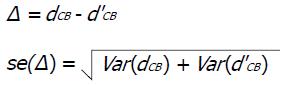Published online Aug 26, 2016. doi: 10.13105/wjma.v4.i4.77
Peer-review started: April 26, 2016
First decision: June 16, 2016
Revised: June 28, 2016
Accepted: July 14, 2016
Article in press: July 18, 2016
Published online: August 26, 2016
Processing time: 123 Days and 2.2 Hours
To compare different antibiotics for eradicating the carriage of Neisseria meningitidis (N. meningitidis), and to investigate heterogeneity and evidence inconsistency.
From a search of PubMed and published systematic reviews, we identified 23 trials evaluating 15 antibiotics that could be connected in a trial network. The outcome of interest is the eradication of N. meningitidis. We used WinBUGS to conduct random-effects, mixed treatment comparisons. Heterogeneity and evidence inconsistency was investigated by meta-regression modelling and examining characteristics of trial participants and interventions evaluated.
Rifampin, ciprofloxacin, minocycline, ceftriaxone, and azythromycin were statistically significantly (P < 0.05) more effective than placebo. The probability of being the best was 67.0% for a combination of rifampin and minocycline, 25.0% for ceftriaxone, 1.7% for azythromycin, and below 1% for the remaining regimens. Significant inconsistency between the direct and indirect estimates was observed for the comparison of rifampin and ciprofloxacin (P < 0.01), which may be caused by different types of carriers and different doses of ciprofloxacin.
A range of prophylactic antibiotic regimens are effective for eradicating meningococcal carriages, and treatment choice will depend on the individual priorities of the patients and physicians. In clinical situations where complete eradication is considered to be of the utmost importance, a combination of rifampin and minocycline seems to offer the highest likelihood of success. Ceftriaxone as a single intramuscular injection is also likely to be more effective as compared with the other two antibiotics (ciprofloxacin or rifampin) recommended by the current guidelines.
Core tip: This network meta-analysis found that a range of prophylactic antibiotic regimens are effective for eradicating meningococcal carriages. A combination of rifampin and minocycline seems the most efficacious, and ceftriaxone is also likely to be more effective than ciprofloxacin or rifampin alone. Careful investigation of significant inconsistency between direct and indirect comparison of rifampin and ciprofloxacin found that it was mainly caused by different types of carriers (persistent or any) and the varying doses of ciprofloxacin in the included trials. Detailed examination of characteristics of relevant studies should be conducted for investigating causes of inconsistency in network meta-analysis.
- Citation: Abdelhamid AS, Loke YK, Abubakar I, Song F. Antibiotics for eradicating meningococcal carriages: Network meta-analysis and investigation of evidence inconsistency. World J Meta-Anal 2016; 4(4): 77-87
- URL: https://www.wjgnet.com/2308-3840/full/v4/i4/77.htm
- DOI: https://dx.doi.org/10.13105/wjma.v4.i4.77
Neisseria meningitidis (N. meningitidis), a Gram-negative bacterium, is a normal inhabitant of the human pharynx. Transmission from person to person happens by droplets from the upper respiratory tract causing meningococcal disease; the severest forms of which are meningitis and septicaemia[1]. Meningococcal disease occurs usually sporadically or in small clusters all over the world as in the African “meningitis belt”, from Ethiopia to Senegal, and also in overcrowded places or wherever large population movements exist[2].
Prevalence of meningococcal carriage varies greatly, from 8% to 25% in random samples of healthy individuals, and as high as 36% to 71% in military recruits, and shows a massive increase in overcrowded places[1]. Current public health guidelines recommend chemoprophylaxis to be offered to close contacts of cases irrespective of vaccination status[3-6]. The evidence behind these recommendations were mainly from published systematic reviews[7,8]. However, there is no definite evidence from the available direct comparison trials, as to which antibiotic is more effective in preventing secondary meningococcal disease cases[9].
With the ever increasing number of competing interventions and a shortage of direct comparison trials, methods for indirect comparison and network meta-analysis have been developed to compare different treatment options[10-13]. Because of limited evidence from direct comparison trials, we conducted a network meta-analysis of randomised controlled trials that evaluated different antibiotics for eradicating carriages of N. meningitidis. We also reported the methodological experience obtained from this work for appropriately investigating causes of evidence inconsistencies in network meta-analysis.
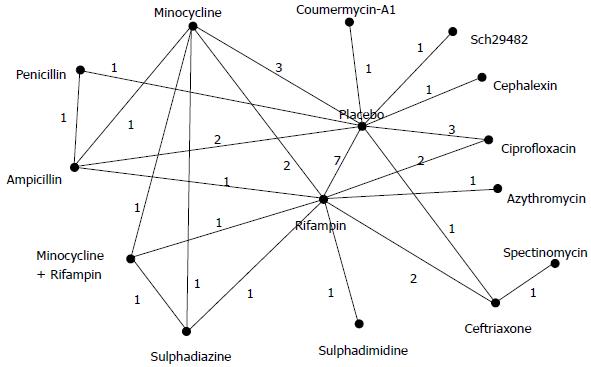
We included randomised controlled trials that evaluated effects of antimicrobial interventions for the prevention of meningococcal infections. Eligible studies were selected according to the following criteria: (1) it was a randomised controlled study; (2) included participants who exposed to patients with meningococcal disease or N. meningitidis carriers; (3) evaluated chemoprophylaxis interventions using any antibiotic regimens; and (4) reported data on eradication of meningococcal carriage. We checked references of previous systematic reviews and conducted additional literature search to identify relevant studies for this meta-analysis. Two recently published high quality systematic reviews (with pair-wise meta-analysis only) were identified, in which the literature searches were updated or conducted in June 2013[7] and in December 2013[8] respectively. We assessed the eligibility of studies included in these two reviews. To identify additional eligible studies possibly published after theses systematic reviews, one reviewer (Song F) conducted a search of PubMed in April 2016. The PubMed search used the following key words: “meningococcal” or “meningitis” combined with “chemoprevent*” or “chemoprophyl*” or antibiotic*” or antimicrobial*”. In addition, the search was limited to “clinical trial” and published in the last 5 years. However, all relevant studies in the current meta-analysis could be identified from existing systematic reviews, and no new eligible studies were identified from the search of PubMed. Eventually, we included 23 trials[14-35], in which 15 different antibiotics (or combinations of antibiotics) could be connected in a network of trials (Figure 1).
