Published online Feb 26, 2015. doi: 10.13105/wjma.v3.i1.43
Peer-review started: July 29, 2014
First decision: September 16, 2014
Revised: December 23, 2014
Accepted: December 29, 2014
Article in press: December 31, 2014
Published online: February 26, 2015
Processing time: 175 Days and 7.3 Hours
AIM: To examine the efficacy of supervised aerobic exercise training on aerobic capacity in survivors of cancer.
METHODS: We conducted a systematic search identifying randomized controlled trials of supervised aerobic exercise interventions among adult cancer survivors with aerobic capacity (VO2max/peak) as the primary outcome. We calculated pooled effect sizes and performed multiple regression moderator analysis.
RESULTS: We identified 18 studies including 1149 survivors of cancer. Studies included mixed cancer groups (4 studies), breast cancer (10 studies), hematological cancers (2 studies), lung cancer (1 study) and liver cancer (1 study). Survivors of cancer who participated in supervised aerobic exercise training improved aerobic capacity (VO2peak) more than controls (18 comparisons, 1093 participants; standardized mean effect: 0.74; 95%CI: 0.52, 0.96; P < 0.001). However, there was significant heterogeneity among the included trials (I2: 63%; P < 0.001). Sixty-six percent of the between-study heterogeneity was explained by differences in exercise adherence and total exercise workload among studies (R2: 65.8%; P < 0.04).
CONCLUSION: Supervised aerobic exercise training provides a moderate-to-large beneficial effect on aerobic capacity among survivors of cancer. Aerobic capacity was improved to a greater degree in exercise studies with better participant attendance and higher overall exercise workload.
Core tip: The optimal exercise prescription for survivors of cancer is unknown and the effect of variations in exercise training parameters on cancer-specific outcomes are poorly understood. Therefore, questions remain over how to best tailor exercise prescriptions to optimize the health outcomes of survivors who are at different time points in their cancer care. We performed a meta-analysis of data from randomized controlled trials examining the effect of supervised aerobic exercise training on aerobic capacity in cancer survivors. We found that aerobic capacity was improved to a greater extent in exercise studies that prescribed a higher exercise workload and had better participant adherence.
- Citation: Beaudry R, Kruger C, Liang Y, Parliament M, Haykowsky M, McNeely ML. Effect of supervised exercise on aerobic capacity in cancer survivors: Adherence and workload predict variance in effect. World J Meta-Anal 2015; 3(1): 43-53
- URL: https://www.wjgnet.com/2308-3840/full/v3/i1/43.htm
- DOI: https://dx.doi.org/10.13105/wjma.v3.i1.43
The burden of cancer continues to increase worldwide due to population growth and aging[1]. More effective cancer screening and novel treatment therapies have resulted in improved detection, earlier treatment and better disease free and overall survival, with the numbers of cancer survivors growing disproportionately to the number of new cancer cases and deaths[2]. Many cancer survivors experience symptoms and side effects related to their cancer or cancer treatment. As many of these effects go undetected and/or untreated, the survivor is placed at increased risk for other health issues such as declining functional status and cardiovascular disease[3,4]. As a result, there is an emerging need for the integration of services and interventions to address the long-term health of survivors[3].
Exercise training is gaining recognition as an important intervention to address acute, late and long-term effects of cancer, and is becoming more widely acceptable as confidence in safety is now established. Importantly, evidence is accumulating to support the benefit of exercise to improve the physical functioning and quality of life of survivors. Currently, the optimal exercise prescription is unknown and the effect of variations in exercise training parameters on cancer-specific outcomes are poorly understood[5]. Therefore, questions remain over how to best tailor exercise prescriptions to optimize the health outcomes of survivors at different times through the cancer continuum[5].
Cardiorespiratory fitness, measured objectively as the highest oxygen consumed during maximal aerobic exercise, provides a means to evaluate associations with disease outcomes. Aerobic capacity is inversely related to the risk of a cardiovascular event and all-cause mortality in healthy individuals and cancer patients[6-10]. Aerobic capacity is best increased by habitual aerobic exercise training that is of a moderate-to-vigorous intensity[11].
Aerobic capacity (VO2max) is the maximum volume of oxygen that the body can consume during maximal exercise, using at least 60% of the musculature, and while breathing air at sea level[12]. This volume is expressed as an absolute rate in litres per minute (L/min) or as a relative rate in millilitres per kilogram of bodyweight per minute (mL/kg per minute). VO2peak is the term used most commonly in clinical populations when a true maximal value is not attained[12]. For example, the test is described as VO2peak rather than VO2max when the test is carried out on a cycle ergometer (bike) rather than a treadmill, or when the highest value reached on the test is limited by the participant’s symptoms.
A meta-analysis by Jones and colleagues included data from six randomized controlled trials (RCTs) and reported a significant benefit from supervised aerobic exercise training, compared with usual care, on VO2peak (2.90 mL/kg per minute; 95%CI: 1.16, 4.64; P = 0.01)[13]. However, statistical and clinical heterogeneity was found among the exercise trials included in their review, leading them to recommend further research to build on and extend the current knowledge in the field. Since this publication, a number of newer studies have been published. Given this amount of new data, we contend that an updated review is warranted.
The primary purpose of this meta-analysis was to examine the efficacy of supervised aerobic exercise training programs on VO2peak in survivors of cancer. Quality of life was analyzed as a secondary outcome measure. As well we aimed to explore heterogeneity in study findings through subgroup analyses and meta-regression where appropriate.
The review conforms to the requirements of PRISMA reporting standards. The published protocol for the review can be found at: http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42013006215#.U1cOnle9aw).
Studies were considered eligible for inclusion if they were RCTs comparing supervised aerobic exercise training with a placebo, controlled comparison or standard care. For the purposes of the review, exercise was defined as a form of leisure-time physical activity that was performed on a repeated basis over an extended period of time, with the intention of improving fitness, performance or health[14]. Studies with an additional treatment arm or combined intervention (e.g., exercise with diet modification) were included only if the effects of exercise could be isolated. A priori, we excluded reports that were available only in abstract form.
Trials were included if they involved adults (17 years and older) diagnosed with cancer who were actively receiving cancer treatment or off treatment. Included studies were required to measure maximal, peak, or estimated maximal oxygen consumption (VO2max/peak) as a study outcome.
A search was performed of the databases including OVID MEDLINE (1948 to October 2013), PubMed (1975 to October 2013), SCOPUS (1950 to October 2013), Web of Science (1950 to October 2013), EMBASE (1988 to October 2013), Cochrane Central Registry of Controlled Trials (1991 to October 2013), and LILACS (1982-October 2013). The search strategy was developed and approved by a librarian with extensive database searching knowledge and experience. We searched terms related to cancer (e.g., neoplasms, tumor), exercise (e.g., exercise, exercise therapy/or motion therapy, aerobic training), publication type (e.g., random allocation, clinical trial), and aerobic capacity (e.g., VO2). The search strategy was modified as necessary for each database. Non-English language publications were eligible for inclusion. To locate unpublished research, we reviewed clinical trial registries and websites housing theses and dissertations. Fourteen experts in the field of cancer and exercise were contacted in order to identify any research that was not published or was pending publication. Table 1 includes an example of the MEDLINE search strategy.
| (1) Exp neoplasms/ |
| (2) (Cancer* or neoplasm* or (tumor* not tumor necrosis factor) or (tumour* not tumour necrosis factor) or malignan* or carcino* or leukaemia* or leukemi* or lymphoma* or myeloma* or adenocarcinoma*).mp. |
| (3) (1) or (2) |
| (4) Exercise therapy/or motion therapy, continuous passive/or muscle stretching exercises/or plyometric exercise/ |
| (5) (Aerobic* or exercise or running or treadmill* or training).mp. [mp = title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] |
| (6) (4) or (5) |
| (7) (3) and (6) |
| (8) (VO2 or Aerobic capacity).mp. [mp = title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] |
| (9) (7) and (8) |
| (10) Limit (9) to clinical trial, all |
The titles and abstracts were screened for eligibility by two independent evaluators (C.K. and R.B.), and coded for exclusion or potential inclusion. Potentially eligible manuscripts were obtained and the same evaluators performed a second round of screening to evaluate full eligibility criteria. Any disagreements were resolved by consensus (C.K., R.B., and M.M.). The two evaluators (C.K. and R.B.) then independently abstracted data on study participants, the intervention and control (usual care) protocols, and study outcomes, and assessed for quality. Studies were evaluated using the quality assessment framework for RCTs developed by the Cochrane Collaboration[15] to assess risk of bias in the individual studies. Sensitivity analyses were conducted to examine the effect of including studies with high risk of bias.
For the purpose of evaluating exercise prescription variables, exercise intensity was standardized to a single %VO2max value[16-18]. For studies that used %VO2max as the intensity prescription the average of the range was used; time spent at different intensities was factored in to create the mean value. High intensity intervals were weighted at 50% of the contributing time. Resistance exercise was not included in intensity ratings. Total exercise workload, or intensity-minutes, was calculated by multiplying the exercise intensity by the prescribed exercise volume (program duration, minutes per session and sessions per week).
Study results were pooled using random effects models. For continuous outcomes, pooled statistics were calculated using mean differences (MD) when data were on a uniform scale and using standardized MD (SMD) when data were on different scales. All results were calculated with 95%CI. The SMD was interpreted as 0.2, 0.5 and 0.8 representing small, medium and large effects on outcomes respectively[19]. Statistical heterogeneity was assessed using a χ2 test that considered a P-value of less than 0.10 to indicate significant heterogeneity. I2 values, ranging from 0% (homogeneity) to 100% (heterogeneity) were also calculated to quantify variability in study effect and values of 25%, 50% and 75% were used to describe low, moderate and high heterogeneity respectively[20]. Subgroup analyses and multiple regression moderator analyses were performed to explore and explain heterogeneity among studies. A priori subgroup analyses included examining the pooled effect estimate by level of supervision of exercise (group or individual), the timing of the intervention (on or off treatment), and cancer type. Meta-regression was performed to explore exercise variables of frequency, time, intensity, duration and adherence on effect estimate.
A biomedical statistician (Y.L.) provided oversight on the statistical methods, and performed the meta-regression analyses. All data were entered into Review Manager 5.2 and analyzed with SPSS v15 software utilizing meta-regression scripts created by Lipsey and Wilson and Stata/SE (version 13.0)[21]. Figures were created using Comprehensive Meta-Analysis (version 3: http://www.meta-analysis.com/index.php).
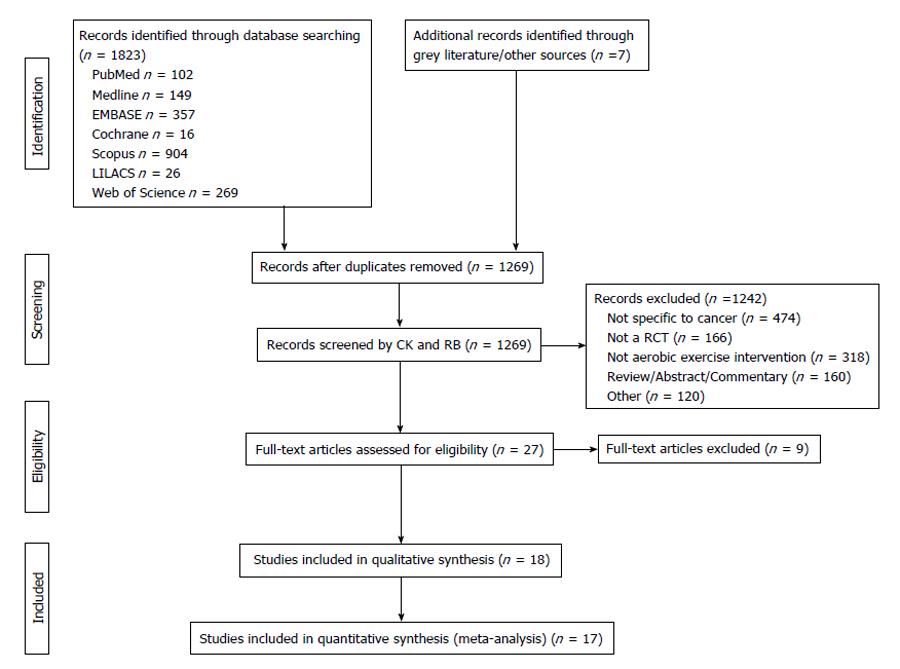
The search protocol yielded 1269 eligible studies; after removal of duplicates and screening of abstracts, 23 studies remained. Reference tracking and contacting of experts accounted for 4 additional studies. Grey literature and trial register searches yielded no further articles. Full text review of the 27 studies excluded a further 9, leaving 18 studies for qualitative and quantitative synthesis[22-39]. One study was not used for the quantitative analyses due to missing data[32] and one study was divided into two comparison groups as it involved both on and off treatment subgroups[27] (unpublished data provided by author). The remaining 17 studies, generating 18 comparisons, were included in the meta-analyses (Figure 1). Kappa statistics for the inclusion of studies was 0.9 (P < 0.001). Following discussion there was 100% agreement in scores between evaluators.
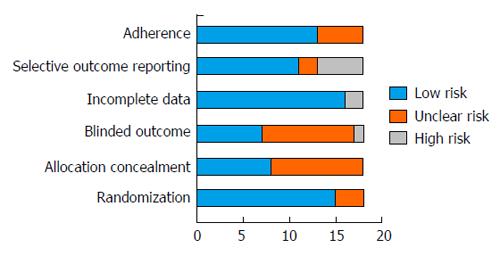
In general there was high or unclear risk of bias for selection (allocation concealment) and detection bias (lack of blinding of outcome assessors) and low risk of bias for attrition (handling of incomplete data) and reporting bias (outcome reporting) among the included studies (Figure 2). Sensitivity analyses were performed after excluding studies with a high or unclear risk of bias for allocation concealment (n = 10)[24,31-39] and for use of blinded outcome assessment (n = 11)[24,26,27,31-36,38,39]. The results showed minimal differences in the pooled effect estimates for aerobic capacity based on risk of bias. For allocation concealment, the pooled effect estimate increased by 0.6 (SMD: 0.80; 95%CI: 0.51, 1.25) whilst for blinding of outcome assessment the estimate decreased by 0.4 (SMD: 0.7; 95%CI: 0.35, 1.05). After excluding studies with a high or unclear risk of bias for any factor (n = 13), the pooled effect estimate decreased by 0.8 (SMD: 0.66, 95%CI: 0.22, 1.11).
The 18 included studies involved 1149 participants of which 576 were randomized to receive an aerobic exercise intervention and the remaining 573 received usual care or no exercise. Participants were on average 53 years of age and 76% were female. Survivors of breast cancer were most commonly studied in both breast cancer specific trials and mixed cancer type trials (14 studies)[22-26,28,29,33-39] accounting for 686 participants (60%) of the total participants in the review. Further details on the included studies are provided in Table 2.
| Ref. | Sample size/ | Age | Gender | Intervention | Comparison | Key outcomes | Adverse |
| cancer type | (SD/range) | (F/M) | group | group | events | ||
| On treatment studies/subgroups | |||||||
| Adamsen et al[22], 2009 Denmark | n = 117 Mixed Cancer Groups | 47.2 (± 6.7) yr | F: 78 M: 39 | Aerobic Training with High-intensity Intervals + Resistance Exercise + Relaxation + Massage | Usual care: allowed to freely increase physical activity | Estimated VO2max | Seizure (n = 1) |
| Courneya et al[26], 2007 Canada | n = 133 Breast Cancer | 49 yr (26-78) | F: 133 | Aerobic Training | Usual care: continue usual activities | VO2peak QoL: FACT-Anemia | Hypotension (n = 1) Dizziness (n = 1) |
| 1Courneya et al[27], 2009b Canada | n = 54 NHL, HL | 253.2 yr (18-80) | 2F: 50 M: 72 | Aerobic Training with High-intensity Intervals | Usual Care: continue usual activities | VO2peak QoL: FACT-B/Ac/An | Back (n = 1), hip (n = 1) and knee (n = 1) pain |
| Hornsby et al[29], 2013 United States | n = 20 Breast Cancer | 51 (± 6) yr | F: 10 | Aerobic Training with High-intensity Intervals | Control: Continue usual exercise levels | VO2peak FACT-B Adverse Events | Leg pain (n = 1) |
| Hwang et al[30], 2012 Taiwan | n = 24 Lung | 61 (± 6.3) | F: 12 M: 12 | Aerobic Training | Usual Care: general patient education | VO2peak QoL: EORTC | Not reported |
| Jarden et al[31], 2009 Denmark | n = 42 Mixed Cancer Groups | 39.1 (12.2) | F: 16 M: 26 | Aerobic Training + Resistance Exercise + Flexibility | Usual Care | Estimated VO2max QoL: EORTC, FACT-An | None |
| Kim et al[33], 2006 United States | n = 41 Breast Cancer | 51.3 (6.7) yr | F: 41 | Aerobic Training | Waitlist Control | VO2peak | Not reported |
| MacVicar et al[34], 1989 United States | n = 34 Breast Cancer | 45.4 (10.2) yr | F: 34 | Aerobic Training with High-intensity Intervals | Control: Continue normal activities | VO2max L/min | Not reported |
| Segal et al[38], 2001 Canada | n = 66 Breast Cancer | 51 (± 8.7) yr | F: 66 | Aerobic Training | Control group encouraged to exercise | Estimated VO2max QoL: SF36 | Not reported |
| Off treatment studies/comparisons | |||||||
| Broderick et al[23], 2013 Ireland | n = 43 Mixed Cancer Groups | 52.3 (8.3) yr | F: 37 M: 6 | Aerobic training | Usual Care | Estimated VO2max QoL: FACT-G, SF36 | Not reported |
| Burnham et al[24], 2000 United States | n = 18 Mixed Cancer Groups | 54.2 (8.1) yr | F: 15 M: 3 | Aerobic training | Control | VO2peak QoL: LASA | Not reported |
| Courneya et al[25], 2003 Canada | n = 50 Breast Cancer | 59 (± 6) yr | F: 54 | Aerobic training | No exercise | VO2peak QoL: FACT-Breast | Lymphedema (n = 3) Gynecological complication (n = 1) |
| 1Courneya et al[27], 2009a Canada | n = 68 NHL, HL | 2As per Courneya, 2009b | 2As per Courneya, 2009b | 2As per Courneya, 2009b | 2As per Courneya, 2009b | 2As per Courneya, 2009b | 2As per Courneya, 2009b |
| Herrero et al[28], 2005 Spain | n = 16 Breast Cancer | 51 (10) yr | F: 16 | Aerobic plus Resistance Training | No Exercise | VO2peak QoL: EORTC | Not reported |
| Kaibori et al[32], 2013 Japan | n = 51 Liver Cancer | 68 (9.1) yr | F: 15 M: 36 | Aerobic Training + Stretching + Diet Intervention | Diet Intervention | VO2peak | Not reported |
| Mehnert et al[35], 2011 Germany | n = 58 Breast Cancer | 53 (7.4) yr | F: 58 | Aerobic Training + Physiotherapeutic Exercises + Relaxation | Waitlist Control | VO2max QoL: BIQ | Not reported |
| Naumann et al[36], 2011 Australia | n = 21 Breast Cancer | 49 (10) yr | F: 21 | Aerobic Training + Resistance Exercise + Flexibility | Usual Care | Estimated VO2max QoL: FACT-B | Not reported |
| Rahnama et al[37], 2010 Iran | n = 29 Breast Cancer | 58.3 (6.3) yr | F: 29 | Aerobic Training + Resistance Exercise | No exercise | Estimated VO2max | Not reported |
| Thorsen et al[39], 2005 Norway | n = 111 Mixed Cancer Groups | 39 (8.4) yr | F: 36 M: 75 | Aerobic Training + Resistance Exercise | Usual Care | Estimated VO2max QoL: EORTC | Not reported |
Ten studies consisted exclusively of aerobic exercise training[23-27,29,30,33,34,38], six studies included a resistance exercise component with or without flexibility training[22,28,31,36,37,39], one included physiotherapy exercises and relaxation[35], and one included flexibility training plus a dietary intervention[32]. Exercise interventions consisted primarily of cycling[23-31,34,39] or walking/jogging[23,24,32,35,37-39]. Five studies[22,23,28,35,38] offered exercise programs in a class setting (group exercise format) and the remaining 13 studies[24-27,29-34,36,37,39] were individualized exercise programs, although further detail on the level of supervision was not often provided. Eight studies were carried out during active cancer treatment[22,26,29-31,33,34,38], nine in the post treatment phase[23-25,28,32,35-37,39] and one included participants both on and off treatment[27]. The duration of exercise programs ranged from 4-6 wk to 26 wk with individual exercise sessions ranging from 20-90 min including warm up and cool down. Seventeen studies prescribed aerobic exercise that was of moderate intensity with 4 of these studies[22,27,29,34] including high intensity intervals. One study combined both low and moderate intensity intervention groups into a single intervention group for their analysis due to the small sample size of the study[24]. Further information on the exercise prescription variables is provided in Table 3.
| Ref. | Study duration | Days/week | Mins/session | Volume | Standardized intensity | Workload | Adherence |
| (wk) | (mean) | (mean) | (intensity minutes) | (attendance) | |||
| Adamsen et al[22] | 6 | 3 | 15 | 270 | 0.83 | 224 | 71% |
| Broderick et al[23] | 8 | 2 | 30 | 480 | 0.57 | 274 | 78% |
| Burnham et al[24] | 10 | 3 | 23 | 690 | 0.41 | 281 | 70% |
| Courneya et al[25] | 15 | 3 | 25 | 1125 | 0.73 | 816 | 98% |
| Courneya et al[26] | 12 | 3 | 30 | 1080 | 0.70 | 756 | 70% |
| Courneya et al[27] (1) | 12 | 3 | 30 | 1080 | 0.79 | 858 | 84% |
| Courneya et al[27] (2) | 12 | 3 | 30 | 1080 | 0.79 | 858 | 71% |
| Herrero et al[28] | 8 | 3 | 25 | 600 | 0.59 | 351 | 91% |
| Hornsby et al[29] | 12 | 3 | 23 | 828 | 0.79 | 657 | 82% |
| Hwang et al[30] | 8 | 3 | 20 | 480 | 0.60 | 288 | 71% |
| Jarden et al[31] | 5 | 5 | 22.5 | 563 | 0.72 | 405 | 80% |
| Kim et al[33] | 8 | 3 | 30 | 720 | 0.65 | 468 | 78% |
| MacVicar et al[34] | 10 | 3 | NR | - | 0.73 | - | NR |
| Mehnert et al[35] | 10 | 2 | 30 | 600 | 0.60 | 360 | NR |
| Naumann et al[36] | 8 | 3 | 53 | 1272 | 0.50 | 636 | 84% |
| Rahnama et al[37] | 15 | 2 | 35 | 1050 | 0.28 | 289 | NR |
| Segal et al[38] | 26 | 3 | NR | - | 0.55 | - | 72% |
| Thorsen et al[39] | 14 | 2 | 30 | 840 | 0.62 | 518 | NR |
All eighteen studies reported VO2peak, with 13 studies (14 comparisons) indexing this outcome to body weight (mL/kg per minute)[23-30,35-39], 4 studies measuring absolute (L/min)[22,31,33,34], and 1 study measuring percent change in VO2peak (mL/kg per minute)[32]. The study measuring percent change in VO2peak was excluded from analysis due to insufficient data on measures of variability.
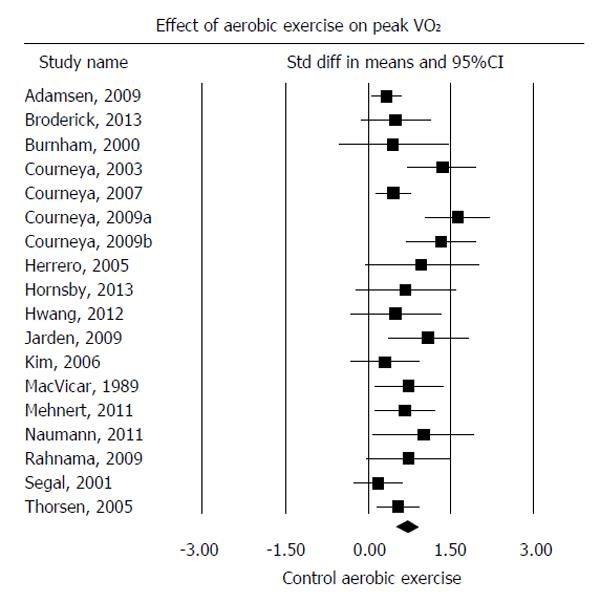
Pooling of all 18 comparisons showed a moderate-to-large effect estimate (SMD: 0.74; 95%CI: 0.52, 0.96; P < 0.001) in favour of supervised aerobic exercise training; however, moderate heterogeneity was found among the included studies (I2 = 63%; P < 0.001) (Figure 3). Pooling of the 13 studies (14 comparisons) reporting VO2peak (mL/kg per minute) showed a statistically significant mean difference in VO2peak of 3.13 mL/kg per minute (95%CI: 2.21, 4.05; P < 0.001) in favour of supervised aerobic exercise training; however, again moderate heterogeneity was found among the included studies (I2 = 58%; P < 0.001).
Subgroup analyses were performed for level of supervision, treatment timing and cancer type (Table 4). A significantly smaller effect estimate (P = 0.003) was found for group/ class-led exercise studies[22,23,35,38] (SMD: 0.36; 95%CI: 0.17, 0.56) when compared to studies involving individualized exercise programs[24-31,33,34,36,37,39] (SMD: 0.87; 95%CI: 0.60, 1.15). Non-significant effects (P = 0.11) were observed between on and off treatment studies. Statistically significant differences in pooled effect estimates were observed between cancer types with a significantly larger beneficial effect found among studies including survivors with hematological cancers (P < 0.001)[27,31] when compared to other cancer tumor groups (breast cancer, lung cancer and mixed cancer).
| Subgroup category | Subgroup | No. studies | Mean Difference in | P value between | No. studies | Standardized mean | P value between |
| mL/kg per minute (95%CI) | subgroups | difference (95%CI) | subgroups | ||||
| Level of exercise supervision | Group Exercise Class | 3 | 1.77 (0.04, 3.51) | P = 0.07 | 4 | 0.36 (0.17, 0.56) | P = 0.003 |
| Individual Exercise | 11 | 3.53 (2.64, 4.43) | 14 | 0.87 (0.60, 1.15) | |||
| Treatment status | On Treatment | 5 | 2.59 (0.7, 4.48) | P = 0.26 | 9 | 0.56 (0.32, 0.81) | P = 0.11 |
| Off Treatment | 9 | 3.74 (3.06, 4.42) | 9 | 0.92 (0.56, 1.29) | |||
| Cancer tumor group | Breast | 8 | 2.41 (1.5, 3.31) | 10 | 0.64 (0.34, 0.88) | ||
| Hematologic | 3 | 5.08 (4.01, 6.16) | 3 | 1.55 (1.09, 2.02) | |||
| Lung | 1 | 2.10 (-1.36, 5.56) | P = 0.002 | 1 | 0.48 (-0.34, 1.30) | P = 0.0002 | |
| Mixed Cancers | 3 | 3.17 (1.34, 5.0) | 4 | 0.41 (0.21, 0.61) |
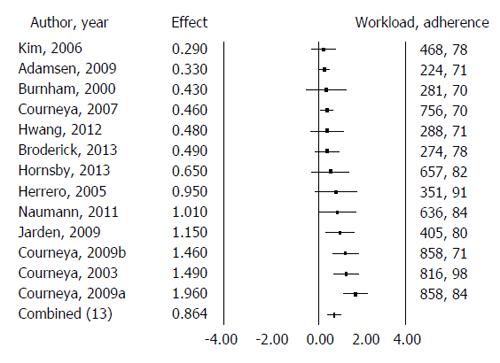
Meta regression was performed analyzing the effect estimate with exercise parameters of exercise workload and participant adherence as potential moderators. These two variables, workload and adherence, explained 65.8% (P = 0.04) of the between-study variance in effect estimate among the included studies (Figure 4).
Nine studies reported data for health-related quality of life as measured by the Functional Assessment of Cancer Therapy-General (FACT-G) scale[23,25,29,31], the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire: EORTC-QLQ-C30[22,28,30,39] and Medical Outcomes Survey: Short Form: SF36[38]. Pooling of all nine studies demonstrated a non-significant effect on quality of life (SMD: 0.3; 95%CI: -0.11, 0.71; P = 0.16), with high heterogeneity found among studies (I2 = 80%; P < 0.001). Further details are provided in Table 5.
| Quality of life measure | No. of studies | Mean difference | P value between | Standardized mean | P value |
| (95%CI) | groups | difference (95%CI) | between groups | ||
| All combined | 9 | Not applicable | - | 0.3 (-0.12, 0.70) | P = 0.16 |
| EORTC Global | 4 | 1.45 (0.58, 2.32) | P = 0.001 | 0.13 (-0.06, 0.33) | P = 0.17 |
| FACT-G | 4 | 3.25 (-0.41, 6.92) | P = 0.08 | 0.47 (0.14, 0.79) | P = 0.005 |
| MOS SF36 | 1 | 2.2 (1.34, 3.06) | P < 0.001 | 1.22 (0.69, 1.74) | P < 0.001 |
This meta-analysis found that supervised aerobic exercise resulted in a moderate-to-large significant benefit on VO2peak in survivors of cancers. The pooled mean difference showed an improvement in VO2peak of 3.13 mL/kg per minute, which is close to one metabolic equivalent (MET) improvement in fitness and similar to the 2.9 mL/kg per minute increase reported by Jones et al[13]. In the general population, each one MET increase in fitness has been found to translate to a 12% decrease in mortality in men[6] and a 17% decrease in women[40]. In the cancer population, a number of studies have reported an inverse correlation between VO2peak and all-cause mortality, including cardiovascular, lung and breast cancer related deaths[41-43].
We did not find an overall significant effect of supervised aerobic exercise interventions on quality of life. Studies in our review used a variety of quality of life measures and when data were pooled significantly high heterogeneity was found. This finding suggests that the differences between study populations and/or differences inherent in the quality of life questionnaires may be factors. Supporting this premise, the pooled data from four studies using the FACT-General scale showed both statistical homogeneity and significant benefit on quality of life.
Our results showed that survivors of cancer participating in individually-based exercise experienced greater improvement in VO2peak than those participating in group or class-led exercise. A reported advantage to group or class-led exercise is the social interaction and group support that may foster improvements in quality of life among survivors. Similar to our findings, a previous meta-analysis comparing group to individual exercise on quality of life in survivors of breast cancer reported that group exercise showed no benefit over individual exercise[44]. While the findings of our review appear to support individually based exercise programs for the outcome of aerobic capacity, we found that data were generally lacking on the ratio of the exercise participant to exercise specialist to allow for closer examination of impact of the level of supervision.
In contrast to the meta-analysis by Jones et al[13] we did not find a significant difference between groups based on the timing of the intervention relative to cancer treatment. Inspection of adherence across studies revealed a bimodal distribution with clusters in the 70-75 and 85-98 percent ranges. This bimodal distribution appeared to reflect on/off treatment status, as better adherence and larger effects were generally seen from exercise intervention studies carried out after completion of cancer treatment. Moreover, the direction of exercise effects compared to usual care may differ in relation to treatment status. For example, Jarden et al[31] demonstrated that exercise during active cancer treatment prevented a decline in VO2peak when compared to usual care, whereas Kim et al[33] found that exercise following cancer treatment increased VO2peak over usual care. More research is required to elucidate the influence of the timing of the exercise intervention through the continuum of cancer treatment and survivorship.
While our overall findings support the benefit of supervised aerobic exercise on VO2peak, the relative benefit varied significantly across studies. As the number of research studies in the area has increased we were able to examine the influence of exercise prescription variables on aerobic capacity. Our analyses showed that VO2peak improved to a larger extent in studies examining survivors of haematological cancers over other cancer groups. However, this finding was based on data from only 2 studies (3 comparisons) and thus, while compelling; further research is needed within this particular cancer subgroup. Of note, significant improvements were found within the subgroups of both breast cancer and mixed cancer groups; however, the effect was smaller.
Better participant adherence and overall exercise workload emerged as important predictors of intervention efficacy. Adherence, in this review, represented attendance to exercise sessions. Data on adherence to intensity and exercise volume were not reported in the majority of trials. Attendance to exercise sessions may reflect the impact of treatment-related side effects, patient motivation, or aspects of the study protocol such as opportunities for making up missed sessions. High adherence to the exercise prescription is critical for ensuring an adequate training stimulus to induce physiological change in cardiorespiratory function. Better reporting of adherence to prescription factors of intensity and duration would allow for more precise examination of the dose response to exercise[5].
Previous meta-analyses examining exercise interventions have reported benefit from more intense aerobic exercise interventions for both quality of life and depressive symptoms[45,46]. In the present meta-analysis, however, overall workload rather than intensity alone was found to predict response to exercise. We found that the majority of studies in the review prescribed moderate intensity exercise training, although some included high intensity interval work. Multiplying the exercise volume by the prescribed intensity provided a workload metric (i.e., intensity-minutes) for discriminating between trials finding large effects from those with small effects. While some studies prescribing lower exercise volumes showed benefit, a target workload (intensity-minutes) of around 600 intensity-minutes (e.g., 10 wk program of 90 min per week of supervised exercise at 70% VO2peak) appears to represent the threshold workload required to obtain a clinically significant large improvement (effect size > 1.0) in VO2peak. A recent meta-analysis by Carayol et al[47] examined the effect of exercise on fatigue and quality of life and found a workload in the range of 90-120 min of moderate intensity exercise was more beneficial in improving fatigue and quality of life than higher volumes of exercise. Our findings suggest that improvements in aerobic capacity can be attained at an exercise workload level that, in theory, should not negatively impact fatigue and quality of life.
The major limitations of this meta-analysis were the assumptions revolving around exercise prescription factors. All intensity values represented average values obtained and were standardized to an estimated %VO2max value. Conversions are imperfect as are average values created from studies using intervals and step protocols. Therefore we acknowledge that there is some associated error in our intensity estimates. As well, no data were provided on actual adherence to intensity among participants in the individual studies to allow more precise estimation of intensity. Thus our crude estimates of targeted intensity functioned merely as a means to determine relative ranking for between study comparisons. Assumptions were also made that resistance exercise provided minimal contributions to VO2peak. A further limitation of our meta-analysis was the small number of included studies, which permitted the analysis of only two moderator variables. Thus, further research is needed particularly in survivors of cancers other than breast cancer.
Studies included in this review were generally of good methodological quality with low risk of bias. However, further attention to study quality is needed, as many studies did not adequately report methods for allocation concealment and use of blinded assessment, limiting our ability to evaluate the impact of risk of bias across studies. Of note, the estimated effect size was lower when excluding studies at high risk of bias; thus, our findings may represent an overestimate of the effect of supervised exercise on aerobic capacity.
A final limitation is that the mechanism(s) responsible for the improvement in VO2peak along the oxygen cascade were not studied in any of the studies included in our review; thus, the favourable finding in VO2peak may be due to improved convective and/or diffusive oxygen transport coupled with improved oxygen utilization by the active muscles[48].
Supervised aerobic exercise training was found to have a moderate-to-large beneficial effect on VO2peak. Aerobic capacity increased in a dose response fashion with overall workload, with larger effects found in studies prescribing a higher overall workload of aerobic exercise. Larger benefits were also seen in studies with better participant attendance and among survivors of haematological cancers. There is a need for further randomized controlled trials examining supervised aerobic exercise interventions in understudied but common cancers such as prostate, lung and colorectal cancer.
Evidence is accumulating to support the benefit of exercise to improve the physical functioning and quality of life of survivors. Currently, the optimal exercise prescription is unknown and the effect of variations in exercise training parameters on cancer-specific outcomes are poorly understood. Therefore, questions remain over how to best tailor exercise prescriptions to optimize the health outcomes of survivors at different times through the cancer continuum.
A previous meta-analysis included data from six randomized controlled trials and reported a significant benefit from supervised aerobic exercise training, compared with usual care, on VO2peak (2.90 mL/kg per minute; 95%CI: 1.16, 4.64; P = 0.01). However, statistical and clinical heterogeneity was found among the exercise trials included in their review, and therefore further research was indicated to build on and extend the current knowledge in the field.
Pooling of the 13 studies (14 comparisons) reporting VO2peak (mL/kg per minute) showed a statistically significant mean difference in VO2peak of 3.13 mL/kg per minute (95%CI: 2.21, 4.05; P < 0.001) in favour of supervised aerobic exercise training; however, again moderate heterogeneity was found among the included studies (I2 = 58%; P < 0.001). Meta-regression was performed analyzing the effect estimate with exercise parameters of exercise workload and participant adherence as potential moderators. These two variables, workload and adherence, explained 65.8% (P = 0.04) of the between-study variance in effect estimate among the included studies.
Supervised aerobic exercise training is an effective intervention to improve aerobic capacity in survivors of cancer. Aerobic capacity increased in a dose response fashion with overall workload, with larger effects found in studies prescribing a higher overall workload of aerobic exercise. Larger benefits were also seen in studies with better participant attendance and among survivors of haematological cancers.
Aerobic capacity (VO2max) is the maximum volume of oxygen that the body can consume during maximal exercise, using at least 60% of the musculature, and while breathing air at sea level. Aerobic capacity is best increased by habitual aerobic exercise training that is of a moderate-to-vigorous intensity.
An excellent systematic review.
P- Reviewer: Lim ECW, Tokuhashi Y S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25540] [Article Influence: 1824.3] [Reference Citation Analysis (7)] |
| 2. | Stubblefield MD, Hubbard G, Cheville A, Koch U, Schmitz KH, Dalton SO. Current perspectives and emerging issues on cancer rehabilitation. Cancer. 2013;119 Suppl 11:2170-2178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 3. | Silver JK, Baima J, Mayer RS. Impairment-driven cancer rehabilitation: an essential component of quality care and survivorship. CA Cancer J Clin. 2013;63:295-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 271] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 4. | Schmitz KH, Speck RM. Risks and benefits of physical activity among breast cancer survivors who have completed treatment. Womens Health (Lond Engl). 2010;6:221-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Winters-Stone KM, Neil SE, Campbell KL. Attention to principles of exercise training: a review of exercise studies for survivors of cancers other than breast. Br J Sports Med. 2014;48:987-995. [PubMed] |
| 6. | Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346:793-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2663] [Cited by in RCA: 2680] [Article Influence: 116.5] [Reference Citation Analysis (0)] |
| 7. | Kaleth AS, Kaleth J. Demonstrating functional outcomes for health and fitness. Baltimore, MD: Lippincott Williams & Wilkins 2010; . |
| 8. | Mora S, Redberg RF, Cui Y, Whiteman MK, Flaws JA, Sharrett AR, Blumenthal RS. Ability of exercise testing to predict cardiovascular and all-cause death in asymptomatic women: a 20-year follow-up of the lipid research clinics prevalence study. JAMA. 2003;290:1600-1607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 359] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 9. | Jones LW, Courneya KS, Mackey JR, Muss HB, Pituskin EN, Scott JM, Hornsby WE, Coan AD, Herndon JE, Douglas PS. Cardiopulmonary function and age-related decline across the breast cancer survivorship continuum. J Clin Oncol. 2012;30:2530-2537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 324] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 10. | Jones LW. Physical activity and lung cancer survivorship. Recent Results Cancer Res. 2011;186:255-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Tabata I, Nishimura K, Kouzaki M, Hirai Y, Ogita F, Miyachi M, Yamamoto K. Effects of moderate-intensity endurance and high-intensity intermittent training on anaerobic capacity and VO2max. Med Sci Sports Exerc. 1996;28:1327-1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 318] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 12. | Myers J, Nieman DC, American College of Sports Medicine. Acsm’s resources for clinical exercise physiology: Musculoskeletal, neuromuscular, neoplastic, immunologic, and hematologic conditions. 2nd ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins Health 2010; . |
| 13. | Jones LW, Liang Y, Pituskin EN, Battaglini CL, Scott JM, Hornsby WE, Haykowsky M. Effect of exercise training on peak oxygen consumption in patients with cancer: a meta-analysis. Oncologist. 2011;16:112-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 209] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 14. | Shephard RJ. Aerobic fitness and health/roy j. Shephard. Champaign, IL: Human Kinetics, c1994 1994; . |
| 15. | Higgins JPT, Green S. Assessing risk of bias in included studies. 2008;187. |
| 16. | Swain DP, Abernathy KS, Smith CS, Lee SJ, Bunn SA. Target heart rates for the development of cardiorespiratory fitness. Med Sci Sports Exerc. 1994;26:112-116. [PubMed] |
| 17. | Dalleck LC, Kravitz L. Relationship Between %Heart Rate Reserve And %VO2 Reserve During Elliptical Crosstrainer Exercise. J Sports Sci Med. 2006;5:662-671. [PubMed] |
| 18. | Arts FJ, Kuipers H. The relation between power output, oxygen uptake and heart rate in male athletes. Int J Sports Med. 1994;15:228-231. [PubMed] |
| 19. | Cohen J. A power primer. Psychol Bull. 1992;112:155-159. [PubMed] |
| 20. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 46470] [Article Influence: 2112.3] [Reference Citation Analysis (3)] |
| 21. | Lipsey MW, Wilson DB. Practical meta-analysis. Thousand Oaks, Calif. : Sage Publications 2001; 247. |
| 22. | Adamsen L, Quist M, Andersen C, Møller T, Herrstedt J, Kronborg D, Baadsgaard MT, Vistisen K, Midtgaard J, Christiansen B. Effect of a multimodal high intensity exercise intervention in cancer patients undergoing chemotherapy: randomised controlled trial. BMJ. 2009;339:b3410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 330] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 23. | Broderick JM, Guinan E, Kennedy MJ, Hollywood D, Courneya KS, Culos-Reed SN, Bennett K, O’ Donnell DM, Hussey J. Feasibility and efficacy of a supervised exercise intervention in de-conditioned cancer survivors during the early survivorship phase: the PEACH trial. J Cancer Surviv. 2013;7:551-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Burnham TR. The effects of exercise on physiological and psychological variables in cancer patients following clinical treatment. : Oregon State University 2000; 56. |
| 25. | Courneya KS, Mackey JR, Bell GJ, Jones LW, Field CJ, Fairey AS. Randomized controlled trial of exercise training in postmenopausal breast cancer survivors: cardiopulmonary and quality of life outcomes. J Clin Oncol. 2003;21:1660-1668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 530] [Cited by in RCA: 528] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 26. | Courneya KS, Segal RJ, Mackey JR, Gelmon K, Reid RD, Friedenreich CM, Ladha AB, Proulx C, Vallance JK, Lane K. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. J Clin Oncol. 2007;25:4396-4404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Courneya KS, Sellar CM, Stevinson C, McNeely ML, Peddle CJ, Friedenreich CM, Tankel K, Basi S, Chua N, Mazurek A. Randomized controlled trial of the effects of aerobic exercise on physical functioning and quality of life in lymphoma patients. J Clin Oncol. 2009;27:4605-4612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 272] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 28. | Herrero F, San Juan AF, Fleck SJ, Balmer J, Pérez M, Cañete S, Earnest CP, Foster C, Lucía A. Combined aerobic and resistance training in breast cancer survivors: A randomized, controlled pilot trial. Int J Sports Med. 2006;27:573-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 121] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 29. | Hornsby WE, Douglas PS, West MJ, Kenjale AA, Lane AR, Schwitzer ER, Ray KA, Herndon JE, Coan A, Gutierrez A. Safety and efficacy of aerobic training in operable breast cancer patients receiving neoadjuvant chemotherapy: a phase II randomized trial. Acta Oncol. 2014;53:65-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 148] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 30. | Hwang CL, Yu CJ, Shih JY, Yang PC, Wu YT. Effects of exercise training on exercise capacity in patients with non-small cell lung cancer receiving targeted therapy. Support Care Cancer. 2012;20:3169-3177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 111] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 31. | Jarden M, Baadsgaard MT, Hovgaard DJ, Boesen E, Adamsen L. A randomized trial on the effect of a multimodal intervention on physical capacity, functional performance and quality of life in adult patients undergoing allogeneic SCT. Bone Marrow Transplant. 2009;43:725-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 165] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 32. | Kaibori M, Ishizaki M, Matsui K, Nakatake R, Yoshiuchi S, Kimura Y, Kwon AH. Perioperative exercise for chronic liver injury patients with hepatocellular carcinoma undergoing hepatectomy. Am J Surg. 2013;206:202-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 33. | Kim CJ, Kang DH, Smith BA, Landers KA. Cardiopulmonary responses and adherence to exercise in women newly diagnosed with breast cancer undergoing adjuvant therapy. Cancer Nurs. 2006;29:156-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 34. | MacVicar MG, Winningham ML, Nickel JL. Effects of aerobic interval training on cancer patients’ functional capacity. Nurs Res. 1989;38:348-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 203] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 35. | Mehnert A, Veers S, Howaldt D, Braumann KM, Koch U, Schulz KH. Effects of a physical exercise rehabilitation group program on anxiety, depression, body image, and health-related quality of life among breast cancer patients. Onkologie. 2011;34:248-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 36. | Naumann F, Martin E, Philpott M, Smith C, Groff D, Battaglini C. Can counseling add value to an exercise intervention for improving quality of life in breast cancer survivors? A feasibility study. J Support Oncol. 2012;10:188-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 37. | Rahnama N, Nouri R, Rahmaninia F, Damirchi A, Emami H. The effects of exercise training on maximum aerobic capacity, resting heart rate, blood pressure and anthropometric variables of postmenopausal women with breast cancer. J Res Med Sci. 2010;15:78-83. [PubMed] |
| 38. | Segal R, Evans W, Johnson D, Smith J, Colletta S, Gayton J, Woodard S, Wells G, Reid R. Structured exercise improves physical functioning in women with stages I and II breast cancer: results of a randomized controlled trial. J Clin Oncol. 2001;19:657-665. [PubMed] |
| 39. | Thorsen L, Skovlund E, Strømme SB, Hornslien K, Dahl AA, Fosså SD. Effectiveness of physical activity on cardiorespiratory fitness and health-related quality of life in young and middle-aged cancer patients shortly after chemotherapy. J Clin Oncol. 2005;23:2378-2388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 153] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 40. | Gulati M, Pandey DK, Arnsdorf MF, Lauderdale DS, Thisted RA, Wicklund RH, Al-Hani AJ, Black HR. Exercise capacity and the risk of death in women: the St James Women Take Heart Project. Circulation. 2003;108:1554-1559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 516] [Cited by in RCA: 507] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 41. | Peel JB, Sui X, Adams SA, Hébert JR, Hardin JW, Blair SN. A prospective study of cardiorespiratory fitness and breast cancer mortality. Med Sci Sports Exerc. 2009;41:742-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 42. | Burnett D, Kluding P, Porter C, Fabian C, Klemp J. Cardiorespiratory fitness in breast cancer survivors. Springerplus. 2013;2:68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 105] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 43. | Sui X, Lee DC, Matthews CE, Adams SA, Hébert JR, Church TS, Lee CD, Blair SN. Influence of cardiorespiratory fitness on lung cancer mortality. Med Sci Sports Exerc. 2010;42:872-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 44. | Floyd A, Moyer A. Group vs. individual exercise interventions for women with breast cancer: a meta-analysis. Health Psychol Rev. 2009;4:22-41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 45. | Ferrer RA, Huedo-Medina TB, Johnson BT, Ryan S, Pescatello LS. Exercise interventions for cancer survivors: a meta-analysis of quality of life outcomes. Ann Behav Med. 2011;41:32-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 181] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 46. | Brown JC, Huedo-Medina TB, Pescatello LS, Ryan SM, Pescatello SM, Moker E, LaCroix JM, Ferrer RA, Johnson BT. The efficacy of exercise in reducing depressive symptoms among cancer survivors: a meta-analysis. PLoS One. 2012;7:e30955. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 47. | Carayol M, Bernard P, Boiché J, Riou F, Mercier B, Cousson-Gélie F, Romain AJ, Delpierre C, Ninot G. Psychological effect of exercise in women with breast cancer receiving adjuvant therapy: what is the optimal dose needed? Ann Oncol. 2013;24:291-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 121] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 48. | Jones LW, Eves ND, Haykowsky M, Freedland SJ, Mackey JR. Exercise intolerance in cancer and the role of exercise therapy to reverse dysfunction. Lancet Oncol. 2009;10:598-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 226] [Article Influence: 14.1] [Reference Citation Analysis (0)] |