Published online Jun 30, 2019. doi: 10.13105/wjma.v7.i6.309
Peer-review started: April 15, 2019
First decision: May 16, 2019
Revised: June 4, 2019
Accepted: June 10, 2019
Article in press: June 10, 2019
Published online: June 30, 2019
Processing time: 78 Days and 9.6 Hours
Pancreatic cancer is one of the most common and lethal malignancies worldwide. The common treatment options for resectable pancreatic cancer include surgery alone, neoadjuvant chemotherapy (CT), neoadjuvant chemoradiotherapy (CRT), adjuvant CT, and adjuvant CRT. However, the optimal treatment is still controversial.
To identify the most effective approach for pancreatic cancer using network meta-analysis.
Eligible studies were searched from PubMed, MEDLINE, EMBASE, Cochrane database, and Google scholar. We searched and included randomized controlled trials reporting on neoadjuvant and adjuvant therapies. For direct comparisons, standard pairwise meta-analysis was performed using the inverse variance DerSimonian-Laird random-effects model. For indirect comparisons, Bayesian network meta-analysis was used to combine direct and indirect evidence. We used relative hazard ratios (HRs) to estimate death difference of different treatments, and relative odds ratios (ORs) for toxic effects. Treatment effects were ranked based on their efficacy for improving survival or reducing toxicity using rankogram. The quality of evidence of estimates from direct comparison and network meta-analysis was evaluated following the GRADE approach.
We included 13 high quality trials with 1591 participants in this network meta-analysis. Compared with surgery alone [pooled HR = 0.7, 95% confidence interval (CI): 0.62-0.79] and surgery with adjuvant CRT (pooled HR = 0.6, 95%CI: 0.54-0.72), surgery with adjuvant CT had a higher rate of overall survival. In contrast, standard pairwise meta-analysis showed a statistically significant survival advantage of surgery with adjuvant CT compared with surgery alone (pooled HR = 0.75, 95%CI: 0.63-0.89; P < 0.001). Rankogram showed that surgery with adjuvant CT was most likely to rank the best in terms of overall survival (probability: 94.2%), followed by surgery alone (probability: 5.8%). No significant differences in overall toxicity or haematological toxicity were found between all the therapies. High quality evidence supported surgery with adjuvant CT over surgery alone for increasing overall survival. Moderate quality evidence supported surgery with adjuvant CT over surgery with adjuvant CRT for increasing overall survival.
Surgery with adjuvant CT prolongs overall survival compared with surgery alone and surgery with adjuvant CRT, suggesting surgery with adjuvant CT is the optimal treatment for resectable pancreatic cancer.
Core tip: No consensus is available in previous studies about the most beneficial treatment option for resectable pancreatic cancer. This is the first network meta-analysis comparing the efficiency of surgery alone, neoadjuvant chemotherapy (CT), neoadjuvant chemoradiotherapy (CRT), adjuvant CT, and adjuvant CRT. We investigated these treatment options in terms of overall survival and toxicity. We found that surgery with adjuvant CT prolonged overall survival compared with surgery alone and surgery with adjuvant CRT. Surgery with adjuvant CT is the optimal treatment for resectable pancreatic cancer.
- Citation: Shen P, Huang KJ, Zhang CZ, Xiao L, Zhang T. Surgery with adjuvant or neoadjuvant treatment vs surgery alone for resectable pancreatic cancer: A network meta-analysis. World J Meta-Anal 2019; 7(6): 309-322
- URL: https://www.wjgnet.com/2308-3840/full/v7/i6/309.htm
- DOI: https://dx.doi.org/10.13105/wjma.v7.i6.309
Pancreatic cancer is one of the most common and lethal malignancies[1]. Surgical resection is the only potential curative treatment for pancreatic cancer. However, even after radical removal of the tumor (R0), the prognosis remained poor, with the 5-year survival rate being less than 25% and the median survival time being 14-21 mo[2-4]. High incidence of both locoregional and distant recurrences is responsible for the poor prognosis. Thus, a multimodal approach is needed to decrease the high recur-rence rate as well as increase overall survival[5,6].
Several neoadjuvant or adjuvant therapies have been shown to be beneficial in selected patients. These therapies are neoadjuvant chemotherapy (CT), neoadjuvant chemoradiotherapy (CRT), adjuvant CT, and adjuvant CRT. However, there are de-bates over which therapy can benefit patients mostly. Regarding neoadjuvant therapy, recent meta-analysis found no significant difference in the overall survival between neoadjuvant CRT and surgery[7]. With regard to adjuvant therapy, the benefit of adjuvant therapy for resectable pancreatic cancer is still controversial, especially the impact of adjuvant CRT. Adjuvant CRT using fluorouracil is considered standard of care in the United States. However, the EORTC trial demonstrated no benefit of adjuvant CRT over observation in patients with resected pancreatic cancer (median survival: 1.3 year vs 1.0 year)[8]. Thus, more powerful and comprehensive evidence is needed to evaluate the best treatment strategy for resectable pancreatic cancer.
There have been several traditional meta-analyses comparing the benefit of neoadjuvant therapy or adjuvant therapy. However, all of the previous meta-analyses only addressed neoadjuvant therapy[7,9-11] or adjuvant therapy alone[12-14]. Thus, it is interesting and meaningful for us to perform this network meta-analysis, that is, to compare both neoadjuvant and adjuvant therapies with surgery alone. The advantage of network meta-analysis is that it can compare different treatments without direct clinical trials. That is, if we have only clinical trials comparing A to B and B to C, we can estimate A to C using network met-analysis. Besides, treatment options can be ranked based on their efficacy for improving survival or reducing toxicity in network meta-analysis.
The aim of this network meta-analysis was to identify the most effective treatment for resectable pancreatic cancer by comparing overall survival and toxic effects after neoadjuvant or adjuvant CT and CRT.
The protocol of this network meta-analysis was registered with the prospective register of systematic reviews, PROSPERO (CRD42017057053). This network meta-analysis was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses[15] and Cochrane guidelines[16].
Eligible studies were searched from PubMed, Medline, EMBASE, Cochrane database, and Google scholar, using a combination of following terms “pancreatic cancer”, “pancreatic neoplasm”, “neoadjuvant therapy”, and “adjuvant therapy”. A manual search through published articles was performed additionally. No publication year was restricted in the search. The search was carried out independently by two au-thors.
The following inclusion criteria were used: (a) Randomized controlled trials; (b) Studies investigating surgery alone, neoadjuvant therapies, or adjuvant therapies for resectable pancreatic cancer; and (c) Studies that had at least one of the following outcomes: Survival and toxicity. Single-arm studies, nonrandomized cohort studies, and studies comparing different ways of adjuvant or neoadjuvant treatment were not included in this network meta-analysis.
The information on study design, methods, patient characteristics, treatment protocols, and outcome (overall survival and toxicity) was extracted independently by two authors. We extracted reported adjusted hazard ratios (HRs) to measure overall survival. When HRs were not reported, we estimated them from summary statistics (Kaplan-Meier curves) in accordance with practical methods for incorporating summary time-to-event data into meta-analysis[17]. If there was no enough information to estimate HRs, median survival durations would be used in this network meta-analysis[18]. Only grade 3 or 4 toxicities (overall toxicities and haematological toxicities) were extracted and analyzed in this network meta-analysis. The quality of ran-domized control study was assessed by the Cochrane Collaboration’s tool[19]. Data collection and study quality assessment were performed following the Quality of Reporting of Meta-Analyses statement.
The study outcomes were overall survival and toxicity after neoadjuvant or adjuvant therapies. For network meta-analysis of overall survival, the preferred outcome measure was reported HRs, followed by estimated HRs and median survival du-rations. Relative treatment effects (HRs) in multi-arm trials were converted to arm-specific outcomes[18]. For network meta-analysis of toxicity (overall toxicity and haematological toxicities), we used odds ratios (ORs) as outcome measures. ORs were calculated from the summary number of reported toxicity events and summary number of exposure patients in each trial. Since the definition and reporting type of toxicity were diverse in the included studies, we only summarize seven toxicity events [nausea/vomiting, infection/fever, asthenia/fatigue, diarrhea, hematological toxicity (leukopenia, thrombopenia, and anemia)] as overall toxicity.
For direct comparisons, standard pairwise meta-analysis was performed using the inverse variance DerSimonian-Laird random-effects model. Heterogeneity was quantified using I-squared statistic. Publication bias was evaluated using the funnel plot. Traditional pairwise meta-analysis was performed using REVIEW MANAGER (version 5.0 for Windows; the Cochrane Collaboration, Oxford, United Kingdom).
For indirect comparisons, we conducted random-effects Bayesian network meta-analysis using Markov chain Monte Carlo methods in The R Programming Language 3.3.2 [R Core Team (2016), R Foundation for Statistical Computing, Vienna, Austria]. Network meta-analysis assumes “consistency” of treatment effects across all included randomized trials, that is, the direct and indirect estimates are the same effects. Network consistency was evaluated by comparing the direct estimates to the indirect estimates using the node splitting model. We used non-informative uniform and normal prior distributions in network meta-analysis. And we used a thinning interval of 500 for each chain and yielded 5000 iterations to obtain the posterior distributions of model parameters. Convergence of iterations was assessed using Gelman-Rubin-Brooks statistic. Trace plot and density plot were used to assess the convergence of the model. The summary effect of each comparison will be presented as point estimate (HR) and the corresponding 95% confidence interval (CI). The probability of each arm achieving the best rank among all the options was calculated and is presented as rankogram. The efficacy of different treatments was ranked using rankogram.
We evaluated the quality of evidence of estimates from direct comparison and network meta-analysis following the GRADE approach. The quality of evidence has four levels orderly: High, moderate, low, and very low quality. In this approach, the quality of direct evidence from RCTs is high initially and can be rated down based on risk of bias, indirectness, imprecision, inconsistency, or publication bias. The quality of indirect evidence starts at the lowest level of direct evidence that contributes as the preferred loops to the indirect evidence, and can be rated down based on imprecision or intransitivity. Network meta-analysis combines both direct and indirect evidence to reach a more comprehensive result, thus, the quality of evidence from network meta-analysis is assigned with the higher level of the direct and indirect evidence.
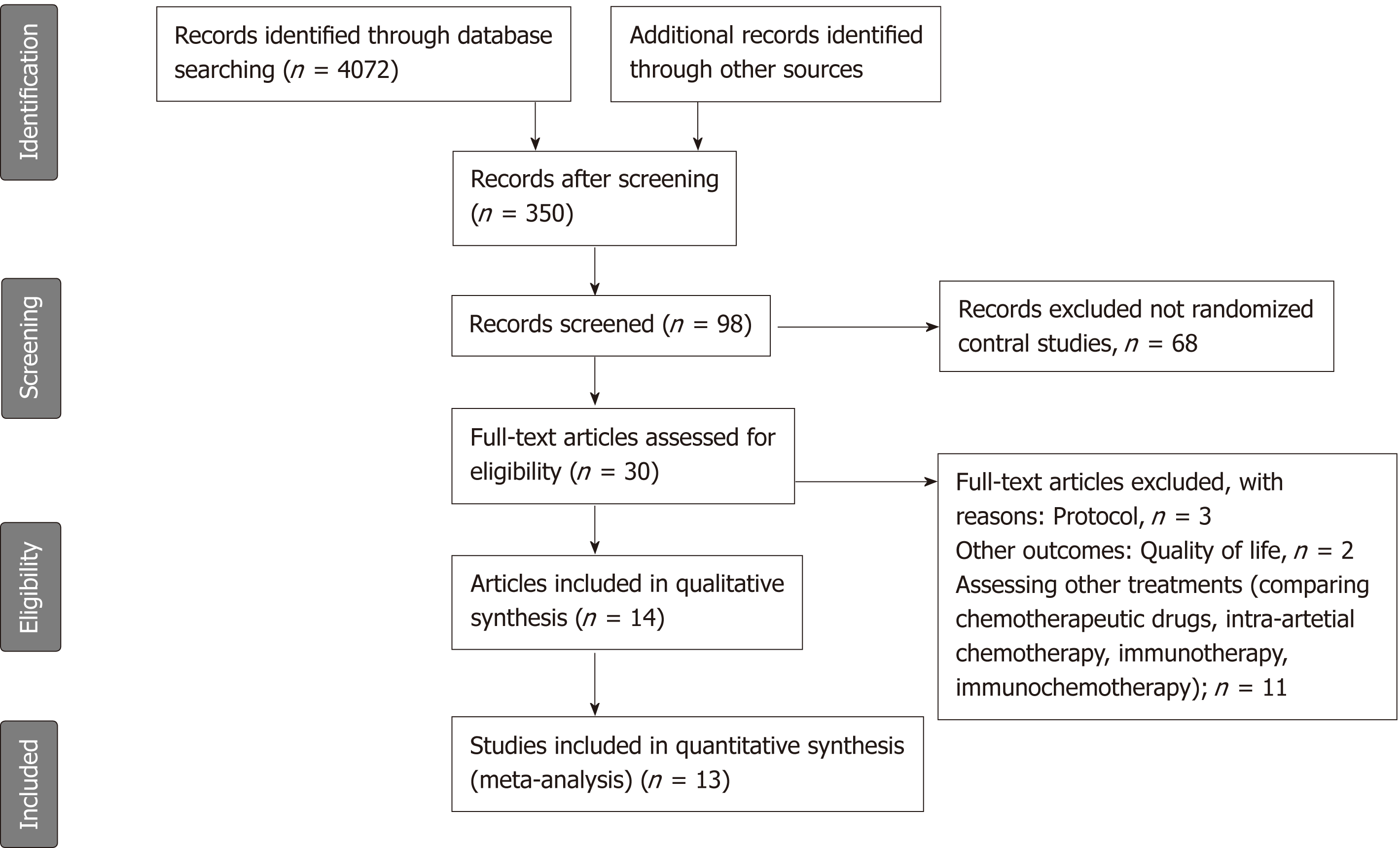
We identified 350 potentially relevant articles without duplicates from database searches and manual searches. After initial screening of these records, we excluded 252 articles because they investigated neither neoadjuvant nor adjuvant therapy of pancreatic cancer. We detailedly assessed the remaining 98 articles by abstracts and excluded 68 not reporting randomized control studies. After assessing full texts of the potential eligible 30 articles, we included 14 articles[8,20-32] (13 trials) in the network meta-analysis (Figure 1). If a single trial was reported in different publications, we combined the data of the different publications. And if a single outcome in a same trial was reported in different publications, the result of the latest publication would be used. The ESPAC-1 trial[29] included three subgroups, as the subgroup with two-by-two factorial design was updated in the following report[28]; this subgroup comparison was recognized as ESPAC-plus trial[28] and the last two subgroups as ESPAC-1 trial[29] in this meta-analysis. Also, we included data from ESPAC-3-v1[25] which was not included in the ESPAC-1 trial to avoid duplication.
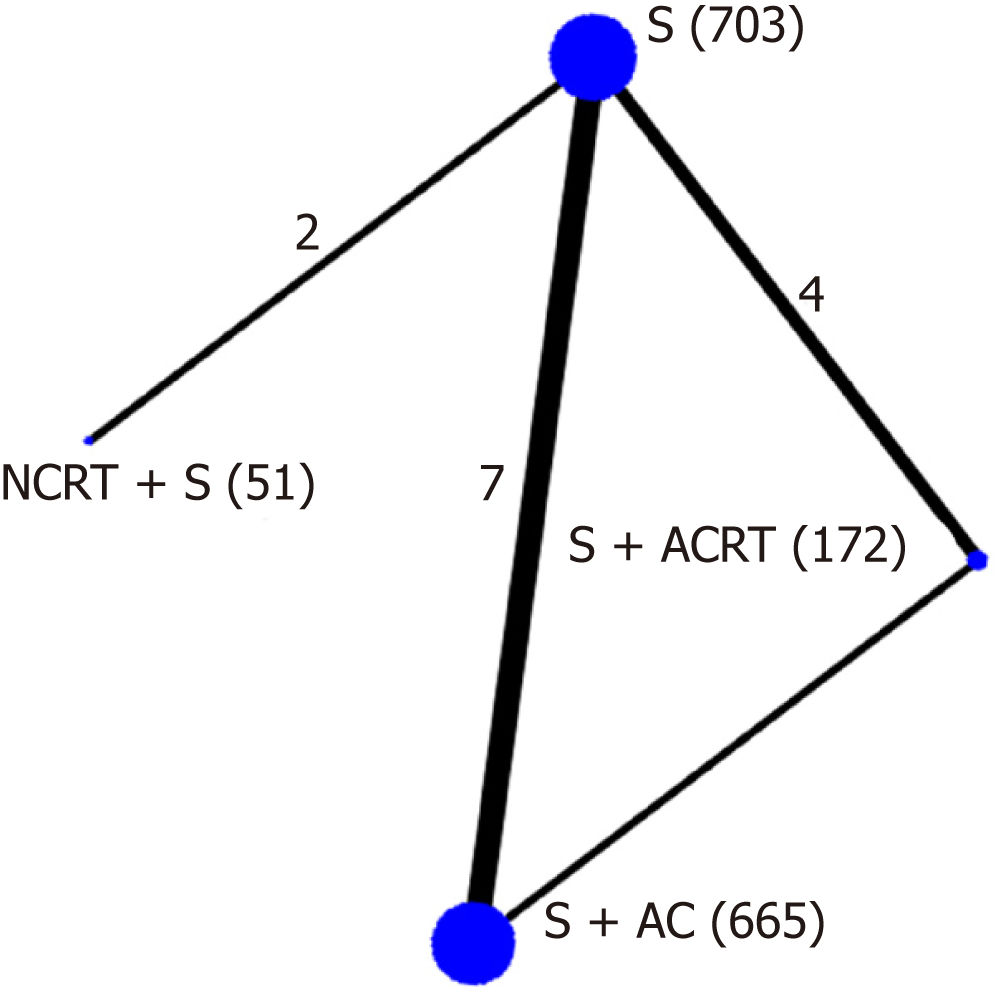
The methodological quality of the included 13 trials was high (Supplemental Table 1). Four trials did not report sequence and six trials did not report allocation concealment. Although blinding was not reported in any trial, the primary outcome (overall survival) would not be affected by blinding or not, and a low risk of bias was recognized. Finally, we included 13 high quality trials with 1591 participants in this network meta-analysis (Figure 2). A total of 1591 participants were randomized to receive either neoadjuvant CRT with surgery (n = 51), surgery alone (n = 703), surgery with adjuvant CT (n = 665), or surgery with adjuvant CRT (n = 172).
| Study | Arms | Number | Period | Country | Schedule |
| Casadei et al[20], 2015 | NCRT + S | 18 | 2007-2014 | Italy | 2 cycles of gemcitabine 1000 mg/m2 on days 1 and 8 every 21 d, then 45 Gy radiation with gemcitabine 50 mg/m2 twice weekly for 6 wk |
| Surgery | 20 | ||||
| Golcher et al[21], 2015 | NCRT + S | 33 | 2003-2009 | Germany, Switzerland | 8 Gy to 55.8 Gy (tumor) or 50.4 Gy (regional lymph nodes) radiation with gemcitabine 300 mg/m2 and cisplatin 30 mg/m2 on days 1, 8, 22, and 29 |
| Surgery | 33 | ||||
| Oettle et al[22,26], 2007, 2013 | S + ACT | 179 | 1998-2004 | Germany, Austria | 3 cycles of gemcitabine 1000 mg/m2 on days 1, 8, and 15 every 4 wk |
| Surgery | 175 | ||||
| Kosuge et al[27], 2006 | S + ACT | 45 | 1992-2000 | Japan | 2 courses of cisplatin 80 mg/m2 on the first day; 5-fluorouracil 500 mg/m2 daily for the first 5 d |
| Surgery | 44 | ||||
| Ueno et al[24], 2009 | S + ACT | 58 | 2002-2005 | Japan | 3 cycles of gemcitabine 1000 mg/m2 on days 1, 8, and 15 every 4 wk |
| Surgery | 60 | ||||
| Bakkevold et al[31], 1993 | S + ACT | 30 | 1984-1987 | Norway | 6 cycles of 5-fluorouracil 500 mg/m2, doxorubicin 40 mg/m2, and mitomycin C 6 mg/m2 once every 3 wk |
| Surgery | 31 | ||||
| Smeenk et al[8], 2007 Klinkenbijl et al[30], 1999 | S + ACDT | 110 | 1987-1995 | Europe | 2 courses of 20 Gy radiotherapy (2 Gy/d, 5 d/wk at weeks 1-2 and 5-6) and 25 mg/kg 5- fluorouracil daily for 5 d |
| Surgery | 108 | ||||
| Kalser et al[32], 1985 | S + ACDT | 21 | 1974-1982 | USA | 2 courses of 20 Gy (5 d a week) radiotherapy and 500 mg/m2 fluorouracil daily for 3 d |
| Surgery | 22 | ||||
| Van Laethem et al[23], 2010 | S + ACDT | 45 | 2004-2007 | France | 2 cycles of gemcitabine 1000 mg/m2 weekly for 3 wk; followed by 50.4 Gy radiotherapy and 300 mg/m2 gemcitabine weekly for two weeks |
| S + ACT | 45 | 4 cycles of gemcitabine 1000 mg/m2 weekly for 3 wk | |||
| Neoptolemos et al[25,28,29], 2001, 2004, 2009 (ESPAC-1) | S + ACDT | 73 | 1994-2000 | Europe | 2 courses of 20 Gy radiotherapy and 500 mg/m2 fluorouracil on days 1-3 |
| S + ACT | 75 | 6 courses of fluorouracil 425 mg/m2 and folinic acid 20 mg/m2 daily for 5 d | |||
| S + ACT + ACDT | 72 | 2 courses of 20 Gy radiotherapy and 500 mg/m2 fluorouracil on days 1-3; then 6 courses of fluorouracil 425 mg/m2 and folinic acid 20 mg/m2 daily for 5 d | |||
| Surgery | 69 | ||||
| ESPAC-1 plus | S + ACDT | 33 | 1994-2000 | Europe | 2 courses of 20 Gy radiotherapy and 500 mg/m2 fluorouracil on days 1–3 |
| Surgery | 36 | ||||
| S + ACT | 97 | 6 courses of fluorouracil 425 mg/m2 and folinic acid 20 mg/m2 daily for 5 d | |||
| Surgery | 95 | ||||
| ESPAC-3 (V1) | S + ACT | 61 | 1994-2000 | Europe | 6 courses of fluorouracil 425 mg/m2 and folinic acid 20 mg/m2 daily for 5 d |
| Surgery | 61 |
The characteristics of the 13 included trials are summarized in Table 1 and Supplemental Table 2. All of the included trials were two-arm studies except the ESPAC-1 plus trial[28], which was a four-arm trial using a two-by-two factorial design. The recruitment period ranged from 3 to 8 years. Both pancreatic adenocarcinoma and invasive ductal pancreatic cancer were included in this meta-analysis. For trials including periampullary carcinoma, the data about periampullary cancer were excluded[8]. The median age ranged from 57 to 71.5 years old. Most (> 90%) of included participants had primary tumor stage T1-T3, and most of them had nodal status N0-N1. The schedule of CT or CRT can be recognized briefly in Table 1.
| Intervention | Direct meta-analysis | Network meta-analysis | ||
| HR | Evidence | HR | Evidence | |
| Compared to surgery alone | ||||
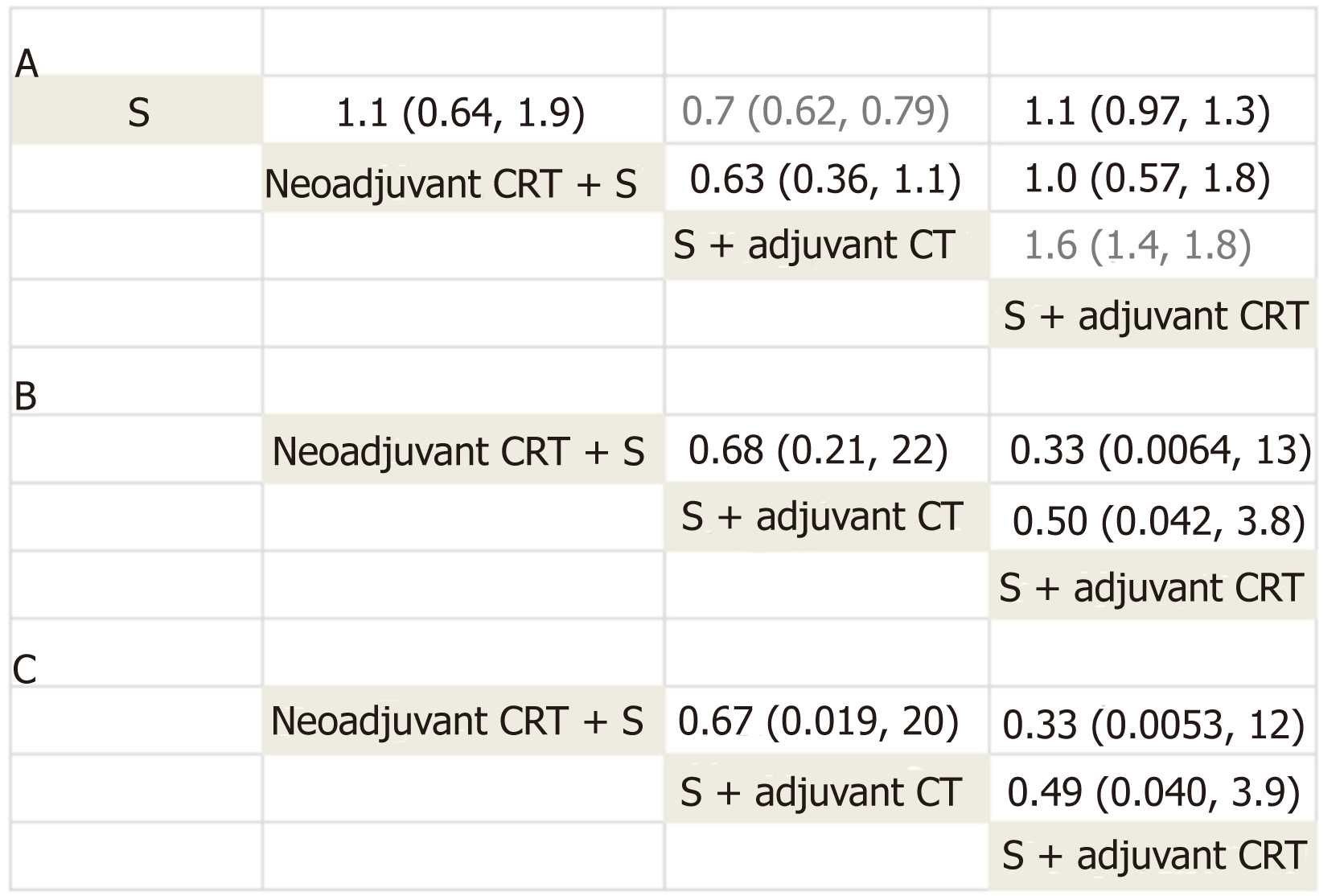
| Neoadjuvant CRT + S | 0.96 (0.68, 1.37) | ⊝⊝ Low1,2 | 1.10 (0.64, 1.90) | Low |
| S + adjuvant CT | 0.75 (0.63, 0.89) | High | 0.70 (0.62, 0.79) | High |
| S + adjuvant CRT | 0.88 (0.51, 1.54) | ⊝Moderate3 | 1.10 (0.97, 1.30) | Moderate |
| Compared to neoadjuvant CRT + S | ||||
| S + adjuvant CT | - | - | 0.63 (0.36, 1.10) | Low |
| S + adjuvant CRT | - | - | 1.00 (0.57, 1.80) | Low |
| Compared to S + adjuvant CT | ||||
| S + adjuvant CRT | 0.98(0.59, 1.64) | ⊝⊝ Low4 | 1.6 (1.40, 1.80) | Moderate |
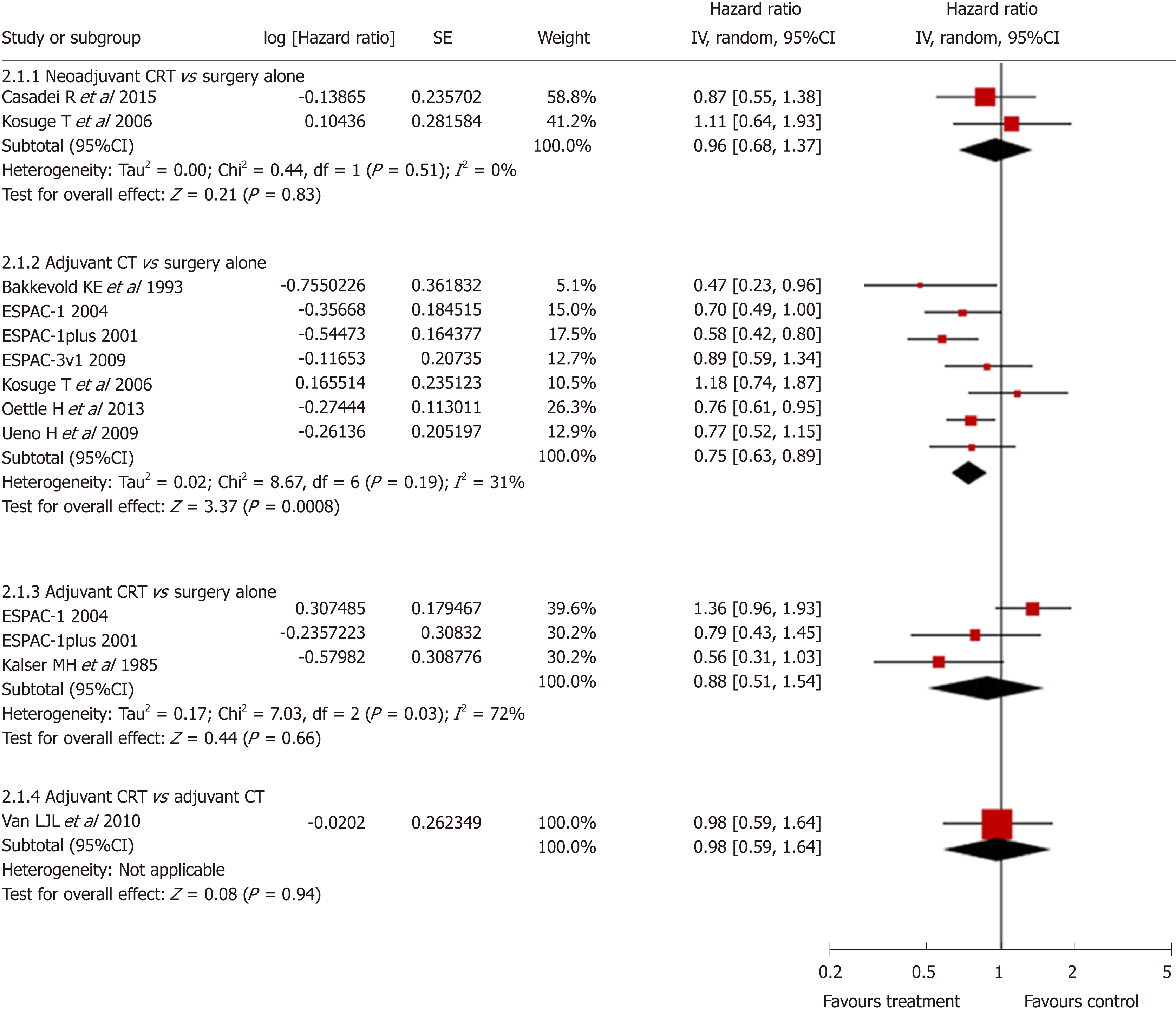
Standard pairwise meta-analysis of direct comparisons was feasible for the following comparisons: neoadjuvant CRT with surgery vs surgery alone (2 trials, n = 104), surgery with adjuvant CT vs surgery alone (7 trials, n = 1080), and surgery with adjuvant CRT vs surgery alone (3 trials, n = 254), and surgery with adjuvant CRT vs surgery with adjuvant CT (1 trial, n = 90). Only surgery with adjuvant CT showed a statistically significant survival advantage compared with surgery alone (pooled HR = 0.75, 95%CI: 0.63-0.89; P < 0.001) (Figure 3). No statistical difference was found in other direct comparisons. Heterogeneity was found only in the comparison of surgery with adjuvant CRT vs surgery alone (I2 = 72%). No publication bias was found using the funnel plot.
All 12 trials reported information on survival and were included for Bayesian network meta-analysis. Density plot, trace plot, and Brooks-Gelman-Rubin diagnosis plot in Bayesian network meta-analysis of overall survival showed satisfied convergence of network plot model (Supplemental Figure 1). We summarize the result of network meta-analysis of overall survival in Figure 4. Surgery with adjuvant CT showed statistically better overall survival compared with surgery alone (pooled HR = 0.7, 95%CI: 0.62-0.79), which is similar to the results in direct comparison. Surgery with adjuvant CT also statistically improved survival compared with surgery with adjuvant CRT (pooled HR = 0.6, 95%CI: 0.54-0.72). No significant results were found between other comparisons (neoadjuvant CRT with surgery vs surgery alone, surgery with adjuvant CRT vs surgery alone, surgery with adjuvant CT vs neoadjuvant CRT with surgery, and surgery with adjuvant CRT vs surgery with adjuvant CT) (Figure 4).
Network meta-analysis results are consistent with the results from traditional pairwise meta-analysis, suggesting no inconsistency between direct and indirect evidence. We also compared the results of direct and corresponding indirect com-parison using node-splitting model. No inconsistency was found (surgery with adjuvant CT vs surgery alone, P = 0.789; surgery with adjuvant CRT vs surgery alone, P = 0.562; and surgery with adjuvant CT vs surgery with adjuvant CRT P = 0.205). Heterogeneity between studies was found using the random-effects model (I2pair = 59.9; I2cons = 67.6).
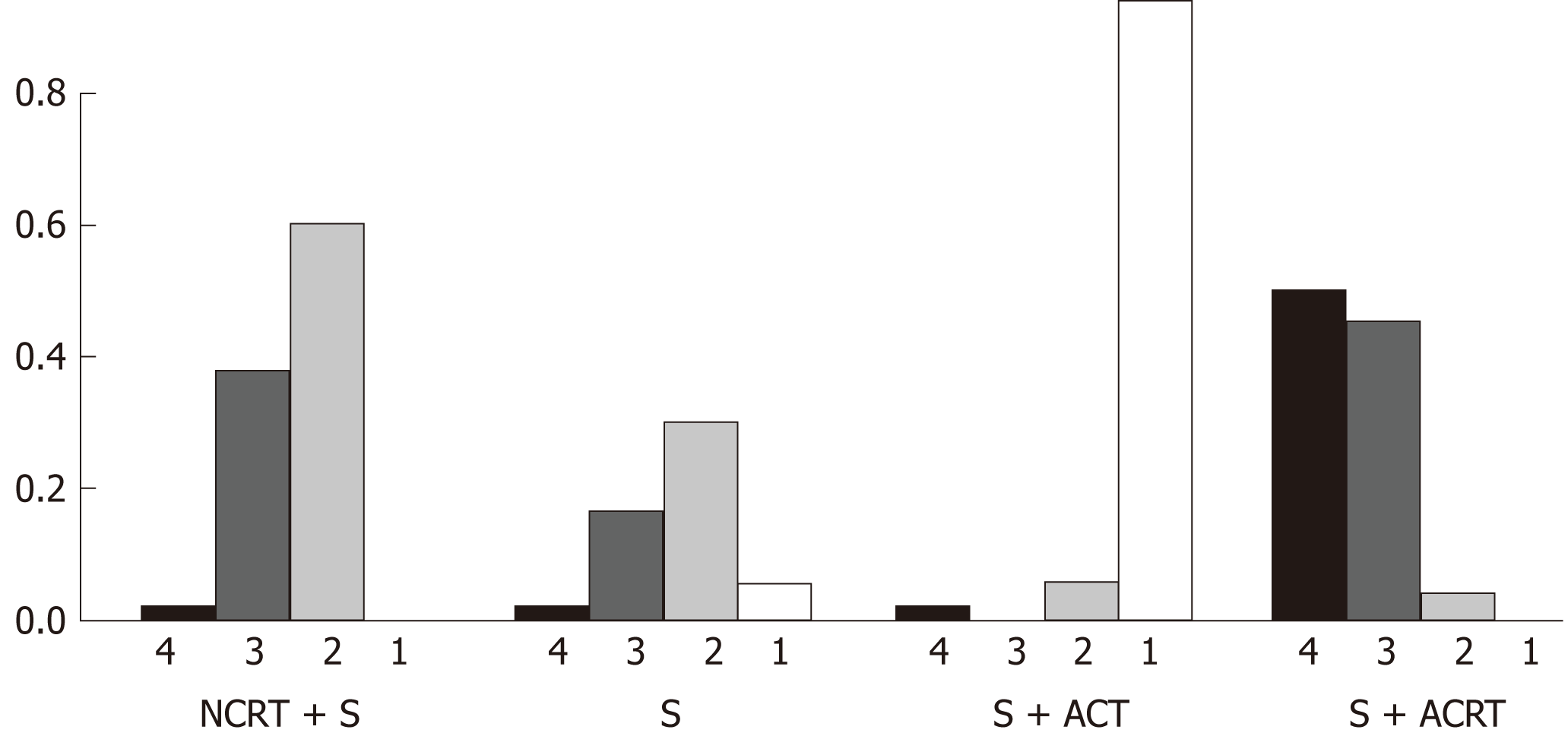
Rankogram (Figure 5) summarizes the ranking probability of the four treatment strategies in terms of overall survival. Surgery with adjuvant CT had the highest probability (94.2%) to rank the best in terms of improving overall survival, followed by surgery alone (5.8%), neoadjuvant CRT with surgery (0%), and surgery with adjuvant CRT (0%).
The results of grading the quality of evidence for overall survival are summarized in Table 2. Based on network meta-analysis, high quality evidence supported surgery with adjuvant CT over surgery alone for increasing overall survival. Moderate quality evidence supported surgery with adjuvant CT over surgery with adjuvant CRT for increasing overall survival.
Data on toxicity were available in seven trials. We summarize all the reported toxicity events [nausea/vomiting, infection/Fever, asthenia/Fatigue, diarrhea, and he-matological toxicity (leukopenia, thrombopenia, and anemia)] in Supplemental Table 3. Neoadjuvant or adjuvant CT and CRT were well tolerated, and grade 3 or 4 toxicities occurred infrequently. We summarize the result of network meta-analysis on overall toxicity and haematological toxicity in Figure 4. Density plot, trace plot, and Brooks-Gelman-Rubin diagnosis plot showed satisfied convergence of network plot model (Supplemental Figure 2). No significant differences in overall toxicity or haematological toxicity were found between all the comparisons (neoadjuvant CRT with surgery, surgery with adjuvant CRT, and surgery with adjuvant CT) (Figure 4).
This study is the first analysis to compare efficacy of neoadjuvant therapies, adjuvant therapies, and surgery alone for resectable pancreatic cancer together in a single analysis. In our network meta-analysis, we included 13 high quality trials with 1591 participants. We demonstrated three principal findings in our analysis: surgery with adjuvant CT has better survival compared with surgery alone and surgery with adjuvant CRT; neoadjuvant CRT with surgery shows no significant difference in survival compared with surgery alone and adjuvant therapies; and toxicities after CT or CRT are well tolerated and show no significant difference among the treatment strategies included in this meta-analysis.
In our network meta-analysis, high quality evidence confirmed the survival advantage of adjuvant CT over surgery alone. Although overall survival associated with adjuvant CT had been evaluated in several head-to-head comparisons[22,24,25,27,31], the absence of statistical significance led to equivocal conclusions[24,31]. Previous meta-analysis also demonstrated a survival difference when comparing surgery alone and surgery with adjuvant CT[12-14]. However, the most recent meta-analysis[13] was performed in 2007 and only included five randomized control studies. Moreover, it used only median survival time and 5-year survival rate instead of HRs to estimate survival difference, which was less precise. In our study, we estimated the survival difference by combining direct and indirect comparisons of different treatments. Moreover, we used both reported HRs and estimated HRs from all the included studies to minimize the selection bias. Thus, we provided the most powerful and reliable evidence that adjuvant CT is better than surgery alone in increasing overall survival for resectable pancreatic cancer.
The survival difference between adjuvant CT and adjuvant CRT for resectable pancreatic cancer remains controversial. Only a few studies demonstrated the survival difference between adjuvant CT and adjuvant CRT[23,29]. A phase II ran-domized controlled study involving 90 participants compared the toxicity and survival between adjuvant gemcitabine alone and gemcitabine-based CRT, and no significant difference was found in survival due to small sample size[23]. The ESPAC-1 trial compared the survival using a two-by-two factorial design (observation, CRT alone, CT alone, or both)[29]. However, the trial was not powered to compare these four groups directly, and only found a potential benefit of adjuvant CT but not adjuvant CRT. In our study, moderate quality evidence supported surgery with adjuvant CT over surgery with adjuvant CRT for increasing overall survival. We confirmed the survival benefit of adjuvant CT over surgery with adjuvant CRT for the first time. Pancreatic cancer is a systemic disease and micrometastasis after surgery may be responsible for high recurrence and low survival. Thus, adjuvant CT but not CRT can benefit the survival of pancreatic cancer patients after surgery. However, CRT in the included studies was performed mainly using external beam, and more highly targeted radiotherapy is now available. The survival benefit between highly targeted radiotherapy and adjuvant CT should be reevaluated in the future study.
CT agents for adjuvant CT are diverse. It is still controversial regarding the best CT agents for adjuvant CT. The ESPAC-3 trial demonstrated that fluorouracil plus folinic acid resulted in similar overall survival to gemcitabine in patients after complete resection of pancreatic cancer[33]. A recent network meta-analysis showed that adjuvant CT with fluorouracil or gemcitabine provided better overall survival than observation[34]. S-1 is another new CT agent for pancreatic cancer. Recent randomized control trials showed that S-1 was superior to gemcitabine, suggesting that S-1 is a new standard care for resected pancreatic cancer[35-37]. In our study, CT agents for adjuvant therapy included gemcitabine[22,24], cisplatin[27], 5-fluorouracil plus doxorubicin plus mitomycin C[31], and fluorouracil plus folinic acid[31]. We combined all of the adjuvant CT with different CT agents in a single arm in this network meta-analysis, because we assumed that the effect of different CT agents for adjuvant CT was consistent. Besides, we tried to compare the effect difference of adjuvant CT with adjuvant CRT and neoadjuvant CRT, and the effect difference was not affected by different CT agents.
The necessity and survival benefit of neoadjuvant therapy for pancreatic cancer is controversial. Borderline pancreatic cancer recently emerged as a category clinically distinct from resectable or locally advanced disease. Neoadjuvant therapy is currently recommended for borderline resectable disease in the National Comprehensive Cancer Network guidelines[38,39]. However, only two reported RCTs access neo-adjuvant CRT for resectable pancreatic cancer so far, and both two RCTs found no survival benefit of neoadjuvant CRT. One of the included RCTs involving 38 participants chose R0 resection as the primary endpoint[20], and another RCT involving 66 patients was terminated early due to slow recruiting[21]. Neoadjuvant therapy is also assessed in our network meta-analysis. Only neoadjuvant CRT with surgery was assessed, as no RCTs about neoadjuvant CT can be found. We found no significant result when comparing neoadjuvant CRT with surgery alone, adjuvant CT, and adjuvant CRT. Now, several randomized controlled trials are ongoing to investigate the survival benefit of neoadjuvant CRT for the treatment of borderline and resectable pancreatic cancer[40-43]. Our result showed no survival benefit of neoadjuvant CRT. Thus, we should be cautious with using neoadjuvant CRT for resectable pancreatic cancer until other powerful evidence exists.
Our network meta-analysis has several strengths. It is the first comprehensive analysis of all the major treatment strategies for resectable pancreatic cancer including neoadjuvant therapy, surgery, and adjuvant therapy. We combined both direct and indirect evidence to reach more precise conclusions, which also allowed us to compare therapies indirectly and rank different therapies clearly. Furthermore, we assessed both overall survival and toxicity of all the therapies. Our meta-analysis provides comprehensive and clear evidence for the treatment of resectable pancreatic cancer, which is great important and meaningful in clinical care.
The limitations of this meta-analysis also need to be acknowledged. First of all, the RCTs included in this analysis were conducted over four decades, and changes in CRT schedule, CT agents, schedules, and surgery techniques may affect the results. However, transitivity assumption was met and there was no evidence of statistically significant inconsistency in this network. This may have less effect on the result. Second, we included both neoadjuvant and adjuvant therapies to offer a com-prehensive overview. However, we included only a limited number of trials (n = 13), and only two trials evaluated neoadjuvant therapies. Thus, although no sig-nificant result about overall survival was found when comparing neoadjuvant therapies with other treatments, this conclusion about neoadjuvant therapies should be interpreted with some caution. Finally, since the definition and reporting type of toxicity were diverse in the included studies, we only summarized seven typical toxicity events as overall toxicity. Although some toxicity events may be neglected in this analysis, the results should still provide effective estimates.
In conclusion, our network meta-analysis show that surgery with adjuvant CT prolongs overall survival compared with surgery alone and surgery with adjuvant CRT. Therefore, we recommend surgery with adjuvant CT as the optimal care for resectable pancreatic cancer. Later research should be focused on the best agents for adjuvant CT.
Pancreatic cancer is one of the most common and lethal malignancies worldwide. The common treatment options for resectable pancreatic cancer include surgery alone, neoadjuvant chemo-therapy (CT), neoadjuvant chemoradiotherapy (CRT), adjuvant CT, and adjuvant CRT. However, the optimal treatment is still controversial.
The optimal treatment for resectable pancreatic cancer is still controversial.
This study aimed to identify the most effective approach for resectable pancreatic cancer using network meta-analysis.
Eligible studies were searched from PubMed, Medline, EMBASE, Cochrane database, and Google scholar. We searched and included randomized controlled trials reporting on neo-adjuvant and adjuvant therapies. For direct comparisons, standard pairwise meta-analysis was performed using the inverse variance DerSimonian-Laird random-effects model. For indirect comparisons, Bayesian network meta-analysis was used to combine direct and indirect evidence. We used relative hazard ratios (HRs) to estimate survival difference between different treat-ments, and relative odds ratios (ORs) for toxic effects. Treatment effects were ranked based on their efficacy for improving survival or reducing toxicity using rankogram. The quality of evidence of estimates from direct comparison and network meta-analysis were evaluated following the GRADE approach.
We included 13 high quality trials with 1591 participants in this network meta-analysis. Com-pared with surgery alone (pooled HR = 0.7, 95%CI: 0.62-0.79) and surgery with adjuvant CRT (pooled HR = 0.6, 95%CI: 0.54-0.72), surgery with adjuvant CT had a higher rate of overall survival. In contrast, standard pairwise meta-analysis only showed a statistically significant survival advantage of surgery with adjuvant CT compared with surgery alone (pooled HR = 0.75, 95%CI: 0.63-0.89; P < 0.001). Rankogram showed that surgery with adjuvant CT was most likely to rank the best in terms of overall survival (probability: 94.2%), followed by surgery alone (probability: 5.8%). No significant differences in overall toxicity or haematological toxicity were found between all the therapies. High quality evidence supported surgery with adjuvant CT over surgery alone for increasing overall survival. Moderate quality evidence supported surgery with adjuvant CT over surgery with adjuvant CRT for increasing overall survival.
Our network meta-analysis show that surgery with adjuvant CT prolongs overall survival compared with surgery alone and surgery with adjuvant CRT.
We recommend surgery with adjuvant CT as the optimal care for resectable pancreatic cancer. Later research should be focused on the best agents for adjuvant CT.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Hara K, Ahmed M, Fabozzi M, Bramhall S S-Editor: Dou Y L-Editor: Wang TQ E-Editor: Ma YJ
| 1. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20511] [Article Influence: 2051.1] [Reference Citation Analysis (20)] |
| 2. | Carpelan-Holmström M, Nordling S, Pukkala E, Sankila R, Lüttges J, Klöppel G, Haglund C. Does anyone survive pancreatic ductal adenocarcinoma? A nationwide study re-evaluating the data of the Finnish Cancer Registry. Gut. 2005;54:385-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 233] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 3. | Gooiker GA, van Gijn W, Wouters MW, Post PN, van de Velde CJ, Tollenaar RA; Signalling Committee Cancer of the Dutch Cancer Society. Systematic review and meta-analysis of the volume-outcome relationship in pancreatic surgery. Br J Surg. 2011;98:485-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 273] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 4. | Richter A, Niedergethmann M, Sturm JW, Lorenz D, Post S, Trede M. Long-term results of partial pancreaticoduodenectomy for ductal adenocarcinoma of the pancreatic head: 25-year experience. World J Surg. 2003;27:324-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 286] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 5. | Silvestris N, Brunetti O, Vasile E, Cellini F, Cataldo I, Pusceddu V, Cattaneo M, Partelli S, Scartozzi M, Aprile G, Casadei Gardini A, Morganti AG, Valentini V, Scarpa A, Falconi M, Calabrese A, Lorusso V, Reni M, Cascinu S. Multimodal treatment of resectable pancreatic ductal adenocarcinoma. Crit Rev Oncol Hematol. 2017;111:152-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Kim SM, Eads JR. Adjuvant and Neoadjuvant Therapy for Resectable Pancreatic and Periampullary Cancer. Surg Clin North Am. 2016;96:1287-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Liu W, Fu XL, Yang JY, Liu DJ, Li J, Zhang JF, Huo YM, Yang MW, Hua R, Sun YW. Efficacy of Neo-Adjuvant Chemoradiotherapy for Resectable Pancreatic Adenocarcinoma: A PRISMA-Compliant Meta-Analysis and Systematic Review. Medicine (Baltimore). 2016;95:e3009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Smeenk HG, van Eijck CH, Hop WC, Erdmann J, Tran KC, Debois M, van Cutsem E, van Dekken H, Klinkenbijl JH, Jeekel J. Long-term survival and metastatic pattern of pancreatic and periampullary cancer after adjuvant chemoradiation or observation: long-term results of EORTC trial 40891. Ann Surg. 2007;246:734-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 225] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 9. | Tang K, Lu W, Qin W, Wu Y. Neoadjuvant therapy for patients with borderline resectable pancreatic cancer: A systematic review and meta-analysis of response and resection percentages. Pancreatology. 2016;16:28-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 104] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 10. | Petrelli F, Coinu A, Borgonovo K, Cabiddu M, Ghilardi M, Lonati V, Aitini E, Barni S; Gruppo Italiano per lo Studio dei Carcinomi dell’Apparato Digerente (GISCAD). FOLFIRINOX-based neoadjuvant therapy in borderline resectable or unresectable pancreatic cancer: a meta-analytical review of published studies. Pancreas. 2015;44:515-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 142] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 11. | Festa V, Andriulli A, Valvano MR, Uomo G, Perri F, Andriulli N, Corrao S, Koch M. Neoadjuvant chemo-radiotherapy for patients with borderline resectable pancreatic cancer: a meta-analytical evaluation of prospective studies. JOP. 2013;14:618-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 12. | Yu Z, Zhong W, Tan ZM, Wang LY, Yuan YH. Gemcitabine Adjuvant Therapy for Resected Pancreatic Cancer: A Meta-analysis. Am J Clin Oncol. 2015;38:322-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Boeck S, Ankerst DP, Heinemann V. The role of adjuvant chemotherapy for patients with resected pancreatic cancer: systematic review of randomized controlled trials and meta-analysis. Oncology. 2007;72:314-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Stocken DD, Büchler MW, Dervenis C, Bassi C, Jeekel H, Klinkenbijl JH, Bakkevold KE, Takada T, Amano H, Neoptolemos JP; Pancreatic Cancer Meta-analysis Group. Meta-analysis of randomised adjuvant therapy trials for pancreatic cancer. Br J Cancer. 2005;92:1372-1381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 268] [Cited by in RCA: 240] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 15. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13930] [Cited by in RCA: 13343] [Article Influence: 833.9] [Reference Citation Analysis (0)] |
| 16. | Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. Available from: URL: http://handbook-5-1.cochrane.org/. |
| 17. | Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4738] [Cited by in RCA: 4951] [Article Influence: 275.1] [Reference Citation Analysis (0)] |
| 18. | Woods BS, Hawkins N, Scott DA. Network meta-analysis on the log-hazard scale, combining count and hazard ratio statistics accounting for multi-arm trials: a tutorial. BMC Med Res Methodol. 2010;10:54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 245] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 19. | Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18487] [Cited by in RCA: 24807] [Article Influence: 1771.9] [Reference Citation Analysis (3)] |
| 20. | Casadei R, Di Marco M, Ricci C, Santini D, Serra C, Calculli L, D'Ambra M, Guido A, Morselli-Labate AM, Minni F. Neoadjuvant Chemoradiotherapy and Surgery Versus Surgery Alone in Resectable Pancreatic Cancer: A Single-Center Prospective, Randomized, Controlled Trial Which Failed to Achieve Accrual Targets. J Gastrointest Surg. 2015;19:1802-1812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 160] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 21. | Golcher H, Brunner TB, Witzigmann H, Marti L, Bechstein WO, Bruns C, Jungnickel H, Schreiber S, Grabenbauer GG, Meyer T, Merkel S, Fietkau R, Hohenberger W. Neoadjuvant chemoradiation therapy with gemcitabine/cisplatin and surgery versus immediate surgery in resectable pancreatic cancer: results of the first prospective randomized phase II trial. Strahlenther Onkol. 2015;191:7-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 197] [Cited by in RCA: 237] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 22. | Oettle H, Neuhaus P, Hochhaus A, Hartmann JT, Gellert K, Ridwelski K, Niedergethmann M, Zülke C, Fahlke J, Arning MB, Sinn M, Hinke A, Riess H. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013;310:1473-1481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1180] [Cited by in RCA: 1358] [Article Influence: 113.2] [Reference Citation Analysis (0)] |
| 23. | Van Laethem JL, Hammel P, Mornex F, Azria D, Van Tienhoven G, Vergauwe P, Peeters M, Polus M, Praet M, Mauer M, Collette L, Budach V, Lutz M, Van Cutsem E, Haustermans K. Adjuvant gemcitabine alone versus gemcitabine-based chemoradiotherapy after curative resection for pancreatic cancer: a randomized EORTC-40013-22012/FFCD-9203/GERCOR phase II study. J Clin Oncol. 2010;28:4450-4456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 199] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 24. | Ueno H, Kosuge T, Matsuyama Y, Yamamoto J, Nakao A, Egawa S, Doi R, Monden M, Hatori T, Tanaka M, Shimada M, Kanemitsu K. A randomised phase III trial comparing gemcitabine with surgery-only in patients with resected pancreatic cancer: Japanese Study Group of Adjuvant Therapy for Pancreatic Cancer. Br J Cancer. 2009;101:908-915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 348] [Cited by in RCA: 320] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 25. | Neoptolemos JP, Stocken DD, Tudur Smith C, Bassi C, Ghaneh P, Owen E, Moore M, Padbury R, Doi R, Smith D, Büchler MW. Adjuvant 5-fluorouracil and folinic acid vs observation for pancreatic cancer: composite data from the ESPAC-1 and -3(v1) trials. Br J Cancer. 2009;100:246-250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 159] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 26. | Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, Schramm H, Fahlke J, Zuelke C, Burkart C, Gutberlet K, Kettner E, Schmalenberg H, Weigang-Koehler K, Bechstein WO, Niedergethmann M, Schmidt-Wolf I, Roll L, Doerken B, Riess H. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297:267-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1779] [Cited by in RCA: 1763] [Article Influence: 97.9] [Reference Citation Analysis (0)] |
| 27. | Kosuge T, Kiuchi T, Mukai K, Kakizoe T; Japanese Study Group of Adjuvant Therapy for Pancreatic Cancer (JSAP). A multicenter randomized controlled trial to evaluate the effect of adjuvant cisplatin and 5-fluorouracil therapy after curative resection in cases of pancreatic cancer. Jpn J Clin Oncol. 2006;36:159-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 28. | Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, Beger H, Fernandez-Cruz L, Dervenis C, Lacaine F, Falconi M, Pederzoli P, Pap A, Spooner D, Kerr DJ, Büchler MW; European Study Group for Pancreatic Cancer. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1945] [Cited by in RCA: 1907] [Article Influence: 90.8] [Reference Citation Analysis (0)] |
| 29. | Neoptolemos JP, Dunn JA, Stocken DD, Almond J, Link K, Beger H, Bassi C, Falconi M, Pederzoli P, Dervenis C, Fernandez-Cruz L, Lacaine F, Pap A, Spooner D, Kerr DJ, Friess H, Büchler MW; European Study Group for Pancreatic Cancer. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomised controlled trial. Lancet. 2001;358:1576-1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 878] [Cited by in RCA: 753] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 30. | Klinkenbijl JH, Jeekel J, Sahmoud T, van Pel R, Couvreur ML, Veenhof CH, Arnaud JP, Gonzalez DG, de Wit LT, Hennipman A, Wils J. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg. 1999;230:776-82; discussion 782-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 947] [Cited by in RCA: 896] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 31. | Bakkevold KE, Arnesjø B, Dahl O, Kambestad B. Adjuvant combination chemotherapy (AMF) following radical resection of carcinoma of the pancreas and papilla of Vater--results of a controlled, prospective, randomised multicentre study. Eur J Cancer. 1993;29A:698-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 217] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 32. | Kalser MH, Ellenberg SS. Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg. 1985;120:899-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1024] [Cited by in RCA: 951] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 33. | Neoptolemos JP, Stocken DD, Bassi C, Ghaneh P, Cunningham D, Goldstein D, Padbury R, Moore MJ, Gallinger S, Mariette C, Wente MN, Izbicki JR, Friess H, Lerch MM, Dervenis C, Oláh A, Butturini G, Doi R, Lind PA, Smith D, Valle JW, Palmer DH, Buckels JA, Thompson J, McKay CJ, Rawcliffe CL, Büchler MW; European Study Group for Pancreatic Cancer. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA. 2010;304:1073-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1144] [Cited by in RCA: 999] [Article Influence: 66.6] [Reference Citation Analysis (0)] |
| 34. | Liao WC, Chien KL, Lin YL, Wu MS, Lin JT, Wang HP, Tu YK. Adjuvant treatments for resected pancreatic adenocarcinoma: a systematic review and network meta-analysis. Lancet Oncol. 2013;14:1095-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 164] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 35. | Uesaka K, Boku N, Fukutomi A, Okamura Y, Konishi M, Matsumoto I, Kaneoka Y, Shimizu Y, Nakamori S, Sakamoto H, Morinaga S, Kainuma O, Imai K, Sata N, Hishinuma S, Ojima H, Yamaguchi R, Hirano S, Sudo T, Ohashi Y; JASPAC 01 Study Group. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet. 2016;388:248-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 635] [Cited by in RCA: 783] [Article Influence: 87.0] [Reference Citation Analysis (0)] |
| 36. | Shimoda M, Kubota K, Shimizu T, Katoh M. Randomized clinical trial of adjuvant chemotherapy with S-1 versus gemcitabine after pancreatic cancer resection. Br J Surg. 2015;102:746-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 37. | Hagiwara Y, Ohashi Y, Uesaka K, Boku N, Fukutomi A, Okamura Y, Konishi M, Matsumoto I, Kaneoka Y, Shimizu Y, Nakamori S, Sakamoto H, Morinaga S, Kainuma O, Imai K, Sata N, Hishinuma S, Ojima H, Yamaguchi R, Hirano S, Sudo T; JASPAC 01 Study Group. Health-related quality of life of adjuvant chemotherapy with S-1 versus gemcitabine for resected pancreatic cancer: Results from a randomised phase III trial (JASPAC 01). Eur J Cancer. 2018;93:79-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 38. | Belli C, Cereda S, Anand S, Reni M. Neoadjuvant therapy in resectable pancreatic cancer: a critical review. Cancer Treat Rev. 2013;39:518-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 39. | Kelly KJ, Winslow E, Kooby D, Lad NL, Parikh AA, Scoggins CR, Ahmad S, Martin RC, Maithel SK, Kim HJ, Merchant NB, Cho CS, Weber SM. Vein involvement during pancreaticoduodenectomy: is there a need for redefinition of "borderline resectable disease"? J Gastrointest Surg. 2013;17:1209-17; discussion 1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 40. | Takahashi S, Ohno I, Ikeda M, Kobayashi T, Akimoto T, Kojima M, Konishi M, Uesaka K. Neoadjuvant S-1 with concurrent radiotherapy followed by surgery for borderline resectable pancreatic cancer: study protocol for an open-label, multicentre, prospective phase II trial (JASPAC05). BMJ Open. 2017;7:e018445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 41. | Labori KJ, Lassen K, Hoem D, Grønbech JE, Søreide JA, Mortensen K, Smaaland R, Sorbye H, Verbeke C, Dueland S. Neoadjuvant chemotherapy versus surgery first for resectable pancreatic cancer (Norwegian Pancreatic Cancer Trial - 1 (NorPACT-1)) - study protocol for a national multicentre randomized controlled trial. BMC Surg. 2017;17:94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 42. | Okada KI, Shimokawa T, Hirono S, Kawai M, Sho M, Satoi S, Matsumoto I, Eguchi H, Murakami Y, Yamada S, Doi M, Yamaue H; NAC-GA investigators. Effect of Neoadjuvant Nab-Paclitaxel plus Gemcitabine Therapy on Overall Survival in Patients with Borderline Resectable Pancreatic Cancer: A Prospective Multicenter Phase II Trial (NAC-GA Trial). Oncology. 2017;93:343-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 43. | Versteijne E, van Eijck CH, Punt CJ, Suker M, Zwinderman AH, Dohmen MA, Groothuis KB, Busch OR, Besselink MG, de Hingh IH, Ten Tije AJ, Patijn GA, Bonsing BA, de Vos-Geelen J, Klaase JM, Festen S, Boerma D, Erdmann JI, Molenaar IQ, van der Harst E, van der Kolk MB, Rasch CR, van Tienhoven G; Dutch Pancreatic Cancer Group (DPCG). Preoperative radiochemotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer (PREOPANC trial): study protocol for a multicentre randomized controlled trial. Trials. 2016;17:127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 130] [Article Influence: 14.4] [Reference Citation Analysis (0)] |