The outcome of interest in this network meta-analysis is failure to eradicate meningococcal carriage up to one week, although only the 2-wk outcome was reported in one trial[14]. From the included studies, two independent reviewers (Asmaa S Abdelhamid and Fujian Song) extracted the following data: Antibiotics evaluated, the number of carriers, the number of carriers with failed eradication at one week after antibiotic prophylaxis, study population, carrier status, reported serogroup, susceptibility of meningococci to antibiotics, study design, adequate or inadequate allocation concealment, and open or blinded. Disagreements between the two reviewers were resolved by discussion.
In contrast to within-trial direct comparisons, adjusted indirect comparison is a cross-trial comparison of different treatments, based on a common treatment (for example, placebo), so that the advantage of within-trial randomisation could be partially preserved[10]. Mixed treatment comparison refers to a combination of evidence from direct comparison trials and evidence based on indirect comparisons[12]. The validity of indirect and mixed treatment comparison depends on whether some basic assumptions could be fulfilled. The basic assumptions include homogeneity assumption for conventional pair-wise meta-analysis, trial similarity assumption for adjusted indirect comparison, and consistency assumption for combining direct and indirect evidence[36]. Among these basic assumptions, heterogeneity in conventional meta-analysis and inconsistency between direct and indirect evidence can be quantitatively assessed.
Markov chain Monte Carlo methods in WinBUGS (MRC Biostatistics Unit, Cambridge, United Kingdom) were used to conduct the random-effects, mixed treatment comparisons based on consistency assumption[37]. The WinBUGS code for Bayesian analysis is available from a report by Dias et al[37,38]. We used non-informative or vague priors, and obtained results by 200000 iterations after a burn-in of 100000.
When different antibiotics could be compared both directly and indirectly, we calculated the inconsistency (Δ) between the direct and indirect evidence by the following:
Math 6
Where dCB and d’CB are the treatment effects (e.g., log odds ratio) by direct and indirect comparison of treatment C and B; se(Δ) is the standard error of the estimated inconsistency; Var(dCB) and Var(d’CB) are estimated variances of the treatments effects.
We used a statistical model suggested by Cooper et al[39] to explore treatment by covariate interactions in the network meta-analysis. It estimates a regression coefficient by assuming a single interaction term for the relative effects of all the treatments vs the reference treatment (i.e., placebo)[38]. The effects of the following study-level covariates were investigated: Persistent carriers vs any carriers, household contacts vs other carriers, cluster/quasi randomised controlled trials vs randomised trials, adequate vs inadequate sequence generation, and open vs blinded design.
We also conducted narrative investigation of causes of inconsistency, which was focused on detailed comparison of rifampin and ciprofloxacin (reasons for this will be provided later). The assessment of clinical diversity and similarity among different sets of trials is a process of identifying possible effect modifiers, which was conducted by answering the following two questions[40]. First, we examined whether there were noticeable differences in study characteristics between different sets of trials. Then, we considered whether any of the observed differences in study characteristics between trials may have modified the relative treatment effects. In this study, we examined individual trials for effect modifiers with special attention to carriage status, dose of antibiotic used and length of intervention.
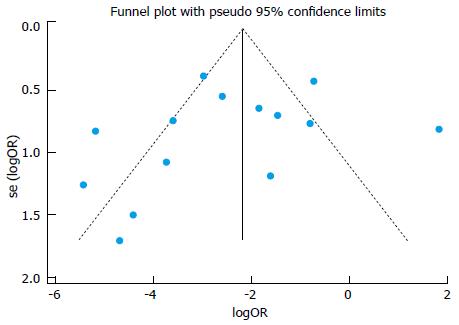
There were 14 trials that compared antibiotics and placebo. Using data from these placebo-controlled trials, we produced a funnel plot to investigate risk of publication bias. Asymmetry of the funnel plot was statistically tested using Harbord’s test for small-study effects[41]. All statistical analyses were conducted and checked by the corresponding author (Fujian Song) who has training and experience in statistical methods.
The main characteristics of the 23 trials are presented in Table 1, and data used in network meta-analyses are shown in Table 2. There are 20 two-arm trials, one three-arm trial, and two four-arm trials. The 15 antibiotics evaluated in these trials are: Placebo, rifampin, ciprofloxacin, minocycline, minocycline plus rifampin, penicillin, ampicillin, ceftriaxone, sulphadiazine, sulphadimidine, azythromycin, spectinomycin, cephalexin, “Sch29482”, and coumermycin A1 (Figure 1).
| Ref. | Antibiotics | Country and population | Carrier status | Serogroups and susceptibility | Study design | Sequence generation | Allocation concealment |
| Blakebrough et al[14] | Rifampin: 4 × 75 mg for 0-2 yr, 4 × 150 mg for 2-4 yr, 4 × 300 mg for 5-14 yr, 4 × 600 mg for > 15 yr (bid, 2 d) Sulphadimidine: 4 × 250 mg for 0-4 yr, 4 × 500 mg for 5-14 yr, 4 × 1 g for > 15 yr (bid, 2 d) | Nigeria Household contacts | Any carriers | Group A Susceptibility tested | Cluster quasi-RCT | Inadequate | Inadequate |
| Borgoño et al[15] | Rifampin: 2 × 10 mg/kg Placebo | Chile Children | Any carriers | Group unknown Susceptibility not tested | RCT | Unclear | Unclear |
| Cuevas et al[16] | Rifampin: 4 × 600 mg for > 18 yr, 4 × 20 mg/kg for 2-18 yr (bid, 2 d) Ciprofloxacin: 1 × 750 mg for > 18 yr, 1 × 15 mg/kg for 2-18 yr | Malawi Household contacts | Any carriers | Group A: 51% (unknown 49%) Susceptibility tested | Cluster RCT | Unclear | Unclear |
| Deal et al[17] | Rifampin: 4 × 600 mg (4 d) Placebo | United States Healthy students | Heavy/ Persistent (3 positive cultures) | Group B Susceptibility tested | RCT | Adequate | Adequate |
| Deal et al[18] | Cephalexin: 12 × 500 mg (tid, 4 d) Placebo | United States Students | Persistent (3 positive cultures) | Group B Susceptibility tested | RCT | Adequate | Adequate |
| Deviatkina et al[19] | Rifampin: 4 × 300 mg (4 d) Placebo | Russia Unclear | Unknown | Group unknown Susceptibility tested | RCT | Unclear | Unclear |
| Devine et al[20] | Rifampin: 4 × 600 mg (4 d) Placebo | United States Army recruits | Any carriers | Group Y: 79% Susceptibility tested | RCT | Adequate | Unclear |
| Devine et al[21] | Coumermycin A1: 14 × 50 mg (bid, 7 d) Placebo | United States Army recruits | Any carriers | Group unknown Susceptibility tested | RCT | Adequate | Unclear |
| Devine et al[22] | Minocycline: 1 × 200 mg + 9 × 100 mg (bid, 5 d) Placebo | United States Army recruits | Any carriers | Group Y: 63% Susceptibility tested | RCT | Adequate | Unclear |
| Devine et al[22] | Minocycline: 4 × 200 mg (bid, 2 d) No antibiotic | United States Army recruits | Any carriers | Group Y: Most Susceptibility tested | RCT | Adequate | Unclear |
| Dowd et al[23] | Ampicillin: 30 × 500 mg (tid, 10 d) Penicillin: 30 × 462 mg (tid, 10 d) Placebo | United States Amy recruits | Any carriers | Group B and sulfadiazine-resistant | RCT | Unclear | Unclear |
| Dworzack et al[24] | Ciprofloxacin: 1 × 750 mg Placebo | United States Young adults | Persistent (3 positive cultures) | Group B: 41%, Z: 33% Susceptibility tested | RCT | Unclear | Unclear |
| Girgis et al[25] | Rifampin: 4 × 600 mg (bid, 2 d) Azithromycin: 1 × 500 mg | Egypt Nursing students | Any carriers | Group A: 37%; B: 33% Susceptibility tested | RCT | Adequate | Unclear |
| Guttler et al[26] | Rifampin: 5 × 600 mg (5 d) Minocycline 10 × 100 mg (bid, 5 d) Ampicillin 10 × 500 mg (bid, 5 d) Placebo | United States Army recruits | Any carriers | Group B or C: 31% (non- groupable 67%) Susceptibility tested | Cluster RCT | Adequate | Unclear |
| Judson et al[27] | Ceftriaxone: im 1 × 125 mg Spectinomycin: im 1 × 2 g | United States Patients with gonorrhoea | Any carriers | Group unknown Susceptibility tested | RCT | Unclear | Unclear |
| Kaiser et al[28] | Rifampin: 4 × 600 mg for weight ≥ 66 lb, or 4 × 300 mg for weight < 66 lb (4 d) Placebo | United States Household contacts | Any carriers | Group C: 35% Susceptibility tested | RCT | Adequate | Unclear |
| Kaya et al[29] | Rifampin: 4 × 600 mg (bid, 2 d) Ciprofloxacin: 1 × 750 mg | Turkey Healthy adults | Any carriers | Group unknown Susceptibility not tested | Quasi RCT | Inadequate | Inadequate |
| Munford et al[30] | Rifampin: 4 × 600 mg (bid, 2 d) Minocycline: 1 × 200 mg + 5 × 100 mg (bid, 3 d) Rifampin + Minocycline: as above Sulphadiazine: 4 × 1 g (bid, 2 d) | Brazil Household contacts | Any carriers | Group C: Most Susceptibility tested | Cluster quasi-RCT | Inadequate | Inadequate |
| Pugsley et al[32] | Sch29482: 16 × 250 mg (every 6 h for 4 d) Placebo | United States | Persistent carriers (2 positive cultures) | Group Z: 36%; B: 24% | RCT | Adequate | Unclear |
| Pugsley et al[31] | Ciprofloxacin: 10 × 500 mg (bid, 5 d) Placebo | Young men United States | Persistent (2 positive cultures) | Susceptibility tested Group B: 79% | RCT | Adequate | Unclear |
| Renkonen et al[33] | Ciprofloxacin: 4 × 250 mg (bid, 2 d) Placebo | Young adults Finland | Heavy (> 100 colonies per plate) | Susceptibility tested Group B: 45% | RCT | Adequate | Adequate |
| Schwartz et al[34] | Rifampin: 4 × 600 mg or 4 × 10 mg/kg (bid, 2 d) | Army recruits Saudi Arabia | Any carriers | Susceptibility tested Group A | Cluster RCT | Unclear | Unclear |
| Simmons et al[35] | Ceftriaxone: im 1 × 250 mg (or 125 mg for < 15 yr) Rifampin: 4 × 600 mg for adults, 4 × 5 mg/kg for children < 1 mo, and 4 × 10 mg for children > 1 mo (bid, 2 d) Ceftriaxone: im 1 × 250 mg, or 1 × 125 mg for < 12 yr | Household contacts New Zealand Household contacts | Any carriers | Susceptibility tested Group B: 53% Susceptibility tested | RCT | Unclear | Unclear |
| Trial | Regimen | n | Failure to eradicate |
| Guttler et al[26] | Placebo | 18 (146) | 8 (65) |
| Rifampin | 18 (147) | 2 (13) | |
| Minocycline | 18 (147) | 1 (12) | |
| Ampicillin | 18 (147) | 3 (22) | |
| Munford et al[30] | Rifampin | 65 (67) | 6 (6) |
| Sulphadiazine | 79 (82) | 37 (38) | |
| Minocycline | 56 (58) | 6 (6) | |
| Rifampin + Minocycline | 59 (61) | 0 (0) | |
| Schwartz et al[34] | Rifampin | 34 (36) | 9 (9) |
| Ceftriaxone | 65 (68) | 2 (2) | |
| Dowd et al[23] | Placebo | 47 | 26 |
| Penicillin | 20 | 9 | |
| Ampicillin | 26 | 8 | |
| Borgoño et al[15] | Placebo | 110 | 71 |
| Rifampin | 118 | 10 | |
| Deal et al[17] | Placebo | 15 | 13 |
| Rifampin | 15 | 2 | |
| Deviatkina et al[19] | Placebo | 43 | 10 |
| Rifampin | 46 | 3 | |
| Devine et al[20] | Placebo | 28 | 25 |
| Rifampin | 38 | 7 | |
| Kaiser et al[28] | Placebo | 6 | 6 |
| Rifampin | 13 | 1 | |
| Dworzack et al[24] | Placebo | 22 | 20 |
| Ciprofloxacin | 24 | 1 | |
| Pugsley et al[31] | Placebo | 21 | 14 |
| Ciprofloxacin | 21 | 0 | |
| Renkonen et al[33] | Placebo | 53 | 46 |
| Ciprofloxacin | 56 | 2 | |
| Deal et al[18] | Placebo | 15 | 14 |
| Cephalexin | 15 | 11 | |
| Devine et al[22] | Placebo | 48 | 42 |
| Minocycline | 41 | 14 | |
| Devine et al[22] | Placebo | 29 | 27 |
| Minocycline | 53 | 16 | |
| Devine et al[21] | Placebo | 39 | 28 |
| Coumermycin A1 | 33 | 31 | |
| Pugsley et al[32] | Placebo | 29 | 26 |
| Sch29482 | 29 | 23 | |
| Cuevas et al[16] | Rifampin | 84 (88) | 3 (3) |
| Ciprofloxacin | 75 (79) | 9 (9) | |
| Kaya et al[29] | Rifampin | 25 | 1 |
| Ciprofloxacin | 26 | 2 | |
| Girgis et al[25] | Rifampin | 59 | 3 |
| Azythromycin | 60 | 4 | |
| Simmons et al[35] | Rifampin | 82 | 4 |
| Ceftriaxone | 100 | 3 | |
| Blakebrough et al[14] | Rifampin | 46 (48) | 11 (11) |
| Sulphadimidine | 33 (34) | 33 (34) | |
| Judson et al[27] | Ceftriaxone | 29 | 0 |
| Spectinomycin | 9 | 8 |
Carriers were mainly from household contacts of cases (six trials), military recruits (seven trials), and students or young people (six trials). Six trials recruited heavy or persistent carriers (defined as two or more sequential positive cultures before antibiotic prophylaxis). The test of susceptibility to antibiotics was done in most of the studies. The sequence generation was inadequate or unclear in 11 trials. Blinding was performed in 12 trials, and allocation concealment was adequate in only three trials (Table 1).
There were five cluster randomised trials. We could not find empirical data on intra-cluster correlation coefficient (ICC) for the included cluster randomised trials, and therefore estimated the effective sample sizes by assuming an ICC of 0.05[42].
Funnel plot using data from 14 placebo-controlled trials is shown in Figure 2. The funnel plot was not statistically significantly asymmetric (P = 0.610), indicating no concern about risk of small-study effects.
The results of the network meta-analysis are shown in Table 3. Rifampin, ciprofloxacin, minocycline, ceftriaxone and azythromycin were significantly (P < 0.05) more effective than placebo. The probability of being the most efficacious was 67.0% for a combination of rifampin and minocycline, 25.0% for ceftriaxone, 1.7% for azythromycin, and less than 1% for the remaining antibiotics. According to evidence from the full network of trials, the combination of rifampin and minocycline was the most efficacious intervention, and ceftriaxone the second (Table 3).
| 2 Rifampin | 3 Ciprofloxacin | 4 Cephalexin | 5 Minocycline | 6 Ampicillin | 7 Penicillin | 8 Ceftriaxone | 9 Rifampin + minocycline | 10 Azythromycin | 11 Spectinomycin | 12 Coumermycin A1 | 13 Sch29482 | 14 Sulphadiazine | 15 Sulphadimidine | |
| 1 Placebo | 0.038a | 0.020a | 0.274 | 0.058a | 0.267 | 0.611 | 0.009a | 0.004a | 0.071a | 5.971 | 5.524 | 0.498 | 0.487 | 23.17 |
| 2 Rifampin | 0.53 | 7.201 | 1.536 | 7.028a | 16.20a | 0.247 | 0.098 | 1.89 | 156.2a | 146.2a | 13.15a | 12.88a | 601a | |
| 3 Ciprofloxacin | 13.7 | 2.911 | 13.29a | 30.67a | 0.467 | 0.184 | 3.54 | 301.0a | 278.0a | 24.87a | 24.4a | 1174a | ||
| 4 Cephalexin | 0.214 | 0.980 | 2.26 | 0.035a | 0.013a | 0.262 | 22.42 | 20.6 | 1.826 | 1.825 | 91.1a | |||
| 5 Minocycline | 4.577 | 10.52 | 0.161 | 0.064 | 1.212 | 102.3a | 95.57a | 8.53 | 8.42a | 396a | ||||
| 6 Ampicillin | 2.291 | 0.035a | 0.014a | 0.266 | 22.54 | 20.85a | 1.864 | 1.84 | 88.6a | |||||
| 7 Penicillin | 0.015a | 0.006a | 0.116 | 9.808 | 9.128 | 0.81 | 0.8 | 39.09 | ||||||
| 8 Ceftriaxone | 0.385 | 7.566 | 620a | 597a | 53.17a | 52.94a | 2493a | |||||||
| 9 Rifampin + minocycline | 20.15 | 1776a | 1584a | 140.2a | 134.3a | 7088a | ||||||||
| 10 Azythromycin | 83.64a | 78.9a | 7.03 | 7 | 334a | |||||||||
| 11 Spectinomycin | 0.924 | 0.082 | 0.084 | 4.032 | ||||||||||
| 12 Coumermycin A1 | 0.089 | 0.088 | 4.372 | |||||||||||
| 13 Sch29482 | 0.992 | 48.75 | ||||||||||||
| 14 Sulphadiazine | 47.14 |
The covariate effects in the network meta-analysis are shown in Table 4. Trials with persistent carriers or household contacts of cases reported significantly greater treatment effects as compared with trials of any carriers or non-household contacts of cases, while the remaining regression coefficients were not statistically significant. When the effect of persistent carrier was incorporated into the network meta-analysis, the between-study variation (τ = 0.434) was much reduced as compared with the between-study variation without significant covariate adjustment (τ > 0.937). Therefore, type of carriers (persistent vs any) may be an effect modifier[39]. However, the between-study variation was not reduced when the effect of household contacts was included in the analysis (τ = 0.975).
| Covariate | Regression coefficient, β (95%CI) | Between-study variation (τ) |
| Persistent carrier (1) vs any carriers (0) | -2.904 (-4.695 to -1.186) | 0.434 |
| Household (1) vs other (0) | -6.178 (-16.79 to -0.069) | 0.975 |
| Cluster/quasi RCT (1) vs RCT (0) | 0.405 (-2.235 to 2.881) | 1.082 |
| Sequence generation inadequate (1) vs adequate (0) | 0.461 (-1.301 to 2.014) | 1.025 |
| Open design (1) vs blinded (0) | 0.055 (-1.877 to 1.662) | 1.087 |
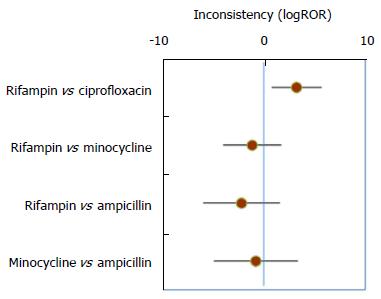
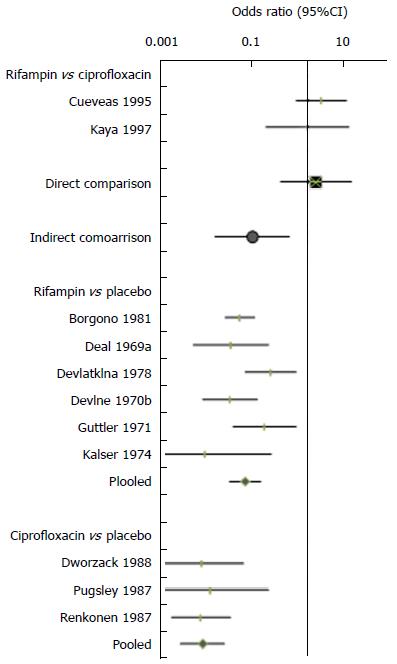
There is sufficient data for both direct and indirect comparisons of four pairs of antibiotics (Table 5), and the estimated inconsistencies between the direct and indirect estimates are shown in Figure 3. A statistically significant inconsistency was observed for the comparison of rifampin and ciprofloxacin. The indirect comparison based on 21 trials found that rifampin was significantly better than ciprofloxacin (OR = 0.09, 95%CI: 0.017-0.40 for failure to eradicate). In contrast, the pooling of two direct comparison trials suggested that rifampin therapy was less effective than ciprofloxacin, with a greater likelihood (non-statistically significant) of failure to eradicate (OR = 2.51, 95%CI: 0.36-15.64).
| MTC estimate | Direct estimate | Indirect estimate | ||||
| Comparison | No. of trials | OR (95%CrI) | No. of trials | OR (95%CrI) | No. of trials | OR (95%CrI) |
| Rifampin vs ciprofloxacin | 23 | 0.52 (0.13, 1.89) | 2 | 2.51 (0.36, 15.64) | 21 | 0.09 (0.017, 0.40) |
| Rifampin vs minocycline | 23 | 1.55 (0.40, 6.07) | 2 | 0.85 (0.11, 5.59) | 21 | 2.27 (0.28, 19.89) |
| Rifampin vs ampicillin | 23 | 6.94 (1.21, 37.53) | 1 | 1.62 (0.09, 29.82) | 20 | 12.23 (1.04, 146.9) |
| Minocycline vs ampicillin | 23 | 4.52 (0.67, 28.30) | 1 | 3.46 (0.16, 91.10) | 20 | 6.50 (0.41, 93.6) |
Our further investigation of causes of inconsistency was therefore focused on the comparison of rifampin and ciprofloxacin. These are also the antibiotics recommended in the current clinical guidelines. The inconsistency investigation was using data from two direct comparison trials[16,29], six placebo-controlled trials of rifampin[15,17,19,20,26,28] and three placebo-controlled trials of ciprofloxacin[24,31,33]. Figure 4 shows the results of the individual trials, with the overall estimates of direct and indirect comparisons.
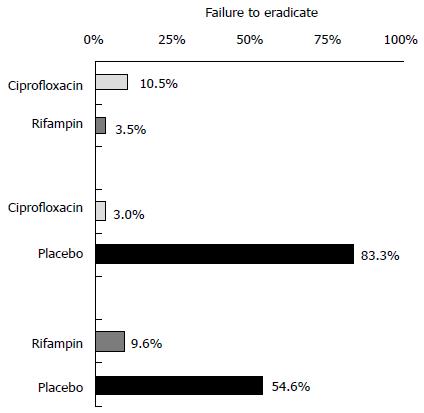
While placebo controlled trials of rifampin included mostly any carriers, three placebo controlled trials of ciprofloxacin included heavy or persistent carriers (Table 1). Consequently, as shown in Figure 5, the proportion of patients with failed eradication in the placebo arm was much higher in trials of ciprofloxacin than that in trials of rifampin (83% vs 55%). If the absolute results of antibiotic interventions were not influenced by the proportion of participants with persistent carriage, trials that included persistent carriers will show greater relative treatment effects purely because of the high failure rates in the placebo group (Figure 5). Therefore, imbalanced distribution of types of carriers across different sets of trials may invalid the similarity assumption in the network meta-analysis, which raises a question whether the indirect comparison is valid in this case.
In addition, the use of ciprofloxacin in the direct comparison trials[16,29] was different from its use in the placebo-controlled trials of ciprofloxacin[24,31,33]. A single dose of ciprofloxacin was compared with multiple doses of rifampin in the two direct comparison trials, while two of the three placebo-controlled trials of ciprofloxacin compared placebo and multiple doses of ciprofloxacin (Table 1). Therefore, the effect of ciprofloxacin (with multiple doses) in the placebo-controlled trials may be enhanced as compared to the single dose in the two direct comparison trials. The eradication failure in the ciprofloxacin arm at one week was 10.5% in the direct comparison trials, as compare with only 3.0% in the placebo-controlled trials (Figure 5). The different doses of ciprofloxacin used in the direct comparison trials and in the placebo-controlled trials also contributed to the significant inconsistency observed.
According to this network meta-analysis, a range of antibiotic regimens are effective for preventing meningococcal infections in carriers. The simultaneous analysis of all randomised controlled trials that could be connected in a coherent network provided results that were not available from the conventional pair-wise meta-analysis[43]. The network meta-analysis revealed that a combination of rifampin and minocycline seems the most efficacious, and ceftriaxone is also likely to be more effective than the antibiotics (ciprofloxacin or rifampin) recommended by the current guidelines[4-6]. The network meta-analysis also revealed significant inconsistency between direct and indirect estimates for the comparison of rifampin and ciprofloxacin. We investigated causes of the observed inconsistency and found that it was likely due to the following two effect modifiers: Types of carriers (persistent vs any), and the varying doses of ciprofloxacin.
The superior efficacy of rifampin and minocycline means it should be considered for areas where there is high degree of resistance to other agents, or in groups of patients where high rates of eradication are considered to be essential. The most efficacious regimen (rifampin and minocycline) was reported to have a significantly increased risk of adverse effects as compared to either drug alone[30]. Headache, dizziness, nausea, or vomiting were specific adverse effects noted more frequently in patients receiving the rifampin-minocycline combination. Nevertheless, patients who consider eradication of carriage to be their top priority may choose to put up with these adverse effects in order to have the best chance of treatment success.
Equally, the effectiveness of single dose intramuscular ceftriaxone, without any need to worry about patient adherence to oral regimens, makes it particularly suitable for patients when there are concerns surrounding the likelihood of the patient being able to regularly take several oral doses as prescribed. For instance, ceftriaxone would be an efficacious option in younger children who have difficulty taking tablets. Moreover, a single dose of ceftriaxone would appear to be less risky option than either ciprofloxacin or rifampin in women who are pregnant or breastfeeding. Use of ceftriaxone in both of these patient groups would be in-line with the United States CDC recommendations[3], and our network meta-analysis now provides the relevant evidence base to support this guidance.
Although the current guidelines in the United Kingdom recommend ciprofloxacin because it can be conveniently used as a single dose regimen, the results of inconsistency investigation indicate that single dose ciprofloxacin may be less effective than either multiple dose ciprofloxacin or rifampin. A regimen of multiple doses of ciprofloxacin seems preferable for persistent carriers (according to evidence from placebo-controlled trials). However, the emergence of ciprofloxacin-resistant N. meningitidis should also be taken into consideration[44].
Choice of optimal antibiotic strategy will be inevitably influenced by considering many factors such as cost, convenience, adherence, tolerability and bacterial resistance in a trade-off against the rate of failed eradication. For example, rifampin has been an important antibiotic agent in tuberculosis treatment, and to minimise the risk of bacterial resistance, it is not recommended as a prophylactic agent for household contacts in sub-Saharan Africa[6].
One of the main advantages of network meta-analysis is pooling of all connected trials into a coherent network of evidence. However, a study found that the inconsistency between direct and indirect evidence may be more prevalent than previously observed[45], and it has been generally accepted that causes of inconsistency in network meta-analysis should be carefully investigated[36,46-48]. In the current study, statistical meta-regression analyses found that the type of carriers (persistent vs any, and household contacts vs other) may be a cause of heterogeneity in the network meta-analysis. However, the usefulness of statistical methods for investigating causes of inconsistency is often limited because of the small number of trials, inadequate reporting of relevant variables, and modelling complexity.
The narrative investigation of causes of inconsistency is difficult for a complex network. The existence of evidence inconsistencies in a network meta-analysis does not mean that the whole network is inconsistent[46]. Therefore, we focused on the investigation of statistically significant inconsistencies. To further simplify the narrative investigation, a sub-network of trials was formed after excluding those that are only remotely connected to the target comparison.
We demonstrated that focused examination of characteristics of trial participants and interventions evaluated may reveal the clinically meaningful causes of inconsistency in network meta-analysis. The detailed examination of trial participants and interventions evaluated is similar to the investigation of heterogeneity in conventional pair-wise meta-analysis. Although the type of carriers (persistent vs any) can be identified by both statistical covariate analysis and narrative investigation, the difference in doses of ciprofloxacin as a possible cause of inconsistency could not be investigated by the statistical models we used. However, the narrative investigation mainly relies on subjective judgement, is restricted by available data from published studies, and a good understanding of the topic is required.
In order to include as many studies as possible in the trial network, we focused on eradication failure and did not consider other important outcomes such as adverse effects and new cases of meningococcal disease. Included studies were mostly conducted in 1970s or 1980s, and the most recent study was published in 2000[35]. Therefore, it is a question about whether the results of previous randomised controlled trials are applicable to the present. Although we included only randomised controlled trials, the quality of the included trials was poor, with considerable risk of bias. According to the results of meta-regression analyses (Table 4), the treatment effects were not significantly associated with whether a trial was cluster or quasi randomised, whether the sequence generation was inadequate, and whether it was blinded. In addition, publication and outcome reporting bias was possible. Funnel plot using data from placebo-controlled trials indicated that there was no statistically significant small-study effect.
The network meta-analysis confirms that a range of prophylactic antibiotic regimens are effective for eradicating meningococcal carriages, and treatment choice will depend on the individual priorities of the patients and physicians. In clinical situations where complete eradication is considered to be of the utmost importance, a combination of rifampin and minocycline seems to offer the highest likelihood of success. Ceftriaxone as a single intramuscular injection is also likely to be more effective as compared with the two recommended antibiotics (ciprofloxacin or rifampin) by the current guidelines. Variation in the type of carriage and dosage regimens of ciprofloxacin may account for the observed inconsistency in the direct and indirect comparisons of rifampin and ciprofloxacin. Detailed examination of characteristics of relevant studies should be conducted for investigating causes of inconsistency in network meta-analysis.
The current public health guidelines recommend chemoprophylaxis to be offered to close contacts of cases of meningococcal meningitis. Because of limited evidence from direct comparison trials, the authors conducted a network meta-analysis of randomised controlled trials that evaluated different antibiotics for eradicating carriages of Neisseria meningitidis (N. meningitidis).
With the ever increasing number of competing interventions and a shortage of direct comparison trials, methods for indirect comparison and network meta-analysis have been widely used to compare different treatment options.
This is the first network meta-analysis to compare the efficacy of competing antibiotics for eradicating the carriage of N. meningitidis. Methodological experience obtained from this network meta-analysis was also reported.
For eradicating meningococcal carriages, a combination of rifampin and minocycline seems the most efficacious, and ceftriaxone is also likely to be more effective than ciprofloxacin or rifampin alone. Detailed examination of characteristics of relevant studies should be conducted for investigating causes of inconsistency in all network meta-analysis.
Network meta-analysis can be used to combine evidence from direct comparison trials and evidence based on indirect comparisons.
This is a well-performed network meta-analysis regarding the effects of antibiotics for eradicating carriages of N. meningitidis. The methodology is clear, the meta-analysis was performed well, the article was well-written, and the limitations of the study have been adequately discussed. The findings of this meta-analysis should be useful for the scientific and clinical community.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Ayieko J, Chen GS S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Stephens DS, Greenwood B, Brandtzaeg P. Epidemic meningitis, meningococcaemia, and Neisseria meningitidis. Lancet. 2007;369:2196-2210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 675] [Cited by in RCA: 658] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 2. | Harrison LH, Trotter CL, Ramsay ME. Global epidemiology of meningococcal disease. Vaccine. 2009;27 Suppl 2:B51-B63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 531] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 3. | ACIP. Prevention and control of meningococcal disease. Recommendations of the Advisary Committee on Immunization Practice. Morbidity and Mortality Weekly Report. Atlanta: Department of Health and Human Services, Centers for Disease Control and Prevention 2005; . |
| 4. | ECDC. Public health management of sporadic cases of invasive meningococcal disease and their contacts. Stockholm: ECDC Guidance Stockholm European Centre for Disease Prevention and Control 2010; . |
| 5. | HPA. Guidance for public health management of meningococcal disease in the UK (Version 01.4-2011). In: London: Health Protection Agency 2011; . |
| 6. | WHO. Meningitis Outbreak Response in Sub-Saharan Africa: WHO Guideline. Geneva: World Health Organization 2014; . |
| 7. | Zalmanovici Trestioreanu A, Fraser A, Gafter-Gvili A, Paul M, Leibovici L. Antibiotics for preventing meningococcal infections. Cochrane Database Syst Rev. 2013;CD004785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Telisinghe L, Waite TD, Gobin M, Ronveaux O, Fernandez K, Stuart JM, Scholten RJ. Chemoprophylaxis and vaccination in preventing subsequent cases of meningococcal disease in household contacts of a case of meningococcal disease: a systematic review. Epidemiol Infect. 2015;143:2259-2268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Purcell B, Samuelsson S, Hahné SJ, Ehrhard I, Heuberger S, Camaroni I, Charlett A, Stuart JM. Effectiveness of antibiotics in preventing meningococcal disease after a case: systematic review. BMJ. 2004;328:1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50:683-691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Higgins JP, Whitehead A. Borrowing strength from external trials in a meta-analysis. Stat Med. 1996;15:2733-2749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med. 2004;23:3105-3124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1684] [Cited by in RCA: 1639] [Article Influence: 78.0] [Reference Citation Analysis (0)] |
| 13. | Lumley T. Network meta-analysis for indirect treatment comparisons. Stat Med. 2002;21:2313-2324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 782] [Cited by in RCA: 860] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 14. | Blakebrough IS, Gilles HM. The effect of rifampicin on meningococcal carriage in family contacts in northern Nigeria. J Infect. 1980;2:137-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Borgoño JM, Rodríguez H, García J, Cánepa I. Efficacy of rifampicin in the treatment of Meningococcus carriers. Rev Chil Pediatr. 1981;52:146-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 16. | Cuevas LE, Kazembe P, Mughogho GK, Tillotson GS, Hart CA. Eradication of nasopharyngeal carriage of Neisseria meningitidis in children and adults in rural Africa: a comparison of ciprofloxacin and rifampicin. J Infect Dis. 1995;171:728-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 38] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Deal WB, Sanders E. Efficacy of rifampin in treatment of meningococcal carriers. N Engl J Med. 1969;281:641-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 65] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Deal WB, Sanders E. Therapeutic trial of cephalexin in meningococcal carriers. Antimicrob Agents Chemother (Bethesda). 1969;9:441-444. [PubMed] |
| 19. | Deviatkina NP, Demina AA, Orlova EV, Timina VP, Petrova IS. Evaluation of the sanative action of rifampicin on the meningococcal carrier state. Antibiotiki. 1978;23:794-797. [PubMed] |
| 20. | Devine LF, Johnson DP, Hagerman CR, Pierce WE, Rhode SL, Peckinpaugh RO. Rifampin. Levels in serum and saliva and effect on the meningococcal carrier state. JAMA. 1970;214:1055-1059. [PubMed] [DOI] [Full Text] |
| 21. | Devine LF, Johnson DP, Hagerman CR, Pierce WE, Rhode SL, Peckinpaugh RO. The effect of coumermycin A on the meningococcal carrier state. Am J Med Sci. 1970;260:165-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 22. | Devine LF, Johnson DP, Hagerman CR, Pierce WE, Rhode SL, Peckinpaugh RO. The effect of minocycline on meningococcal nasopharyngeal carrier state in naval personnel. Am J Epidemiol. 1971;93:337-345. [PubMed] |
| 23. | Dowd JM, Blink D, Miller CH, Frank PF, Pierce WE. Antibiotic prophylaxis of carriers of sulfadiazine-resistant meningococci. J Infect Dis. 1966;116:473-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Dworzack DL, Sanders CC, Horowitz EA, Allais JM, Sookpranee M, Sanders WE, Ferraro FM. Evaluation of single-dose ciprofloxacin in the eradication of Neisseria meningitidis from nasopharyngeal carriers. Antimicrob Agents Chemother. 1988;32:1740-1741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Girgis N, Sultan Y, Frenck RW, El-Gendy A, Farid Z, Mateczun A. Azithromycin compared with rifampin for eradication of nasopharyngeal colonization by Neisseria meningitidis. Pediatr Infect Dis J. 1998;17:816-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 47] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Guttler RB, Counts GW, Avent CK, Beaty HN. Effect of rifampin and minocycline on meningococcal carrier rates. J Infect Dis. 1971;124:199-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 65] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Judson FN, Ehret JM. Single-dose ceftriaxone to eradicate pharyngeal Neisseria meningitidis. Lancet. 1984;2:1462-1463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 28. | Kaiser AB, Hennekens CH, Saslaw MS, Hayes PS, Bennett JV. Seroepidemiology and chemoprophylaxis disease due to sulfonamide-resistant Neisseria meningitidis in a civillian population. J Infect Dis. 1974;130:217-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 29. | Kaya A, Tasyaran MA, Celebi S, Yilmaz S. Efficacy of a single dose of ciprofloxacin vs. rifampicin in eradicating the nasopharyngeal carriage of Neisseria meningitidis. Turkish J Med Sci. 1997;27:153-155. |
| 30. | Munford RS, Sussuarana de Vasconcelos ZJ, Phillips CJ, Gelli DS, Gorman GW, Risi JB, Feldman RA. Eradication of carriage of Neisseria meningitidis in families: a study in Brazil. J Infect Dis. 1974;129:644-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 42] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Pugsley MP, Dworzack DL, Horowitz EA, Cuevas TA, Sanders WE, Sanders CC. Efficacy of ciprofloxacin in the treatment of nasopharyngeal carriers of Neisseria meningitidis. J Infect Dis. 1987;156:211-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Pugsley MP, Dworzack DL, Sanders CC, Sanders WE. Evaluation of Sch 29,482 in the eradication of Neisseria meningitidis from nasopharyngeal carriers. Antimicrob Agents Chemother. 1984;25:494-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 33. | Renkonen OV, Sivonen A, Visakorpi R. Effect of ciprofloxacin on carrier rate of Neisseria meningitidis in army recruits in Finland. Antimicrob Agents Chemother. 1987;31:962-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 34. | Schwartz B, Al-Tobaiqi A, Al-Ruwais A, Fontaine RE, A’ashi J, Hightower AW, Broome CV, Music SI. Comparative efficacy of ceftriaxone and rifampicin in eradicating pharyngeal carriage of group A Neisseria meningitidis. Lancet. 1988;1:1239-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 59] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 35. | Simmons G, Jones N, Calder L. Equivalence of ceftriaxone and rifampicin in eliminating nasopharyngeal carriage of serogroup B Neisseria meningitidis. J Antimicrob Chemother. 2000;45:909-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 36. | Song F, Loke YK, Walsh T, Glenny AM, Eastwood AJ, Altman DG. Methodological problems in the use of indirect comparisons for evaluating healthcare interventions: survey of published systematic reviews. BMJ. 2009;338:b1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 37. | Dias S, Sutton AJ, Ades AE, Welton NJ. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med Decis Making. 2013;33:607-617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 924] [Cited by in RCA: 943] [Article Influence: 78.6] [Reference Citation Analysis (0)] |
| 38. | Dias S, Sutton AJ, Welton NJ, Ades AE. Evidence synthesis for decision making 3: heterogeneity--subgroups, meta-regression, bias, and bias-adjustment. Med Decis Making. 2013;33:618-640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 329] [Cited by in RCA: 369] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 39. | Cooper NJ, Sutton AJ, Morris D, Ades AE, Welton NJ. Addressing between-study heterogeneity and inconsistency in mixed treatment comparisons: Application to stroke prevention treatments in individuals with non-rheumatic atrial fibrillation. Stat Med. 2009;28:1861-1881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 172] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 40. | Xiong T, Parekh-Bhurke S, Loke YK, Abdelhamid A, Sutton AJ, Eastwood AJ, Holland R, Chen YF, Walsh T, Glenny AM. Overall similarity and consistency assessment scores are not sufficiently accurate for predicting discrepancy between direct and indirect comparison estimates. J Clin Epidemiol. 2013;66:184-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 41. | Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med. 2006;25:3443-3457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1463] [Cited by in RCA: 1703] [Article Influence: 89.6] [Reference Citation Analysis (0)] |
| 42. | Higgins JPT, Deeks JJ, Altman DG. Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration 2011; Available from: http//www.cochrane-handbook.org. |
| 43. | Zalmanovici Trestioreanu A, Fraser A, Gafter-Gvili A, Paul M, Leibovici L. Antibiotics for preventing meningococcal infections. Cochrane Database Syst Rev. 2011;CD004785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 44. | Wu HM, Harcourt BH, Hatcher CP, Wei SC, Novak RT, Wang X, Juni BA, Glennen A, Boxrud DJ, Rainbow J. Emergence of ciprofloxacin-resistant Neisseria meningitidis in North America. N Engl J Med. 2009;360:886-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 45. | Song F, Xiong T, Parekh-Bhurke S, Loke YK, Sutton AJ, Eastwood AJ, Holland R, Chen YF, Glenny AM, Deeks JJ. Inconsistency between direct and indirect comparisons of competing interventions: meta-epidemiological study. BMJ. 2011;343:d4909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 143] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 46. | Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades AE. Evidence synthesis for decision making 4: inconsistency in networks of evidence based on randomized controlled trials. Med Decis Making. 2013;33:641-656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 467] [Cited by in RCA: 583] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 47. | Donegan S, Williamson P, Gamble C, Tudur-Smith C. Indirect comparisons: a review of reporting and methodological quality. PLoS One. 2010;5:e11054. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 48. | Jansen JP, Fleurence R, Devine B, Itzler R, Barrett A, Hawkins N, Lee K, Boersma C, Annemans L, Cappelleri JC. Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 1. Value Health. 2011;14:417-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 804] [Article Influence: 57.4] [Reference Citation Analysis (0)] |