Published online Feb 22, 2019. doi: 10.13105/wjma.v7.i2.51
Peer-review started: January 16, 2019
First decision: January 30, 2019
Revised: February 7, 2019
Accepted: February 13, 2019
Article in press: February 14, 2019
Published online: February 22, 2019
Processing time: 35 Days and 20.7 Hours
It is of vital importance to find radiologic biomarkers that can accurately predict treatment response. Usually, the initiation of antiangiogenic therapy causes a rapid decrease in the contrast enhancing tumor. However, the treatment response is observed only in a fraction of patients due to the partial radiological response secondary to stabilization of abnormal vessels which does not essentially indicate a true antitumor effect. Perfusion-weighted magnetic resonance imaging (PW-MRI) techniques have shown implicitness as a strong imaging biomarker for gliomas since they give hemodynamic information of blood vessels. Hence, there is a rapid expansion of PW-MRI related studies and clinical applications.
To determine the diagnostic performance of PW-MRI techniques including: (A) dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI); and (B) dynamic susceptibility contrast magnetic resonance imaging (DSC-MRI) for evaluating response to antiangiogenic therapy in patients with recurrent gliomas.
Databases such as PubMed (MEDLINE included), EMBASE, and Google Scholar were searched for relevant original articles. The included studies were assessed for methodological quality with the Quality Assessment of Diagnostic Accuracy Studies 2 tool. Medical imaging follow-up or histopathological analysis was used as the reference standard. The data were extracted by two reviewers independently, and then the sensitivity, specificity, summary receiver operating characteristic curve, area under the curve (AUC), and heterogeneity were calculated using Meta-Disc 1.4 software.
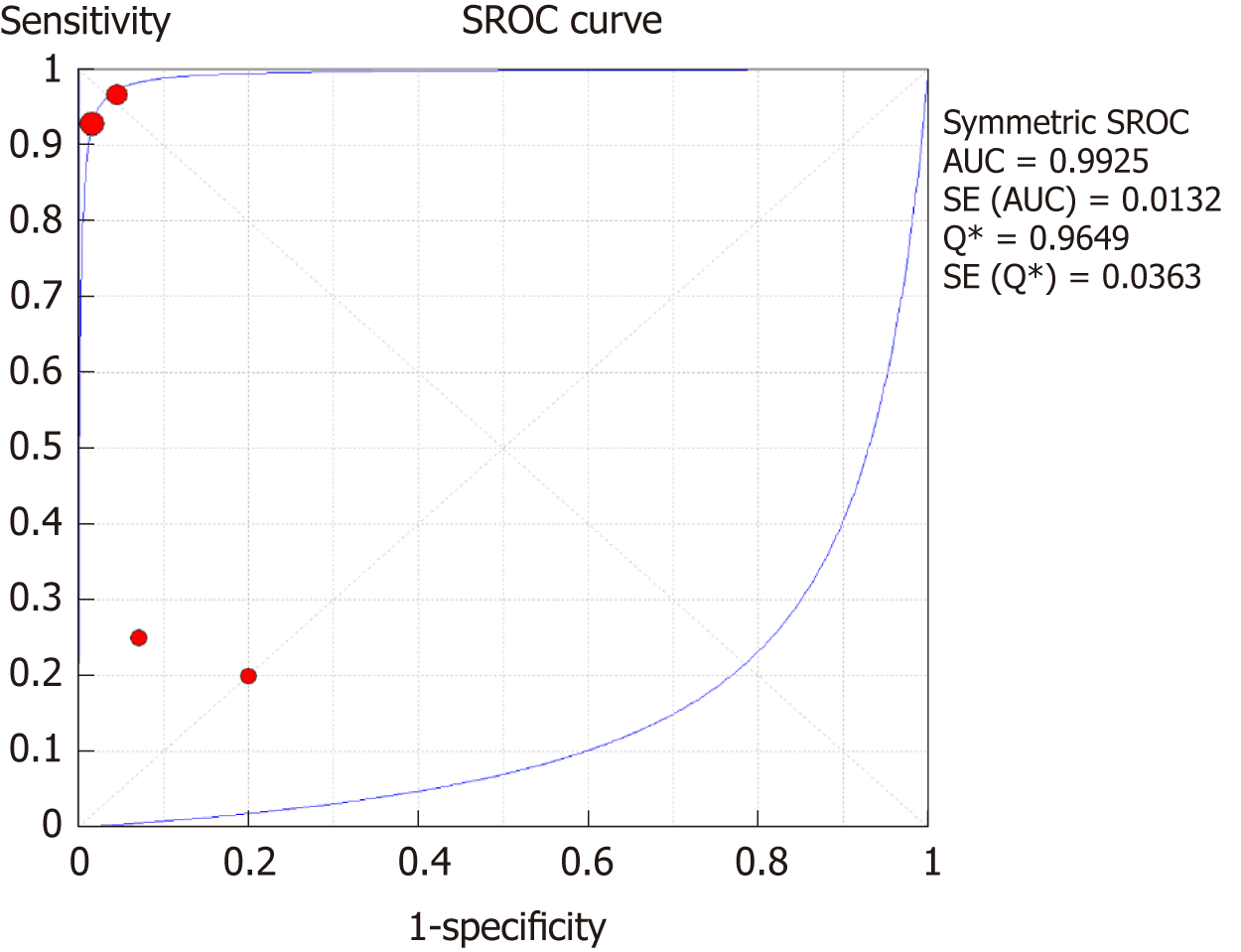
This study analyzed a total of six articles. The overall sensitivity for DCE-MRI and DSC-MRI was 0.69 [95% confidence interval (CI): 0.53-0.82], and the specificity was 0.99 (95%CI: 0.93-1) by a random effects model (DerSimonianee-Laird model). The likelihood ratio (LR) +, LR-, and diagnostic odds ratio (DOR) were 12.84 (4.54-36.28), 0.35 (0.22-0.53), and 24.44 (7.19-83.06), respectively. The AUC (± SE) was 0.9921 (± 0.0120), and the Q* index (± SE) was 0.9640 (± 0.0323). For DSC-MRI, the sensitivity was 0.73, the specificity was 0.98, the LR+ was 7.82, the LR- was 0.32, the DOR was 31.65, the AUC (± SE) was 0.9925 (± 0.0132), and the Q* index was 0.9649 (± 0.0363). For DCE-MRI, the sensitivity was 0.41, the specificity was 0.97, the LR+ was 5.34, the LR- was 0.71, the DOR was 8.76, the AUC (± SE) was 0.9922 (± 0.2218), and the Q* index was 0.8935 (± 0.3037).
This meta-analysis demonstrated a beneficial value of PW-MRI (DSC-MRI and DCE-MRI) in monitoring the response of recurrent gliomas to antiangiogenic therapy, with reasonable sensitivity, specificity, +LR, and -LR.
Core tip: Perfusion-weighted magnetic resonance imaging is one of the advanced magnetic resonance (MR) techniques which offer non-invasive and effective ways of grading, differentiating, and assessing therapeutic response and prognosis of brain tumors. This meta-analysis evaluates the clinical applicability of this MR technique in the assessment of the response of recurrent gliomas to antiangiogenic therapy.
- Citation: Kasenene A, Baidya A, Shams S, Xu HB. Evaluation of tumor response to antiangiogenic therapy in patients with recurrent gliomas using contrast-enhanced perfusion-weighted magnetic resonance imaging techniques: A meta-analysis. World J Meta-Anal 2019; 7(2): 51-65
- URL: https://www.wjgnet.com/2308-3840/full/v7/i2/51.htm
- DOI: https://dx.doi.org/10.13105/wjma.v7.i2.51
Gliomas are the most common primary brain neoplasms in adults[1]. They overgrow and are very invasive and angiogenic with a high rate of recurrence and dismal median 15-mo survival regardless of multi-modality management including surgical resection, radiotherapy, and chemotherapy[2]. Glioma-associated neovascularization peculiar assembly is an essential factor which contributes to several biological patterns like tumor development, invasiveness, and treatment response[3].
Thanks to the recognition of different signaling pathways and growth factors imperative in tumor angiogenesis, a couple of new anti-angiogenic drugs have so far been manufactured[4]. Vascular endothelial growth factor (VEGF) is an arch controller of angiogenesis, vessel development, and vessel permeability[5]. The angiogenic action of VEGF is promoted by VEGF receptor-2 (VEGFR-2) which is also the central target for anti-angiogenic therapies, even though added studies have highlighted the significance of signaling via VEGFR-1[6].
Bevacizumab (BEV), a humanized monoclonal antibody to the VEGF-A, was the first VEGF inhibitor accredited for cancer treatment by the food and drug authority[7]. Also, other multiple approaches for inhibiting VEGF were researched over the past decade. These approaches include neutralizing antibodies to VEGF[8], low-molecular-weight VEGFR tyrosine kinase inhibitors (TKIs)[9,10], and soluble VEGFR constructs (VEGF-Trap)[11]. Although antiangiogenic drugs result in a rapid decrease in the contrast enhancing tumor, the treatment response is observed in just a fraction of patients, because the radiological response may partially result from stabilization of abnormal vessels and does not inherently indicate a true antitumor effect[12,13]. Therefore, it is of vital importance to find radiologic biomarkers that can accurately predict treatment response or measure response after the initiation of anti-VEGF therapy[14].
The current imaging modality of choice for clinical use in brain tumors is magnetic resonance imaging (MRI)[15]. Although the conventional MRI techniques show excellent anatomical information of tumors, they cannot evaluate quantitatively the blood vessel physiology and exhibit biological characteristics within the tumor molecules and cells, which play a part in tumor grading[16], treatment evaluation[17], and prognosis prediction[18]. Moreover, non-enhancing regions representative of edema surrounding the tumor cannot be seen on conventional MRI, hampering the total safe tumor excision and treatment response evaluation[19,20]. Perfusion-weighted MRI (PW-MRI) techniques, including dynamic contrast-enhanced MRI (DCE-MRI) and dynamic susceptibility contrast MRI (DSC-MRI), have shown implicitness as a reliable imaging biomarker for gliomas since they give hemodynamic information of blood vessels[21-23]. Hence, there is a rapid expansion of PW-MRI application range which explores the association of imaging parameters and the distinctive features of gliomas non-invasively[24].
Recently, a meta-analysis containing 35 studies assessed the diagnostic accuracy of MRI techniques in the evaluation of treatment responses in patients with high-grade gliomas[25]. In 23 studies, DSC-MRI and DCE-MRI scans were carried out. The sensitivity and specificity of DSC-MRI (18 reviews, 708 patients) were 87% (82-91%) and 86%, respectively. The pooled analysis showed that the sensitivity and specificity of DCE- MRI (five studies, 207 patients) were 92% (73-98%) and 85%, respectively. However, there is no meta-analysis on the use of PW-MRI in the assessment of the response of recurrent gliomas to antiangiogenic therapy. Therefore, we believe that a meta-analysis of the relevant studies is required to better clarify the role of PW-MRI in predicting and assessing the response of recurrent gliomas to antiangiogenic drugs.
The present Meta-analysis was done as per the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement[26].
Studies included in this analysis fulfilled these requirements: (A) the study researched the performance of DSC-MRI or DCE-MRI for evaluating antiangiogenic therapy response in recurrent glioma patients; (B) the patients underwent tumor resection and chemotherapy treatment before recurrence and were treated with antiangiogenic therapy after recurrence; (C) The reference standard was either pathology reports or clinical observation or by imaging follow-up; and (D) the primary data were enough to compute the true positive (TP), true negative (TN), false positive (FP), and false negative (FN).
The studies were excluded for the following reasons: (A) reviews, pre-clinical studies, titles, abstracts, and expert opinions; (B) studies assessing PW-MRI in glioma grading; and (C) inadequate data to generate a 2 × 2 table.
We conducted a comprehensive search in EMBASE, Google Scholar, and Medline (PubMed) for studies up to December 31, 2017 that evaluated the use of PW-MRI in the detection of antiangiogenic response for recurrent gliomas. For PubMed which was the primary database we used to search for studies, MeSH headings used were “MRI”, “DCE-MRI”, “DSC-MRI”, “PW-MRI”, “Glioma”, “Glioblastoma”, “GBM”, “Anti-VEGF”, “Antiangiogenic”, and their combinations.
Data extraction was carried out by two reviewers (Kasenene A and Baidya A) from each of the selected studies using an Excel spreadsheet.
The extracted data included general characteristics (i.e., first author and year of publication for each study, country, number of patients, median age, study design, imaging modality, imaging biomarkers, MR scanner tesla intensity, type of anti-angiogenic therapy given, and the treatment response gold standard used), TP, TN, FP, and FN. Disagreements between investigators concerning outcomes of interest were reviewed later, and the consensus was reached by discussion.
The Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) tool[27] was used to assess the quality of the included studies, where we scored all 14 QUDAS items used to determine the risk of bias as “low risk”, “high risk”, or “uncertain risk”.
We constructed 2 × 2 tables for each study independently. Data from the original publications were used to calculate the sensitivity, specificity, positive likelihood ratio (LR+), negative likelihood ratio (LR-), diagnostic odds ratio (DOR), 95% confidence intervals (CI), summary receiver operating characteristic (SROC) curves, and Q* index. Wherever any of TP, FP, FN, and TN values was zero, we added 0.5 to every cell of each study.
We adopted a random effects model (DerSimonianee-Laird model) in this study because of the difference in sample size, anti-angiogenic therapy, and reference standards among the included studies.
The analysis was done using Meta-Disc (version 1.4) software and Microsoft Excel 2016 (Microsoft, Seattle, WA, USA)[28].
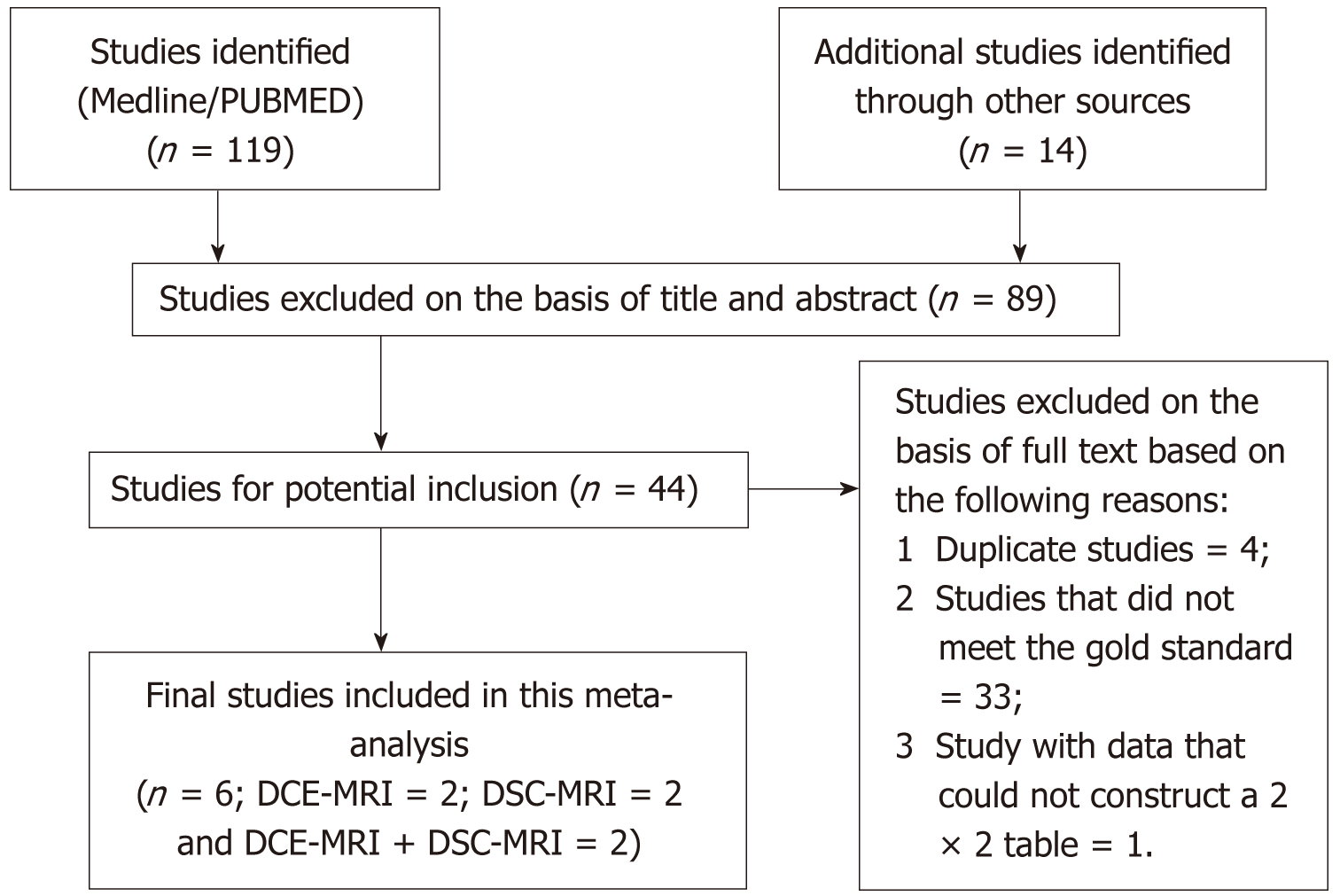
This meta-analysis included studies which met the selection criteria successfully and were published before December 31, 2017. The search strategy generated a total of 133 clinical studies, of which 44 were investigated further. Out of the 44 studies, we excluded 38 studies, and finally, in this meta-analysis, we included only six studies. The flowchart outlining the process of study selection and exclusion reasons is showed in Figure 1.
The quality assessment was performed as per the QUADAS-2 tool and the findings are shown in Table 1. Among the six included studies, four were conducted prospectively, and two were conducted retrospectively. None of the included studies checked all 14 QUDAS risk assessment items.
| Study | Risk of bias | Applicability concerns | ||||||
| Patient selection | Index test | Reference standard | Flow and timing | Patient selection | Index test | Reference standard | ||
| Iwamoto et al[29], 2010 | L | U | H | L | L | L | L | |
| Piludu et al[30], 2015 | L | U | U | L | L | L | L | |
| Kalpathy-Cramer et al[31], 2017 | L | U | U | L | L | L | L | |
| O’Neill et al[32], 2016 | L | U | H | L | L | L | L | |
| Schmainda et al[4], 2014 | L | U | U | L | L | L | L | |
| Hilario et al[33], 2016 | L | U | U | L | L | L | L | |
The characteristics and the clinical responses of the six studies encompassed in this meta-analysis are delineated in Tables 2 and 3. The studies were all in English, containing 120 patients all together with their age ranging from 25-78 years. The sample size of the studies was 10 to 36 patients.
| Studies | Country | Patients, n | Median age | Study design | Imaging modality | Imaging biomarkers | MR scanner tesla | Anti-angiogenic treatment | Clinical question | Standard reference | TP | FP | FN | TN |
| Iwamoto et al[29], 2010 | United States | 11 | 53 (29-73) | Pro-spective | DSC, DCE | Ktrans, rCBV | NR | Pazopanib | DSC and DCE vs response | Macdonald criteria RANO criteria | 1 | 0 | 6 | 4 |
| Piludu et al[30], 2015 | Germany | 27 | 54 (33-77) | Pro-spective | DCE | nIAUGC, Ktrans | 3.0 T | Bevacizumab | DCE vs response | RANO criteria | 6 | 0 | 5 | 16 |
| Kalpathy-Cramer et al[31], 2017 | United States | 10 | 62 (51-74) | Pro-spective | DSC, DCE | Ktrans, rCBV, rCBF | 3.0 T | Tivozanib | DSC and DCE vs response | RANO criteria | 1 | 1 | 4 | 4 |
| O’Neill et al[32], 2016 | United States | 12 | NR | Pro-spective | DCE | Ktrans, Ve | 1.5 T | VEGF Trap | DCE vs response | Macdonald criteria | 1 | 0 | 0 | 11 |
| Schmainda et al[4], 2014 | United States | 36 | 34 (30-68) | Retro-spective | DSC | rCBV, stdrCBV | 1.5 T or 3.0 T | Bevacizumab | DSC vs response | Macdonald criteria RANO criteria | 6 | 0 | 0 | 30 |
| Hilario et al[33], 2016 | Spain | 24 | 52.5 (31-74) | Retro-spective | DSC | Leakage volume (CBV-LCCBV) | 1.5 T | Bevacizumab | DSC vs response | RANO criteria | 14 | 0 | 0 | 10 |
| Study | Patient, n | RANO criteria | Macdonald criteria | ||||||
| Responders | Non-responders | Responders | Non-responders | ||||||
| CR | PR | SD | PD | CR | PR | SD | PD | ||
| O’Neill et al[32], 2016 | 12 | - | - | - | - | 0 | 1 | 0 | 11 |
| Piludu et al[30], 2015 | 27 | 0 | 6 | 5 | 16 | - | - | - | - |
| Schmainda et al[4], 2014 | 36 | 0 | 6 | 0 | 30 | - | - | - | - |
| Iwamoto et al[29], 2010 | 11 | - | - | - | - | 0 | 1 | 6 | 4 |
| Hilario et al[33], 2016 | 24 | 0 | 14 | 0 | 10 | - | - | - | - |
| Kalpathy-Cramer et al[31], 2017 | 10 | 1 | 1 | 4 | 4 | - | - | - | - |
There were two studies on DCE-MRI, two studies on DSC-MRI, and two combined both. All studies performed a sequential DCE-MRI, DSC-MRI, or both before and after the antiangiogenic treatment. Four different types of antiangiogenic therapies were used as the treatment of choice whereby BEV, which is a recombinant humanized monoclonal antibody targeting the VEGF, was used in three studies. VEGF Trap, which acts as a trap by inhibiting the activity of the VEGF subtypes VEGF-A and VEGF-B, was used in one study. Pazopanib, a potent and selective multi-targeted receptor TKI which stops the growth of the tumor and halts angiogenesis, was used in one study. Tivozanib, which is also a TKI, was used in the remaining one study. In four studies, a decrease in volume transfer constant (Ktrans) value was used as the cutoff criterion for response to antiangiogenic therapy. The other studies relied on a reduction of relative cerebral blood volume (rCBV), extracellular volume fraction (EES Ve) value, vessel leakage reduction, decrease in tumor volume, tissue oxygenation, or vasogenic edema criteria for therapy response.
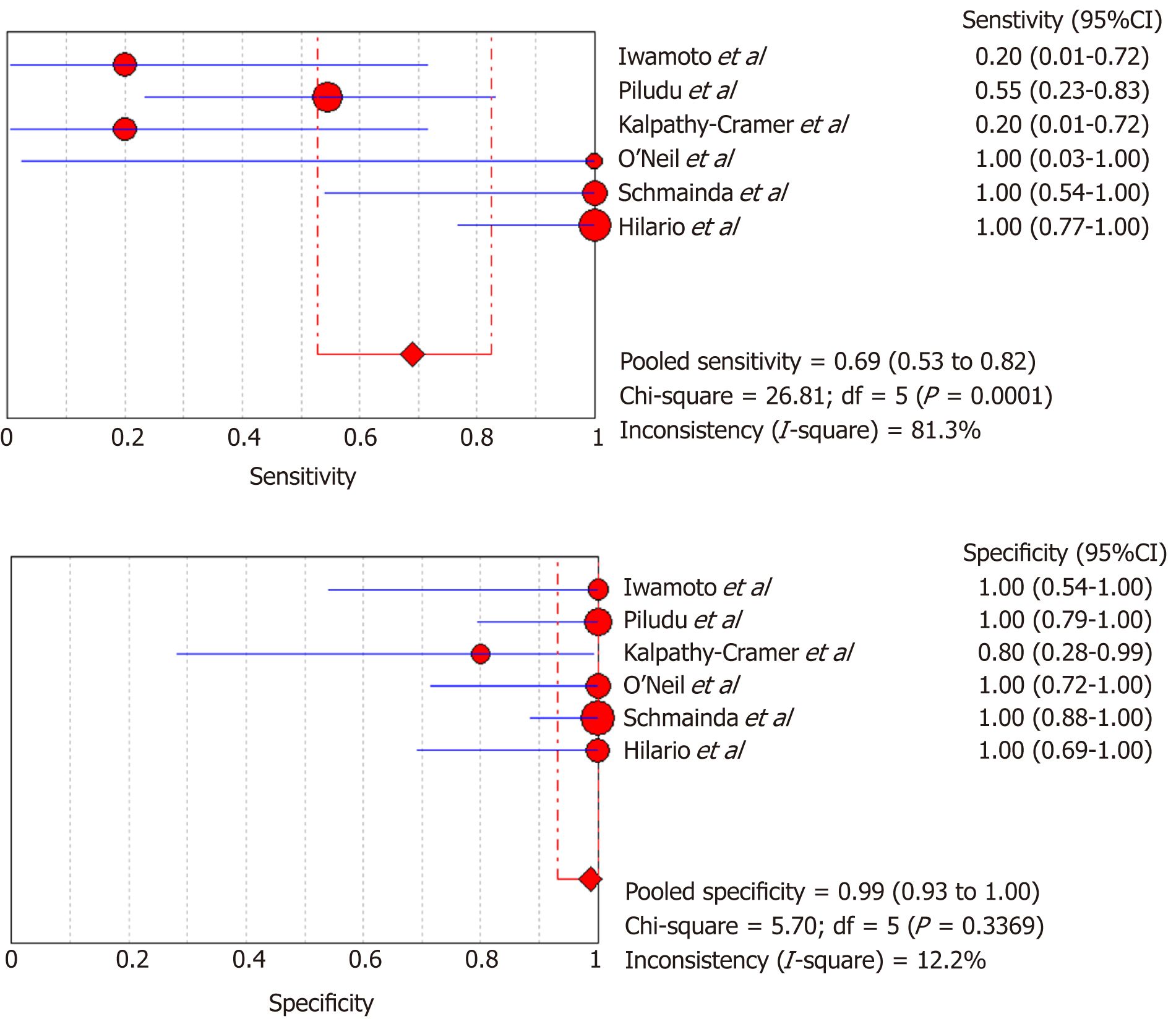
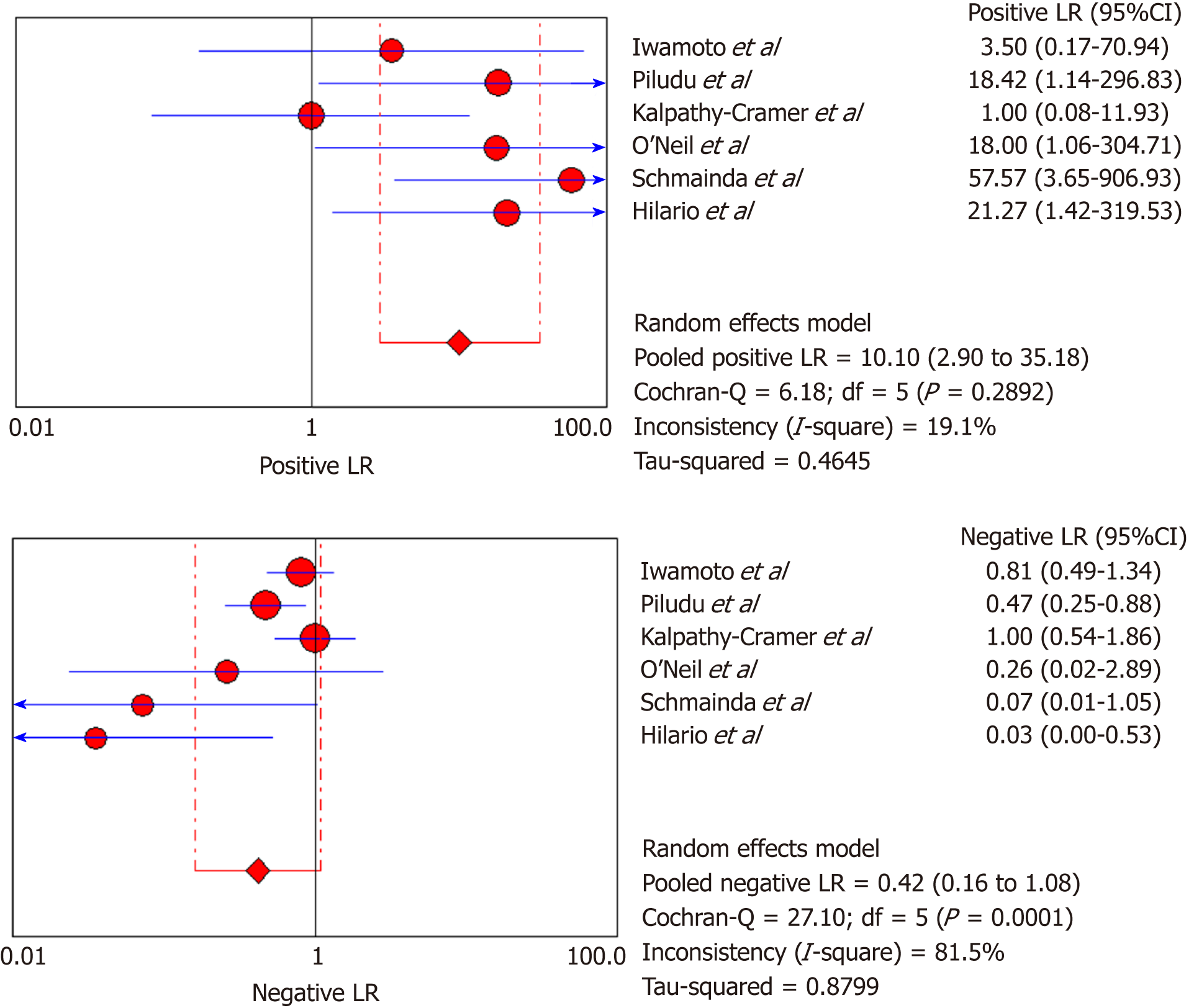
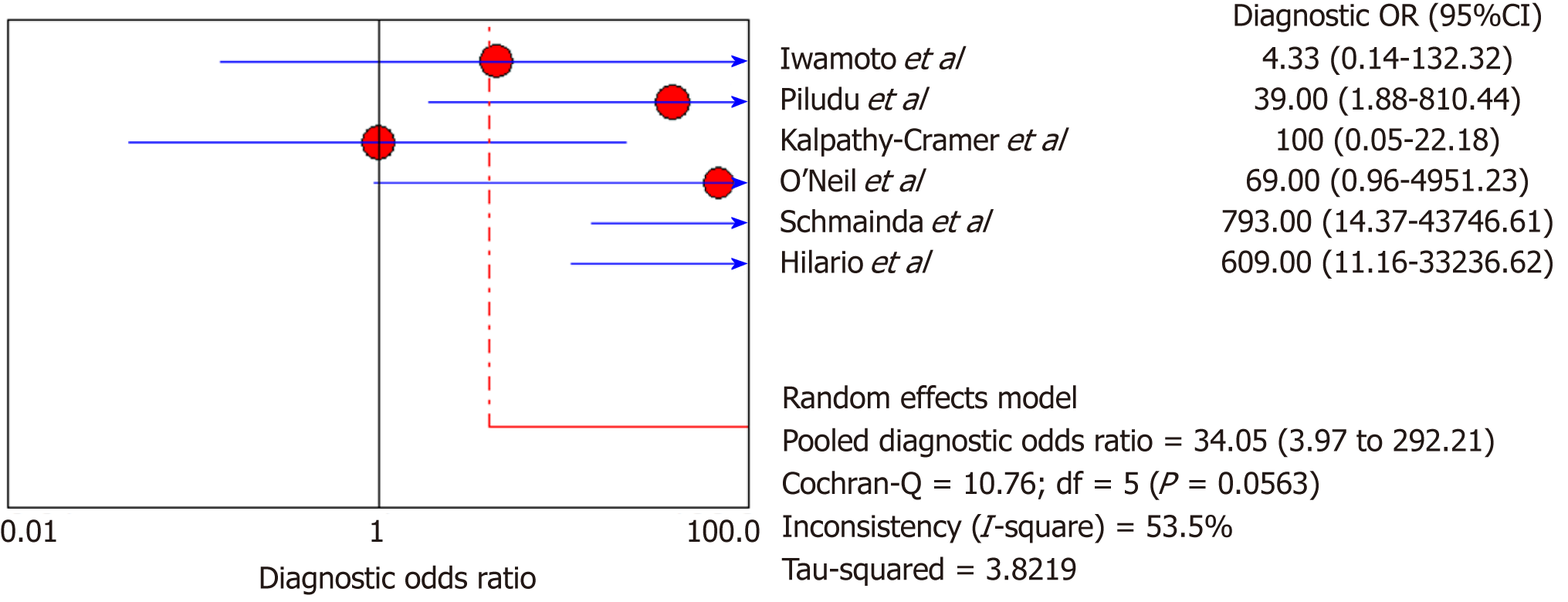
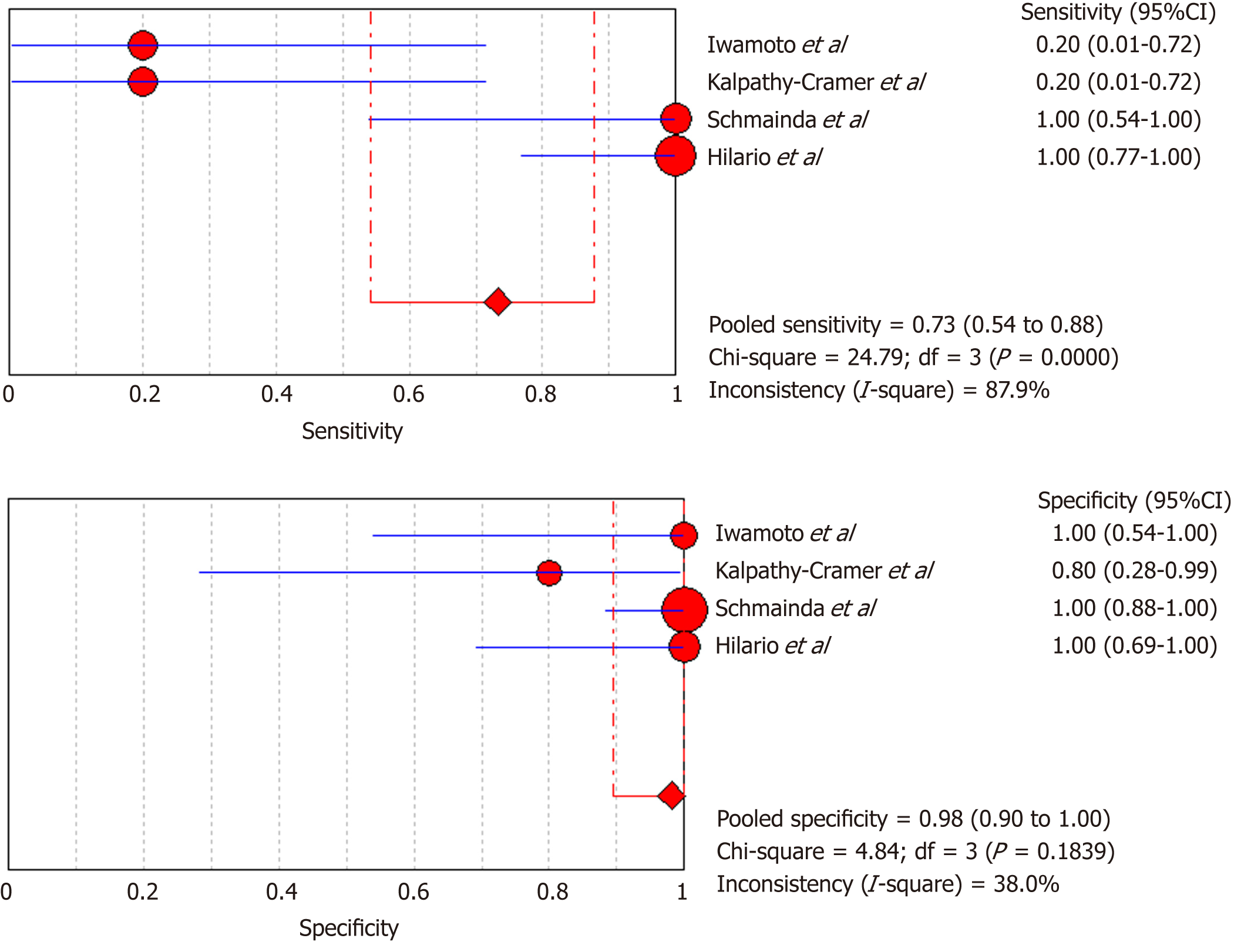
DSC-MRI and DCE-MRI: Due to the heterogeneity across all six included studies which evaluated DCE-MRI or DSC-MRI or both, a random effects model (DerSimonianee-Laird model) was used to compute the sensitivity, specificity, LR+, LR-, and DOR (Figures 2-4). The overall sensitivity of DCE-MRI and DSC-MRI was 69%. The specificity was 99%. The detailed sensitivity, specificity, LR+, LR-, and DOR with 95%CI for individual studies are presented in Table 4.
| Study | TP | FP | FN | TN | Sensitivity (95%CI) | Specificity (95%CI) | LR+ (95%CI) | LR- (95%CI) | DOR (95%CI) |
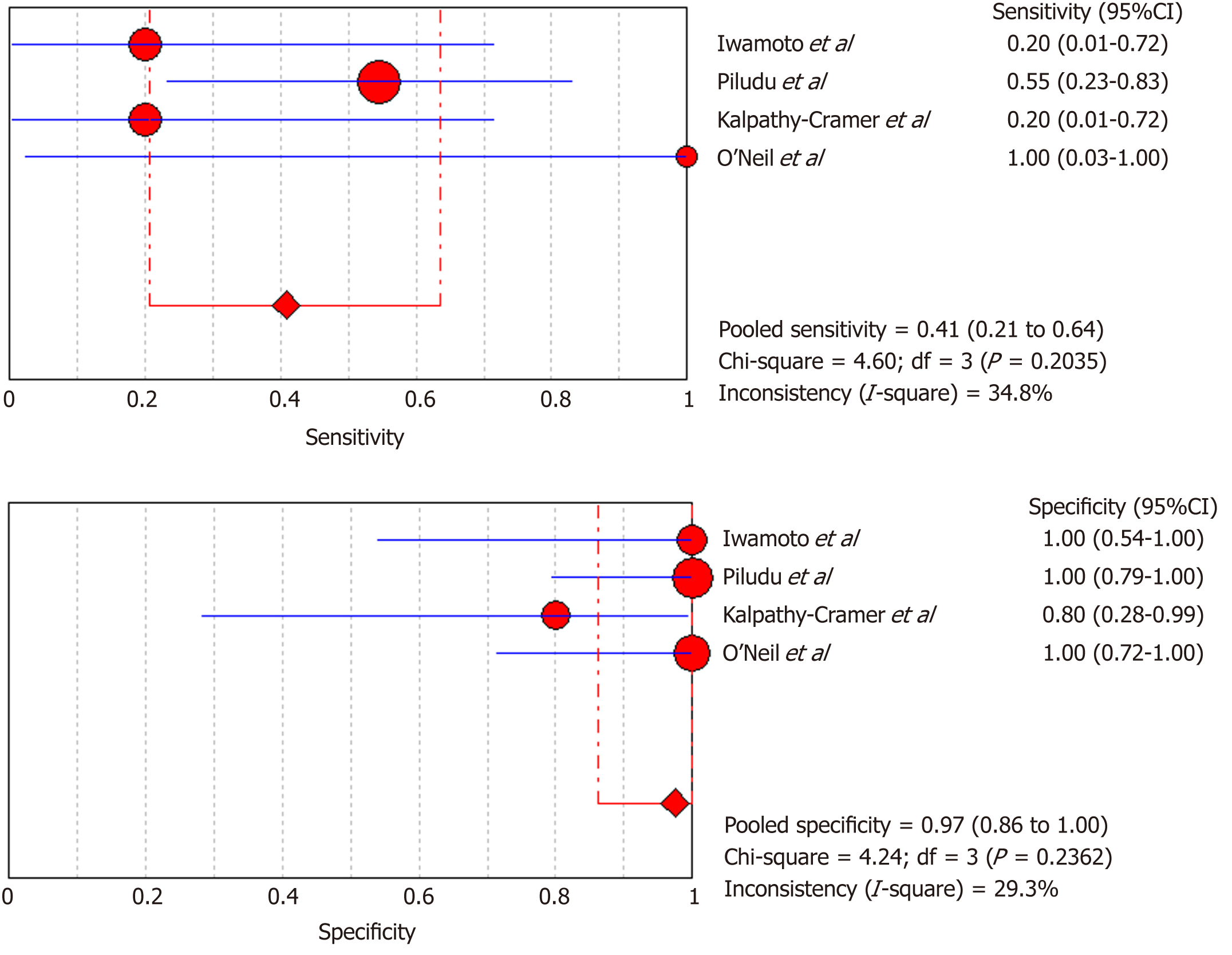
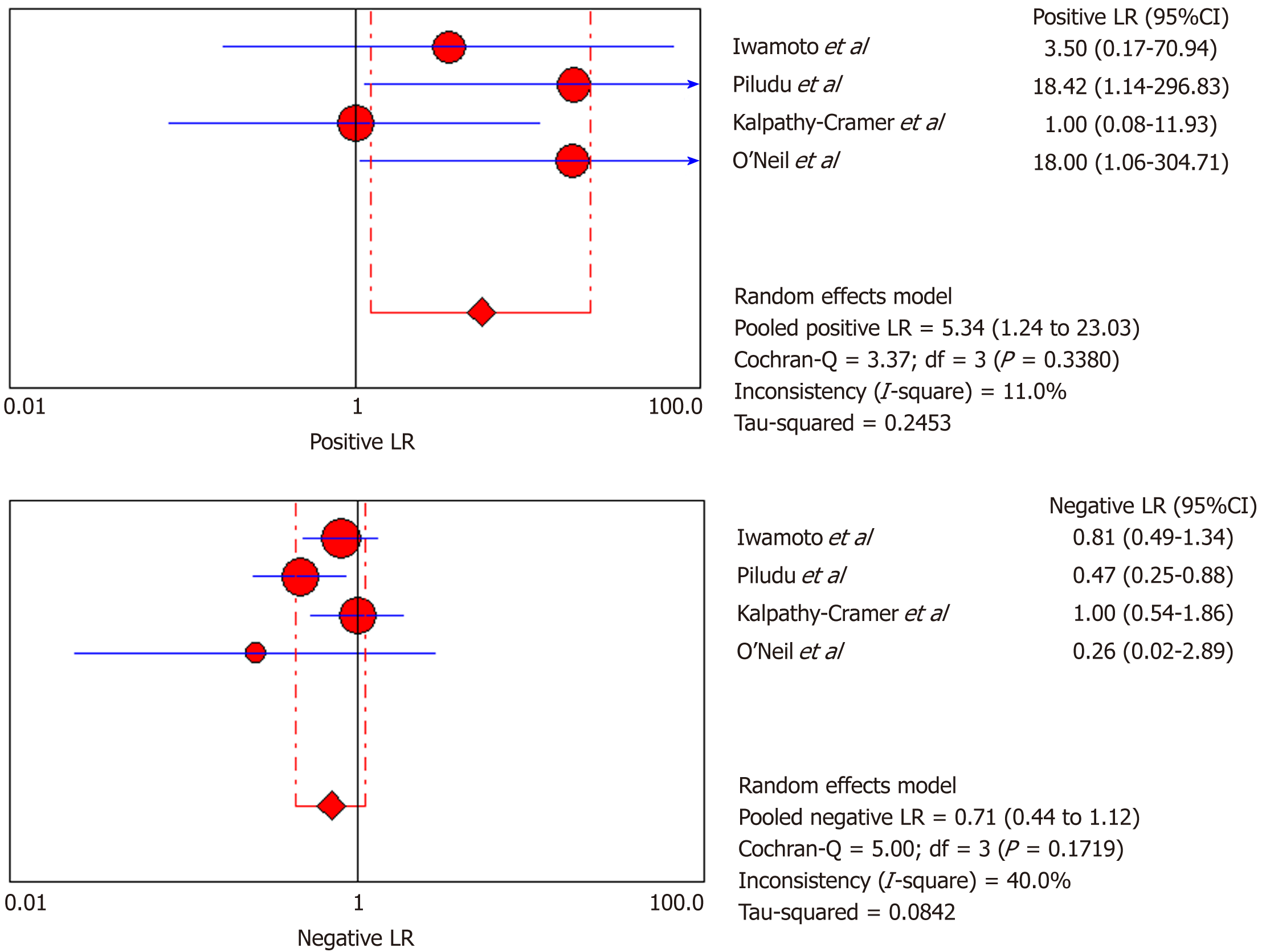
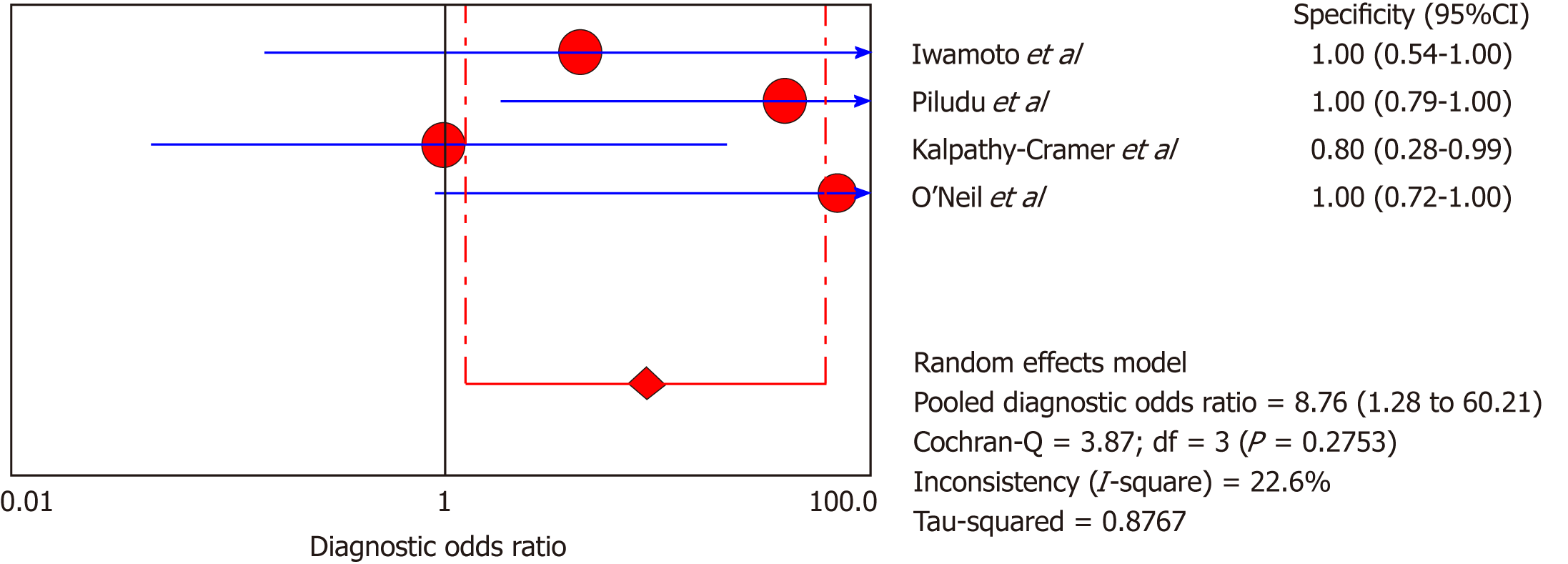
| Iwamoto et al[29], 2010 | 1 | 0 | 4 | 6 | 0.2 (0.01-0.72) | 1 (0.54-1) | 3.5 (0.17-70.94) | 0.81 (0.49-1.340) | 4.33 (0.14-132.32) |
| Piludu et al[30], 2015 | 6 | 0 | 5 | 16 | 0.55 (0.23-0.83) | 1 (0.79-1) | 18.42 (1.14-296.83) | 0.47 (0.25-0.880 | 39 (1.88-810.44) |
| Kalpathy-Cramer et al[31], 2017 | 1 | 1 | 4 | 4 | 0.2 (0.01-0.72) | 0.8 (0.28-0.99) | 1 (0.08-11.93) | 1 (0.54-1.86) | 1 (0.05-22.18) |
| O’Neill et al[32], 2016 | 1 | 0 | 0 | 11 | 1 (0.03-1) | 1 (0.72-1) | 18 (1.06-304.71) | 0.26 (0.02-2.89) | 69 (0.96-4951.23) |
| Schmainda et al[4], 2014 | 6 | 0 | 0 | 30 | 1 (0.54-1) | 1 (0.88-1) | 57.57 (3.65-906.93) | 0.07 (0.01-1.05) | 793 (14.37-43,746.61) |
| Hilario et al[33], 2016 | 14 | 0 | 0 | 10 | 1 (0.77-1) | 1 (0.69-1) | 21.27 (1.42-319.53) | 0.03 (0-0.53) | 609 (911.16-33,236.62) |
| Pooled results | 29 | 1 | 13 | 77 | 0.69 (0.53-0.82) | 0.99 (0.93-1) | 12.84 (4.54-36.28) | 0.35 (0.22-0.53) | 24.44 (7.19-83.06) |
| Heterogeneity test | I2 = 81.30%, P = 0.0001 | I2 = 12.2%, P = 0.3369 | I2 = 19.1%, P = 0.2892 | I2 = 81.5%, P = 0.0001 | I2 = 53.5%, P = 0.0563 | ||||
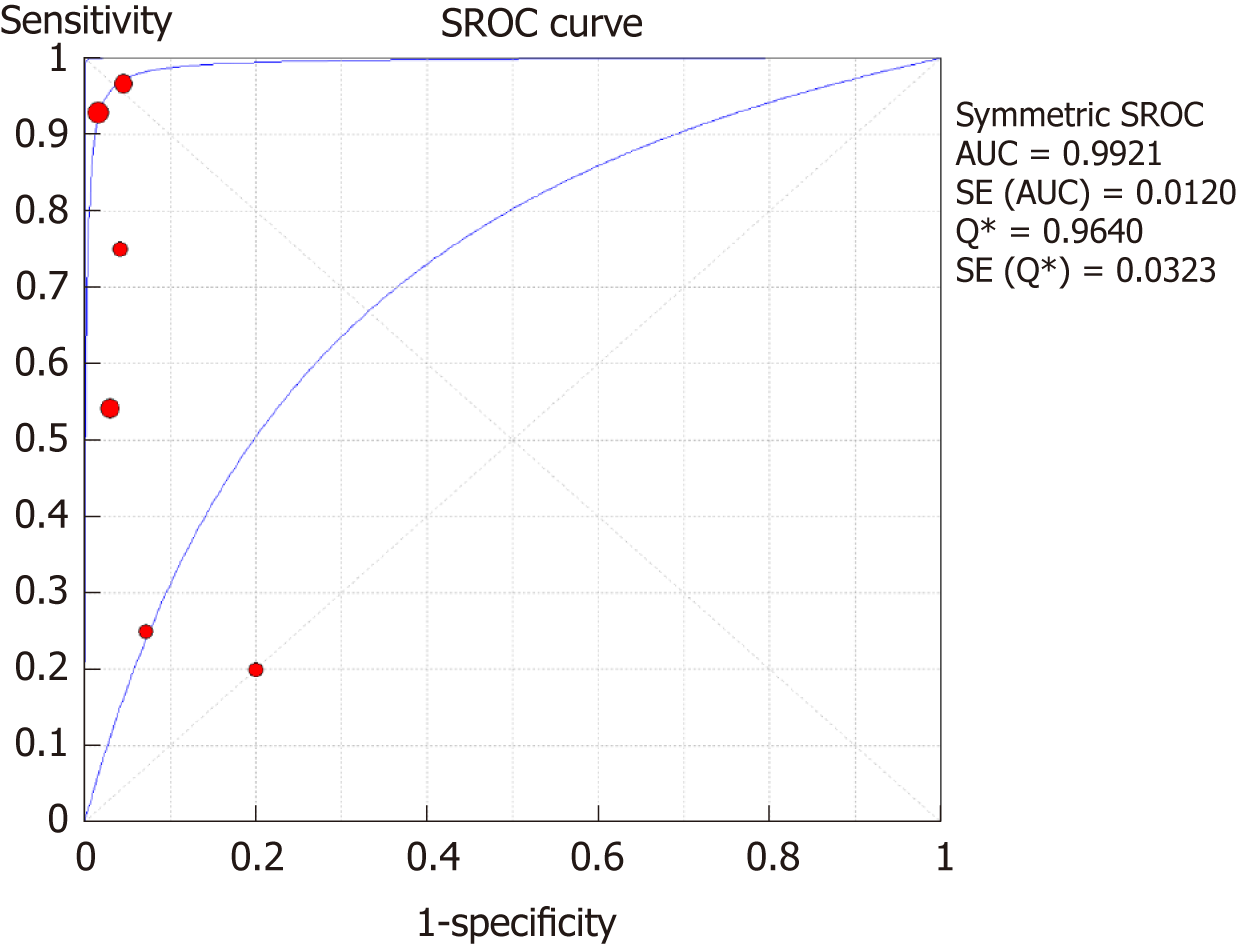
The SROC curves with the Q* index are shown in Figure 5. Of all six studies, the area under the curve (AUC) (± SE) was 0.9921 (± 0.0120), and the Q* index was 0.9640 (± 0.0323).
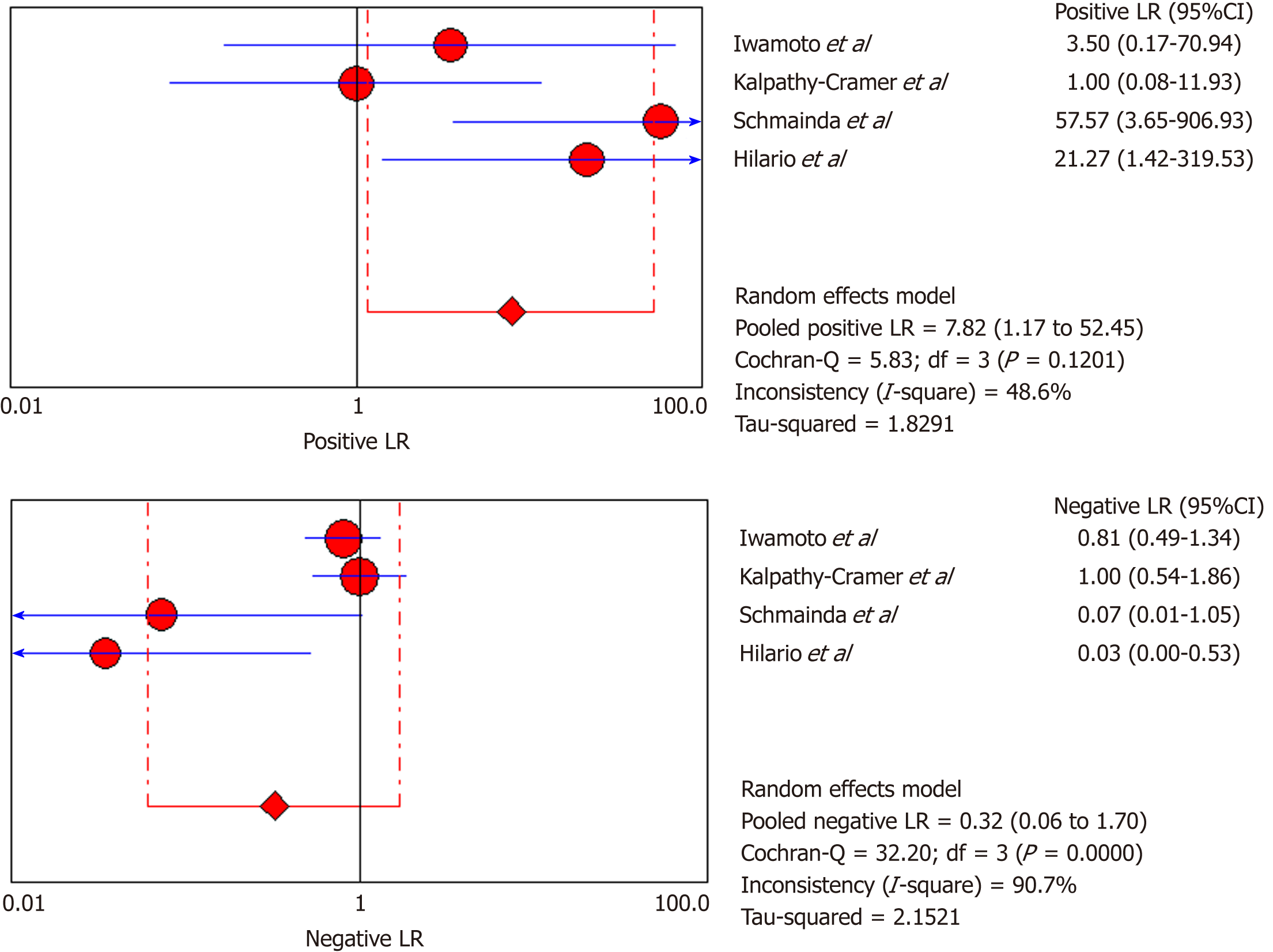
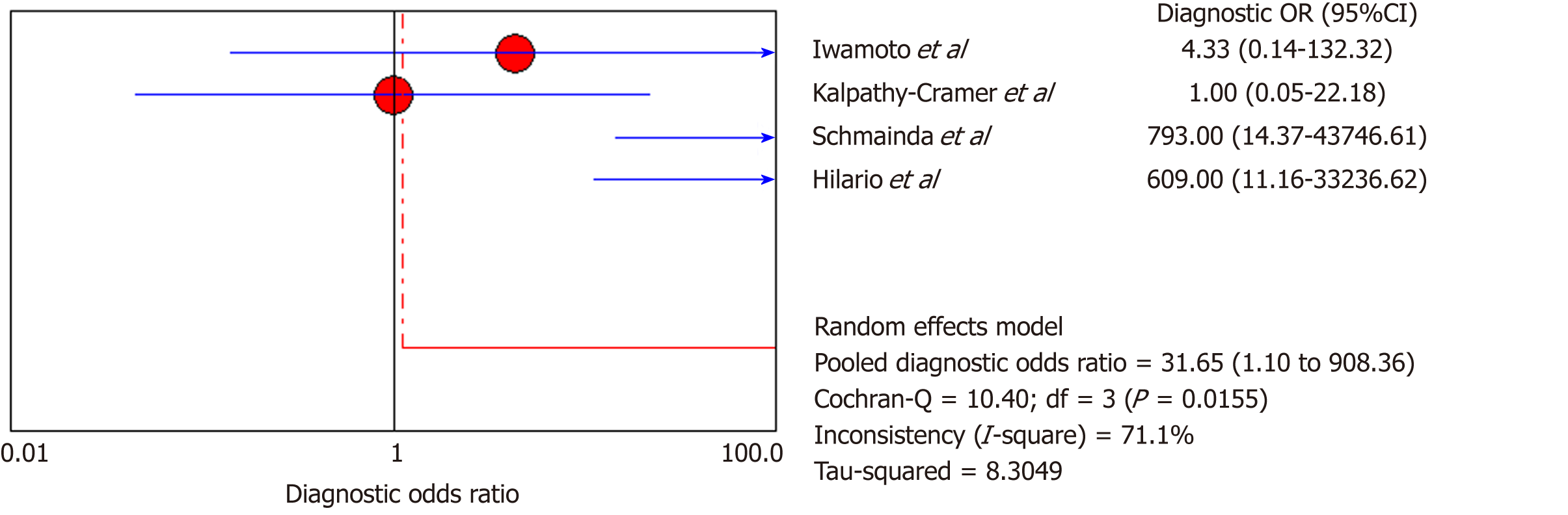
DSC-MRI: Four included studies evaluated DSC-MRI. Also, the random effects model (DerSimonianee-Laird model) was used to calculate sensitivity, specificity, LR+, LR-, and DOR (Figures 6-8). The sensitivity of DSC-MRI to assess antiangiogenic treatment response in gliomas was 73%. The specificity was 98%. The pooled LR+ was 7.82; LR- was 0.32. The pooled DOR was 31.65.
The SROC curves with the Q* index are shown in Figure 9. Of all four studies, the AUC (± SE) was 0.9925 (± 0.0132), and the Q* index was 0.9649 (± 0.0363).
DCE-MRI: Four included studies evaluated DCE-MRI, again the random effects model (DerSimonianee-Laird model) was used to calculate sensitivity, specificity, LR+, LR-, and DOR (Figures 10-12). The sensitivity of DCE-MRI to assess antiangiogenic treatment response in gliomas ranged from 21% to 64%. The specificity was 97%. The pooled LR+ was 5.34; LR- was 0.71. The pooled DOR was 8.76.
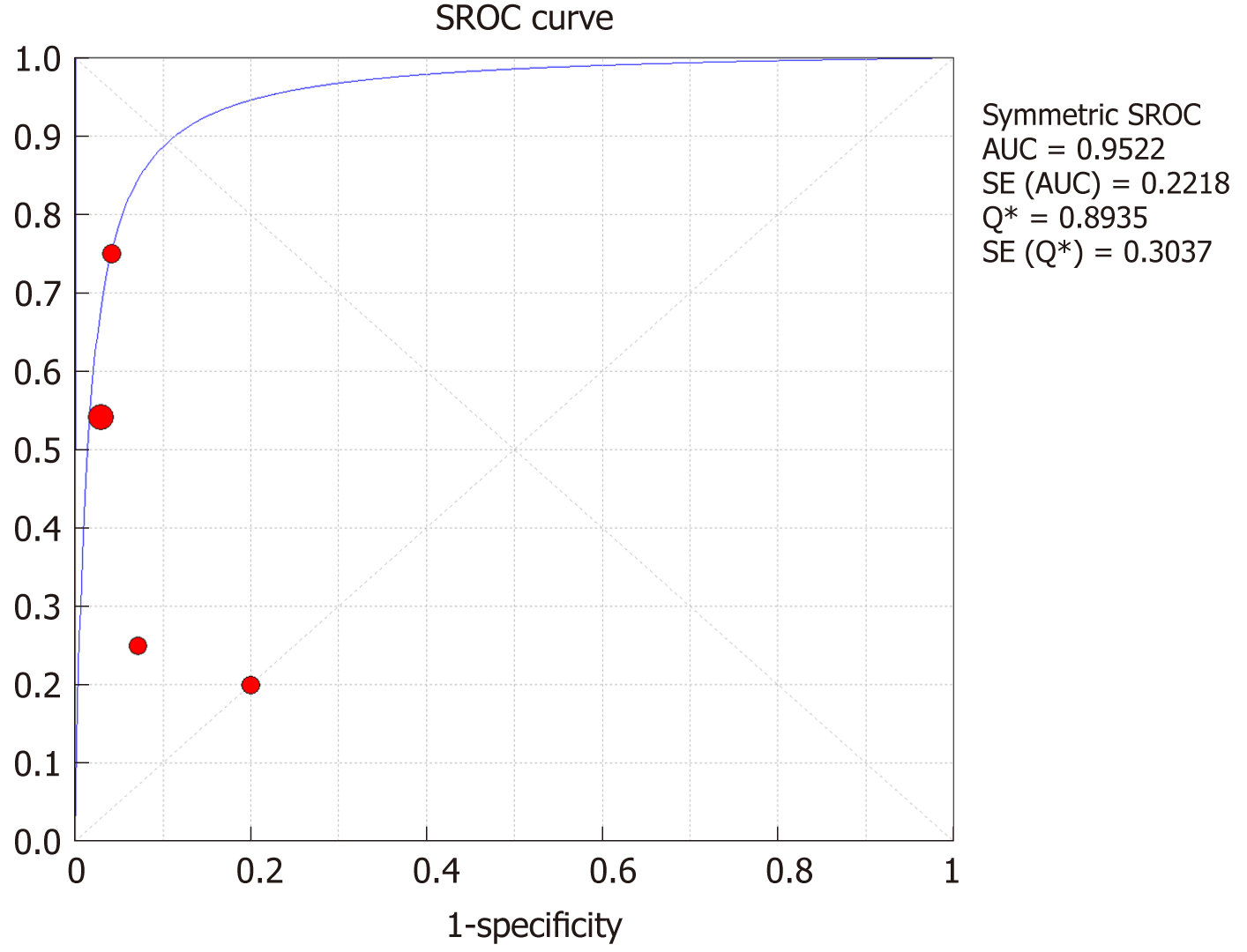
The SROC curves with the Q* index are shown in Figure 13. Of all four studies, the AUC (± SE) was 0.9922 (± 0.2218), and the Q* index was 0.8935 (± 0.3037).
PW-MRI is a non-invasive imaging technique which is increasingly used in clinical evaluation and treatment response assessment of brain tumors. The current meta-analysis was conducted to assess the diagnostic performance of PW-MRI techniques (DCE-MRI and DSC-MRI) in the evaluation of glioma response to antiangiogenic therapy.
The overall sensitivity and specificity for DSC-MRI and DCE-MRI were 0.69 and 0.99, respectively. Although the pooled sensitivity for the present study was relatively low, the statistical results still showed that PW-MRI (DSC-MRI and DCE-MRI) techniques are effective ways to assess the response of gliomas to antiangiogenic therapy. On the SROC, the summary points were closer to the left hand, and the AUC was 0.9921, which indicates an excellent diagnostic accuracy. Likelihood ratios are other measures of the diagnostic accuracy, with LR greater than 1 meaning that the test result is associated with the disease presence, and the LR ratio less than 1 meaning that the test result is related to the disease absence. The higher the LR are from 1, the stronger the evidence for the presence or absence of disease; LR > 10 and < 0.1 are thought to provide strong evidence to declare the presence or absence of the disease in most cases[34]. The LR+ for the DSC-MRI and DCE-MRI group was 12.84, indicating that there were an approximately 13 times chance of PWI-MRI showing clinically meaningful changes as the result of antiangiogenic therapy. On the other hand, the LR- was 0.35, meaning it was below the cut-off value, indicating a chance of not showing any changes was approximately 0.4 times.
Moreover, for the DSC-MRI group, the sensitivity and specificity were 0.73 and 0.98, respectively, where the sensitivity was slightly higher than in the DSC-MRI and DCE-MRI group. The pooled LR+ was 7.82, which means the chance of DSC-MRI indicating the changes associated with antiangiogenic therapy was approximately 8 times; LR- was 0.32 and below the cut-off point, which suggests that the chance of DSC-MRI not showing treatment-associated changes was 0.3 times, and AUC was 0.9925, indicating an excellent diagnostic accuracy. While for the DCE-MRI group, the sensitivity and specificity were 0.41 and 0.97, respectively; the sensitivity of the DCE-group was lower as compared to the previous two groups, and this might be due to the patient sample size variation among the studies in this group. The LR+ was 5.34, indicating a 5 times chance of showing treatment-associated changes; LR- was 0.71 and below the cut-off value, indicating a 0.7 times chance of not showing anything, and the AUC was 0.9922, indicating a higher diagnostic accuracy. All of these values suggest that both DSC-MRI and DCE-MRI have diagnostic value in treatment evaluation of recurrent gliomas.
First, some included studies were too small, which may have led to imprecise and inconclusive results. Second, only articles published in English were included, which may have led to publication bias. Third, there was verification bias or workup bias, because patients were subjected to different reference tests and antiangiogenic medications.
Regardless of some limitations, this meta-analysis demonstrated a beneficial value of PW-MRI (DSC-MRI and DCE-MRI) in monitoring the response to antiangiogenic therapy in recurrent gliomas, with reasonable sensitivity, specificity, +LR, and -LR.
The perfusion-weighted imaging is an advanced magnetic resonance (MR) technique capable of giving a detailed hemodynamic status of the tumors; this has prompted a surge of studies both in vitro and in vivo with some of these studies done on humans. The current meta-analysis is exploring the clinical application of this method in recurrent glioma patients after antiangiogenic therapy.
So far, biopsy, which is too invasive, is the gold standard for diagnosing brain tumors; perfusion-weighted magnetic resonance imaging (PW-MRI) offers a non-invasive way for diagnosing, grading, and assessing progression of brain tumors. Therefore, scientifically proving that this method works is of paramount importance in the spirit of encouraging its incorporation to the daily clinical practices.
The primary objective of this study was to assess the use of PW-MRI in evaluating the response of recurrent gliomas to antiangiogenic treatment based on the already available literature, and the finding of this study showed that both dynamic contrast-enhanced MRI and dynamic susceptibility contrast MRI could be used for this purpose. These findings would warrant further human-based studies on this MR technique to achieve its universal acceptability for clinical use.
Studies related to this topic were searched in PubMed, Google Scholar, and other scientific search engines. The selected studies were sorted according to the Preferred Reporting Items for Systematic Reviews guidelines, and only six studies meeting the inclusion criteria were included in the current meta-analysis. Meta-Disc 1.4 software and Microsoft Excel were used to analyze the data.
The sensitivity and specificity of this study proved that PW-MR techniques are capable of evaluating treatment response in patients with brain tumors, i.e., recurrent gliomas, and these findings, together with other related studies, mean that we are getting a step closer to its complete adaption for clinical use. The only concern is what should be done to improve and perfect this technique.
This meta-analysis showed that PW-MRI could be used to assess brain tumor therapeutic response. However, more studies must be conducted because that is the only way we will be sure of its clinical efficacy in diagnosing, follow-up, and treatment of brain tumors.
While searching for studies, we realized that very few studies had been carried out on human subjects; this tells us that there is more to be done until it is adopted universally for clinical use. There are many studies carried on animals, which mean the technique is being perfected and improved with each passing day. We need more studies assessing its application on human subjects.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Petretta M S- Editor: Dou Y L- Editor: Wang TQ E- Editor: Bian YN
| 1. | Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131:803-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10993] [Cited by in RCA: 10821] [Article Influence: 1202.3] [Reference Citation Analysis (0)] |
| 2. | Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14033] [Cited by in RCA: 15745] [Article Influence: 787.3] [Reference Citation Analysis (0)] |
| 3. | Hardee ME, Zagzag D. Mechanisms of glioma-associated neovascularization. Am J Pathol. 2012;181:1126-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 336] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 4. | Schmainda KM, Prah M, Connelly J, Rand SD, Hoffman RG, Mueller W, Malkin MG. Dynamic-susceptibility contrast agent MRI measures of relative cerebral blood volume predict response to bevacizumab in recurrent high-grade glioma. Neuro Oncol. 2014;16:880-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 5. | Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92:735-745. [PubMed] |
| 6. | Carmeliet P, Moons L, Luttun A, Vincenti V, Compernolle V, De Mol M, Wu Y, Bono F, Devy L, Beck H, Scholz D, Acker T, DiPalma T, Dewerchin M, Noel A, Stalmans I, Barra A, Blacher S, VandenDriessche T, Ponten A, Eriksson U, Plate KH, Foidart JM, Schaper W, Charnock-Jones DS, Hicklin DJ, Herbert JM, Collen D, Persico MG. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med. 2001;7:575-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1211] [Cited by in RCA: 1196] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 7. | Food and Drug Administration. Drug Safety and Availability - Avastin (bevacizumab) Information. Available from: URL: https://www.fda.gov/Drugs/DrugSafety/ucm193900.htm. |
| 8. | Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335-2342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7832] [Cited by in RCA: 7721] [Article Influence: 367.7] [Reference Citation Analysis (1)] |
| 9. | Motzer RJ, Michaelson MD, Redman BG, Hudes GR, Wilding G, Figlin RA, Ginsberg MS, Kim ST, Baum CM, DePrimo SE, Li JZ, Bello CL, Theuer CP, George DJ, Rini BI. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24:16-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1299] [Cited by in RCA: 1246] [Article Influence: 62.3] [Reference Citation Analysis (0)] |
| 10. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10237] [Article Influence: 602.2] [Reference Citation Analysis (2)] |
| 11. | Gomez-Manzano C, Holash J, Fueyo J, Xu J, Conrad CA, Aldape KD, de Groot JF, Bekele BN, Yung WK. VEGF Trap induces antiglioma effect at different stages of disease. Neuro Oncol. 2008;10:940-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 12. | Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, Degroot J, Wick W, Gilbert MR, Lassman AB, Tsien C, Mikkelsen T, Wong ET, Chamberlain MC, Stupp R, Lamborn KR, Vogelbaum MA, van den Bent MJ, Chang SM. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28:1963-1972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2528] [Cited by in RCA: 2918] [Article Influence: 194.5] [Reference Citation Analysis (0)] |
| 13. | Leu K, Pope WB, Cloughesy TF, Lai A, Nghiemphu PL, Chen W, Liau LM, Ellingson BM. Imaging biomarkers for antiangiogenic therapy in malignant gliomas. CNS Oncol. 2013;2:33-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Kickingereder P, Wiestler B, Graf M, Heiland S, Schlemmer HP, Wick W, Wick A, Bendszus M, Radbruch A. Evaluation of dynamic contrast-enhanced MRI derived microvascular permeability in recurrent glioblastoma treated with bevacizumab. J Neurooncol. 2015;121:373-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Kimura M, da Cruz LCH. Multiparametric MR Imaging in the Assessment of Brain Tumors. Magn Reson Imaging Clin N Am. 2016;24:87-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 16. | Jain R, Gutierrez J, Narang J, Scarpace L, Schultz LR, Lemke N, Patel SC, Mikkelsen T, Rock JP. In vivo correlation of tumor blood volume and permeability with histologic and molecular angiogenic markers in gliomas. AJNR Am J Neuroradiol. 2011;32:388-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 17. | Jiang W, Huang Y, An Y, Kim BY. Remodeling Tumor Vasculature to Enhance Delivery of Intermediate-Sized Nanoparticles. ACS Nano. 2015;9:8689-8696. [PubMed] [DOI] [Full Text] |
| 18. | Sorensen AG, Emblem KE, Polaskova P, Jennings D, Kim H, Ancukiewicz M, Wang M, Wen PY, Ivy P, Batchelor TT, Jain RK. Increased survival of glioblastoma patients who respond to antiangiogenic therapy with elevated blood perfusion. Cancer Res. 2012;72:402-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 244] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 19. | Lemée JM, Clavreul A, Menei P. Intratumoral heterogeneity in glioblastoma: don't forget the peritumoral brain zone. Neuro Oncol. 2015;17:1322-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 213] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 20. | Ellingson BM, Wen PY, van den Bent MJ, Cloughesy TF. Pros and cons of current brain tumor imaging. Neuro Oncol. 2014;16 Suppl 7:vii2-vi11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 21. | Jain R. Measurements of tumor vascular leakiness using DCE in brain tumors: clinical applications. NMR Biomed. 2013;26:1042-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 22. | O'Connor JP, Jackson A, Parker GJ, Roberts C, Jayson GC. Dynamic contrast-enhanced MRI in clinical trials of antivascular therapies. Nat Rev Clin Oncol. 2012;9:167-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 284] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 23. | Barajas RF, Cha S. Benefits of dynamic susceptibility-weighted contrast-enhanced perfusion MRI for glioma diagnosis and therapy. CNS Oncol. 2014;3:407-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Chung C, Metser U, Ménard C. Advances in Magnetic Resonance Imaging and Positron Emission Tomography Imaging for Grading and Molecular Characterization of Glioma. Semin Radiat Oncol. 2015;25:164-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | van Dijken BRJ, van Laar PJ, Holtman GA, van der Hoorn A. Diagnostic accuracy of magnetic resonance imaging techniques for treatment response evaluation in patients with high-grade glioma, a systematic review and meta-analysis. Eur Radiol. 2017;27:4129-4144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 179] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 26. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18665] [Cited by in RCA: 17477] [Article Influence: 1092.3] [Reference Citation Analysis (1)] |
| 27. | Zeng X, Zhang Y, Kwong JS, Zhang C, Li S, Sun F, Niu Y, Du L. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med. 2015;8:2-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1492] [Cited by in RCA: 1432] [Article Influence: 143.2] [Reference Citation Analysis (0)] |
| 28. | Zamora J, Abraira V, Muriel A, Khan K, Coomarasamy A. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol. 2006;6:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1446] [Cited by in RCA: 1571] [Article Influence: 82.7] [Reference Citation Analysis (0)] |
| 29. | Iwamoto FM, Lamborn KR, Robins HI, Mehta MP, Chang SM, Butowski NA, Deangelis LM, Abrey LE, Zhang WT, Prados MD, Fine HA. Phase II trial of pazopanib (GW786034), an oral multi-targeted angiogenesis inhibitor, for adults with recurrent glioblastoma (North American Brain Tumor Consortium Study 06-02). Neuro Oncol. 2010;12:855-861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 156] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 30. | Piludu F, Marzi S, Pace A, Villani V, Fabi A, Carapella CM, Terrenato I, Antenucci A, Vidiri A. Early biomarkers from dynamic contrast-enhanced magnetic resonance imaging to predict the response to antiangiogenic therapy in high-grade gliomas. Neuroradiology. 2015;57:1269-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 31. | Kalpathy-Cramer J, Chandra V, Da X, Ou Y, Emblem KE, Muzikansky A, Cai X, Douw L, Evans JG, Dietrich J, Chi AS, Wen PY, Stufflebeam S, Rosen B, Duda DG, Jain RK, Batchelor TT, Gerstner ER. Phase II study of tivozanib, an oral VEGFR inhibitor, in patients with recurrent glioblastoma. J Neurooncol. 2017;131:603-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 32. | O'Neill AF, Qin L, Wen PY, de Groot JF, Van den Abbeele AD, Yap JT. Demonstration of DCE-MRI as an early pharmacodynamic biomarker of response to VEGF Trap in glioblastoma. J Neurooncol. 2016;130:495-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Hilario A, Sepulveda JM, Hernandez-Lain A, Salvador E, Koren L, Manneh R, Ruano Y, Perez-Nuñez A, Lagares A, Ramos A. Leakage decrease detected by dynamic susceptibility-weighted contrast-enhanced perfusion MRI predicts survival in recurrent glioblastoma treated with bevacizumab. Clin Transl Oncol. 2017;19:51-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Um O, Cn O. Evaluating Measures of Indicators of Diagnostic Test Performance: Fundamental Meanings and Formulars. J Biom Biostat. 2012;3. [RCA] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (1)] |