Published online Jun 26, 2016. doi: 10.13105/wjma.v4.i3.69
Peer-review started: January 3, 2016
First decision: February 29, 2016
Revised: March 23, 2016
Accepted: April 21, 2016
Article in press: April 22, 2016
Published online: June 26, 2016
Processing time: 165 Days and 3 Hours
AIM: To conduct a systematic review and meta-analysis into the efficacy, safety, and dosage regimens of degarelix for treating prostate cancer (PCa).
METHODS: PubMed, EMBASE, the Cochrane Library, and Web of Science was systematically searched to identify randomized controlled trials (RCTs) comparing degarelix (240/80 mg vs 240/160 mg) to the gonadotropin-releasing hormone agonists, goserelin and leuprolide, for the treatment of PCa. Two independent reviewers screened putative studies, assessed the risk of bias, and then extracted pertinent data. Analyses were performed using Review Manager 5.2.
RESULTS: Seven papers from six RCTs, involving 1204 patients, were identified. The present meta-analysis showed that treatment with 240/160 mg degarelix is more effective and has fewer adverse events (AEs) relative to conventional 240/80 mg regimen. Degarelix significantly decreased International Prostate Symptom Scores [standardized mean differences (SMD) = -0.32, 95%CI: -0.51 to -0.12, P = 0.02] and caused fewer AEs (SMD = -0.28, 95%CI: -0.48 to -0.07, P = 0.008) than goserelin. Degarelix suppressed testosterone and prostate-specific antigen significantly faster than leuprolide.
CONCLUSION: Degarelix is a useful option in the treatment of advanced PCa. Degarelix 240/160 mg regimen was superior to a 240/80 mg regimen. More rigorously designed RCTs are urgently needed to confirm the efficacy of degarelix.
Core tip: This meta-analysis and systematic review aimed to compare the efficacy, safety, and dosage regimens of degarelix for prostate cancer. A total of seven papers from 6 randomized controlled trials were identified, involving 1204 patients. Degarelix was an useful option in the treatment of advanced prostate cancer, and degarelix 240/160 mg regimen was superior to 240/80 mg regimen.
- Citation: Fang C, Wu CL, Liu SS, Ge L, Bai JL. Efficacy, safety, and dose comparison of degarelix for the treatment of prostate cancer: A systematic review and meta-analysis. World J Meta-Anal 2016; 4(3): 69-76
- URL: https://www.wjgnet.com/2308-3840/full/v4/i3/69.htm
- DOI: https://dx.doi.org/10.13105/wjma.v4.i3.69
Prostate cancer (PCa) is one of the most common malignant neoplasm in men. The mortality rates associated with PCa has reduced in many developed countries due to improvements in curative treatment[1]. However, the incidence of PCa and related mortality rates are increasing in many developing countries[1-3].
PCa is hormone-sensitive[4] and is the most common initial treatment regime for PCa is androgen deprivation therapy (ADT)[5]. Androgen deprivation may be achieved by either surgical or medical intervention[4]. Gonadotropin-releasing hormone (GnRH) agonists and antagonists have been approved for ADT in treating advanced PCa[6]. GnRH agonists and antagonists ultimately act by suppressing testosterone to castration levels[7]. GnRH antagonists bind directly to GnRH receptors, blocking the effect of GnRH on the pituitary, producing an immediate suppression of luteinising hormone, follicle stimulating hormone, and testosterone. GnRH antagonists are likely to replace GnRH agonists as first-line ADT in the future[8].
Degarelix, a GnRH antagonist and first-line therapy for androgen-sensitive advanced PCa, causes a rapid and sustained testosterone suppression to castrate levels without a surge[6]. Degarelix has demonstrated a significantly superior progression-free survival and overall survival rates related to GnRH agonists in a recent pooled individual patient data analysis[9]. The conventional monthly degarelix regimen of 240/80 mg (initial dosage/maintenance dosage) has been approved by the Food and Drug Administration (FDA) and the European Medicines Agency (EMA)[10]. The results of phase II and III studies show that the efficacy and safety of the 240/80 mg and 240/160 mg degarelix regimens are not markedly different[10,11]. However, the dosage-funding study by Van Poppel et al[12] suggested a regimen of dosage 240 mg and 160 mg is preferred.
The study aims to conduct a systematic review and meta-analysis to compare the efficacy and safety of degarelix (240/80 mg and 240/160 mg) vs GnRH agonists for the treatment of advanced PCa.
PubMed (1966-July 2014), EMBASE.com (1974-July 2014), Cochrane Library (CENTRAL, Issue 6 of 12, June 2014), and Web of Science (2000 - July 2014) were searched to identified all relevant RCTs, the search was performed in July 8, 2014. No restrictions as to language, publication data, and publication status were applied. The search strategy was independently conducted by two reviewers. And the search strategy of PubMed is following: [“Prostatic Neoplasms”(Mesh) OR “Prostatic Neoplasms, Castration-Resistant”(Mesh)] OR [prostatic cancer* OR prostatic tumor* OR prostatic carcinoma* OR prostatic neoplasm* OR prostate cancer* OR prostate tumor* OR prostate carcinoma* OR prostate neoplasm* (Title/Abstract)] AND [“acetyl-2-naphthylalanyl-3-chlorophenylalanyl-1-oxohexadecyl-seryl-4-aminophenylalanyl(hydroorotyl)-4-aminophenylalanyl(carbamoyl)-leucyl-ILys-prolyl-alaninamide” (Supplementary Concept) OR degarelix OR firmagon] AND [random* OR randomized con–trolled trial* OR randomized trial* OR Randomized Controlled Trial(ptyp) OR “Randomized Controlled Trials as Topic”]. We also tracked the references of included studies and reviews to find potentially eligible studies.
RCTs met the following criteria were included: (1) study participants were ≥ 18 years old, had a histological confirmation of PCa (all stages), for whom endocrine treatment was indicated, and any previous or current hormonal management of PCa had been discontinued for > 6 mo before enrolment; (2) RCT or “random group” was mentioned in the methodology section; and (3) reported outcomes included the mean percentage changes of total prostate volume (TPV), quality of life (QoL) related to urinary symptoms, International Prostate Symptom Score (IPSS), adverse events (AEs), the testosterone response rates (cumulative proportion of patients with serum testosterone suppression ≤ 0.5 ng/mL), the incidence of prostate-specific antigen (PSA) failure (defined as an increase in PSA of ≥ 50% from nadir or ≥ 5 ng/mL on two consecutive occasions at least two weeks apart), the incidence of death, and PSA, luteinizing hormone (LH), follicle-stimulating hormone (FSH) level.
Exclusion criteria were studies reporting: (1) on patients who had received hormonal treatments for PCa within 6 mo; (2) where the intervention was not degarelix; and (3) animal studies, case-reports, reviews, abstracts, corres or letters to the journal editors.
Two reviewers independently examined studies for eligibility according to the eligibility criteria. Conflicts were resolved by a third reviewer.
A standard data extraction form was designed, which included fields for the first authors, publication year, intervention regimen, study size, tumor stage, Gleason score, dosage, duration, and outcomes. The methodological quality was evaluated according to the Cochrane Handbook version 5.1.0[13], namely on criteria of: Random sequence generation (selection bias), allocation concealment (selection bias), blinding (performance bias and detection bias), incomplete outcome data (detection bias), selective reporting (detection bias), and other biases. Judgments for each entry involved stratifying the risk of bias as “low risk”, “high risk”, or “unclear risk”. Data extraction and quality assessment were performed by two independent reviewers, conflicts were resolved by a third reviewer.
The standardized mean differences (SMD) with 95%CI were calculated for continuous variables (mean percentage changes of TPV, mean IPSS). OR with 95%CI were calculated for dichotomous variables (AEs, etc.). The heterogeneity between trials was evaluated using c2 statistic, where an I2≤ 50% and a P-value ≥ 0.10 was indicative of no statistical heterogeneity, upon which a fixed-effects model was applied. All analyses were conducted using Review Manager 5.2 software.
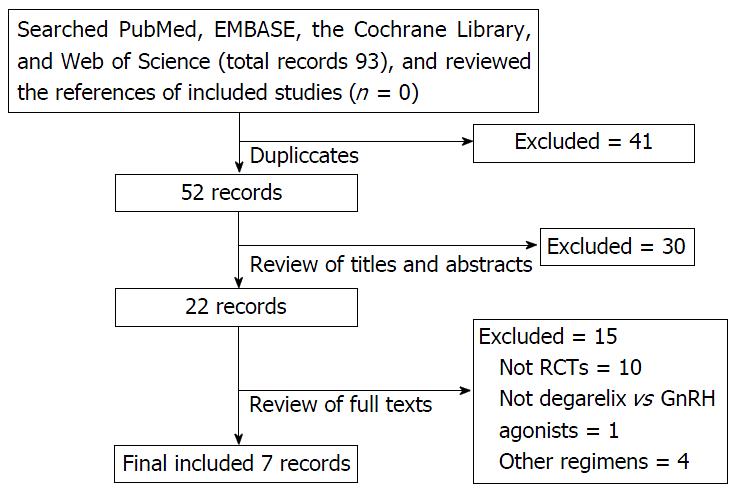
A total of 93 studies were identified. Forty-one studies were excluded duo to duplication. After screening the title and abstract of the remaining 52 studies, 30 studies were excluded for not being a RCT, not involving degarelix, not treating PCa, or for being an abstract, letter. After screening the full-text versions of 22 studies, 7 studies were excluded for not being a RCT (n = 3), for not reporting on degarelix vs GnRH agonists (n = 1) or 240/80 mg vs 240/160 mg regimens (n = 4), or for reporting identical results as a previous RCT (n = 3), or for being a review (n = 2) or a cost-effectiveness analysis (n = 2). Finally, 7 papers reporting on 6 RCTs, involving 1204 patients, were included in the present meta-analysis. The details of identifying studies could be found in Figure 1. Three RCTs reported on degarelix vs goserelin[14-16], 1 on degarelix vs leuprolide[11], and 3 on 240/80 mg vs 240/160 mg degarelix regimens[10-12]. The baseline characteristics are presented in Table 1.
| Ref. | Study arms (dose, Initial dose (mg)/monthly maintenance dosage (mg) | Regimen | Sample | Age (yr) | BMI (kg/m2) | T stage | Tumour stage | Gleason score | ECOG ( ≤ 2) | Duration (mo) | ||||||
| T1/2 | T3/4 | Localized | Locally advanced | Metastatic | Not classifiable | 2-6 | 7 | 8-10 | ||||||||
| Axcrona et al[14] | Degarelix (240/80) | Monthly | 82 | 71.9 ± 7.7 | 26.8 ± 4.1 | 35 | 47 | 24 | 30 | 22 | 6 | 17 | 24 | 41 | 82 | 3 |
| Goserelin (3.6) | Goserelin 12 wk + 50 mg/d bicalutamide during the initial 28 d | 97 | 73.0 ± 7.1 | 26.5 ± 3.7 | 42 | 55 | 32 | 23 | 31 | 11 | 16 | 31 | 50 | 96 | ||
| Anderson et al[15] | Degarelix (240/80) | 240 mg for 1 mo, 80 mg/mo | 27 | 68 (53-87) | NR | 5 | 21 | NR | 2 | 25 | 27 | 3 | ||||
| Goserelin (3.6) | 3.6 mg/mo goserelin + 50 mg/d bicalutamide | 13 | 72 (57-85) | NR | 2 | 11 | 0 | 13 | 13 | |||||||
| Mason et al[16] | Degarelix (240/80) | 240 mg day 0 + 80 mg day 28 and 56 | 180 | 70.6 ± 6.37 | 27.8 ± 3.99 | 116 | 64 | 111 | 63 | NR | 6 | 41 | 97 | 42 | 180 | 3 |
| Goserelin (3.6) | Goserelin 3.6 mg day 3, 31 and 59 | 64 | 70.8 ± 5.96 | 26.8 ± 3.69 | 42 | 21 | 41 | 20 | NR | 3 | 12 | 42 | 10 | 64 | ||
| Klotz L et al[11] | Degarelix (240/80, 240/160) | 240 mg for 1 mo + 80/160 mg monthly | 207 | 72 (51-89) | 26.7 ± 4.2 | 69 | 64 | 69 | 64 | 37 | 37 | 88 | 63 | 56 | 195 | 12 |
| Leuprolide (7.5) | Leuprolide 7.5 mg/mo | 201 | 74 (50-88) | 26.9 ± 3.9 | 63 | 52 | 63 | 52 | 47 | 39 | 87 | 62 | 51 | 190 | ||
| Ozono et al[10] | Degarelix (240/80) | 240 mg/dose + 80 mg/mo | 136 | 74.7 ± 6.76 | NR | 61 | 42 | 61 | 42 | 33 | 0 | 19 | 117 | NR | 12 | |
| Degarelix (240/160) | 240 mg/dose + 160 mg/mo | 137 | 74.2 ± 7.19 | NR | 64 | 41 | 64 | 41 | 31 | 1 | 23 | 114 | NR | |||
| Van Poppel et al[12] | Degarelix (240/80) | 240 mg/dose + 80 mg/mo | 30 | 70 (57-88) | 26 (18-41) | 5 | 12 | 5 | 12 | 5 | 8 | 19 | 11 | NR | 12 | |
| Degarelix (240/160) | 240 mg/dose + 160 mg/mo | 30 | 73 (52-82) | 25 (20-30) | 5 | 10 | 5 | 10 | 7 | 8 | 15 | 15 | NR | |||
All of the included RCTs were conducted using a multicenter, randomized, parallel-group, open-label, comparative design. Only 2 studies mentioned the methods of randomization and allocation concealment (a validated computer program and central allocation, respectively). However, all 6 of the RCTs failed to report the use of blinding. All studies were considered low risk for selective reporting (Table 2).
| Ref. | Adequate sequence generation | Adequate allocation concealment | Blinding | Incomplete outcome data addressed | Free of selective reporting |
| Axcrona et al[14] | Unclear | Unclear | High risk | Low risk | Low risk |
| Anderson et al[15] | Unclear | Unclear | High risk | Low risk | Low risk |
| Mason et al[16] | Unclear | Unclear | High risk | Low risk | Low risk |
| Klotz et al[11] | Validated computer program | Low risk | High risk | Low risk | Low risk |
| Ozono et al[10] | Central allocation | Low risk | High risk | Low risk | Low risk |
| Van Poppel et al[12] | Unclear | Unclear | High risk | Low risk | Low risk |
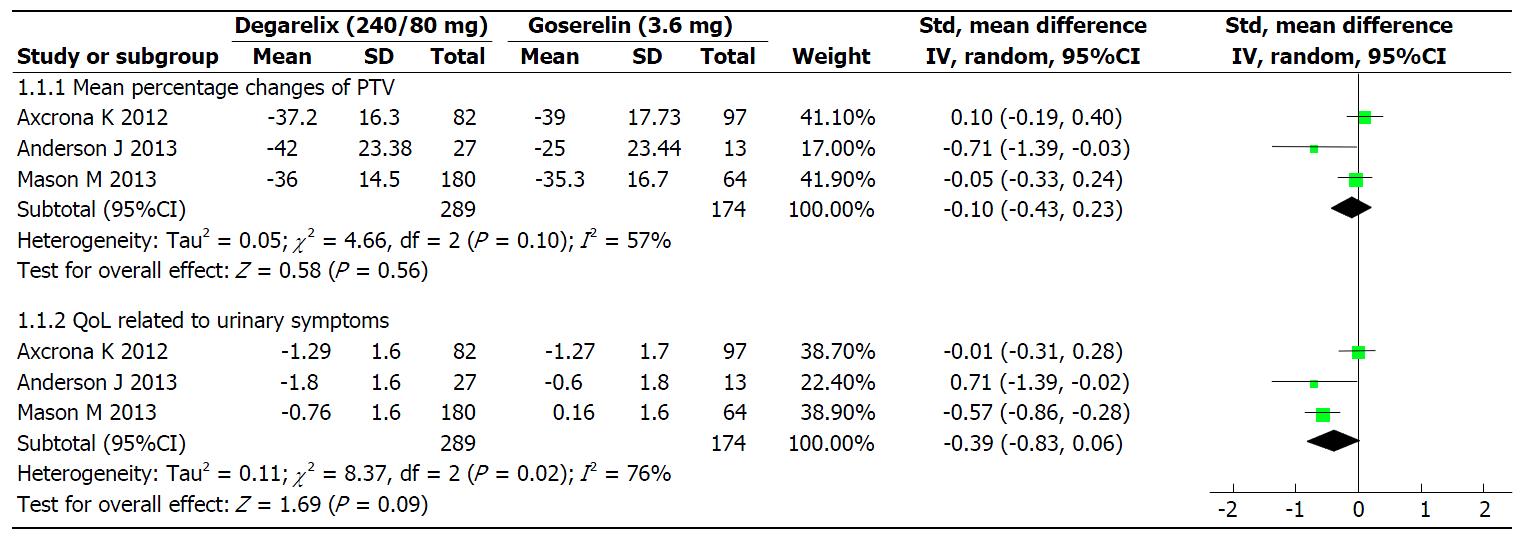
Mean percentage changes of TPV: Three studies[14-16], involving 463 patients, reported TPV. The results of heterogeneity evaluation between the three studies showed that I2 was 57%, P = 0.10. The results were modelled with random effects. The efficacy of degarelix in terms of mean percentage decreases in TPV was similar to that of goserelin (SMD = -0.10; 95%CI: -0.43 to 0.23; P = 0.56; Figure 2).
QoL related to urinary symptoms: Three studies[14-16], involving 463 patients, reported on QoL. The heterogeneity (I2) between three studies was I2 = 76% (P = 0.02). The results were modelled with random effects. The improvement of QoL related to urinary symptoms in degarelix group was similar to goserelin (SMD = -0.391; 95%CI: -0.83 to 0.06; P = 0.09; Figure 2).
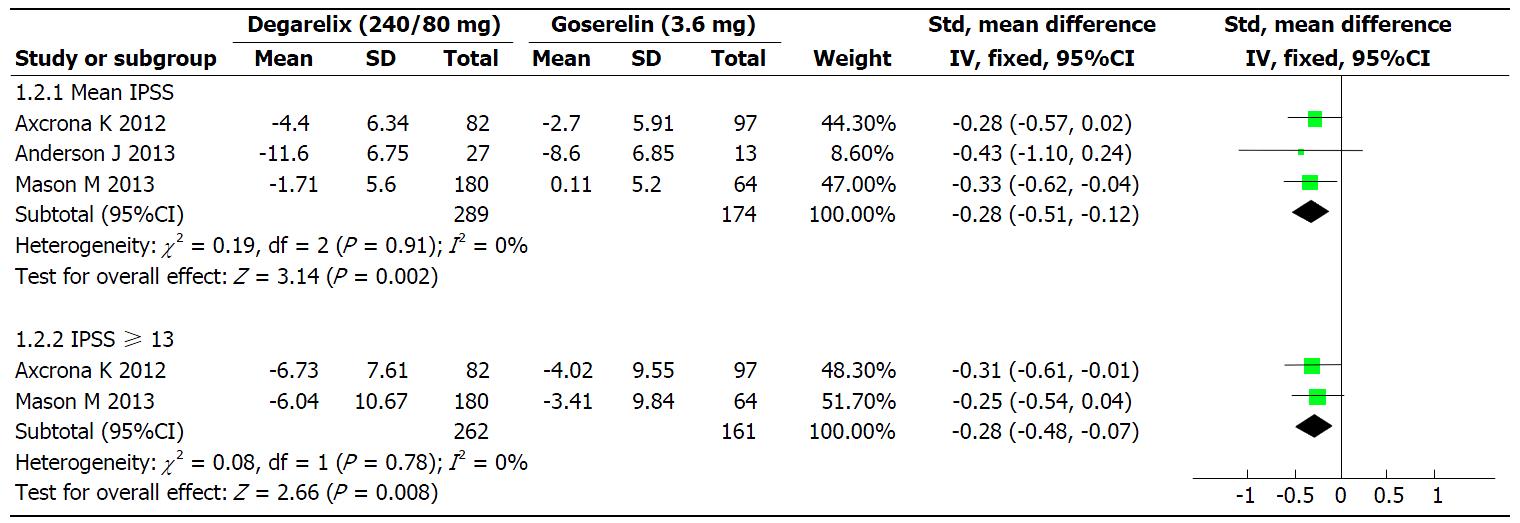
Mean IPSS and IPSS ≥ 13: The mean decrease of IPSS scores from baseline level were reported in three studies[14-16]. A fixed-effect model was used for meta-analysis since there was no statistical heterogeneity (I2 = 0%; P = 0.91). The mean decrease in IPSS scores in the degarelix group was significantly greater than in the goserelin group (SMD = -0.32; 95%CI: -0.51 to -0.12; P = 0.02; Figure 3). The heterogeneity (I2) between the two studies for a decrease in IPSS of ≥ 13 (moderate/severe) from baseline level was I2 = 0% (P = 0.78). After a fixed-effect model was applied, the results of the meta-analysis indicated that the decrease in IPSS ≥ 13 was greater in the degarelix group than within the goserelin group (SMD = -0.28; 95%CI: -0.48 to -0.07; P = 0.008; Figure 3).
Changes from baseline in serum testosterone and PSA: Three studies[14-16] reported the levels of testosterone and PSA. All patients within the degarelix group maintained serum testosterone at or below castrate levels (0.5 ng/mL) at 4, 8 and 12 wk. Patients within the goserelin group, however, only achieved castrate levels of serum testosterone at 8 and 12 wk. Mean PSA levels at 12 wk were reduced by 91.07% in the degarelix group and by 95.77% within the goserelin group. There were no differences between the two groups for serum testosterone and PSA levels.
The present search strategy only identified one RCT[11] comparing the use of degarelix (240/80 mg) vs leuprolide (7.5 mg) for the treatment of PCa. The testosterone response rates from day 28 through day 364 were similar between degarelix and leuprolide groups (97.2% vs 96.4%; P = 0.53). By day 3, the median testosterone levels were ≤ 0.5 ng/mL in 96.1% in the degarelix groups. By contrast, the median testosterone levels increased by 65% from baseline by day 3 in the leuprolide groups (median testosterone level 6.30 ng/mL; P < 0.001). The median rate of decrease in PSA levels up to day 14 and day 28 were greater in degarelix groups than in the leuprolide group (64% vs 18% and 85% vs 68%, respectively). The incidence of PSA failure was higher in the degarelix than within the goserelin groups (8.70% vs 13.93%; P = 0.10). The median reduction in FSH at the day 364 compared with baseline levels were 88.50% and 54.8% within the degarelix and goserelin groups, respectively.
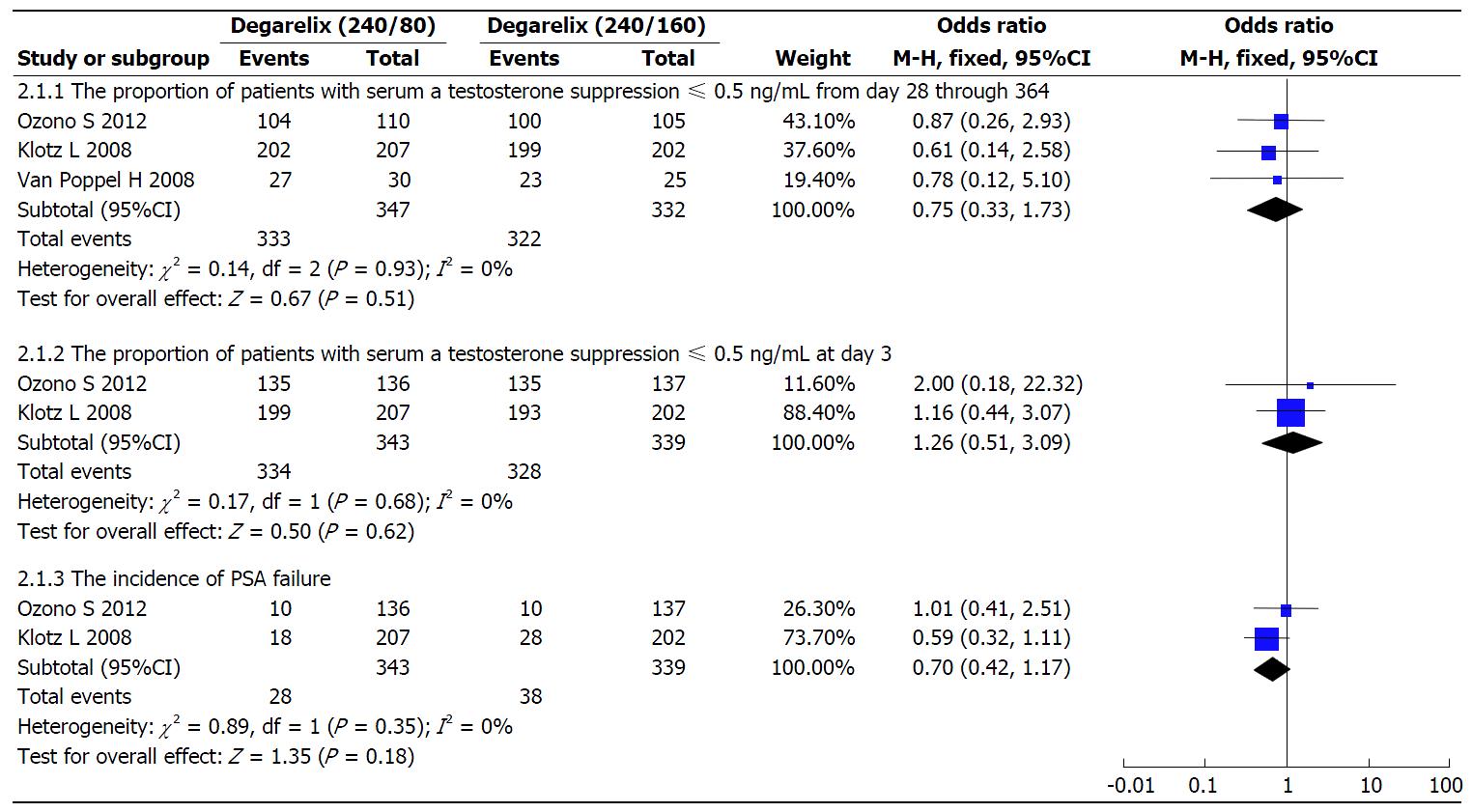
The testosterone response rates from day 28 through to day 364: Three studies[10-12] reported this outcome. There was no statistical heterogeneity between these three studies (I2 = 0%; P = 0.93). The data was modelled with fixed-effects. There were no statistical differences for two groups in the testosterone response rates (OR = 0.75; 95%CI: 0.33-1.73; P = 0.51; Figure 4).
The testosterone response rates at day 3 and the incidence of PSA failure: Two studies[10,11] reported these outcomes. The heterogeneity (I2) between the studies were 0% (P > 0.10). The testosterone response rates at day 3 (OR = 1.26; 95%CI: 0.51-3.09; P = 0.62) and the incidence of PSA failure (OR = 0.70; 95%CI: 0.42-1.17; P = 0.18) in the degarelix 240/80 mg were similar to degarelix 240/160 mg (Figure 4).
Other outcomes: There were no differences between the two groups in the median rate of change from baseline levels in PSA up to day 14 (63.24% vs 63.51%) and day 28 (82.57% vs 81.26%), or in the median reduction in LH at day 1 (85.00% vs 84.15%) and at the end of treatment (93.96% vs 93.95%). The median reduction in FSH at the end compared with baseline (86.03% vs 85.42%).
The AEs associated with the treatment regimens are presented in Table 3. The incidences of AEs due to treatment in patients treated with degarelix 240/80 mg were lower than those treated with goserelin 3.6 mg (OR = 0.62; 95%CI: 0.40-0.95; P = 0.03), and were similar in those treated with leuprolide 7.5 mg (OR = 1.07; 95%CI: 0.67-1.71; P = 0.78) and degarelix 240/160 mg (OR = 0.80; 95%CI: 0.53-1.2; P = 0.29). The incidences of injection site reactions were higher in the degarelix 240/80 mg group than within the goserelin 3.6 mg (OR = 33.08; 95%CI: 15.01-72.93; P < 0.00001) and leuprolide 7.5 mg groups (OR = 108.96; 95%CI: 14.96-793.44; P < 0.00001). The incidence of injection site reaction were slightly fewer in the degarelix 240/80 mg group than 240/160 mg group (OR = 0.81; 95%CI: 0.60-1.09; P = 0.16).
| AEs | Degarelix (240/80 mg) vs goserelin (3.6 mg) | Degarelix (240/80 mg) vs leuprolide (7.5 mg) | Degarelix (240/80 mg) vs degarelix (240/160 mg) | |||||||||
| Stusies (n) | Heterogeneity | OR (95%CI) | P-value | Studies (n) | Heterogeneity | OR (95%CI) | P-value | Studies (n) | Heterogeneity | OR (95%CI) | P-value | |
| Treatment-emergent AEs | 3 | I² = 0%, P = 0.64 | 0.62 (0.40, 0.95) | 0.03 | 1 | Not applicable | 1.07 (0.67, 1.71) | 0.78 | 3 | I² = 0%, P = 0.97 | 0.80 (0.53, 1.2) | 0.29 |
| Injection site reactions | 3 | I² = 0%, P = 0.79 | 33.08 (15.01, 72.93) | < 0.00001 | 1 | Not applicable | 108.96 (14.96, 793.44) | < 0.00001 | 3 | I² = 44%, P = 0.17 | 0.81 (0.60, 1.09) | 0.16 |
| Hot flush | 3 | I² = 0%, P = 0.59 | 0.80 (0.50, 1.28) | 0.35 | 1 | Not applicable | 1.26 (0.80, 2.00) | 0.32 | 3 | I² = 11%, P = 0.32 | 1.23 (0.89, 1.70) | 0.22 |
| Weight increase | - | - | - | - | 1 | Not applicable | 0.70 (0.37, 1.34) | 0.28 | 3 | I² = 17%, P = 0.30 | 1.13 (0.72, 1.76) | 0.6 |
| Hypertension | - | - | - | - | 1 | Not applicable | 1.48 (0.59, 3.71) | 0.4 | 2 | I² = 0%, P = 0.88 | 0.79 (0.44, 1.42) | 0.44 |
| Constipation | - | - | - | - | 1 | Not applicable | 0.57 (0.20, 1.60) | 0.29 | 2 | I² = 0%, P = 0.45 | 0.40 (0.19, 0.84) | 0.02 |
| UTI | 1 | Not applicable | 0.08 (0.00-1.88) | 0.12 | 1 | Not applicable | 0.52 (0.23, 1.15) | 0.1 | 2 | I² = 0%, P = 0.45 | 3.05 (0.97, 9.61) | 0.06 |
| Incidence of PSA recurrence | - | - | - | - | 1 | Not applicable | 0.56 (0.29, 1.09) | 0.09 | - | - | - | - |
| Incidence of death | - | - | - | - | 1 | Not applicable | 0.53 (0.17, 1.60) | 0.26 | - | - | - | - |
Summary of key findings: The present study conducted a comprehensive systematic review and meta-analysis to assess the effectiveness of a degarelix 240/80 mg regimen for the treatment of PCa. The results of the systematic review and meta-analysis show that, compared with goserelin 3.6 mg, treatment with degarelix 240/80 mg resulted in a similar decrease in TPV and QoL related to urinary symptoms; and that treatment with degarelix 240/80 mg was preferential in term of the decreasing IPSS scores and reducing treatment-emergent AEs. Our findings were similar to the pooled analysis of individual patient data of degarelix vs luteinising hormone releasing hormone agonists by Klotz et al[9]. In addition, treatment with degarelix 240/80 mg was not inferior to leuprolide 7.5 mg at maintaining low testosterone levels over a 1-year treatment period. Furthermore degarelix induced testosterone and PSA suppression significantly faster than leuprolide[11]. Both degarelix dosage regimens (240/80 mg and 240/160 mg) maintained castrate levels of testosterone; however, the testosterone suppression was not statistically different between doses. The degarelix 240/80 mg regimen had slightly fewer incidences of treatment-emergent AEs and injection site reactions within PCa patients, but more patients reported with hot flush, weight increase, and UTIs than within those receiving 240/160 mg degarelix.
This is the first systematic review and meta-analysis to comprehensively and systematically compare the clinical effectiveness and safety of degarelix vs GnRH agonists (goserelin and leuprolide) for treating PCa, and to decide the best dosage regimen for degarelix treatment. However, there were some limitations. Firstly, though we performed a systematic literature search of common databases and other sources, only 6 RCTs were identified and published in English, which could lead to a publication bias. Secondly, although degarelix has already been widely used as first-line therapy for PCa in the United States, European Union, and Japan[4], evidence in the form of RCTs towards its impact remain limited. Therefore, only a small number of studies could have been included in our review. Thirdly, 4 of the 6 RCTs included in our study failed to report on sequence generation and allocation concealment, and furthermore, were all open-label trials, which might have resulted in an overestimation of the effect[17]. Fourthly, only two dosage regimens of degarelix (240/80 mg and 240/160 mg) were compared for the treatment of PCa. Other dosage regimens (200/80 mg, 200/120 mg, and 200/160 mg) may be superior, and therefore more studies are needed to confirm. Finally, due to the data limitation of included studies, we could not do a meta-analysis on the survival, and we still don’t know the influences of degarelix on 3-year, 5-year and overall survival, while these data are important in cancer.
Our meta-analysis showed that a degarelix 240/160 mg regimen was more effective and had fewer AEs than the conventional 240/80 mg regimen although 240/80 mg regimen approved by the FDA and EMA. Furthermore, degarelix was statistically superior to goserelin in decreasing IPSS scores and treatment-emergent AEs, and suppressed testosterone and PSA levels significantly faster than leuprolide. Degarelix is therefore a useful option for the treatment of PCa.
Based on the failure of the included studies to report on their methods of randomization and use of blinding, future RCTs should be rigorously designed to ensure such methodology is addressed in future, thereby improving upon the quality of evidence for the use of degarelix in the treatment of PCa. A meta-analysis of dosage-funding and cost-effectiveness studies is needed to confirm the best dosage regimens from an economic point of view. For editors, instructions to authors of meta-analyses should require the disclosure of related item and the CONSORT checklist[18].
Prostate cancer is one of the most common malignant neoplasm in men. The incidence of prostate cancer and related mortality rates are increasing in many developing countries. Prostate cancer is hormone-sensitive and is the most common initial treatment regime for prostate cancer is androgen deprivation therapy (ADT). Gonadotropin-releasing hormone (GnRH) agonists and antagonists have been approved for ADT in treating advanced prostate cancer. However, degarelix has demonstrated a significantly superior progression-free survival and overall survival rates related to GnRH agonists in a recent pooled individual patient data analysis.
The conventional monthly degarelix regimen of 240/80 mg (initial dosage/maintenance dosage) has been approved by the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for treating advanced prostate cancer. However, present results of phase II and III studies were inconsistent.
The results of phase II and III studies show that the efficacy and safety of the 240/80 mg and 240/160 mg degarelix regimens are not markedly different. However, the dosage-funding study by Van Poppel et al suggested a regimen of dosage 240 mg and 160 mg is preferred. This is the first systematic review and meta-analysis to comprehensively and systematically compare the clinical effectiveness and safety of degarelix vs GnRH agonists (goserelin and leuprolide) for treating prostate cancer, and to decide the best dosage regimen for degarelix treatment.
This meta-analysis showed that a degarelix 240/160 mg regimen was more effective and had fewer adverse events than the conventional 240/80 mg regimen although 240/80 mg regimen approved by the FDA and EMA. Furthermore, degarelix was statistically superior to goserelin in decreasing International Prostate Symptom Scores and treatment-emergent adverse events, and suppressed testosterone and prostate-specific antigen levels significantly faster than leuprolide.
A systematic review attempts to collate all empirical evidence that fits pre-specified eligibility criteria in order to answer a specific research question. It uses explicit, systematic methods that are selected with a view to minimizing bias, thus providing more reliable findings from which conclusions can be drawn and decisions made. Meta-analysis is the use of statistical methods to summarize the results of independent studies. By combining information from all relevant studies, meta-analyses can provide more precise estimates of the effects of health care than those derived from the individual studies included within a review.
It is a well written analysis of the existing evidence regarding degarelix in prostate cancer.
P- Reviewer: Creta M, Goluboff ET, Ilie CP S- Editor: Gong XM L- Editor: A E- Editor: Li D
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25541] [Article Influence: 1824.4] [Reference Citation Analysis (7)] |
| 2. | Moore MA, Ariyaratne Y, Badar F, Bhurgri Y, Datta K, Mathew A, Gangadharan P, Nandakumar A, Pradhananga KK, Talukder MH. Cancer epidemiology in South Asia - past, present and future. Asian Pac J Cancer Prev. 2010;11 Suppl 2:49-66. [PubMed] |
| 3. | Kang DI, Chung JI, Ha HK, Min K, Yoon J, Kim W, Seo WI, Kang Pm, Jung SJ, Kim IY. Korean prostate cancer patients have worse disease characteristics than their American counterparts. Asian Pac J Cancer Prev. 2013;14:6913-6917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Carter NJ, Keam SJ. Degarelix: a review of its use in patients with prostate cancer. Drugs. 2014;74:699-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Zhou ZR, Liu SX, Zhang TS, Xia J, Li B. Abiraterone for treatment of metastatic castration-resistant prostate cancer: a systematic review and meta-analysis. Asian Pac J Cancer Prev. 2014;15:1313-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Boccon-Gibod L, Albers P, Morote J, van Poppel H, de la Rosette J, Villers A, Malmberg A, Neijber A, Montorsi F. Degarelix as an intermittent androgen deprivation therapy for one or more treatment cycles in patients with prostate cancer. Eur Urol. 2014;66:655-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Mottet N, Bellmunt J, Bolla M, Joniau S, Mason M, Matveev V, Schmid HP, Van der Kwast T, Wiegel T, Zattoni F. EAU guidelines on prostate cancer. Part II: Treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2011;59:572-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 405] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 8. | Van Poppel H, Klotz L. Gonadotropin-releasing hormone: an update review of the antagonists versus agonists. Int J Urol. 2012;19:594-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 9. | Klotz L, Miller K, Crawford ED, Shore N, Tombal B, Karup C, Malmberg A, Persson BE. Disease control outcomes from analysis of pooled individual patient data from five comparative randomised clinical trials of degarelix versus luteinising hormone-releasing hormone agonists. Eur Urol. 2014;66:1101-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 10. | Ozono S, Ueda T, Hoshi S, Yamaguchi A, Maeda H, Fukuyama Y, Takeda K, Ohashi Y, Tsukamoto T, Naito S. The efficacy and safety of degarelix, a GnRH antagonist: a 12-month, multicentre, randomized, maintenance dose-finding phase II study in Japanese patients with prostate cancer. Jpn J Clin Oncol. 2012;42:477-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Klotz L, Boccon-Gibod L, Shore ND, Andreou C, Persson BE, Cantor P, Jensen JK, Olesen TK, Schröder FH. The efficacy and safety of degarelix: a 12-month, comparative, randomized, open-label, parallel-group phase III study in patients with prostate cancer. BJU Int. 2008;102:1531-1538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 415] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 12. | Van Poppel H, Tombal B, de la Rosette JJ, Persson BE, Jensen JK, Kold Olesen T. Degarelix: a novel gonadotropin-releasing hormone (GnRH) receptor blocker--results from a 1-yr, multicentre, randomised, phase 2 dosage-finding study in the treatment of prostate cancer. Eur Urol. 2008;54:805-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 96] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 13. | Higgins J, Green SE. Cochrance Handbook for Systematic Reviews of Interventions Version 5.1.0 [EB/OL]. The Cochrane Collaboration, 2011. . |
| 14. | Axcrona K, Aaltomaa S, da Silva CM, Ozen H, Damber JE, Tankó LB, Colli E, Klarskov P. Androgen deprivation therapy for volume reduction, lower urinary tract symptom relief and quality of life improvement in patients with prostate cancer: degarelix vs goserelin plus bicalutamide. BJU Int. 2012;110:1721-1728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 15. | Anderson J, Al-Ali G, Wirth M, Gual JB, Gomez Veiga F, Colli E, van der Meulen E, Persson BE. Degarelix versus goserelin (+ antiandrogen flare protection) in the relief of lower urinary tract symptoms secondary to prostate cancer: results from a phase IIIb study (NCT00831233). Urol Int. 2013;90:321-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Mason M, Maldonado Pijoan X, Steidle C, Guerif S, Wiegel T, van der Meulen E, Bergqvist PB, Khoo V. Neoadjuvant androgen deprivation therapy for prostate volume reduction, lower urinary tract symptom relief and quality of life improvement in men with intermediate- to high-risk prostate cancer: a randomised non-inferiority trial of degarelix versus goserelin plus bicalutamide. Clin Oncol (R Coll Radiol). 2013;25:190-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Di BS, Wei KP, Tian JH, Xiao XJ, Li Y, Zhang XH, Yu Q, Yang KH, Ge L, Huang WH. Effectiveness and safety of pemetrexed versus docetaxel as a treatment for advanced non-small cell lung cancer: a systematic review and meta-analysis. Asian Pac J Cancer Prev. 2014;15:3419-3424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Wang JC, Tian JH, Ge L, Gan YH, Yang KH. Which is the best Chinese herb injection based on the FOLFOX regimen for gastric cancer? A network meta- analysis of randomized controlled trials. Asian Pac J Cancer Prev. 2014;15:4795-4800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |