Published online Apr 26, 2016. doi: 10.13105/wjma.v4.i2.44
Peer-review started: May 20, 2015
First decision: June 19, 2015
Revised: July 18, 2015
Accepted: November 9, 2015
Article in press: January 4, 2016
Published online: April 26, 2016
Processing time: 329 Days and 20.1 Hours
AIM: To compare the outcomes of endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) of colorectal lesions.
METHODS: An electronic systematic literature search of four computerized databases was performed in July 2014 identifying studies reporting the outcomes of colorectal ESD and EMR. The primary outcome measures were en-bloc resection rate, endoscopic clearance rate and lesion recurrence rate of the patients followed up. The secondary outcome was the complication rate (including bleeding, perforation and surgery post EMR or ESD rate). Statistical pooling and random effects modelling of the studies calculating risk difference, heterogeneity and assessment of bias and quality were performed.
RESULTS: Six observational studies reporting the outcomes of 1324 procedures were included. The en-bloc resection rate was 50% higher in the ESD group than in the EMR group (95%CI: 0.17-0.83, P < 0.0001, I2 = 99.7%). Endoscopic clearance rates were also significantly higher in the ESD group (95%CI: -0.06-0.02, P < 0.0001, I2 = 92.5%). The perforation rate was 7% higher in the ESD group than the EMR group (95%CI: 0.05-0.09, P > 0.05, I2 = 41.1%) and the rate of recurrence was 50% higher in the EMR group than in the ESD group (95%CI: 0.20-0.79, P < 0.001, I2 = 99.5%). Heterogeneity remained consistent when subgroup analysis of high quality studies was performed (with the exception of piecemeal resection rate), and overall effect sizes remained unchanged for all outcomes.
CONCLUSION: ESD demonstrates higher en-bloc resection rates and lower recurrence rates compared to colorectal EMR. Differences in outcomes may benefit from increased assessment through well-designed comparative studies.
Core tip: Endoscopic mucosal resection (EMR) is the conventional resection method of colorectal polyps. However certain lesions such as large sessile polyps can be challenging. Piecemeal resection has been shown to result in a high recurrence rate requiring further endoscopic sessions or surgery. Colorectal endoscopic submucosal dissection (ESD) is still at a relatively early stage, there are very few studies directly comparing the two modalities, few randomised controlled trials and fewer still reporting longer-term outcomes. This meta-analysis reports mid-term follow-up outcomes of colorectal ESD and EMR. ESD demonstrates higher en-bloc resection rates and lower mid-term recurrence rates compared to colorectal EMR albeit with higher complication rates.
- Citation: Patel N, Alexander J, Ashrafian H, Athanasiou T, Darzi A, Teare J. Meta-analysis comparing differing methods of endoscopic therapy for colorectal lesions. World J Meta-Anal 2016; 4(2): 44-54
- URL: https://www.wjgnet.com/2308-3840/full/v4/i2/44.htm
- DOI: https://dx.doi.org/10.13105/wjma.v4.i2.44
Colorectal cancer is the fourth most common cancer in the world with an incidence of 9.7% and a 8.5% mortality rate[1]. The introduction of colorectal cancer screening programmes, particularly in the western world, and advancements in endoscopic imaging are likely to result in a greater number of early cancers and polyps detected.
The conventional endoscopic treatment of colorectal polyps is polypectomy or endoscopic mucosal resection (EMR) which is performed worldwide[2,3]. Performing EMR on lesions such as laterally spreading tumours or complex sessile polyps can be challenging and may require a number of endoscopic sessions or surgery resulting in extra cost, potential in-patient hospital stays, increased complication rates and stress to the patient[4,5]. Furthermore, piecemeal resection makes histopathological assessment of whether the procedure was curative or not difficult and has also been shown to result in a high recurrence rate[6-8].
As a result of the drive towards minimally invasive surgery, endoscopic submucosal dissection (ESD) has emerged as a viable endoscopic alternative for early colorectal cancers or polyps, which would otherwise have been treated surgically or endoscopically. The technique pioneered in Japan for early gastric cancer, has been used with great success particularly in East Asia[9-11] where it is now the standard of care. Given the success of the technique, the indications are now expanding and the technique is increasingly being used to treat colorectal lesions[5,12,13]. ESD has improved en-bloc resection rates for early gastric cancer compared to EMR[14-16]. However, the technique is also associated with long procedure times, greater complication rates as well as the need for a highly skilled endoscopist[5,17].
The uptake of colorectal ESD has been slow for a number of reasons. It is a more challenging technique than EMR and gastric ESD due to the long colonic lumen which has a thin luminal wall and comprises of flexures and folds resulting in an already technically demanding and complex technique becoming even more so.
Whether ESD outcomes, which have been so successful for early gastric cancer, can translate to colorectal lesions is not yet clear[18]. There are few studies directly comparing these techniques for colorectal lesions with insufficient information and varying short and mid-term outcomes[2,13,19-21].
The objective of this meta-analysis is to compare the outcomes of colorectal EMR and ESD from the literature to date. The efficacy of the techniques was determined by establishing the following primary outcomes: En-bloc resection rate, endoscopic completeness rate and recurrence rate. Secondary outcome measures include the complication rate including perforation, bleeding and surgery after EMR or ESD.
