Published online Jun 30, 2019. doi: 10.13105/wjma.v7.i6.297
Peer-review started: April 23, 2019
First decision: May 24, 2019
Revised: May 31, 2019
Accepted: June 10, 2019
Article in press: June 10, 2019
Published online: June 30, 2019
Processing time: 68 Days and 23.5 Hours
Rumination syndrome (RS) is characterized by recurrent effortless postprandial regurgitation of recently ingested food from the stomach to the oral cavity and has been associated with quality of life impairment and malnutrition. There is a general lack of consensus on the most appropriate treatment options for RS.
To summarize the literature on treatment options for RS.
We conducted a systematic review according to PRISMA guidelines. We searched Medline (1946 to February 2019), EMBASE (1947 to February 2019), PsycINFO (1806 to February 2019) and Cochrane central register of controlled trials for articles discussing treatment options for adult patients (> 18 years) with RS. All relevant articles were accessed in full text. We extracted data on study designs, patient profiles, duration of symptoms, follow up periods, date, diagnostic criteria, interventions and outcomes. Risk of bias assessment was carried out independently by 3 reviewers via Cochrane Risk of Bias tool and Newcastle Ottawa Scale for randomized controlled trials and Cohort studies respectively.
Twelve articles were identified. A total of 254 patients were included in the analysis, with a mean age of 36.1 (range 18-89). 185 patients (72.8%) were females. 5 studies looked into behavioral therapies, primarily diaphragmatic breathing (DB) 2 studies looked at baclofen, 1 fundoplication and 1 supportive lifestyle changes. 3 studies looked at a combination of therapies involving pharmacological, behavioral and psychotherapies.
Although evidence for treatment options is still limited, the strongest evidence point towards the use of DB and Baclofen, and both should be considered depending on their availabilities.
Core tip: Rumination syndrome (RS) is a relatively common but underdiagnosed gastroenterological condition. Due to recent advances in research, we have decided to perform the first systematic review on treatment options for RS. Our results show that diaphragmatic breathing has the strongest data for efficacy in this condition.
- Citation: Ong AML, Tay SW, Wang YT. Treatment options for rumination syndrome: A systematic review. World J Meta-Anal 2019; 7(6): 297-308
- URL: https://www.wjgnet.com/2308-3840/full/v7/i6/297.htm
- DOI: https://dx.doi.org/10.13105/wjma.v7.i6.297
Rumination syndrome (RS) is characterized by recurrent effortless postprandial regurgitation of recently ingested food from the stomach to the oral cavity[1,2]. Although rumination was historically described mainly in children or adults with impaired mental development, it is now recognized in adults regardless of mental state[2,3]. Although not considered to be life-threatening, RS has been associated with quality of life impairment and even malnutrition[2].
Currently, the diagnosis of RS in adults is based on careful history and sub-sequently applying the Rome IV criteria[4], often also supported by postprandial High Resolution Impedance Manometry (HRIM) findings of reflux episodes associated with a preceding abdominal pressure increase of > 30 mmHg[5]. These findings also allow RS to be discriminated from gastroesophageal reflux disease (GERD), a common competing diagnosis in these patients, often resulting in several years of delay before the diagnosis of RS is made.
There is a general lack of consensus on the most appropriate treatment options for RS. Therefore, the aim of this systematic review is to summarize the literature on studies that looked into treatment options for adult RS patients, and ascertain what is most evidence-based approach in treating them.
We followed PRISMA guidelines and the medical literature was searched using OVID within the databases Medline (1946 to February 2019), EMBASE (1947 to February 2019), PsycINFO (1806 to February 2019) and Cochrane central register of controlled trials. Searches were based on controlled vocabulary including medical subject heading terms (MeSH) where possible (e.g., “rumination syndrome” and “eructa-tion”). In addition, a combination of keywords, free text terms and database-specific subject headings for rumination, RS, eructation, postprandial regurgitation, feeding disorder, treatment, therapy, behavioral therapy, non-pharmacological treatment were included. In case of multiple reports of one trial were found, we selected the most updated one.
Abstracts of the papers identified by the initial search were evaluated by the lead reviewer (AO) for appropriateness to the study question, and all potentially relevant papers were obtained and evaluated in detail. In order to identify potentially eligible studies published only in abstract form, conference proceedings (Digestive Diseases Week, American College of Gastroenterology, United European Gastroenterology Week, Federation of Neurogastroenterology and Motility) between 2001 and 2019 were also hand-searched.
The bibliographies of studies included in the final analysis as well as relevant reviews were also screened for additional relevant articles. The website Clinical-Trials.gov was also searched to look for trials not included in the mentioned databases.
We included studies evaluating management of adults over 18 with a diagnosis of RS. Articles involving pediatrics or patients with eating disorders, and those with no mention of treatment strategies or without identifiable outcomes were excluded. Editorials, case reports of single cases, letters, qualitative studies, clinical guidelines and narrative reviews were also excluded. Articles were restricted to English language. Titles and abstracts were then screened by 3 independent reviewers (Ong AML, Wang YT, Tay SW) onto a Microsoft (Richmond, VA) Excel spreadsheet. Using a standardized form, the 3 reviewers independently extracted data and assessed study risk of bias and quality using the Cochrane Risk of Bias tool[6] for randomized controlled trials (RCTs) and Newcastle Ottawa Scale (NOS)[7] for cohort studies. A trial was judged with low risk of bias when all six domains of the Cochrane risk of bias tool were classified as low risk of bias for RCTs. Studies that achieved at least six stars for the NOS were considered studies of high quality[7]. No attempts at assessing study quality was made for studies with case series. Any disagreements were resolved by consensus. No studies in the search were discarded because of assessed quality.
Data on study design, location, patient profile, duration of symptoms, follow up periods, date, diagnostic criteria, intervention, outcome, and follow-up were extracted. Due to significant heterogeneity among studies such as study design, treatment and outcome measurements, no head to head comparisons or meta-analysis was performed.
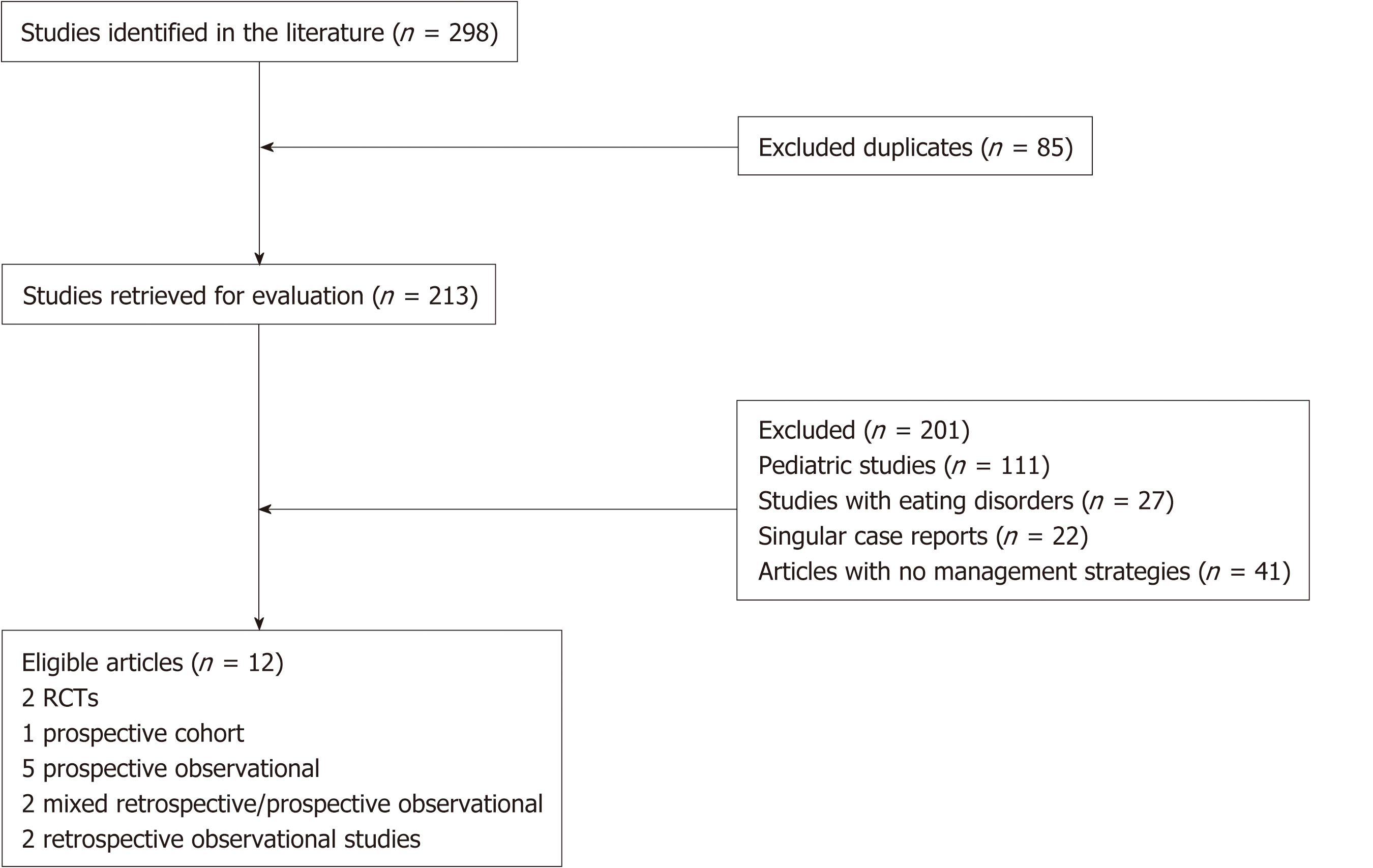
We retrieved 298 articles based on our search criteria (Figure 1). After excluding duplicates (n = 85), pediatric studies (n = 111), studies involving eating disorders (n = 27), singular case reports (n = 22) and studies without mention of treatment strategies (n = 41), we arrived at 12 studies for analysis (Figure 1). These studies consist of 2 RCTs, 1 prospective cohort, 5 prospective observational, 2 mixed retro-spective/pro-spective observational and 2 retrospective observational studies.
A total of 254 patients were included in the analysis, with a mean age of 36.1 (range 18-89). 185 patients (72.8%) were females. 5 studies looked into behavioral therapies, primarily diaphragmatic breathing (DB), where 2 studies were done with electromyo-graphy (EMG) guidance[8,9], 1 study was done with HRiM guidance[10] and the other 2 without any visual guidance[11,12]. 2 studies looked at the utility of baclofen[13,14], 1 looked at utility of fundoplication[15] and 1 looked at supportive lifestyle changes[16]. 3 studies looked at a combination of therapies involving pharmaco-logical, behavioral and psychotherapies[2,17,18]. Characteristics of the studies can be found in Table 1.
| Study | Site | Type of study | n | Fem (%) | Age (yr) (range) | Diagnostic criteria | Physiological tests done | Treatment | Description of treatment | Primary outcome | Main results | Proposed mechanism of action | Follow up period (mo) |
| Barba et al[8] | Spain | RCT, Placebo controlled | 12 | 7 (58) | Median 42 (19-69) | Rome 3 rumination syndrome | EMG+ activity of abdomino-thoracic muscles, done PRE and POST | EMG+ guided biofeedback | Pre-meals, patients were trained to control the activity of the abdomino-thoracic muscles under visual control of EMG+ recordings displayed on a monitor. Specifically, they were instructed to voluntarily reduce the activity of intercostal and anterior abdominal muscles and to increase the activity of the diaphragm. After each biofeedback session, patients were instructed to perform the same exercises daily at home for 5 min before and after breakfast, lunch, and dinner. At the end of the treatment period, patients were encouraged to continue practicing these same exercises over time. 3 such sessions performed over 10 d | Reduction in rumination episodes measured over 10 d, patient reported | Regurgitation episodes decreased by 74 ± 6% in the biofeedback group (n = 12) but only by 1 ± 14% in the placebo group (n = 11; P < 0.001). Biofeedback significantly reduced the activity of the abdominothoracic muscles, whereas the placebo had no effect; Number of daily rumination episodes decreased to 7.7 ± 1.9 immediately after biofeedback, 3.0 ± 1.1 by 1 mo, 1.2 ± 0.5 by 3 mo, and 0.7 ± 0.4 by 6 mo (P < 0.001) | Modified basal postprandial muscular tone; Possibly increase awareness in patients to suppress rumination | 6 mo |
| Pauwels et al[14] | Belgium | RCT, Placebo controlled | 10 | 6 (60) | Mean 42 (18-61) | Rome 4 rumination syndrome and/or supragastric belching | Oesophageal HRiM+ done PRE and POST | Baclofen | 5 mg tds first week then increased to 10 mg tds second week, followed by 1 wk washout period, before 2 wk crossover to alternative treatment | Number of symptoms of regurgitation via event marker on HRiM+ and overall treatment evaluation (OTE) | Median number of times that the “regurgitation” marker was pushed significantly lower in baclofen group compared to placebo [4 (0–14) vs 6 (0–19), P = 0.04] Patients reported significantly better OTE ratings after baclofen compared to placebo [mean score 1 (0–2) vs 0 (−1–1), P = 0.03]. On baclofen treatment, 63% of patients improved on Baclofen compared to 26% on placebo (P < 0.0001) | Increased LES+ pressure: Postprandial LES+ pressure significantly higher in the baclofen arm compared to placebo [17.79 (12.72–22.68) vs 13.06 (7.16–16.91) mm Hg (P = 0.0002)]. Borderline negative correlation between postprandial LES pressure and the number of rumination episodes in the baclofen condition (P = 0.056, r = −0.54). Reduced TLESR+: Postprandial TLESRs was significantly lower after baclofen compared to placebo [4 (1–8) vs 7 (3–12), P = 0.017]. | No long term follows up |
| Barba et al[9] | Spain | Prospective cohort with controls | 24 | 17 (71) | 14-76 | Rome 3 rumination syndrome | EMG+ activity, done PRE and POST treatment | EMG+ guided biofeedback | Pre-meals, patients were trained to control the activity of the abdomino-thoracic muscles under visual control of EMG+ recordings displayed on a monitor. Specifically, they were instructed to voluntarily reduce the activity of intercostal and anterior abdominal muscles and to increase the activity of the diaphragm. After each biofeedback session, patients were instructed to perform the same exercises daily at home for 5 min before and after breakfast, lunch, and dinner. At the end of the treatment period, patients were encouraged to continue practicing these same exercises over time. 3 such sessions performed over 10 d | Not defined | Post-biofeedback session, patients experienced a decrease in the number of regurgitation events (8 recorded vs 18 in the basal challenge test; P < 0.001). The improvement observed during the first biofeedback session was strengthened by the following biofeedback sessions. Regurgitation events had decreased by 70% (P < 0.001). By the end of the 3 biofeedback sessions, postprandial abdominal symptoms were reduced (1.6 score; P < 0.001 vs basal). Further reductions in the number of rumination events during the 6-mo observation period while controls had no changes | Modified basal postprandial muscular tone; Possibly increase awareness in patients to suppress rumination | 6 mo |
| Halland et al[10] | United States | Prospective observational | 16 | 9 (56) | Mean 37 | Rome 3 rumination | Oesophageal HRiM+ done PRE, during and POST treatment | HRM+ guided biofeedback therapy | Behavioral therapy delivered by a single subspecialist gastroenterologist where he placed his hand on the patient’s abdomen and instructed patients in diaphragmatic breathing, which entails abdominal rather than chest motion. Patients were also instructed to observe the HRM+ monitor to observe the impact of DB on reduction in gastric pressurizations and regurgitation. | Not defined | Rumination episodes reduced from a median of 5 (2–10) to 1 (0–2) (P < 0.001) during, and 3 (1–5) after (P < 0.001 vs during) diaphragmatic breathing. | Diaphragmatic breathing increased EGJ pressure (P < 0.001) and restored a negative gastroesophageal pressure gradient [20 mmHg (80-7)] by reducing postprandial intragastric pressure. DB may also alter vagal acticity and reduce TLESR whilst increasing LES tone | Nil |
| O’Brien et al[2] | United States | Retrospective and Prospective observational | 36 | 29 (81) | Mean 27 | Not elaborated | All had oesophageal manometry, 20 had pH studies. Tests done PRE treatment | Various | 6 prokinetics7 antacids3 behavioural therapy (e.g. biofeedback); 2 psychotherapy; 2 combined behavioural and psychotherapy | Not defined | 12/16 patients reported subjected improvement, but not broken down to individual treatment options. No therapy deemed effective enough compared to another | N/A | Mean 25 (7-74) |
| Soykan et al[18] | United States | Retrospective and Prospective observational | 10 | 6 (60) | Mean 28.5 (16-63) | Rome 2 for rumination syndrome | All had oesophageal manometry, electrogastrography, gastric emptying study. All done PRE treatment | Various | 5 biofeedback;2 prokinetics;1 prokinetic and acid blockade; 1 leuprolide acetate and antacid; 1 no treatment | Not defined | all 5 undergoing biofeedback improved, 1 taking prokinetic improved | N/A | Mean 31.2 (6-72) |
| Vijayvargiya et al[12] | United States | Retrospective observational | 57 | 54 (95) | Mean 30.3 (14-62) | Rome 3 for rumination syndrome and rectal evacuation disorder | 11 oesophageal manometry, 45 gastric emptying, 3 pH studies, 6 barium oesophagogram, 12 SPECT+. All done PRE treatment | Diaphragmatic breathing | Via behavioural psychologist with instructions onf diaphragmatic breathing to abort or control regurgitation | Not defined | Not reported | N/A | N/A |
| Tucker et al[11] | United Kingdom | Prospective observational | 46 | 34 (74) | 18-68 | HRM+ criteria (Rommel) | All had oesophageal HRM+ PRE treatment | Diaphragmatic breathing | All patients received a 20 min behavioural intervention immediately after HRM+ investigation. This included a description of the abnormal findings, cause of symptoms and explanation of the rationale for behavioural therapy. Behavioural instruction was focused on deep muscle relaxation and diaphragmatic breathing | Not defined | Complete improvement in rumination in 20/46 (43%). Partial improvement in 13 (28%) | N/A | Median 5 (3-11) |
| Lee et al[17] | South Korea | Prospective observational | 21 | 8 (38.1%) | Mean 41.9 | Modified Rome 2 for rumination ayndrome | All had oesophageal HRM+, pH study and gastric emptying tests PRE treatment | various | all given levosulpride 25 mg TDS+; supportive psychotherapy, education and reassurance given monthly, with 15 min sessions over a minimum of 6 mo via therapists experienced in eating disorders | Not defined | 8 (38.1%) showed improvement, 47.6% unchanged while 3 (14.3%) worsened. Those who improved were statistically more likely to have undergone treatment for > 6 mo and less likely to have low mean LES+ pressure | N/A | Mean 19 (15-24) |
| Oelschlager et al[15] | United States | Prospective observational | 5 | 4 (80%) | Mean 40.6 (18-61) | Rome 2 for rumination syndrome | All had oesophageal manometry and pH studies PRE treatment | Fundoplication | 1 laparoscopic, 4 open Nissen fundoplication | Not defined | All had resolution of symptoms;3/5 had pathological acid exposure, 4/5 had hypotensive LES+, 3/5 had hiatal hernias | Restoration of LES+ dysfunction | Median 6 mo, 2 wk - 1 yr |
| Blondeau et al[13] | Belgium | Prospective observational | 12 | 8 (67) | 45 (18-89) | Clinical diagnosis | All had oesophageal HRiM+ PRE and POST treatment | Baclofen | 10 mg TDS+ for a week | Not defined | Patients on baclofen recorded significantly fewer symptoms during the study [6 (2–22); P 0.01). The number of symptom markers for regurgitation was significantly reduced from 9 (0 –11) to 1 (0 –13) (P 0.01); The total number of flow events was significantly reduced from 473 to 282 (39.2%) during baclofen treatment (P 0.02) | Increase in LES+ function and reduction in TLESR+; Possible central mechanism of action to reduce sensitivity of stomach during distension and reduction of compulsive behaviour of straining; The number of TLESR+s during the postprandial period was significantly reduced from 15 (9-19) in baseline conditions to 7 (6-15) during baclofen treatment (P 0.03). The number of strains was reduced from 32 (17-48) in baseline conditions to 17 (2–70) during baclofen treatment (P 0.1). | No long term follows up |
| Johnson et al[16] | United States | Retrospective observational | 5 | 3 (60) | Mean 26.8 (18-43) | Clinical diagnosis | 1 barium oesophagogram; 1 gastric emptying test; all done PRE | Lifestyle changes | All advised to eat slowly, chew completely, avoid food triggers, regular exercises, weight reduction, stress management strategies | Not defined | All 5 had complete cessation of symptoms | Reduction in behavioural and cognitive processes that may develop and maintain symptoms; improvement in coping mechanisms for symptoms | Mean 34.4 (22-43) |
Using the Cochrane risk of bias tool[6], the RCT by Barbal[8] had low risk of bias for the following domains: Allocation, missing outcome data, outcome measures, selection of reported results. However, there were some concerns of bias via deviations from intended interventions as study patients may be aware, they were given placebo. Also, the interaction with a health care professional may improve symptoms and adherence to treatment. There was also no mention of compliance rates in treatment as well as some concerns of bias in outcome measurement due to lack of blinding of outcome assessors. For the RCT by Pauwels[14], there was low risk of bias for the following domains: Allocation, deviation from intended intervention, missing outcome data, outcome measurement and selection of reported results. The cohort study by Barba[9] scored 6 on the NOS with a star given for the following domains: representativeness, selection, ascertainment of exposure, demonstration of outcome not present as start, adequate follow up duration and adequacy of follow up.
We performed a systematic review looking at adult patients diagnosed with RS, and identified 12 articles evaluating the efficacy of various treatment modalities for RS and ranked them in order of level of evidence (Table 2).
| Treatment | Strength of evidence | Treatment outcome |
| Diaphragmatic Breathing | RCT[8] | Regurgitation episodes decreased by 74% in the biofeedback group compared to 1% in placebo (P < 0.001) |
| Prospective cohort with controls[9] | Regurgitation events decreased by 70% (P < 0.001). | |
| Prospective observational[10] | Median rumination episodes reduced from 5 (2–10) to 1 (0–2) (P < 0.001) | |
| Retrospective observational[12] | Not reported | |
| Prospective observational[11] | Complete improvement in rumination in 43%. Partial improvement in 28% | |
| Baclofen | RCT[14] | Median regurgitation events lower with baclofen compared to placebo [4 (0–14) vs 6 (0–19), P = 0.04] |
| Prospective observational[13] | Median regurgitation events significantly reduced from 9 (0-11) to 1 (0-13) (P 0.01) | |
| Surgery | Prospective observational[15] | 100% (5/5) resolution of symptoms |
| Psychotherapy | Prospective observational[17] | 38.1% showed improvement. 47.6% unchanged. |
| Retrospective observational[16] | 100% (5/5) resolution of symptoms |
The studies with the strongest evidence were the 2 RCTs looking at EMG-guided biofeedback and Baclofen. In the former[8], 12 patients who underwent 3 biofeedback sessions had 74% reduction in rumination symptoms compared to 1% reduction in the placebo group with oral simethicone. The improvements with biofeedback appeared sustainable in the long-term with improvement of symptoms at each subsequent follow up. The study when assessed for quality based on the Cochrane Risk of Bias tool[6] had generally low risk of bias, although there were some concerns about the lack of blinding of outcome assessors as well as the placebo group being aware they were in the placebo arm. The other RCT[14] showed that Baclofen at 5-10 mg three times daily reduced the number of symptoms of regurgitation via event markers on HRIM and overall treatment evaluation via questionnaire where 63% of patients improved on Baclofen compared to 26% of patients on placebo treatment (P < 0.0001). The study quality was assessed to also have low risk of bias apart from having a heterogonous population of RS and supragastric belching, although this more accurately mimics real-world situations where there is significant overlap in presenta-tion for these 2 conditions.
The exercises that were part of the biofeedback protocols[8,9] were essentially ab-dominal breathing exercises otherwise known as DB[19]. DB was further supported by other non-RCT studies included in this analysis. There were variations on how these were performed, from using EMG guidance[8], HRiM guidance[10] or just delivered without visual aids[11,12]. Some of the studies showed that even a single session of DB training can improve symptoms, but as these symptoms tend to recur over time, compliance to home exercises is likely important to maintain sustainability of response.
Many of the studies analyzed performed physiological tests prior to the treatment and post-treatment, thus allowing an insight into possible mechanisms of the origin of symptoms in these patients. Although the exact pathogenesis of RS is still unknown, the primary initiating mechanism is commonly a post-prandial gastric pressuri-zation[10,11] that possibly results from anterior abdominal muscle contractions[9]. However, a low esophageal sphincter (LES) pressure is also required to facilitate the upward movement of gastric contents as Halland[10] showed that high intragastric pressure waves led to rumination episodes only when accompanied by reduction in esophagogastric junction (EGJ) pressure. Furthermore, post-prandially patients demonstrated contraction of intercostals muscles to facilitate a negative intra-thoracic pressure[9]. It is likely this combination of increased intra-abdominal pressure coupled with negative intra-thoracic pressure and a permissive EGJ that allows rumination to take place. The significance of a low LES pressure has also been highlighted in some of the studies. Patients with low LES baseline pressures were shown to have a poorer outcome to treatment for RS[17]. Reasons for this low LES pressure can be a learned prolonged postprandial voluntary relaxation of the diaphragmatic crura or increased TLESRs[20]. Other suggested possibilities include increased abdominal pressure displacing the EGJ proximally away from the crura thus losing the crural contribution to the EGJ. An unrecognized central mechanism may also be involved since healthy adults are not able to induce rumination[21].
The physiology tests also allow us to understand the rationale for these treatment options, especially in DB, where the evidence appears strongest in terms of quantity and quality. In the study by Halland[10], they demonstrated that DB may improve crural function via several mechanisms. DB can directly augment the tone of the LES by voluntary contraction of the crural diaphragm. DB can also prevent the increased intra-gastric pressure from displacing the EGJ proximally, thus not allowing a permissive EGJ during such episodes. Also, DB may alter vagal activity and prevent TLESRs from happening and thus maintain a more prolonged high pressure LES tone. DB also likely competes with the need for the learned behavior of gastric straining, and this abolishes the trigger to ruminate when performed post meals[22]. Barba[8] showed that EMG guided-biofeedback, of which DB was part of the intervention, significantly reduced the activity of the abdominothoracic muscles, whereas the placebo had no effect, and this correlated with reduction of rumination symptoms. Based on their EMG findings pre and post, they postulate that patients with RS have an abnormal level of abdominothoracic muscular tone. They then showed it was possible to specifically target the relevant muscles and unlearn this coordinated abdominothoracic maneuver which generates rumination (e.g., reducing activity of intercostals and anterior abdominal muscle while increasing activity of diaphragm reduces rumination symptoms).
Other studies not involving DB also shed light on mechanisms of RS and its treatment. 2 studies[13,14] showed that baclofen reduced the number of rumination episodes possibly by reduction in TLESRs and increasing postprandial LES pressure which were both significantly different in the intervention group compared to placebo. These mechanisms are similar in those postulated to take place post-DB. Baclofen may have other mechanisms of action as well, as shown by[13] where baclofen reduced voluntary gastric straining events and the authors postulate that this could be either related to central mechanisms of reducing compulsive behaviours of straining or by reducing the mechanosensitivity of the stomach as studies[23] have shown that patients with RS often have increased gastric sensitivity to distension.
There are likely psychosocial and cognitive processes in play that initiate and perpetuate symptoms in RS as evident by patients reporting onset of symptoms following acute illness[24], surgeries[18], psychological stress[18] and major life events[11,18,24]. Comorbid psychiatric disturbances such as depression, anxiety and somatoform disorders[12,18] were frequently found in RS patients, and it is not entirely clear whether these are causes or consequences of RS. Pediatric studies[25] have shown that successfully treating psychiatric disorders, when present, is helpful for RS. It is not unreasonable to think that the same applies to adult patients as patients with psychiatric disorders may have a lack of motivation which interferes with compliance to behavioral treatments[12], and therefore needs to be addressed. Behavioral treatments targeting stress reduction and improving coping mechanisms to symptoms have also been shown to be helpful in reducing symptoms in RS[16]. A single open label study looked at 21 adults with RS[17] and looked the effect of supportive psychotherapy together with a prokinetic leveosulpiride. Only 38% of patients showed improvement, so perhaps psychotherapy itself is not efficacious but possibly, a more targeted form of psychotherapy in association with behavioral treatments may be effective. As such, investigators are currently actively recruiting patients and looking into using a form of Cognitive Behavioral Therapy to treat this condition (https://clinicaltrials.gov/ct2/show/NCT03113682).
Interestingly, it has been suggested that refractory cases of rumination be treated with surgery such as fundoplication[15]. In this study, all 5 patients had complete cessation of symptoms post-surgery, although 4 out of 5 patients had a hypotensive LES while 3 out of 5 had hiatal hernias and pathological acid exposure, thus improvement in their symptoms could have been due to improvement in their GERD. It is therefore not recommended to treat RS patients with surgery without con-comitant GERD or structural abnormalities at this point without further evidence. However, this study showed the likely contribution of an incompetent LES in the overall picture manifestation of RS.
There were some limitations to our analysis. We only included studies in English, and we also excluded pediatric studies since our focus was on adult patients. However, some of the results from pediatric studies could still be relevant in understanding the efficacy of RS treatment. Even though most of the studies included some form of physiological testing, the studies were heterogenous and tests such as gastric emptying studies or 24-h pH impedance studies were often not performed. Thus, the diagnosis of GERD and gastroparesis may be missed in some of these patients labeled as RS, and therefore caution needs to be exercised in interpreting some of the study results. It was difficult to make strong conclusions based on the strength of the data as only 3 studies were controlled and only 2 were randomized interventions. In view of the limited literature available in this field, we retained observational studies despite knowing that they were prone to bias, and thus our recommendations are not based on strong evidence, but rather a summary of what is available in the literature (Tables 2 and 3).
| Condition | Treatment |
| Initial treatment | Extensive explanation of condition and underlying mechanism together with reassurance of benign nature of condition[2,20] |
| Diaphragmatic breathing by trained personnel (with EMG guidance or HRiM if available) | |
| If no response to diaphragmatic breathing after ensuring compliance, Baclofen 5-10 mg three times daily | |
| For refractory cases | Consider alternative diagnosis (GERD, gastroparesis, functional dyspepsia, supragastric belching) and treat appropriately |
| Since both DB and baclofen appear to be effective and work via different mechanisms, we postulate that a switching to the other therapy or a combination of these therapies could be useful in cases refractory to either treatments | |
| Address psychological illness, if present. Consider adjunctive psychological therapies to correct cognitive processes that may perpetuate symptoms |
In conclusion, RS may present similarly to other conditions such as GERD and gastroparesis and is likely under-recognized, therefore clinicians need to be aware of this syndrome in their differential diagnosis. Although evidence for treatment options is still limited, the strongest evidence points towards the use of DB and Baclofen, and both should be considered depending on their availabilities. Most of the studies analyze are limited by the small sample sizes and variability in delivery of biofeed-back. Therefore, further studies are needed to tackle these knowledge gaps.
Rumination syndrome (RS) is a relatively common yet underdiagnosed condition.
There is no consensus on how to treat patients diagnosed with runination syndrome.
Our objectives are to systematically review the literature on the efficacy of treatment options for adults with RS.
We conducted a systematic review according to PRISMA guidelines. We searched Medline (1946 to February 2019), EMBASE (1947 to February 2019), PsycINFO (1806 to February 2019) and Cochrane central register of controlled trials for articles discussing treatment options for adult patients (> 18 years) with RS. All relevant articles were accessed in full text. We extracted data on study designs, patient profiles, duration of symptoms, follow up periods, date, diagnostic criteria, interventions and outcomes. Risk of bias assessment was carried out independently by 3 reviewers via Cochrane Risk of Bias tool and Newcastle Ottawa Scale for RCTs and Cohort studies respectively.
12 articles were identified. The strongest evidence pointed towards diaphragmatic breathing (DB), and less so for baclofen. A total of 254 patients were included in the analysis.
DB has the strongest evidence for efficacy in adults with RS.
The quality of the evidence is still weak. More research needs to be done in this field.
Manuscript source: Invited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: Singapore
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): D, D, D
Grade E (Poor): 0
P-Reviewer: Iliescu L, Wang YP, Koulaouzidis A, Tarnawski AS, Kato J, Cerwenka H S-Editor: Dou Y L-Editor: A E-Editor: Liu JH
| 1. | Malcolm A, Thumshirn MB, Camilleri M, Williams DE. Rumination Syndrome. Mayo Clin Proc. 1997;72:646-652. [RCA] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 38] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | O’Brien MD, Bruce BK, Camilleri M. The rumination syndrome: Clinical features rather than manometric diagnosis. Gastroenterology. 1995;108:1024-1029. [RCA] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 96] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 3. | Chial HJ, Camilleri M, Williams DE, Litzinger K, Perrault J. Rumination syndrome in children and adolescents: diagnosis, treatment, and prognosis. Pediatrics. 2003;111:158-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 108] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 4. | Stanghellini V, Chan FK, Hasler WL, Malagelada JR, Suzuki H, Tack J, Talley NJ. Gastroduodenal Disorders. Gastroenterology. 2016;150:1380-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 818] [Cited by in RCA: 975] [Article Influence: 108.3] [Reference Citation Analysis (0)] |
| 5. | Kessing BF, Bredenoord AJ, Smout AJ. Objective manometric criteria for the rumination syndrome. Am J Gastroenterol. 2014;109:52-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 6. | Higgins JPT, Savović J, Page MJ, Sterne JAC. Revised Cochrane risk of bias tool for randomized trials (RoB 2.0). 2018;52. |
| 7. | Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. |
| 8. | Barba E, Accarino A, Soldevilla A, Malagelada JR, Azpiroz F. Randomized, Placebo-Controlled Trial of Biofeedback for the Treatment of Rumination. Am J Gastroenterol. 2016;111:1007-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Barba E, Burri E, Accarino A, Malagelada C, Rodriguez-Urrutia A, Soldevilla A, Malagelada JR, Azpiroz F. Biofeedback-guided control of abdominothoracic muscular activity reduces regurgitation episodes in patients with rumination. Clin Gastroenterol Hepatol. 2015;13:100-6.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | Halland M, Parthasarathy G, Bharucha AE, Katzka DA. Diaphragmatic breathing for rumination syndrome: efficacy and mechanisms of action. Neurogastroenterol Motil. 2016;28:384-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 11. | Tucker E, Knowles K, Wright J, Fox MR. Rumination variations: aetiology and classification of abnormal behavioural responses to digestive symptoms based on high-resolution manometry studies. Aliment Pharmacol Ther. 2013;37:263-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 12. | Vijayvargiya P, Iturrino J, Camilleri M, Shin A, Vazquez-Roque M, Katzka DA, Snuggerud JR, Seime RJ. Novel Association of Rectal Evacuation Disorder and Rumination Syndrome: Diagnosis, Co-morbidities and Treatment. United European Gastroenterol J. 2014;2:38-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Blondeau K, Boecxstaens V, Rommel N, Farré R, Depeyper S, Holvoet L, Boeckxstaens G, Tack JF. Baclofen improves symptoms and reduces postprandial flow events in patients with rumination and supragastric belching. Clin Gastroenterol Hepatol. 2012;10:379-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 14. | Pauwels A, Broers C, Van Houtte B, Rommel N, Vanuytsel T, Tack J. A Randomized Double-Blind, Placebo-Controlled, Cross-Over Study Using Baclofen in the Treatment of Rumination Syndrome. Am J Gastroenterol. 2018;113:97-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 15. | Oelschlager BK, Chan MM, Eubanks TR, Pope CE, Pellegrini CA. Effective treatment of rumination with Nissen fundoplication. J Gastrointest Surg. 2002;6:638-644. [RCA] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Johnson WG, Corrigan SA, Crusco AH, Jarrell MP. Behavioral assessment and treatment of postprandial regurgitation. J Clin Gastroenterol. 1987;9:679-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Lee H, Rhee PL, Park EH, Kim JH, Son HJ, Kim JJ, Rhee JC. Clinical outcome of rumination syndrome in adults without psychiatric illness: a prospective study. J Gastroenterol Hepatol. 2007;22:1741-1747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Soykan I, Chen J, Kendall BJ, McCallum RW. The rumination syndrome: clinical and manometric profile, therapy, and long-term outcome. Dig Dis Sci. 1997;42:1866-1872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Chitkara DK, Van Tilburg M, Whitehead WE, Talley NJ. Teaching diaphragmatic breathing for rumination syndrome. Am J Gastroenterol. 2006;101:2449-2452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 20. | Tack J, Blondeau K, Boecxstaens V, Rommel N. Review article: the pathophysiology, differential diagnosis and management of rumination syndrome. Aliment Pharmacol Ther. 2011;33:782-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Halland M, Pandolfino J, Barba E. Diagnosis and Treatment of Rumination Syndrome. Clin Gastroenterol Hepatol. 2018;16:1549-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 22. | Hejazi RA, McCallum RW. Rumination syndrome: a review of current concepts and treatments. Am J Med Sci. 2014;348:324-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Thumshirn M, Camilleri M, Hanson RB, Williams DE, Schei AJ, Kammer PP. Gastric mechanosensory and lower esophageal sphincter function in rumination syndrome. Am J Physiol. 1998;275:G314-G321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Amarnath RP, Abell TL, Malagelada JR. The rumination syndrome in adults. A characteristic manometric pattern. Ann Intern Med. 1986;105:513-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 73] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Green AD, Alioto A, Mousa H, Di Lorenzo C. Severe pediatric rumination syndrome: successful interdisciplinary inpatient management. J Pediatr Gastroenterol Nutr. 2011;52:414-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |