Published online Aug 26, 2014. doi: 10.13105/wjma.v2.i3.78
Revised: May 5, 2014
Accepted: May 28, 2014
Published online: August 26, 2014
Processing time: 302 Days and 3.6 Hours
AIM: To determine the efficacy of therapeutic ultrasound vs sham for improving pain and physical function immediately post-intervention in people with knee osteoarthritis (OA).
METHODS: We hand searched meta-analyses on the topic published in 2010 and updated the search in three electronic databases (MEDLINE, EMBASE, CINAHL) January 1, 2009 to September 5, 2013 to identify relevant studies. The inclusion criteria were human randomized controlled trials published in the English language in which active therapeutic ultrasound was compared to sham ultrasound, data for people with knee OA were reported separately, participants were blinded to treatment allocation and outcomes assessed before and after treatment included pain, self-reported physical function and performance-based physical function. Two reviewers independently screened titles and abstracts retrieved in the search to identify trials suitable for full text review. Data extraction and risk of bias assessment of the identified trials were completed independently by two reviewers. Pooled analyses were conducted using inverse-variance random effects models.
RESULTS: We screened 1013 titles and abstracts. Meta-analysis of pain outcomes from 5 small trials (281 participants/OA knees) showed that, compared to sham ultrasound, therapeutic ultrasound improves pain [standardized mean difference (SMD) (95%CI) = -0.39 (-0.70, -0.08); P = 0.01] but not physical function [self-reported in 3 trials (130 participants/OA knees): SMD (95%CI) = -0.21 (-0.55, 0.14), P = 0.24; walking performance in 4 trials (130 participants/OA knees): SMD (95%CI) = -0.11 (-0.59, 0.37), P = 0.65). For the walking performance outcome, the dispersion of the estimated effects exceeded that expected due to sampling error (χ2 = 8.37, P = 0.04, I² = 64%). Subgroup analyses of three trials that administered high dose ultrasound improved the consistency (I2 = 28%) but the treatment effect remained insignificant.
CONCLUSION: Meta-analyzed double-blind placebo-controlled randomized trials provide low-strength evidence that therapeutic ultrasound decreases knee OA pain and very low-strength evidence that it does not improve physical function.
Core tip: Controversy exists regarding the efficacy of therapeutic ultrasound in the management of knee osteoarthritis (OA). Lack of participant blinding in effectiveness trials introduces bias known to exaggerate treatment effect estimates particularly for outcomes such as pain and self-reported physical function. We meta-analyzed data from double- and triple-blind trials only and high level evidence shows that therapeutic ultrasound decreases knee OA pain but does not increase physical function immediately following treatment. Due to the methodological quality of the included trials, we conclude that a large well-designed trial is required before this clinical question can be answered definitively.
- Citation: MacIntyre NJ, Negm A, Loyola-Sánchez A, Bhandari M. Efficacy of therapeutic ultrasound vs sham ultrasound on pain and physical function in people with knee osteoarthritis: A meta-analysis of randomized controlled trials. World J Meta-Anal 2014; 2(3): 78-90
- URL: https://www.wjgnet.com/2308-3840/full/v2/i3/78.htm
- DOI: https://dx.doi.org/10.13105/wjma.v2.i3.78
Knee osteoarthritis (OA) is a highly prevalent chronic condition and a leading cause of lower extremity disability in community-dwelling older adults in North America[1]. To date, no treatment exists which modifies the disease and, despite symptom management, pain and functional limitations may progress to the point where total joint replacement is required[2]. As the population ages and the prevalence of obesity increases, the associated economic and personal burden associated with knee OA is expected to rise[2]. Current clinical practice guidelines for managing knee OA recommend a combination of pharmacologic and nonpharmacologic treatment in order to decrease the need for surgical replacement of the damaged joint[3,4].
Therapeutic ultrasound is widely used in clinical settings for various musculoskeletal conditions[5,6]. In a provincial survey of 123 Canadian physical therapists treating clients with knee OA, 81% reported at least some use of ultrasound therapy in their multicomponent management of knee OA[7]. Recent meta-analyses demonstrate that 10 to 24 sessions of continuous or pulsed ultrasound reduces knee OA pain[8-10]. Using two different methods for investigating the effect of dose, two meta-analyses reported that low dose ultrasound achieved using the pulsed mode to administer low intensity (0.375-0.625 W/cm2) sound waves produced greater pain relief than high dose ultrasound[8,9]. The authors of the most recent meta-analysis back transformed the effect estimate for pain to a visual analogue scale (VAS) score and found a difference of -16.3 [95%CI: -20.9-(-11.7)] mm which was judged to reflect a clinically important change[10]. The effect of therapeutic ultrasound on physical function (self-reported and walking performance) was not significant in the two meta-analyses published in 2010[8,9]. However, the meta-analysis published in 2012 (6 trials, 387 participants) found clinical and statistically significant effects on composite physical function and gait function outcomes[10]. Although these systematic reviews provide evidence for the efficacy of therapeutic ultrasound in the management of knee OA pain and physical disability, all reported that the few, small trials with low methodological quality eligible for inclusion limit the confidence in the effect estimates[8-10].
Meta-analyses of randomized trials provide the highest level of evidence for evaluating the effectiveness of clinical interventions such as therapeutic ultrasound. However the quality of this evidence depends on study design characteristics that yield comparable intervention and control groups. A meta-epidemiologic study of 1973 trials found that intervention effect estimates in trials using subjective outcomes such as self-reported measures were inflated and heterogeneity between trials was increased when double blinding was absent or unclear[11]. It appears that bias due to lack of double blinding exaggerates the effect estimates and heterogeneity for subjectively assessed outcomes more than other study design flaws such as inadequate/unclear random sequence generation and inadequate/unclear allocation concealment[11]. In at least half of the trials included in previous systematic reviews investigating the efficacy of ultrasound therapy on pain and physical function in people with knee OA, no attempt was made to administer sham ultrasound[8-10]. As a result, between-trial heterogeneity and the estimates of the effect of ultrasound on self-reported pain and physical function outcomes published in the highest level evidence available to date are likely to be inflated due to a lack of or unclear blinding of participants with knee OA.
Therefore the objective of this systematic review and meta-analysis was to determine if therapeutic ultrasound vs sham ultrasound is effective in improving pain and physical function immediately following the intervention period in people with knee OA blinded to treatment allocation. As a secondary objective, we determined treatment safety based on reported side effects and adverse events.
Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) recommendations were followed to ensure full and transparent reporting of this review[12]. This systematic review was conducted following a pre-determined protocol which is available from the authors upon request. This protocol was not registered and is not publicly available.
We updated the search for relevant papers completed for previous systematic reviews on this topic (to February 2009)[8,9], by searching OVID MEDLINE, OVID EMBASE, and CINAHL (through EBSCOhost) electronic databases from January 1, 2009 to September 5, 2013. The search strategy combined Medical Subject Headings (MeSH) terms and text words related to design (randomized controlled trial), condition (knee osteoarthritis), and intervention (therapeutic ultrasound). We reproduced the specific search strategy used for each database published in the previous systematic review focusing on clinical outcomes[9]. (Although previously published, the replicated strategy used to search the Medline electronic database is shown in Table 1 to comply with the PRISMA checklist.) No language limit was placed on the search in order to increase sensitivity. Duplicates were removed after all databases were searched. We searched for published trials only and attempted to contact primary authors to request additional information if necessary.
| 1 | Exp Osteoarthritis/ |
| 2 | osteoarthr$.ti,ab,sh. |
| 3 | gonarthr$.ti,ab,sh. |
| 4 | coxarthr$.ti,ab,sh. |
| 5 | arthr$.ti,ab. |
| 6 | [(knee$ or hip$ or joint$) adj3 (pain$ or ach$ or discomfort$)].ti,ab. |
| 7 | [(knee$ or hip$ or joint$) adj3 stiff$].ti,ab. |
| 8 | Exp Ultrasonic Therapy/ |
| 9 | Exp Ultrasonography/ |
| 10 | us.fs. |
| 11 | (ultrasound$ or ultrasonic$).tw. |
| 12 | short wave therapy.tw. |
| 13 | ultrasonograph$.tw. |
| 14 | randomized controlled trial.pt. |
| 15 | controlled clinical trial.pt. |
| 16 | randomized controlled trial.sh. |
| 17 | random allocation.sh. |
| 18 | double blind method.sh. |
| 19 | single blind method.sh. |
| 20 | clinical trial.pt. |
| 21 | Exp Clinical Trial/ |
| 22 | (clin$ adj25 trial$).ti,ab. |
| 23 | [(singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)].ti,ab. |
| 24 | placebos.sh. |
| 25 | placebo$.ti,ab. |
| 26 | random$.ti,ab. |
| 27 | research design.sh. |
| 28 | comparative study.sh. |
| 29 | exp evaluation studies/ |
| 30 | follow up studies.sh. |
| 31 | prospective studies.sh. |
| 32 | (control$ or prospectiv$ or volunteer$).ti,ab. |
| 33 | 1 or 2 or 3 or 4 or 5 or 6 or 7 |
| 34 | 8 or 9 or 10 or 11 or 12 or 13 |
| 35 | 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 |
| 36 | 33 and 34 and 35 |
| 37 | animal/ |
| 38 | animal/ and human/ |
| 39 | 37 not 38 |
| 40 | 36 not 39 |
| 41 | Limit 40 to yr = ”2009-Current” |
Two reviewers (NM, AL) independently screened the titles and abstracts for all citations identified by the search and retrieved all parallel-group randomized sham-controlled trials assessing the effect of ultrasound on pain or physical function in people with knee OA published in English. Studies examining other joints were only included if the data for people with knee OA were reported separately. Trials that included other interventions in addition to active ultrasound were included as long as the sham ultrasound group received the same combination of interventions. Lack of blinding of study participants was an exclusion criterion because we considered this to be a major source of biased results regarding ultrasound efficacy on self-reported pain and physical function outcomes[11]. Abstracts published as conference proceedings and not as full trials were excluded due to insufficient reporting of data for extraction. Cohen’s unweighted kappa (κ) was used to measure agreement between reviewers. Disagreement was solved by consensus including a third reviewer (AN). A final list of eligible studies was prepared for full text review after title and abstract screening.
The data collection form used for our previous systematic review[8] was modified for this updated review to reflect the narrowed research question focusing on patient-centred outcomes of pain and physical function. The form was independently pilot-tested on two randomly-selected studies by two reviewers (NM, AN) to ensure consistency in coding instructions. A double extraction method was followed using the refined data collection form. A physical therapist with expertise in research methodology and OA rehabilitation (NM) and an orthopaedic surgeon with expertise in OA (AN) reviewed the papers and extracted the data in a standardized manner. As recommended in the Cochrane Handbook[13], the reviewers were not blinded to any aspect of the trials during data extraction. Any disagreement was resolved through consensus.
The information extracted from each study included: (1) characteristics of the study participants (age, gender, diagnostic criteria, joint involvement, and knee OA severity); (2) characteristics of the therapeutic ultrasound intervention (device, frequency, mode, intensity, effective radiating area, surface area treated, application protocol, number and length of treatment sessions); (3) description of co-interventions; (4) description of pain and physical function outcomes and corresponding data; (5) reported adverse events and reasons for loss to follow up; and (6) general characteristics of the studies (location, clinical setting and funding source). The ultrasound dose was calculated as for our previous review[8] using the following formula:
Energy (J/cm2) = [(average temporal intensity) (time) (effective radiating area)]/treated surface area
Group means for outcomes at baseline, post treatment, change from baseline and standard deviations (SDs), or the information from which SDs could be derived, were extracted. For trials that included two groups receiving active ultrasound (continuous and pulsed mode), the formulae for combining means and SDs for two groups published in the Cochrane Handbook were used for estimating the effect of active ultrasound on the clinical outcomes of interest. (See Table 7.7a in the Cochrane Handbook 2005[13]). When a trial presented outcomes at time points other than pre- and post-intervention, we extracted the data for all time points, however, the mean values at the end of the treatment period were used in the meta-analysis. Heterogeneity in trial outcomes was minimized by pooling the data for the same outcome measure where possible, or pooling those that were most similar in terms of constructs assessed (e.g., pain with movement measured by VAS or numeric rating scale, NRS).
Two reviewers (NM, AN) independently assessed risk of bias for each study and the level of agreement was determined using linear weighted kappa (κ). Any disagreement regarding risk of bias was resolved by consensus. The methodological domains recommended by The Cochrane Collaboration were assessed: randomization, treatment allocation concealment, blinding of participants, care givers and outcome assessors, completeness of outcome data, selective outcome reporting and other potential threats to validity[14]. Table 2 illustrates the criteria for assessing risk of bias in these domains. An overall risk of bias was considered for each study and across all studies based on the criteria outlined in the Cochrane Handbook[14]. We assigned studies as having high risk of bias if at least one criterion was not met. The quality of the body of evidence for each outcome was determined considering within-trial risk of bias, directness of evidence, heterogeneity, risk of publication bias and precision of effect estimates as recommended by the Grading of Recommendations, Assessment Development and Evaluation (GRADE) working group[15]. We defined treatment effects as precise when pooled estimates had reasonably narrow 95%CIs and the pooled sample size was greater than 400[15].
| Random sequence generation | |
| Low risk | Referring to a random number table; Using a computer random number generator; Coin tossing; Shuffling cards or envelopes; Throwing dice; Drawing of lots |
| High risk | Sequence generated by odd or even date of birth; Sequence generated by some rule based on date (or day) of admission; Sequence generated by some rule based on hospital or clinic record number; Allocation by judgment of the clinician; Allocation by preference of the participant; Allocation based on the results of a laboratory test or a series of tests; Allocation by availability of the intervention |
| Unclear | Insufficient information |
| Allocation concealment | |
| Low risk | Central allocation (including telephone, web-based); Sequentially numbered, opaque, sealed envelopes; An equivalent method was used to conceal allocation |
| High risk | Using an open random allocation schedule (e.g., a list of random numbers); Assignment envelopes were used without appropriate safeguards (e.g., if envelopes were unsealed or nonopaque or not sequentially numbered); Alternation or rotation; Date of birth; Case record number; Any other explicitly unconcealed procedure |
| Unclear | Insufficient information |
| Blinding of each of: participant, care provider, outcome assessor | |
| Low risk | Unlikely that the blinding could have been broken |
| High risk | Likely that the blinding could have been broken |
| Unclear | Insufficient information |
| Completeness of outcome data collection | |
| Low risk | No missing outcome data; Reasons for missing outcome data unlikely to be related to true outcome; Missing outcome data balanced in numbers across all groups, with similar reasons for missing data across groups; Missing data have been imputed using appropriate methods; For continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size |
| High risk | Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; "As-treated" analysis done with substantial departure of the intervention received from that assigned at randomization. Potentially inappropriate application of simple imputation. For continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size |
| Unclear | Insufficient information |
| Completeness of outcome reporting | |
| Low risk | The study protocol is available and all of the study’s pre-specified outcomes that are of interest in the review have been reported in the pre-specified way; The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre-specified |
| High risk | Not all of the study’s pre-specified primary outcomes have been reported; One or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g., subscales) that were not pre-specified; One or more reported primary outcomes were not pre-specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); One or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta-analysis; The study report fails to include results for a key outcome that would be expected to have been reported for such a study |
| Unclear | Insufficient information |
| Other potential sources | |
| Low risk | The study appears to be free of other sources of bias |
| High risk | There is at least one important risk of bias. For example, the study had a potential source of bias related to the specific study design used or has been claimed to be fraudulent, or has some other problem |
| Unclear | Insufficient information |
We focused on patient-centred outcomes of pain and physical function. Follow up duration was to the end of the ultrasound therapy treatment. Review Manager Version 5 was used for data analyses. Effect size for each outcome (by study and overall) was estimated using the standardized mean difference (SMD) calculated as the raw mean difference divided by the pooled variance for each outcome to allow comparison of estimates of effect. Where the same outcome measure was used, the mean difference was also calculated. We used inverse-variance random-effects models to pool results to account for the inevitable variation in patient populations, concomitant treatments, and specific components of the physical therapy intervention as recommended when the number of studies is small and the reasons for heterogeneity across the studies cannot be reliably evaluated[16]. We evaluated the consistency of findings by comparing the direction and strength of effect along with the degree of statistical heterogeneity (based on the χ2 and I2 statistical tests) in effects across studies. χ2 values with P≥ 0.1 and I² < 60% were considered to be acceptable homogeneity for pooling the data[16]. We used subgroup analyses and planned to conduct meta-regression to evaluate the effects of a priori-defined clinical characteristics (OA severity) and study characteristics (ultrasound dose) on pain and physical function outcomes. These subgroups were of interest because our previous meta-analysis demonstrated that the effect estimates for pain reduction were increased for those receiving low dose therapeutic ultrasound and heterogeneity observed for physical function and walking performance was reduced in the subgroup of trials including participants with mild knee OA severity[8]. We hypothesize that therapeutic ultrasound will be more effective in people with less severe knee OA in whom tissue damage is less advanced[17]. Confidence intervals (CI) at the 95% level were calculated for pooled estimates for each outcome and the Z test was used for determining the treatment effect. Statistical significance was considered at P≤ 0.05.
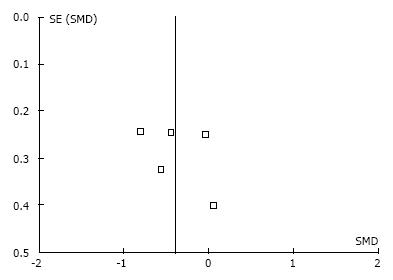
For each study reporting each of the three clinical outcomes of interest, we evaluated funnel plots for asymmetry in the standard error of the SMD as a function of the SMD. We did not use statistical tests for publication bias given that we did not include unpublished data sources in our search strategy.
The study was funded, in part, by CIHR (NM, MB). CIHR had no role in the development of the question or the review protocol, literature search, data extraction and analysis, interpretation of the results, or preparation of the manuscript.
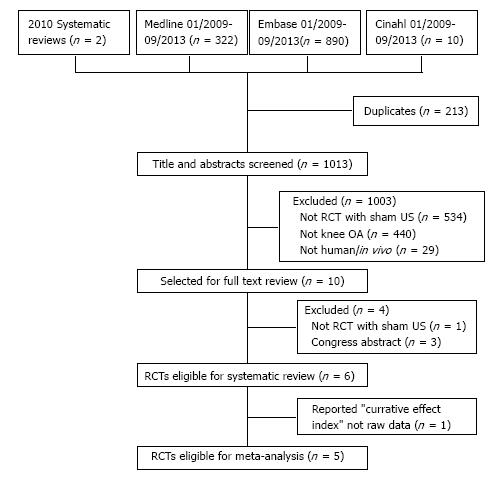
Figure 1 is a flowchart of the results of search strategy. The initial database and hand searches retrieved 1226 citations (n = 213 duplicates). After title and abstract screening, 10 randomized controlled trials were identified as eligible and retrieved for full text review. Of these, six were eligible for inclusion in the systematic review and five provided published data that permitted pooling. Reasons for exclusion are shown in Figure 1. The agreement between the reviewers in identifying the studies eligible for full text review and for inclusion in this review was κ = 1.0.
Characteristics of the six parallel-group, randomized, placebo-controlled trials eligible for inclusion in the systematic review are summarized in Table 1[18-23]. The RCTs were published between 1992 and 2012. Five trials included two arms (active ultrasound and sham ultrasound)[18,19,21-23], whereas the trial by Tascioglu et al[20] included three arms in which one arm received active pulsed ultrasound (low dose), one arm received active continuous ultrasound (high dose), and one arm received sham ultrasound. Three trials[19,20,23] were conducted in Turkey and one trial was conducted in each of the United States of America[18], Canada[22], and China[21]. The duration of the intervention varied from 5 d to 8 wk and the dosage varied from 26 J/cm2 to 196.3 J/cm2 (Table 3). The control groups in all the studies received sham ultrasound and the participants were blinded to the allocated treatment. Only one trial confirmed that treatment providers were blinded to the intervention allocation[22]. In the trial conducted by Yang et al[21] it was unclear how the sham ultrasound was delivered. Furthermore, the trial by Yang et al[21] reported a “curative effect score” for the 87 participants (100 knees) randomized to active or sham ultrasound groups rather than reporting group means (SD) for pain and physical function outcomes and attempts to contact the authors to secure the raw data were unsuccessful. This calculated efficacy index was significantly improved for pain (P < 0.001) and physical function (P < 0.001)[21]. Nevertheless, the data from this trial[21] could not be included in the meta-analysis. The five trials eligible for meta-analysis reported data for a total of 281 participants and OA knees[18-20,22,23]. Table 3 shows that the number of participants providing data for analyses was lower than the number of participants randomized (varying from 2 to 8 participants per trial) in all trials meta-analyzed. Reasons given for loss to follow up included protocol violation (used analgesics)[19,20], illness[18], dissatisfaction with treatment[18], lack of time to attend/lack of regular attendance at sessions[20,23], transportation problems[18], or incomplete baseline assessment before withdrawing from the study[22]. The average age across the studies was approximately 61 years with the majority of participants being female (Table 3). Four trials[19,20,22,23] confirmed that participants had mild to moderate radiographic knee OA whereas one included trial[18] reported results for 69 participants/knees with unknown radiographic severity. (The latter trial included eight participants with a history of total knee replacement and all 69 had restricted knee range of motion for more than 6 mo[18].) Three trials reported that the majority of participants had bilateral knee OA[18,22,23] with one of these trials including only participants with bilateral knee OA[23]. Two of the five meta-analyzed trials applied active and sham ultrasound in combination with concurrent treatments (including isometric quadriceps exercises) that were identical for both study groups[18,23]. In contrast, two trials[19,20] did not allow any concurrent treatments including the use of analgesics during the intervention period. Three trials[18,20,22] administered low dose ultrasound (< 150 J/cm2) and three trials[19,20,23] administered high dose ultrasound (≥ 150 J/cm2). Three trials[19,22,23] reported scores on more than 1 pain outcome measure and two of these trials[22,23] reported scores on more than 1 self-reported physical function outcome measure. Table 3 specifies the pain and self-reported physical function outcomes selected for inclusion in the meta-analysis in order to reduce heterogeneity arising from differences in outcomes used across studies. Three trials[19,22,23] administered the WOMAC LK 3.1 questionnaire and reported scores on the physical function subscale. One trial[20], administering the WOMAC LK3.1 to assess the effect of ultrasound therapy on disability, reported only the total score which was not included in the meta-analysis for the self-reported physical function outcome. Another trial[21] administered the Lequesne Severity Index to assess the effect of ultrasound therapy on self-reported physical function but did not report the group means in a format that permitted pooling as mentioned previously. Walking performance was measured as walking speed (time to walk a specified distance) in four trials[18-20,23], and as distance walked (metres walked in six minutes) in one trial[22]. One trial[18] reported means for walking velocity (metres/s) pre and post treatment for the total sample only. We were unsuccessful in obtaining group means from the primary author and therefore these walking performance data could not be included in the meta-analysis. The units of measurement for the meta-analysed walking performance values were time (in seconds), where lower values indicate better scores, and distance (in metres), where higher values indicate better scores. Therefore, the group means for distance walked in six minutes were entered into the meta-analysis as negative values to adjust for the directional difference in interpreting better scores for this outcome.
| Falconer et al[18] | Ozgönenel et al[19] | Tascioglu et al[20] | Yang et al[21] | Loyola-Sánchez et al[22] | Ulus et al[23] | |
| Trial registry number | Not available | Not available | Not available | Not available | NTC00931749 | Not available |
| Trial duration | 4-6 wk (12 sessions 2-3 x/wk) | 2 wk (10 sessions 5 x/wk) | 2 wk (10 sessions 5x/wk) | 5 d (5 sessions); + 1 mo follow up | 8 wk (24 sessions 3 x/wk) | 3 wk (15 sessions 5 x/wk) |
| Sample size | Randomized: 74; analyzed: 69 (35 CG) | Randomized: 67; analyzed: 65 (31 CG) | Randomized: 90; analyzed: 82 (27 CG) | Randomized and analyzed: 87 (100 knees; 50 CG) | Randomized: 27; analyzed: 25 (13 CG) | Randomized: 42; analyzed: 40 (20 CG) |
| Sample characteristics (Mean SD/n reported) | Age approximately 67.5 (11) yr; 50 F; All restricted knee ROM ≥ 6 mo; 8 knee joint replacement; 51 bilateral OA | Age approximately 55 (7.5) yr; 54 F; Newly diagnosed; 31 mild OA, 36 moderate OA | Age approximately 60 (3) yr; 56 F; Disease duration 6.5 yr; 48 mild OA, 34 moderate OA | Age 58.3 yr; 72 F; Disease duration 2.8 yr | Age approximately 61.8 (10) yr; 21 F; 8 had mild OA, 19 had moderate OA; 24 bilateral OA | Age approximately 60.5 (9.5) yr; 34 F; disease duration 8.9(8.7) yr; 17 mild OA, 23 moderate OA; all bilateral OA |
| Ultrasound device | Chattanooga Intellect 200 | Peterson .250 | Sonopuls 434 | NERCUM | Chattanooga Intellect Mobile | Sonopuls 434 |
| Application protocol | 12 min; 1 MHz; intensity: 1.7 W/cm2, continuous mode (n = 34) | 5 min; 1 MHz; intensity 1 W/cm2; continuous mode (n = 34) | 5 min; 1 MHz; intensity 1 W/cm2, continuous mode (n = 27); pulsed (duty cycle 20%, n = 28) | No details: 15min treatment model then 20min rehabilitation model (n = 50 knees) | 9.5 min; 1MHz; intensity 1 W/cm2; pulsed mode (duty cycle 20%, n = 12) | 10 min; 1 MHz; intensity 1 W/cm2; continuous mode (n = 20) |
| Sham Application | Start button not pushed | Applicator disconnected from back of device | No output delivered | No output delivered | Ceramic crystal removed from soundhead | No output delivered and applicator disconnected from back of device |
| Application site | Knee flexed or extended per most restricted motion; treated surface area 100 cm2 | Patellofemoral and tibiofemoral borders; treated surface area 25 cm2 | Antero-medial and lateral parts of extended knee; treated surface area 60 cm2 | Knee extended; 4 soundheads fixed on joint line; treated surface area not reported | Knee flexed to 90°; Soundhead fixed at antero-medial joint line. treated surface area 5 cm2 | Antero-medial and lateral parts of extended knee; treated surface area 60 cm2 |
| Dosage | 26 J/cm2 | 150.7 J/cm2 | 196.3 J/cm2 39.3 J/cm2 | Unable to calculate | 114 J/cm2 | 196.3 J/cm2 |
| Concurrent treatment | Stretching, joint mobilizations, exercises (ROM, bridging, isometric quads, home program) | none | none | none | None reported; use of analgesics not reported | Hotpacks (20 min); IFC (10 min); exercises (isometric quads); analgesics except during physio |
| Outcomes included in meta-analysis | Pain – 10 cm VAS | Pain on movement in past wk – 10 cm VAS WOMAC LK 3.1 Physical Function subscale Walking speed [time (s) to walk 50 m] | Pain on movement in past wk – 10 cm VAS Walking speed [time (s) to walk 20 m] | none | Pain following walking test – 11 point NRS WOMAC LK 3.1 Physical Function subscale Distance (m) walked in 6 min | Pain on activity – 10 cm VAS WOMAC LK 3.1 Physical Function subscale Walking speed [time (s) to walk 50 m] |
| Funding source | Non-profit | Not reported | Not reported | NERCUM, institutional | Government | Unfunded |
| Comments | Trial author confirmed mode was continuous; pain data extracted from graphs; request for pain and walking data was unsuccessful | Treated surface area not reported; estimated to be 3x the sound head size (based on parts of knee treated) | Attempts to contact authors for pain and physical function data that could be pooled were unsuccessful | 1 of the 2 reviewers co-authored the trial; outcomes pooled in this review were secondary outcomes in trial | Treated surface area not reported; estimated to be 3x the sound head size (based on parts of knee treated) |
The agreement between reviewers in determining risk of bias was κ = 0.81. Table 4 summarizes the methodological quality assessment of the six trials retrieved for full text review. Two trials[22,23] reported adequate random sequence generation and allocation concealment. Only one trial[21] did not report the blinding of participants adequately. Only one trial[22] confirmed that care providers were blinded (triple blind) and all five of the meta-analyzed trials confirmed that assessors were blinded to treatment allocation (double blind)[18-20,22,23]. In two included trials[19,20], it was unclear if complete data were collected. In one trial[18] included in the meta-analysis, data reporting was incomplete. Only one trial[21] (not included in the meta-analysis) was deemed to have other potential sources of bias due to randomization of more than one knee from the same participant. Based on our criteria for judging the overall methodological quality assessment for each trial, risk of bias was low in one trial and high in five trials (Table 4). Therefore, risk of bias across the studies is high. Slight asymmetry is noted in the funnel plots for each of the outcomes as illustrated in Figure 2 for the pain outcome.
| Ref. | Random Sequence Generation | Allocation Concealment | Blinding of Participant | Blinding of Care Provider | Blinding of Assessor | Data Collection Complete | Complete Outcome Reporting | Free of Other Potential Bias | Risk of Bias1 |
| Falconer et al[18] | Unclear | Unclear | Yes | No | Yes | Yes | No | Yes | High |
| Ozgönenel et al[19] | Unclear | Unclear | Yes | No | Yes | Unclear | Yes | Yes | High |
| Tascioglu et al[20] | Unclear | Unclear | Yes | No | Yes | Unclear | Yes | Yes | High |
| Yang et al[21] | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | No | No | High |
| Loyola-Sánchez et al[22] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Ulus et al[23] | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | High |
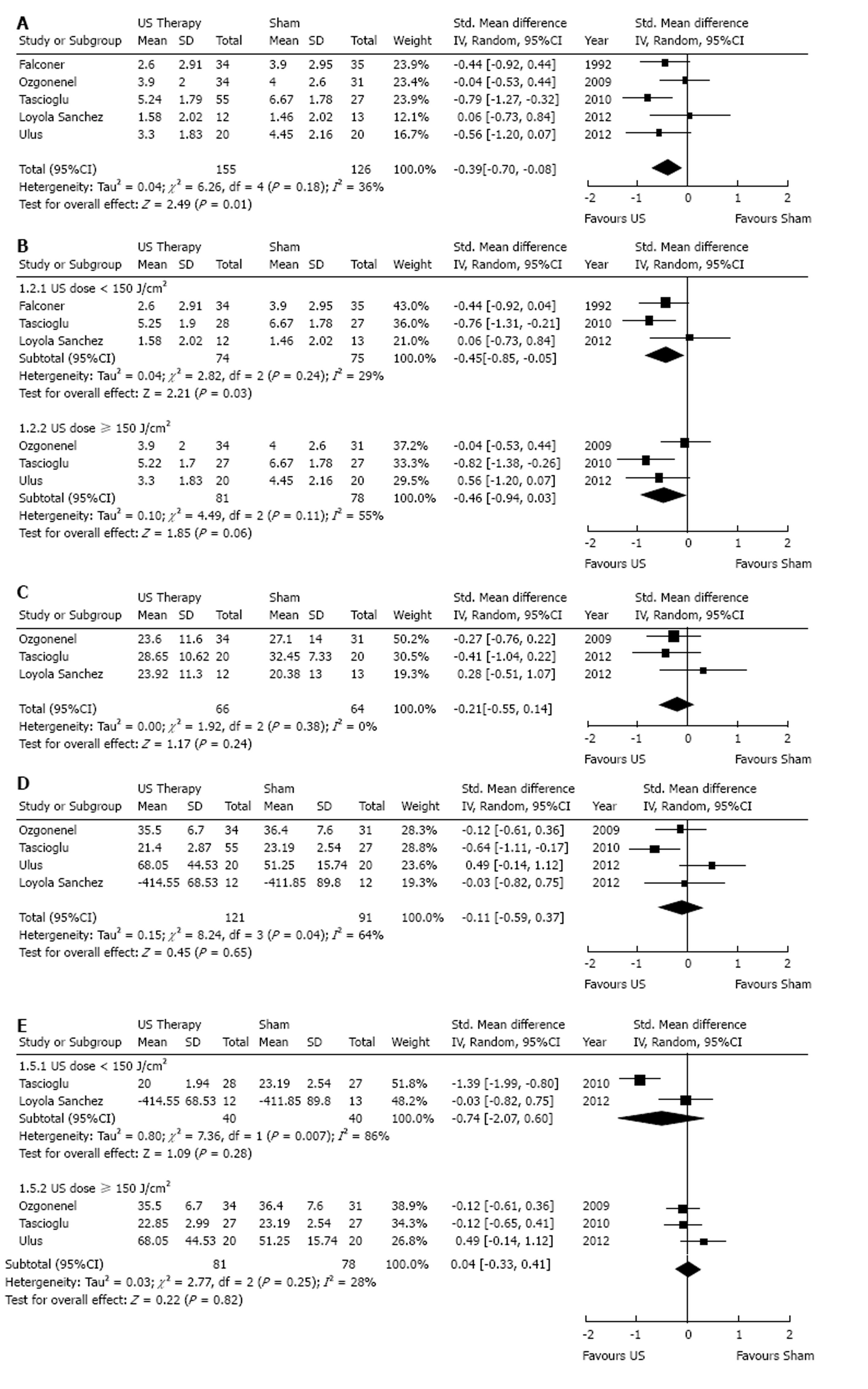
In the five trials included in the analysis assessing efficacy of therapeutic ultrasound on knee OA pain, a total of 281 participants were randomised: 155 to an active ultrasound group and 126 to a sham ultrasound group[18-20,22,23]. Figure 3A illustrates that therapeutic ultrasound (pulsed or continuous) was effective in reducing pain compared to sham ultrasound [SMD = -0.39 (-0.70, -0.08); P = 0.01]. The proportion of the dispersion in effect estimates across studies fell within that expected due to sampling error (I2 = 36%) and this low heterogeneity is unlikely to represent a true difference in effects in the studies (χ2 = 6.26, P = 0.18). Subgroup analyses based on dosage planned a priori demonstrated that low dose therapeutic ultrasound resulted in a significant decrease in pain [3 trials; SMD = -0.45 (-0.85, -0.05); P = 0.03; Figure 3B] and reduced the heterogeneity further (χ2 = 2.82, P = 0.24; I2 = 29%). In contrast, pooling the three studies administering high dose ultrasound vs sham ultrasound yielded a statistically insignificant decrease in pain and, although still acceptable, the heterogeneity increased [3 trials, SMD = -0.46 (-0.94, 0.03); P = 0.06; χ2 = 4.49, P = 0.11; I2 = 55%]. We were unable to perform subgroup analyses based on OA severity because all five either included participants with mild to moderate radiographic knee OA or did not report radiographic OA severity. Meta-regression analyses were not performed due to the small number of trials available for subgroup comparisons.
Three trials (130 participants and OA knees) assessed the effect of therapeutic ultrasound on self-reported physical function. Figure 3C illustrates that the point estimate for self-reported physical function in people with knee OA favours therapeutic ultrasound, however, the 95%CI crosses the point of no difference (SMD= -0.21, 95%CI: -0.55, 0.14, P = 0.24). Homogeneity for pooling the data across studies was acceptable (χ2 = 1.92, P = 0.24, I² = 0%). Due to the small numbers of studies that reported self-reported physical function outcomes, we could not perform meta-regression analyses.
Four trials assessed the effect of therapeutic ultrasound on physical function with respect to walking performance in people with knee OA. Figure 3D illustrates that walking performance in the group who received active ultrasound was not significantly different from that in the group who received sham ultrasound (SMD= -0.11, 95%CI: -0.59, 0.37, P = 0.65). In this analyses, the dispersion of the estimated effects exceeded that expected due to sampling error (χ2 = 8.37, P = 0.04, I² = 64%). Figure 3E illustrates that only the subgroup analysis of three trials administering high dose therapeutic ultrasound vs sham reduced the heterogeneity (I2 = 28%). The sample size was very small and the small effect estimate was not statistically significant (SMD = 0.04, 95%CI: -0.33-0.41, P = 0.82). Of the four trials available for meta-analysis, all included participants with mild to moderate knee OA. Meta-regression analyses planned a priori were not performed due to the small number of trials available for subgroup comparisons.
Two trials reported that no major complaints were reported[18,23]. Two trials reported no side effects although in one trial[19] two participants in the sham ultrasound group had to use analgesics and were dropped from the study and in another trial[20] six out of 90 participants (one in the active pulsed ultrasound group, two in the active continuous ultrasound group and three in the sham ultrasound group) had to use nonsteroidal anti-inflammatory drugs or analgeiscs because of intolerable pain and were withdrawn from the study. In one trial[21], adverse effects included dizziness (n = 3), mental stress, palpitation or fatigue however it was not stated if these participants received active or sham ultrasound. In the triple blind trial[22], two participants reported a “stinging” sensation during active ultrasound treatment and two participants receiving sham ultrasound reported a similar ‘stinging’ sensation during treatment.
The strength of the body of evidence for pain reduction and improvement in self-reported physical function and walking performance is summarized in Table 5. The finding that therapeutic ultrasound reduces pain is based on low-strength evidence (Table 5). For the self-reported physical function outcome, the mean difference in score is shown in Table 5 since all three of the trials provided data for the WOMAC LK3.1 physical function subscale that permitted pooling. The finding that therapeutic ultrasound does not improve self-reported physical function is based on very low strength evidence (Table 5). We report the findings for walking performance obtained when we pooled all four trials that assessed this outcome although our criteria for pooling data were not met (P = 0.04 for χ2 and I² = 64%). Acceptable homogeneity was achieved when we meta-analyzed the 3 trials (159 participants and knees) applying high dose ultrasound (χ2 = 2.77, P = 0.25, I² = 28%). We did not report the results of this subgroup analysis in the Summary of Findings Table because we felt the very small sample size contributed to the imprecision of the effect estimate for walking performance to a similar degree as the inconsistency thus the strength of the body of evidence could not be upgraded.
| Outcomes | Difference1 in ultrasound group mean relative to the control group mean (95%CI) | No of Participants and knees (studies) | Strength of the body of evidence2 | Inconsistency (I2) | Outcome specific risk of bias |
| Pain VAS; NRS Follow-up: 2-8 wk | 0.39 standard deviations lower [-0.70-(-0.08)] | 281 (5 studies) | Low | 36% | High risk of bias of the included studies, imprecision due to small sample size and wide CI |
| Self-reported physical function WOMAC® LK 3.1 Physical function; Follow-up: 2-8 wk | 2.49 points lower (-0.55-0.14) | 130 (3 studies) | Very low | 0% | High risk of bias of the included studies, imprecision due to very small sample size and wide CI |
| Walking performance 50 m walk speed (s); 20 m walk speed (s); 6MWT (m) Follow-up: 2-8 wks | 0.11 standard deviations lower (-0.59-0.37) | 212 (4 studies) | Very low | 64% | High risk of bias in the included studies, imprecision due to small sample size and wide CI, inconsistent |
This systematic review provides a meta-analysis of the efficacy of therapeutic ultrasound vs sham ultrasound for decreasing pain and improving physical function in people with knee OA who were blinded to treatment allocation. The main finding is that therapeutic ultrasound treatment decreases pain but does not improve physical function significantly in this patient population. Our results confirm those of recent meta-analyses[8-10]. Confidence in the treatment effect estimates is increased by including only those studies in which participants were blinded to the treatment they received[11]. Whereas we did remove an important source of subjective assessment bias, the strength of the evidence for pain and physical function outcomes is low and very low, respectively. Few trial participants from either the active or sham ultrasound group reported adverse events or side effects. These findings demonstrate that therapeutic ultrasound is a safe and effective treatment for pain in people with knee OA and further research is needed to determine if physical function improves.
In our systematic review, the treatment effect estimate is lower compared to those reported previously [SMD = -0.39 (-0.70, -0.08); P = 0.01] demonstrating the importance of study design characteristics of the trials included in the meta-analyses. Of the three new trails added to our meta-analysis, two had low risk of bias due to randomized sequence generation, allocation concealment and blinding of participants to this meta-analysis (Table 4). In one trial[22], the person administering the intervention was also blinded and the overall risk of bias was low. In contrast, trials published prior to 2011 had unclear risks of bias due to random sequence generation and allocation concealment and the care providers were not blinded. Given that the two outcomes of interest in our meta-analyses are self-reported, the estimates of treatment effect and between-trial heterogeneity may still be inflated[11] since these studies contribute 216 participants/OA knees to the total sample of 281 participants and OA knees. Our sample was too small to determine whether ultrasound dose influences the treatment effect estimates and all participants had mild to moderate OA so the influence of radiographic OA severity could not be investigated. Nevertheless, our results confirm the findings of previous meta-analyses[8-10] reporting that therapeutic ultrasound reduces knee OA pain and further research will clarify if there is a beneficial treatment effect with respect to physical function outcomes.
While recently published trials have ensured adequate blinding of the participants, the trials included in the current meta-analysis lack methodological rigour in terms of registering the protocol, assuring that risk of bias is low, and recruiting a sample size large enough to ensure precise estimates of efficacy. One trial[22] addressed the feasibility of recruitment and describes the burden of attending a centre for a passive treatment unproven in terms of efficacy as a barrier to participation and retention. Given the growing prevalence of knee OA, strategies to increase sample size and minimize loss to follow up is required in order to conduct a definitive high quality trial addressing this clinical question. If high quality evidence confirms that ultrasound is an effective conservative treatment for knee OA, the wide availability of the treatment ensures rapid translation of this evidence into clinical practice[6,7].
Apart from the walking performance outcome, between-trial homogeneity suggests that the interventions provided in the five trials were comparable. However, subgroup analyses pooling only the trials administering low dose ultrasound reduced the heterogeneity in the pain outcome from 36% to 29%. Table 3 highlights other differences in the interventions administered. Data from two trials that included co-interventions were pooled with data from three trials that did not describe co-interventions. Co-interventions included exercise in both trials[18,23] and the use of analgesics (except during physiotherapy) in one[23] of these trials. These treatments have proven efficacy in the treatment of knee OA and, clinical practice guidelines recommend multicomponent physical therapy for this population[3,4]. However, the interactions between these co-interventions and ultrasound therapy are not known. Another source of variability in the intervention was the application site. Of the five trials included in the meta-analyses, three trials[19,20,23] administered therapeutic ultrasound using a moving sound head applied to the anteromedial and lateral parts of the knee; one trial[18] used a moving sound head applied to the soft tissue around the knee which limited joint range of motion the most; one trial[22] used a stationary sound head applied to the anteromedial tibiofemoral joint line. Despite these differences, the data for pain and self-reported physical function outcomes had acceptable homogeneity for pooling. Taken together, these interventions were effective in reducing knee OA pain. However, further research is required to identify the critical components before a specific protocol for administering therapeutic ultrasound can be recommended.
We chose to focus this review on published trials that compared active and sham ultrasound in order to identify and synthesize the highest quality evidence available in the English-language literature. The funnel plots may be interpreted as evidence of publication bias however asymmetry is attenuated due to the small number of trials and the similar small sample size in each trial. We expected publication bias and therefore we did not plan to perform statistical tests to confirm this. Given that the Cochrane Handbook recommends a minimum of 10 studies in order to run the inferential analysis[24], we could not have conducted statistical testing anyway.
Whereas our systematic review provides high level evidence that therapeutic ultrasound reduces knee OA pain and may improve physical function based on trials in which participants did not know if they received active or sham ultrasound, this review was not without limitations. Random sequence generation and allocation concealment were inadequately described in three of the five meta-analyzed trials. However, it has been suggested that the selection bias which may be present as a result of these study design characteristics are most problematic when the participants’ prognosis is easy to assess at the time of randomization[11]. Since the progression of clinical symptoms in chronic knee OA is difficult to predict, we believe that the influence of selection bias on the effect estimates in the current review is minimal. Inadequate design and/or reporting of trial methods in the trials included in our systematic review limit the strength of the body of evidence which increases the uncertainty in the results. Finally, we chose to focus this review on double blind trials published in English; we did not request data for the three trials published only as abstracts in conference proceedings nor did we search trial registries to identify unpublished data. Furthermore, we were unsuccessful in our attempts to contact two of the primary authors to clarify trial methodology and secure study data suitable for pooling. The inclusion of these unpublished data may have yielded different results.
In conclusion, our meta-analysis suggests that therapeutic ultrasound decreases pain in people with knee OA; however, it is very likely that this conclusion will change when more research is conducted. Therapeutic ultrasound appears to be no better than sham ultrasound for improving self-reported physical function or walking performance. However, the very low strength of the body of evidence for the physical function outcomes leaves us very uncertain about these estimates and further research is necessary to answer this question.
We thank Heather N deBoer for her assistance with updating the literature search. This review protocol was designed and initiated while NJ MacIntyre was completing the Canadian Institutes of Health Research Randomized Controlled Trials Mentorship Program under the mentorship of M. Bhandari.
Knee osteoarthritis (OA) is a common problem resulting in pain and mobility limitations in people around the globe. Often symptoms progress to the point where joint replacement is required. Interventions are required that will delay or alleviate the need for surgical interventions. Multiple non-surgical interventions are used to treat patient-reported symptoms of pain and activity limitations and the efficacy of a single treatment component is difficult to evaluate.
Past systematic reviews evaluating the efficacy of therapeutic ultrasound which pooled data from studies in which participants did not know if there received active ultrasound therapy (blinded) or received the same interventions with the exception of ultrasound therapy (not blinded) suggest ultrasound is effective in reducing knee OA pain whereas reductions in physical function are not statistically significant. Efficacy of ultrasound with respect to patient-reported pain and physical function outcomes should be evaluated in randomized controlled trials in which participants are blinded.
Based on this meta-analysis, including only participants who were blinded to treatment allocation, knee OA pain decreases but physical function is not significantly improved following a course of therapeutic ultrasound treatment. These findings strengthen our confidence in similar findings reported in previous systematic reviews.
Therapeutic ultrasound appears to be an effective treatment to include in the multi-component non-surgical management of the important clinical problem of knee OA pain. Physical function was not statistically improved immediately following a course of therapeutic ultrasound treatment. Large, methodologically rigorous randomized trials in which the participants, health care providers, and assessors are blinded to treatment allocation and followed up over a longer period are needed to provide a clear answer to this clinical question.
Knee osteoarthritis is characterized by degenerative changes to cartilage and other tissues in and around the joint. Therapeutic ultrasound delivers high frequency sound waves and is a physical agent commonly applied by physical therapists to reduce inflammation and/or enhance tissue healing. These effects are postulated to result in improvements in patient-reported pain and physical function (self-reported or performance-based).
This meta-analysis was used to determine the efficacy of therapeutic ultrasound versus sham for improving pain and physical function immediately post-intervention in people with knee OA. This is an interesting meta-analysis.
P- Reviewer: Dai SM, Guo X S- Editor: Wen LL L- Editor: A E- Editor: Lu YJ
| 1. | Guccione AA, Felson DT, Anderson JJ, Anthony JM, Zhang Y, Wilson PW, Kelly-Hayes M, Wolf PA, Kreger BE, Kannel WB. The effects of specific medical conditions on the functional limitations of elders in the Framingham Study. Am J Public Health. 1994;84:351-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1052] [Cited by in RCA: 1098] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 2. | Felson DT. An update on the pathogenesis and epidemiology of osteoarthritis. Radiol Clin North Am. 2004;42:1-9, v. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 344] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 3. | Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, Towheed T, Welch V, Wells G, Tugwell P. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken). 2012;64:465-474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Richmond J, Hunter D, Irrgang J, Jones MH, Snyder-Mackler L, Van Durme D, Rubin C, Matzkin EG, Marx RG, Levy BA. American Academy of Orthopaedic Surgeons clinical practice guideline on the treatment of osteoarthritis (OA) of the knee. J Bone Joint Surg Am. 2010;92:990-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 134] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 5. | Bélanger AY. Ultrasound. Evidence-based guide to therapeutic physical agents. USA: Lippincott Williams & Wilkins 2003; 223-261. |
| 6. | Nussbaum EL, Burke S, Johnstone L, Lahiffe G, Robitaille E, Yoshida K. Use of electrophysical agents: Findings and implications of a survey of practice in metro Toronto. Physiother Can. 2007;59:118-131. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | MacIntyre NJ, Busse JW, Bhandari M. Physical therapists in primary care are interested in high quality evidence regarding efficacy of therapeutic ultrasound for knee osteoarthritis: a provincial survey. ScientificWorldJournal. 2013;2013:348014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Loyola-Sánchez A, Richardson J, MacIntyre NJ. Efficacy of ultrasound therapy for the management of knee osteoarthritis: a systematic review with meta-analysis. Osteoarthritis Cartilage. 2010;18:1117-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Rutjes AW, Nüesch E, Sterchi R, Jüni P. Therapeutic ultrasound for osteoarthritis of the knee or hip. Cochrane Database Syst Rev. 2010;CD003132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 10. | Wang SY, Olson-Kellogg B, Shamliyan TA, Choi JY, Ramakrishnan R, Kane RL. Physical therapy interventions for knee pain secondary to osteoarthritis: a systematic review. Ann Intern Med. 2012;157:632-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 11. | Savović J, Jones H, Altman D, Harris R, Jűni P, Pildal J, Als-Nielsen B, Balk E, Gluud C, Gluud L. Influence of reported study design characteristics on intervention effect estimates from randomised controlled trials: combined analysis of meta-epidemiological studies. Health Technol Assess. 2012;16:1-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 439] [Cited by in RCA: 515] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 12. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6253] [Cited by in RCA: 7632] [Article Influence: 477.0] [Reference Citation Analysis (1)] |
| 13. | Higgins JPT, Deeks JJ. Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration 2011; Available from: http://www.cochrane-handbook.org. |
| 14. | Higgins JPT, Altman DG. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration 2011; Available from: http://www.cochrane-handbook.org. |
| 15. | Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11058] [Cited by in RCA: 14870] [Article Influence: 874.7] [Reference Citation Analysis (0)] |
| 16. | Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration 2011; Available from: http:// www.cochrane-handbook.org. |
| 17. | Gurkan I, Ranganathan A, Yang X, Horton WE, Todman M, Huckle J, Pleshko N, Spencer RG. Modification of osteoarthritis in the guinea pig with pulsed low-intensity ultrasound treatment. Osteoarthritis Cartilage. 2010;18:724-733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Falconer J, Hayes KW, Chang RW. Effect of ultrasound on mobility in osteoarthritis of the knee. A randomized clinical trial. Arthritis Care Res. 1992;5:29-35. [PubMed] |
| 19. | Ozgönenel L, Aytekin E, Durmuşoglu G. A double-blind trial of clinical effects of therapeutic ultrasound in knee osteoarthritis. Ultrasound Med Biol. 2009;35:44-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Tascioglu F, Kuzgun S, Armagan O, Ogutler G. Short-term effectiveness of ultrasound therapy in knee osteoarthritis. J Int Med Res. 2010;38:1233-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 21. | Yang PF, Li D, Zhang SM, Wu Q, Tang J, Huang LK, Liu W, Xu XD, Chen SR. Efficacy of ultrasound in the treatment of osteoarthritis of the knee. Orthop Surg. 2011;3:181-187. [PubMed] |
| 22. | Loyola-Sánchez A, Richardson J, Beattie KA, Otero-Fuentes C, Adachi JD, MacIntyre NJ. Effect of low-intensity pulsed ultrasound on the cartilage repair in people with mild to moderate knee osteoarthritis: a double-blinded, randomized, placebo-controlled pilot study. Arch Phys Med Rehabil. 2012;93:35-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Ulus Y, Tander B, Akyol Y, Durmus D, Buyukakıncak O, Gul U, Canturk F, Bilgici A, Kuru O. Therapeutic ultrasound versus sham ultrasound for the management of patients with knee osteoarthritis: a randomized double-blind controlled clinical study. Int J Rheum Dis. 2012;15:197-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 24. | Sterne JAC, Egger M, Moher , D . Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration 2011; Available from: http://www.cochrane-handbook.org. |