Published online May 31, 2019. doi: 10.13105/wjma.v7.i5.218
Peer-review started: March 18, 2019
First decision: April 13, 2019
Revised: April 27, 2019
Accepted: May 1, 2019
Article in press: May 1, 2019
Published online: May 31, 2019
Processing time: 75 Days and 15.8 Hours
Autoimmune pancreatitis (AIP) is defined as pancreatitis caused by irregular narrowing of the pancreatic duct accompanied by pancreatic swelling, fibrosis and lymphocyte infiltration, events that are related to autoimmune mechanisms. The 2010 International Consensus Diagnostic Criteria for AIP defined pancreatitis as “type 1” when increased levels of serum IgG4 were present and other organs were involved; lymphoplasmacytic sclerosing pancreatitis was the main histological characteristic. Apart from surgery, endoscopic ultrasonography-guided fine needle aspiration (EUS-FNA) is the only method for the histological diagnosis of AIP; however, this method is difficult. The use of larger-diameter FNA needles and trucut biopsy did not improve the diagnostic performance of EUS-FNA, but it has improved gradually. In this review, we look back at past efforts to improve the diagnostic performance of EUS-FNA and reveal the present state of EUS-FNA for the histological diagnosis of AIP type 1.
Core tip: Apart from surgery, endoscopic ultrasonography-guided fine needle aspiration (EUS-FNA) is the only method for the histological diagnosis of autoimmune pancreatitis (AIP). However, this method is difficult. Several attempts to improve the diagnostic performance of EUS-FNA have been undertaken, with gradual success. In this review, we examine past efforts and discuss the present state of EUS-FNA for the histological diagnosis of AIP type 1.
- Citation: Sugimoto M, Takagi T, Suzuki R, Konno N, Asama H, Sato Y, Irie H, Watanabe K, Nakamura J, Kikuchi H, Takasumi M, Hashimoto M, Hikichi T, Ohira H. Present state of endoscopic ultrasonography-guided fine needle aspiration for the diagnosis of autoimmune pancreatitis type 1. World J Meta-Anal 2019; 7(5): 218-223
- URL: https://www.wjgnet.com/2308-3840/full/v7/i5/218.htm
- DOI: https://dx.doi.org/10.13105/wjma.v7.i5.218
In 1993, a case accompanied by lymphadenopathy and IgG4 hypergamma-globulinemia was reported by Suzuki et al[1]. Thereafter, the concept of IgG4-related disease (IgG4-RD) was established as follows: IgG4-RD is characterized by elevated serum IgG4 accompanied by the swelling of multiple organs or tumoral lesions infiltrated by lymphocytes, with IgG4-positive plasma cells and fibrosis observed throughout the body[2,3]. In gastroenterology, autoimmune pancreatitis (AIP) type 1[4,5] and IgG4-related sclerosing cholangitis (IgG4-SC)[6-8] are the primary presentations.
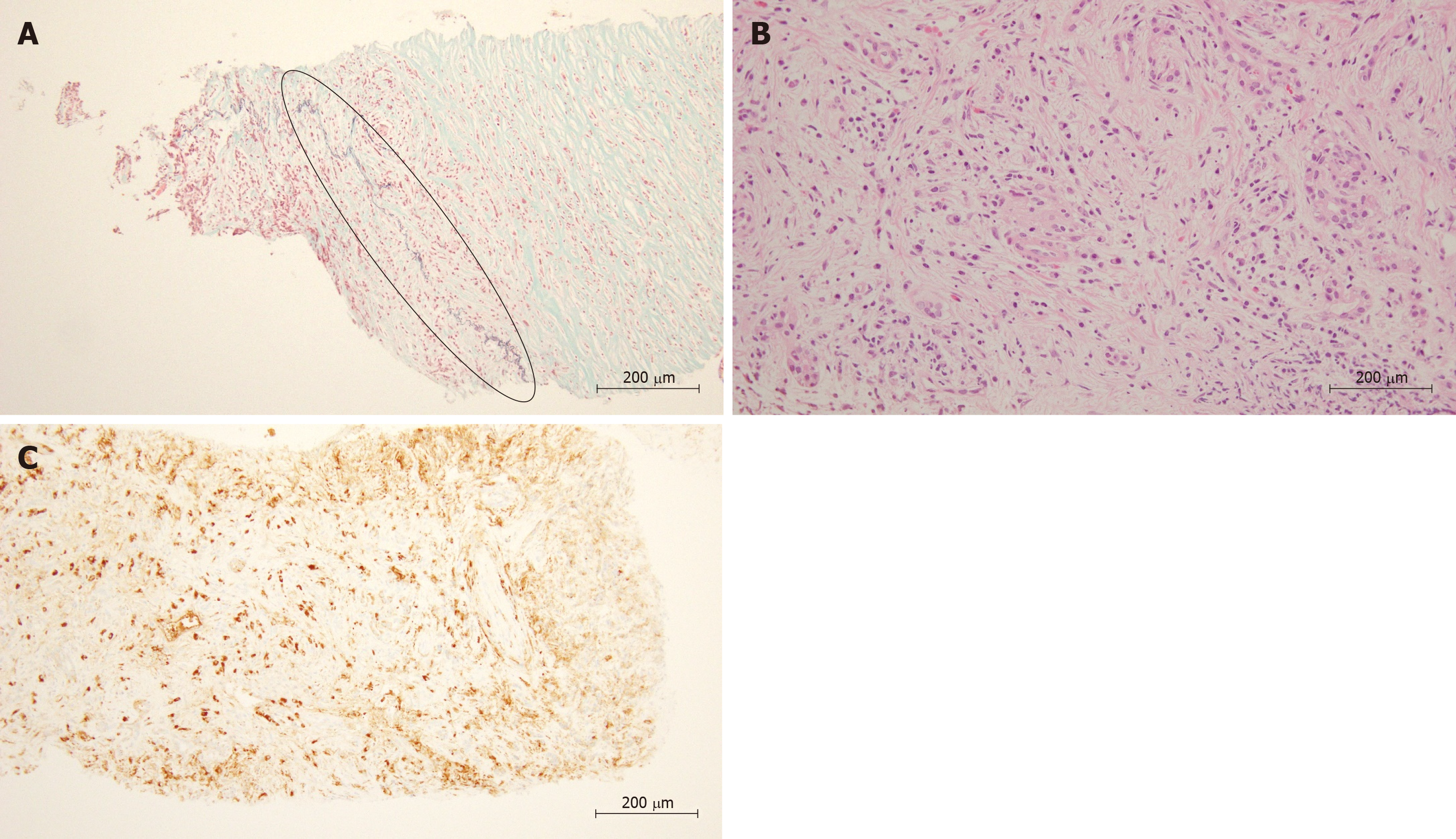
AIP was defined by Yoshida et al[9] as pancreatitis caused by irregular narrowing of the pancreatic duct accompanied by pancreatic swelling, fibrosis and lymphocyte infiltration, events that are related to autoimmune mechanisms. Moreover, Hamano et al[4] reported elevated levels of serum IgG4 in AIP patients. The 2010 International Consensus Diagnostic Criteria (ICDC) for AIP defined pancreatitis as “type 1” when the levels of serum IgG4 are elevated and other organs are involved; lympho-plasmacytic sclerosing pancreatitis (LPSP) is considered the main histological characteristic[10]. Four characteristics have been identified as important for diagnosing LPSP, namely, periductal lymphoplasmacytic infiltrate without granulocytic infiltration, obliterative phlebitis, storiform fibrosis, and abundant (> 10 cells/HPF) IgG4-positive cells (Figure 1). The presence of three of these four characteristics is defined as level 1 histological findings. The presence of only two of these characteristics is defined as level 2 histological findings.
AIP can be diagnosed by imaging and increased serum IgG4 levels or by other methods[11]. However, a histological diagnosis of AIP requires level 1 pancreatic histological findings. Apart from surgery, endoscopic ultrasonography-guided fine needle aspiration (EUS-FNA) is the only method for the histological diagnosis of AIP. Furthermore, AIP cases associated with pancreatic cancer have been reported[12-16]. Therefore, EUS-FNA is important for the safe and noninvasive distinction of AIP from pancreatic cancer. In this report, we discuss the efficacy and present diagnostic performance of EUS-FNA for AIP type 1.
Reports were searched in PubMed and the Cochrane Library by using the following keywords: “autoimmune pancreatitis” and “endoscopic ultrasonography-guided fine needle aspiration”. Among the searched reports, only original articles were included in this review. Furthermore, we performed a manual search and added such articles to this review as necessary.
We introduce the achievements in the included reports in the following order: Before and after the ICDC, conventional EUS-FNA (22-gauge, 19-gauge), EUS-TCB, multicenter studies, and special needles.
Before the ICDC, few reports described the use of EUS-FNA for the histological diagnosis of AIP type 1. In 2005, Deshpande et al[17] performed EUS-FNA in 16 AIP patients. Among these patients, three were found to have false-positive cytological diagnoses (one adenocarcinoma, one solid pseudopapillary neoplasm, and one mucinous neoplasm). The cellularity of the stromal fragments was significantly higher in the AIP samples than in the control samples (adenocarcinoma, chronic pancreatitis, etc.,). In the same year, researchers performed EUS-guided trucut biopsy (EUS-TCB) in three AIP patients. In two of the three patients, fibrosis and lymphoplasmacytic infiltration were observed. In 2009, Mizuno et al[18] performed EUS-TCB, and among nine AIP patients, four were diagnosed with probable LPSP. In 2011, Khalid et al[19] reported the diagnosis of two of 14 AIP patients with LPSP. In this period, the performance of EUS-FNA for the histological diagnosis of AIP was very poor.
After the ICDC were established, several reports described the use of EUS-FNA for the diagnosis of AIP. The results of these studies (excluding case reports) are shown in Table 1. In the following sections, we describe the details of these studies.
| Ref. | Yr | Needle (G) | No. of needle passes | n | LPI | OP | SF | IgG4 (+) PC | Level 1 HF | Level 1 or 2 HF |
| Conventional EUS-FNA of single-center study | ||||||||||
| Imai et al[20] | 2011 | 22 | 3.5 ± 0.9 | 21 | 11 | 0 | 0 | 0 | 0 | 0 |
| Ishikawa et al[21] | 2012 | 22 | 2.0 ± 0.48 | 39 | 16 | 0 | 34 | 10 | 9 | 14 |
| Cao et al[22] | 2018 | 22 | 3.59 ± 1.28 | 27 | 18 | 0 | 18 | 8 | 5 | 17 |
| Iwashita et al[23] | 2012 | 19 | 3.0 | 44 | 23 | 21 | 38 | 5 | 17 | 19 |
| Multicenter study | ||||||||||
| Morishima et al[24] | 2016 | 22 | 2.02 ± 0.48 | 41 | 36 | 0 | 0 | 27 | 0 | 27 |
| Kanno et al[25] | 2016 | 22 | 3.4 ± 1.3 | 78 | 43 | 38 | 49 | 19 | 32 | 45 |
| EUS-FNA by automated spring-loaded Powershot needle | ||||||||||
| Kanno et al[26] | 2012 | 22 | 3-7 | 25 | 23 | 4 | 20 | 9 | 14 | 20 |
First, the results of EUS-FNA using a 22-gauge needle were described. In 2011, Imai et al[20] reported the results of 21 AIP patients who underwent EUS-FNA. The AIP patients could not be histologically diagnosed with AIP. In 2012, Ishikawa et al[21] reported that level 1 histological findings as defined by the ICDC were observed in 9 of 39 EUS-FNA specimens from AIP type 1 patients, and level 1 or level 2 histological findings were observed in 14 of 39 patients. In 2018, Cao et al[22] reported the results of EUS-FNA in 27 AIP patients: 18.5% (5/27) of the AIP patients showed level 1 histological findings, and 62.96% (17/27) showed level 1 or level 2 histological findings. Although these results were somewhat insufficient, they represented a gradual improvement.
In 2012, Iwashita et al[23] reported the results of 44 AIP patients who underwent EUS-FNA using a 19-gauge needle. In this report, 39% (17/44) showed level 1 histological findings, and 43% (19/44) showed level 1 or level 2 histological findings. Although the diameter of the needle was larger, more specimens could not be sampled.
As mentioned above, EUS-TCB achieved somewhat modest results before the ICDC were announced. Before the ICDC, almost all of the reports on using EUS-FNA to identify AIP type 1 asserted that AIP could not be histologically diagnosed by EUS-FNA. However, Mizuno et al[18] reported the efficacy of EUS-TCB (referred to in the section REPORTS BEFORE THE ICDC).
Because AIP is a relatively rare disease, studies of EUS-FNA in AIP patients have had limited sample sizes. However, in 2016, two multicenter studies were performed, both of which used a 22-gauge needle for EUS-FNA.
Morishima et al[24] performed a multicenter study in which 18 hospitals took part, and 41 AIP type 1 patients were entered in the study. The number of needle passes was 2.01 ± 0.48 (1-4). In this report, 65.8% (27/41) of the patients showed level 2 histological findings. However, none of the patients showed level 1 histological findings. In addition, storiform fibrosis and obliterative phlebitis were not observed.
Kanno et al[25] performed a multicenter study in which twelve institutions participated. The report included 78 AIP type 1 patients. The number of needle passes was 3.4 ± 1.3. In this report, 41.0% (32/78) of patients showed level 1 histological findings, and 57.7% (45/78) showed level 1 or level 2 histological findings. Twenty-four (19/78) patients showed abundant (> 10 cells/HPF) IgG4-positive cells. A total of 62.8% (49/78) of patients showed storiform fibrosis, and 48.7% (38/78) of patients showed obliterative phlebitis. These two reports provided sufficient evidence that EUS-FNA could be used for the diagnosis of level 2 histological findings.
Due to the difficulty of the histological diagnosis of AIP, EUS-FNA was performed using special needles. Kanno et al[26] used an automated spring-loaded Powershot needle (NA11J-KB; Olympus, Tokyo, Japan). In this report, 56% (14/25) of patients showed level 1 histological findings, and 80% (20/25) showed level 1 or level 2 histological findings. Obliterative phlebitis was observed in 40% (10/25) of patients. Although it is very difficult to prove obliterative phlebitis by EUS-FNA, this report showed promising results.
Recently, the use of SharkCore (Medtronic, Sunnydale, Calif) needles for EUS-guided fine needle biopsy (EUS-FNB) in AIP patients was reported. According to a report written by Detlefsen et al[27] in 2017, one AIP type 1 patient showed level 2 histological findings. However, obliterative phlebitis was observed in this patient. In the same year, Runge et al[28] reported EUS-FNB in two patients. Both patients showed a marked increase in IgG4-positive plasma cells. Although storiform fibrosis and obliterative phlebitis were not reported, these two patients likely showed level 2 or higher histological findings. In 2018, Bhattacharya et al[29] reported two patients who showed level 1 histological findings by EUS-FNB. Although the three reports on EUS-FNB using the SharkCore needle were case reports, all cases showed some histological findings as defined by the ICDC. The diagnostic performance of EUS-FNA for AIP could be improved by the further development of such needles.
Diagnosing AIP type 1 by EUS-FNA is currently difficult. However, the diagnostic performance of EUS-FNA for AIP is gradually improving over time and with the further development of special needles. In the future, further advancement of FNA needles and puncture methods will be warranted to improve the histological diagnosis of AIP.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Chow WK, Fouad YM, Friedel D, Morelli L S-Editor: Ji FF L-Editor: A E-Editor: Wu YXJ
| 1. | Suzuki S, Kida S, Ohira Y, Ohba T, Miyata M, Nishimaki T, Morito T, Kasukawa R, Hojyo H, Wakasa H. [A case of Sjögren's syndrome accompanied by lymphadenopathy and IgG4 hypergammaglobulinemia]. Ryumachi. 1993;33:249-254. [PubMed] |
| 2. | Umehara H, Okazaki K, Masaki Y, Kawano M, Yamamoto M, Saeki T, Matsui S, Yoshino T, Nakamura S, Kawa S, Hamano H, Kamisawa T, Shimosegawa T, Shimatsu A, Nakamura S, Ito T, Notohara K, Sumida T, Tanaka Y, Mimori T, Chiba T, Mishima M, Hibi T, Tsubouchi H, Inui K, Ohara H. Comprehensive diagnostic criteria for IgG4-related disease (IgG4-RD), 2011. Mod Rheumatol. 2012;22:21-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 625] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 3. | Stone JH, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med. 2012;366:539-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1856] [Cited by in RCA: 1870] [Article Influence: 143.8] [Reference Citation Analysis (83)] |
| 4. | Hamano H, Kawa S, Horiuchi A, Unno H, Furuya N, Akamatsu T, Fukushima M, Nikaido T, Nakayama K, Usuda N, Kiyosawa K. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med. 2001;344:732-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2026] [Cited by in RCA: 1878] [Article Influence: 78.3] [Reference Citation Analysis (0)] |
| 5. | Okazaki K, Uchida K, Koyabu M, Miyoshi H, Takaoka M. Recent advances in the concept and diagnosis of autoimmune pancreatitis and IgG4-related disease. J Gastroenterol. 2011;46:277-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 205] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 6. | Zen Y, Harada K, Sasaki M, Sato Y, Tsuneyama K, Haratake J, Kurumaya H, Katayanagi K, Masuda S, Niwa H, Morimoto H, Miwa A, Uchiyama A, Portmann BC, Nakanuma Y. IgG4-related sclerosing cholangitis with and without hepatic inflammatory pseudotumor, and sclerosing pancreatitis-associated sclerosing cholangitis: do they belong to a spectrum of sclerosing pancreatitis? Am J Surg Pathol. 2004;28:1193-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 389] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 7. | Hamano H, Kawa S, Uehara T, Ochi Y, Takayama M, Komatsu K, Muraki T, Umino J, Kiyosawa K, Miyagawa S. Immunoglobulin G4-related lymphoplasmacytic sclerosing cholangitis that mimics infiltrating hilar cholangiocarcinoma: part of a spectrum of autoimmune pancreatitis? Gastrointest Endosc. 2005;62:152-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 130] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 8. | Naitoh I, Nakazawa T, Ohara H, Ando T, Hayashi K, Tanaka H, Okumura F, Takahashi S, Joh T. Endoscopic transpapillary intraductal ultrasonography and biopsy in the diagnosis of IgG4-related sclerosing cholangitis. J Gastroenterol. 2009;44:1147-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 110] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 9. | Yoshida K, Toki F, Takeuchi T, Watanabe S, Shiratori K, Hayashi N. Chronic pancreatitis caused by an autoimmune abnormality. Proposal of the concept of autoimmune pancreatitis. Dig Dis Sci. 1995;40:1561-1568. [PubMed] |
| 10. | Shimosegawa T, Chari ST, Frulloni L, Kamisawa T, Kawa S, Mino-Kenudson M, Kim MH, Klöppel G, Lerch MM, Löhr M, Notohara K, Okazaki K, Schneider A, Zhang L; International Association of Pancreatology. International consensus diagnostic criteria for autoimmune pancreatitis: guidelines of the International Association of Pancreatology. Pancreas. 2011;40:352-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1050] [Cited by in RCA: 1056] [Article Influence: 75.4] [Reference Citation Analysis (0)] |
| 11. | Sugimoto M, Takagi T, Suzuki R, Konno N, Asama H, Watanabe K, Nakamura J, Kikuchi H, Waragai Y, Takasumi M, Sato Y, Hikichi T, Ohira H. Endoscopic Ultrasonography-Guided Fine Needle Aspiration Can Be Used to Rule Out Malignancy in Autoimmune Pancreatitis Patients. J Ultrasound Med. 2017;36:2237-2244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Inoue H, Miyatani H, Sawada Y, Yoshida Y. A case of pancreas cancer with autoimmune pancreatitis. Pancreas. 2006;33:208-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 85] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 13. | Fukui T, Mitsuyama T, Takaoka M, Uchida K, Matsushita M, Okazaki K. Pancreatic cancer associated with autoimmune pancreatitis in remission. Intern Med. 2008;47:151-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 70] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 14. | Matsubayashi H, Matsunaga K, Uesaka K, Fukutomi A, Sasaki K, Furukawa H, Ono H. A case of pancreatic carcinoma with suspected autoimmune pancreatitis. Clin J Gastroenterol. 2009;2:59-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Motosugi U, Ichikawa T, Yamaguchi H, Nakazawa T, Katoh R, Itakura J, Fujii H, Sato T, Araki T, Shimizu M. Small invasive ductal adenocarcinoma of the pancreas associated with lymphoplasmacytic sclerosing pancreatitis. Pathol Int. 2009;59:744-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Chandrasegaram MD, Chiam SC, Nguyen NQ, Ruszkiewicz A, Chung A, Neo EL, Chen JW, Worthley CS, Brooke-Smith ME. A case of pancreatic cancer in the setting of autoimmune pancreatitis with nondiagnostic serum markers. Case Rep Surg. 2013;2013:809023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Deshpande V, Mino-Kenudson M, Brugge WR, Pitman MB, Fernandez-del Castillo C, Warshaw AL, Lauwers GY. Endoscopic ultrasound guided fine needle aspiration biopsy of autoimmune pancreatitis: diagnostic criteria and pitfalls. Am J Surg Pathol. 2005;29:1464-1471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 102] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 18. | Mizuno N, Bhatia V, Hosoda W, Sawaki A, Hoki N, Hara K, Takagi T, Ko SB, Yatabe Y, Goto H, Yamao K. Histological diagnosis of autoimmune pancreatitis using EUS-guided trucut biopsy: a comparison study with EUS-FNA. J Gastroenterol. 2009;44:742-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 131] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 19. | Khalid A, Dewitt J, Ohori NP, Chen JH, Fasanella KE, Sanders M, McGrath KM, Nikiforova M. EUS-FNA mutational analysis in differentiating autoimmune pancreatitis and pancreatic cancer. Pancreatology. 2011;11:482-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Imai K, Matsubayashi H, Fukutomi A, Uesaka K, Sasaki K, Ono H. Endoscopic ultrasonography-guided fine needle aspiration biopsy using 22-gauge needle in diagnosis of autoimmune pancreatitis. Dig Liver Dis. 2011;43:869-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 21. | Ishikawa T, Itoh A, Kawashima H, Ohno E, Matsubara H, Itoh Y, Nakamura Y, Hiramatsu T, Nakamura M, Miyahara R, Ohmiya N, Goto H, Hirooka Y. Endoscopic ultrasound-guided fine needle aspiration in the differentiation of type 1 and type 2 autoimmune pancreatitis. World J Gastroenterol. 2012;18:3883-3888. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 66] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | Cao L, Wang Y, Wang J, Guo Q, Chen Q, Wu X, Tang SJ, Cheng B. The role of EUS-guided fine needle aspiration in autoimmune pancreatitis: a single center prospective study. Scand J Gastroenterol. 2018;53:1604-1610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Iwashita T, Yasuda I, Doi S, Ando N, Nakashima M, Adachi S, Hirose Y, Mukai T, Iwata K, Tomita E, Itoi T, Moriwaki H. Use of samples from endoscopic ultrasound-guided 19-gauge fine-needle aspiration in diagnosis of autoimmune pancreatitis. Clin Gastroenterol Hepatol. 2012;10:316-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 24. | Morishima T, Kawashima H, Ohno E, Yamamura T, Funasaka K, Nakamura M, Miyahara R, Watanabe O, Ishigami M, Shimoyama Y, Nakamura S, Hashimoto S, Goto H, Hirooka Y. Prospective multicenter study on the usefulness of EUS-guided FNA biopsy for the diagnosis of autoimmune pancreatitis. Gastrointest Endosc. 2016;84:241-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 25. | Kanno A, Masamune A, Fujishima F, Iwashita T, Kodama Y, Katanuma A, Ohara H, Kitano M, Inoue H, Itoi T, Mizuno N, Miyakawa H, Mikata R, Irisawa A, Sato S, Notohara K, Shimosegawa T. Diagnosis of autoimmune pancreatitis by EUS-guided FNA using a 22-gauge needle: a prospective multicenter study. Gastrointest Endosc. 2016;84:797-804.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 91] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 26. | Kanno A, Ishida K, Hamada S, Fujishima F, Unno J, Kume K, Kikuta K, Hirota M, Masamune A, Satoh K, Notohara K, Shimosegawa T. Diagnosis of autoimmune pancreatitis by EUS-FNA by using a 22-gauge needle based on the International Consensus Diagnostic Criteria. Gastrointest Endosc. 2012;76:594-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 27. | Detlefsen S, Joergensen MT, Mortensen MB. Microscopic findings in EUS-guided fine needle (SharkCore) biopsies with type 1 and type 2 autoimmune pancreatitis. Pathol Int. 2017;67:514-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Runge TM, Hart PA, Sasatomi E, Baron TH. Diagnosis of autoimmune pancreatitis using new, flexible EUS core biopsy needles: report of 2 cases. Gastrointest Endosc. 2017;85:1311-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 29. | Bhattacharya A, Cruise M, Chahal P. Endoscopic ultrasound guided 22 gauge core needle biopsy for the diagnosis of Autoimmune pancreatitis. Pancreatology. 2018;18:168-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |