Published online May 26, 2014. doi: 10.13105/wjma.v2.i2.36
Revised: January 3, 2014
Accepted: February 16, 2014
Published online: May 26, 2014
Processing time: 208 Days and 4.1 Hours
AIM: To investigate the clinical characteristics of pancreatic malignant melanoma.
METHODS: Here we report a case of pancreatic malignant melanoma and present a meta-analysis of pancreatic melanoma based on data from well-documented case reports.
RESULTS: A 32-year-old male presented with abdominal pain and jaundice. Computed tomography showed a non-discrete mass in the head of the pancreas. A pancreatoduodenectomy was performed. Histology revealed pancreatic malignant melanoma. Immunostaining showed that the neoplastic cells were positive for Melan A and S100, and that they expressed HMB-45. A review of 13 publications reporting 15 cases identified main clinical symptoms as abdominal pain (nine cases) and jaundice (three cases). Nine patients had a history of extra-abdominal malignant melanoma. The overall median survival time was 10 mo.
CONCLUSION: Pancreatic malignant melanoma is not typified by any special clinical presentation. The diagnosis depends on biopsy or specimen histology. Surgical resection remains the best treatment choice.
Core tip: Pancreatic malignant melanoma is rare and only few well-documented cases have been reported. Consequently only sparse information regarding its clinical features and biologic behavior is available. Here we report a case of pancreatic malignant melanoma and present a meta-analysis of pancreatic melanoma based on data from well-documented case reports.
- Citation: Yuan ZN, Liu FH, Tang Y. Overview and a meta-analysis of pancreatic melanoma based on case reports. World J Meta-Anal 2014; 2(2): 36-41
- URL: https://www.wjgnet.com/2308-3840/full/v2/i2/36.htm
- DOI: https://dx.doi.org/10.13105/wjma.v2.i2.36
Malignant melanoma is a form of neoplasm which originates from melanocytes. Melanocytes are pigmented cells located in the dermis or epidermis of the skin, squamous mucous membranes, the retina and leptomeninges. Most melanomas occur at the junction of the skin and mucous membranes[1]. Pancreatic melanoma occurs in approximately 50% of cases in which there is a disseminated neoplastic disease[2]. The formation of melanoma from isolated pancreatic metastasis occurs in no more than 2% of patients with visceral metastases[1,3,4]. Consequently the clinical features of pancreatic melanoma remain unclear.
Here we report a case of pancreatic melanoma treated at our own center. We also provide an overview and meta-analysis of data of published case reports.
A review of the literature was performed using MEDLINE (1966 to November 2012), CNKI (1994 to November 2012) and Springer Link (1989 to November 2012) databases, using the key words “pancreas” and “melanoma” to search for case reports and reviews. References cited in case series and case reports were searched to obtain all relevant case reports of pancreatic melanoma. From these, we compiled data on age, gender, symptoms, diagnostic methods, treatment protocols, and survival time. Statistical analysis was performed using Kaplan-Meier curves.
A 32-year-old man presented with a 2-wk history of abdominal pain and jaundice and was admitted to Tianjin Cancer Hospital. In 2011, the patient had been diagnosed with malignant skin melanoma which was successfully treated with two cycles of chemotherapy (BUCN and DTIC) at another center. One year ago, ultrasound and computed tomography (CT) revealed a mass in the pancreatic head. At that time the patient showed no sign of jaundice. The mass was successfully treated using gamma knife radiation.
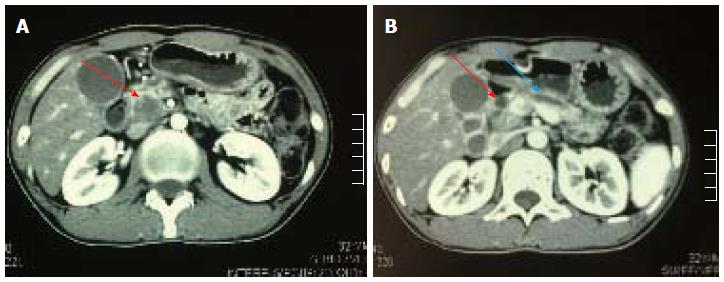
Laboratory tests showed that total bilirubin and direct bilirubin were both elevated (372.3 μmol/L and 257.9 μmol/L, respectively), ALT was 284 μmol/L, AST was 93 μmol/L and alkaline phosphate was 745 μmol/L. Carcinoembryonic antigen and carbohydrate antigen 19.9 (CA 19.9) were normal. Abdominal CT showed a hypodense mass in the head of the pancreas together with dilation of both the common bile duct and pancreatic duct (Figure 1A and B). There was no evidence of lymphadenopathy or vascular encasement.
Based on these findings, pancreatoduodenectomy was performed. Intraoperatively, a lesion 3.5 cm in diameter was found in the pancreatic head, but there were no signs of other abdominal metastases. Histopathology of the resected specimen confirmed the presence of metastatic melanoma.
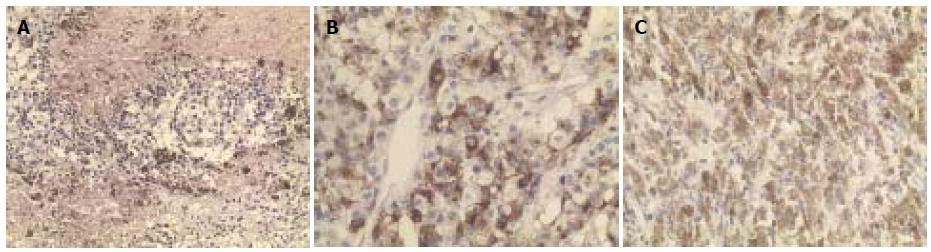
Immunostaining showed that the neoplastic cells were positive for Melan A, S100 and HMB-45 (Figure 2A, B and C). All resection margins were clear and there was no lymph node involvement. The patient achieved complete remission and was given one cycle of adjuvant chemotherapy (CCNU and DTIC). Liver metastases were found 2 mo postoperatively. The patient was still alive with tumors 6 mo later.
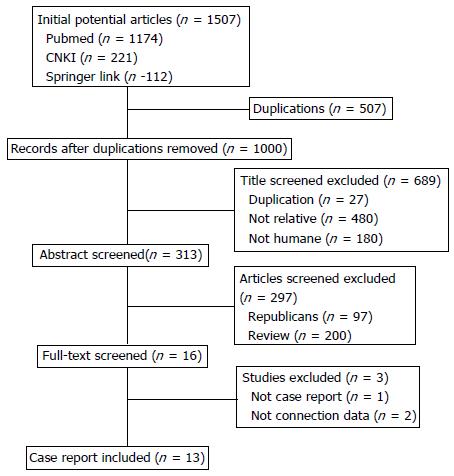
A total of 313 articles reporting pancreatic malignant melanoma were initially identified. Thirteen of them were case reports that included complete data (Figure 3). The 13 publications provided case data on 15 cases of malignant pancreatic melanoma (Table 1). The mean age of the patients was 53.2 years (range, 22 to 83 years) and the male-to-female ratio was 1.2:1. The most common clinical symptoms were abdominal pain (nine cases) and jaundice (three cases). Nine of the 15 had a history of extra-abdominal malignant melanoma.
| Ref. | Age (yr) | Gender | Symptoms | History | Imaging | Treatment | Prognosis (mo) |
| Lei et al[5] | 64 | M | Abdominal pain, fatigue, weight loss | - | CT and MR showed a lesion located in the body and tail of the pancreas. | Surgical resection | - |
| Wu et al[6] | 57 | M | Abdominal pain, weight loss | - | CT showed a mass in the head of the pancreas | TP | 6 + |
| Bergmann et al[7] | 46 | M | - | - | CT and MRI showed a lesion in the head of the pancreas | Whipple operation | 4 |
| Yang et al[8] | 58 | M | Abdominal pain, fatigue, weight loss | - | Ultrasound and CT showed atumor in the pancreas | TP | - |
| Solomons et al[9] | 60 | F | Nausea, abdominal pain, obstructive jaundice | - | Ultrasound and CT and MRI revealed a mass in the region of the pancreatic head | Bile duct stenting | 8 |
| Nikfarjam et al[10] | 45 | F | Abdominal discomfort | Ocular melanoma 12 yr ago | CT, PET-CT and MRI showed a mass in the head of pancreas | Pylorus-preserving pancreato-duodenectomy and segmental liver resection | 6 + |
| 55 | M | Abdominal pain | Ocular melanoma 13 yr ago | CT and PET-CT showed a mass in the head of the pancreas | TP | 7 + | |
| Kitamura et al[11] | 74 | M | - | - | Ultrasound and ERCP disclosed a cyst in the pancreas, communicating with a crater in the stomach. | Local resection | - |
| Stauch et al[12] | 68 | M | Obstructive jaundice | Melanoma in right ear 13 yr ago | Ultrasound. ERCP and CT revealed a mass in the head of the pancreas | Whipple operation | - |
| DeWitt et al[13] | 33 | M | Abdominal discomfort, obstructive jaundice | Ultrasound and CT demonstrated a mass in the head of the pancreas | Laparotomy | 6 | |
| 83 | F | Abdominal pain, hematochezia | CT and EUS found multiple lesions in the body and the tail of the pancreas | Supportive treatment | 10 | ||
| Dumitrascu et al[14] | 43 | F | - | Melanoma in the right eye 4 yr ago | Ultrasound found three metastases: (one in the body of pancreas and two in the right inferior pulmonary lobe) | Central pancreatectomy, right inferior pulmonary lobectomy | 12 + |
| Minoguchi et al[15] | 35 | F | _ | Melanoma in the vagina 2 yr ago, and metastasis to the lung 1 yr ago | CT and MRI found a mass in the head of the pancreas | Chemotherapy | 4 |
| Belagyi et al[16] | 22 | F | - | Melanoma in the back 9 yr ago, and metastasis to the right ovary 4 yr ago | CT showed the metastases tothe right ovary, the body of the pancreas, bowel and distal stomach, respectively | Resection of all metastases | 4 |
| Camp et al[17] | 56 | F | Right upper abdominal pain | Uveal melanoma of the left eye 6 yr ago | CT and PET-CT revealed a hepatic metastasis and a metastasis in the midbody of the pancreas | DP, mesohepatectomy and biochemotherapy with dacarbazine and interferon alpha | 20 + |
The tumor imaging features were similar to those of other types of pancreatic carcinoma. In eight cases the lesions were located in the head, and three patients had lesions in the pancreatic body or tail. In two cases the lesions diffused throughout the entire pancreas and in two other patients pancreatic lesions were accompanied by other organ metastasis.
Ten of the 15 patients underwent surgery: four had local resections, three had Whipple operations, and the remaining three underwent total pancreatectomy. The overall median survival was 10 mo (Figure 4).
As the pancreatic melanoma is rare, we have attempted to review its clinical presentations by analysis of published cases. The results of our meta-analysis show that the clinical presentations of pancreatic malignant melanoma are similar to those of other pancreatic malignant tumors and that diagnosis is reliant on biopsy or pathology after surgery. There is evidence to suggest that a history of malignant melanoma is associated with the diagnosis
Malignant melanoma rarely affects intra-abdominal organs, and most cases of visceral melanoma are the result of the spread of advanced malignant melanoma. A previously published autopsy review reported that the most common sites for metastatic gastrointestinal melanoma were the liver (68%), small bowel (58%), colon (22%) and stomach (20%). The duodenum, rectum, esophagus and anus accounted for 1% to 12% of cases[15]. Other researchers have shown that single organ involvement is present in approximately one third of all cases[2,4] of gastrointestinal melanoma, whereas extra-abdominal melanoma spreading to the pancreas only accounts for about 2% of all cases[14].
The lesions of pancreatic malignant melanoma are often located in the head of the pancreas, and the symptoms often mimic those of other pancreatic tumors (including abdominal pain, lack of strength and jaundice). The initial diagnosis of pancreatic malignant melanoma is, therefore, dependent on CT, magnetic resonance imaging or ultrasonography[8,9]. Recently, positron emission tomography has been used to identify sites of metastatic melanoma and for qualitative diagnosis[18,19].
A confirmatory diagnosis of pancreatic malignant melanoma depends on biopsy or pathology of the resected specimen. On gross inspection the pancreatic tumor is often present as a sharply demarcated mass. Histologically, the tumor displays white and brownish, grey or black microcystic areas[7], and is composed of a heterogeneous mix of spindle-shaped cells, plump, polygonal cells with an epithelioid appearance, and multinucleated giant cells. The cells are arranged in fascicles, whorls, nodular patterns, or are of a myxoid-cystic appearance. Most of the tumor cells contain melanin pigmentation. However, there is no recognizable growth pattern.
Immunohistochemical staining is useful in confirming the diagnosis of metastatic melanoma, and often reveals the presence of focal staining for S100 and HMB-45 within the pigmented areas[8-10].
Currently there is no satisfactory non-surgical treatment for metastatic melanoma. All of the metastatic tissue needs to be excised if surgery is to offer survival benefit. Complete pancreatic resection has been shown to improve survival even in patients with multiple metastases and in some cases is curative[14-16,20,21-24].
It is estimated that only 1% of all pancreatic resections are performed for metastases[20]. A study of 60 patients with metastatic diseases involving intra-abdominal solid organs showed that the 5-year overall survival (OS) was significantly improved after complete resection (24%) vs incomplete resection (0%), but median OS after complete resection was not significantly different for single-site vs synchronous multisite metastases[25]. Median disease-free survival (DFS) after complete resection was 15 mo. The 2-year DFS rate after complete resection was 53% for synchronous multi-site metastases vs 26% for single-site metastases[25].
It is unclear whether adjuvant therapy helps to improve the survival benefit achieved with surgery. Evidence from uncontrolled phase 2 studies suggests a modest survival benefit with such an approach, but the survival benefit of adjuvant therapy has yet to be demonstrated in randomized trials[26].
For patients unable to undergo resection, combination chemotherapy with dacarbazine, cisplatin, interferon-α-2α and interleukin-2 has been shown to be effective, resulting in a partial response rate of 24% with stable disease being maintained in 48% of cases[27].
The survival outcome of patients with visceral metastases from melanoma is dismal, regardless of histopathological features, with a median survival of 6-12 mo[25,28,29]. Five-year survival rates from several series is less than 10%[19,30-32].
In summary, the present case report and the review of literature show the difficulty in diagnosing a metastatic melanoma in the pancreas, due to its insidious clinical manifestations . In patients with a history of melanoma, a high index of suspicion for metastasis needs to be maintained if they present with seemingly unrelated symptoms. Diagnosis requires histology after surgery or biopsy with special immunohistochemical staining (HMB-45 and S100). This malignancy has a poor prognosis with a median survival of 6 to 12 mo. Surgery is the only effective treatment, and it is unclear whether an adjuvant therapy helps to improve the survival benefit.
Malignant melanoma is a form of neoplasm which originates from melanocytes. Most melanomas occur at the junction of the skin and mucous membranes. Pancreatic melanoma occurs in approximately 50% of cases in which there is a disseminated neoplastic disease. The formation of melanoma from isolated pancreatic metastasis occurs in no more than 2% of patients with visceral metastases. Consequently the clinical features of pancreatic melanoma remain unclear.
Malignant melanoma rarely affects intra-abdominal organs, and most cases of visceral melanoma are the result of the spread of advanced malignant melanoma. A previously published autopsy review reported that the most common sites for metastatic gastrointestinal melanoma were the liver (68%), small bowel (58%), colon (22%) and stomach (20%). The duodenum, rectum, esophagus and anus accounted for 1% to 12% of cases. Other researchers have shown that single organ involvement is present in approximately one third of all cases of gastrointestinal melanoma, whereas extra-abdominal melanoma spreading to the pancreas only accounts for about 2% of all cases.
A confirmatory diagnosis of pancreatic malignant melanoma depends on biopsy or pathology of the resected specimen. On gross inspection the pancreatic tumor is often present as a sharply demarcated mass. Histologically, the tumor displays white and brownish, grey or black microcystic areas, and is composed of a heterogeneous mix of spindle-shaped cells, plump, polygonal cells with an epithelioid appearance, and multinucleated giant cells. The cells are arranged in fascicles, whorls, nodular patterns, or are of a myxoid–cystic appearance. Most of the tumor cells contain melanin pigmentation. However, there is no recognizable growth pattern. Immunohistochemical staining is useful in confirming the diagnosis of metastatic melanoma, and often reveals the presence of focal staining for S100 and HMB-45 within the pigmented areas.
The study results suggest that a high index of suspicion for metastasis needs to be maintained if they present with seemingly unrelated symptoms. Diagnosis requires histology after surgery or biopsy with special immunohistochemical staining (HMB-45 and S100). This malignancy has a poor prognosis with a median survival of 6-12 mo. Surgery is the only effective treatment, and it is unclear whether an adjuvant therapy helps to improve the survival benefit.
Malignant melanoma is a form of neoplasm which originates from melanocytes. These are pigmented cells located in the dermis or epidermis of the skin, in squamous mucous membranes, the retina and leptomeninges.
The topic of the research is very relevant and interesting to a wide range of readers around the world, because presentations on pancreatic melanoma are very limited.
P- Reviewers: Bashashati M, Kadusevicius E, Wang XH S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Wu HL
| 1. | Patel JK, Didolkar MS, Pickren JW, Moore RH. Metastatic pattern of malignant melanoma. A study of 216 autopsy cases. Am J Surg. 1978;135:807-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 430] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 2. | Dasgupta TK, Brasfield rd. metastatic melanoma of the gastrointestinal tract. Arch Surg. 1964;88:969-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 189] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 3. | Collaborative Ocular Melanoma Study Group. Assessment of metastatic disease status at death in 435 patients with large choroidal melanoma in the Collaborative Ocular Melanoma Study (COMS): COMS report no. 15. Arch Ophthalmol. 2001;119:670-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 283] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 4. | Brodish RJ, McFadden DW. The pancreas as the solitary site of metastasis from melanoma. Pancreas. 1993;8:276-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Lei JQ, Wang XH, Chen Y, Guo SL. Malignant melanoma of the pancreas: case report. Chin J Med Imaging Technol. Chin J Med Imaging Technol. 2009;25:1329. [DOI] [Full Text] |
| 6. | Wu GJ, Yang CQ, Huang MX, Wang ZT. Malignant melanoma of the pancreas: case report. Chin J Hepatobiliary Surg. 2001;7:265. [DOI] [Full Text] |
| 7. | Bergmann F, Hackert T, Mechtersheimer G, Penzel R, Bläker H, Berger I, Esposito I, Büchler MW, Otto HF. Differential diagnosis of non-epithelial tumors of the pancreas: malignant non-epithelial pancreatic tumor with focal pigmentation. Virchows Arch. 2004;444:190-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Yang Y, Li HL, Cao HG. Ultrasonic features of pancreatic malignant melanoma: case report. Chin J Ultrasonogr. 2000;9:45. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Solomons G, Gibson RN, Tello RJ. Pancreatic head melanoma producing obstructive jaundice: magnetic resonance characteristics aiding differentiation from adenocarcinoma. Australas Radiol. 2000;44:471-473. [PubMed] |
| 10. | Nikfarjam M, Evans P, Christophi C. Pancreatic resection for metastatic melanoma. HPB (Oxford). 2003;5:174-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Kitamura Y, Sakue M, Nishiyama K, Matsumoto S, Tamagawa M, Okada K, Chinzei T, Yamashiro K, Bando K, Fujimoto T. A case of metastatic malignant melanoma mimicking pancreatic pseudocyst. Gan No Rinsho. 1987;33:748-752. [PubMed] |
| 12. | Stauch S, Rösch W. Obstructive jaundice caused by metastasis to the head of the pancreas by a malignant melanoma. Bildgebung 1987-. 1989;56:115-117. [PubMed] |
| 13. | DeWitt JM, Chappo J, Sherman S. Endoscopic ultrasound-guided fine-needle aspiration of melanoma metastatic to the pancreas: report of two cases and review. Endoscopy. 2003;35:219-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Dumitraşcu T, Dima S, Popescu C, Gheonea DI, Ciurea T, Saftoiu A, Popescu I. An unusual indication for central pancreatectomy--late pancreatic metastasis of ocular malignant melanoma. Chirurgia (Bucur). 2008;103:479-485. [PubMed] |
| 15. | Minoguchi M, Yanagawa N, Ishikawa C, Sasajima J, Goto M, Okamoto M, Saito Y, Murakami M, Orii Y, Yaosaka T. Pancreatic metastasis of malignant melanoma diagnosed by EUS-guided fine needle aspiration (EUS-FNA). Nihon Shokakibyo Gakkai Zasshi. 2007;104:1082-1087. [PubMed] |
| 16. | Belágyi T, Zsoldos P, Makay R, Issekutz A, Oláh A. Multiorgan resection (including the pancreas) for metastasis of cutaneous malignant melanoma. JOP. 2006;7:234-240. [PubMed] |
| 17. | Camp R, Lind DS, Hemming AW. Combined liver and pancreas resection with biochemotherapy for metastatic ocular melanoma. J Hepatobiliary Pancreat Surg. 2002;9:519-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Harrison LE, Merchant N, Cohen AM, Brennan MF. Pancreaticoduodenectomy for nonperiampullary primary tumors. Am J Surg. 1997;174:393-395. [PubMed] |
| 19. | Damian DL, Fulham MJ, Thompson E, Thompson JF. Positron emission tomography in the detection and management of metastatic melanoma. Melanoma Res. 1996;6:325-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 101] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 20. | Ollila DW, Essner R, Wanek LA, Morton DL. Surgical resection for melanoma metastatic to the gastrointestinal tract. Arch Surg. 1996;131:975-999. [PubMed] |
| 21. | Prichard RS, Hill AD, Skehan SJ, O’Higgins NJ. Positron emission tomography for staging and management of malignant melanoma. Br J Surg. 2002;89:389-396. |
| 22. | Hiotis SP, Klimstra DS, Conlon KC, Brennan MF. Results after pancreatic resection for metastatic lesions. Ann Surg Oncol. 2002;9:675-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 140] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 23. | Roland CF, van Heerden JA. Nonpancreatic primary tumors with metastasis to the pancreas. Surg Gynecol Obstet. 1989;168:345-347. [PubMed] |
| 24. | Hirota T, Tomida T, Iwasa M, Takahashi K, Kaneda M, Tamaki H. Solitary pancreatic metastasis occurring eight years after nephrectomy for renal cell carcinoma. A case report and surgical review. Int J Pancreatol. 1996;19:145-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 41] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Wood TF, DiFronzo LA, Rose DM, Haigh PI, Stern SL, Wanek L, Essner R, Morton DL. Does complete resection of melanoma metastatic to solid intra-abdominal organs improve survival? Ann Surg Oncol. 2001;8:658-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 108] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 26. | Spitler LE, Grossbard ML, Ernstoff MS, Silver G, Jacobs M, Hayes FA, Soong SJ. Adjuvant therapy of stage III and IV malignant melanoma using granulocyte-macrophage colony-stimulating factor. J Clin Oncol. 2000;18:1614-1621. [PubMed] |
| 27. | Proebstle TM, Scheibenbogen C, Sterry W, Keilholz U. A phase II study of dacarbazine, cisplatin, interferon-alpha and high-dose interleukin-2 in ‘poor-risk’ metastatic melanoma. Eur J Cancer. 1996;32A:1530-1533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Sharpless SM, Das Gupta TK. Surgery for metastatic melanoma. Semin Surg Oncol. 1998;14:311-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 29. | Overett TK, Shiu MH. Surgical treatment of distant metastatic melanoma. Indications and results. Cancer. 1985;56:1222-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 30. | Schuchter LM, Green R, Fraker D. Primary and metastatic diseases in malignant melanoma of the gastrointestinal tract. Curr Opin Oncol. 2000;12:181-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 124] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 31. | Balch CM, Soong SJ, Murad TM, Smith JW, Maddox WA, Durant JR. A multifactorial analysis of melanoma. IV. Prognostic factors in 200 melanoma patients with distant metastases (stage III). J Clin Oncol. 1983;1:126-134. [PubMed] |
| 32. | Barth A, Wanek LA, Morton DL. Prognostic factors in 1,521 melanoma patients with distant metastases. J Am Coll Surg. 1995;181:193-201. [PubMed] |