine:2 oxoglutarate aminotransferase':ti,ab OR 'e.c. 2.6.1.2':ti,ab OR 'glutamate alanine transaminase':ti,ab OR 'glutamate pyruvate aminotran-sferase':ti,ab OR 'glutamate pyruvate transaminase':ti,ab OR 'glutamate pyruvatetransaminase':ti,ab OR 'glutamic alanine aminotransferase':ti,ab OR 'glutamic pyruvate transaminase':ti,ab OR 'glutamic pyruvic aminotransferase':ti,ab OR 'glutamic pyruvic transaminase':ti,ab OR 'glutamopyruvic transaminase':ti,ab OR 'l alanine 2 oxoglutarate aminotransferase':ti,ab OR 'l alanine:2 oxoglutarate aminotransferase':ti,ab OR 'alkaline phosphatase'/exp OR 'alcalic phosphatase':ti,ab OR 'alkali phosphatase':ti,ab OR 'alkalic phosphatase':ti,ab OR 'alkaline monophosphoesterase':ti,ab OR 'alkaline phosphatase':ti,ab OR 'alkaline phosphohydrolase':ti,ab OR 'alkaline phosphomonoesterase':ti,ab OR 'alkalinic phosphatase':ti,ab OR 'basic phosphatase':ti,ab OR 'e.c. 3.1.3.1':ti,ab OR 'heat stable alkaline phosphatase':ti,ab OR 'orthophosphoric monoester phosphohydrolase':ti,ab OR 'phosphatase, alkaline':ti,ab OR 'gamma glutamyltransferase'/exp OR '(5 glutamyl) peptide:amino acid 5 glutamyltransferase':ti,ab OR '4 glutamyl transferase':ti,ab OR 'alpha glutamyltranspeptidase':ti,ab OR 'e.c. 2.3.2.2':ti,ab OR 'gamma glutamyl transferase':ti,ab OR 'gamma glutamyl transpeptidase':ti,ab OR 'gamma glutamyltransferase':ti,ab OR 'gamma glutamyltranspeptidase':ti,ab OR 'gamma gt':ti,ab OR 'gammaglutamyltransferase':ti,ab OR 'gammaglutamyl transpeptidase':ti,ab OR 'glutamate transpeptidase':ti,ab OR 'glutamic trans-ferase':ti,ab OR 'glutamyl transferase':ti,ab OR 'glutamyl transpeptidase':ti,ab OR 'glutamyltransferase':ti,ab OR 'glutamyltranspeptidase':ti,ab OR 'levo glutamyl-transpeptidase':ti,ab OR 'transpeptidase':ti,ab OR 'albuminoid'/exp OR 'albumin derivative':ti,ab OR 'albuminoid':ti,ab OR 'albumins':ti,ab OR 'bilirubin'/exp OR '1, 3, 6, 7 tetramethyl 4, 5 dicarboxyethyl 2, 8 divinyl (b 13) dihydrobilenone':ti,ab OR 'bilirubin':ti,ab OR 'bilirubin acid':ti,ab OR 'bilirubin delta':ti,ab OR 'bilirubin ix alpha':ti,ab OR 'bilirubin ixalpha':ti,ab OR 'bilirubin pigment':ti,ab OR 'bilirubin sulfate isomer':ti,ab OR 'bilirubin sulphate isomer':ti,ab OR 'bilirubinate':ti,ab OR 'bilirubine':ti,ab OR 'bilirubinoid':ti,ab OR 'calcium bilirubinate':ti,ab OR 'haematoidin':ti,ab OR 'hematoidin':ti,ab OR 'indirect bilirubin':ti,ab OR 'mesobilirubin':ti,ab OR 'unconjugated bilirubin':ti,ab OR 'dyslipidemia'/exp OR 'dyslipaemia':ti,ab OR 'dyslipemia':ti,ab OR 'dyslipidaemia':ti,ab OR 'dyslipidaemias':ti,ab OR 'dyslipidemia':ti,ab OR 'dyslipidemias':ti,ab OR 'lipidaemia, dys':ti,ab OR 'lipidemia, dys':ti,ab OR 'hyperlipidemia'/exp OR 'hyperlipaemia':ti,ab OR 'hyperlipemia':ti,ab OR 'hyperlipidaemia':ti,ab OR 'hyperlipidaemia type ii':ti,ab OR 'hyperlipidaemia type iii':ti,ab OR 'hyperlipidaemia type v':ti,ab OR 'hyperlipidaemias':ti,ab OR 'hyperlipidemia':ti,ab OR 'hyperlipidemia type ii':ti,ab OR 'hyperlipidemia type iii':ti,ab OR 'hyperlipidemia type v':ti,ab OR 'hyperlipidemias':ti,ab OR 'hyperlipidemic':ti,ab OR 'lipaemia':ti,ab OR 'lipemia':ti,ab OR 'lipidaemia':ti,ab OR 'lipidemia':ti,ab OR 'hyperlipoproteinemia'/exp OR 'essential familial hyperproteinaemia':ti,ab OR 'essential familial hyperproteinemia':ti,ab OR 'familial hyperlipoproteinaemia':ti,ab OR 'familial hyperlipoproteinemia':ti,ab OR 'hereditary hyperlipoproteinaemia':ti,ab OR 'hereditary hyperlipoproteinemia':ti,ab OR 'hyperlipoproteinaemia':ti,ab OR 'hyperlipoproteinaemias':ti,ab OR 'hyperlipoproteinemia':ti,ab OR 'hyperlipoproteinemias':ti,ab OR 'hypertrigly-ceridemia'/exp OR 'hypertriglyceridaemia':ti,ab OR 'hypertriglyceridemia':ti,ab OR 'idiopathic familiary hypertriglyceridaemia':ti,ab OR 'idiopathic familiary hypertriglyceridemia':ti,ab OR 'mckusick 14575':ti,ab OR 'triglyceride storage disease':ti,ab OR 'triglyceridemia':ti,ab OR 'hypercholesterolemia'/exp OR 'cholesteremia':ti,ab OR 'cholesterinemia':ti,ab OR 'cholesterolemia':ti,ab OR 'hypercholesteremia':ti,ab OR 'hypercholesterinaemia':ti,ab OR 'hyperchole-sterinemia':ti,ab OR 'hypercholesterolaemia':ti,ab OR 'hyperchole-sterolemia':ti,ab OR 'cholesterol'/exp OR '3 hydroxy 5 cholestene':ti,ab OR '3beta hydroxy 5 cholestene':ti,ab OR '3beta hydroxycholest 5 ene':ti,ab OR '5 cholesten 3beta ol':ti,ab OR 'beta cholesterol':ti,ab OR 'cholest 5 en 3beta ol':ti,ab OR 'cholest 5 ene 3 ol':ti,ab OR 'cholesterin':ti,ab OR 'cholesterine':ti,ab OR 'cholesterol':ti,ab OR 'cholesterol release':ti,ab OR 'dythol':ti,ab OR 'nsc 8798':ti,ab OR 'plasma lipids':ti,ab OR 'triacylglycerol'/exp OR 'acylglycerol, tri':ti,ab OR 'fatty acid triglyceride':ti,ab OR 'triacyl glyceride':ti,ab OR 'triacylglycerol':ti,ab OR 'triglyceride':ti,ab OR 'triglycerides':ti,ab OR 'tryglyceride':ti,ab OR 'high density lipoprotein'/exp OR 'hdl':ti,ab OR 'alpha 7 lipoprotein':ti,ab OR 'alpha lipoprotein':ti,ab OR 'high density lipoprotein':ti,ab OR 'high density lipoprotein phospholipid':ti,ab OR 'lipoprotein, alpha':ti,ab OR 'lipoprotein, high density':ti,ab OR 'lipoproteins, hdl':ti,ab OR 'pre alpha lipoprotein':ti,ab OR 'very high density lipoprotein':ti,ab OR 'low density lipoprotein'/exp OR 'ldl':ti,ab OR 'beta lipoprotein':ti,ab OR 'lipoprotein, beta':ti,ab OR 'lipoprotein, low density':ti,ab OR 'lipoproteins, ldl':ti,ab OR 'low density lipoprotein':ti,ab OR 'very low density lipoprotein'/exp OR 'vldl':ti,ab OR 'lipoproteins, vldl':ti,ab OR 'pre beta lipoprotein':ti,ab OR 'prebeta lipoprotein':ti,ab OR 'prebetalipoprotein':ti,ab OR 'very low density lipoprotein':ti,ab) AND ('probiotic agent'/exp OR 'probiotic':ti,ab OR 'probiotic agent':ti,ab OR 'probiotics':ti,ab OR 'synbiotic agent'/exp OR 'synbiotic':ti,ab OR 'synbiotic agent':ti,ab OR 'synbiotics':ti,ab OR 'lactobacillales'/exp OR 'lactobacillales':ti,ab OR 'lactobacillus'/exp OR 'betabacterium':ti,ab OR 'lactobacileae':ti,ab OR 'lactobacilleae':ti,ab OR 'lactobacillus':ti,ab OR 'lactobacteria':ti,ab OR 'lactobacilli':ti,ab OR 'pediococcus'/exp OR 'pediococcus':ti,ab OR 'leuconostoc'/exp OR 'leuconostoc':ti,ab OR 'oenococcus'/exp OR 'oenococcus':ti,ab OR 'weissella'/exp OR 'weissella':ti,ab OR 'lactococcus'/exp OR 'lactococcus':ti,ab OR 'streptococcus'/exp OR 'strepto-cocceae':ti,ab OR 'streptococcus':ti,ab OR 'streptococcus species':ti,ab OR 'streptococcal component':ti,ab OR 'streptococci':ti,ab OR 'bifidobacteriales'/exp OR 'bifidobacteriales':ti,ab OR 'bifidobacterium'/exp OR 'bifidobacterium':ti,ab OR bombiscardovia:ti,ab OR galliscardovia:ti,ab OR 'gardnerella'/exp OR 'gardnerella':ti,ab OR neoscardovia:ti,ab OR parascardovia:ti,ab OR pseudoscardovia:ti,ab OR 'scardovia wiggsiae'/exp OR 'saccharomyces'/exp OR 'saccharomyces':ti,ab) AND 'randomized controlled trial'/exp.
A systematic review management software, Covidence, was used. The titles and abs-tracts of the primary studies identified in the electronic search were screened by the same two authors. The duplicate studies were excluded. The following inclusion criteria were set for inclusion in this meta-analysis: (1) Controlled clinical experiments with or without randomization on individuals of any gender at least 18 years of age who received probiotics with or without prebiotics for improving fatty liver, obesity, or dyslipidemia; and (2) Studies containing fibrosis score (kPa), body mass index (BMI; kg/m2), serum lipid profiles: Total cholesterol (TC; mg/dL), high density lipoprotein (HDL; mg/dL), low density lipoprotein (LDL; mg/dL), triglyceride (TG; mg/dL), liver enzymes: Alanine transaminase (ALT; IU/L), aspartate transaminase (AST; IU/L), alkaline phosphatase (ALP; IU/L), gamma-glutamyl transpeptidase (GGT; IU/L), subcutaneous fat (%), subcutaneous fat area (cm2). The following exclusion criteria were set: (1) Review articles, letters, comments and case reports; (2) Studies on food-based probiotics (e.g., yogurt, fermented/sour milk, soy product); (3) Studies where it was impossible to convert the probiotic dosage into colony-forming units (CFUs); and (4) Studies where it was impossible to calculate the outcomes of interest. The trial authors were requested if incomplete data were reported. If the trial authors did not respond within two weeks, only available data were used. Any disagreement was resolved through discussion and the final determination was made by the first author (KP).
The same two authors extracted the data for the following variables: (1) Authors, year of publication, and study type; (2) Genus, species, and characteristics, including dosage of the probiotics; and (3) Clinical outcomes, including fibrosis score, BMI, serum lipid profile, liver enzymes, subcutaneous fat. All relevant text, tables, and figures were examined for data extraction. Discrepancies between the two reviewers were resolved by the first author (KP).
Two authors (KJ and YD) independently assessed the risk of bias in the included trials using the Cochrane Risk of Bias tool 2.0 in the following domains: bias arising from the randomization process; bias due to deviations from intended intervention; bias due to missing outcome data; bias in the measurement of the outcome; and bias in the selection of the reported result. The reviewers resolved any disagreement by discu-ssion and consensus.
The analysis was performed by the following subgroups: Intake duration (< 12 weeks vs 12 weeks), dose per day (< 100x108 CFU vs ≥ 100x108 CFU), and the presence of prebiotics (with vs without).
Mean differences (MD) between the intervention and control groups, along with 95% Confidence Interval (95%CI) were reported for continuous variables. Clinical and methodological heterogeneity was assessed by examining participant characteristics, probiotics type, duration of probiotics usage and dose, outcomes, as well as the design of the study. Statistical heterogeneity was assessed using the I2 and X2 statistics. The level of heterogeneity as defined in Chapter 9 of the Cochrane Handbook for Syste-matic Reviews of Interventions: 0% to 40% might not be important; 30% to 60% may represent moderate heterogeneity; 50% to 90% may represent substantial hetero-geneity; 75% to 100% considerable heterogeneity. For the X2 test, statistical heterogeneity of the included trials was assessed with a p-value of less than 0.05 (statistically significant). The random-effects meta-analysis by DerSimonian and Laird method was used as clinical, methodological, and statistical heterogeneity was encountered. The meta-analysis was performed using Stata/MP software version 15 (StataCorp 2017, College Station, TX, United States).
The literature search yielded 3736 articles. After 287 duplicates were removed, 3449 titles and abstracts were screened, and 3293 irrelevant articles were removed. Of 156 articles selected for full-text screening, 143 were excluded for the following reasons: 23 were not controlled trials, 23 studies targeted irrelevant patient population, 49 studied focused on food-based probiotics and/or mixed probiotics, 12 did not specify the probiotic doses, 36 did not have quantifiable outcomes of interest. Finally, a total of 13 articles, dated between 1997 to 2018, fulfilled the selection criteria and were included in this meta-analysis[12-24] (Figure 1).
Included studies were published between 2006 to 2018 in various countries. All of the included studies are Randomized Controlled Trials (RCTs). Participants were randomly allocated to a control group or intervention group which reduce bias. Participants are 18 years of age or older which was related to inclusion criteria. Treat-ment periods were divided from 63 days to the longest period of 168 days (Table 1).
| First author | Year | Study period | Country | Study design | Participant, n | Age range (yr) | Treatment period (d) | Ref. |
| Simons | 2006 | 2004-2005 | Australia | Randomized, double blind, placebo-controlled | 44 | 30-75 | 70 | [24] |
| Ooi | 2010 | - | Malaysia | Randomized, double blind, placebo-controlled | 32 | 18 years of age or older | 84 | [21] |
| Jones | 2012 | - | Canada | Randomized, double blind, placebo-controlled | 127 | 20-75 | 63 | [19] |
| Fuentes | 2013 | - | Spain | Randomized, double blind, placebo-controlled | 60 | 18-65 | 84 | [15] |
| Sanchez | 2014 | - | Canada | Randomized, double blind, placebo-controlled | 93 | 18-55 | 168 | [23] |
| Childs | 2014 | 2008-2009 | United Kingdom | Randomized, double blind, placebo-controlled, factorial, cross-over | 42 | 25-65 | 21 | [13] |
| Rajkumar | 2015 | - | India | Randomized, single blind, placebo-controlled | 45 | 20-25 | 45 | [22] |
| Ahn | 2015 | 2012-2014 | South Korea | Randomized, double blind, placebo-controlled | 92 | - | 84 | [12] |
| Higashikawa | 2016 | 2013 | Japan | Randomized, double blind, placebo-controlled | 41 | 20-70 | 84 | [17] |
| Fuentes | 2016 | 2010 | Spain | Randomized, double blind, placebo-controlled | 60 | 18-25 | 84 | [16] |
| Kim | 2017 | - | South Korea | Randomized, double blind, placebo-controlled | 66 | - | 84 | [20] |
| Costabile | 2017 | 2015 | United Kingdom | Randomized, double blind, placebo-controlled | 46 | 18-50 | 84 | [14] |
| Inoue | 2018 | - | Japan | Randomized, double blind, placebo-controlled | 38 | 66-78 | 84 | [18] |
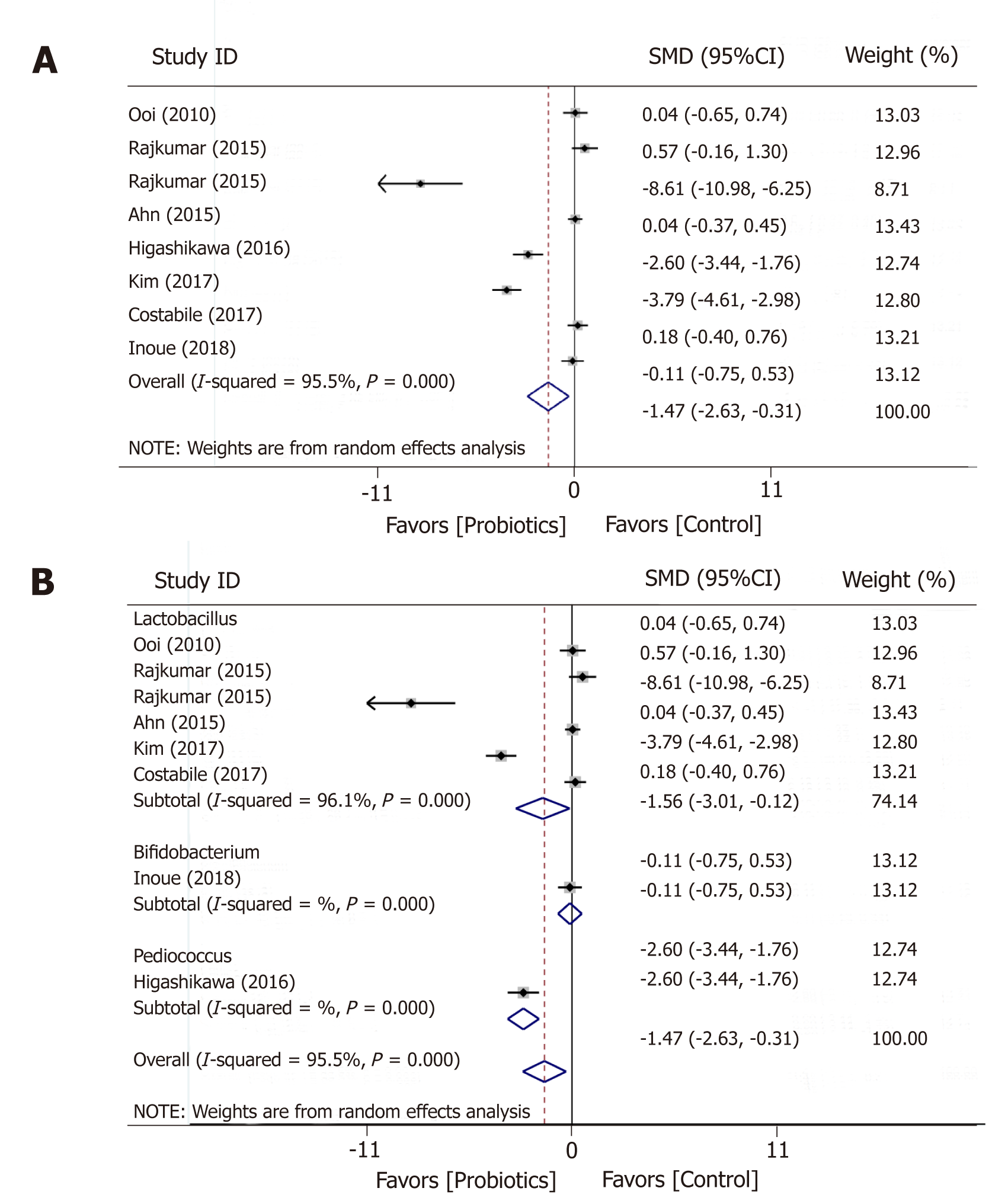
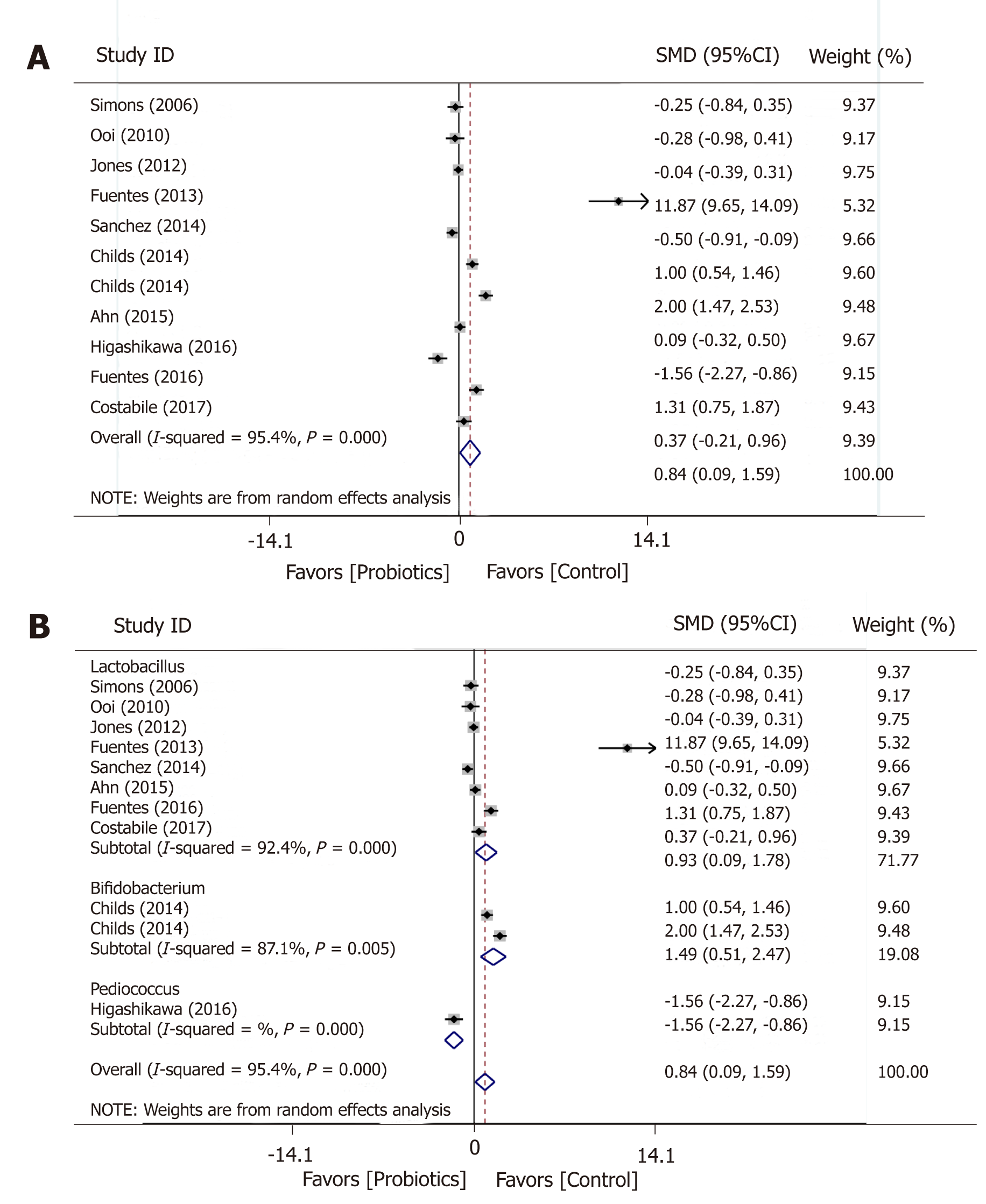
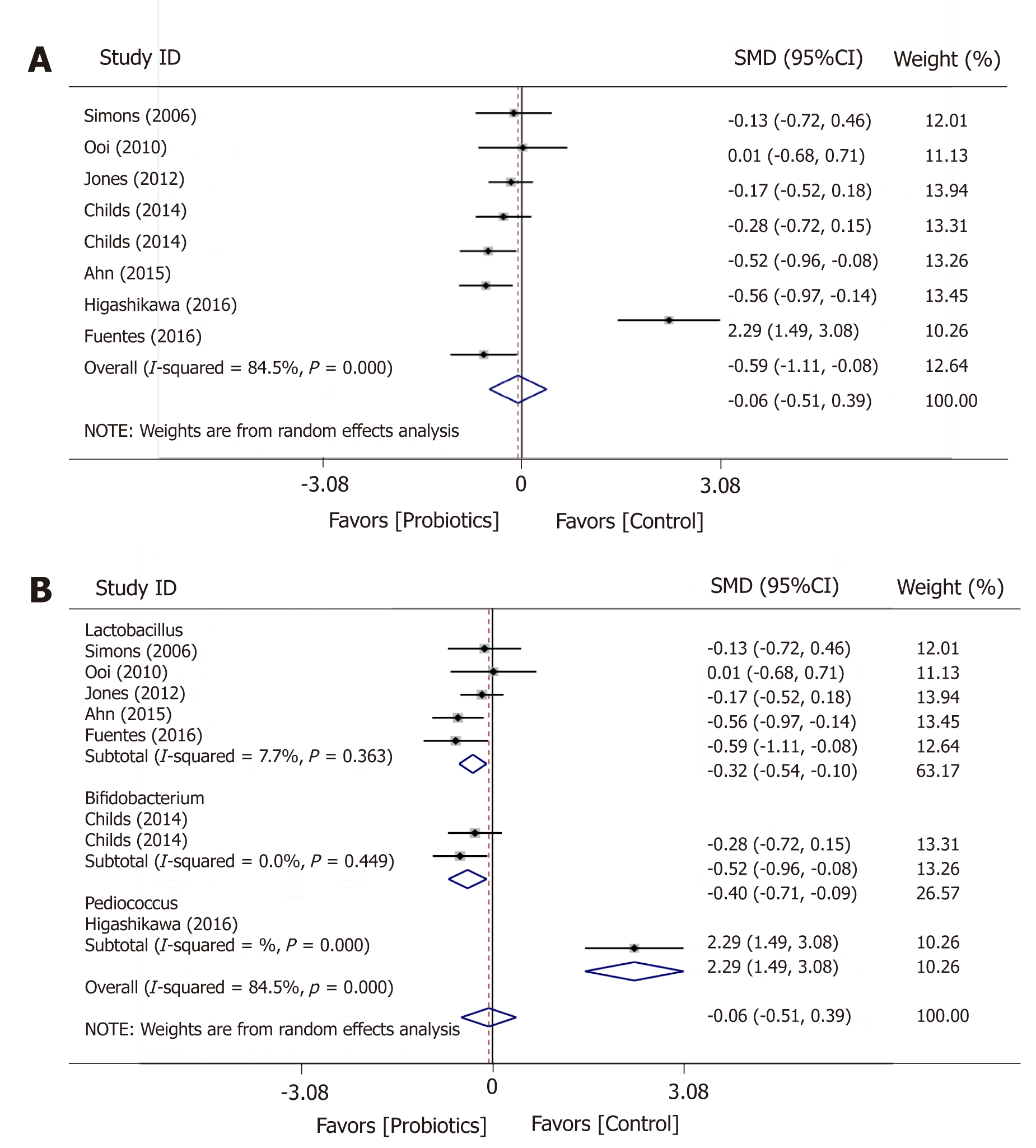
Ten studies used Lactobacillus, two studies used Bifidobacterium, and one study used Pediococcus. Due to the low number of studies accessing the effect of Bifidobacterium and Pediococcus, meta-regression for each genus was not performed. Especially, the study by Childs et al. intervened subjects by Bifidobacterium and Bifidobacterium plus a prebiotic. Meta-regression based on Childs’s study suggested that Bifidobacterium had no significant impact on TC (SMD = 0.219; 95%CI: -0.213 to 0.651; P = 0.320) and LDL (SMD = 0.00; 95%CI: -0.49 to 0.49; P = 1.000). However, Bifidobacterium’ s protective effect on HDL (SMD = 1.49; 95%CI: 0.51-2.47; P = 0.003) and triglycerides (SMD = -0.40; 95%CI: -0.71 to -0.09; P = 0.011) was significant.
BMI was measured in seven trials before and after the administration of probiotic and placebo products (Figure 2). Overall, meta-analysis showed that probiotics significantly reduced BMI compared to placebo (SMD = -1.47; 95%CI: -2.63 to -0.13; P = 0.013); however, between-study heterogeneity was high (I2 = 95.5%; P = 0.000). Subgroup analysis based on the type of probiotic genus revealed that Lactobacillus induced a great reduction in BMI (SMD = -1.56; 95%CI: -3.01 to -0.12; P = 0.034) (Table 2). However, heterogeneity between studies in Lactobacillus was still large (I2 = 96.1%; P = 0.000).
| Subgroup/sensitivity analysis | No. of groups | SMD (95%CI) | P-value | Heterogeneity (I2, P-value) | |
| BMI | |||||
| Intake duration | < 12 wk | 6 | -0.535 (-0.782, -0.289) | 0.000 | 95.1% (0.000) |
| = 12 wk | 2 | -0.220 (-0.920, 0.479) | 0.537 | 98.1% (0.000) | |
| Dose per day | Low-dosage (< 100*108 CFU) | 4 | 0.024 (-0.351, 0.399) | 0.900 | 94.4% (0.000) |
| High-dosage (≥ 100*108 CFU) | 4 | -0.829 (-1.125, -0.532) | 0.00 | 96.7% (0.000) | |
| Combined with or without prebiotics | Probiotic alone | 6 | -0.481 (-0.730, -0.233) | 0.000 | 95.4% (0.000) |
| Combined with prebiotics | 2 | -0.638 (-1.304, 0.027) | 0.060 | 97.9% (0.000) | |
| Total cholesterol | |||||
| Intake duration | < 12 wk | 6 | -0.225 (-0.443, -0.006) | 0.044 | 86.9% (0.000) |
| = 12 wk | 4 | -0.665 (-0.906, -0.424) | 0.000 | 94.9% (0.000) | |
| > 12 wk | 1 | -0.200 (-0.607,0.208) | 0.337 | ||
| Dose per day | Low-dosage (< 100*108 CFU) | 9 | -0.496 (-0.664,0.328) | 0.000 | 93.4% (0.000) |
| High-dosage (≥ 100*108 CFU) | 2 | 0.031 (-0.310, 0.371) | 0.860 | 0.00% (0.336) | |
| Combined with or without prebiotics | Probiotic alone | 9 | -0.466 (-0.630, -0.303) | 0.000 | 92.7% (0.000) |
| Combined with prebiotics | 2 | 0.004 (-0.377, 0.386) | 0.983 | 93.6% (0.000) | |
| LDL | |||||
| Intake duration | < 12 wk | 4 | -0.306 (-0.524, -0.088) | 0.006 | 81.2% (0.000) |
| = 12 wk | 6 | -0.871 (-1.113, -0.628) | 0.000 | 94.1% (0.000) | |
| > 12 wk | 1 | -0.250 (-0.658, 0.159) | 0.213 | ||
| Dose per day | Low-dosage (< 100*108 CFU) | 9 | -0.558 (-0.725, -0.391) | 0.000 | 91.9% (0.000) |
| High-dosage (≥ 100*108 CFU) | 2 | -0.333 (-0.685, 0.020) | 0.064 | 92.7% (0.000) | |
| Combined with or without prebiotics | Probiotic alone | 9 | -0.609 (-0.774, -0.445) | 0.000 | 92.0% (0.000) |
| Combined with prebiotics | 2 | -0.042 (-0.415, 0.331) | 0.826 | 84.8% (0.010) | |
| HDL | |||||
| Intake duration | < 12 wk | 4 | 0.557 (0.329, 0.784) | 0.000 | 94.0% (0.000) |
| = 12 wk | 6 | 0.273 (0.027, 0.520) | 0.030 | 96.6% (0.000) | |
| > 12 wk | 1 | -0.501 (-0.914, -0.087) | 0.018 | ||
| Dose per day | Low-dosage (< 100*108 CFU) | 9 | 0.445 (0.272, 0.617) | 0.000 | 95.7% (0.000) |
| High-dosage (≥ 100*108 CFU) | 2 | -0328 (-0.682, 0.025) | 0.069 | 93.7% (0.000) | |
| Combined with or without prebiotics | Probiotic alone | 9 | 0.162 (-0.004, 0.329) | 0.056 | 95.4% (0.000) |
| Combined with prebiotics | 2 | 1.158 (0.735, 1.581) | 0.000 | 96.2% (0.000) | |
| Triglycerides | |||||
| Intake duration | < 12 wk | 4 | -0.277 (-0.493, -0.061) | 0.000 | 0% (0.614) |
| = 12 wk | 4 | -0.135 (-0.411, 0.140) | 0.336 | 93.0% (0.000) | |
| Dose per day | Low-dosage (< 100*108 CFU) | 6 | -0.298 (-0.489, -0.107) | 0.770 | 97.4% (0.000) |
| High-dosage (≥ 100*108 CFU) | 2 | 0.055 (-0.314, 0.424) | 0.069 | 0.0% (0.567) | |
| Combined with or without prebiotics | Probiotic alone | 6 | -0.185 (-0.376, 0.006) | 0.058 | 88.3% (0.000) |
| Combined with prebiotics | 2 | -0.368 (0.740, 0.004) | 0.052 | 39.4% (0.199) | |
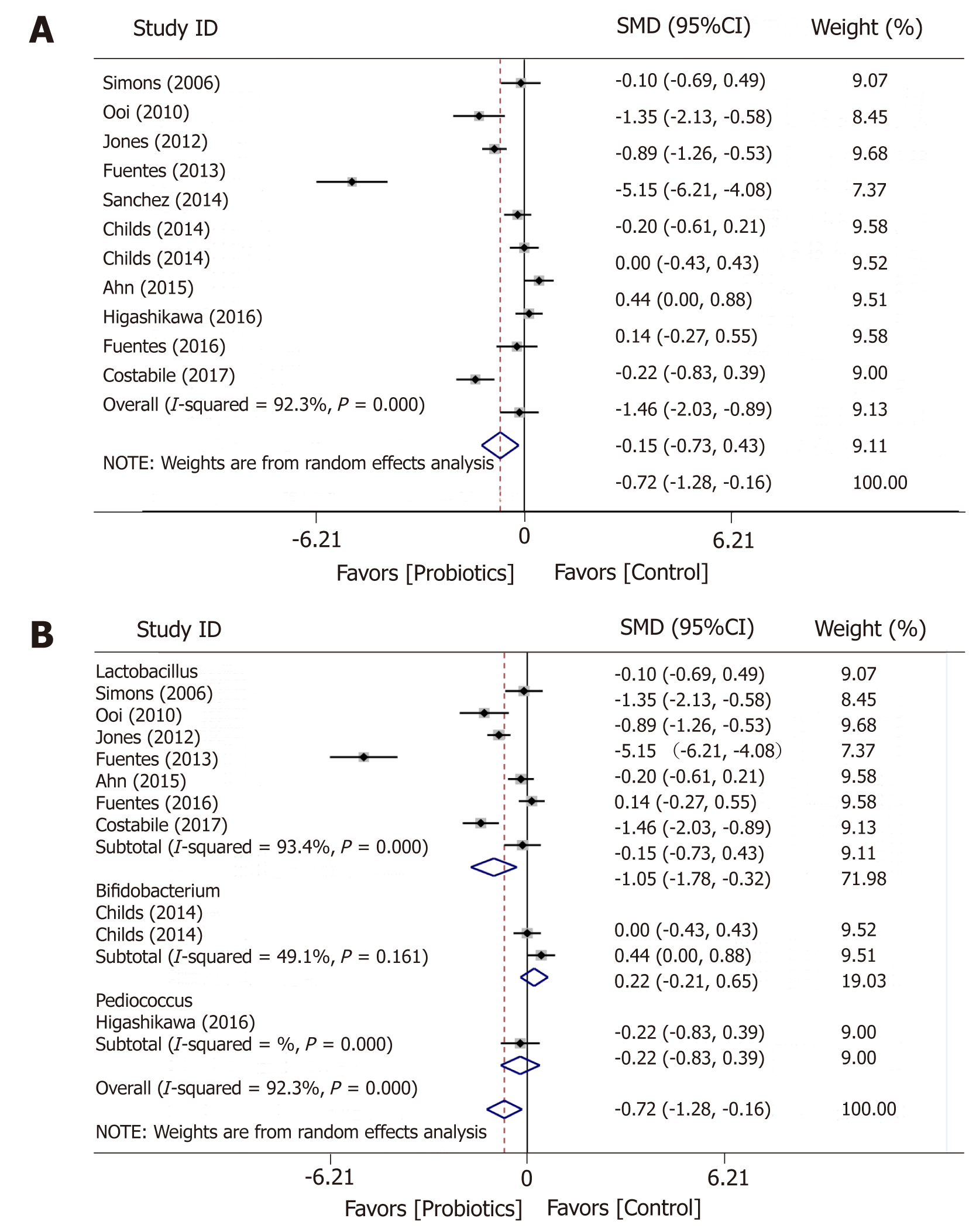
A total of 10 studies examined the effects of probiotic on TC (Figure 3). The administration of probiotics was associated with significant decrease in TC levels (SMD = -0.72; 95%CI: -1.28 to -0.16; P = 0.011), but with high heterogeneity (I2 = 92.3%; P = 0.000) between the studies. Subgroup analysis with regards to probiotic genus was performed. Lactobacillus significantly reduced TC levels (SMD = -0.72; 95%CI: -1.28 to -0.16; P = 0.011). However, it is worth noting the high heterogeneity between studies (I2 = 93.4%; P = 0.000).
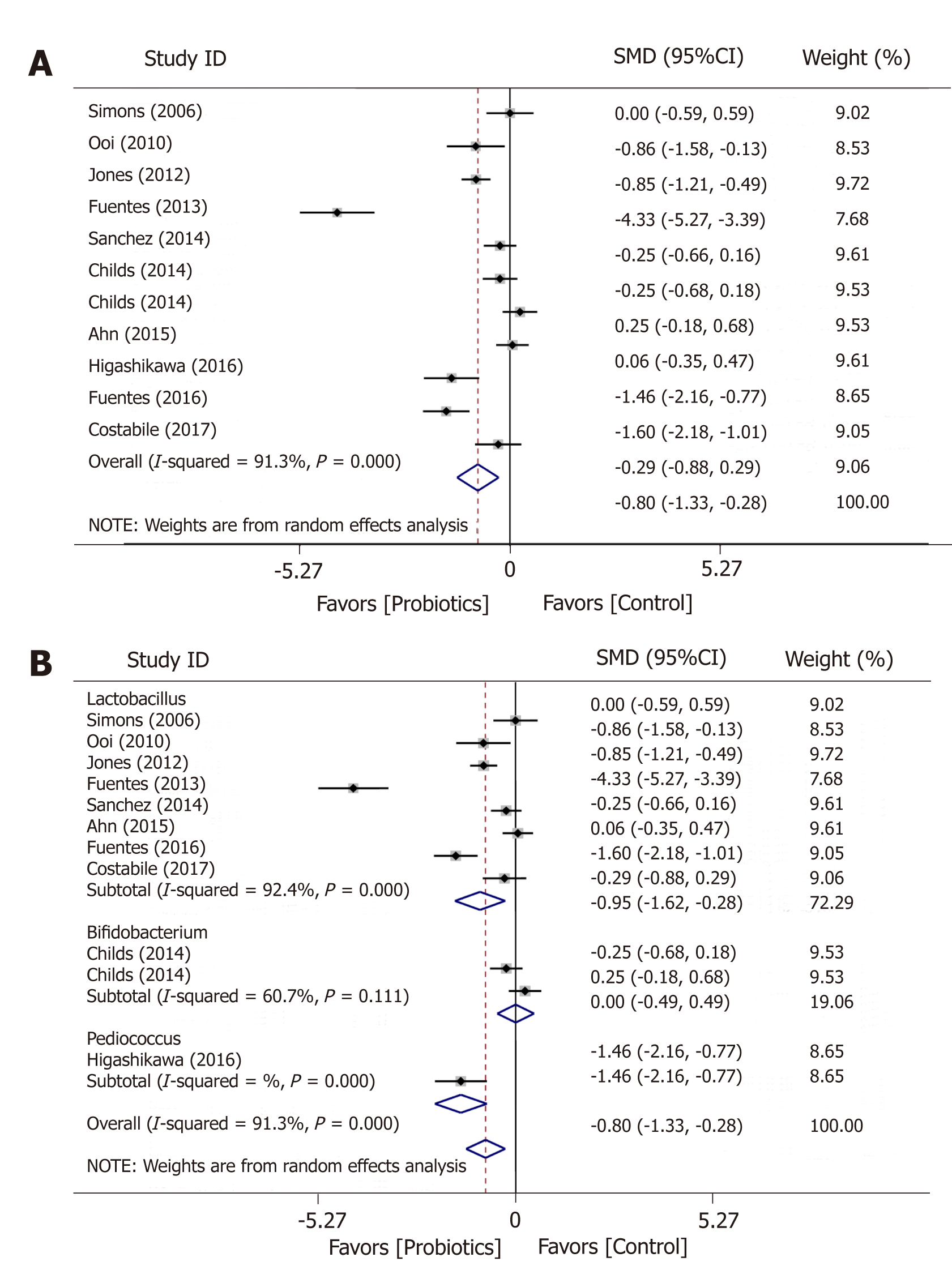
The overall estimate of the ten studies showed a huge reduction in LDL in the treatment groups compared with the placebo groups (SMD = -0.85; 95%CI: -1.33 to -0.28; P = 0.006), but the heterogeneity was large (I2 = 91.3%; P = 0.000) (Figure 4). The effect size was even larger in Lactobacillus group (SMD = -0.95; 95%CI: -1.62 to -0.28; P = 0.006).
An overall significant increase after intervention was reported for HDL levels in ten studies (SMD = 0.84; 95%CI: 0.09-1.59; P = 0.028) (Figure 5). The effect of probiotic on HDL did not change much when it came to subgroup analysis for Lactobacillus (SMD = -0.95; 95%CI: -1.62 to -0.28).
The meta-analysis based on indicated a non-significant change in triglycerides post intervention (SMD = -0.06; 95%CI: -0.505 to 0.385; P = 0.792) (Figure 6). However, an analysis for Lactobacillus showed a significant decrease in triglycerides (SMD = -0.32; 95%CI: -0.54 to -0.095; P = 0.005) and with a low between-study heterogeneity (I2 = 7.7%; P = 0.363).
No included studies reported fibrosis score, liver functions, subcutaneous fat out-comes.
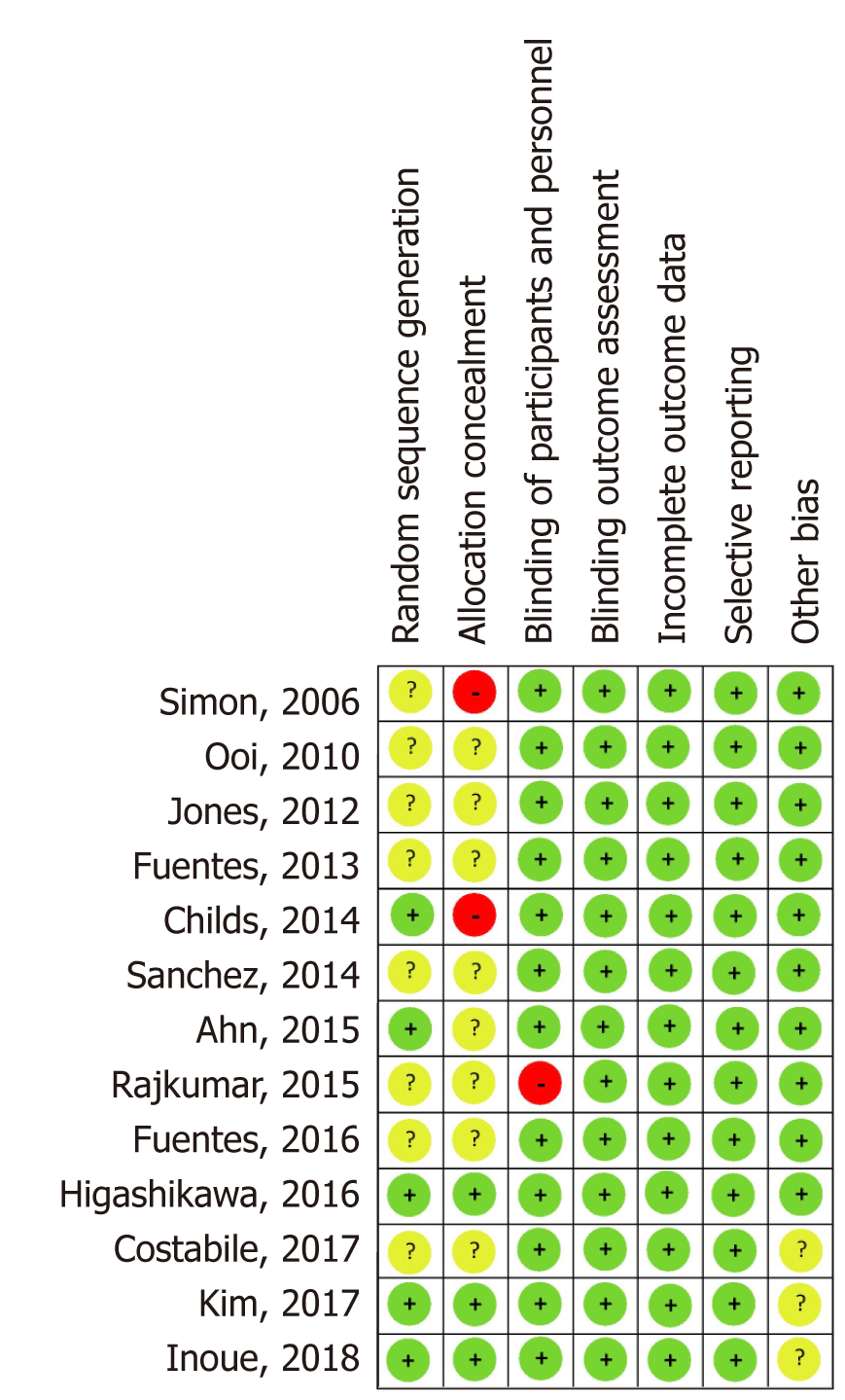
The analyses of the risk of bias of the included studies were summarized in Figure 7. Generally, all studies were classified as low risk of bias. Five articles clearly explained the methods used for randomization, while eight studies did not describe the process of randomizing. Twelve studies blinded the patients, researchers and outcome assessors whereas Rajkumar’s study did not blind the patients and filed staff since the capsules looked different. All studies explicitly explained the methods used for dealing with incomplete outcome data. Three studies worked well in allocation concealment whereas eight studies failed to make the process of allocation concealment clear. Two studies might have a high bias from the predictable allocation of intervention and placebo. At last, all studies had no problem with selective outcome reporting.
This systematic review revealed that probiotics have a potentially beneficial effect on improving obesity and dyslipidemia. Probiotics were found to significantly decrease BMI, levels of TC and LDL as well as increase HDL level. However, the effect of probiotics on TG was not statistically significant.
Our study filled the gap that previous studies assessing the effect of probiotics focused more on multiple probiotic strains by including trials using the single strain as treatment. Our results seem to be different from most of the previous studies. Sun’s meta-analysis found that compared to multiple probiotic strains, a single strain did not have a significant effect on TC, HDL, and triglyceride[25]. However, due to the limited number of studies which intervened with a single strain included in their meta-analysis, caution is required while coming to the conclusion. In our study, 13 studies that used single strain as treatment are enrolled. The considerable number of trials enrolled guarantee more reliable results.
While existing studies showed that probiotics administered in different forms such as fermented milk, bread, tablet, powder or capsule had different effects on lipid profiles and BMI; in this meta-analysis, only studies not using food-based probiotic as interventions are included. Therefore, compared to previous studies, this meta-analysis could isolate the effects of probiotics from other supplements better. Multiple genera of probiotics could have different additive or synergistic effects in comparison with the single genus of probiotics. Hence, caution is needed to extrapolate multiple genera of probiotics’ significant effects on lipid profiles and BMI to a single genus of probiotics. This meta-analysis fills the gap in this area. Thirteen studies are included in this meta-analysis. Compared to previous studies, a sufficient number of studies lead to more reliable conclusions.
Another impacted finding of this study is the daily consumption of probiotics more than 100*108 CFU had a greater benefit on BMI reduction than daily dosage lower than 100*108 CFU. Currently, there are no uniform standard regards to the among of daily intake of probiotics. This study suggests that to ensure an effect on reducing BMI, the number of probiotics may be more than 100*108 CFU.
Another awaiting-to-answer question is the range of intake duration. Among 13 included studies, six trials treated subjects for less than 12 weeks, while the other six trials chose the exact 12 wk for intervention. Only one study intervened subjects for more than 12 wk. Considering studies’ concentration around 12 weeks, we grouped studies into ≤ 12 wk and > 12 wk. Omitting the study that was longer than 12 wk, sensitivity analysis showed that ≤ 2 wk’ intake of probiotic has a significant effect on TC, LDL, and HDL. Hence, although comparison among various lengths of admini-stration terms should be done to further confirm the effect of administration term, we could come to a preliminary conclusion that intake duration of no more than 12 wk could ensure a significant effect of probiotics on TC, LDL, and HDL.
The meta-analysis revealed that probiotics did not significantly reduce the level of triglycerides. Subgroup analysis showed that when restricting studies to those whose duration of intake is less than 12 wk, the effect of probiotics on triglycerides became significant. This result was in agreement with other studies[25,26].
A number of limitations of this study should be acknowledged. First, the findings were limited to fat metabolism but not metabolic syndrome as a whole. Second, the included studies did not report adverse effects, which indicates the safety and tolerance of probiotic capsules. Hence, when making a clinical recommendation of probiotic agents, adverse effects need to be taken into account and be carefully investigated. Third, limited studies reported effects of probiotics combined with other prebiotics. Two of the included studies reported synbiotics’ effects on lipid profiles. Child’s study found that compared with using Bifidobacterium alone, the combination with xylo-oligosaccharides resulted in a significant but modest change in HDL. In addition, Rajkumar’s study showed a superior influence of synbiotics on lipid profiles in comparison to using probiotics alone. A further meta-analysis of more studies is required to confirm the augmentation of the impacts of probiotics alone on serum lipid profiles. Last but not least, crossover studies and parallel studies were included. Crossover studies have more methodological advantages and are easier to control individual-varying confounders compared to parallel RCTs. However, cross-over studies could introduce additional bias when studies have insufficient washout periods. One of our included studies used crossover design with a washout period of 28 d. Whether the washout period is long enough to avoid additional bias needs further study.
An imbalance of the microorganisms could lead to many human diseases including dyslipi-demia, fatty liver, and obesity. Probiotic supplementation has been considered an alternative treatment.
Variety of probiotics has been available as ‘healthy’ products to consumers for many health purposes. These over-the-counter probiotics usually comprised of multiple probiotic strains with some health claims. Given limited evidence on the isolated effect of each probiotic strain, a systematic approach to synthesize current scientific evidence is essential.
This study was aimed to identify clinical trials on the use of single probiotics alone or in combi-nation with prebiotics for improving fatty liver, obesity, and dyslipidemia.
This systematic review and meta-analysis was conducted using a rigorous methodology and supported by the use of systematic review management software. Titles and abstracts of the primary studies listed in PubMed and Embase databases were screened by two assessors using standard sets of inclusion and exclusion criteria. Data from the included articles were extracted in order to synthesize the effect of single probiotics on specific outcome measures.
A total of 13 randomized controlled trials were included. Three probiotics were included: Lactobacillus (10 studies), Bifidobacterium (2 studies), and Pediococcus (1 study). Probiotics significantly reduced BMI, reduced total cholesterol, reduced low-density lipoprotein, and increased high-density lipoprotein, compared to placebo; high study heterogeneities were observed. Only Lactobacillus could decrease triglyceride level with low heterogeneity. No included studies reported fibrosis score, liver functions, or subcutaneous fat outcomes.
This systematic review emphasizes the effects of single genus non-food-based probiotics on decreasing BMI, total cholesterol and low-density lipoprotein as well as increasing high-density lipoprotein levels.
Evidence on single genus probiotics is still limited. Additional clinical trials are needed for each of the single probiotics before combining two or more probiotics could be investigated.
Manuscript source: Invited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: Thailand
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Arslan N, de Oliveira C, Sherif Z, Tarantino G, Tziomalos K S-Editor: Gong ZM L-Editor: A E-Editor: Wang J
| 1. | Dethlefsen L, McFall-Ngai M, Relman DA. An ecological and evolutionary perspective on human-microbe mutualism and disease. Nature. 2007;449:811-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1124] [Cited by in RCA: 1071] [Article Influence: 59.5] [Reference Citation Analysis (0)] |
| 2. | Yoo JY, Kim SS. Probiotics and Prebiotics: Present Status and Future Perspectives on Metabolic Disorders. Nutrients. 2016;8:173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 201] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 3. | Reis SA, Conceição LL, Rosa DD, Siqueira NP, Peluzio MCG. Mechanisms responsible for the hypocholesterolaemic effect of regular consumption of probiotics. Nutr Res Rev. 2017;30:36-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 4. | Manson JE, Tosteson H, Ridker PM, Satterfield S, Hebert P, O'Connor GT, Buring JE, Hennekens CH. The primary prevention of myocardial infarction. N Engl J Med. 1992;326:1406-1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 324] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 5. | Ma J, Zhou Q, Li H. Gut Microbiota and Nonalcoholic Fatty Liver Disease: Insights on Mechanisms and Therapy. Nutrients. 2017;9:pii: E1124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 142] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 6. | NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet. 2016;387:1377-1396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3952] [Cited by in RCA: 3516] [Article Influence: 390.7] [Reference Citation Analysis (0)] |
| 7. | Nieuwdorp M, Gilijamse PW, Pai N, Kaplan LM. Role of the microbiome in energy regulation and metabolism. Gastroenterology. 2014;146:1525-1533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 296] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 8. | Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915-1920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3394] [Cited by in RCA: 3544] [Article Influence: 177.2] [Reference Citation Analysis (5)] |
| 9. | Cotillard A, Kennedy SP, Kong LC, Prifti E, Pons N, Le Chatelier E, Almeida M, Quinquis B, Levenez F, Galleron N, Gougis S, Rizkalla S, Batto JM, Renault P; ANR MicroObes consortium, Doré J, Zucker JD, Clément K, Ehrlich SD. Dietary intervention impact on gut microbial gene richness. Nature. 2013;500:585-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1189] [Cited by in RCA: 1296] [Article Influence: 108.0] [Reference Citation Analysis (0)] |
| 10. | Borgeraas H, Johnson LK, Skattebu J, Hertel JK, Hjelmesaeth J. Effects of probiotics on body weight, body mass index, fat mass and fat percentage in subjects with overweight or obesity: a systematic review and meta-analysis of randomized controlled trials. Obes Rev. 2018;19:219-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 172] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 11. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13930] [Cited by in RCA: 13343] [Article Influence: 833.9] [Reference Citation Analysis (0)] |
| 12. | Ahn HY, Kim M, Ahn YT, Sim JH, Choi ID, Lee SH, Lee JH. The triglyceride-lowering effect of supplementation with dual probiotic strains, Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032: Reduction of fasting plasma lysophosphatidylcholines in nondiabetic and hypertriglyceridemic subjects. Nutr Metab Cardiovasc Dis. 2015;25:724-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 13. | Childs CE, Röytiö H, Alhoniemi E, Fekete AA, Forssten SD, Hudjec N, Lim YN, Steger CJ, Yaqoob P, Tuohy KM, Rastall RA, Ouwehand AC, Gibson GR. Xylo-oligosaccharides alone or in synbiotic combination with Bifidobacterium animalis subsp. lactis induce bifidogenesis and modulate markers of immune function in healthy adults: a double-blind, placebo-controlled, randomised, factorial cross-over study. Br J Nutr. 2014;111:1945-1956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 113] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 14. | Costabile A, Buttarazzi I, Kolida S, Quercia S, Baldini J, Swann JR, Brigidi P, Gibson GR. An in vivo assessment of the cholesterol-lowering efficacy of Lactobacillus plantarum ECGC 13110402 in normal to mildly hypercholesterolaemic adults. PLoS One. 2017;12:e0187964. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 101] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 15. | Fuentes MC, Lajo T, Carrión JM, Cuñé J. Cholesterol-lowering efficacy of Lactobacillus plantarum CECT 7527, 7528 and 7529 in hypercholesterolaemic adults. Br J Nutr. 2013;109:1866-1872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 116] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 16. | Fuentes MC, Lajo T, Carrión JM, Cuñé J. A randomized clinical trial evaluating a proprietary mixture of Lactobacillus plantarum strains for lowering cholesterol 1. Mediterr J Nutr Metab. 2016;9:125-135. [RCA] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Higashikawa F, Noda M, Awaya T, Danshiitsoodol N, Matoba Y, Kumagai T, Sugiyama M. Antiobesity effect of Pediococcus pentosaceus LP28 on overweight subjects: a randomized, double-blind, placebo-controlled clinical trial. Eur J Clin Nutr. 2016;70:582-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 18. | Inoue T, Kobayashi Y, Mori N, Sakagawa M, Xiao JZ, Moritani T, Sakane N, Nagai N. Effect of combined bifidobacteria supplementation and resistance training on cognitive function, body composition and bowel habits of healthy elderly subjects. Benef Microbes. 2018;1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 19. | Jones ML, Martoni CJ, Prakash S. Cholesterol lowering and inhibition of sterol absorption by Lactobacillus reuteri NCIMB 30242: a randomized controlled trial. Eur J Clin Nutr. 2012;66:1234-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 177] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 20. | Kim M, Kim M, Kang M, Yoo HJ, Kim MS, Ahn YT, Sim JH, Jee SH, Lee JH. Effects of weight loss using supplementation with Lactobacillus strains on body fat and medium-chain acylcarnitines in overweight individuals. Food Funct. 2017;8:250-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 21. | Ooi LG, Ahmad R, Yuen KH, Liong MT. Lactobacillus gasseri [corrected] CHO-220 and inulin reduced plasma total cholesterol and low-density lipoprotein cholesterol via alteration of lipid transporters. J Dairy Sci. 2010;93:5048-5058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 22. | Rajkumar H, Kumar M, Das N, Kumar SN, Challa HR, Nagpal R. Effect of Probiotic Lactobacillus salivarius UBL S22 and Prebiotic Fructo-oligosaccharide on Serum Lipids, Inflammatory Markers, Insulin Sensitivity, and Gut Bacteria in Healthy Young Volunteers: A Randomized Controlled Single-Blind Pilot Study. J Cardiovasc Pharmacol Ther. 2015;20:289-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 98] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 23. | Sanchez M, Darimont C, Drapeau V, Emady-Azar S, Lepage M, Rezzonico E, Ngom-Bru C, Berger B, Philippe L, Ammon-Zuffrey C, Leone P, Chevrier G, St-Amand E, Marette A, Doré J, Tremblay A. Effect of Lactobacillus rhamnosus CGMCC1.3724 supplementation on weight loss and maintenance in obese men and women. Br J Nutr. 2014;111:1507-1519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 242] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 24. | Simons LA, Amansec SG, Conway P. Effect of Lactobacillus fermentum on serum lipids in subjects with elevated serum cholesterol. Nutr Metab Cardiovasc Dis. 2006;16:531-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 92] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 25. | Sun J, Buys N. Effects of probiotics consumption on lowering lipids and CVD risk factors: a systematic review and meta-analysis of randomized controlled trials. Ann Med. 2015;47:430-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 135] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 26. | Guo Z, Liu XM, Zhang QX, Shen Z, Tian FW, Zhang H, Sun ZH, Zhang HP, Chen W. Influence of consumption of probiotics on the plasma lipid profile: a meta-analysis of randomised controlled trials. Nutr Metab Cardiovasc Dis. 2011;21:844-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 151] [Article Influence: 10.8] [Reference Citation Analysis (0)] |