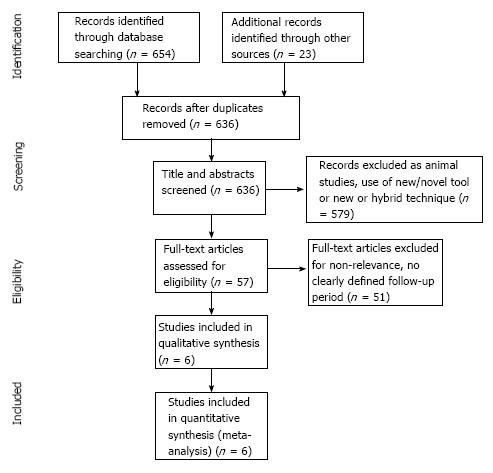
An electronic search was conducted from four computerized databases, MEDLINE (1946 to end July 2014), EMBASE (1974 to end July 2014), Cochrane Central Register of Controlled Trials and systematic reviews (1991 to end July 2014), CINAHL (1937 to end July 2014) using the following search strategy: (Endoscopic mucosal resection OR EMR) AND (Endoscopic submucosal dissection OR ESD) AND (exp colonic polyps OR Colon) AND (exp endoscopic polypectomy OR polypectomy). Additional studies identified through relevant reviews, references cited by included papers and PubMed “related articles” feature were also examined in full text for potential inclusion (Figure 1).
Studies which analysed the outcomes of colonic lesions (early cancers or polyps) removed by EMR and ESD were considered for inclusion in this meta-analysis.
The primary outcome measures were en-bloc resection rate, endoscopic clearance rate and lesion recurrence rate of the patients followed up. The secondary outcome was the complication rate (including bleeding, perforation and surgery post EMR or ESD rate). Both full articles and abstracts were included.
Published abstracts or articles which did not contain a primary outcome variable were excluded. In addition, reviews, editorials, letters, opinions, comments, case reports and surveys were not included. Data which had been published by the same research group or published by the same author were not included; only the most recent data which included the previously published data were included.
Papers which reported data for patients who were treated with ESD or EMR in different time periods or in different sites in the gastrointestinal tract were included if the colorectal data could be easily extracted. Animal studies and endoscopic removal of inflammatory polyps or neuroendocrine tumours were excluded. Studies which reported outcomes from snare-assisted, hybrid ESD, laparoscopic ESD or which used new endoscopic tools were excluded.
Eligible articles were reviewed independently by two investigators (NP and JA); data was extracted into a standardized data extraction form[19,22-26]. Discrepancies were resolved by a third investigator (JT) who made the final decision for eligibility and data extraction.
The following data were extracted where available: Year of publication, study location, patient demographics, operating time, lesion size, en-bloc resection rate, piecemeal resection rate, complete resection rate, length of follow-up, lesion recurrence and treatment, endoscopic completeness rate and complication rate (bleeding, perforation and surgery post ESD) (Table 1, Table 2, Table 3 and Table 4).
| Ref. | Year | Study site | Publication type | Total sample size | EMR | ESD | ||||
| Sample size | Male (%) | Age [mean ± SD (range)] | Sample size | Male (%) | Age [mean/median ± SD (range)] | |||||
| Tajika et al[22] | 2011 | Japan | Full paper | 189 | 104 | 61 | 59.9 ± 10.6 | 85 | 58 | 64.3 ± 9.2 |
| Lee et al[23] | 2012 | South Korea | Full paper | 454 | 140 | 64 | 63 (23-90) | 314 | 55 | 61 (25-85) |
| Kobayashi et al[24] | 2012 | Japan | Full paper | 84 | 56 | 68 | 65.9 ± 9.9 | 28 | 68 | 65.1 ± 9.7 |
| Saito et al[25] | 2010 | Japan | Full paper | 373 | 228 | - | 64 ± 4 | 145 | - | 64 ± 11 |
| Kim et al[26] | 2009 | South Korea | Abstract | 121 | 76 | - | - | 45 | - | - |
| Tamegai et al[19] | 2007 | Japan | Full paper | 103 | 32 | - | - | 71 | 54 | 63.4 |
| Ref. | Lesion size [mean ± SD (range) mm] | Operating time [mean or median ± SD (range) min] | Lesion location (EMR: ESD cases) | Lesion type (EMR:ESD cases) | ||||||||||
| EMR | ESD | EMR | ESD | Left colon | Right colon | Rectum | Sessile | Depressed | Protruding | LST-G | LST-NG | LST-F | Recurrence | |
| Tajika et al[22] | 25.5 ± 6.8 (20-55) | 31.6 ± 9 (20-54) | 29.4 ± 26.1 (3-115) | 87.2 ± 49.7 (19-256) | 41:13 | 35:41 | 28:31 | 0:1 | 68:10 | 28:33 | 7:38 | 1:3 | ||
| Lee et al[23] | 21.7 ± 3.5 (20-40) | 28.9 ± 12.7 (20-145) | - | 54.73 ± 40.9 (6-321) | 41:82 | 82:172 | 0.75 | 36:73 | 49:129 | 55:112 | ||||
| Kobayashi et al[24] | 25 ± 9 | 27.1 ± 10.1 | 11 (2-280) | 140 (45-400) | 26:14 | 15:6 | 15:8 | 12:0 | 22:6 | 22:20 | 0:6 | |||
| Saito et al[25] | 28 ± 8 (20-95) | 37 ± 14 (20-140) | 29 ± 25 (3-120) | 108 ± 7 (15-360) | 52:28 | 89:44 | 110:73 | 80:5 | 0:2 | 114:62 | 34:71 | |||
| Kim et al[26] | - | - | - | - | - | - | - | 28:48 | 6:16 | 22:2 | ||||
| Tamegai et al[19] | 28.7 (20-60) | 32.1 (13-75) | - | 61.1 (7-164) | -:28 | -:26 | -:17 | 0:2 | 12:19 | |||||
| Ref. | En-bloc resection rate (%) | Piecemeal resection rate (%) | R0 lesion margins (%) | Endoscopic completeness rate (%) | Bleeding rate EMR:ESD (%) | Perforation rate EMR:ESD (%) | Total complication rate (%) | Surgery post EMR/ESD (EMR:ESD cases) | |||||
| EMR | ESD | EMR | ESD | EMR | ESD | EMR | ESD | Due to perfor-ation | Due to deep invasion | ||||
| Tajika et al[22] | 48.1 | 83.5 | 52.9 | 16.5 | 39.4 | 83.5 | 97 | 98.8 | 2.9:2.4 | 0:5.9 | 2.9:8.2 | 0:3 | 0 |
| Lee et al[23] | 42.9 | 92.7 | 57.1 | 7.3 | 32.9 | 87.6 | 99.1 | 90.8 | 0:0.6 | 0:8 | 5.7:11.5 | 0:2 | 9:26 |
| Kobayashi et al[24] | 37.5 | 92.9 | 62.5 | 7.1 | - | - | 98.2 | 100 | 1.8:7.1 | 0:10.7 | 1.8:17.9 | 0 | 0 |
| Saito et al[25] | 33 | 84 | 67 | 16 | - | - | 98.7 | 100 | 3.1:1.4 | 1.3:6.2 | 4.4:7.6 | 0 | 0 |
| Kim et al[26] | 72.4 | 80 | 27.6 | 20 | - | - | 100 | 100 | - | - | 3.9:6.7 | - | - |
| Tamegai et al[19] | 0 | 98.6 | 100 | 1.4 | - | 95.6 | 100 | 90.1 | -:0 | -:1.4 | -:1.4 | - | -:7 |
| Ref. | Follow-up time (mean or median ± SD, range) (mo) | Recurrence rate (%) | Piecemeal resection rate of recurrent lesions (%) | Recurrent lesion histology (EMR:ESD cases) | Treatment of recurrent lesion (EMR:ESD cases) | ||||||||
| EMR | ESD | EMR | ESD | EMR | ESD | Adenoma | Non-inv cancer | Sm1 | Invasive cancer | APC | EMR | Surgery | |
| Tajika et al[22] | 53.8 ± 44.6 (3-191 | 14.3 ± 13.4 (3-53) | 15.4 | 1.2 | 94 | 100 | 13/16:0 | 3/16:0 | 0:1/1 | 0:0 | 7/16:0 | 8/16:0 | 1/16:1/1 |
| Lee et al[23] | 26 (IQ range 13-41) | 17 (IQ range 10-23) | 25.7 | 0.8 | 90 | 50 | -:2/2 (serrated) | - | - | - | 0:0 | 28/29:2/2 | 1/29:0 |
| Kobayashi et al[24] | 38 (2.8-112.5) | 19.9 (6.4-43.9) | 21.4 | 0 | 92 | n/a | 8/12:0 | 3/12:0 | 0 | 1/12:0 | 0:0 | 11/12:0 | 1/12:0 |
| Saito et al[25] | 26 ± 17 (6-68) | 20 ± 13 (6-61) | 14 | 2 | 94 | 100 | -:3/3 | - | - | 2/33:0 | 0:0 | 30/33: | 3/33:0 |
| 3/33 | |||||||||||||
| Kim et al[26] | 12 (6-12) | 12 (6-12) | 11.8 | 4.8 | - | 0 | - | 1/1:0 | 0 | 0:0 | - | - | - |
| Tamegai et al[19] | 19.2 (3-34) | 12.2 (3-34) | 6.3 | 0 | 100 | - | - | - | - | - | -:0 | 2/2:0 | 0:0 |
En-bloc resection rate was defined as the removal of a lesion in one piece as observed endoscopically. Piecemeal resection was defined as the removal of a lesion in more than one piece as observed endoscopically. Once removed, resected specimens are evaluated histologically. Specimens with clear lateral and basal margins of tumour were defined as an R0 resection, incomplete (R1) resection was defined as a positive lateral or basal margin for tumour and Rx resection where the margins of the specimen could not be evaluated due to piecemeal resection or as a result of thermal injury during resection. Endoscopic clearance rates were defined as complete endoscopic removal of a lesion en-bloc or piecemeal and at one or more procedures.
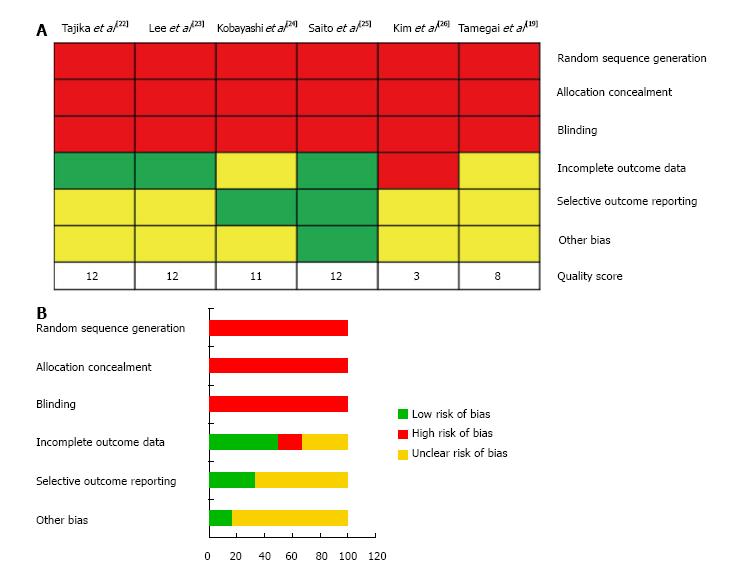
The studies were assessed using the risk of bias tool from the Cochrane Collaboration[27] (Figure 2). The risk of bias assessment domains examined were: (1) adequate sequence generation, determining if the allocation sequence generated by a computer or random numbers was adequate; (2) allocation concealment, determining if the participants and investigators enrolling the patients could foresee the study treatment arms during allocation; (3) blinding, which assessed if the study personnel, participants and assessors had knowledge of the allocation interventions during the study; (4) data reporting, determining if incomplete outcome data were adequately addressed; (5) selective outcome reporting, which is if the study protocols, primary outcomes and analysis methods are reported; and (6) other potential risks to study validity such as a potential source of bias related to the study design, or that the study was prematurely stopped due to a data-dependent process or fraudulent claims.
The quality of included studies was assessed using a modified Newcastle-Ottawa scale (Table 5). The quality domains examined were (1) patient selection; (2) intergroup comparability; and (3) outcome assessment using a star based system (maximum 3, 10 and 2 stars, respectively, total /15). The scoring was independently assessed by two authors (Patel N and Alexander J), with 100% inter-rater agreement (Figure 2).
| Quality Checklist | |
| Selection | |
| 1 | Assignment for treatment-any criteria reported (if yes, 1-star)? |
| 2 | How representative was the reference group (EMR group) in comparison to the general population for colorectal lesions? (If yes, 1-star, no stars if the patients were selected or selection of group was not described) |
| 3 | How representative was the treatment group (ESD group) in comparison to the general population for colorectal lesions? (If drawn from the same community as the reference group, 1-star, no stars if drawn from a different source or selection of group was not described) |
| Comparability | |
| Comparability variables | (1) Age; (2) gender; (3) lesion size; (4) LST; (5) lesion location; (6) LGD; (7) HGD; (8) submucosal tumor; (9)non-invasive cancer; (10) cancer |
| 4 | Groups comparable for 1, 2, 3, 4, 5 (If yes, 1-star was assigned for each of these. No star was assigned if the two groups differed) |
| 5 | Groups comparable for 6, 7, 8, 9, 10 (If yes, 1-star was assigned for each of these. No star was assigned if the two groups differed) |
| Outcome assessment | |
| 6 | Clearly defined outcome of interest (if yes, 1-star) |
| 7 | Follow-up (1-star if described) |
Proportion difference between EMR and ESD outcomes and calculated risk differences were calculated and pooled through DerSimonian and Laird random-effects modelling[27]. This considered both between-study and within-study variances which contributed to study weighting. Pooled values and 95%CIs were computed and represented on funnel plots. Statistical heterogeneity was determined by the I2 statistic; where < 30% is low, 30%-60% is moderate and > 60% is high. Analyses were performed using Stata version 12 (StataCorp LP, College Station, TX, United States).
The literature search identified 677 potential studies (Figure 1). The majority of these were excluded as they reported outcomes from animal studies, the use of new tools or a hybrid technique. Of the 57 studies that were assessed in full text for eligibility, 51 were excluded for the following reasons: No data on all primary outcome measures, no clearly defined follow up period, repeated published data and upper gastrointestinal endoscopic therapy. The final analysis included six studies published from 2007 to 2012 reporting 1324 lesions subjected to analysis, of which 688 were in the ESD group and 636 in the EMR group. Adequate demographic data was reported in three studies[22-24], 59% of patients were men and 41% were women. The mean age was 62.5 years in the EMR group, and 61.9 years in the ESD group (Table 1).
Mean procedure times were reported in four studies[19,22,23,25] (Table 2). The overall mean time was 29 min (range 2-280) for EMR[22,25] and 73 min (range 6-400) for ESD[19,22,23,25]. The mean follow up period was 29.7 mo in the EMR group and 15.9 in the ESD group, as reported in 4 studies[19,22,25,26] (Table 4).
Five studies reported data on the size of lesions[19,22-25] (Table 2). The mean size of lesion was 25.7 mm (range 20-95 mm) in the EMR group and 31.4 mm (range 13-145 mm) in the ESD group. The location of lesions was reported in three studies[22-24] shown in Table 2. In the EMR group, 44% lesions were in the right colon, 36% lesions were in the left colon and 20% were in the rectum. In the ESD group, 51% lesions were in the right colon, 26% were in the left colon and 23% in the rectum. Data on lesion type was available for 93% of all lesion outcomes reported (Table 2). The majority of procedures were carried out on lateral spreading tumour (LST) (365/574 treated by EMR and 535/656 by ESD). In the EMR group, 66% were the granular type (LST-G) and 23% were non-granular (LST-NG). In the ESD group, 52% were LST-G and 48% LST-NG. EMR was performed in a greater number of sessile lesions (20% EMR, 12% ESD) and protruding lesions (16% EMR, 4% ESD). ESD was performed in a greater number of patients with depressed or recurrent lesions (0.2% EMR, 2% ESD).
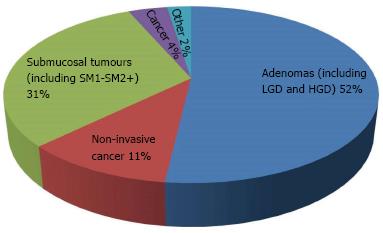
Histologically, 52% of lesions were adenomas (including low grade and high grade dysplasia). Eleven percent of lesions were described as non-invasive mucosal cancers and 4% as cancers. Submucosal tumours (SM1 and SM2+) made up 31% of the lesions resected (Figure 3).
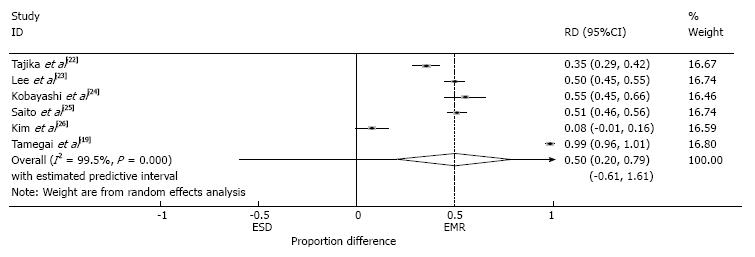
The en-bloc resection rate was reported in all studies (Table 3). This demonstrated a 50% higher en-bloc resection rate in the ESD than the EMR group (95%CI: 0.17-0.83, P < 0.0001, I2 = 99.7 %) (Figure 4).
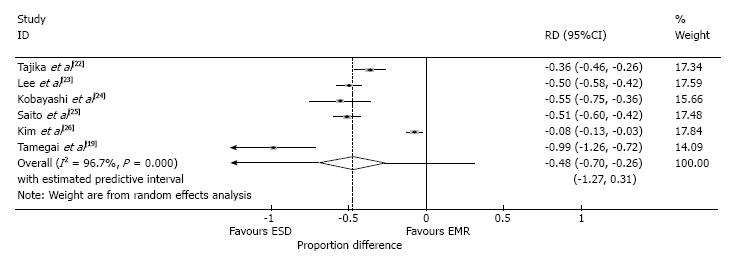
The piecemeal resection rate was also reported in all six studies (Table 3). The rate of piecemeal resection was 48% higher in the EMR group than in the ESD group (95%CI: -0.70-0.26, P < 0.0001, I2 = 96.7%) (Figure 5).
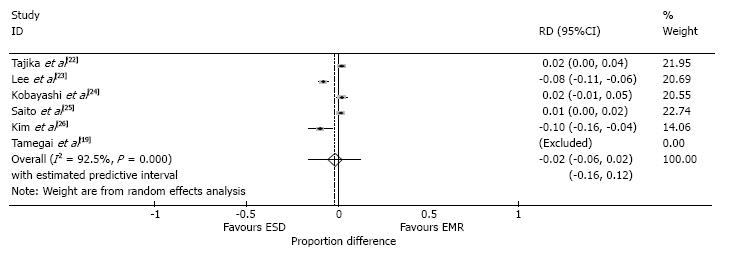
The endoscopic clearance rate was reported in all studies (Table 3). This demonstrated a marginal but significant, 2% higher rate in the ESD group compared to the EMR group (95%CI: -0.06-0.02, P < 0.0001, I2 = 92.5%) (Figure 6).
The R0 rates were reported in both groups in two studies[22,23] and only the ESD group from Tamegai et al[19]. The average R0 rate for the EMR group was 36.2% and 88.9% in the ESD group.
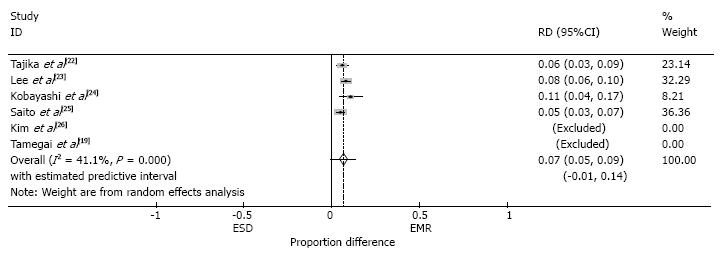
The total reported complication rate, including perforation, bleeding and coagulation syndrome, was 3.9% in the EMR group and 9.2% in the ESD group. The perforation rate for both EMR and ESD was reported in four of the six studies (Tamegai et al[19] only reported perforation rate for ESD). The perforation rate was 7% higher in the ESD group than the EMR group (95%CI: 0.05-0.09, P > 0.05, I2 = 41.1%) (Figure 7). Five patients required surgery due to perforation in the ESD group, compared to none in the EMR group (Table 3).
The recurrence rate was reported in all studies (Table 4). In cases that were followed up, the rate of recurrence was 50% higher in the EMR group than in the ESD group (95%CI: 0.20-0.79, P < 0.001, I2 = 99.5%) (Figure 8). The resected margins were reported in Tajika et al[22]. In the EMR group 7/16 cases had R1 margins and 9/16 Rx margins. In the ESD group, 41/56 cases were R0, 6/56 R1 and 9/56 cases Rx. All studies except Kim et al[26] reported the piecemeal rate in the recurrence groups. 92% (85/92) of cases in the EMR group and 71% (5/7) of cases in the ESD group had been removed by piecemeal. The recurrent lesions in both groups were mainly adenomas (21/32 recurrent EMR cases and 5/6 ESD cases (data not available from Kim et al[26] in the ESD group). There were three invasive cancers reported as recurrent lesions in the EMR group and none in the ESD group. Seventy nine of the recurrent EMR cases were successfully treated with repeat EMR procedures, seven cases with argon photocoagulation and six required surgery (a portion of this group had multiple previous attempts at EMR before technical difficulties or invasive carcinoma were found at a later date) (data not available from Kim et al[26]). In the ESD group, 5 recurrent cases were successfully treated with EMR and one with surgery[22] (Table 4).
All of the included trials had a high risk or unclear risk of bias in one or more of the assessed domains (Figure 2). Random sequence generation, allocation concealment and blinding were the main potential risks of bias in studies included in this meta-analysis. The overall quality scores are shown in Figure 2. Four studies received score of ≥ 10 and were hence deemed to be of relative high quality. These studies were analysed as a sub-group to determine the source of heterogeneity (Table 6). There was no substantial change in heterogeneity when en-bloc resection rate, endoscopic completeness rate and recurrence rates were re-analysed. Piecemeal resection rates however demonstrated a reduction from significant to moderate heterogeneity though effect sizes remained similar throughout. All studies adequately matched both EMR and ESD groups for comparability and outcome assessment.
This is one of the first meta-analyses comparing the outcomes of colorectal ESD and EMR. The pooled outcome results of this meta-analysis (from non-comparative studies) suggest that there may be a perceptible difference in the clinical outcomes colorectal of ESD and EMR. The results for ESD demonstrated higher en-bloc resection rates, endoscopic clearance rates and lower recurrence rates, albeit with higher pooled outcome complication rates. However, any inferences regarding clinical superiority should be taken with caution, as these results do not derive from comparative studies and demonstrate high heterogeneity throughout.
Although EMR is an established technique, it is usually performed for smaller lesions or larger lesions in piecemeal (associated with higher recurrence rates). Piecemeal resection involving multiple smaller resections often makes the endoscopic field difficult to detect residual tissue due to electrocautery burns, blood and local trauma. Further therapeutic procedures may therefore be required with cost, time and increased complication rate implications. In comparison, creating a mucosal incision around the lesion during ESD means that the endoscopic resection margins have already been delineated minimising disruption of the endoscopic field during submucosal dissection.
ESD appears advantageous as it allows accurate histopathological assessment of the resected lesion and resected margins, associated with fewer reported recurrences or residual disease. However, colorectal ESD is technically complex requiring more highly skilled endoscopists compared to upper gastrointestinal ESD. Compared to EMR, the procedure times are longer, more demanding and have higher complication rates.
There are endoscopic tools which have been developed or are in development designed to facilitate ESD and further improve clinical effectiveness, long-term outcomes and safety. For example, hydrodissection in the submucosal plane can be performed using the HybridKnife (ERBE)[28] and a hybrid ESD approach using a snare has also been introduced.
ESD has been shown to result in significantly lower recurrence rates compared to EMR. This may result from greater en-bloc resection rates, lower piecemeal rates and, in the studies that reported the resected margins, a higher R0 rate. However, ESD is more time consuming and associated with significantly greater complication rates. Safety of the technique is an important consideration, particularly if the uptake of ESD is to increase. There are technical difficulties of performing ESD in the colonic environment which is thin-walled containing flexures and folds. However, it will be interesting to monitor the uptake and outcomes in countries other than East Asia such as the Western world where, although the incidence of colorectal cancer is higher, upper gastrointestinal ESD is an infrequent occurrence. In these countries the learning curve is likely to be greater as a result of difficulties with training opportunities resulting from a lack of clinical cases, experience and skilled tutors.
Trans-anal endomicroscopy allows full-thickness resection of rectal lesions with accurate staging albeit with a higher complication rate compared to endoscopic therapy. In addition, conventional rectal surgery is more invasive with the risk of stoma formation and problems with incontinence resulting in a drive for a favourable minimally invasive endoscopic approach. However, differences between rectal and colonic lesion endotherapy outcomes have been reported[29]. This is multifactorial with anatomical and vascular differences between the two sites. The rectum is the first place to start training endoscopists in ESD because it is easily accessible compared to other parts of the colon[30]. Furthermore, rectal insufflation creates a neat and stable workspace to perform ESD compared to a mobile, narrow colon with folds or flexures to consider. Significantly higher recurrence rates have been reported in patients with high-risk submucosal rectal cancers treated with endoscopic therapy compared to colonic lesions[29]. Further analysis of endoscopic therapy comparing these two lesion locations is required to determine whether or not definite surgical measures with lymph node dissection rather than ESD for these higher risk patients is a better longer-term treatment plan. To improve the quality of analysis of colorectal ESD outcomes, prospective randomised controlled trials with appropriate follow-up periods which also accommodate for learning curve effects and include quality of life data are required to validate the technique in the lower gastrointestinal tract.
There are a number of limitations to this analysis which derive from significant clinical and statistical heterogeneity throughout. The significant statistical heterogeneity demonstrated suggests there is a risk the included studies were clinically heterogenous. This may result in the effect size difference being a secondary finding or a high risk for bias finding. The four high quality studies were also studied as a subgroup to determine if the heterogeneity decreases[22-25]. This only decreased from significant to moderate for piecemeal resection and effect sizes remained similar throughout. The quality scores of many of the included studies was moderate, there are few studies directly comparing the outcomes of colorectal ESD and EMR and no randomised controlled trials in the literature to date. The eligibility criteria are often unclear for both techniques, lesions had differing characteristics and size and all of the included studies were retrospective case-control studies or observational studies.
In addition, all the included studies originated from East Asia (Japan and South Korea) where there are a larger number of endoscopists familiar with the technique and hence this may cause bias. In a number of studies the time periods during which EMR and ESD were carried out were different reflecting a change in practice with the introduction of ESD[19,22,23]. The outcomes of the studies may have hence been subject to bias with improvements in endoscopy technique and introduction of ESD tools and devices to facilitate the procedure reflected in the significant heterogeneity of the resulting outcomes. The effect size may also have been affected by the learning curve effect. Five out of the six studies scored poorly for the quality of patient selection, particularly how representative the groups were. The selection of the groups was not described adequately in these studies and may be reflected by the significant heterogeneity of the results.
Follow-up periods also differed in these studies and as a result lead-time and selection biases may have also occurred. Follow-up in some studies was difficult as the procedures were often carried out at tertiary referral centres with follow-up at local hospitals where the outcome data were not reported[24,25].
In conclusion, Whilst ESD for early non-metastatic gastric cancer is now the treatment of choice in East Asia and is gaining popularity worldwide, colorectal ESD is still at a relatively early stage. The adoption of the technique in the West is particularly important given the significantly higher incidence and is another step towards the scarless surgery goal. The colonic environment is more challenging than the upper gastrointestinal tract and there is a learning curve to the technique. However, en-bloc resection has significantly more favourable mid-term outcomes compared to EMR. This is in addition to the benefits of not performing a surgical procedure in terms of recovery, cost and complications.
This meta-analysis reports on mid-term follow-up outcomes. In order to better identify the differences in outcome between these two modalities, case-matched prospective and randomised studies should be carried out with protracted follow-up periods to ascertain longer-term outcomes. The trade-off between safety and risk of perforation also needs to be established, patient selection and analysis of ESD and EMR colorectal registry data will be useful to establish this through more robust data in the future.
The authors are grateful to the Departments of Surgery and Cancer and Gastroenterology at Imperial College London for their discussion regarding this meta-analysis and support during the data collection and writing of this article.
Minimally invasive endosurgical techniques such as endoscopic submucosal dissection (ESD) are gaining popularity worldwide as an alternative to conventional surgery. Whilst ESD for early non-metastatic gastric cancer is the treatment modality of choice in East Asia, the uptake of the technique in the Western world has been slow. This is in part due to the appropriate case load and also due to the high complexity of the technique. Colorectal cancer and polyps are highly prevalent in the Western world and hence endoscopic submucosal dissection should be explored and compared to current endoscopic therapy.
A meta-analysis was used to evaluate the mid-term outcomes of colorectal ESD and endoscopic mucosal resection (EMR).
This is one of the first detailed meta-analysis evaluating immediate and mid-term outcomes for colorectal ESD and EMR. Most of the literature to date report immediate outcomes after endoscopic therapy, there is no longer-term outcome data and little mid-term outcome data reported.
This meta-analysis showed that colorectal ESD demonstrates higher en-bloc resection rates and lower recurrence rates compared to colorectal EMR. Although the complication rates are higher with a significantly increased perforation rate, ESD obviates the need for surgery and reduces the need for further endoscopic procedures. Differences in outcomes may benefit from increased assessment through well-designed comparative studies.
This is a good meta-analysis, suitable for publication. This meta-analyses study reports the comparison between EMR and ESD for colorectal lesions. Although this kind of meta-analyses is not the first report, this is still useful to compare both methods for colorectal tumours.
P- Reviewer: Kiriyama S, Kopacova M, Shibata T S- Editor: Gong ZM L- Editor: A E- Editor: Lu YJ
| 1. | Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013; Available from: http://globocan.iarc.fr. |
| 2. | Kudo S. Endoscopic mucosal resection of flat and depressed types of early colorectal cancer. Endoscopy. 1993;25:455-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 594] [Cited by in RCA: 556] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 3. | Tanaka S, Oka S, Chayama K, Kawashima K. Knack and practical technique of colonoscopic treatment focused on endoscopic mucosal resection using snare. Dig Endosc. 2009;21 Suppl 1:S38-S42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Hurlstone DP, Cross SS, Brown S, Sanders DS, Lobo AJ. A prospective evaluation of high-magnification chromoscopic colonoscopy in predicting completeness of EMR. Gastrointest Endosc. 2004;59:642-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Saito Y, Uraoka T, Matsuda T, Emura F, Ikehara H, Mashimo Y, Kikuchi T, Fu KI, Sano Y, Saito D. Endoscopic treatment of large superficial colorectal tumors: a case series of 200 endoscopic submucosal dissections (with video). Gastrointest Endosc. 2007;66:966-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 291] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 6. | Conio M, Repici A, Demarquay JF, Blanchi S, Dumas R, Filiberti R. EMR of large sessile colorectal polyps. Gastrointest Endosc. 2004;60:234-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 163] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 7. | Fukami N, Lee JH. Endoscopic treatment of large sessile and flat colorectal lesions. Curr Opin Gastroenterol. 2006;22:54-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 8. | Min BH, Lee JH, Kim JJ, Shim SG, Chang DK, Kim YH, Rhee PL, Kim KM, Park CK, Rhee JC. Clinical outcomes of endoscopic submucosal dissection (ESD) for treating early gastric cancer: comparison with endoscopic mucosal resection after circumferential precutting (EMR-P). Dig Liver Dis. 2009;41:201-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 9. | Ono H, Kondo H, Gotoda T, Shirao K, Yamaguchi H, Saito D, Hosokawa K, Shimoda T, Yoshida S. Endoscopic mucosal resection for treatment of early gastric cancer. Gut. 2001;48:225-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1134] [Cited by in RCA: 1149] [Article Influence: 47.9] [Reference Citation Analysis (4)] |
| 10. | Ono H. Endoscopic submucosal dissection for early gastric cancer. Chin J Dig Dis. 2005;6:119-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Fujishiro M, Yahagi N, Kakushima N, Kodashima S, Muraki Y, Ono S, Yamamichi N, Tateishi A, Oka M, Ogura K. Outcomes of endoscopic submucosal dissection for colorectal epithelial neoplasms in 200 consecutive cases. Clin Gastroenterol Hepatol. 2007;5:678-683; quiz 645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 275] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 12. | Yamamoto H, Kawata H, Sunada K, Sasaki A, Nakazawa K, Miyata T, Sekine Y, Yano T, Satoh K, Ido K. Successful en-bloc resection of large superficial tumors in the stomach and colon using sodium hyaluronate and small-caliber-tip transparent hood. Endoscopy. 2003;35:690-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 298] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 13. | Sano Y, Machida H, Fu KI, Ito H, Fuji T. Endoscopic mucosal resection and submucosal dissection method for large colorectal tumours. Dig Endosc. 2004;16:S93-S96. [RCA] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Kakushima N, Fujishiro M. Endoscopic submucosal dissection for gastrointestinal neoplasms. World J Gastroenterol. 2008;14:2962-2967. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 114] [Cited by in RCA: 127] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 15. | Yahagi N, Fujishiro M, Kakushima N, Kobayashi K, Hashimoto T, Oka M, Iguchi M, Enomoto S, Ichinose M, Niwa H. Endoscopic submucosal dissection for early gastric cancer using tip of an electrosurgical snare (thin type). Dig Endosc. 2004;16:34-38. [RCA] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 175] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 16. | Gotoda T, Yamamoto H, Soetikno RM. Endoscopic submucosal dissection of early gastric cancer. J Gastroenterol. 2006;41:929-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 507] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 17. | Yoshida N, Wakabayashi N, Kanemasa K, Sumida Y, Hasegawa D, Inoue K, Morimoto Y, Kashiwa A, Konishi H, Yagi N. Endoscopic submucosal dissection for colorectal tumors: technical difficulties and rate of perforation. Endoscopy. 2009;41:758-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 86] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 18. | Toyanaga T, Man-I M, Ivanov D, Sanuki T, Morita Y, Kutsumi H, Inokuchi H, Azuma T. The results and limitations of endoscopic submucosal dissection for colorectal tumors. Acta Chir Iugosl. 2008;55:17-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Tamegai Y, Saito Y, Masaki N, Hinohara C, Oshima T, Kogure E, Liu Y, Uemura N, Saito K. Endoscopic submucosal dissection: a safe technique for colorectal tumors. Endoscopy. 2007;39:418-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 201] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 20. | Uraoka T, Kato J, Ishikawa S, Harada K, Kuriyama M, Takemoto K, Kawahara Y, Saito Y, Okada H. Thin endoscope-assisted endoscopic submucosal dissection for large colorectal tumors (with videos). Gastrointest Endosc. 2007;66:836-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 75] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | Saito Y, Mashimo Y, Kikuchi T, Ikehara H, Uraoka T, Matsuda T, Fukuzawa M, Saito D. Endoscopic Submucosal Dissection Resulted in Higher En-Bloc Resection Rates and Reduced Lower Recurrence for LSTS 20 mm Compared to Conventional EMR. Gastrointest Endosc. 2007;65:AB273. [DOI] [Full Text] |
| 22. | Tajika M, Niwa Y, Bhatia V, Kondo S, Tanaka T, Mizuno N, Hara K, Hijioka S, Imaoka H, Ogura T. Comparison of endoscopic submucosal dissection and endoscopic mucosal resection for large colorectal tumors. Eur J Gastroenterol Hepatol. 2011;23:1042-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 23. | Lee EJ, Lee JB, Lee SH, Youk EG. Endoscopic treatment of large colorectal tumors: comparison of endoscopic mucosal resection, endoscopic mucosal resection-precutting, and endoscopic submucosal dissection. Surg Endosc. 2012;26:2220-2230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 112] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 24. | Kobayashi N, Yoshitake N, Hirahara Y, Konishi J, Saito Y, Matsuda T, Ishikawa T, Sekiguchi R, Fujimori T. Matched case-control study comparing endoscopic submucosal dissection and endoscopic mucosal resection for colorectal tumors. J Gastroenterol Hepatol. 2012;27:728-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 25. | Saito Y, Fukuzawa M, Matsuda T, Fukunaga S, Sakamoto T, Uraoka T, Nakajima T, Ikehara H, Fu KI, Itoi T. Clinical outcome of endoscopic submucosal dissection versus endoscopic mucosal resection of large colorectal tumors as determined by curative resection. Surg Endosc. 2010;24:343-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 428] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 26. | Kim DU, Song GA, Lee SM, Kim TO, Kim GH, Heo J. Endoscopic Mucosal Resection Versus Endoscopic Submucosal Dissection According to the Sizes and the Subtypes of Laterally Spreading Tumors. Gastrointest Endosc. 2009;69:AB282. [DOI] [Full Text] |
| 27. | DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26739] [Cited by in RCA: 30416] [Article Influence: 779.9] [Reference Citation Analysis (0)] |
| 28. | Schumacher B, Charton JP, Nordmann T, Vieth M, Enderle M, Neuhaus H. Endoscopic submucosal dissection of early gastric neoplasia with a water jet-assisted knife: a Western, single-center experience. Gastrointest Endosc. 2012;75:1166-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 29. | Ikematsu H, Yoda Y, Matsuda T, Yamaguchi Y, Hotta K, Kobayashi N, Fujii T, Oono Y, Sakamoto T, Nakajima T. Long-term outcomes after resection for submucosal invasive colorectal cancers. Gastroenterology. 2013;144:551-559; quiz e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 202] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 30. | Iacopini F, Bella A, Costamagna G, Gotoda T, Saito Y, Elisei W, Grossi C, Rigato P, Scozzarro A. Stepwise training in rectal and colonic endoscopic submucosal dissection with differentiated learning curves. Gastrointest Endosc. 2012;76:1188-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |