Published online Dec 26, 2015. doi: 10.13105/wjma.v3.i6.254
Peer-review started: June 28, 2015
First decision: September 17, 2015
Revised: October 13, 2015
Accepted: December 3, 2015
Article in press: December 4, 2015
Published online: December 26, 2015
Processing time: 181 Days and 2.8 Hours
AIM: To systematically assess risk of pancreatic adverse events with glucagon-like peptide-1 (GLP-1) receptor agonist and dipeptidyl peptidase-4 (DPP-4) inhibitor drugs.
METHODS: We searched PubMed, Embase, CINAHL, Cochrane review of clinical trials, pharmaceutical company clinical trials register, United States Food and Drug Administration website, European Medicines Agency website and ClinicalTrials.gov for randomized controlled trials from inception to October 2013. Randomized control trial studies were selected for inclusion if they reported on pancreatic complication events and/or changes in pancreatic enzyme levels (serum amylase and serum lipase) as adverse events or as serious adverse events for patients who were on GLP-1 receptor agonist and DPP-4 inhibitor drugs. Two independent reviewers extracted data directly. We performed Peto odds ratio (OR) fixed effect meta-analysis of pancreatic adverse events a, and assessed heterogeneity with the I2 statistic.
RESULTS: Sixty-eight randomized controlled trials were eligible. A total of 60720 patients were included in our analysis of the association of risk of pancreatic complication events with GLP-1 agents. A total of 89 pancreatic related adverse events occurred among the GLP-1 agents compared to 74 events among the controls. There was a statistically significant increased risk of elevation of pancreatic enzymes associated with GLP-1 agents compared with control (Peto OR = 3.15, 95%CI: 1.56-6.39, P = 0.001, I2 = 0%). There was no statistically significant difference in the risk of pancreatic adverse event associated with GLP-1 agent compared with controls (Peto OR = 1.00, 95%CI: 0.73-1.37, P = 1.00, I2 = 0%). There were a total of 71 pancreatitis events in patients on GLP-1 agents and 56 pancreatitis events occurred in the control patients. There were 36 reports of pancreatic cancer in these studies. Of these cases, 2 used linagliptin, 2 used alogliptin, 1 used vildagliptin, 7 used saxagliptin while 6 used sitagliptin. The remaining 18 cases occurred among controls.
CONCLUSION: Although GLP-1 based agents are associated with pancreatic enzyme elevation, we were unable to confirm a significant risk of pancreatitis or pancreatic cancer.
Core tip: There is conflicting data on the risk of pancreatitis with glucagon-like peptide-1 (GLP-1) receptor agonists and dipeptidyl peptidase-4 (DPP-4) inhibitors. We performed a meta-analysis of 68 randomized controlled trials of 11 different GLP-1 or DPP-4 targeted drugs. The incidence of pancreatic adverse events in the trials was generally low and we did not find any definitive evidence for pancreatitis or pancreatic cancer amongst the trials. However, we found a significantly raised risk of elevated pancreatic enzymes in a small number of trials that reported such enzyme elevations.
- Citation: Shihab HM, Akande T, Armstrong K, Singh S, Loke YK. Risk of pancreatic adverse events associated with the use of glucagon-like peptide-1 receptor agonist and dipeptidyl peptidase-4 inhibitor drugs: A systematic review and meta-analysis of randomized trials. World J Meta-Anal 2015; 3(6): 254-283
- URL: https://www.wjgnet.com/2308-3840/full/v3/i6/254.htm
- DOI: https://dx.doi.org/10.13105/wjma.v3.i6.254
Glucagon-like peptide-1 (GLP-1) is a naturally occurring gut hormone that is mainly secreted by the intestinal L cell. It is a potent antihyperglycemic hormone, inducing glucose-dependent stimulation of insulin secretion while suppressing glucagon secretion. Once in the circulation, GLP-1 has a half-life of less than 2 min, due to the rapid degradation by the enzyme dipeptidyl peptidase-4 (DPP-4). The GLP-1 based therapies include GLP-1 receptor agonists and DPP-4 inhibitors. As GLP-1 is a gut hormone, it is possible that patients may experience adverse effects on the gastrointestinal system such as nausea or abdominal pain.
There are already several GLP-1 receptor agonists and DPP-4 inhibitor drugs approved by the Food and Drug Administration (FDA) or the European Medicines Agency (EMA), and we are aware of additional agents in development. However, sitagliptin and exenatide have been shown to cause acute pancreatitis in rodent models via amplification of ductal replication and induction of acinar to ductal metaplasia[1-4]. A recent case-control study showed a significant increased risk of hospitalization for acute pancreatitis associated with the use of sitagliptin or exenatide among adult patients with type-2 diabetes mellitus[5]. A meta-analysis of clinical trials reported no difference for sitagliptin use compared with placebo or other oral hypoglycemic in the incidence rates of pancreatitis[6]. Although complications involving the pancreas (acute pancreatitis, chronic pancreatitis and pancreatic cancer) are potentially serious adverse effects of GLP-1 receptor agonist drugs, there is a paucity of data available to clinicians regarding these effects of GLP-1 receptor agonist drugs. A recent meta-analysis[7] suggested that neither exenatide nor liraglutide increases the risk for acute pancreatitis when used in the treatment of type-2 diabetes mellitus. This analysis, however, was based on small studies, non-clinical evaluation of pancreatitis in the included RCTs and residual confounding in the observational studies that were included. None of the previous studies have adequately evaluated the role of pancreatic enzyme elevations. These studies have not evaluated the occurrence of reports of pancreatic cancer in these trials. Finally, the risk of pancreatic complication associated with individual therapies has not been evaluated.
Our objective was to conduct a systematic review to ascertain the risk of pancreatic complications (acute and chronic pancreatitis and pancreatic cancer) and pancreatic enzyme elevations associated with GLP-1 based therapies, as compared to placebo or other oral hypoglycemic drugs in randomized controlled trials of GLP-1 based therapies.
We defined study aims and procedures in the study protocol registered with PROSPERO register of systematic reviews[8].
We searched MEDLINE, EMBASE, CINAHL and the Cochrane database from inception to October 2013 using the search terms: (drug name OR chemical compound OR drug class) AND [“Pancreatic Neoplasms”(Mesh) OR “Pancreatitis”(Mesh) OR “pancreas”(tiab) OR “pancreatitis”(tiab) OR “pancreatic”(tiab) OR “pancreatic cancer”(tiab) OR “serum amylase”(tiab) OR “serum lipase”(tiab) OR “Islet Cell Adenoma”(tiab) OR “Insulinoma”(tiab) OR “Islet Cell Carcinoma”(tiab) OR “Gastrinoma”(tiab) OR “Glucagonoma”(tiab) OR “Somatostatinoma”(tiab) OR “Vipoma”(tiab) OR “Pancreatic Ductal Carcinoma”(tiab) OR “Islet Cell Adenomas”(tiab) OR “Insulinomas”(tiab) OR “Islet Cell Carcinomas”(tiab) OR “Gastrinomas”(tiab) OR “Glucagonomas”(tiab) OR “Somatostatinomas”(tiab) OR “Vipomas”(tiab) OR “Pancreatic Ductal Carcinomas”(tiab)] AND English(lang) NOT [“Animals”(Mesh)] NOT [“Animals”(Mesh) AND “Humans”(Mesh)].
We did not specify any language or population restrictions. To identify any unpublished studies, we keyed in the names of specific drug compounds into the search boxes of all GLP-1 agent pharmaceutical companies, three of which had publicly available clinical trials, these were Boehringer Ingelheim clinical trials register, Novartis clinical trials register and Takeda Pharmaceuticals register. We also searched the FDA, the EMA and ClinicalTrials.gov up to August 2013. Bibliographies of included studies and recent review articles were checked for additional relevant studies.
We selected randomized controlled trials that enrolled participants using GLP-1 agonist and DPP-4 inhibitor drugs and reported on the risk of pancreatic complications either as adverse events or as serious adverse events. We included studies that reported on the use of FDA approved GLP-1 receptor agonists such as Exenatide (Byetta, Bydureon), Liraglutide (Victoza) and Albiglutide (Tanzeum). Other GLP-1 receptor agonists that were studied but have not yet been approved by FDA included Taspoglutide, Lixisenatide (Lyxumia), Dulaglutide and Semaglutide were included. Studies that also used FDA approved DPP-4 inhibitors such as Vidagliptin (Eucreas, Galvus, Icandra, Jalra, Xiliarx, Zomarist), Sitagliptin (Efficib, Januvia, Janumet, Ristaben, Ristfor, Tesavel, Velmetia, Xelevia), Saxagliptin (Komboglyze, Onglyza), Linagliptin (Jentadueto, Trajenta) and Alogliptin (Nesina) were included. Other DPP-4 inhibitors in development were included in our search. These include Septagliptin, Anagliptin, Bisegliptin, Carmegliptin, Denagliptin, Dutogliptin, Gosogliptin, Isoleucine Thiazolidide, Valine pyrrolidide, Evogliptin, Gemigliptin, Melogliptin, Omarigliptin, Tenegliptin and Trelagliptin. We did not restrict studies by healthcare settings, methods of diagnosing pancreatitis or by indication for the drug.
Two reviewers (HMS and TA) evaluated all titles and abstracts for studies that met the inclusion criteria, and excluded any articles that clearly did not meet the selection criteria. Full reports of potentially relevant studies were retrieved and independently checked for eligibility. Data from the included studies were then extracted independently by two reviewers (HMS and TA) who collected information on study design, study location, study population description, drug exposure, pancreatic complication (acute pancreatitis, chronic pancreatitis, pancreatic cancer) events, pancreatic enzyme derangement (elevated serum pancreatic amylase and/or pancreatic serum lipase) data, mortality from pancreatic events, how the pancreatic events were defined and monitored, confounders for pancreatic events and characteristics of participants onto a pre-formatted spreadsheet. Another reviewer (YKL or SS) then checked the data. Any uncertainties or discrepancies were resolved through rechecking against the source papers, and through discussion with a third reviewer.
We used a pre-specified spreadsheet to record the location and duration of the randomized controlled trials (in years), dose and frequency of GLP-1 agonist drug and DPP-4 inhibitor drug and placebo or alternative hypoglycemic agent, mean age and sex of participants, number of pancreatic complication events and confounders.
The Cochrane toolkit was used for the assessment of bias in evaluating each trial for the reporting of randomization, allocation concealment, the use of blinding of participants and staff, and information on loss to follow-up or withdrawal rates[9]. In accordance with the Cochrane handbook of systematic reviews, we assessed the quality of data on adverse events by recording how they were monitored and recorded by the investigators[10]. We aimed to generate funnel plots to assess the possibility of publication bias, provided that there were > 10 studies available in the meta-analysis, with no evidence of substantial statistical heterogeneity[11].
We used RevMan[12] 5.3 to conduct meta-analysis based on the summary statistic of Peto Odds Ratios, which is the recommended approach for rare events[9]. We assumed similarity between the risk ratio and OR because the incidence of adverse outcomes was low[13]. We evaluated both adjusted and unadjusted data from primary studies, although we preferentially used adjusted data where available.
Statistical heterogeneity was assessed using I2 statistic[14], with I2 values of 30%-60% representing a moderate level of heterogeneity. Pre-specified subgroup analysis was performed by evaluating the effect of study design, study setting and outcome ascertainment.
The statistical methods of this study were reviewed by Yoon K Loke, convenor of the Cochrane Adverse Effects Methods Group.
After a review of 3583 citations, we identified 68 randomized controlled trials (Figure 1) with a total of 60811 patients for inclusion in our analysis of the association of risk of pancreatic complication events with the use of GLP-1 agonist and DPP-4 inhibitor drugs.
The study characteristics are listed in Table 1 and quality assessment of the trials in Table 2.
| Ref. | Location (No. of centers) | Year of study completion | Total duration (wk) | Duration of GLP-1 exposure (wk) | Participant disease | Arms | No. of participants | Mean age, yr (SD) | Female, n (%) |
| Ross et al[21] | Multi-national (84 centers in 9 countries) | 2010 | 43 | 12 | Type 2 diabetes | Linagliptin 2.5 mg bid | 223 | 58.7 (9.9) | 85 (38.1) |
| Linagliptin 5 mg qd | 224 | 58.4 (10.6) | 103 (46.0) | ||||||
| Placebo | 44 | 59.9 (10.7) | 23 (52.3) | ||||||
| Haak et al[22] | Multi-national (133 clinics in 14 countries) | 2010 | 73 | 24 | Type 2 diabetes | Linagliptin 5 mg qd | 142 | 56.2 (10.8) | 62 (43.7) |
| Metformin 500 mg bid | 144 | 52.9 (10.4) | 62 (43.1) | ||||||
| Metformin 1000 mg bid | 147 | 55.2 (10.6) | 69 (46.9) | ||||||
| Linagliptin 2.5 mg qd + Metformin 500 mg bid | 143 | 55.6 (11.2) | 73 (49.0) | ||||||
| Linagliptin 2.5 mg qd + Metformin 1000 mg bid | 143 | 56.4 (10.7) | 66 (46.2) | ||||||
| Placebo | 72 | 55.7 (11.0) | 36 (50) | ||||||
| NCT00328172[23] | Multi-national (71 sites in 6 countries) | 2007 | 65 | 12 | Type 2 diabetes | Linagliptin 0.5 mg | 58 | 58.0 (9.4) | 13 (22.4) |
| Linagliptin 2.5 mg | 57 | 59.8 (10.3) | 30 (52.6) | ||||||
| Linagliptin 5.0 mg | 55 | 56.6 (9.6) | 24 (43.6) | ||||||
| Metformin | 65 | 53.7 (10.7) | 26 (40.0) | ||||||
| Placebo | 67 | 58.6 (8.9) | 34 (50.7) | ||||||
| Yki-Jarvinen et al[24,25] | Multi-national (169 sites in 19 countries) | 2011 | 108 | 52 | Type 2 diabetes | Linagliptin 5.0 mg | 631 | 59.7 (9.9) | 302 (47.9) |
| Placebo | 630 | 60.4 (10.0) | 301 (47.8) | ||||||
| NCT00654381[26] | Japan | 2010 | 91 | 12 | Type 2 diabetes | Linagliptin 5.0 mg | 159 | 60.3 (9.4) | 48 (30.2) |
| Linagliptin 10.0 mg | 160 | 61.3 (10.0) | 48 (30.0) | ||||||
| Voglibose | 162 | 58.5 (9.9) | 47 (29.0) | ||||||
| Placebo | 80 | 59.7 (8.9) | 23 (28.7) | ||||||
| NCT00622284[27] | Multi-national (221 sites in 16 countries) | 2010 | 146 | 104 | Type 2 diabetes | Linagliptin | 776 | 59.8 (9.4) | 314 (40.5) |
| Glimepiride | 775 | 59.8 (9.4) | 304 (39.2) | ||||||
| BI Trial No: 1218.15/ U09-2519-01[28] | Multi-national (43 sites in 7 countries) | 2009 | 61 | 24 | Type 2 diabetes | Linagliptin 5 mg + Pioglitazone 30 mg | 259 | NR | NR |
| Pioglitazone 30 mg + Placebo | 130 | NR | NR | ||||||
| BI Trial No: 1218.52/U11-1782-01[29] | Multi-national (101 sites in 14 countries) | 2011 | 102 | 54 | Type 2 diabetes | Linagliptin 2.5 mg + Metformin (500 mg and 1000 mg bid) | 396 | NR | NR |
| Metformin 1000 mg bid | 170 | NR | NR | ||||||
| BI Trial No: 1218.63/U11-1781-02[30] | Multi-national (33 sites in 5 countries) | 2011 | 67 | 24 | Type 2 diabetes | Linagliptin 5 mg | 162 | NR | 46 (28.4) |
| Placebo | 79 | NR | 30 (38.0) | ||||||
| BI Trial No: 1218.75/U12-3204-01[31] | Multi-center study (Black/African American patients only) | 2011 | 55 | 24 | Type 2 diabetes | Linagliptin 5 mg | 106 | NR | NR |
| Placebo | 120 | NR | NR | ||||||
| BI Trial No: 1218.61/U13-3124-01[32] | Multi-national study (4 countries) | 2012 | 123 | 24 | Type 2 diabetes | Linagliptin 5 mg | 183 | NR | NR |
| Placebo | 89 | NR | NR | ||||||
| BI Trial No: 1218.65/U12-2143-01[33] | Multi-national study (19 sites in 3 countries) | 2012 | 74 | 24 | Type 2 diabetes | Linagliptin 5 mg | 205 | 82%(< 65 yr) | NR |
| Placebo | 100 | 83% (< 65 yr) | NR | ||||||
| BI Trial No: 1218.64/U13-1283-01[34] | Multi-national study (52 sites in 9 countries) | 2012 | 117 | 52 | Type 2 diabetes | Linagliptin 5 mg | 113 | NR | 43 (38.1) |
| Placebo (first 12 wk)/ Glimepiride (next 40 wk) | 122 | NR | 43 (35.2) | ||||||
| BI Trial No: 1218.66/U12-2076-01[35] | Multi-national study (19 sites in 3 countries | 2012 | 80 | 24 | Type 2 diabetes | Linagliptin 5 mg | 200 | 84.0% (< 65 yr) | NR |
| Placebo | 99 | 89.9% (< 65 yr) | NR | ||||||
| Rosenstock et al[36] | Multi-national study (110 sites in 13 countries) | 2007 | 65 | 26 | Type 2 diabetes | Alogliptin 12.5 mg | 131 | 55.4 (9.8) | 76 (58) |
| Alogliptin 25 mg | 129 | 55.9 (10.2) | 85 (66) | ||||||
| Placebo | 130 | 55.0 (10.6) | 68 (52) | ||||||
| White et al[37] | Multi-national study (898 centers in 49 countries) | 2013 | 193 | 76 (median) | Type 2 diabetes | Alogliptin | 2701 | 36.0% (≥ 65 yr) | 873 (32.3) |
| Placebo | 2679 | 34.9% (≥ 65 yr) | 856 (32.0) | ||||||
| NCT01318135[38] | Japan (58 sites) | 2010 | 52 | 52 | Type 2 diabetes | Alogliptin 12.5 mg qd + Glimepiride 1-6 mg qd or bid | 150 | 38.0% (≥ 65 yr) | 53 (35.3) |
| Alogliptin 25 mg qd + Glimepiride 1-6 mg qd or bid | 152 | 30.9% (≥ 65 yr) | 52 (34.2) | ||||||
| NCT01289119[39] | Multi-national study (21 sites in 3 countries) | 2011 | 52 | 16 | Type 2 diabetes | Alogliptin monotherapy | 92 | 51.6 (10.41) | 37 (40.2) |
| Metformin | 98 | 53.2 (9.46) | 50 (51.0) | ||||||
| Metformin + Alogliptin Add-on Therapy | 99 | 53.0 (9.88) | 48 (48.5) | ||||||
| Pioglitazone | 63 | 51.8 (10.37) | 24 (38.1) | ||||||
| Pioglitazone + Alogliptin Add-on Therapy | 61 | 52.6 (9.44) | 33 (54.1) | ||||||
| Placebo | 93 | 53.1 (8.88) | 39 (41.9) | ||||||
| NCT01263496[40] | Japan (58 sites) | 2008 | 72 | 52 | Type 2 diabetes | Alogliptin 6.25 mg qd | 96 | 28.1 (≥ 65 yr) | 26 (27.1) |
| Alogliptin 12.5 mg qd | 101 | 33.7 (≥ 6 5 yr) | 29 (28.7) | ||||||
| Alogliptin 25 mg qd | 97 | 34.0 (≥ 65 yr) | 22 (22.7) | ||||||
| Alogliptin 50 mg qd | 97 | 32.9 (≥ 65 yr) | 29 (29.9) | ||||||
| Voglibose 0.2 mg tid | 83 | 38.6 (≥ 65 yr) | 27 (32.5) | ||||||
| NCT00328627[41] | Multi-national study (90 sites in 19 countries) | 2008 | 93 | 26 | Type 2 diabetes | Alogliptin 12.5 mg + Placebo | 128 | 53.1 (9.59) | 61 (47.6) |
| Alogliptin 25 mg + Placebo | 129 | 53.7 (9.31) | 79 (61.2) | ||||||
| Placebo | 129 | 55.2 (9.89) | 68 (52.7) | ||||||
| NCT00395512[42] | Multi-national study (268 sites in 23 countries) | 2008 | 67 | 26 | Type 2 diabetes | Alogliptin 25 mg + Pioglitazone 30 mg | 164 | 52.8 (11.01) | 91 (55.5) |
| Alogliptin 12.5 mg + Pioglitazone 30 mg | 164 | 53.5 (11.37) | 83 (50.6) | ||||||
| Pioglitazone 30 mg | 163 | 51.5 (10.72) | 73 (44.8) | ||||||
| Kikuchi et al[43] | Japan (26 sites) | 2007 | 52 | 12 | Type 2 diabetes | Vildagliptin 50 mg bid + glimepiride | 102 | 59.2 (9.8) | 27 (26.5) |
| Placebo + glimepiride | 100 | 60.3 (10.1) | 31 (31.0) | ||||||
| Lukashevich et al[44] | Multi-national study (12 countries) | 2010 | 291 | 24 | Type 2 diabetes | Vildagliptin 50 mg qd (moderate RI) | 165 | 67.7 (8.8) | 69 (41.8) |
| Placebo (moderate RI) | 129 | 69.7 (7.3) | 49 (38.0) | ||||||
| Vildagliptin 50 mg qd (severe RI) | 124 | 64.1 (9.2) | 59 (47.6) | ||||||
| Placebo (severe RI) | 97 | 64.5 (10.8) | 44 (45.4) | ||||||
| Strain et al[45] | Multi-national study (45 centers in 7 countries) | 2012 | 64 | 24 | Type 2 diabetes | Vildagliptin | 139 | 75.1 (4.3) | 66 (47.5) |
| Placebo | 139 | 74.4 (4.0) | 86 (61.9) | ||||||
| NCT00106340[46] (CLAF237A2308) | Multi-national study (402 centers in 25 countries) | 2008 | 166 | 104 | Type 2 diabetes | Vildagliptin 50 mg bid + Metformin | 1562 | 57.5 (9.07) | 733 (46.9) |
| Glimepiride up to 6 mg qd + Metformin | 1556 | 57.5 (9.19) | 718 (46.1) | ||||||
| NCT00300287[47] (CLAF237A2307) | Multi-national study (69 centers in 6 countries) | 2006 | 85 | 52 | Type 2 diabetes | Vildagliptin 50 mg qd | 156 | 63.27 (10.18) | 63 (40.4) |
| Placebo | 150 | 62.84 (11.03) | 61 (40.7) | ||||||
| CLAF237A1301[48] | Japan (51 centers) | 2007 | 44 | 12 | Type 2 diabetes | Vildagliptin 50 mg bid | 188 | 60.3 (10.48) | 67 (35.6) |
| Voglibose 0.2 mg tid | 192 | 58.0 (9.32) | 62 (32.3) | ||||||
| CLAF237A23119[49] | United States (796 centers) | 2007 | 53 | 12 | Type 2 diabetes | Vildagliptin 100 mg + Metformin | 1776 | 55.3 | 864 (48.6) |
| Thiazolinedione + Metformin | 888 | 56.2 | 467 (52.6) | ||||||
| NCT00110240[50] (CLAF237A2323) | Multi-national study (31 centers in 3 countries) | 2006 | 87 | 24 | Type 2 diabetes | Vildagliptin 50 mg bid | 441 | 51.79 (10.13) | 176 (40.0) |
| Acarbose up to 100 mg tid | 220 | 51.93 (10.34) | 81 (37.0) | ||||||
| NCT00327015[51] | Multi-national study (211 sites in 13 countries) | 2007 | 78 | 24 | Type 2 diabetes | Saxagliptin 5 mg + Metformin 500 mg | 320 | 51.95 (10.43) | 155 (48.4) |
| Saxagliptin 10 mg + Metformin 500 mg | 323 | 52.08 (11.59) | 177 (54.8) | ||||||
| Metformin 500 mg + Placebo | 328 | 51.83 (10.74) | 165 (50.3) | ||||||
| Hollander et al[52] | Multi-national study (133 sites in 7 countries) | 2007 | 82 | 24 | Type 2 diabetes | Saxagliptin 2.5 mg + TZD | 195 | 54.9 (9.7) | 89 (45.6) |
| (NCT00295633) | Saxagliptin 5 mg + TZD | 186 | 53.2 (10.6) | 97 (52.2) | |||||
| Placebo + TZD | 184 | 54.0 (10.1) | 99 (53.8) | ||||||
| NCT00757588[53] | Multi-national study (80 sites in 10 countries) | 2010 | 73 | 24 | Type 2 diabetes | Saxagliptin 5 mg + Insulin | 304 | 57.2 (9.4) | 184 (60.5) |
| Placebo + Insulin | 151 | 57.3 (9.3) | 83 (54.9) | ||||||
| Scirica et al[54] | Multi-national study (788 sites in 26 countries) | 2013 | 156 | 109 | Type 2 diabetes | Saxagliptin | 8280 | 65.1 (8.5) | 2768 (33.4) |
| Placebo | 8212 | 65 (8.6) | 2687 (32.7) | ||||||
| Göke et al[55] | Multi-national study (130 sites in 11 countries) | 2010 | 139 | 104 | Type 2 diabetes | Saxagliptin + Metformin | 428 | 57.5 (10.26) | 216 (50.5) |
| Glipizide + Metformin | 430 | 57.59 (10.37) | 198 (46.1) | ||||||
| NCT00316082[56] | Multi-national study (74 sites in 4 countries) | 2007 | 74 | 24 | Type 2 diabetes | Saxagliptin 2.5/5 mg QAM | 71 | 54.28 (10.93) | 34 (47.9) |
| Saxagliptin 2.5 mg QAM | 74 | 55.24 (10.44) | 49 (66.2) | ||||||
| Saxagliptin 5 mg QAM | 74 | 54.66 (9.71) | 36 (48.6) | ||||||
| Saxagliptin 5 mg QPM | 72 | 55.11 (10.35) | 39 (54.2) | ||||||
| Placebo | 74 | 55.57 (10.32) | 39 (52.7) | ||||||
| NCT00614939[57] | Multi-national study (74 sites in 14 countries) | 2009 | 74 | 12 | Type 2 diabetes | Saxagliptin | 85 | 66.8 (8.3) | 53 (62.4) |
| Placebo | 85 | 66.2 (9.1) | 44 (51.8) | ||||||
| Chan et al[58,59] | Multi-national study (30 sites in 13 countries) | 2006 | NR | 54 | Type 2 diabetes | Sitagliptin 50 mg or 25 mg once daily | 65 | 68.9 (9.8) | 34 (52.3) |
| Glipizide | 26 | 65.3 (9.7) | 10 (38.5) | ||||||
| Kojima et al[60] | Japan (Japanese Red Cross Medical Center) | 2011 | 65 | 12 | Type 2 diabetes | Sitagliptin | 20 | 63.85 (12.92) | 5 (0.25) |
| Nateglinide | 16 | 66.44 (9.02) | 4 (0.25) | ||||||
| NCT00509262 | Multi-national study | 2011 | 178 | 54 | Type 2 diabetes | Sitagliptin | 211 | 64.2 (10.7) | 80 (37.9) |
| (Arjona Ferreira et al[61,62]) | |||||||||
| Glipizide | 212 | 64.2 (9.4) | 90 (42.5) | ||||||
| Henry et al[63,64] | Multi-national study | 2010 | 108 | 54 | Type 2 diabetes | Sitagliptin 100 mg/Pioglitazone 15 mg | 230 | NR | 112 (48.7) |
| Sitagliptin 100 mg/Pioglitazone 30 mg | 231 | NR | 96 (41.6) | ||||||
| Sitagliptin 100 mg/Pioglitazone 45 mg | 230 | NR | |||||||
| 95 (41.3) | |||||||||
| Pioglitazone 15 mg | 230 | NR | 82 (35.7) | ||||||
| Pioglitazone 30 mg | 233 | NR | 106 (45.5) | ||||||
| Pioglitazone 45 mg | 230 | NR | 117 (50.9) | ||||||
| Raz et al[65,66] | Multi-national study (30 sites in 13 countries) | 2007 | 47 | 30 | Type 2 diabetes | Sitagliptin 100 mg | 96 | 53.6 (9.5) | 47 (48.9) |
| Placebo | 94 | 56.1 (9.5) | 55 (58.5) | ||||||
| NCT01131182[67] | NR | 2010 | 22 | 4 | Type 2 diabetes | Sitagliptin | 507 | 55.0 (11.0) | 238 (46.9) |
| Sulfonylurea | 514 | 55.0 (11.0) | 259 (50.4) | ||||||
| Goldstein et al[68,69] | Multi-national study | 2006 | 69 | 54 | Type 2 diabetes | Sitagliptin 50 mg bid + Metformin 500 mg bid | 190 | 54.1 (10.0) | 85 (44.7) |
| Sitagliptin 50 mg bid + Metformin 1000 mg bid | 182 | 53.3 (9.6) | 105 (57.7) | ||||||
| Sitagliptin 50 mg bid + Metformin 1000 mg bid (Open Label Cohort) | 117 | 52.6 (10.0) | 50 (42.7) | ||||||
| Metformin 500 mg bid | 182 | 53.4 (10.2) | 93 (51.1) | ||||||
| Metformin 1000 mg bid | 182 | 53.2 (9.6) | 100 (54.9) | ||||||
| Placebo/Metformin 1000 mg bid | 176 | 53.6 (10.0) | 83 (47.2) | ||||||
| Arechavaleta et al[70,71] | Multi-national study | 2009 | 74 | 30 | Type 2 diabetes | Sitagliptin | 516 | 56.3 (9.7) | 232 (44.9) |
| Glimepiride | 519 | 56.2 (10.1) | 240 (46.2) | ||||||
| NCT00086515 et al[72,73] | Multi-national study | 2007 | 135 | 24 | Type 2 diabetes | Sitagliptin 100 mg | 464 | 54.4 (10.4) | 205 (44.2) |
| Placebo/Glipizide 5 mg | 237 | 54.7 (9.7) | 96 (40.5) | ||||||
| Bergenstal et al[74,75] | Multi-national study (62 sites in 3 countries) | 2009 | 56 | 26 | Type 2 diabetes | Exenatide once weekly | 160 | 52.4 (10.41) | 71 (44.4) |
| Sitagliptin | 166 | 52.2 (10.54) | 80 (48.2) | ||||||
| Pioglitazone | 165 | 53.0 (9.92) | 86 (52.1) | ||||||
| NCT00094757[76] | Multi-national study | 2006 | 78 | 54 | Type 2 diabetes | Sitagliptin 100 mg | 205 | 54.5 (10.0) | 95 (46.3) |
| Sitagliptin 200 mg | 206 | 55.4 (9.2) | 102 (49.5) | ||||||
| Placebo/Pioglitazone | 110 | 55.5 (10.1) | 41 (37.3) | ||||||
| NCT00094770[77] | Multi-national study (173 sites in 27 countries) | 2006 | 139 | 104 | Type 2 diabetes | Sitagliptin 100 mg | 588 | 56.8 (9.3) | 252 (42.8) |
| Glipizide | 584 | 56.6 (9.8) | 226 (38.7) | ||||||
| NCT01137812[78,79] | Multi-national study (182 sites in 17 countries) | 2012 | 87 | 52 | Type 2 diabetes | Sitagliptin 100 mg | 378 | 56.6 (9.33) | 163 (43.1) |
| Canagliflozin 300 mg | 377 | 56.5 (9.62) | 170 (45.1) | ||||||
| NCT00482729[80] | Multi-national study (209 sites in United States) | 2008 | 74 | 44 | Type 2 diabetes | Sitagliptin/Meformin-Fixed Dose Combination | 625 | 49.5 (10.5) | 272 (43.5) |
| Metformin | 621 | 50.0 (10.5) | 266 (42.8) | ||||||
| Bunck et al[81] | Multi-national study (3 sites in 3 countries) | 2007 | 154 | 52 | Type 2 diabetes | Exenatide | 36 | 58.4 (1.4) | 13 (36.1) |
| Insulin glargine | 33 | 58.3 (1.3) | 11 (33.3) | ||||||
| Diamant et al[82] | Multi-national study (72 sites in 7 countries) | 2009 | 53 | 26 | Type 2 diabetes | Exenatide | 233 | 58.0 (10.0) | 113 (48.0) |
| Insulin glargine | 223 | 58 (9.0) | 100 (45.0) | ||||||
| Inagaki et al[83] | Japan (22 sites) | 2010 | 61 | 26 | Type 2 diabetes | Exenatide once weekly | 215 | 57.07 (10.44) | 73 (34.0) |
| Insulin glargine once daily | 212 | 56.44 (11.16) | 64 (30.2) | ||||||
| Russell-Jones et al[84] | Multi-national study (106 sites in 22 countries) | 2010 | 82 | 26 | Type 2 diabetes | Exenatide 2 mg once weekly + Oral placebo | 248 | 53.7 (10.91) | 109 (43.9) |
| Sitagliptin 100 mg/d + SC placebo | 163 | 52.3 (11.05) | 69 (42.3) | ||||||
| Metformin starting at 1000 mg/d + SC placebo | 246 | 53.7 (11.08) | 92 (37.4) | ||||||
| Pioglitazone starting at 30 mg/d+ SC placebo | 163 | 55.3 (10.96) | 66 (40.5) | ||||||
| NCT01003184[85] | 34 sites in Ireland and United Kingdom | 2011 | 91 | 26 | Type 2 diabetes | Exenatide once weekly | 111 | 59.2 (9.86) | 40 (36.04) |
| Insulin Detemir twice daily | 105 | 57.8 (9.48) | 33 (31.4) | ||||||
| Astrup et al[86] | Multi-national study (19 sites in 8 European countries) | 2009 | 117 | 104 | Type 2 diabetes | Liraglutide 1.2 mg | 95 | 47.18 (9.72) | 73 (76.8) |
| Liraglutide 1.8 mg | 90 | 45.53 (10.9) | 68 (75.6) | ||||||
| Liraglutide 2.4 mg | 93 | 45.01 (11.09) | 71 (76.3) | ||||||
| Liraglutide 3.0 mg | 93 | 45.91 (10.71) | 70 (75.3) | ||||||
| Placebo | 98 | 45.86 (10.28) | 74 (75.5) | ||||||
| Garber et al[87] | 126 sites in United States and 12 sites in Mexico | 2007 | 91 | 52 | Type 2 diabetes | Liraglutide 1.2 mg | 251 | 53.7 (11.0) | 134 (53.4) |
| Liraglutide 1.8 mg | 247 | 52.0 (10.8) | 126 (51.0) | ||||||
| Glimepiride 8 mg | 248 | 53.4 (10.9) | 115 (46.4) | ||||||
| Nauck et al[88] | Multi-national study (170 sites in 21 countries) | 2007 | 52 | 26 | Type 2 diabetes | Once daily Liraglutide (0.6 mg) | 242 | 56.0 (11.0) | 91 (37.6) |
| Once daily Liraglutide (1.2 mg) | 241 | 57 (9.0) | 111 (46.1) | ||||||
| Once daily Liraglutide (1.8 mg) | 242 | 57 (9.0) | 100 (41.3) | ||||||
| Once daily Glimepiride (4 mg) | 244 | 57 (9.0) | 103 (42.2) | ||||||
| Placebo | 122 | 56 (9.0) | 49 (40.2) | ||||||
| Marre et al[89] | Multi-national study (116 sites in 21 countries) | NR | NR | 26 | Type 2 diabetes | Liraglutide 0.6 mg | 233 | 55.7 (9.9) | 107 (46.0) |
| Liraglutide 1.2 mg | 228 | 57.7 (9.0) | 125 (55.0) | ||||||
| Liraglutide 1.8 mg | 234 | 55.6 (10.0) | 110 (47.0) | ||||||
| Placebo | 114 | 54.7 (10.0) | 60 (53.0) | ||||||
| Zinman et al[90] | 90 sites in United States and Canada | 2007 | 65 | 26 | Type 2 diabetes | Liraglutide 1.2 mg | 178 | 55.0 (10.0) | 77 (43.0) |
| Liraglutide 1.8 mg | 178 | 55.0 (11.0) | 87 (49.0) | ||||||
| Placebo | 177 | 55.0 (10.0) | 67 (38.0) | ||||||
| Raz et al[91] | Multi-national study (53 centers in 11 countries) | 2011 | 134 | 24 | Type 2 diabetes | Taspoglutide 10 mg | 116 | NR | NR |
| Taspoglutide 20 mg | 129 | NR | NR | ||||||
| Placebo | 123 | NR | NR | ||||||
| Rosenstock et al[92] | Multi-national study (118 sites in 4 countries) | 2008 | 56 | 16 | Type 2 diabetes | Albiglutide 4 mg weekly | 35 | 50.4 (10.3) | 20 (57.1) |
| Albiglutide 15 mg weekly | 35 | 55.5 (10.5) | 17 (48.6) | ||||||
| Albiglutide 30 mg weekly | 31 | 54.2 (9.7) | 23 (74.2) | ||||||
| Albiglutide 15 mg biweekly | 33 | 52.5 (9.6) | 19 (57.6) | ||||||
| Albiglutide 30 mg biweekly | 32 | 55.5 (9.9) | 16 (50.0) | ||||||
| Albiglutide 50 mg biweekly | 35 | 51.1 (10.3) | 16 (45.7) | ||||||
| Albiglutide 50 mg monthly | 35 | 54.1 (11.3) | 18 (51.4) | ||||||
| Albiglutide 100 mg monthly | 34 | 54.4 (9.9) | 15 (44.1) | ||||||
| Placebo | 51 | 54.0 (10.6) | 23 (45.1) | ||||||
| Seino et al[93] | Multi-national study (57 centers in 4 Asian countries) | NR | NR | 24 | Type 2 diabetes | Lixisenatide (10 ug for 1 wk, 15 mg for 1 wk, then 20 mg-maintenance dose) | 154 | 58.7 (10.2) | 85 (55.2) |
| Placebo | 157 | 58.0 (10.1) | 77 (49.0) | ||||||
| Umpierrez et al[94] | 36 sites in United States and 3 in Puerto Rico | 2008 | 39 | 16 | Type 2 diabetes | LY2189265 (LY 0.5/1.0) | 66 | 59.0 (12.0) | 31 (47.0) |
| LY2189265 (LY 1.0/1.0) | 65 | 57.0 (12.0) | 30 (46.0) | ||||||
| LY2189265 (LY 1.0/2.0) | 65 | 54.0 (11.0) | 31 (48.0) | ||||||
| Placebo | 66 | 56.0 (12.0) | 37 (56.0) |
| Ref. | Sequence generation | Blinding | Allocation concealment | Was Pancreatitis an AE or SAE? | Adverse event monitoring | Arms | Withdrawal rate (%) | Loss to follow- up (%) |
| Ross et al[21] | Central computer based; randomization: block in a 5:5:1 ratio | Double blind | Adequate | AE | Safety and tolerability end-points were the incidence of adverse events (including adverse changes observed during physical examinations or ECGs), protocol-specified significant AEs, hypoglycemia and changes from baseline in vital signs, clinical laboratory parameters and body weight | Linagliptin 2.5 mg bid | 7.2 | 0 |
| Linagliptin 5 mg qd | 4.5 | 0 | ||||||
| Placebo | 2.3 | 0 | ||||||
| Haak et al[22] | NR | Double blind | Adequate | AE | Incidence of AEs, serious AEs, discontinuation due to AEs,12-lead ECGs, vital signs and clinical laboratory parameters. The causal relationships between study medications and AEs were evaluated by the investigators at the site | Linagliptin 5 mg qd | 14.8 | 2.1 |
| Metformin 500 mg bid | 11.8 | 2.1 | ||||||
| Metformin 1000 mg bid | 14.3 | 2.7 | ||||||
| Linagliptin 2.5 mg qd + Metformin 500 mg bid | 11.2 | 2.8 | ||||||
| Linagliptin 2.5 mg qd + Metformin 1000 mg bid | 7.7 | 0 | ||||||
| Placebo | 25.0 | 1.4 | ||||||
| NCT00328172[23] | NR | Double blind | NR | SAE | NR | Linagliptin 0.5 mg | 24.1 | 1.7 |
| Linagliptin 2.5 mg | 17.5 | 3.5 | ||||||
| Linagliptin 5.0 mg | 23.6 | 1.8 | ||||||
| Metformin | 7.7 | 1.5 | ||||||
| Placebo | 32.8 | 1.5 | ||||||
| Yki-Jarvinen et al[24,25] | NR | Double blind | NR | SAE | NR | Linagliptin 5.0 mg | 13.9 | 2.2 |
| Placebo | 17.5 | 1.3 | ||||||
| NCT00654381[26] | NR | Double blind | NR | SAE | NR | Linagliptin 5.0 mg | 1.89 | 0 |
| Linagliptin 10.0 mg | 3.13 | 0 | ||||||
| Voglibose | 2.5 | 0 | ||||||
| Placebo | 7.5 | 0 | ||||||
| NCT00622284[27] | NR | Double blind | NR | SAE | NR | Linagliptin | 24.4 | 1.4 |
| Glimepiride | 22.1 | 1.7 | ||||||
| BI Trial No: 1218.15/ U09-2519-01[28] | Randomized into 1;2 ratio to receive either placebo or linagliptin | Double blind | Adequate | SAE | Incidence and intensity of AEs, withdrawals due to AEs, physical examination, 12-lead ECG, vital signs, clinical laboratory parameters | Linagliptin 5 mg + Pioglitazone 30 mg | 5.8 | NR |
| Pioglitazone 30 mg + Placebo | 14.6 | NR | ||||||
| BI Trial No: 1218.52/U11-1782-01[29] | NR | Double blind | NR | SAE | Safety endpoints were the incidence and intensity of AEs, withdrawals due to AEs, clinically relevant new or worsening findings in physical examination, 12-lead ECG, vital signs and clinical laboratory parameters | Linagliptin 2.5 mg + Metformin (500 mg and 1000 mg bid) | 0.0 | NR |
| Metformin 1000 mg bid | 0.6 | NR | ||||||
| BI Trial No: 1218.63/U11-1781-02[30] | NR | Double blind | NR | SAE | Incidence and intensity of AEs, withdrawals due to AEs, physical examination, 12-lead ECG, vital signs, clinical laboratory parameters | Linagliptin 5 mg | 1.23 | NR |
| Placebo | 1.26 | NR | ||||||
| BI Trial No: 1218.75/U12-3204-01[31] | NR | Double blind | NR | AE | Incidence and intensity of AEs, withdrawals due to AEs, clinically relevant changes from baseline in vital signs (blood pressure and pulse rate), clinically relevant new or worsening findings in 12-lead ECG as reported as AEs, clinically relevant changes from baseline in clinical laboratory assessments, cardiac and cerebrovascular events adjudicated CEC | Linagliptin 5 mg | 12.3 | NR |
| Placebo | 12.5 | NR | ||||||
| BI Trial No: 1218.61/U13-3124-01[32] | NR | Double blind | NR | AE | Incidence and intensity of AEs, primarily based on spontaneous AEs; withdrawal due to AEs; clinically relevant new or worsening findings in physical examination reported as AEs; changes from baseline in vital signs (BP and pulse); clinically relevant new or worsening findings in 12 lead ECG reported as AEs; changes from baseline in clinical lab assessments; and hypoglycemic events | Linagliptin 5 mg | 2.2 | NR |
| Placebo | 0.0 | NR | ||||||
| BI Trial No: 1218.65/U12-2143-01[33] | NR | Double blind | NR | SAE | Incidence and intensity of adverse events, withdrawals due to AEs, physical examination, ECGs, change from baseline in clinical lab parameters and cardiovascular events (Clinical Event Committee adjudication results) | Linagliptin 5 mg | 0.98 | NR |
| Placebo | 3.0 | NR | ||||||
| BI Trial No: 1218.64/U13-1283-01[34] | NR | Double blind | NR | AE | Incidence and intensity of adverse events (AEs), withdrawals due to AEs, physical examination, vital signs, 12 lead ECG, change from baseline in clinical lab parameters | Linagliptin 5 mg | 0.0 | NR |
| Placebo (first 12 wk)/ Glimepiride (next 40 wk) | 1.64 | NR | ||||||
| BI Trial No: 1218.66/U12-2076-01[35] | NR | Double blind | NR | SAE | Incidence and intensity of adverse events, withdrawals due to AEs, physical examination and vital signs, 12-lead ECG, clinical laboratory assessments | Linagliptin 5 mg | 5.1 | NR |
| Placebo | 2.0 | NR | ||||||
| Rosenstock et al[36] | Automated interactive voice response system using a randomization schedule | Double blind | NR | SAE | During the treatment period, patients were reviewed for adverse event evaluations. Further safety assessments included clinical examination of skin and digits. Hematology, serum chemistry, vital signs, physical exam and ECG parameters were done | Alogliptin 12.5 mg | 36.6 | 3.05 |
| Alogliptin 25 mg | 40.3 | 2.33 | ||||||
| Placebo | 57.7 | 1.54 | ||||||
| White et al[37] | NR | Double blind | NR | SAE | The principal secondary safety end point was the primary composite end point with the addition of urgent revascularization due to unstable angina within 24 h after hospital admission. Additional safety end points included angioedema, hypoglycemia, pancreatitis, cancer, and the results of laboratory testing | Alogliptin | NR | NR |
| Placebo | NR | NR | ||||||
| NCT01318135[38] | NR | Open Label | Inadequate | SAE (Pancreatic cancer only) | Alogliptin 12.5 mg qd + Glimepiride 1-6 mg qd or bid | NR | NR | |
| Alogliptin 25 mg qd + Glimepiride 1-6 mg qd or bid | NR | NR | ||||||
| NCT01289119[39] | NR | Double blind | NR | SAE | TEAE were defined as any adverse events that started on or after the date of the first dose of double-blind study drug and within 14 d after the date of the last dose of double-blind study drug | Alogliptin monotherapy | 9.78 | 3.26 |
| Metformin | 9.18 | 0 | ||||||
| Metformin + Alogliptin Add-on Therapy | 6.06 | 0 | ||||||
| Pioglitazone | 7.94 | 0 | ||||||
| Pioglitazone + Alogliptin Add-on Therapy | 6.56 | 1.64 | ||||||
| Placebo | 9.78 | 0 | ||||||
| NCT01263496[40] | NR | Open Label | Inadequate | SAE | A TEAE is defined as an adverse event with an onset that occurs after receiving study drug and within 30 d after receiving the last dose of study drug | Alogliptin 6.25 mg qd | NR | NR |
| Alogliptin 12.5 mg qd | NR | NR | ||||||
| Alogliptin 25 mg qd | NR | NR | ||||||
| Alogliptin 50 mg qd | NR | NR | ||||||
| Voglibose 0.2 mg tid | NR | NR | ||||||
| NCT00328627[41] | NR | Double blind | NR | SAE | NR | Alogliptin 12.5 mg + Placebo | 24.2 | 1.56 |
| Alogliptin 25 mg + Placebo | 21.7 | 1.55 | ||||||
| Placebo | 45.7 | 3.1 | ||||||
| NCT00395512[42] | NR | Double blind | Adequate | SAE | NR | Alogliptin 25 mg + Pioglitazone 30 mg | 17.1 | 3.05 |
| Alogliptin 12.5 mg + Pioglitazone 30 mg | 23.2 | 3.05 | ||||||
| Pioglitazone 30 mg | 22.7 | 3.68 | ||||||
| Kikuchi et al[43] | Dynamic randomization | Double blind | NR | SAE | Adverse events were recorded at each visit, and these AEs were assessed for severity and suspected relationship to the study drug. Hematology, biochemistry and urinalysis were performed at each scheduled visit. All laboratory assessments were processed at a central testing to ensure consistency | Vildagliptin 50 mg bid + glimepiride | 2.9 | NR |
| Placebo + glimepiride | 4 | NR | ||||||
| Lukashevich et al[44] | NR | Double blind | NR | SAE | All treatment emergent AEs were recorded and assessed by the investigator as to severity and potential relationship to study drug. Particular attention was paid to hepatic, infections, skin, pancreatitis as well as edema and cardiovascular safety | Vildagliptin 50 mg qd (moderate RI) | 10.3 | 2.4 |
| Placebo (moderate RI) | 10.9 | 1.6 | ||||||
| Vildagliptin 50 mg qd (severe RI) | 13.7 | 1.6 | ||||||
| Placebo (severe RI) | 13.4 | 2.1 | ||||||
| Strain et al[45] | Validated automated system | Double blind | Adequate | AE | All AEs and their severity, serious AEs, and their presumed relation with the study drug were monitored and recorded at each study visit | Vildagliptin | 5.8 | 0.72 |
| Placebo | 5.8 | 0 | ||||||
| NCT00106340[46] (CLAF237A2308) | NR | Double blind | NR | SAE | Safety assessments included monitoring and recording all AEs, SAEs and pregnancies; regular monitoring of hematology, blood chemistry, and urine (performed at a central lab); and regular assessments of vital signs, ECG, physical condition and body weight. Severity and relationship to study drug were recorded for all AEs and SAEs | Vildagliptin 50 mg bid + Metformin | 36.4 | 0 |
| Glimepiride up to 6 mg qd + Metformin | 38.8 | 0 | ||||||
| NCT00300287[47] | NR | Double blind | NR | SAE | Safety assessments included monitoring and recording all AEs, SAEs with their severity and presumed relationship to study drug and pregnancies, recording of hypoglycemic events, the regular monitoring of hematology, blood chemistry and urine, and regular assessments of vital signs, physical condition, body weight, and ECGs | Vildagliptin 50 mg qd | 14.7 | 0.6 |
| Placebo | 12.7 | 0.7 | ||||||
| (CLAF237A2307) CLAF237A1301[48] | NR | Double blind | NR | AE (elevated pancreatic enzymes) | Safety assessments included monitoring and recording all AEs, SAEs with their severity and presumed relationship to study drug and pregnancies, recording of hypoglycemic events, the regular monitoring of hematology, blood chemistry and urine, and regular assessments of vital signs, physical condition, body weight, and ECGs | Vildagliptin | 4.8 | NR |
| 50 mg bid Voglibose 0.2 mg tid | 5.2 | NR | ||||||
| CLAF237A23119[49] | NR | Open Label | NA | SAE | Safety assessments included monitoring and recording all AEs, SAEs with their severity and presumed relationship to study drug and pregnancies, recording of hypoglycemic events, the regular monitoring of hematology, blood chemistry and urine, and regular assessments of vital signs, physical condition, body weight, and ECGs | Vildagliptin 100 mg + Metformin | 10.4 | 2.5 |
| Thiazolinedione + Metformin | 11.8 | 2.1 | ||||||
| NCT00110240[50] (CLAF237A2323) | NR | Double Blind | NR | SAE | Safety assessments included adverse events, hypoglycemic events and serious adverse events, physical examination, vital signs, laboratory evaluations, and ECGs | Vildagliptin 50 mg bid | 9.5 | 1.6 |
| Acarbose up to 100 mg tid | 12.7 | 1.4 | ||||||
| NCT00327015[51] | NR | Double Blind | NR | SAE | Safety and tolerability end-points included incidence of AEs, SAEs, discontinuation due to AEs, physical and ECG examinations, vital signs and results of clinical laboratory tests | Saxagliptin 5 mg + Metformin 500 mg | 28.4 | 6.9 |
| Saxagliptin 10 mg + Metformin 500 mg | 28.5 | 7.1 | ||||||
| Metformin 500 mg + Placebo | 33.2 | 6.7 | ||||||
| Hollander et al[52] (NCT00295633) | NR | Double Blind | NR | SAE | Safety assessments included incidence of AEs, SAEs and discontinuation due to AEs, changes from baseline lab parameters; changes from baseline vital signs; and incidence of marked clinical laboratory abnormalities | Saxagliptin 2.5 mg + TZD | 31.8 | NR |
| Saxagliptin 5 mg + TZD | 36 | NR | ||||||
| Placebo + TZD | 41.3 | NR | ||||||
| NCT00757588[53] | Interactive voice response system | Double Blind | NR | SAE | Safety end points included AEs, hypoglycemia and weight gain | Saxagliptin 5 mg + Insulin | 11.8 | 0.98 |
| Placebo + Insulin | 11.3 | 3.31 | ||||||
| Scirica et al[54] | Central computerized telephone or web based system | Double Blind | NR | NR (Safety End Point) | A clinical events committee comprising specialists in cardiovascular and pancreatic medicine, all of whom were unaware of the study group assignments, adjudicated | Saxagliptin | NR | NR |
| Placebo | NR | NR | ||||||
| Goke et al[55] | NR | Double Blind | NR | SAE | Safety and tolerability assessments included AEs and SAEs, lab measurements, vital signs, physical examination and ECG testing | Saxagliptin + Metformin | 61.4 | 0.23 |
| Glipizide + Metformin | 65.8 | 0.69 | ||||||
| NCT00316082[56] | NR | Double Blind | NR | SAE | NR | Saxagliptin 2.5/5 mg QAM | 38.0 | 9.9 |
| Saxagliptin 2.5 mg QAM | 44.6 | 9.5 | ||||||
| Saxagliptin 5 mg QAM | 29.7 | 8.1 | ||||||
| Saxagliptin 5 mg QPM | 36.1 | 11.1 | ||||||
| Placebo | 35.1 | 8.1 | ||||||
| NCT00614939[57] | Interactive voice response system | Double Blind | NR | SAE | Safety and tolerability assessments included AEs, SAEs, treatment-related AEs, discontinuations of randomized study medication because of AEs, deaths, AEs of special interest and hypoglycemic events | Saxagliptin | 71.8 | NR |
| Placebo | 80.0 | NR | ||||||
| Chan et al[58,59] | Computer generated randomization schedule | Double Blind | Adequate | SAE | Assessment of safety and tolerability included evaluation of the data from physical examinations, vital signs and ECGs collected at specified study visits. All adverse experiences were rated by the investigators for intensity and relationship to study drug | Sitagliptin 50 mg or 25 mg once daily | 29.2 | NR |
| Placebo/Glipizide | 23.1 | NR | ||||||
| Kojima et al[60] | Random allocation sequence performed centrally | Open label | NA | AE | NR | Sitagliptin | NR | NR |
| Nateglinide | NR | NR | ||||||
| NCT00509262 (Arjona Ferreira JC et al[61,62]) | Computer generated randomization schedule | Double | NR | SAE | Safety measurements included evaluation of AEs, physical exam and vital signs, and ECG. Lab safety studies included serum chemistry, hematology and urinalysis. All AEs were rated by the investigator for intensity and relationship to study drug | Sitagliptin | 210 | |
| Glipizide | 212 | |||||||
| Henry RR et al[63,64] | NR | Blind Double blind | NR | SAE | Safety and tolerability were evaluated throughout the study by physical examination, monitoring of vital signs and safety lab measurements that included serum chemistry, hematology and urinalysis. AEs were monitored and evaluated by the investigators for intensity (severity), duration, outcome and relationship to study drug | Sitagliptin 100 mg/Pioglitazone 15 mg | 20.9 | 3.5 |
| Sitagliptin 100 mg/Pioglitazone 30 mg | 22.9 | 6.9 | ||||||
| Sitagliptin 100 mg/Pioglitazone 45 mg | 22.2 | 5.7 | ||||||
| Pioglitazone 15 mg | 31.3 | 6.1 | ||||||
| Pioglitazone 30 mg | 27.9 | 9 | ||||||
| Pioglitazone 45 mg | 27.4 | 5.7 | ||||||
| Raz I et al[65,66] | Computer generated schedule | Double blind | NR | SAE | Safety and tolerability were evaluated by physical examination, vital signs and lab measurements that included routine serum chemistry, hematology, urinalysis and pregnancy testing. AEs were monitored through the study for intensity, duration, outcome, relationship to study drug and level of severity | Sitagliptin 100 mg | 17.7 | |
| 3.13 | ||||||||
| Placebo | 14.9 | 3.19 | ||||||
| NCT01131182[67] | NR | Open label | NA | SAE | NR | Sitagliptin | NR | NR |
| Sulfonylurea | NR | NR | ||||||
| Goldstein et al[68,69] | NR | Double blind | NR | SAE | Data were collected regarding AEs, physical exam, vital signs, ECGs and body weight throughout the study. All AEs were rated by investigators for intensity and relationship to study drug | Sitagliptin 50 mg bid + Metformin 500 mg bid | 22.1 | 2.6 |
| Sitagliptin 50 mg bid + Metformin 1000 mg bid | 22.5 | 5.5 | ||||||
| Sitagliptin 50 mg bid + Metformin 1000 mg bid (OLC) | 32.5 | 2.6 | ||||||
| Metformin 500 mg bid | 30.8 | 2.2 | ||||||
| Metformin 1000 mg bid | 25.8 | 3.8 | ||||||
| Placebo/Metformin 1000 mg bid | 34.7 | 5.1 | ||||||
| Arechavaleta et al[70,71] | Concealed computer-generated allocation schedule | Double blind | Adequate | SAE | Safety and tolerability were assessed by a review of all safety parameters including adverse experiences, laboratory safety parameters, body weight and vital signs | Sitagliptin | 9.3 | 1.7 |
| Glimepiride | 9.8 | 1.7 | ||||||
| NCT00086515 et al[72,73] | NR | Double blind | NR | SAE | Safety and tolerability were assessed throughout the study. Monitoring for adverse experiences, physical examinations, vital signs, body weight, 12-lead ECGs (read at a central reading laboratory), and safety laboratory measurements comprising routine hematology, serum chemistry, and urinalysis were performed | Sitagliptin 100 mg | 10.6 | 0.86 |
| Placebo/Glipizide 5 mg | 18.9 | 2.11 | ||||||
| Bergenstal et al[74,75] | Interactive voice response system | Double blind | Adequate | SAE | NR | Exenatide once weekly | 26.9 | 5 |
| Sitagliptin | 16.9 | 5.4 | ||||||
| Pioglitazone | 24.8 | 7.8 | ||||||
| NCT00094757[76] | NR | Double blind | NR | SAE | Data for adverse experiences, physical examinations, vital signs, ECGs, and body weight were collected throughout the study | Sitagliptin 100 mg | 25.8 | 1.5 |
| Sitagliptin 200 mg | 30.1 | 2.4 | ||||||
| Placebo/Pioglitazone | 27.3 | 5.4 | ||||||
| NCT00094770[77] | NR | Double blind | NR | SAE | Data on adverse experiences, physical examinations, vital signs, ECGs and body weight were collected throughout the study. All adverse experiences were rated by the study site investigators for intensity and relationship to study drug. Laboratory safety evaluations included blood chemistry, haematology and urinalysis | Sitagliptin 100 mg | 34.4 | 3.2 |
| Glipizide | 29.5 | 1.7 | ||||||
| NCT01137812[78,79] | Interactive Voice Response System/Interactive Web Response System | Double blind | Adequate | SAE | Safety evaluations included AEs, clinical laboratory tests, vital sign measurements, physical examinations, self-monitored blood glucose, 12-lead electrocardiograms, and documentation of hypoglycemic episodes | Sitagliptin 100 mg | 44.4 | 2.1 |
| Canagliflozin 300 mg | 32.6 | 1.6 | ||||||
| NCT00482729[80] | NR | Double blind | NR | SAE | NR | Sitagliptin/Meformin-Fixed Dose Combination | 34.7 (217/626) | 13.7 (86/626) |
| Metformin | 34.9 (218/624) | 10.6 (66/624) | ||||||
| Bunck et al[81] | NR | Open label | NA | SAE | NR | Exenatide | 16.7 | 0 |
| Insulin glargine | 9.1 | 3.03 | ||||||
| Diamant et al[82] | Computer generated randomization sequence | Open label | NA | SAE | Safety endpoints were adverse events, clinical lab assessments, vital signs, and hypoglycemia. We defined adverse events as those occurring at or after randomization or worsening during the study | Exenatide | 10.3 | 0.86 |
| Insulin glargine | 6.3 | 0.45 | ||||||
| Inagaki et al[83] | Computer generated randomization sequence | Open label | NA | AE | Safety profile end points included AEs and hypoglycemia | Exenatide once weekly | 10.2 | 0.47 |
| Insulin glargine once daily | 5.2 | 0 | ||||||
| Russell-Jones et al[84] | Computer generated randomization sequence | Double blind | Adequate | SAE | Safety end points were adverse events, clinical lab assessments, vital signs, hypoglycemia and antibodies to exenatide. Treatment emergent adverse events were defined as those occurring or worsening after the first dose of study drug | Exenatide 2 mg once weekly + Oral placebo | 15.3 | 1.6 |
| Sitagliptin 100 mg/d + SC placebo | 14.1 | 2.4 | ||||||
| Metformin starting at 1000 mg/d + SC placebo | 13.4 | 0.4 | ||||||
| Pioglitazone starting at 30 mg/d + SC placebo | 1.8 | 1.8 | ||||||
| NCT01003184[85] | NR | Open label | NR | SAE | NR | Exenatide once weekly | 17.1 | 0.9 |
| Insulin Detemir twice daily | 11.4 | 0 | ||||||
| Astrup et al[86] | NR | Double blind (first 20 wk) Weeks 20-104: Open label | NR | SAE | Safety assessments included adverse events, recorded at every visit, standard lab tests and serum liraglutide antibodies. A safety committee for data surveillance was established | Liraglutide 1.2 mg | 10.5 | 0 |
| Liraglutide 1.8 mg | 17.8 | 0 | ||||||
| Liraglutide 2.4 mg | 21.5 | 0 | ||||||
| Liraglutide 3.0 mg | 11.8 | 0 | ||||||
| Placebo | 19.4 | 0 | ||||||
| Garber et al[87] | Telephone based or web-based systems | Double blind | Adequate | SAE | Key safety assessments were tolerability (including nausea and other gastrointestinal adverse events), serum calcitonin and hypoglycemic episodes | Liraglutide 1.2 mg | 35.5 | NR |
| Liraglutide 1.8 mg | 29.7 | NR | ||||||
| Glimepiride 8 mg | 38.7 | NR | ||||||
| Nauck et al[88] | Telephone based or web-based randomization systems | Double blind | Adequate | SAE | Safety variables included adverse events, vital signs, ECG, biochemical and hematology measures and subject reported hypoglycemic episodes | Once daily Liraglutide (0.6 mg) | 14.0 | 0 |
| Once daily Liraglutide (1.2 mg) | 18.0 | 0.4 | ||||||
| Once daily Liraglutide (1.8 mg) | 21.0 | 0 | ||||||
| Once daily Glimepiride (4 mg) | 14.0 | 0 | ||||||
| Placebo | 39.0 | 0 | ||||||
| Marre et al[89] | NR | Double blind | NR | SAE | Safety variables included hypoglycemic episodes, liraglutide antibodies, tolerability (gastrointestinal complaints) and pulse. AEs, vital signs, ECG, biochemical and hematology measures including calcitonin were also monitored | Liraglutide 0.6 mg | 10.7 | NR |
| Liraglutide 1.2 mg | 14.0 | NR | ||||||
| Liraglutide 1.8 mg | 8.9 | NR | ||||||
| Placebo | 27.2 | NR | ||||||
| Zinman et al[90] | Telephone based or web-based randomization systems | Double blind | Adequate | SAE | Safety variables included AEs, vital signs, ECG, biochemical and hematology measures and subject reported hypoglycemic episodes | Liraglutide 1.2 mg | 14.0 | NR |
| Liraglutide 1.8 mg | 25.0 | NR | ||||||
| Placebo | 32.0 | NR | ||||||
| Raz et al[91] | NR | Double blind | NR | SAE | Safety assessments included AEs, vital signs, physical examinations, clinical lab tests, ECG and hypoglycemia | Taspoglutide 10 mg | 11.2 | NR |
| Taspoglutide 20 mg | 13.2 | NR | ||||||
| Placebo | 3.3 | NR | ||||||
| Rosenstock et al[92] | NR | Double blind | NR | SAE | Adverse event assessments and safety analyses were conducted throughout the study | Albiglutide 4 mg weekly | 48.6 | 5.7 |
| Albiglutide 15 mg weekly | 31.4 | 8.6 | ||||||
| Albiglutide 30 mg weekly | 32.3 | 3.2 | ||||||
| Albiglutide 15 mg biweekly | 45.5 | 9.1 | ||||||
| Albiglutide 30 mg biweekly | 24.2 | 0 | ||||||
| Albiglutide 50 mg biweekly | 42.9 | 2.9 | ||||||
| Albiglutide 50 mg monthly | 14.3 | 2.9 | ||||||
| Albiglutide 100 mg monthly | 44.1 | 2.9 | ||||||
| Placebo | 23.5 | 0 | ||||||
| Seino et al[93] | Interactive voice response system | Double blind | Adequate | SAE | Safety and tolerability included reported AEs and other safety information such as symptomatic hypoglycemia | Lixisenatide (10 ug for 1 wk, 15 ug for 1 wk, then 20 ug-maintenance dose) | NR | NR |
| Placebo | NR | NR | ||||||
| Umpierrez et al[94] | Computer generated random sequence | Double blind | Adequate | SAE | Safety measures included AEs, vital signs, hypoglycemia events and lab tests | LY2189265 (LY 0.5/1.0) | 12.1 | 1.5 |
| LY2189265 (LY 1.0/1.0) | 10.8 | 1.5 | ||||||
| LY2189265 (LY 1.0/2.0) | 13.8 | 1.5 | ||||||
| Placebo | 9.1 | 1.5 |
Of these 69 studies, data was abstracted from 28 published reports, 32 studies from clinicaltrials.gov and 9 studies were abstracted from pharmaceutical company databases (Boehringer Ingelheim, Novartis and Takeda Pharmaceutical Company). Almost all the trials were multicenter or multinational studies in patients with type II diabetes mellitus.
The majority of studies did not report on the method of generating the random sequence, or on the means of concealing allocation. However, most of the trials (n = 55) were double-blinded, thus reducing risk of bias in the diagnosis of pancreatic adverse events. We found that most of the trials, except for two, did not specific pancreatitis as a part of their safety monitoring protocol. As such, there is a strong possibility that pancreatic adverse events may have been missed or wrongly diagnosed. Moreover, the included studies did not specify whether they applied similar criteria in defining cases of pancreatitis.
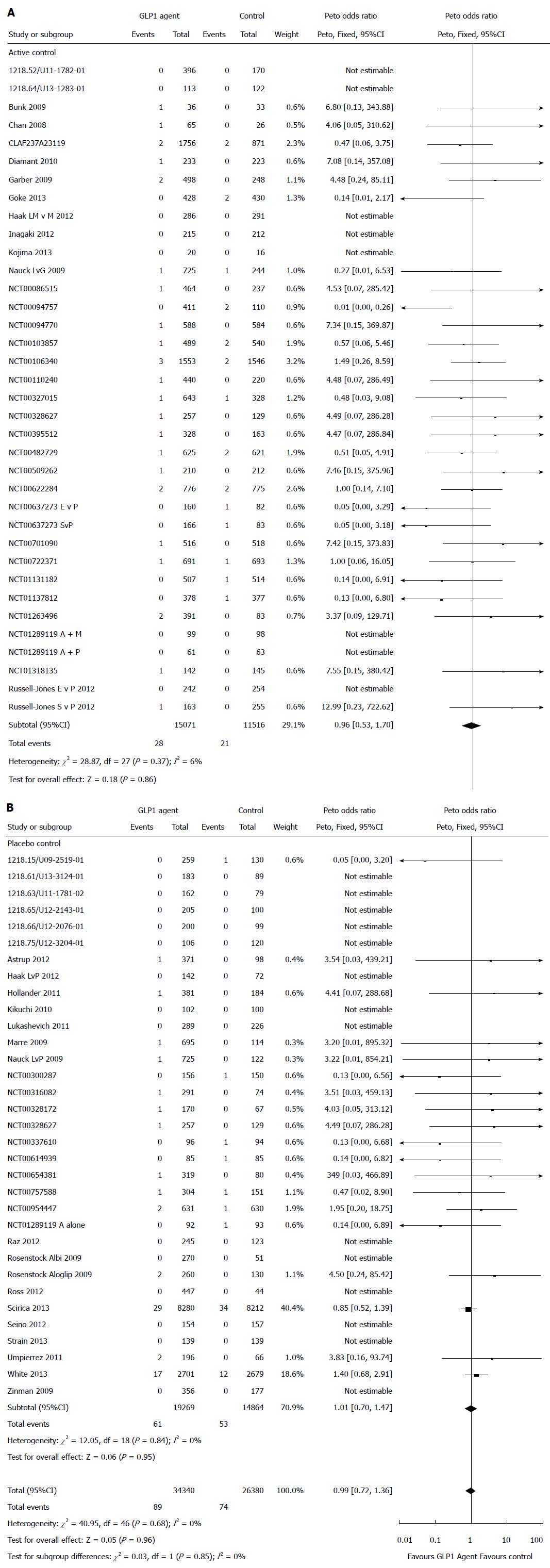
Total pancreatic related adverse events: With a total of 89 pancreatic related adverse events among the 34340 number of patients receiving GLP-1 agents and 74 events among 26380 patients receiving the control agents, there was no statistically significant difference in the risk of pancreatic adverse event associated with GLP-1 agent compared with controls (Peto OR = 0.99, 95%CI: 0.72-1.36, P = 0.96; I2 = 0%) (Figure 2).
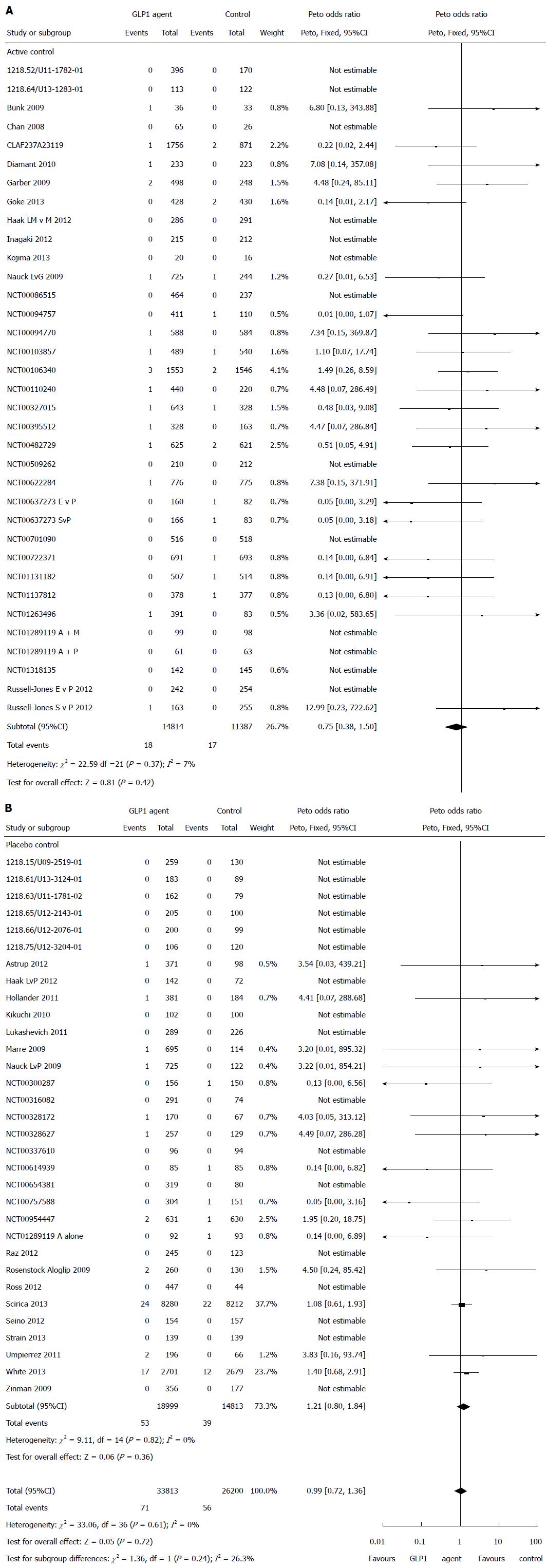
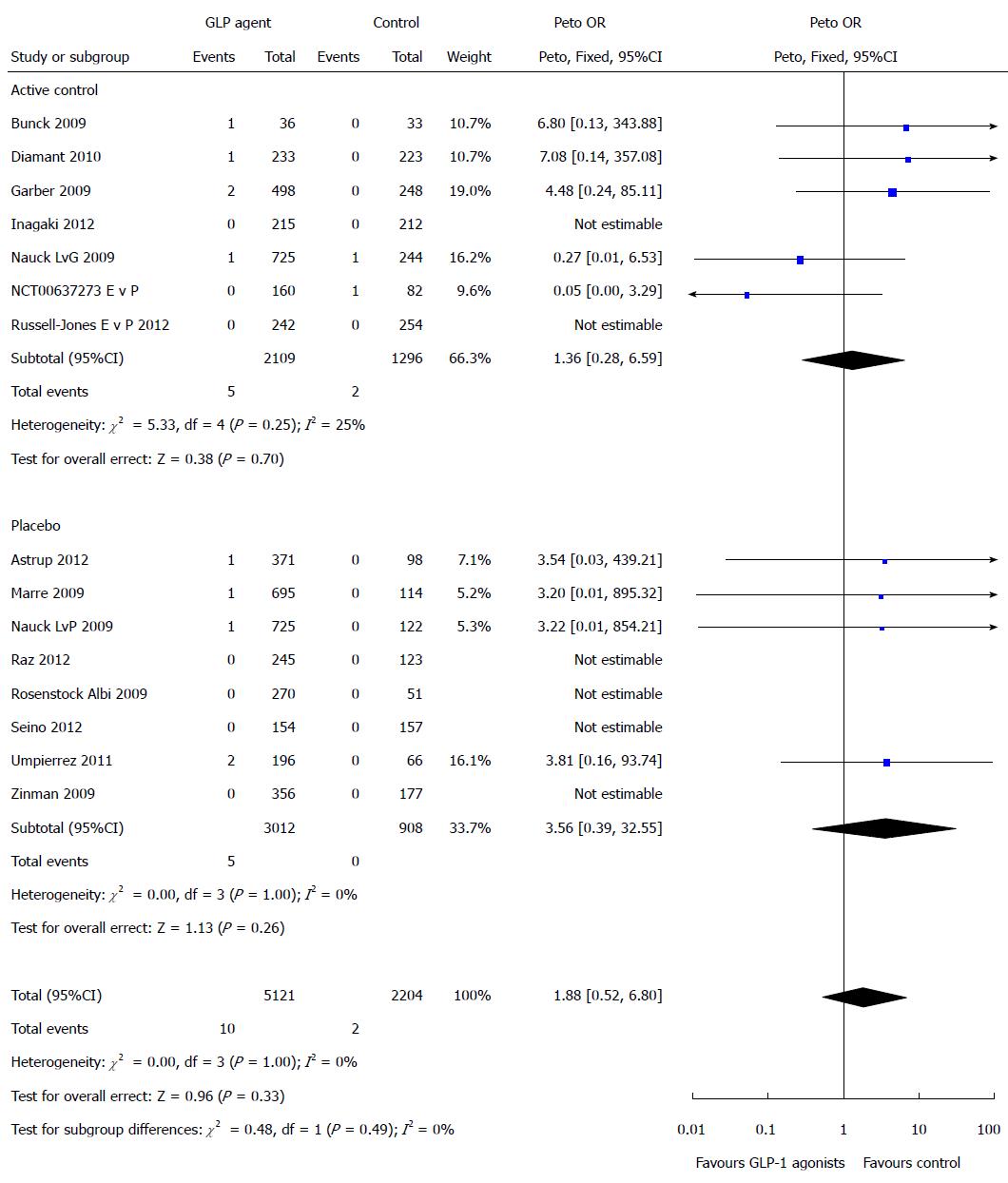
Pancreatitis: There were a total of 71 pancreatitis events in patients on GLP-1 agents and 56 pancreatitis events occurred in the control patients. There was no statistically significant difference in the risk of pancreatic adverse event associated with GLP-1 agent compared with controls (Peto OR = 1.07, 95%CI: 0.75-1.52, P = 0.72, I2 = 0%) (Figure 3).
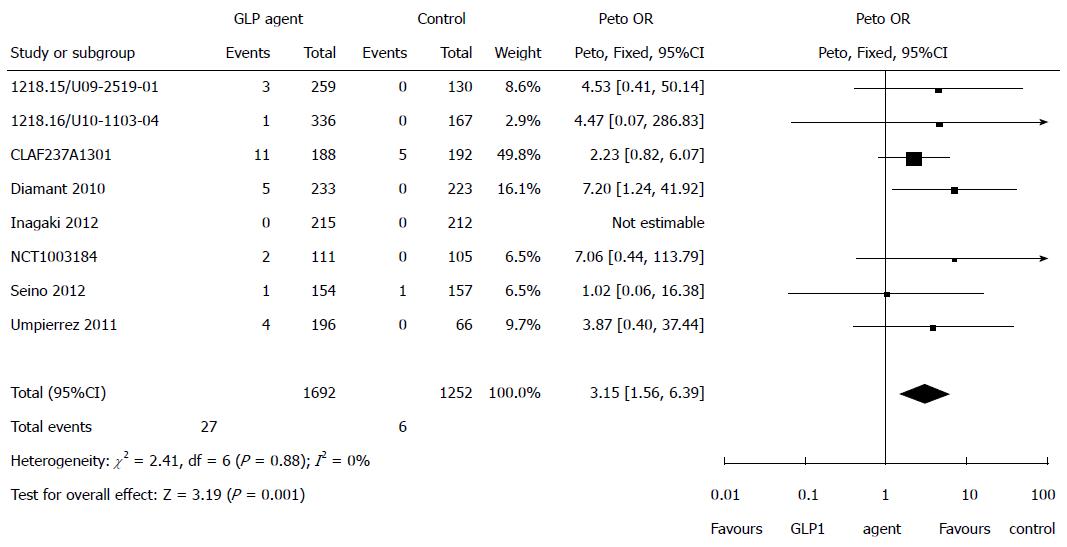
Elevated pancreatic enzymes: Eight studies reported on elevated pancreatic enzymes. There was a statistically significant increased risk of elevation of pancreatic enzymes associated with GLP-1 agents compared with control (Peto OR = 3.15, 95%CI: 1.56-6.39, P = 0.001, I2 = 0%) (Figure 4).
Pancreatic cancer: Eighteen studies reported on pancreatic cancer (Table 3). There were a total of 35 cases of pancreatic cancer reported from studies that used GLP-1 agents. Seventeen cases of pancreatic occurred among 18259 patients taking GLP-1 agents compared to 18 cases among 15785 controls. Of these cases, 2 used linagliptin, 2 used alogliptin, 1 used vildagliptin, 7 used saxagliptin while 5 used sitagliptin. The remaining 18 cases occurred among controls.
| Ref. | Duration of GLP-1 exposure (wk) | Arms | No. of participants | No. of cases |
| NCT00654381[26] | 52 | Linagliptin 5 mg | 159 | 0 |
| Linagliptin 10 mg | 160 | 1 | ||
| Voglibose | 162 | 0 | ||
| Placebo | 80 | 0 | ||
| NCT00622284[27] | 104 | Linagliptin | 776 | 1 |
| Glimepiride | 775 | 2 | ||
| BI Trial No: 1218.15/U09-2519-01[28] | 24 | Linagliptin 5 mg + Pioglitazone 30 mg | 130 | 0 |
| Pioglitazone 30 mg + Placebo | 259 | 1 | ||
| White et al[37] | 76 | Alogliptin | 2701 | 0 |
| Placebo | 2679 | 0 | ||
| NCT01318135[38] | 52 | Alogliptin 12.5 mg qd + Metformin 500 mg bid or 750 mg tid | 142 | 1 |
| Metformin 500 mg bid or 750 mg tid | 145 | 0 | ||
| NCT01263496[40] | 52 | Alogliptin 6.25 mg qd | 96 | 0 |
| Alogliptin 12.5 mg qd | 101 | 0 | ||
| Alogliptin 25 mg qd | 97 | 1 | ||
| Alogliptin 50 mg qd | 97 | 0 | ||
| Voglibose 0.2 mg tid | 83 | 0 | ||
| CLAF237A23119[49] | 12 | Vildagliptin 100 mg + Metformin | 1756 | 1 |
| Thiazolinedione + Metformin | 871 | NR | ||
| NCT00757588[53] | 52 | Saxagliptin 5 mg + Insulin | 304 | 1 |
| Placebo + Insulin | 151 | 0 | ||
| Scirica et al[54] | 109 | Saxagliptin | 8280 | 5 |
| Placebo | 8212 | 12 | ||
| NCT00316082[56] | 24 | Saxagliptin 2.5/5 mg QAM | 71 | 1 |
| Saxagliptin 2.5 mg QAM | 74 | 0 | ||
| Saxagliptin 5 mg QAM | 74 | 0 | ||
| Saxagliptin 5 mg QPM | 72 | 0 | ||
| Placebo | 74 | 0 | ||
| Chan et al[58,59] | 54 | Sitagliptin 50 mg or 25 mg once daily | 65 | 1 |
| Placebo/Glipizide | 26 | 0 | ||
| Ferreira et al[61,62] | 54 | Sitagliptin | 210 | 1 |
| Glipizide | 212 | 0 | ||
| Henry et al[63,64] | 54 | Pioglitazone 15 mg | 230 | 0 |
| Pioglitazone 30 mg | 233 | 0 | ||
| Pioglitazone 45 mg | 230 | 0 | ||
| Sitagliptin 100 mg/Pioglitazone 15 mg | 230 | 0 | ||
| Sitagliptin 100 mg/Pioglitazone 30 mg | 231 | 1 | ||
| Sitagliptin 100 mg/Pioglitazone 45 mg | 230 | 0 | ||
| Raz et al[65,66] | 30 | Sitagliptin 100 mg | 96 | 0 |
| Placebo | 94 | 1 | ||
| Goldstein et al[68,69] | 104 | Metformin 500 mg bid | 182 | 0 |
| Metformin 1000 mg bid | 182 | 0 | ||
| Sitagliptin 50 mg bid + Metformin 500 mg bid | 190 | 0 | ||
| Sitagliptin 50 mg bid + Metformin 1000 mg bid | 182 | 0 | ||
| Sitagliptin 50 mg bid + Metformin 1000 mg bid | 117 | 0 | ||
| Placebo/Metformin 1000 mg bid | 176 | 1 | ||
| Arechavaleta et al[70,71] | 30 | Sitagliptin | 516 | 1 |
| Glimepiride | 518 | 0 | ||
| Charbonnel et al[72,73] | 104 | Sitagliptin 100 mg | 464 | 1 |
| Placebo/Glipizide 5 mg | 237 | 0 | ||
| NCT00094757[76] | 54 | Sitagliptin 100 mg | 205 | 0 |
| Sitagliptin 200 mg | 206 | 0 | ||
| Placebo/Pioglitazone | 110 | 1 |
DPP-4 inhibitors: (1) Linagliptin: Fifteen studies that used Linagliptin had a total of 7263 patients. There was no statistically significant difference in the risk of pancreatic adverse event (Peto OR = 1.14, 95%CI: 0.32-4.13) or pancreatitis (Peto OR = 2.90, 95%CI: 0.49-17.36) associated with linagliptin compared with controls; (2) Alogliptin: Nine studies that used Alogliptin had a total of 7914 patients. In comparison with control, there was no increased risk of having a pancreatic adverse event (Peto OR = 1.59, 95%CI: 0.82-3.07) or pancreatitis (Peto OR = 1.50, 95%CI: 0.77-2.94) with alogiptin; (3) Vildagliptin: Seven studies that used Vildagliptin had a total of 7687 patients. In comparison with control, there was no statistically significant difference in the risk of pancreatic adverse event (Peto OR = 0.87, 95%CI: 0.26-2.94) or pancreatitis (Peto OR = 0.75, 95%CI: 0.21-2.67) with vildagliptin; (4) Saxagliptin: Seven studies that used Saxagliptin had a total of 19876 patients. In comparison with control, there was no statistically significant difference in the risk of pancreatic adverse event (Peto OR = 0.79, 95%CI: 0.49-1.25) or pancreatitis (Peto OR 0.91, 95%CI: 0.53-1.56) with saxaglipitin; and (5) sitagliptin: Sixteen studies that used Sitagliptin had a total of 10360 patients. In comparison with control, there was no statistically significant difference in the risk of pancreatic adverse event (Peto OR = 0.66, 95%CI: 0.27-1.63) or pancreatitis (Peto OR = 0.45, 95%CI: 0.14-1.43) with sitagliptin.
Exenatide: Five studies that used Exenatide had a total of 1690 patients. In comparison with control, there was no statistically significant difference in the risk of pancreatic adverse event (Peto OR = 1.53, 95%CI: 0.15-15.29) or pancreatitis (Peto OR = 1.53, 95%CI: 0.15-15.29) with exenatide.
Liraglutide: Six studies that used Liraglutide had a total of 4373 patients. In comparison with control, there was no statistically significant difference in the risk of pancreatic adverse event (Peto OR = 1.71, 95%CI: 0.29-10.04) or pancreatitis (Peto OR = 1.71, 95%CI: 0.29-10.04 with liraglutide.
Dulaglutide: One study that used Dulaglutide had 262 patients. In comparison with control, there was no statistically significant difference in the risk of pancreatic adverse event (Peto OR = 3.83, 95%CI: 0.16-93.74) or pancreatitis (Peto OR = 3.83, 95%CI: 0.16-93.74) with dulaglutide.
Taspoglutide, albiglutide and lixisenatide: Taspoglutide, Albiglutide and Lixisenatide all had 1 study each with 368, 321 and 311 patients each. The effect estimates were not estimable due to the small number of events.
In a post-hoc analysis, we examined whether there was any difference between DPP-4 inhibitors and GLP-1 based therapies. The results showed that neither the DPP-4 inhibitors nor the GLP- 1 based therapies were associated with a risk of pancreatic complications (Figure 5).
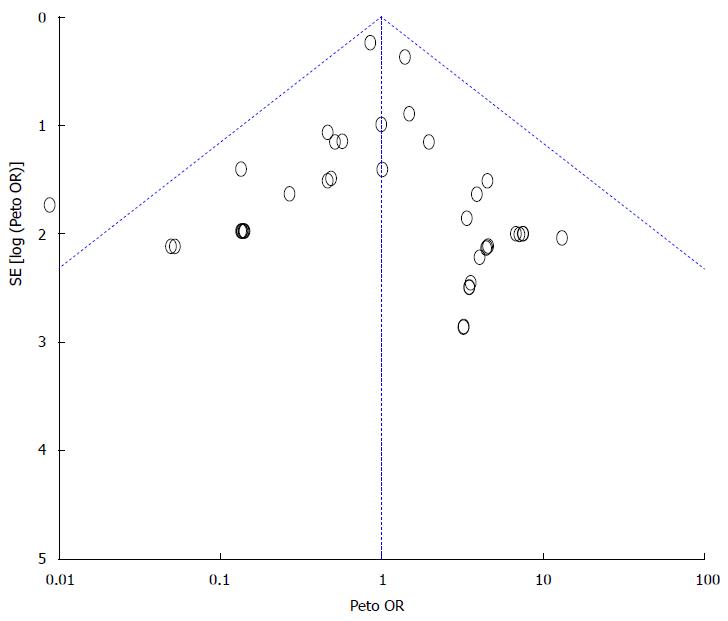
We did not detect any publication bias in the funnel plot (Figure 6).
Our study showed a significantly increased risk of pancreatic enzyme elevation with GLP-1 based therapies. However, the use of GLP-1 based therapies was not associated with a statistically significant increased risk of pancreatic complication events in patients with type 2 diabetes in randomized controlled trials. Additionally, when we examined individual agents, none of the DPP-4 inhibitors or GLP-1 agonists was associated with a statistically significant increased risk of pancreatitis (Figure 3). Despite the lack of statistical significance the upper bounds of the CI in several analyses, particularly for the GLP-1 receptor agonists (exenatide, liraglutide and albiglutide) exceeded 1 and could not rule out a clinically significant hazard. There were an insufficient number of cases of pancreatic cancer to allow for the estimation of meaningful differences between GLP-1 based agents and controls.
These discordant results-no significant effect on the outcome of acute pancreatitis but significant increase in the risk of pancreatic enzyme elevation associated with GLP-1 based therapies in a small number of studies may have two alternative explanations.
These could indicate that injury with GLP-1 based therapies is sub-threshold and result in pancreatic inflammation that may not reach the level of acute pancreatitis. Alternatively, the ascertainment of pancreatic adverse events/complications may have been more complete in this subset of studies showing an elevation in pancreatic enzymes. It was not clear whether pancreatitis adverse events were rigorously defined or captured in an objective rather than subjective manner across the trials, potentially biasing towards the null due to misclassification. In contrast, measurement of elevated pancreatic enzymes is a more objective measure, serial enzyme measurements should be regularly checked in trial participants on GLP-1 agents who present with gastrointestinal symptoms. Lack of awareness for the need to assess pancreatic enzymes could lead to under-ascertainment of pancreatic adverse events in patients presenting with upper abdominal symptoms. Among patients with type 2 diabetes, one previous study reported an increase in enzyme associated with DPP-4 inhibitors compared to controls (36% vs 18%), suggesting that this adverse reaction deserves further investigation[15].
Our meta-analysis should be seen in the light of other recent studies. A recent review reported a slightly increased trend for reporting of acute pancreatitis associated with GLP-1 receptor agonists but not with DPP-IV inhibitors[16]. Two other systematic reviews reported no increased risk of acute pancreatitis, but with very wide confidence intervals that could not rule out a significant increase[6,17]. However, one such meta-analysis included observational studies, which may be prone to confounding[17]. The difference in meta-analysis should reflect differences in inclusion of trials and ascertainment of events. Importantly none of the previous meta-analysis have reported on elevations in pancreatic enyzmes associated with GLP-1 based therapies. However, the CIs were wide in all meta-analyses and we could not rule out a significant increase in the risk of pancreatitis with GLP-1 based therapies. The lack of statistical significance may reflect incomplete ascertainment of pancreatic adverse events in clinical trials of GLP-1 based therapies or inadequate statistical power to detect rare but serious complication such as pancreatitis. Observational studies have also shown inconsistent results between GLP-1 based therapies and acute pancreatitis due to incomplete ascertainment of covariates, or poor performance of the diagnostic codes for acute pancreatitis[5,18-20]. It is also unclear whether the inflammatory process from recurrent or chronic pancreatitis is a predisposing factor to subsequent development of pancreatic cancer.
Our study has some limitations. We limited our analysis to published RCTs. However; there may be unpublished studies that report on this outcome. We did not have access to data to conduct individual patient data meta-analysis and ascertain time to the occurrence of pancreatic enzyme elevations. Importantly, clinical trials may not have ascertained the occurrence of pancreatitis on participants who withdrew from the trial (as a result of the complication). This may bias our estimates towards the null. The availability of sponsors of individual patient data to independent investigators may allow for further analyses.
Our meta-analysis shows a three-fold increased risk of pancreatic enzyme elevation with GLP-1 based agents compared to controls, without an a significant increased risk of pancreatitis or pancreatic cancer due to small number of cases. Future adequately powered observational studies with well validated codes for pancreatitis and pancreatic cancer and careful control of confounding are needed to evaluate the risk of pancreatic enzyme elevation, pancreatitis and pancreatic cancer with GLP-1 based therapies.
Recent developments have led to an increasingly wide range of glucose lowering drugs being trialed for treatment of type II diabetes mellitus. However, a variety of concerns have been raised regarding the safety of these new agents for long-term chronic use. This has led to tightening of the regulatory landscape and closer scrutiny of data regarding serious rare adverse events.
Many trials have been conducted to demonstrate the efficacy of glucagon-like-peptide-1 (GLP-1) agonists and dipeptidyl peptidase-4 (DPP-4) inhibitors in reducing blood glucose levels. However, there have been suggestions of a potential increase in risk of pancreatic adverse events with these drugs due to a postulated proliferative effect on pancreatic cells. The existing evidence base is conflicting, and difficult to interpret due to the very low incidence of pancreatic adverse events.
The findings of this meta-analysis are that risks of pancreatitis or pancreatic cancer have not been definitively established with any of the GLP-1 agonists or DPP-4 inhibitors. However, there is a signal suggesting increased risk of elevated pancreatic enzymes, which has not previously been described in other systematic reviews.
GLP-1 agonists or DPP-4 inhibitors may have some relationship with elevations in the pancreatic enzyme levels. Further large scale studies are needed to determine if these elevations may or may not be associated with adverse clinical outcomes.
GLP-1 belongs to the incretin group of hormones which act to stimulate insulin secretion dependent on glucose levels. GLP-1 receptor agonists are drugs developed as incretin-mimetics. DPP-4 is an enzyme that breaks down GLP-1, thus causing GLP-1 to have a short half-life. Drugs that inhibit DPP-4 would be expected to increase the availability of endogenous GLP-1.
This manuscript has a great collecting data about this topic.
P- Reviewer: Rungsakulkij N S- Editor: Qi Y L- Editor: A E- Editor: Jiao XK
| 1. | Butler PC, Dry S, Elashoff R. GLP-1-based therapy for diabetes: what you do not know can hurt you. Diabetes Care. 2010;33:453-455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 2. | Drucker DJ, Sherman SI, Gorelick FS, Bergenstal RM, Sherwin RS, Buse JB. Incretin-based therapies for the treatment of type 2 diabetes: evaluation of the risks and benefits. Diabetes Care. 2010;33:428-433. [PubMed] |
| 3. | Nachnani JS, Bulchandani DG, Nookala A, Herndon B, Molteni A, Pandya P, Taylor R, Quinn T, Weide L, Alba LM. Biochemical and histological effects of exendin-4 (exenatide) on the rat pancreas. Diabetologia. 2010;53:153-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 154] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 4. | Matveyenko AV, Dry S, Cox HI, Moshtaghian A, Gurlo T, Galasso R, Butler AE, Butler PC. Beneficial endocrine but adverse exocrine effects of sitagliptin in the human islet amyloid polypeptide transgenic rat model of type 2 diabetes: interactions with metformin. Diabetes. 2009;58:1604-1615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 188] [Cited by in RCA: 191] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 5. | Singh S, Chang HY, Richards TM, Weiner JP, Clark JM, Segal JB. Glucagonlike peptide 1-based therapies and risk of hospitalization for acute pancreatitis in type 2 diabetes mellitus: a population-based matched case-control study. JAMA Intern Med. 2013;173:534-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 306] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 6. | Monami M, Dicembrini I, Martelli D, Mannucci E. Safety of dipeptidyl peptidase-4 inhibitors: a meta-analysis of randomized clinical trials. Curr Med Res Opin. 2011;27 Suppl 3:57-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 162] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 7. | Alves C, Batel-Marques F, Macedo AF. A meta-analysis of serious adverse events reported with exenatide and liraglutide: acute pancreatitis and cancer. Diabetes Res Clin Pract. 2012;98:271-284. [PubMed] |
| 8. | Shihab HM, Akande T, Loke YK, Singh S. Risk of pancreatic complication events associated with the use of GLP-1 receptor agonist and DPP-4 inhibitor drugs: A systematic review and meta-analysis. PROSPERO: International prospective register of systematic reviews. Available from: http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42013004742. Accessed June 23, 2014. |
| 9. | Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; 2011. . |
| 10. | Loke YK, Price D, Herxheimer A. Chapter 14: Adverse Effects. Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley and Sons 2008; . |
| 11. | Ioannidis JP, Trikalinos TA. The appropriateness of asymmetry tests for publication bias in meta-analyses: a large survey. CMAJ. 2007;176:1091-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 662] [Cited by in RCA: 754] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 12. | Review Manager (RevMan) [computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration 2014; . |
| 13. | Davies HT, Crombie IK, Tavakoli M. When can odds ratios mislead? BMJ. 1998;316:989-991. [PubMed] |
| 14. | Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21630] [Cited by in RCA: 25798] [Article Influence: 1121.7] [Reference Citation Analysis (0)] |
| 15. | Lando HM, Alattar M, Dua AP. Elevated amylase and lipase levels in patients using glucagonlike peptide-1 receptor agonists or dipeptidyl-peptidase-4 inhibitors in the outpatient setting. Endocr Pract. 2012;18:472-477. [PubMed] |
| 16. | Meier JJ, Nauck MA. Risk of pancreatitis in patients treated with incretin-based therapies. Diabetologia. 2014;57:1320-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 17. | Li L, Shen J, Bala MM, Busse JW, Ebrahim S, Vandvik PO, Rios LP, Malaga G, Wong E, Sohani Z. Incretin treatment and risk of pancreatitis in patients with type 2 diabetes mellitus: systematic review and meta-analysis of randomised and non-randomised studies. BMJ. 2014;348:g2366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 175] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 18. | Garg R, Chen W, Pendergrass M. Acute pancreatitis in type 2 diabetes treated with exenatide or sitagliptin: a retrospective observational pharmacy claims analysis. Diabetes Care. 2010;33:2349-2354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 233] [Cited by in RCA: 234] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 19. | Dore DD, Hussein M, Hoffman C, Pelletier EM, Smith DB, Seeger JD. A pooled analysis of exenatide use and risk of acute pancreatitis. Curr Med Res Opin. 2013;29:1577-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Chou HC, Chen WW, Hsiao FY. Acute pancreatitis in patients with type 2 diabetes mellitus treated with dipeptidyl peptidase-4 inhibitors: a population-based nested case-control study. Drug Saf. 2014;37:521-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Ross SA, Rafeiro E, Meinicke T, Toorawa R, Weber-Born S, Woerle HJ. Efficacy and safety of linagliptin 2.5 mg twice daily versus 5 mg once daily in patients with type 2 diabetes inadequately controlled on metformin: a randomised, double-blind, placebo-controlled trial. Curr Med Res Opin. 2012;28:1465-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Haak T, Meinicke T, Jones R, Weber S, von Eynatten M, Woerle HJ. Initial combination of linagliptin and metformin in patients with type 2 diabetes: efficacy and safety in a randomised, double-blind 1-year extension study. Int J Clin Pract. 2013;67:1283-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 117] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 23. | Boehringer Ingelheim Pharmaceuticals. Efficacy and safety of 3 doses of BI1356 (linagliptin) in type 2 diabetes patients. gov [Internet]. Bethesda (MD): National Library of Medicine (US). [accessed 2014; Jun 8] Available from: https://clinicaltrials.gov/ct2/show/NCT00328172 NLM Identifier: NCT00328172. |
| 24. | Yki-Järvinen H, Rosenstock J, Durán-Garcia S, Pinnetti S, Bhattacharya S, Thiemann S, Patel S, Woerle HJ. Effects of adding linagliptin to basal insulin regimen for inadequately controlled type 2 diabetes: a ≥52-week randomized, double-blind study. Diabetes Care. 2013;36:3875-3881. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 111] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 25. | Boehringer Ingelheim Pharmaceuticals, Eli Lilly and Company. Efficacy and safety of linagliptin in combination with insulin in patients with type 2 diabetes. gov [Internet]. Bethesda (MD): National Library of Medicine (US). [accessed 2014; Jun 8] Available from: https://clinicaltrials.gov/ct2/show/NCT00954447 NLM Identifier: NCT00954447. |
| 26. | Boehringer Ingelheim Pharmaceuticals. Japanese P III vs voglibose and placebo. gov [Internet]. Bethesda (MD): National Library of Medicine (US). [accessed 2014; Jun 8] Available from: https://clinicaltrials.gov/ct2/show/NCT00654381 NLM Identifier: NCT00654381. |
| 27. | Boehringer Ingelheim Pharmaceuticals. Efficacy and safety of BI 1356 in combination with metformin in patients with type 2 diabetes. gov [Internet]. Bethesda (MD): National Library of Medicine (US). [accessed 2014; Jun 8] Available from: https://clinicaltrials.gov/ct2/show/NCT00622284 NLM Identifier: NCT00622284. |
| 28. | Boehringer Ingelheim Pharmaceuticals. A randomised, double-blind, placebo controlled, parallel group 24 week study to assess the efficacy and safety of linagliptin (5mg) in combination with 30mg pioglitazone (both administered orally once daily), compared to 30mg pioglitazone plus placebo in drug-naive or previously treated type 2 diabetic patients with insufficient glycaemic control. [accessed 2014 Jun 8]. Available from: http://trials.boehringer-ingelheim.com/content/dam/internet/opu/clinicaltrial/com_EN/results/1218/1218.15_U09-2519.pdf. |
| 29. | Boehringer Ingelheim Pharmaceuticals. A phase III randomised, double-blind parallel group extension study to investigate the safety and efficacy of twice daily administration of the free combination of linagliptin 2.5 mg metformin 500 mg or of linagliptin 2.5 mg metformin 1000mg versus monotherapy with metformin 1000 mg twice daily over 54 weeks in type 2 diabetic patients previously completing the double-blind part of study 1218.46. [accessed 2014 Jun 8]. Available from: http://trials.boehringer-ingelheim.com/content/dam/internet/opu/clinicaltrial/com_EN/results/1218/1218.52_U11-1782-01.pdf. |
| 30. | Boehringer Ingelheim Pharmaceuticals. A phase III randomised, double-blind, placebo-controlled, parallel group, efficacy and safety study of linagliptin (5mg), administered orally once daily over 24 weeks in type 2 diabetic patients (age 70 years) with insufficient glycaemic control (HbA1c 7.0%) despite metformin and/or sulphonylurea and/or insulin therapy. [accessed 2014 Jun 8]. Available from: http://trials.boehringer-ingelheim.com/content/dam/internet/opu/clinicaltrial/com_EN/results/1218/1218.63_U11-1781-02.pdf. |
| 31. | Boehringer Ingelheim Pharmaceuticals. A phase IIIb, 24 week, randomised, placebo-controlled, double-blinded, efficacy and safety study of linagliptin in black/african american patients with type 2 diabetes with a MTT sub-study. [accessed 2014Jun 8]. Available from: http://trials.boehringer-ingelheim.com/content/dam/internet/opu/clinicaltrial/com_EN/results/1218/1218.75_U12-3204-01.pdf. |
| 32. | Boehringer Ingelheim Pharmaceuticals. A phase III, randomised, double-blind, placebo-controlled parallel group efficacy and safety study of linagliptin 5mg administered orally once daily over 24 weeks in type 2 diabetic patients with insufficient glycaemic control despite a therapy of metformin in combination with pioglitazone. [accessed 2014 Jun 8]. Available from: http://trials.boehringer-ingelheim.com/content/dam/internet/opu/clinicaltrial/com_EN/results/1218/1218.61_U13-3124-01.pdf. |
| 33. | Boehringer Ingelheim Pharmaceuticals. A randomised, double-blind, placebo-controlled parallel group efficacy and safety study of linagliptin (5mg administered orally once daily) over 24 weeks in type 2 diabetic patients with insufficient glycaemic control despite metformin therapy in asian population. [accessed 2014 Jun 8]. Available from: http://trials.boehringer-ingelheim.com/content/dam/internet/opu/clinicaltrial/com_EN/results/1218/1218.65_U12-2143-01.pdf. |
| 34. | Boehringer Ingelheim Pharmaceuticals. A phase III, randomised, double-blind, placebo-controlled parallel group safety and efficacy study of linagliptin (5mg administered orally once daily) over 12 weeks followed by a 40 week double-blind extension period (placebo patients switched to glimepiride) in drug naive or previously treated type 2 diabetic patients with moderate to severe renal impairment and insufficient glycaemic control. [accessed 2014 Jun 8]. Available from: http://trials.boehringer-ingelheim.com/content/dam/internet/opu/clinicaltrial/com_EN/results/1218/1218.64_U13-1283-01-DS.pdf. |
| 35. | Boehringer Ingelheim Pharmaceuticals. A randomised, double-blind, placebo-controlled parallel group, efficacy and safety study of linagliptin (5mg administered orally once daily) over 24 weeks, in drug naive or previously treated type 2 diabetic patients with insufficient glycaemic control. [accessed 2014 Jun 8]. Available from: http://trials.boehringer-ingelheim.com/content/dam/internet/opu/clinicaltrial/com_EN/results/1218/1218.66_U12-2076-01.pdf. |
| 36. | Rosenstock J, Rendell MS, Gross JL, Fleck PR, Wilson CA, Mekki Q. Alogliptin added to insulin therapy in patients with type 2 diabetes reduces HbA(1C) without causing weight gain or increased hypoglycaemia. Diabetes Obes Metab. 2009;11:1145-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 144] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 37. | White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, Perez AT, Fleck PR, Mehta CR, Kupfer S. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327-1335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1927] [Cited by in RCA: 1898] [Article Influence: 158.2] [Reference Citation Analysis (0)] |
| 38. | White WB; Takeda. Long-term safety study of alogliptin used in combination with sulfonylurea or metformin in participants with type 2 diabetes in japan. gov [Internet]. Bethesda (MD): National Library of Medicine (US). [accessed 2014; Jun 22] Available from: https://clinicaltrials.gov/ct2/show/NCT01318135 NLM Identifier: NCT01318135. |
| 39. | White WB; Takeda. Efficacy and safety of alogliptin in participants with type 2 diabetes. gov [Internet]. Bethesda (MD): National Library of Medicine (US). [accessed 2014; Jun 22] Available from: https://clinicaltrials.gov/ct2/show/NCT01289119 NLM Identifier: NCT01289119. |
| 40. | White WB; Takeda. Long-term safety study of alogliptin in participants with type 2 diabetes in japan. gov [Internet]. Bethesda (MD): National Library of Medicine (US). [accessed 2014; Jun 22] Available from: https://clinicaltrials.gov/ct2/results?term=NCT01263496 NLM Identifier: NCT01263496. |
| 41. | White WB; Takeda. Efficacy and safety of alogliptin combined with pioglitazone in treating subjects with type 2 diabetes mellitus. gov [Internet]. Bethesda (MD): National Library of Medicine (US). [accessed 2014; Jun 22] Available from: https://clinicaltrials.gov/ct2/show/NCT00328627 NLM Identifier: NCT00328627. |
| 42. | White WB; Takeda. Efficacy of alogliptin with pioglitazone (actos) in subjects with type 2 diabetes mellitus. gov [Internet]. Bethesda (MD): National Library of Medicine (US). [accessed 2014; Jun 22] Available from: https://clinicaltrials.gov/ct2/show/NCT00395512 NLM Identifier: NCT00395512. |
| 43. | Kikuchi M, Haneda M, Koya D, Tobe K, Onishi Y, Couturier A, Mimori N, Inaba Y, Goodman M. Efficacy and tolerability of vildagliptin as an add-on to glimepiride in Japanese patients with Type 2 diabetes mellitus. Diabetes Res Clin Pract. 2010;89:216-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 44. | Lukashevich V, Schweizer A, Shao Q, Groop PH, Kothny W. Safety and efficacy of vildagliptin versus placebo in patients with type 2 diabetes and moderate or severe renal impairment: a prospective 24-week randomized placebo-controlled trial. Diabetes Obes Metab. 2011;13:947-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 110] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 45. | Strain WD, Lukashevich V, Kothny W, Hoellinger MJ, Paldánius PM. Individualised treatment targets for elderly patients with type 2 diabetes using vildagliptin add-on or lone therapy (INTERVAL): a 24 week, randomised, double-blind, placebo-controlled study. Lancet. 2013;382:409-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 112] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 46. | Novartis Pharmaceuticals. Vildagliptin compared to glimepiride in combination with metformin in patients with type 2 diabetes. gov [Internet]. Bethesda (MD): National Library of Medicine (US). [accessed 2014; Jun 22] Available from: https://clinicaltrials.gov/ct2/show/NCT00106340 NLM Identifier: NCT00106340. |
| 47. | Novartis Pharmaceuticals. A 56-week extension to a clinical study to assess the efficacy and safety of vildagliptin compared to placebo in drug naive patients with type 2 diabetes and mild hyperglycemia. gov [Internet]. Bethesda (MD): National Library of Medicine (US). [accessed 2014; Jun 22] Available from: https://clinicaltrials.gov/ct2/show/NCT00300287 NLM Identifier: NCT00300287. |
| 48. | Novartis Pharmaceuticals. A multicenter, randomized, double-blind, active-controlled study to compare the effects of 12 weeks treatment with vildagliptin 50 mg b.i.d. to voglibose 0.2 mg t.i.d. in patients with type 2 diabetes. [accessed Jun 22]. Available from: http://www.novctrd.com/ctrdWebApp/clinicaltrialrepository/displayFile.do?trialResult=2524. |
| 49. | Novartis Pharmaceuticals. A multi-center, randomized, open-label, active controlled, parallel arm study to compare the efficacy of 12 weeks of treatment with vildagliptin 100 mg, once daily (qd) to thiazolidinedione (TZD) as add-on therapy in patients with type 2 diabetes inadequately controlled with metformin monotherapy in a community-based practice setting. [accessed Jun 22]. Available from: http://www.novctrd.com/ctrdWebApp/clinicaltrialrepository/displayFile.do?trialResult=2567.. |
| 50. | Novartis Pharmaceuticals. Efficacy and safety of vildagliptin compared to acarbose in drug naive patients with type 2 diabetes. gov [Internet]. Bethesda (MD): National Library of Medicine (US). [accessed 2014; https://clinicaltrials.gov/ct2/show/NCT00110240 NLM Identifier: NCT00110240. |
| 51. | Novartis Pharmaceuticals; AstraZeneca. A phase 3 study of BMS-477118 in combination with metformin in subjects with type 2 diabetes who are not controlled with diet and exercise. gov [Internet]. Bethesda (MD): National Library of Medicine (US). [accessed 2014; Jun 22] Available from: https://clinicaltrials.gov/ct2/show/NCT00327015 NLM Identifier: NCT00327015. |
| 52. | Hollander PL, Li J, Frederich R, Allen E, Chen R. Safety and efficacy of saxagliptin added to thiazolidinedione over 76 weeks in patients with type 2 diabetes mellitus. Diab Vasc Dis Res. 2011;8:125-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 53. | Hollander PL; AstraZeneca. Safety and efficacy of saxagliptin plus insulin with or without metformin. gov [Internet]. Bethesda (MD): National Library of Medicine (US). [accessed 2014; Jun 22] Available from: https://clinicaltrials.gov/ct2/show/NCT00757588 NLM Identifier: NCT00757588. |
| 54. | Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, Ohman P, Frederich R, Wiviott SD, Hoffman EB. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2804] [Cited by in RCA: 2583] [Article Influence: 215.3] [Reference Citation Analysis (0)] |
| 55. | Göke B, Gallwitz B, Eriksson JG, Hellqvist Å, Gause-Nilsson I. Saxagliptin vs. glipizide as add-on therapy in patients with type 2 diabetes mellitus inadequately controlled on metformin alone: long-term (52-week) extension of a 52-week randomised controlled trial. Int J Clin Pract. 2013;67:307-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 56. | Göke B; AstraZeneca. Study of BMS-477118 as monotherapy with titration in subjects with type 2 diabetes who are not controlled with diet and exercise. gov [Internet]. Bethesda (MD): National Library of Medicine (US). [accessed 2014; https://clinicaltrials.gov/ct2/show/NCT00316082 NLM Identifier: NCT00316082. |
| 57. | Göke B; AstraZeneca. Treatment effect of saxagliptin compared with placebo in patients with type 2 diabetes and renal impairment. gov [Internet]. Bethesda (MD): National Library of Medicine (US). [accessed 2014; Jun 22] Available from: https://clinicaltrials.gov/ct2/show/NCT00614939 NLM Identifier: NCT00614939. |
| 58. | Merck Sharp and Dohme Corp. An investigational drug in patients with type 2 diabetes mellitus and chronic renal insufficiency. gov [Internet]. Bethesda (MD): National Library of Medicine (US). [accessed 2014; Jun 22] Available from: https://clinicaltrials.gov/ct2/show/NCT00095056 NLM Identifier: NCT00095056. |
| 59. | Chan JC, Scott R, Arjona Ferreira JC, Sheng D, Gonzalez E, Davies MJ, Stein PP, Kaufman KD, Amatruda JM, Williams-Herman D. Safety and efficacy of sitagliptin in patients with type 2 diabetes and chronic renal insufficiency. Diabetes Obes Metab. 2008;10:545-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 181] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 60. | Kojima Y, Kaga H, Hayashi S, Kitazawa T, Iimura Y, Ohno M, Yoshitsugu M, Fujiwara M, Hiyoshi T. Comparison between sitagliptin and nateglinide on postprandial lipid levels: The STANDARD study. World J Diabetes. 2013;4:8-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 61. | Arjona Ferreira JC, Marre M, Barzilai N, Guo H, Golm GT, Sisk CM, Kaufman KD, Goldstein BJ. Efficacy and safety of sitagliptin versus glipizide in patients with type 2 diabetes and moderate-to-severe chronic renal insufficiency. Diabetes Care. 2013;36:1067-1073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 122] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 62. | Merck Sharp and Dohme Corp. Sitagliptin versus glipizide in participants with type 2 diabetes mellitus and chronic renal insufficiency (MK-0431-063 AM1). gov [Internet]. Bethesda (MD): National Library of Medicine (US). [accessed 2014; Jun 22] Available from: https://clinicaltrials.gov/ct2/show/NCT00509262 NLM Identifier: NCT00509262. |
| 63. | Merck Sharp and Dohme Corp. MK0431 and pioglitazone co-administration factorial study in patients with type 2 diabetes mellitus (0431-102 AM2). gov [Internet]. Bethesda (MD): National Library of Medicine (US). [accessed 2014; Jun 22] Available from: https://clinicaltrials.gov/ct2/show/NCT00722371 NLM Identifier: NCT00722371. |
| 64. | Henry RR, Staels B, Fonseca VA, Chou MZ, Teng R, Golm GT, Langdon RB, Kaufman KD, Steinberg H, Goldstein BJ. Efficacy and safety of initial combination treatment with sitagliptin and pioglitazone--a factorial study. Diabetes Obes Metab. 2014;16:223-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 65. | Merck Sharp and Dohme Corp. Sitagliptin metformin add-on study in patients with TYpe 2 diabetes mellitus. gov [Internet]. Bethesda (MD): National Library of Medicine (US). [accessed 2014; Jun 22] Available from: https://clinicaltrials.gov/ct2/show/NCT00337610 NLM Identifier: NCT00337610. |
| 66. | Raz I, Chen Y, Wu M, Hussain S, Kaufman KD, Amatruda JM, Langdon RB, Stein PP, Alba M. Efficacy and safety of sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes. Curr Med Res Opin. 2008;24:537-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 180] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 67. | Merck Sharp and Dohme Corp. Study of sitagliptin treatment in patients with type 2 diabetes during ramadhan (0431-263). gov [Internet]. Bethesda (MD): National Library of Medicine (US). [accessed 2014; Jun 22] Available from: https://clinicaltrials.gov/ct2/show/NCT01131182 NLM Identifier: NCT01131182. |
| 68. | Goldstein BJ, Feinglos MN, Lunceford JK, Johnson J, Williams-Herman DE. Effect of initial combination therapy with sitagliptin, a dipeptidyl peptidase-4 inhibitor, and metformin on glycemic control in patients with type 2 diabetes. Diabetes Care. 2007;30:1979-1987. [PubMed] |
| 69. | Merck Sharp and Dohme Corp. MK0431 (sitagliptin) and metformin co-administration factorial study in patients with type 2 diabetes mellitus (0431-036). gov [Internet]. Bethesda (MD): National Library of Medicine (US). [accessed 2014; Jun 22] Available from: https://clinicaltrials.gov/ct2/show/NCT00103857 NLM Identifier: NCT00103857. |
| 70. | Merck Sharp and Dohme Corp. A study to test the safety and efficacy of sitagliptin compared to glimepiride in patients with type 2 diabetes on a stable dose of metformin (0431-803). gov [Internet]. Bethesda (MD): National Library of Medicine (US). [accessed 2014; Jun 22] Available from: https://clinicaltrials.gov/ct2/show/NCT00701090 NLM Identifier: NCT00701090. |
| 71. | Arechavaleta R, Seck T, Chen Y, Krobot KJ, O’Neill EA, Duran L, Kaufman KD, Williams-Herman D, Goldstein BJ. Efficacy and safety of treatment with sitagliptin or glimepiride in patients with type 2 diabetes inadequately controlled on metformin monotherapy: a randomized, double-blind, non-inferiority trial. Diabetes Obes Metab. 2011;13:160-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 121] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 72. | Merck Sharp and Dohme Corp. Metformin add-on study in patients with type 2 diabetes mellitus (0431-020). gov [Internet]. Bethesda (MD): National Library of Medicine (US). [accessed 2014; Jun 22] Available from: https://clinicaltrials.gov/ct2/show/NCT00086515 NLM Identifier: NCT00086515. |
| 73. | Charbonnel B, Karasik A, Liu J, Wu M, Meininger G. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care. 2006;29:2638-2643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 555] [Cited by in RCA: 533] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 74. | Merck Sharp and Dohme Corp. A study to compare the glycemic effects, safety, and tolerability of exenatide once weekly to those of sitagliptin and pioglitazone in subjects with type 2 diabetes treated with metformin (DURATION-2). gov [Internet]. Bethesda (MD): National Library of Medicine (US). [accessed 2014; Jun 22] Available from: https://clinicaltrials.gov/ct2/show/NCT00637273 NLM Identifier: NCT00637273. |
| 75. | Bergenstal RM, Wysham C, Macconell L, Malloy J, Walsh B, Yan P, Wilhelm K, Malone J, Porter LE. Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION-2): a randomised trial. Lancet. 2010;376:431-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 476] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 76. | Merck Sharp and Dohme Corp. An investigational drug study in patients with type 2 diabetes mellitus (MK0431-023). gov [Internet]. Bethesda (MD): National Library of Medicine (US). [accessed 2014; Jun 22] Available from: https://clinicaltrials.gov/ct2/show/NCT00094757 NLM Identifier: NCT00094757. |
| 77. | Merck Sharp and Dohme Corp. An investigational drug study in patients with type 2 diabetes mellitus (0431-024). gov [Internet]. Bethesda (MD): National Library of Medicine (US). [accessed 2014; Jun 22] Available from: https://clinicaltrials.gov/ct2/show/NCT00094770 NLM Identifier: NCT00094770. |
| 78. | ClinicalTrials ; Janssen Research and Development, LLC. The CANTATA-D2 trial (CANagliflozin treatment and trial analysis-DPP-4 inhibitor second comparator trial). gov [Internet]. Bethesda (MD): National Library of Medicine (US). [accessed 2014; Jun 22] Available from: https://clinicaltrials.gov/ct2/show/NCT01137812 NLM Identifier: NCT01137812. |
| 79. | Schernthaner G, Gross JL, Rosenstock J, Guarisco M, Fu M, Yee J, Kawaguchi M, Canovatchel W, Meininger G. Canagliflozin compared with sitagliptin for patients with type 2 diabetes who do not have adequate glycemic control with metformin plus sulfonylurea: a 52-week randomized trial. Diabetes Care. 2013;36:2508-2515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 362] [Cited by in RCA: 390] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 80. | Merck Sharp and Dohme Corp. MK0431 A comparative study in patients with type 2 diabetes (0431A-079). gov [Internet]. Bethesda (MD): National Library of Medicine (US). [accessed 2014; Jun 22] Available from: https://clinicaltrials.gov/ct2/show/NCT00482729 NLM Identifier: NCT00482729. |
| 81. | Bunck MC, Diamant M, Cornér A, Eliasson B, Malloy JL, Shaginian RM, Deng W, Kendall DM, Taskinen MR, Smith U. One-year treatment with exenatide improves beta-cell function, compared with insulin glargine, in metformin-treated type 2 diabetic patients: a randomized, controlled trial. Diabetes Care. 2009;32:762-768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 299] [Cited by in RCA: 306] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 82. | Diamant M, Van Gaal L, Stranks S, Northrup J, Cao D, Taylor K, Trautmann M. Once weekly exenatide compared with insulin glargine titrated to target in patients with type 2 diabetes (DURATION-3): an open-label randomised trial. Lancet. 2010;375:2234-2243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 346] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 83. | Inagaki N, Atsumi Y, Oura T, Saito H, Imaoka T. Efficacy and safety profile of exenatide once weekly compared with insulin once daily in Japanese patients with type 2 diabetes treated with oral antidiabetes drug(s): results from a 26-week, randomized, open-label, parallel-group, multicenter, noninferiority study. Clin Ther. 2012;34:1892-1908.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 84. | Russell-Jones D, Cuddihy RM, Hanefeld M, Kumar A, González JG, Chan M, Wolka AM, Boardman MK. Efficacy and safety of exenatide once weekly versus metformin, pioglitazone, and sitagliptin used as monotherapy in drug-naive patients with type 2 diabetes (DURATION-4): a 26-week double-blind study. Diabetes Care. 2012;35:252-258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 275] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 85. | Russell-Jones D; AstraZeneca. Efficacy of once-weekly exenatide versus once or twice daily insulin detemir in patients with type 2 diabetes. gov [Internet]. Bethesda (MD): National Library of Medicine (US). [accessed 2014; Jun 22] Available from: https://clinicaltrials.gov/ct2/show/NCT01003184 NLM Identifier: NCT01003184. |
| 86. | Astrup A, Carraro R, Finer N, Harper A, Kunesova M, Lean ME, Niskanen L, Rasmussen MF, Rissanen A, Rössner S. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int J Obes (Lond). 2012;36:843-854. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 393] [Cited by in RCA: 479] [Article Influence: 34.2] [Reference Citation Analysis (1)] |
| 87. | Garber A, Henry R, Ratner R, Garcia-Hernandez PA, Rodriguez-Pattzi H, Olvera-Alvarez I, Hale PM, Zdravkovic M, Bode B; LEAD-3 (Mono) Study Group. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 mono): A randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373:473-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 752] [Cited by in RCA: 792] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 88. | Nauck M, Frid A, Hermansen K, Shah NS, Tankova T, Mitha IH, Zdravkovic M, Düring M, Matthews DR. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care. 2009;32:84-90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 805] [Cited by in RCA: 854] [Article Influence: 53.4] [Reference Citation Analysis (0)] |
| 89. | Marre M, Shaw J, Brändle M, Bebakar WM, Kamaruddin NA, Strand J, Zdravkovic M, Le Thi TD, Colagiuri S. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with Type 2 diabetes (LEAD-1 SU). Diabet Med. 2009;26:268-278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 603] [Cited by in RCA: 628] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 90. | Zinman B, Gerich J, Buse JB, Lewin A, Schwartz S, Raskin P, Hale PM, Zdravkovic M, Blonde L. Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met+TZD). Diabetes Care. 2009;32:1224-1230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 629] [Cited by in RCA: 651] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 91. | Raz I, Fonseca V, Kipnes M, Durrwell L, Hoekstra J, Boldrin M, Balena R. Efficacy and safety of taspoglutide monotherapy in drug-naive type 2 diabetic patients after 24 weeks of treatment: results of a randomized, double-blind, placebo-controlled phase 3 study (T-emerge 1). Diabetes Care. 2012;35:485-487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 92. | Rosenstock J, Reusch J, Bush M, Yang F, Stewart M. Potential of albiglutide, a long-acting GLP-1 receptor agonist, in type 2 diabetes: a randomized controlled trial exploring weekly, biweekly, and monthly dosing. Diabetes Care. 2009;32:1880-1886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 192] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 93. | Seino Y, Min KW, Niemoeller E, Takami A. Randomized, double-blind, placebo-controlled trial of the once-daily GLP-1 receptor agonist lixisenatide in Asian patients with type 2 diabetes insufficiently controlled on basal insulin with or without a sulfonylurea (GetGoal-L-Asia). Diabetes Obes Metab. 2012;14:910-917. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 210] [Cited by in RCA: 222] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 94. | Umpierrez GE, Blevins T, Rosenstock J, Cheng C, Anderson JH, Bastyr EJ. The effects of LY2189265, a long-acting glucagon-like peptide-1 analogue, in a randomized, placebo-controlled, double-blind study of overweight/obese patients with type 2 diabetes: the EGO study. Diabetes Obes Metab. 2011;13:418-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |