Published online Feb 26, 2016. doi: 10.13105/wjma.v4.i1.1
Peer-review started: September 3, 2015
First decision: November 13, 2015
Revised: December 1, 2015
Accepted: January 29, 2016
Article in press: January 31, 2016
Published online: February 26, 2016
Processing time: 168 Days and 3.8 Hours
AIM: To obtain an accurate evaluation of the association between high expression of epithelial cellular adhesion molecule (EpCAM) and gastric cancer (GC) risk.
METHODS: Studies that had examined the association between high expression of EpCAM and GC risk were identified by searching electronic databases PubMed, EMBASE, Cochrane library and Chinese Biomedical Literature database. Risk ratios (RRs) together with their 95%CIs were used to assess the association between high expression of EpCAM and GC risk. We selected eligible studies based on inclusion criteria. RevMan 5.3 software was used to calculate the pooled values.
RESULTS: A total of 14 studies were included in this meta-analysis. EpCAM-positive cases were significantly associated with tumor size (RR: 1.68, 95%CI: 1.47-1.91, P < 0.00001 fixed-effect), depth of invasion (RR: 1.37, 95%CI: 1.11-1.68, P = 0.003 random-effect), TNM stage (RR: 2.02, 95%CI: 1.35-3.02, P = 0.0007 random-effect), tumor location (RR: 0.80, 95%CI: 0.71-0.91, P = 0.0007 fixed-effect), histologic differentiation (RR: 1.23, 95%CI: 1.13-1.33, P < 0.00001 fixed-effect) and lymph node metastasis (RR: 1.89, 95%CI: 1.28-2.80, P = 0.001 random-effect). However, we did not observe any significant association between the presence of EpCAM with age, gender, distant metastasis, Borrmann type or Lauren classification. Additionally, EpCAM expression was not associated with the overall survival rate. The pooled HR of the overall effect was 1.39 (95%CI: 0.30-6.48, P = 0.67 random-effect).
CONCLUSION: Our meta-analysis indicates that EpCAM contributes to GC risk, which acts as a prognostic factor and a marker of poor outcome.
Core tip: This meta-analysis aimed to obtain an accurate evaluation of the association between high expression of epithelial cellular adhesion molecule (EpCAM) and gastric cancer (GC) risk. EpCAM-positive cases were significantly associated with tumor size, depth of invasion, TNM stage, tumor location, histologic differentiation and lymph node metastasis. EpCAM contributed to GC risk, and acted as a prognostic factor and a marker of poor outcome.
- Citation: Xiao YB, Xi HQ, Li JY, Chen L. Expression of epithelial cellular adhesion molecule in gastric cancer: A meta-analysis. World J Meta-Anal 2016; 4(1): 1-9
- URL: https://www.wjgnet.com/2308-3840/full/v4/i1/1.htm
- DOI: https://dx.doi.org/10.13105/wjma.v4.i1.1
Although gastric cancer (GC) rates have decreased substantially in the past few decades, it remains the second most common cause of cancer-related death worldwide[1]. Patients with GC have a poor prognosis, especially those with advanced stage disease. The most common cause of this phenomenon is the advanced stage of most cases at the time of initial diagnosis. Additionally, tumor cell spread has occurred in some cases[1,2]. Currently, surgery is the primary treatment strategy for localized advanced GC, with an average 5-year survival rate of 20%-30%; however, for unresectable disease such as metastatic or recurrent GC, chemotherapy is regarded as a basic therapeutic approach[1].
The efficacy of current chemotherapeutic agents is still unsatisfactory and these agents with poor specificity have significant side effects. Consequently, multimodality therapy options are needed to improve the prognosis of GC. This necessitates finding new adjuvant therapeutic targets and prognostic markers for GC patients.
The epithelial cellular adhesion molecule (EpCAM) is a 37-42 KDa, 314-amino-acid type I transmembrane glycoprotein with two epidermal growth factor-like repeats in the external domain and two α-actin binding sites for actin cytoskeleton linkage in the intracellular domain[2]. The 9-exon gene TACSTD1, which has been mapped to chromosome 2p21, encodes it. EpCAM functions as a homotypic intracellular adhesion molecule. It is interconnected with E-cadherin during the process of epithelial cell adhesion[2,3].
EpCAM is expressed in most normal epithelial tissues on the basolateral membrane and overexpression of EpCAM has been detected in a variety of epithelial cancers[4]. EpCAM was found to be overexpressed in colon cancer tissues, breast cancer squamous cells, ovarian carcinomas and most human adenocarcinomas. Because its overexpression has effects on differentiation, cell proliferation, signaling and migration, EpCAM can be used as a marker to predict recurrence and metastasis of the tumor and influence survival of cancer patients[5].
Furthermore, EpCAM has been considered as a target antigen for a number of specific immunotherapies because of its frequent and high-level expression[6,7]. Catumaxomab, an EpCAM monoclonal antibody, has been used for the intraperitoneal treatment of malignant effusion in patients with EpCAM-positive cells since 2009. Catumaxomab also had a significant overall survival (OS) benefit in GC patients[8]. However, the role of EpCAM in GC is still unclear. Although several studies showed high expression of EpCAM in GC[6-10], which was related to cancer progression and survival prognosis, there is no comprehensive study on the correlation of EpCAM expression with survival prognosis or the effects of EpCAM expression on clinicopathologic characteristics in GC patients. Thus, this meta-analysis was conducted to determine the association between high expression of EpCAM and clinicopathological features and progression as well as prognosis of GC.
We conducted a comprehensive literature search in PubMed, EMBASE, Cochrane library and Chinese Biomedical Literature databases. There was no restriction on time period, sample size, population or languages. The search terms included “Stomach Neoplasms” OR “Gastric Neoplasms” OR “Stomach Cancer” OR “GC” OR “Stomach Carcinoma” OR “Gastric Carcinoma” AND “EpCAM” OR “epithelial cellular adhesion molecule”. The search was limited to studies in humans. All eligible studies were retrieved and their references were scanned for other relevant studies. Two reviewers (Yi-Bin Xiao and Hong-Qing Xi) independently screened titles and abstracts of all citations. When multiple articles were reported on the same or overlapping data, we selected the study that investigated the most individuals or the most recent study.
Articles were considered if: (1) they provided information on GC verified by pathological examination; (2) they provided information on case control or cohort studies that evaluated the association between EpCAM expression and GC; (3) no preoperative chemotherapy and/or radiotherapy was administered to patients; (4) they had available data for estimating risk ratio (RR) (95%CI); and (5) the control population did not contain patients with malignant tumors.
Studies were excluded if they: (1) had no control population; (2) were duplicates of an earlier publication; (3) reported insufficient data; (4) had cell or animal experiments; and (5) were letters, reviews, case reports and conference abstracts without original data or articles published in a book.
Two investigators (Yi-Bin Xiao and Hong-Qing Xi) reviewed all articles. Then the first investigator extracted the following information according to the prespecified selection criteria: (1) Publication details, including first author’s name, year of publication and publication journal; (2) Characteristics of the studied population, including country, ethnicity, number of cases and controls; and (3) Number and characteristics of different clinical and pathologic parameters of both the gastric patients and their control group, including age, gender, tumor size, depth of invasion, TNM stage, tumor location, distant metastasis, Borrmann type, Lauren classification, histologic differentiation and lymph node metastasis.
Discrepancies between the two investigators were resolved through consensus discussion.
The quality of the studies was assessed according to the Newcastle-Ottawa Scale (NOS) by two investigators (Yi-Bin Xiao and Hong-Qing Xi) independently. The scale includes three major classifications: Selection, comparability and outcome. A maximum score of 1 was graded for each item, except for comparability, where a score of 2 was allowed to be graded. Scores ranged from 0 (lowest) to 9 (highest) and studies that scored equal to or higher than 7 points were assigned as “high-quality” studies, whereas those with scores less than 7 were considered “low-quality” studies. Any disagreement was resolved through consensus discussion.
The association between EpCAM and GC risk was evaluated using hazard ratio (HR, 95%CIs) for time-to-event data (OS) and (RR, 95%CIs) for dichotomous data (various adverse events). Cochran’s χ2-based Q test and Higgins I2 statistics were used to check heterogeneity among studies. I2 lay between 0 and 10%, and a value of 0% meant no observed heterogeneity, with larger values indicating increasing heterogeneity. P < 0.05 or I2 >50% was considered statistically significant. A value of 0% indicated no observed heterogeneity, and larger values showed increasing heterogeneity, with 25% indicating low, 50% indicating moderate, and 75% indicating high heterogeneity (Higgins, Thompson, Deeks, and Altman, 2003).
We selected the fixed-effect model (the Mantel-Haenszel method) if there was no significant heterogeneity. Otherwise, we selected the random-effect model (the DerSimonian and Laird method) if heterogeneity existed and could not be explained or corrected. Begg’s funnel plots were used to examine potential publication bias in this study. For the pooled analysis of the correlation between EpCAM expression and clinicopathological features, RRs and their 95%CIs were used to assess the effect. All the statistical tests were performed using RevMan5.3 (Cochrane collaboration, Oxford, United Kingdom) software. Kaplan-Meier curves were read using an Engauge Digitizer 4.1. P < 0.05 was considered statistically significant. HRs or RRs > 1 meant a worse prognosis for GC patients with EpCAM overexpression and were considered to be statistically significant if the 95%CI did not overlap 1. In addition, sensitivity analysis was conducted by sequential omission of individual studies to evaluate the stability of the results.
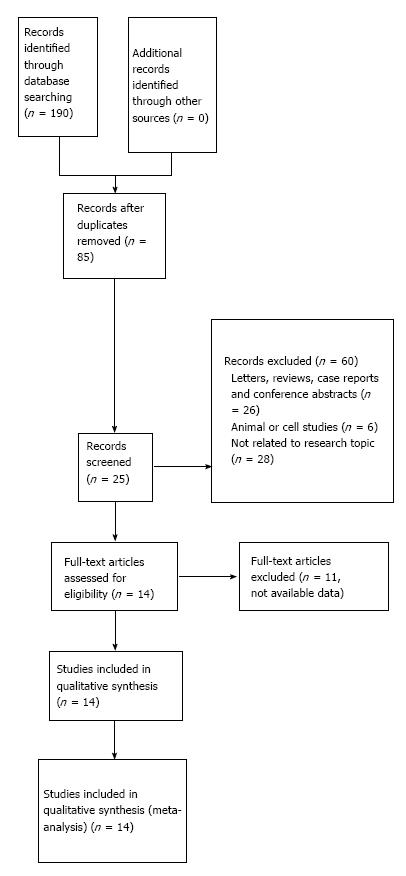
A flow diagram of the literature search is shown in Figure 1. The initial search yielded a total of 190 studies according to the search criteria. A total of 28 potential relevant studies were recruited into this meta-analysis. Of these studies, three were excluded because they contained overlapping data. Another 11 studies were excluded because they were unable to offer EpCAM-specific data for calculating HRs or RRs according to the described method. A total of 14 studies that met the inclusion and exclusion criteria were included. Three studies reported an association between EpCAM and the 5-year survival rate[7,11,12], and 13 studies[1-11,13,14] were chosen to demonstrate the connection between EpCAM expression and clinical features. As a result, we did not find any additional articles using a manual search of references cited in the published studies. The details of the articles are summarized in Tables 1 and 2.
| First author | Country | Year | Ethnics | Age (< 50:≥50) | No. of patients (male:female) | No. of patients (EpCAM+: EpCAM-) | Diagnosis of GC (Histo-, Patho-, NR) | Study quality (NOS) |
| Zhang et al | China | 2011 | Asian | 11:31 | 24:18 | 34:8 | Patho- | 8 |
| Sun et al | China | 2010 | Asian | 31:29 | 48:12 | 46:14 | Patho- | 8 |
| Fang et al | China | 2010 | Asian | 27:31 | 39:19 | 46:12 | Patho- | 8 |
| Lu et al | China | 2011 | Asian | 43:48 | 70:21 | 84:7 | Patho- | 9 |
| Yang et al | China | 2014 | Asian | 33:39 | 57:15 | 48:24 | Patho- | 9 |
| Peng et al | China | 2011 | Asian | 20:11 | 18:13 | 21:10 | Patho- | 9 |
| Yang et al | China | 2012 | Asian | 33:62 | 66:29 | 56:39 | Patho- | 8 |
| Zhang et al | China | 2014 | Asian | 17:25 | 24:18 | 37:5 | Patho- | 7 |
| Li et al | China | 2012 | Asian | NR | 311:125 | 179:257 | Patho- | 7 |
| Du et al | China | 2009 | Asian | 26:74 | 61:39 | 74:26 | Patho- | 8 |
| Went et al | Switzerland | 2006 | Caucasian | NR | 311:117 | NR | Patho- | 7 |
| Kroepil et al | Germany | 2013 | Caucasian | NR | NR | 126:37 | Patho- | 8 |
| Wang et al | China | 2013 | Asian | NR | 428:173 | 247:354 | NR | 8 |
| Songun et al | The Netherlands | 2005 | Caucasian | NR | NR | NR | Patho- | 7 |
| First author | Tumor size ( ≤5 cm:> 5 cm) | Depth of invasio-n (T1-T2: T3-T4) | TNM stage (I-II:III-IV) | Tumor location (upper:middle:lower) | Distant metastasis (yes: no) | Borrma-nn type (I:II:III:IV) | Lauren classificatio-n (intestinal:diffuse: mixed) | Histologic differentiate-on (high: moderate: low) | Lymph node metastasis (N0: N1/2/3) |
| Zhang et al | NR2 | NR | NR | NR | 23:19 | 8:12:22 | 20:22 | ||
| Sun et al | NR | 11:49 | NR | NR | NR | NR | NR | 20:20:20 | NR |
| Fang et al | NR | 17:41 | 17:41 | NR | 18:40 | NR | NR | 11:17:30 | 15:43 |
| Lu et al | 41:50 | 19:72 | 34:57 | 41:25:25 | NR | 8:12:59:11 | NR | 3:24:64 | 31:60 |
| Yang et al | 45:27 | 35:37 | 35:37 | NR | NR | NR | NR | NR | 25:47 |
| Peng et al | NR | 19:12 | 19:12 | 12:13:6 | NR | NR | NR | 15:13:3 | NR |
| Yang et al | NR | 7:88 | NR | 29:26:40 | NR | 3:12:61:19 | NR | 6:19:70 | 29:66 |
| Zhang et al | 14:28 | 13:29 | 13:29 | NR | NR | NR | NR | 16:26:0 | 11:31 |
| Li et al | 256:180 | 166:270 | 194:242 | 55:163:218 | 61:375 | NR | 223:213:0 | 141:295 | 166:270 |
| Du et al | NR | NR | NR | NR | NR | NR | 91:19 | 25:42:33 | 50:50 |
| Went et al | NR | 42:372 | NR | NR | 25:445 | NR | NR | NR | 153:316 |
| Kroepil et al | NR | 107:56 | NR | NR | 9:154 | NR | 62:61:40 | NR | 41:122 |
| Wang et al | 350:251 | 221:380 | 262:339 | 84:223:294 | 91:510 | NR | 299:302 | 17:175:409 | 220:381 |
| Songun et al1 | NR | NR | NR | NR | NR | NR | NR | NR | NR |
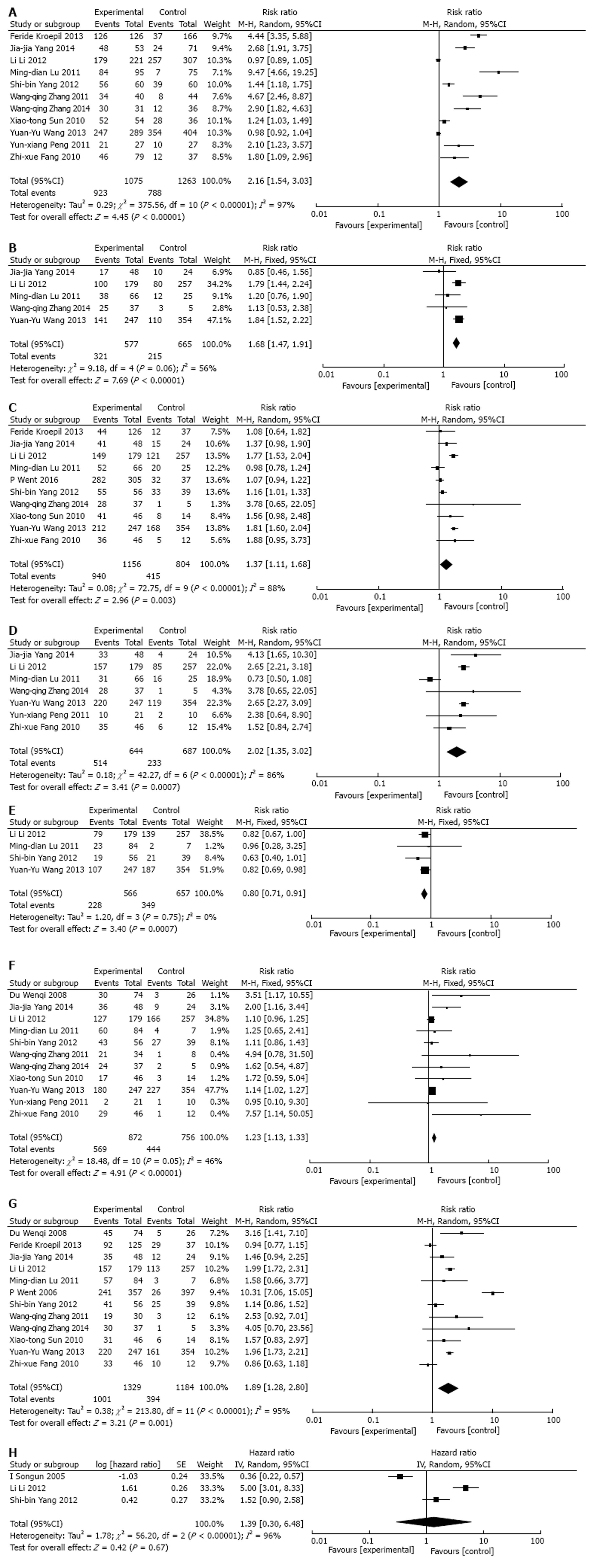
Thirteen studies reported correlations between EpCAM expression and some clinical characteristics of GC (including age, gender, tumor size, depth of invasion, TNM stage, tumor location, distant metastasis, Borrmann type, Lauren classification, histologic differentiation and lymph node metastasis). These were pooled to calculate the RRs.
In our study, the expression level of EpCAM was higher in samples of GC than in normal ones (pooled RR = 2.16, 95%CI: 1.54-3.03, P < 0.00001 random-effect) (Figure 2A). In addition, EpCAM expression was significantly associated with tumor size (pooled RR = 1.68, 95%CI: 1.47-1.91, P < 0.00001 fixed-effect) (Figure 2B), depth of invasion (pooled RR = 1.37, 95%CI: 1.11-1.68, P = 0.003 random-effect) (Figure 2C), TNM stage (pooled RR = 2.02, 95%CI: 1.35-3.02, P = 0.0007 random-effect) (Figure 2D), tumor location (pooled RR = 0.80, 95%CI: 0.71-0.91, P = 0.0007 fixed-effect) (Figure 2E), histologic differentiation (pooled RR: 1.23, 95%CI: 1.13-1.33, P < 0.00001 fixed-effect) (Figure 2F), and lymph node metastasis (pooled RR = 1.89, 95%CI: 1.28-2.80, P = 0.001 random-effect) (Figure 2G). However, EpCAM expression in GC was not associated with age (pooled RR = 1.12, 95%CI: 0.93-1.35, P = 0.24 fixed-effect), gender (pooled RR = 0.97, 95%CI: 0.91-1.04, P = 0.37 fixed-effect), distant metastasis (pooled RR = 2.25, 95%CI: 0.77-6.61, P = 0.14, random-effect), Borrmann type (pooled RR = 1.03, 95%CI: 0.89-1.19, P = 0.70 fixed-effect), or Lauren classification (pooled RR = 1.64, 95%CI: 0.75-3.60, P = 0.21 random-effect).
Meta-analysis of the association between EpCAM expression and OS was determined in three studies. The pooled RR was analyzed using previously described methods. EpCAM expression was not associated with the OS rate. The pooled HR of the overall effect was 1.39 (95%CI: 0.30-6.48, P = 0.67) in the random-effect model (Figure 2H).
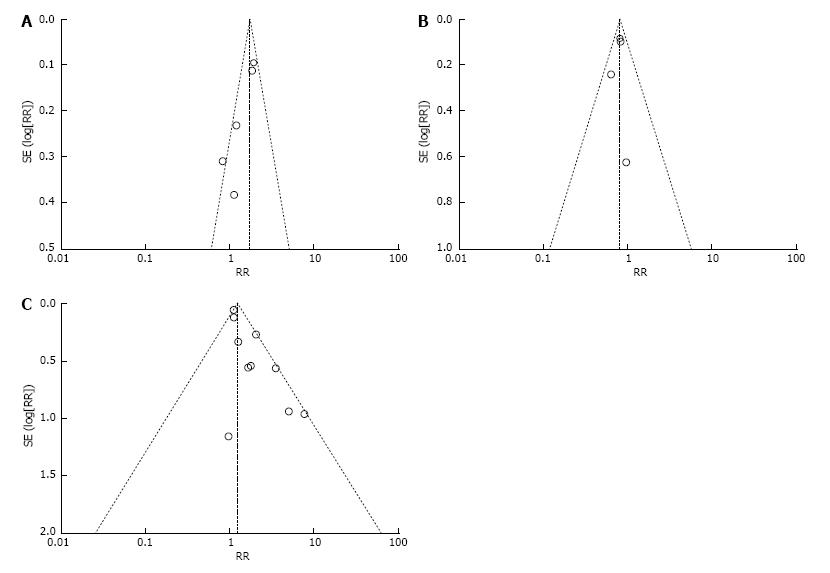
The funnel plot test recommended for meta-analyses was used to examine publication bias (Figure 3). We inspected its asymmetry visually and found that there was almost no potential for publication bias.
One included study was excluded at each time to investigate the influence of the individual data on the overall results. The pooled RR or HR estimates were recalculated for the remaining studies. The statistical significance of the overall results was not changed when any individual study was excluded, which indicates the reliability of our results.
In recent years, many cell adhesion molecules (CAMs) have proven to be responsible for tumorigenesis and metastasis[2,3]. The role of EpCAM is not only limited to cell adhesion but is also involved in other cellular processes including signaling, cell migration, proliferation and differentiation[15]. EpCAM is a potent signal transducer, which can use components of the Wnt pathway and is involved in the regulation of cell proliferation and cell cycle progression[5,13,16]. It is overexpressed in many solid cancers including esophageal, pancreatic, prostate and gastric[12,17], and it has recently been identified as a type of cancer stem cell marker[14,18,19].
Identification of a prognostic factor such as EpCAM is necessary for high-risk patients for whom specific therapy might be necessary[20,21]. However, conflicting data on the prognostic impact of EpCAM have been reported. Wenqi et al[22] reported that EpCAM was overexpressed in gastric cell lines and tumor tissues and downregulation of EpCAM resulted in a decrease in cell proliferation and suppressed tumor formation. In contrast, Songun et al[23] reported that 93% of 300 GC patients were EpCAM-positive and the loss of EpCAM expression indicated tumor aggression, especially in patients with stage I and II disease. Thus, the prognostic role of EpCAM in GC is still unclear and the association between clinical characteristics of GC patients and EpCAM expression levels needs to be further elucidated. These conflicting data were likely due to the small sample size and intratumoral heterogeneity of GC, which was observed in the studies.
This meta-analysis is the first study to systematically estimate EpCAM expression and its relationship with clinicopathological characteristics and OS rates in GC patients. We calculated pooled RRs to study the correlation of EpCAM with patient clinical characteristics. This showed that EpCAM expression was positively related with poor histological type, lymph node metastasis, high-grade of TNM stage and tumor size (> 5 cm), depth of invasion (T3-T4) and tumor location (lower part of the stomach) in GC patients. This suggests that GC patients with the above-mentioned clinical characteristics were more likely to have a poorer prognosis after the diagnosis was made.
The biological function of EpCAM may be implicated in the relationship between EpCAM expression and cancer outcome mentioned above. Recently, studies have reported that overexpression of EpCAM occurs in a variety of cancers, for example colon, breast and ovarian, and most human adenocarcinomas. Furthermore, it has effects on differentiation, proliferation and migration of cancer cells.
There are certain limitations in the present meta-analysis that need to be pointed out. First, although we tried to avoid biases in performing this meta-analysis, publication bias may have occurred because only published studies were included in the meta-analysis even if the statistical test did not show it. Second, we did not find any significant association between EpCAM expression and OS in GC patients. It is very likely that limited research has been done on EpCAM and its relationship with prognosis. Only three studies were included in the OS meta-analysis, with a relatively small sample size of 831 patients. Finally, there was heterogeneity between studies present in this article, with a P-value < 0.05, especially in the evaluation of the relationship between EpCAM expression and some adverse clinical parameters. This was related to insufficient sample size and a lack of certain original data. To adjust for this, we used a trim-and-fill method in the random-effect model to make the outcomes statistically credible.
In conclusion, this meta-analysis suggests that the expression of EpCAM is associated with poor clinicopathological features of GC. However, because of the heterogeneity of included studies and bias of meta-analysis, our conclusions need to be interpreted with caution. More clinical studies will be required to determine the association between the expression of EpCAM and GC prognosis.
Although gastric cancer (GC) rates have decreased substantially in the past few decades, it remains the second most common cause of cancer-related death worldwide. Although several studies showed high expression of epithelial cellular adhesion molecule (EpCAM) in GC, which was related to cancer progression and survival prognosis, there is no comprehensive study on the correlation of EpCAM expression with survival prognosis or the effects of EpCAM expression on clinicopathologic characteristics in GC patients.
This meta-analysis was conducted to determine the association between high expression of EpCAM and clinicopathological features and progression as well as prognosis of GC.
Studies that had examined the association between high expression of EpCAM and GC risk were identified by searching electronic databases PubMed, EMBASE, Cochrane library and Chinese Biomedical Literature database.
This meta-analysis indicates that EpCAM contributes to GC risk, which acts as a prognostic factor and a marker of poor outcome.
The authors reported the “Expression of epithelial cellular adhesion molecule in gastric cancer: A meta-analysis”. These findings are important to those with closely related research interests. It is well organized and systemically analysed.
P- Reviewer: Lee HW, Matsuo Y S- Editor: Wang JL L- Editor: A E- Editor: Lu YJ
| 1. | Chan AO, Wong BC, Lam SK. Gastric cancer: past, present and future. Can J Gastroenterol. 2001;15:469-474. [PubMed] |
| 2. | Kroepil F, Dulian A, Vallböhmer D, Geddert H, Krieg A, Vay C, Topp SA, am Esch JS, Baldus SE, Gires O. High EpCAM expression is linked to proliferation and lauren classification in gastric cancer. BMC Res Notes. 2013;6:253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Spizzo G, Fong D, Wurm M, Ensinger C, Obrist P, Hofer C, Mazzoleni G, Gastl G, Went P. EpCAM expression in primary tumour tissues and metastases: an immunohistochemical analysis. J Clin Pathol. 2011;64:415-420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 218] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 4. | Martowicz A, Spizzo G, Gastl G, Untergasser G. Phenotype-dependent effects of EpCAM expression on growth and invasion of human breast cancer cell lines. BMC Cancer. 2012;12:501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 5. | Went P, Vasei M, Bubendorf L, Terracciano L, Tornillo L, Riede U, Kononen J, Simon R, Sauter G, Baeuerle PA. Frequent high-level expression of the immunotherapeutic target Ep-CAM in colon, stomach, prostate and lung cancers. Br J Cancer. 2006;94:128-135. [PubMed] |
| 6. | Imano M, Itoh T, Satou T, Yasuda A, Nishiki K, Kato H, Shiraishi O, Peng YF, Shinkai M, Tsubaki M. High expression of epithelial cellular adhesion molecule in peritoneal metastasis of gastric cancer. Target Oncol. 2013;8:231-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Horikawa M, Iinuma H, Inoue T, Ogawa E, Fukushima R. Clinical significance of intraperitoneal CD44 mRNA levels of magnetically separated CD45-negative EpCAM-positive cells for peritoneal recurrence and prognosis in stage II and III gastric cancer patients. Oncol Rep. 2011;25:1413-1420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Du W, Ji H, Cao S, Wang L, Bai F, Liu J, Fan D. EpCAM: a potential antimetastatic target for gastric cancer. Dig Dis Sci. 2010;55:2165-2171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Warneke VS, Behrens HM, Haag J, Krüger S, Simon E, Mathiak M, Ebert MP, Röcken C. Members of the EpCAM signalling pathway are expressed in gastric cancer tissue and are correlated with patient prognosis. Br J Cancer. 2013;109:2217-2227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Heideman DA, Snijders PJ, Craanen ME, Bloemena E, Meijer CJ, Meuwissen SG, van Beusechem VW, Pinedo HM, Curiel DT, Haisma HJ. Selective gene delivery toward gastric and esophageal adenocarcinoma cells via EpCAM-targeted adenoviral vectors. Cancer Gene Ther. 2001;8:342-351. [PubMed] |
| 11. | Fong D, Seeber A, Terracciano L, Kasal A, Mazzoleni G, Lehne F, Gastl G, Spizzo G. Expression of EpCAM(MF) and EpCAM(MT) variants in human carcinomas. J Clin Pathol. 2014;67:408-414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 12. | van der Gun BT, Melchers LJ, Ruiters MH, de Leij LF, McLaughlin PM, Rots MG. EpCAM in carcinogenesis: the good, the bad or the ugly. Carcinogenesis. 2010;31:1913-1921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 250] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 13. | Imano M, Okuno K. Treatment strategies for gastric cancer patients with peritoneal metastasis. Surg Today. 2014;44:399-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Okada T, Nakamura T, Watanabe T, Onoda N, Ashida A, Okuyama R, Ito K. Coexpression of EpCAM, CD44 variant isoforms and claudin-7 in anaplastic thyroid carcinoma. PLoS One. 2014;9:e94487. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Ralhan R, Cao J, Lim T, Macmillan C, Freeman JL, Walfish PG. EpCAM nuclear localization identifies aggressive thyroid cancer and is a marker for poor prognosis. BMC Cancer. 2010;10:331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 16. | Wang YY, Li L, Zhao ZS, Wang YX, Ye ZY, Tao HQ. L1 and epithelial cell adhesion molecules associated with gastric cancer progression and prognosis in examination of specimens from 601 patients. J Exp Clin Cancer Res. 2013;32:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Balzar M, Winter MJ, de Boer CJ, Litvinov SV. The biology of the 17-1A antigen (Ep-CAM). J Mol Med (Berl). 1999;77:699-712. [PubMed] |
| 18. | Varga M, Obrist P, Schneeberger S, Mühlmann G, Felgel-Farnholz C, Fong D, Zitt M, Brunhuber T, Schäfer G, Gastl G. Overexpression of epithelial cell adhesion molecule antigen in gallbladder carcinoma is an independent marker for poor survival. Clin Cancer Res. 2004;10:3131-3136. [PubMed] |
| 19. | Soysal SD, Muenst S, Barbie T, Fleming T, Gao F, Spizzo G, Oertli D, Viehl CT, Obermann EC, Gillanders WE. EpCAM expression varies significantly and is differentially associated with prognosis in the luminal B HER2(+), basal-like, and HER2 intrinsic subtypes of breast cancer. Br J Cancer. 2013;108:1480-1487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 20. | Chaudry MA, Sales K, Ruf P, Lindhofer H, Winslet MC. EpCAM an immunotherapeutic target for gastrointestinal malignancy: current experience and future challenges. Br J Cancer. 2007;96:1013-1019. [PubMed] |
| 21. | Went PT, Lugli A, Meier S, Bundi M, Mirlacher M, Sauter G, Dirnhofer S. Frequent EpCam protein expression in human carcinomas. Hum Pathol. 2004;35:122-128. [PubMed] |
| 22. | Wenqi D, Li W, Shanshan C, Bei C, Yafei Z, Feihu B, Jie L, Daiming F. EpCAM is overexpressed in gastric cancer and its downregulation suppresses proliferation of gastric cancer. J Cancer Res Clin Oncol. 2009;135:1277-1285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 23. | Songun I, Litvinov SV, van de Velde CJ, Pals ST, Hermans J, van Krieken JH. Loss of Ep-CAM (CO17-1A) expression predicts survival in patients with gastric cancer. Br J Cancer. 2005;92:1767-1772. [PubMed] |