Published online Jun 28, 2018. doi: 10.13105/wjma.v6.i2.9
Peer-review started: March 23, 2018
First decision: April 18, 2018
Revised: April 26, 2018
Accepted: May 15, 2018
Article in press: May 15, 2018
Published online: June 28, 2018
Processing time: 97 Days and 14.7 Hours
To provide a comprehensive examination of the existing evidence of the antitumor effect of long-acting octreotide in neuroendocrine tumors (NETs).
A systematic literature review of clinical trials and observational studies was conducted in PubMed, EMBASE, and Cochrane through January 18, 2017. Conference abstracts for 2015 and 2016 from 5 scientific meetings were also searched.
Of 41 articles/abstracts identified, 13 unique studies compared octreotide with active or no treatment. Two of the 13 studies were clinical trials; the remaining were observational studies. The phase 3 Placebo-Controlled, Double-Blind, Prospective, Randomized Study of the Effect of Octreotide long-acting repeatable (LAR) in the Control of Tumor Growth in Patients with Metastatic Neuroendocrine Midgut Tumors clinical trial showed that long-acting octreotide significantly prolonged time to tumor progression compared with placebo in patients with functionally active and inactive metastatic midgut NETs; no statistically significant difference in overall survival (OS) was observed, possibly due to the crossover of placebo patients to octreotide. Retrospective observational studies found that long-acting octreotide use was associated with significantly longer OS than no octreotide use for patients with distant metastases although not for those with local/regional disease.
The clinical trial and observational studies with informative evidence support long-acting octreotide’s antitumor effect on time to tumor progression and OS. This review showed the rarity of existing studies assessing octreotide’s antitumor effect and recommends that future research is warranted.
Core tip: This review comprehensively summarizes the existing clinical trial and observational studies that have assessed long-acting octreotide’s tumor control effect. The comparative studies of relatively large sample size support long-acting octreotide’s antitumor effect on time to tumor progression and overall survival. This review shows the rarity of existing studies assessing octreotide’s antitumor effect; future research is warranted.
- Citation: Barrows SM, Cai B, Copley-Merriman C, Wright KR, Castro CV, Soufi-Mahjoubi R. Systematic literature review of the antitumor effect of octreotide in neuroendocrine tumors. World J Meta-Anal 2018; 6(2): 9-20
- URL: https://www.wjgnet.com/2308-3840/full/v6/i2/9.htm
- DOI: https://dx.doi.org/10.13105/wjma.v6.i2.9
Neuroendocrine tumors (NETs) are rare, slow-growing neoplasms[1] that most commonly arise in the gastrointestinal tract, lung, and pancreas[2]. Neuroendocrine tumors account for only 0.5% of all malignancies, with an estimated annual incidence of approximately 2/100000[3]. However, the incidence has been rising, possibly due to increased awareness, improved diagnosis, or evolving definition[3]. Using Surveillance, Epidemiology, and End Results (SEER) data, Dasari et al[4] reported an increase in the annual age-adjusted incidence from 1973 (1.09/100000) to 2012 (6.98/100000). Survival for patients with NETs depends on the stage at diagnosis and site of disease. Dasari et al[4] reported a median overall survival (OS) for all stages of NETs of 9.3 years. The authors observed that patients with localized NETs had a better median OS (> 30 years) compared with patients with regional NETS (10.2 years) and distant NETs (12 mo). Further, Dasari et al[4] observed improvements in OS over time: survival for patients with NETs who were diagnosed in 2009-2012 improved compared with patients with NETs who were diagnosed in 2000-2004 [hazard ratio (HR): 0.79; 95%CI: 0.73-0.85]. Over these same 3 time intervals (2000-2004; 2005-2008; and 2009-2012), improvements in OS were observed for patients with distant-stage gastrointestinal (GI) NETs (HR: 0.71; 95%CI: 0.62-0.81) and in distant-stage pancreatic NETs (HR: 0.56; 95%CI: 0.44-0.70)[4].
Current National Comprehensive Cancer Network (NCCN) guidelines for treatment of NETs recommend the use of somatostatin analogs (SSAs; octreotide and lanreotide) as first-line treatment in patients with advanced NETs[5]. Additional treatment options are based on patient symptoms and the primary tumor location. For patients with unresectable NETs of the pancreas and/or distant metastases who have progressed on treatment with an SSA, octreotide or lanreotide may be continued in combination with everolimus, sunitinib, or chemotherapy[5]. In a review of the available clinical data of octreotide and lanreotide as antitumor agents, the authors concluded that both octreotide and lanreotide have comparable antitumor efficacy and, thus, are interchangeable[6].
Although approved in the United States only for carcinoid symptom (severe diarrhea/flushing episodes) control and not for tumor control, octreotide has been a mainstay of NET therapy for nearly 3 decades[7]. In December 2014, another SSA, lanreotide, was approved for tumor control (i.e., “treatment of patients with unresectable, well- or moderately differentiated, locally advanced or metastatic gastroenteropancreatic NETs to improve progression-free survival”)[8].
Sidéris et al[2] reviewed literature indexed in MEDLINE (search dates not provided) that identified prospective clinical trials examining the antitumor effects of octreotide and lanreotide in patients with NETs[2]. Six studies published from 1991-1999 showed that 15%[9] to 85.7%[10] of patients with advanced NETs reported stable disease with subcutaneous octreotide[2]. Sidéris et al[2] reported that, after the introduction of long-acting octreotide, overall stable disease was observed in 26% to 87.5%[11] of patients with advanced, functioning or nonfunctioning NETs. In those studies that reported partial response, up to 31% of patients receiving subcutaneous octreotide[9] and up to 11% of patients receiving long-acting octreotide experienced a partial response[2].
Broder et al[1] conducted a systematic review of literature indexed in PubMed and Cochrane from 1998-2012 to evaluate the efficacy and safety of long-acting octreotide used at higher doses than the United States Food and Drug Administration-approved 30 mg per month. The authors concluded that a summary of the data suggests a trend supporting the use of high-dose, long-acting octreotide for control of symptoms and limited data supporting the use of high-dose, long-acting octreotide for control of tumor progression in patients with NETs[1]. Several publications provided expert opinion statements that mostly endorsed the use of above-label doses of long-acting octreotide for patients with symptom or tumor progression when lower doses were inadequate to control disease[1]. Most expert opinion publications suggested that higher doses should be used in cases where there is tumor progression or lack of symptom control on lower doses[1]. A recently published review of escalated-dose SSAs in gastroenteropancreatic NETs by Chan et al[12] also found evidence of octreotide’s antiproliferative effects.
These previous reviews focused on escalated doses of SSAs[1,12] and clinical trials of the antitumor effect of SSAs[2]. At the time of the current review, no systematic reviews summarizing both clinical trial and observational data had been published. Our objective was to provide a systematic and comprehensive review of the existing evidence on the antitumor effect of long-acting octreotide in NETs regardless of dosing and to broaden the search to include real-world evidence and clinical trials.
We searched PubMed, EMBASE, and the Cochrane Library databases for prospective and retrospective studies evaluating the antitumor effect of octreotide in patients with NETs. Additional studies not published in the peer-reviewed literature were identified by searching online conference abstracts of 5 professional societies: American Society of Clinical Oncology (ASCO), European Society of Medical Oncology, North American Neuroendocrine Tumor Society, European Neuroendocrine Tumor Society, and ASCO-Gastrointestinal Cancers Symposium.
To supplement our search, we also reviewed the bibliographic reference lists of relevant systematic review articles.
The search terms for the medical library databases included Medical Subject Heading, Emtree, and free-text terms of “neuroendocrine tumors,” “neuroendocrine neoplasms,” “neuroendocrine malignanc*,” “neuroendocrine carcinoma,” “carcinoid,” “octreotide,” “Sandostatin,” “SMS 201-995,” various terms to identify specific antitumor and antiproliferative effect and other outcomes of interest, and terms to identify observational studies, randomized controlled trials, clinical trials, and case series studies. The search was limited to English language studies of humans but had no date limit.
Two independent reviewers screened the titles and abstracts according to predefined inclusion and exclusion criteria (Supplementary Table 1). Full-text articles of selected records were obtained, and the 2 independent reviewers further screened each article according to the same predefined inclusion and exclusion criteria.
| First author | Study design | No. of patients | Tumor type | Treatment and dose | Treatment duration/observation period | TTP, mo | SD, % | PR, % | OS, mo | 5-yr survival | PFS, mo |
| Octreotide vs placebo or no treatment | |||||||||||
| Rinke et al[13] (2009) PROMID study | RCT | 85 | Well-differentiated, advanced NET with midgut or unknown origin. Functional and nonfunctional | LA OCT 30 mg every 28 d (n = 42) vs PBO (n = 43) | Patients enrolled between March 2001 and Jan 2008; followed until June 2008 | Median OCT: 14.3 vs PBO: 6.0 HR: 0.34; 95%CI: 0.20-0.59; P = 0.000072 | At 6 mo: OCT: 66.7 vs PBO: 37.2 (P = 0.0079) | At 6 mo: 1 in each group | Interim analysis: Median: OCT: Not reached (> 77.4) vs PBO: 73.7 HR: 0.81; 95%CI: 0.30-2.18; P = 0.77 | - | - |
| Rinke et al[14] (2017) PROMID study | RCT | 85 | Well-differentiated, advanced NET with midgut or unknown origin. Functional and nonfunctional | LA OCT 30 mg every 28 d (n = 42) vs PBO (n = 43) | Patients enrolled between March 2001 and Jan 2008; followed until May 2014 | - | - | - | Final analysis Median: OCT: 84.7 vs PBO: 83.7 HR: 0.83; 95%CI: 0.47-1.46; P = 0.51 | - | - |
| Shen et al[15]1 (2014) | RWE | 1291 | Distant and local/regional disease; well, moderately, and unknown differentiated tumors with various origin Functional NETs | LA OCT (dose not defined) vs no LA OCT | Cohort entry: July 1999-Dec 2007 Follow-up through Dec 2009 | - | - | - | Distant stage: OCT: 2.11 y vs no OCT: 1.25 y; P = 0.002 Local/regional stage: “no significant survival benefit” | Distant-stage: HR: 0.61; 95%CI: 0.47-0.79; P ≤ 0.001 Local/regional stage: HR: 0.88; 95%CI: 0.57-1.36; P = 0.563 | - |
| Shen et al[16]2 (2015) | RWE | 6940 | Distant and local/regional disease; well, moderately, and unknown. differentiated tumors with various origin Functional and nonfunctional NETs | LA OCT and no LA OCT distant stage (n = 1176) local/regional stage (n = 5764) | Cohort entry: Jan 1999-Dec 2009 Follow-up through Dec 2011 | - | - | - | Distant stage: OCT: 35.22 vs no OCT: 19.15 HR: 0.68; 95%CI: 0.554-0.840; P < 0.001 Local/regional stage: OCT: 64.85 vs no OCT: 104.97 HR: 1.253; 95%CI: 0.928-1.692; P = 0.1415 | - | - |
| Anthony and Vinik[19] (2011) | RWE | 392 | Tumor pathology: NR. Localized/metastatic/ unknown disease with various origin | Without carcinoid syndrome (n = 106) With carcinoid syndrome (n = 260) Carcinoid syndrome after initiation of treatment (n = 24) Overall population initial dose: LA OCT 20 mg: 49% LA OCT 30 mg: 39% | NR | - | 57 (any dose) | 6 (any dose) | - | - | - |
| Chadha et al[20] (2009) | RWE | 54 | Tumor pathology: NR Metastatic disease with GEP origin | OCT conventional dose (20 or 30 mg every month; n = 24) OCT high dose (40-90 mg3; n = 30) | Median follow-up, mo: OCT conventional dose: 35.8 OCT high dose: 44.1 | - | - | - | 1 yr OS: 0.77 vs 0.88; P = 0.4777) | - | - |
| Ferolla et al[17] (2012) | CT | 28 | Well differentiated functional and nonfunctional NET with various origin | LA OCT 30 mg every 28 d (n = 28) LA OCT 30 mg every 21 d (n = 28) | NR | - | - vs 93 | - vs 7 | - | - | - |
| Jann et al[21] (2013) | RWE | 43 | G1/G2/Unknown KI-67 index Functional and nonfunctional metastatic tumors with pancreas origin | LA OCT 30 mg (n = 19) LA OCT ≤ 20 mg (n = 16) OCT (dose unknown(n = 8) | Median follow-up: 58 mo | - | 37 (any dose) | 5 (any dose) | Median, 98 | - | - |
| Shen et al[18]2 (2016) | RWE | 222 | Well, moderately, or poorly differentiated Functional and nonfunctional distant-stage NETs with various origin | LA OCT every 28 d by dose: ≤ 20 mg (n = 81) 21-30 mg (n = 82) > 30 mg (n = 59) | Cohort entry: Jan 1999-Dec 2009 Follow-up through Dec 2011 | - | - | - | ≤ 20 mg: 20.8 21-30 mg: 32.6 > 30 mg: 36.3 ≤ 20 mg vs 21-30 mg: HR: 2.000; 95%CI: 1.318-3.035; P = 0.0011 > 30 mg vs 21-30 mg: HR: 1.094; 95%CI: 0.671-1.788; P = 0.7193 | - | - |
| Octreotide monotherapy vs another monotherapy | |||||||||||
| Bongiovanni et al[23] (2017) | RWE | 30 | Well or moderately differentiated locally advanced/metastatic tumors with lung origin | LA OCT 30 mg every 28 d (n = 20) LAN 120 mg every 28 d (n = 10) | Median follow-up , 40 mo | - | - | - | - | 65.6% vs 87.5% (P = 0.864) | 11.1 vs 10.1 (P = 0.769) |
| Creutzfeldt et al[24] (1991) | CT | 33 | Tumor pathology: NR Metastatic gastrointestinal tumors | IFN-α2c (2 × 106 IU/m2 daily; n = 17) OCT (200 μg 3 times daily, 500 μg 3 times daily if tumor progressed; n = 16) | NR | - | 85.7 vs 37.5 Comparison NR | - | - | - | - |
| Wolin et al[22] (2015) | RCT | 110 | Well, moderate, or poorly differentiated Locally advanced/metastatic tumors with various origin | PAS LAR 60 mg every 28 d (n = 53) LA OCT 40 mg every 28 d (n = 57) | NR | - | 70.6 vs 73.1 Comparison NR | 2.0 vs 1.9 Comparison NR | - | - | - |
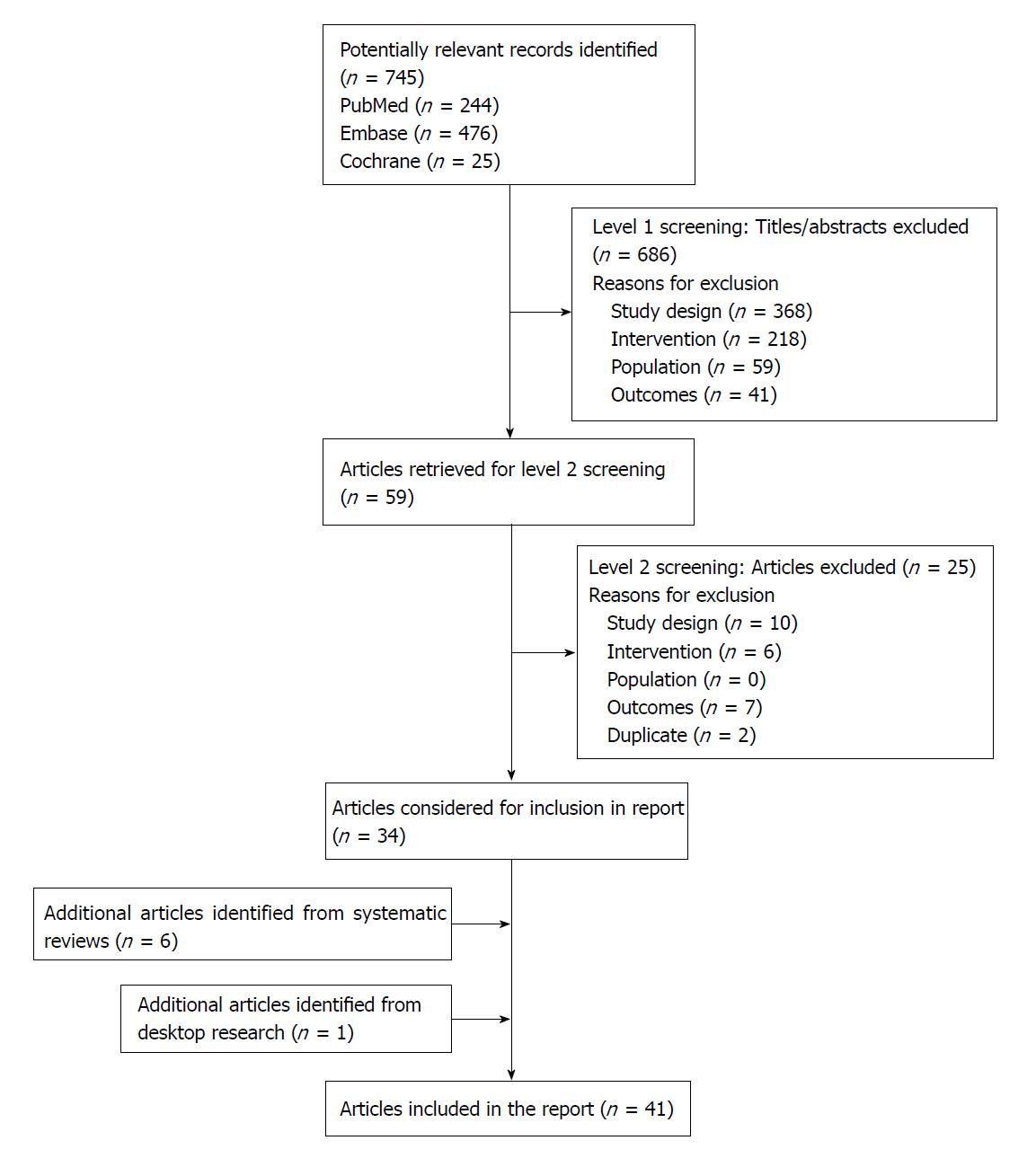
The literature database search identified 745 unique records. Six additional articles were identified following a review of the bibliographic reference lists of relevant systematic review articles. One additional abstract was identified from the search of professional societies and associated conferences. A total of 41 publications met inclusion criteria (Figure 1). Of the 41 publications, 20 reported comparative analyses, and 21 reported single-arm studies.
A total of 20 publications of comparative analyses were identified based on 13 unique studies. Two of the 13 studies were clinical trials, and the remaining were observational studies.
Four publications reported results of comparisons of long-acting octreotide to placebo or no treatment. This included 2 prospective analyses of the Placebo-Controlled, Double-Blind, Prospective, Randomized Study of the Effect of Octreotide long-acting repeatable (LAR) in the Control of Tumor Growth in Patients with Metastatic Neuroendocrine Midgut Tumors (PROMID) study[13,14] and 2 retrospective analyses of the SEER database[15,16].
Evidence of an antitumor effect of octreotide in patients with midgut NETs was confirmed with the results of the phase 3 PROMID study[13]. Long-acting octreotide significantly lengthened time to tumor progression compared with placebo in patients with functionally active and inactive metastatic midgut NETs. Median time to tumor progression for the long-acting octreotide (n = 42) group was 14.3 mo compared with 6 mo in the placebo (n = 43) group (HR: 0.34; 95%CI: 0.20-0.59; P = 0.000072). After 6 mo of treatment, stable disease was observed in 66.7% of octreotide-treated patients vs 37.2% of patients in the placebo group[13]. Rinke et al[14] reported final results of median OS for long-acting octreotide and placebo in the PROMID trial as 84.7 and 83.7 mo, respectively (HR: 0.83; 95%CI: 0.47-1.46; P = 0.51). There was a trend toward improved survival in patients with low hepatic tumor load receiving long-acting octreotide vs placebo (median not reached vs 87.2 mo; HR: 0.59; 95%CI: 0.29-1.2; P = 0.142). Crossover of the majority of placebo patients to long-acting octreotide may have confounded the OS data[14].
Two long-term retrospective analyses were conducted using overlapping periods within the SEER-Medicare database[15,16]. Patients were at least 65 years of age and had functional and nonfunctional NETs originating at varying sites. In both studies, long-acting octreotide (dose not defined) was compared with no octreotide treatment[15,16]. Shen et al[16] (cohort entry July 1999-December 2009 with follow-up through December 2011) reported that in patients with functional or nonfunctional NETs and distant-stage disease, median OS for patients who started long-acting octreotide within 12 mo of diagnosis was significantly longer (35.22 mo; 95%CI: 27.96-47.77) than for those who did not receive octreotide (19.15 mo, 95%CI: 16.36-22.80; HR: 0.68, 95%CI: 0.554-0.840; P < 0.001)[16]. In patients with local/regional disease, median OS was 64.85 mo in patients who received long-acting octreotide compared with 104.97 mo in patients who did not receive octreotide (HR: 1.253; 95%CI: 0.928-1.692; P = 0.1415)[16]. Shen et al[16] further reported a significant survival benefit in the subgroups of patients with distant-stage disease with (HR: 0.65; P = 0.003) and without carcinoid syndrome (HR: 0.55; P = 0.002). In the analysis reported by Shen et al[15] (cohort entry July 1999-December 2007 with follow-up through December 2009), patients with functional NETs and distant-stage disease who received long-acting octreotide within 6 mo of diagnosis had significantly longer median OS (2.11 years; 95%CI: 1.73-2.84 years) than patients who did not receive long-acting octreotide (1.25 years; 95%CI: 0.72-1.71 years; P = 0.002). No significant survival benefit was found among the group of patients with NETs of local/regional stage. Further analysis demonstrated that long-acting octreotide was associated with significant improvement in 5-year survival for the subgroup of patients with distant-stage disease (HR: 0.61; 95%CI: 0.47-0.79; P ≤ 0.001). There was no significant benefit observed for patients with local/regional stage disease (HR: 0.88; 95%CI: 0.57-1.36; P = 0.563).
Five studies involving 28 to 392 patients compared different dose regimens or frequency of dosing for long-acting octreotide; of these, 1 study was prospective and 4 were retrospective. A prospective study examined retrospective data of patients who had been treated with standard-dose long-acting octreotide 30 mg every 28 d and compared it with the same patients after switching to long-acting octreotide 30 mg every 21 days. The shorter dose interval (i.e., 21 d vs 28 d) showed a longer time to tumor progression (30 mo vs 9 mo, P < 0.0001), and 93% of patients on the 21 d schedule had stable disease[17]. Using the SEER-Medicare database, Shen et al[18] estimated the 5-year survival of patients with NETs who received long-acting octreotide within 12 mo of diagnosis. Multivariate analysis showed that, compared with a medium long-acting octreotide dose (21-30 mg), a low dose (≤ 20 mg) was associated with significantly worse survival (HR: 2.000; P = 0.0011), whereas a high initial dose (> 30 mg) did not show additional survival benefits over that observed with a medium dose (HR: 1.094; P = 0.7193)[18].
Anthony and Vinik[19] (2011) conducted a retrospective medical record review comparing different doses of long-acting octreotide (20, 30, 40, and 60 mg) in which 390 patients were evaluated for tumor response. At the most common dose (long-acting octreotide 30 mg), the rates of complete and partial tumor response were 1% and 8%, respectively. Logistic regression analysis identified no statistically significant correlation between tumor progression and response and the patient’s dose, sex, carcinoid syndrome status, and change in dose[19].
In another retrospective medical record review (n = 54), Chadha et al[20] reported that, in patients with gastroenteropancreatic NETs (GEP-NET), conventional long-acting octreotide (20-30 mg) demonstrated lower estimated 1-year survival and time to any other intervention vs high-dose (median, 40 mg) long-acting octreotide, but the results were not statistically significant[20].
In a retrospective medical record review conducted in 43 patients with pancreatic NETs treated with long-acting octreotide, a comparison of low-dose (≤ 20 mg) vs medium-dose (30 mg) long-acting octreotide showed longer time to tumor progression for medium dose though, again, the results were not statistically significant[21].
Three studies (1 prospective, 1 retrospective, and 1 indirect comparison) in 30-110 patients compared octreotide monotherapy to another monotherapy treatment[22-24]. A phase 3 trial comparing long-acting pasireotide 60 mg every 28 d (n = 53) and long-acting octreotide 40 mg every 28 d (n = 57) showed a higher tumor control rate and median PFS for long-acting pasireotide than long-acting octreotide, but the results were not statistically significant[22]. In a small retrospective medical record review, octreotide 30 mg (n = 20) vs lanreotide 120 mg (n = 10) showed no statistically significant differences in median PFS or 5-year OS[23]. Median PFS was 11.1 mo (95%CI: 7.0-15.2) in the octreotide group vs 10.1 mo (95%CI: 4.3-17.0) in the lanreotide group (P = 0.769). Five-year OS was 65.6% (95%CI: 29.4-86.6) in the octreotide group and 87.5% (95%CI: 38.7-98.1) in the lanreotide group (P = 0.864)[23]. In a study that indirectly compared 182 mo of treatment with recombinant interferon α-2c (2 × 106 IU/m2 daily; n = 17) and octreotide (3 × 200 µg subcutaneous daily; n = 16), stable disease was reported in 85.7% of patients treated with recombinant interferon α-2c and 37.5% of patients treated with subcutaneous octreotide[24].
Table 1 summarizes the 21 publications comparing long-acting octreotide with no treatment or placebo, different octreotide doses, or other monotherapy treatment.
Eight prospective studies compared octreotide combination therapy with octreotide monotherapy[25-32]. Five of the studies were based on the RADIANT-2 study[25-29], 2 studies compared subcutaneous octreotide plus interferon α with subcutaneous octreotide monotherapy[30,31], and 1 study compared long-acting octreotide plus 177Lu-Dotatate with long-acting octreotide monotherapy[32]. The results did not inform the main question of interest for this study (i.e., an antitumor effect of octreotide). Further information pertaining to these studies can be found in the online supplement and Supplementary Table 2.
A total of 21 studies were identified as single-arm studies that evaluated the antitumor effect of octreotide. The studies had varying sample sizes (n = 7-254), tumor types, and octreotide dosing regimens. The results did not inform the main question of interest for this study (i.e., an antitumor effect of octreotide). Further information pertaining to these studies can be found in the online supplement and Supplementary Table 3[9-11,33-50].
This review identified existing clinical trials and observational studies that assessed the antitumor effect of octreotide in patients with NETs. The strongest clinical trial evidence supporting octreotide’s antitumor effect was in the phase 3, randomized, placebo-controlled PROMID clinical trial; compared with placebo, long-acting octreotide demonstrated significantly longer time to tumor progression in patients with functionally active or inactive metastatic midgut NETs with or without secretory symptoms[13]. OS did not appear to be significantly different between the two arms, possibly because most patients in the placebo group crossed over to the octreotide arm. There was a trend toward improved OS in patients with a low hepatic tumor load receiving long-acting octreotide compared with placebo[14].
Three retrospective analyses of overlapping periods of SEER-Medicare data provide the strongest retrospective evidence for an antitumor effect of long-acting octreotide, indicating that use of long-acting octreotide was associated with significantly longer OS than no octreotide treatment among patients with distant metastases of various origin, and that standard dosing (21-30 mg) seems to be associated with better OS than low dose (≤ 20 mg)[15,16,18]. These studies provided unique and valuable real-world evidence in the association between long-acting octreotide and OS in tumors of various origin. In the real-world clinical setting, accurate assessment of tumor progression may be challenging due to the rare use of a consistent tumor progression measure (e.g., Response Evaluation Criteria in Solid Tumors, or RECIST); therefore, OS, defined by verified mortality data, is a more consistent study endpoint. SEER-Medicare data allow the long-term follow-up from diagnosis to mortality (longest time period: cohort entry July 1999 to December 2009 with follow-up through December 2011), regardless of changes in providers or health plans. The large sample size and long-term follow-up complements the limitation of clinical studies, which are typically small in sample size and are not powered to assess OS, especially when subject to majority crossover between arms. However, these observational studies assessed only the Medicare population, which is not nationally representative of the NET population, and the crossover between the octreotide and placebo groups may underestimate the OS difference[16].
Current NCCN guidelines for treatment of NETs recommend the use of SSAs (octreotide or lanreotide) as first-line treatment in patients with advanced NETs. Additional subsequent-line therapy options are based on patient symptoms and tumor location (e.g., GI, lung, thymus, pancreas). For patients with unresectable NETs of the pancreas and/or distant metastases who have progressed on treatment with an SSA, octreotide or lanreotide may be continued in combination with everolimus, sunitinib, or chemotherapy[5]. In addition, octreotide and lanreotide have been shown to have comparable antitumor efficacy and thus can be considered interchangeable in regard to antitumor activity[6].
This study adds to previous reviews published in 2012[2], 2015[1], and 2017[12] on this topic by broadening the search in multiple databases and not restricting by dose level, study type (i.e., clinical trial or retrospective study), or date of publication. This review suggests that data from the PROMID trial, combined with real-world effectiveness data[15,16,18], support an antitumor effect of octreotide in NETs, thereby fulfilling an unmet need. The strength of this review lies in its comprehensive search, review, and synthesis of the findings, as well as its rigorous methodology.
Many of the studies included in our review exhibit limitations, including small sample sizes, the absence of a comparative arm, and crossover study designs. Additional studies with large sample sizes and a control arm that does not include octreotide are needed to confirm octreotide’s antitumor effect. In addition, future studies should include patients with NETs of various origins.
This study systematically provides the most comprehensive review, to our knowledge, on the clinical trial and retrospective studies that have assessed octreotide’s antitumor effect. The clinical trial and observational studies with larger sample sizes support the antitumor effect of long-acting octreotide on time to tumor progression and OS. Most existing studies in this area feature small sample sizes or were not designed to comparatively assess octreotide’s antitumor effect. This review identified the rarity of existing studies assessing octreotide’s antitumor effect and the need for further research using larger sample sizes and well-controlled study designs.
Neuroendocrine tumors (NETs) are rare, slow-growing neoplasms that most commonly arise in the gastrointestinal tract, lung, and pancreas. Although approved in the United States only for carcinoid symptom (severe diarrhea/flushing episodes) control and not for tumor control, octreotide has been a mainstay of NET therapy for nearly 3 decades.
Previous literature reviews focused on escalated doses of somatostatin analogs (SSAs) and clinical trials of the antitumor effect of SSAs. At the time of the current review, no systematic reviews summarizing both clinical trial and observational data had been published.
The objective of this literature review was to provide a systematic and comprehensive examination of the existing evidence of the antitumor effect of long-acting octreotide in NETs regardless of dosing and to broaden the search to include real-world evidence and clinical trials.
A systematic literature review of clinical trials and observational studies was conducted in PubMed, EMBASE, and Cochrane through January 18, 2017. Conference abstracts for 2015 and 2016 from 5 scientific meetings were also searched. To supplement the search, the bibliographic reference lists of relevant systematic review articles were also reviewed. Two independent reviewers screened the titles and abstracts according to predefined inclusion and exclusion criteria. Full-text articles of selected records were obtained, and the 2 independent reviewers further screened each article according to the same predefined inclusion and exclusion criteria.
Of 41 articles/abstracts identified, 13 unique studies compared octreotide with active or no treatment. Two of the 13 studies were clinical trials; the remaining were observational studies. The phase 3 Placebo-Controlled, Double-Blind, Prospective, Randomized Study of the Effect of Octreotide long-acting repeatable (LAR) in the Control of Tumor Growth in Patients with Metastatic Neuroendocrine Midgut Tumors clinical trial showed that long-acting octreotide significantly prolonged time to tumor progression compared with placebo in patients with functionally active and inactive metastatic midgut NETs; no statistically significant difference in overall survival (OS) was observed, possibly due to the crossover of placebo patients to octreotide. Retrospective observational studies found that long-acting octreotide use was associated with significantly longer OS than no octreotide use for patients with distant metastases although not for those with local/regional disease.
The clinical trial and observational studies with informative evidence support long-acting octreotide’s antitumor effect on time to tumor progression and OS. This review showed the rarity of existing studies assessing octreotide’s antitumor effect and recommends that future research is warranted.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: De Raffele E, Hijioka S S- Editor: Wang XJ L- Editor: A E- Editor: Tan WW
| 1. | Broder MS, Beenhouwer D, Strosberg JR, Neary MP, Cherepanov D. Gastrointestinal neuroendocrine tumors treated with high dose octreotide-LAR: a systematic literature review. World J Gastroenterol. 2015;21:1945-1955. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 59] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 2. | Sidéris L, Dubé P, Rinke A. Antitumor effects of somatostatin analogs in neuroendocrine tumors. Oncologist. 2012;17:747-755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 3. | Taal BG, Visser O. Epidemiology of neuroendocrine tumours. Neuroendocrinology. 2004;80 Suppl 1:3-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 263] [Article Influence: 12.5] [Reference Citation Analysis (3)] |
| 4. | Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, Shih T, Yao JC. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017;3:1335-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1510] [Cited by in RCA: 2489] [Article Influence: 311.1] [Reference Citation Analysis (4)] |
| 5. | National Comprehensive Cancer Network. Clinical practice guidelines: neuroendocrine tumors. Version 3.2017. National Comprehensive Cancer Network. 2017; Available from: https://www.nccn.org/professionals/physician_gls/pdf/neuroendocrine.pdf. |
| 6. | Enzler T, Fojo T. Long-acting somatostatin analogues in the treatment of unresectable/metastatic neuroendocrine tumors. Semin Oncol. 2017;44:141-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Sandostatin LAR package insert. United States: Novartis; July 1, 2016. Available from: https://www.pharma.us.novartis.com/sites/www.pharma.us.novartis.com/files/sandostatin_lar.pdf. |
| 8. | Somatuline depot (lanreotide) injection prescribing information. Ipsen Biopharmaceuticals, Inc.; December. 2014; Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/022074s011lbl.pdf. |
| 9. | Anthony L, Johnson D, Hande K, Shaff M, Winn S, Krozely M, Oates J. Somatostatin analogue phase I trials in neuroendocrine neoplasms. Acta Oncol. 1993;32:217-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 76] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Angeletti S, Corleto VD, Schillaci O, Moretti A, Panzuto F, Annibale B, Delle Fave G. Single dose of octreotide stabilize metastatic gastro-entero-pancreatic endocrine tumours. Ital J Gastroenterol Hepatol. 1999;31:23-27. [PubMed] |
| 11. | Tomassetti P, Migliori M, Corinaldesi R, Gullo L. Treatment of gastroenteropancreatic neuroendocrine tumours with octreotide LAR. Aliment Pharmacol Ther. 2000;14:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 82] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Chan DL, Ferone D, Albertelli M, Pavlakis N, Segelov E, Singh S. Escalated-dose somatostatin analogues for antiproliferative effect in GEPNETS: a systematic review. Endocrine. 2017;57:366-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Rinke A, Müller HH, Schade-Brittinger C, Klose KJ, Barth P, Wied M, Mayer C, Aminossadati B, Pape UF, Bläker M. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol. 2009;27:4656-4663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1609] [Cited by in RCA: 1742] [Article Influence: 108.9] [Reference Citation Analysis (0)] |
| 14. | Rinke A, Wittenberg M, Schade-Brittinger C, Aminossadati B, Ronicke E, Gress TM, Müller HH, Arnold R; PROMID Study Group. Placebo-Controlled, Double-Blind, Prospective, Randomized Study on the Effect of Octreotide LAR in the Control of Tumor Growth in Patients with Metastatic Neuroendocrine Midgut Tumors (PROMID): Results of Long-Term Survival. Neuroendocrinology. 2017;104:26-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 259] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 15. | Shen C, Shih YC, Xu Y, Yao JC. Octreotide long-acting repeatable use among elderly patients with carcinoid syndrome and survival outcomes: a population-based analysis. Cancer. 2014;120:2039-2049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Shen C, Shih YC, Xu Y, Yao JC. Octreotide long-acting repeatable among elderly patients with neuroendocrine tumors: a survival analysis of SEER-Medicare data. Cancer Epidemiol Biomarkers Prev. 2015;24:1656-1665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Ferolla P, Faggiano A, Grimaldi F, Ferone D, Scarpelli G, Ramundo V, Severino R, Bellucci MC, Camera LM, Lombardi G. Shortened interval of long-acting octreotide administration is effective in patients with well-differentiated neuroendocrine carcinomas in progression on standard doses. J Endocrinol Invest. 2012;35:326-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 18. | Shen C, Xu Y, Dasari A, Shih YC, Yao JC. Octreotide LAR Dosage and Survival Among Elderly Patients With Distant-Stage Neuroendocrine Tumors. Oncologist. 2016;21:308-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Anthony L, Vinik AI. Evaluating the characteristics and the management of patients with neuroendocrine tumors receiving octreotide LAR during a 6-year period. Pancreas. 2011;40:987-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Chadha MK, Lombardo J, Mashtare T, Wilding GE, Litwin A, Raczyk C, Gibbs JF, Kuvshinoff B, Javle MM, Iyer RV. High-dose octreotide acetate for management of gastroenteropancreatic neuroendocrine tumors. Anticancer Res. 2009;29:4127-4130. [PubMed] |
| 21. | Jann H, Denecke T, Koch M, Pape UF, Wiedenmann B, Pavel M. Impact of octreotide long-acting release on tumour growth control as a first-line treatment in neuroendocrine tumours of pancreatic origin. Neuroendocrinology. 2013;98:137-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Wolin EM, Jarzab B, Eriksson B, Walter T, Toumpanakis C, Morse MA, Tomassetti P, Weber MM, Fogelman DR, Ramage J. Phase III study of pasireotide long-acting release in patients with metastatic neuroendocrine tumors and carcinoid symptoms refractory to available somatostatin analogues. Drug Des Devel Ther. 2015;9:5075-5086. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 147] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 23. | Bongiovanni A, Recine F, Riva N, Foca F, Liverani C, Mercatali L, Nicolini S, Pieri F, Amadori D, Ibrahim T. Outcome Analysis of First-line Somatostatin Analog Treatment in Metastatic Pulmonary Neuroendocrine Tumors and Prognostic Significance of 18 FDG-PET/CT. Clin Lung Cancer. 2017;18:415-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Creutzfeldt W, Bartsch HH, Jacubaschke U, Stöckmann F. Treatment of gastrointestinal endocrine tumours with interferon-alpha and octreotide. Acta Oncol. 1991;30:529-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 40] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Pavel ME, Hainsworth JD, Baudin E, Peeters M, Horsch D, Winkler RE, Klimovsky J, Lebwohl D, Jehl V, Wolin EM. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): a randomised, placebo-controlled, phase 3 study. Lancet. 2011;378:2005-2012. [RCA] [DOI] [Full Text] [Cited by in Crossref: 814] [Cited by in RCA: 754] [Article Influence: 53.9] [Reference Citation Analysis (0)] |
| 26. | Anthony LB, Pavel ME, Hainsworth JD, Kvols LK, Segal S, Hörsch D, Van Cutsem E, Öberg K, Yao JC. Impact of Previous Somatostatin Analogue Use on the Activity of Everolimus in Patients with Advanced Neuroendocrine Tumors: Analysis from the Phase III RADIANT-2 Trial. Neuroendocrinology. 2015;102:18-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 27. | Castellano D, Bajetta E, Panneerselvam A, Saletan S, Kocha W, O'Dorisio T, Anthony LB, Hobday T; RADIANT-2 Study Group. Everolimus plus octreotide long-acting repeatable in patients with colorectal neuroendocrine tumors: a subgroup analysis of the phase III RADIANT-2 study. Oncologist. 2013;18:46-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 28. | Fazio N, Granberg D, Grossman A, Saletan S, Klimovsky J, Panneerselvam A, Wolin EM. Everolimus plus octreotide long-acting repeatable in patients with advanced lung neuroendocrine tumors: analysis of the phase 3, randomized, placebo-controlled RADIANT-2 study. Chest. 2013;143:955-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 29. | Strosberg JR, Yao JC, Bajetta E, Aout M, Bakker B, Hainsworth JD, Ruszniewski PB, Van Cutsem E, Öberg K, Pavel ME. Efficacy of octreotide long-acting repeatable in neuroendocrine tumors: RADIANT-2 placebo arm post hoc analysis. Endocr Relat Cancer. 2015;22:933-940. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 30. | Arnold R, Rinke A, Klose KJ, Muller HH, Wied M, Zamzow K, Schmidt C, Schade-Brittinger C, Barth P, Moll R. Octreotide versus octreotide plus interferon-alpha in endocrine gastroenteropancreatic tumors: a randomized trial. Clin Gastroenterol Hepatol. 2005;3:761-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 188] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 31. | Kölby L, Persson G, Franzén S, Ahrén B. Randomized clinical trial of the effect of interferon alpha on survival in patients with disseminated midgut carcinoid tumours. Br J Surg. 2003;90:687-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 147] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 32. | Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, Mittra E, Kunz PL, Kulke MH, Jacene H. Phase 3 Trial of 177 Lu-Dotatate for Midgut Neuroendocrine Tumors. N Engl J Med. 2017;376:125-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1702] [Cited by in RCA: 2236] [Article Influence: 279.5] [Reference Citation Analysis (0)] |
| 33. | Butturini G, Bettini R, Missiaglia E, Mantovani W, Dalai I, Capelli P, Ferdeghini M, Pederzoli P, Scarpa A, Falconi M. Predictive factors of efficacy of the somatostatin analogue octreotide as first line therapy for advanced pancreatic endocrine carcinoma. Endocr Relat Cancer. 2006;13:1213-1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 77] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 34. | Shojamanesh H, Gibril F, Louie A, Ojeaburu JV, Bashir S, Abou-Saif A, Jensen RT. Prospective study of the antitumor efficacy of long-term octreotide treatment in patients with progressive metastatic gastrinoma. Cancer. 2002;94:331-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 135] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 35. | Welin SV, Janson ET, Sundin A, Stridsberg M, Lavenius E, Granberg D, Skogseid B, Oberg KE, Eriksson BK. High-dose treatment with a long-acting somatostatin analogue in patients with advanced midgut carcinoid tumours. Eur J Endocrinol. 2004;151:107-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 93] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 36. | Saglam S, Hacisahinogullari H, Ozturk N, Kapran Y, Gulluoglu M, Turkmen C, Adalet I, Orhan Bilge A, Cem Balci N. Outcomes of first-line long-acting octreotide treatment in non-functional, advanced gastroenteropancreatic neuroendocrine tumors. J BUON. 2015;20:1201-1205. [PubMed] |
| 37. | Arnold R, Trautmann ME, Creutzfeldt W, Benning R, Benning M, Neuhaus C, Jürgensen R, Stein K, Schäfer H, Bruns C. Somatostatin analogue octreotide and inhibition of tumour growth in metastatic endocrine gastroenteropancreatic tumours. Gut. 1996;38:430-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 226] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 38. | Arnold R, Benning R, Neuhaus C, Rolwage M, Trautmann ME. Gastroenteropancreatic endocrine tumours: effect of Sandostatin on tumour growth. The German Sandostatin Study Group. Digestion. 1993;54 Suppl 1:72-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 39. | Bajetta E, Catena L, Procopio G, Bichisao E, Ferrari L, Della Torre S, De Dosso S, Iacobelli S, Buzzoni R, Mariani L. Is the new WHO classification of neuroendocrine tumours useful for selecting an appropriate treatment? Ann Oncol. 2005;16:1374-1380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 49] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 40. | Chung MH, Pisegna J, Spirt M, Giuliano AE, Ye W, Ramming KP, Bilchik AJ. Hepatic cytoreduction followed by a novel long-acting somatostatin analog: a paradigm for intractable neuroendocrine tumors metastatic to the liver. Surgery. 2001;130:954-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 51] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 41. | di Bartolomeo M, Bajetta E, Buzzoni R, Mariani L, Carnaghi C, Somma L, Zilembo N, di Leo A. Clinical efficacy of octreotide in the treatment of metastatic neuroendocrine tumors. A study by the Italian Trials in Medical Oncology Group. Cancer. 1996;77:402-408. [RCA] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 42. | Janson ET, Oberg K. Long-term management of the carcinoid syndrome. Treatment with octreotide alone and in combination with alpha-interferon. Acta Oncol. 1993;32:225-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 141] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 43. | Panzuto F, Di Fonzo M, Iannicelli E, Sciuto R, Maini CL, Capurso G, Milione M, Cattaruzza MS, Falconi M, David V. Long-term clinical outcome of somatostatin analogues for treatment of progressive, metastatic, well-differentiated entero-pancreatic endocrine carcinoma. Ann Oncol. 2006;17:461-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 95] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 44. | Ricci S, Antonuzzo A, Galli L, Ferdeghini M, Bodei L, Orlandini C, Conte PF. Octreotide acetate long-acting release in patients with metastatic neuroendocrine tumors pretreated with lanreotide. Ann Oncol. 2000;11:1127-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 64] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 45. | Saltz L, Trochanowski B, Buckley M, Heffernan B, Niedzwiecki D, Tao Y, Kelsen D. Octreotide as an antineoplastic agent in the treatment of functional and nonfunctional neuroendocrine tumors. Cancer. 1993;72:244-248. [RCA] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 46. | Al-Efraij K, Aljama MA, Kennecke HF. Association of dose escalation of octreotide long-acting release on clinical symptoms and tumor markers and response among patients with neuroendocrine tumors. Cancer Med. 2015;4:864-870. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 47. | Laskaratos FM, Walker M, Naik K, Maragkoudakis E, Oikonomopoulos N, Grant L, Meyer T, Caplin M, Toumpanakis C. Predictive factors of antiproliferative activity of octreotide LAR as first-line therapy for advanced neuroendocrine tumours. Br J Cancer. 2016;115:1321-1327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 48. | Öberg K, Norheim I, Theodorsson E. Treatment of malignant midgut carcinoid tumours with a long-acting somatostatin analogue octreotide. Acta Oncol. 1991;30:503-507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 49. | Ramundo V, Del Prete M, Marotta V, Marciello F, Camera L, Napolitano V, De Luca L, Circelli L, Colantuoni V, Di Sarno A. Impact of long-acting octreotide in patients with early-stage MEN1-related duodeno-pancreatic neuroendocrine tumours. Clin Endocrinol (Oxf). 2014;80:850-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 50. | Wang Y, Wang W, Jin K, Fang C, Lin Y, Xue L, Feng S, Zhou Z, Shao C, Chen M. Somatostatin receptor expression indicates improved prognosis in gastroenteropancreatic neuroendocrine neoplasm, and octreotide long-acting release is effective and safe in Chinese patients with advanced gastroenteropancreatic neuroendocrine tumors. Oncol Lett. 2017;13:1165-1174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |