Published online Feb 26, 2014. doi: 10.13105/wjma.v2.i1.1
Revised: November 20, 2013
Accepted: December 17, 2013
Published online: February 26, 2014
Processing time: 123 Days and 19.6 Hours
Heart failure is a dynamic condition with high morbidity and mortality and its prognosis should be reassessed frequently, particularly in patients for whom critical treatment decisions may depend on the results of prognostication. In patients with heart failure, nuclear cardiology techniques are useful to establish the etiology and the severity of the disease, while fewer studies have explored the potential capability of nuclear cardiology to guide cardiac resynchronization therapy (CRT) and to select patients for implantable cardioverter defibrillators (ICD). Left ventricular synchrony may be assessed by radionuclide angiography or gated single-photon emission computed tomography myocardial perfusion scintigraphy. These modalities have shown promise as predictors of CRT outcome using phase analysis. Combined assessment of myocardial viability and left ventricular dyssynchrony is feasible using positron emission tomography and could improve conventional response prediction criteria for CRT. Preliminary data also exists on integrated positron emission tomography/computed tomography approach for assessing myocardial viability, identifying the location of biventricular pacemaker leads, and obtaining left ventricular functional data, including contractile phase analysis. Finally, cardiac imaging with autonomic radiotracers may be useful in predicting CRT response and for identifying patients at risk for sudden cardiac death, therefore potentially offering a way to select patients for both CRT and ICD therapy. Prospective trials where imaging is combined with image-test driven therapy are needed to better define the role of nuclear cardiology for guiding device therapy in patients with heart failure.
Core tip: This article focuses on the potential capability of nuclear cardiology techniques to guide cardiac resynchronization therapy and to select patients for implantable cardioverter defibrillators. Radionuclide angiography and gated single-photon emission computed tomography myocardial perfusion imaging have shown promise as predictors of outcome after device therapies. Combined positron emission tomography/computed tomography may identify the location of biventricular pacemaker leads and obtain left ventricular functional data, including contractile phase analysis. Cardiac imaging with autonomic radiotracers may predict cardiac resynchronization therapy response and may also identify patients at risk for sudden cardiac death, therefore potentially offering a way to select patients for these device treatments.
- Citation: Petretta M, Petretta A, Pellegrino T, Nappi C, Cantoni V, Cuocolo A. Role of nuclear cardiology for guiding device therapy in patients with heart failure. World J Meta-Anal 2014; 2(1): 1-16
- URL: https://www.wjgnet.com/2308-3840/full/v2/i1/1.htm
- DOI: https://dx.doi.org/10.13105/wjma.v2.i1.1
The diagnostic and prognostic utility of nuclear cardiology techniques in patients with suspected or known coronary artery disease (CAD) is well established[1-5]. In patients with heart failure (HF), stress myocardial perfusion imaging is useful for establishing the etiology and the severity of the disease, while fewer studies have explored the potential capability of nuclear cardiology for guiding device therapy. Two aspects seem particularly relevant: (1) guiding cardiac resynchronization therapy (CRT) and (2) selecting patients for implantable cardioverter defibrillators (ICD).
In the United States, HF incidence has remained stable over the past several decades, with > 650000 new cases diagnosed annually[6-8]. It has been estimated that approximately 2% of the adult population in developed countries has HF; most patients will be aged > 70 years and about half will have a left ventricular (LV) ejection fraction (EF) < 50%[9]. Epidemiological and clinical trial data suggest that about 60% to 70% of HF patients have CAD[10]. An estimated 5.1 million Americans ≥ 20 years of age has HF[6]. Projections show that by 2030, the total cost of HF will increase almost 120% to $70 billion from the 2013 estimated total cost of $32 billion[6,11].
Patients with CAD-related LV systolic dysfunction have a worse prognosis than those with nonischemic cardiomyopathy[12,13]. In patients with LV systolic dysfunction, it may be useful to distinguish those with extensive CAD from those with more limited disease. The latter are considered to have coexisting but not causally related CAD and their prognosis seems to be similar to patients with nonischemic cardiomyopathy and better than those with extensive CAD[14]. However, in a cohort of 2331 patients enrolled in the HF-ACTION (Heart Failure: A Controlled Trial Investigating Outcomes of Exercise TraiNing) study, ischemic etiology was not a key determinant of a worse prognosis[15]. The analysis from the HF-ACTION clinical trial database represents the first risk prediction model for patients with HF due to systolic dysfunction that were treated with a high degree of evidence-based therapy (β-blockade, 95%; angiotensin-converting enzyme inhibitor, 74%; ICD, 40%; CRT, 18%). This analysis supports the consideration that because HF is a dynamic condition with high morbidity and mortality, its prognosis should be reassessed frequently, particularly in patients for whom critical treatment decisions may depend on the results of prognostication[16].
In about one third of HF patients there is marked prolongation of the QRS interval[17-19]. This sign has been associated with ventricular electromechanical delay (dyssynchrony) and QRS duration and dyssynchrony are both predictors of worsening HF, sudden cardiac death and total death[20,21]. The worse prognosis cannot be only explained by a lower LVEF[22-25]. Several randomized clinical trials have shown that CRT is associated with decreases in recurrent HF hospitalization, improvement in quality of life, LV remodeling and survival[26-31]. In the REVERSE study (Resynchronization reverses Remodeling in Systolic left vEntricular dysfunction), which included New York Heart Association (NYHA) class I or II HF patients and QRS prolongation, CRT-induced reverse remodeling was comparable in subjects with LVEF > 30% and in those with more severe LV systolic dysfunction[32]. However, about 30% of patients who receive CRT based on QRS duration do not derive symptom improvement or demonstrate reverse remodeling[33-36]. CRT seems to be useful only in a small proportion (5%-10%) of HF patients, nevertheless this is still a large number of individuals[37]. Based on data from two EuroHeart Failure surveys and extrapolating from hospital discharge statistics[38-40], it has been estimated that about 400 patients per million population per year might be suitable for CRT, or up to 400000 patients per year in European countries[41].
The American College of Cardiology/American Heart Association/Heart Rhythm Society guidelines recommend CRT for patients who have LVEF ≤ 35%, sinus rhythm, left bundle-branch block (LBBB) with a QRS duration ≥ 150 ms, and NYHA class II, III, or ambulatory IV symptoms on guideline-directed medical therapy (GDMT) (class I, level of evidence A)[42]. For these guidelines, class IIa recommendations are: (1) CRT can be useful for patients who have LVEF ≤ 35%, sinus rhythm, LBBB with a QRS duration 120 to 149 ms, and NYHA class II, III or ambulatory IV symptoms on GDMT (level of evidence B); (2) CRT can be useful for patients who have LVEF ≤ 35%, sinus rhythm, a non-LBBB pattern with a QRS duration ≥ 150 ms, and NYHA class III/ambulatory class IV symptoms on GDMT (level of evidence A); (3) CRT can be useful in patients with atrial fibrillation and LVEF ≤ 35% on GDMT if the patient requires ventricular pacing or otherwise meets CRT criteria and atrioventricular nodal ablation or pharmacological rate control will allow nearly 100% ventricular pacing with CRT (level of evidence B); and (4) CRT can be useful for patients on GDMT who have LVEF ≤ 35% and are undergoing new or replacement device placement with anticipated requirement for significant (> 40%) ventricular pacing (level of evidence C).
The European Society of Cardiology in collaboration with the European Heart Rhythm Association also developed guidelines for CRT[41]. According to these guidelines, CRT is recommended in chronic HF patients with LBBB and QRS duration > 150 ms and LVEF ≤ 35% who remain in NYHA functional class II, III and ambulatory IV despite adequate medical treatment (class I, level of evidence A). In chronic HF patients with LBBB with QRS duration 120-150 ms and LVEF ≤ 35% who remain in NYHA functional class II, III and ambulatory IV despite adequate medical treatment, CRT is also recommended (class I, level of evidence B). For non-LBBB with QRS duration > 150 ms, CRT should be considered in chronic HF patients and LVEF ≤ 35% that remain in NYHA functional class II, III and ambulatory IV despite adequate medical treatment (class IIa, level of evidence B). These recommendations represent a majority view of the task force but not all who contributed agreed. Several statements were based on subgroup analyses of randomized clinical trials that pose many problems with interpretation (interrelationship between QRS morphology and QRS duration, gender differences in response, prognostic benefit in ischemic vs non-ischemic patients) or with areas of uncertainty that are still the objects of investigation (potential role of echocardiographic dyssynchrony in narrow QRS). Thus, future studies might change knowledge and recommendations.
It must be outlined that, also using stringent criteria of selection, 20% to 40% of patients fail to respond to CRT[21,27,43-46]. It has been hypothesized that electrical dyssynchrony, as indicated by a prolonged QRS interval, does not necessarily reflect mechanical dyssynchrony, probably explaining why the patients in the above trials did not respond to CRT[47-49]. However, the recent findings do negate the use of echocardiographic measures of mechanical dyssynchrony in patients with QRS duration < 130 ms for identifying those who are most likely to have a good response to CRT[50]. In fact, in patients with systolic HF and a QRS duration < 130 ms, CRT does not reduce the rate of death or hospitalization for HF and may increase mortality[51].
It has been hypothesized that as a wide QRS duration (i.e., electrical dyssynchrony) does not necessarily reflect mechanical dyssynchrony, characterization and quantification of resting mechanical dyssynchrony may help to improve the clinical response to CRT[52,53]. Accordingly, it has been reported that imaging techniques may be able to identify those patients who will respond favorably to CRT[54-56]; however, no modality can be considered the gold standard (Table 1). A multicenter study evaluated eight echocardiographic parameters of dyssynchrony, demonstrating a considerable variability among various techniques that assess prevalence of mechanical dyssynchrony and in identification of the latest mechanical LV contracting region[57]. Moreover, in the PROSPECT (Predictors Of Response to Cardiac Resynchronization Therapy) trial, echocardiographic parameters of cardiac mechanical dyssynchrony showed a modest accuracy to predict response to CRT, defined by improvement in the composite clinical score and ≥ 15% reduction in LV end-systolic volume[58] and only a sub-study of this trial showed that the extent of LV reverse remodeling was associated with the extent of baseline interventricular and intra-LV mechanical dyssynchrony[59]. However, a recent single center study reported that a multiparametric echocardiographic score, including LV end-diastolic dimension, LV global longitudinal strain, left atrium area, right ventricular end-diastolic area, right ventricular fractional area change and right atrium area, is helpful in selecting patients likely to undergo reverse remodeling post-CRT[60]. This score was also able to predict clinical outcomes, showing a direct inverse correlation with adverse cardiac events and all cause mortality.
| Techniques | Advantages | Limitations |
| Echocardiography | Widely available; good spatial resolution; assessment of left and right ventricular volumes and function, scar burden and mitral regurgitation; no ionizing radiation; relatively low cost | Low reproducibility, limited by hemodynamic variations, operator experience, machine settings, available acoustic window, and angle of incidence; geometrical models may provide sources of error; complex interpretation due to too many indices; the time needed to perform extensive measurements may limit application in routine clinical practice |
| Cardiac computed tomography | Especially useful to guide endocardial left ventricular lead placement; pre-procedural use to characterize venous anatomy aids in lead placement; fusion imaging modalities available | Low temporal and spatial resolution, improved by the advent of dual-source multidetector; prolonged procedure times may increase risk for periprocedural complications and radiation exposure; low availability; limited clinical experience; intermediate cost |
| Cardiac magnetic resonance imaging | High spatial resolution and tissue characterization; accurate quantification of chamber size, ventricular function and 3-dimensional myocardial strain; high reproducibility with low operator dependency; no ionizing radiation; fusion imaging modalities available | Long acquisition times, potential magnetic resonance hazards of implanted cardiac devices; complex post-processing techniques; low availability; limited clinical experience; high cost |
| Radionuclide imaging | Widely available; simplicity of interpretation; provides data on scar burden and location, left ventricular function and site of latest contraction, and mechanical dyssynchrony from a single scan; fusion imaging modalities available | No role in identifying coronary venous anatomy; ionizing radiations; intermediate cost |
Other imaging techniques, including cardiac magnetic resonance, speckle tracking echocardiography, tissue-Doppler strain imaging and nuclear cardiology, have been investigated, yielding several parameters of LV mechanical dyssynchrony that have been demonstrated to be independent predictors of CRT response and long-term outcome in observational studies[55,61-64]. Approaches utilizing cardiac magnetic resonance and cardiac X-ray computed tomography (CT) have been used to assess LV dyssynchrony, also providing venous anatomy visualization and scar burden[65,66]. Therefore, these techniques have the potential for increased clinical use with further development and validation of adequate tools.
Radionuclide-based methods to measure LV synchrony include: (1) planar radionuclide angiography; (2) single-photon emission computed tomography (SPECT) radionuclide angiography; and (3) gated SPECT myocardial perfusion scintigraphy (MPS). These modalities have all shown promise as predictors of CRT outcome using phase analysis[62,67,68]. In particular, studies have shown that the degree of dyssynchrony on planar and SPECT radionuclide angiography has prognostic value and may be useful for predicting response to CRT[69,70]. However, these techniques are unable to provide simultaneous information on myocardial perfusion and scar location and extent that might compromise the response to CRT[55,71].
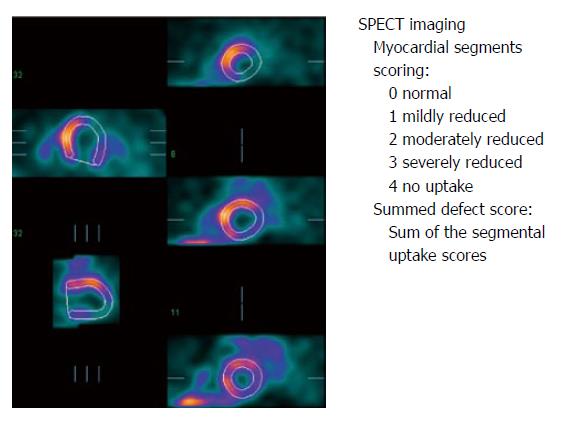
Phase analysis of gated MPS is a promising modality for several reasons: stress MPS is widely available and phase analysis can be performed on a study that is acquired using standard methods. Gated MPS also provides additional information on LV perfusion, function, scar location and extent[72]. Gated MPS produces a number of three-dimensional LV frames corresponding to different time points during the cardiac cycle. As these frames progress from the R wave, both location and intensity of each myocardial segment change periodically. The change in location of each myocardial segment allows assessment of regional wall motion and a change in the intensity indicates regional wall thickening as a result of the partial-volume effect[73,74]. Another technique of dyssynchrony calculation includes thickening and wall motion dyssynchrony calculations[75].
Phase analysis of gated MPS is based on Fourier transformation of the time-activity curve of each myocardial sample to derive the first harmonic function[76]. The first harmonic function is displayed as a continuous curve, representing thickening of the myocardial. The phase information is related to the time interval when a myocardial region starts to contract, providing information as to how uniform or inhomogeneous the distribution of these time intervals is for the entire LV. In particular, the temporal onset of mechanical contraction during the cardiac cycle of each myocardial sample is considered to be the phase of the inflection point of the thickening curve on a horizontal line representing the average myocardial count over a cardiac cycle. The phase and amplitude distribution from LV myocardial samples is displayed in histogram and polar map formats. The frequency distribution of Fourier phase angles is also tabulated in global and regional histograms, using amplitude information to eliminate phase measurements for which the corresponding amplitude was deemed too low to yield accurate measurements, as the Fourier phase angle is not well defined for a signal that exhibits low temporal variations[77]. Two validated quantitative indices of LV dyssynchrony (both expressed in degree) include the phase standard deviation (the standard deviation of the onset of mechanical contraction) and the phase histogram bandwidth (the width of the histogram band including 95% of the myocardium initiates contraction)[78]. Entropy is a further variability measure derived from information theory specifically addressed by Cedars-Sinai’s QGS software[68]. For a normal subject, the entire LV starts contraction almost at the same time so that the phase polar map is nearly uniform and the phase histogram is narrow and highly peaked. Normal limits of phase standard deviation and phase histogram bandwidth have been reported[79]. Advantages are that the number of frames (usually 8 or 16) have no major effect on the calculations[76] and that reproducibility and repeatability are high due to the automated nature of the processing[80].
Scar defined by MPS, in terms of both overall scar burden and scar localized near the LV lead, has been shown to predict the lack of clinical response and failure to improve ventricular function after CRT in small studies with follow-up limited to 6 mo[81,82]. A subsequent study evaluated 620 NYHA classes III-IV HF patients with LVEF ≤ 35% and QRS duration ≥ 120 ms referred for CRT[83]. During a mean follow-up of 2.1 years, ischemic cardiomyopathy patients had worse survival and less LVEF improvement than nonischemic patients. Ischemic patients with low scar burden had favorable survival and LVEF improvement, similar to nonischemic patients. Baseline echocardiographic dyssynchrony analysis, performed in a subgroup of patients, was not predictive of outcome. Thus, extensive scar burden unfavorably affects clinical and LV functional outcomes after CRT, regardless of baseline dyssynchrony measures. Ischemic patients and lower scar burden had significantly better outcomes, similar to nonischemic patients.
Although gated MPS may be used to quantify the scar burden, there are some limitations because it might overestimate the extent of necrosis due to low spatial resolution of the images, particularly when assessing viability in dilated ventricles with thin walls[72]. There is evidence that dyssynchrony during the relaxation phase of the LV, which causes LV filling abnormalities, may adversely affect CRT response and long-term clinical outcomes in HF patients[84,85]. Conversely, in patients with impaired relaxation, the improvement in echocardiographic assessed diastolic filling time after CRT implantation was associated with a significant reduction in all-cause mortality and HF admissions[86]. Diastolic dyssynchrony can also be assessed by nuclear cardiology techniques using a multi-harmonic fit of the thickening curve[87]. In patients with end-stage HF with LVEF of ≤ 35%, phase analysis on gated MPS showed a good intra- and interobserver reproducibility for the determination of diastolic phase standard deviation and diastolic histogram bandwidth. Moreover, a good agreement was found between tissue Doppler imaging dyssynchrony and gated MPS diastolic phase histograms[88]. Diastolic dyssynchrony was also found to be more prevalent (65%) than systolic dyssynchrony (47%) in patients with end-stage renal disease[89].
Positron emission tomography (PET) imaging is performed with higher tracer counts and better spatial resolution[90,91]. However, there are limited data on using PET to quantify dyssynchrony and predict CRT response. A mismatch pattern (preserved glucose metabolism with reduced perfusion) is observed in ischemic, hibernating but viable myocardium[92], whereas a reverse mismatch pattern (reduced glucose metabolism compared to perfusion) is often observed in patients with recent myocardial infarction, chronic stable CAD or LBBB[93,94]. Also, if many patients with LBBB and LV dysfunction have a reverse mismatch pattern, the precise mechanism remains unclear and altered transmembrane glucose transport or phosphorylation kinetics has been proposed[95]. A reverse mismatch pattern is a sign of reversible myocardial dysfunction. In a pilot study, Inoue et al[96] found that a reverse mismatch pattern in the septum can predict a good prognosis. Birnie et al[97] evaluated the relationship between septal reverse mismatch and response to CRT in patients with pre-implant PET scanning and found that reduced septal glucose metabolism predicts the 3 mo response to CRT in the nonischemic cardiomyopathy subset. To evaluate if the probability of a CRT response increases with the presence of high amounts of viable and dyssynchronous myocardium, Lehner et al[98] studied patients who underwent gated PET before CRT device implantation, followed for 6 mo. A significantly higher amount of viable and dyssynchronous myocardium was found before CRT in responders than in nonresponders. These preliminary studies indicate that combined assessment of myocardial viability and LV dyssynchrony is feasible using PET and could improve conventional response prediction criteria for CRT.
Preliminary data also exists on integrated PET/CT approach for assessing LV viability, identifying the location of biventricular pacemaker leads, and obtaining LV functional data, including contractile phase analysis. Uebleis et al[99] compared seven consecutive CRT nonresponders with 7 age- and sex-matched CRT responders. Besides PET/CT, the authors measured brain natriuretic peptide levels and assessed dyssynchrony using transthoracic echocardiography. Compared with nonresponders, CRT responders showed significant differences in the declines of LV end-systolic volume and brain natriuretic peptide and in LV dyssynchrony, extent of the myocardial scar burden, and biventricular pacemaker leads positioned within viable myocardial regions. Among the nonresponders, further therapy management was guided by the PET/CT results in 4 of 7 patients. From this pilot study, it appears that cardiac hybrid imaging using gated PET/CT might identify potential reasons for non-response to CRT, which can guide subsequent therapy.
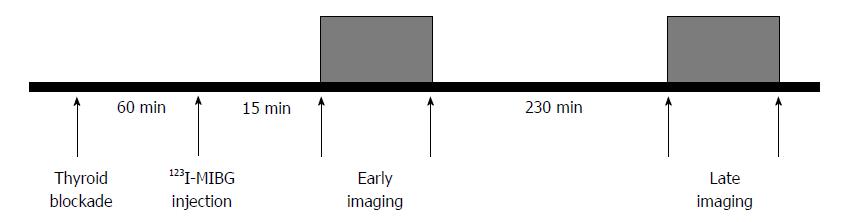
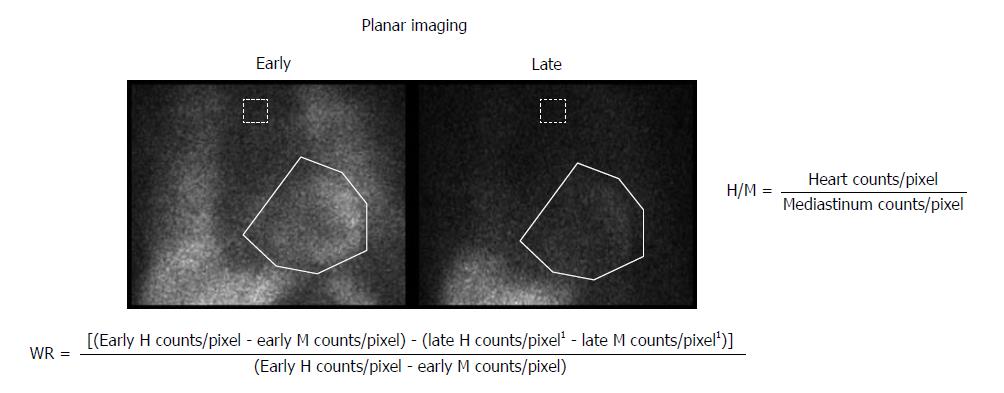
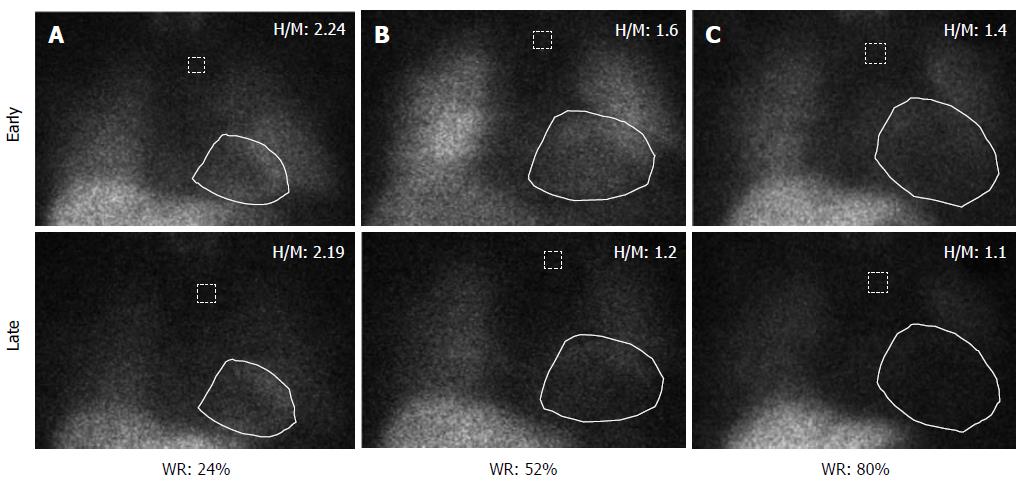
Patients with HF show increased activation of the sympathetic nervous system, as reflected by an increase in plasma norepinephrine levels. In addition, neuronal uptake of norepinephrine is impaired in the failing myocardium. Both the enhanced release of norepinephrine and changes in its cardiac neuronal uptake may be responsible for the observed downregulation of adrenoreceptors in patients with HF[100]. Myocardial innervation imaging with 123I-metaiodobenzylguanidine (MIBG) scintigraphy provides a noninvasive tool for the investigation of cardiac sympathetic innervation. This technique can also demonstrate drug-induced changes in cardiac adrenergic activity[101]. MIBG is an analog of guanethidine and is taken up by the postganglionic pre-synaptic nerve endings of the adrenergic nervous system[102]. After depolarization, MIBG is released into the synaptic cleft like norepinephrine but is not metabolized. Labeling MIBG with 123I allows the visualization of adrenergic innervation in vivo; MIBG scintigraphy not only displays the presence of noradrenergic innervation but also its functional capability[103,104]. Thus, radiolabeled MIBG is a sympathetic neuron-imaging agent useful to study the organs richly innervated by the sympathetic nervous system, demonstrating storage, transport and reuptake characteristics similar to norepinephrine in sympathetic neurons. Uptake of MIBG into neurons from the synaptic cleft is achieved mainly through the human norepinephrine transporter 1 (hNET1). This homeostatic energy-dependent system responsible for the reuptake of norepinephrine is known as uptake-1[105]. Unlike norepinephrine, MIBG is not metabolized, allowing it to be imaged. The uptake-1 mechanism is one of the main norepinephrine disposal systems and its malfunction may lead to abnormal catecholamine concentration in the synaptic cleft. Cardiac hNET1 function appears to be reduced in common heart diseases, such as congestive HF, ischemic heart disease and stress-induced cardiomyopathy[106]. Increased norepinephrine turnover and pre-synaptic norepinephrine deficits can be identified as an increased MIBG washout rate from the heart and decreased MIBG activity quantified as the heart-to-mediastinum (H/M) ratio[107]. Whether in HF hNET1 dysfunction is only a consequence of progressive disease or if it may contribute to worsening of the disease is still unclear[102]. A standard MIBG imaging protocol is illustrated in Figure 1. Myocardial uptake and distribution of the tracer can be visually assessed and also semiquantified by calculating H/M ratio after drawing regions of interest over the heart and mediastinum (Figure 2). By comparing early and late activities, the washout rate from the myocardium can be derived, providing an index of retention of norepinephrine by sympathetic neurons[108]. In addition, polar maps of the myocardium can be constructed from the SPECT images, allowing assessment of the defect extent and severity (Figure 3). This approach provides highly reproducible indices of cardiac sympathetic activity[109,110]. Figure 4 illustrates early and late MIBG imaging in a control subject and in patients with moderate or severe HF. As shown, H/M ratio progressively decreased and WR progressively accelerated from control subject to severe heart failure patient. MIBG SPECT image can also be compared with MPS to examine differences between regional innervation and perfusion, taking into account the differences between normal innervation and perfusion patterns, such as lower uptake of MIBG in the inferior wall, especially in elderly subjects[111,112].
Many studies have been designed to evaluate the role of MIBG cardiac scintigraphy in evaluating the effectiveness of pharmacological treatments in HF patients[113]. Moreover, in patients with severe cardiomyopathy, worsening of MIBG images 6 mo after optimal medical therapy was an independent predictor of cardiac death with brain natriuretic peptide [114]. Other studies also investigated the effects of CRT on myocardial innervation[103,115]. In patients with permanent dual-chamber pacemakers for complete heart block, the asynchronous ventricular activation from the apex of the right ventricle leads to regional disturbances of the adrenergic innervation of the LV myocardium, as assessed by MIBG activity[116]. Differently, Erol-Yilmaz et al[117] found in a very small number of HF patients that the significant improvement of NYHA class, QRS width and echocardiographic parameters after 6 mo of CRT was associated with an increase in late H/M ratio, as well as with a reduction in washout rate. Similarly, Gould et al[118] found that the activation of biventricular pacing was associated with an increased early and late H/M ratio in 10 patients with HF undergoing CRT. However, whether MIBG cardiac imaging has the potential to specifically direct CRT therapy in patients with HF remains uncertain. Tanaka et al[119] assessed H/M ratio in 50 HF patients who underwent CRT. Patients with dyssynchrony and high H/M ratio (i.e., H/M ratio ≥ 1.6) had the highest frequency of response to CRT and a favorable outcome over 3 years. Based on the results of this study, it appears that dyssynchrony is associated with lower H/M ratio and higher washout rate and that MIBG scintigraphy may be valuable for predicting the response to CRT. However, this study covered a small number of patients in a single center. Thus, larger prospective studies are needed to address the role of MIBG in predicting CRT response and in appropriate selection of HF patients.
Approximately 90% of HF patients die from cardiovascular causes. In particular, 50% die from progressive HF and the remainder die suddenly from ischemic events and/or arrhythmias, mainly due to ventricular tachycardia, ventricular fibrillation and bradycardia[120,121]. The decision to use an ICD is largely based on the patient’s LVEF and NYHA functional class, the parameters most commonly used to determine the risk for sudden cardiac death[42,122,123].
According to the European Society of Cardiology guidelines for the diagnosis and treatment of acute and chronic HF[9], to reduce the risk of sudden death, an ICD is recommended in secondary prevention in patients with ventricular arrhythmias causing hemodynamic instability who are expected to survive for at least 1 year with good functional status (class of recommendation I; level of evidence A) and in primary prevention in patients with symptomatic HF (NYHA class II-III) and a LVEF ≤ 35% despite ≥ 3 mo of treatment with optimal pharmacological therapy who are expected to survive for at least 1 year with good functional status (class of recommendation I and level of evidence A for patients with ischemic etiology and > 40 d after acute myocardial infarction and B for patients with nonischemic etiology).
Widespread use of ICD therapy has increased the number of patients, medical costs and also unfavorable effects related to this device, especially in patients at a lower risk for sudden cardiac death[124-127] and in older patients, in whom underlying mortality may be increased because of comorbid conditions and competing mortality risks[128]. In many patients who receive an ICD on the basis of a reduced LVEF (i.e., < 35%), the device never has to deliver therapy. An analysis from the MADIT-II (Multicenter Automatic Defibrillator Implantation Trial) database[129] reported that of 720 patients with an ICD during an average follow-up of 21 mo, only 169 received 1 or more successful device therapies and among baseline characteristics, only NYHA class ≥ II and electrophysiological inducibility distinguished patients who received or not appropriate ICD therapy for their first ventricular tachyarrhythmic episode. Conversely, most patients who die suddenly have a higher LVEF and thus by current guidelines do not qualify for ICD placement. Therefore, a parameter other than LVEF is needed to better select patients at risk for lethal ventricular arrhythmias who need an ICD[130].
A retrospective study investigated the relationship between LV dyssynchrony and cardiovascular events in 70 patients with ICD and LVEF < 40%, evaluated by gated MPS within 6 wk of the device implantation[131]. At 1 year, 8 patients died or had ICD shocks. The patients with events had significantly higher dyssynchrony than those without events. All patients with events had a phase standard deviation ≥ 50°, while none of the patients with a phase standard deviation < 50° had an event. This study indicated that the severity of LV mechanical dyssynchrony by phase analysis in patients with LV dysfunction and ICD is associated with increased risk of death and appropriate ICD shock[78]. However, these preliminary findings need to be validated prospectively using a large patient population.
The autonomic nervous system plays a central role in the pathophysiology of HF and in the pathogenesis of potentially lethal ventricular arrhythmias[132] and the electrophysiological and potentially arrhythmogenic effects of catecholamines have been shown to be one of the main causes of ventricular tachycardia and sudden cardiac death in patients with autonomic dysfunction and sympathetic hyperactivity[133].
The importance of non-sudden cardiac death risk in predicting benefit from ICD therapy must be considered[134]. Considering the high economic cost of widespread ICD use, it is mandatory to identify a high-risk population who will benefit most from these devices. A number of non-LVEF risk stratification tests for predicting a variety of outcomes (overall mortality, arrhythmic events/mortality, ICD shocks and mortality benefit from ICD) have been evaluated[135,136]. These tests include signal-averaged electrocardiogram, microvolt T wave alternans, electrophysiological testing, serum markers (including brain natriuretic peptide), and autonomic function evaluation (including heart rate variability, baroreflex sensitivity, heart rate turbulence and deceleration capacity of heart rate). However, these tests have not demonstrated sufficiently high predictive value for arrhythmic death or arrhythmic events[137]. Thus, it has been hypothesized that cardiac radionuclide imaging may be useful for identifying patients at risk for sudden cardiac death from ventricular arrhythmias, therefore potentially offering a way to better select patients for ICD therapy.
The extent of myocardial scar and stress-induced ischemia, alone or in combination, are both predictive of sudden cardiac death[138,139], providing incremental prognostic information beyond the LVEF[140]. The extent of stress perfusion defects is also associated with an increased risk of sudden cardiac death in patients with CAD and LVEF > 35%[141]. Moreover, myocardial scar seems to provide a substrate for reentrant ventricular arrhythmias and sudden cardiac death in patients with nonischemic cardiomyopathy[142,143].
As autonomic nervous system activation is often the trigger for life-threatening ventricular arrhythmias[144,145], an appealing hypothesis is that cardiac imaging with autonomic radiotracers may be useful for identifying patients at risk for sudden cardiac death, therefore potentially offering a way to better select patients for ICD therapy[146]. Several studies[147-150], a meta-analysis[151] and a pooled-data analysis of 1328 patients[152] have shown that the assessment of the cardiac autonomic state with MIBG scintigraphy can help to estimate the prognosis and to monitor the effects of therapeutic interventions in HF.
The ADMIRE-HF (AdreView Myocardial Imaging for Risk Evaluation in Heart Failure) study consisted of 2 identical open-label phase III studies to provide prospective validation of the prognostic role of quantitation of sympathetic innervation of the myocardium using MIBG scintigraphy[150,153]. The combined primary efficacy findings from the two ADMIRE-HF studies provide the first large, prospective confirmation of the strong prognostic value of quantitation of cardiac adrenergic neuronal activity in HF patients. The results also suggest a potential application for the MIBG imaging for identifying HF patients at very low and very high risks for near-term morbidity and mortality. MIBG imaging also provided additional discrimination in analyses of interactions among β-type natriuretic peptide, LVEF and H/M ratio. Combining the values of these parameters, the highest rate events were among the subjects with all three abnormal parameters (i.e., H/M ratio ≤ 1.30, LVEF ≤ 23%, BNP > 311 ng/L) and the lowest in the subjects with normal parameters (Table 2). During follow-up (median 17 mo), 9% of the subjects experienced nonfatal arrhythmic events or sudden cardiac death. These combined “arrhythmic” events were significantly more common in subjects with a late H/M ratio < 1.60 (reflecting both denervated myocardium and accelerated washout rate from increased sympathetic nerve activity). The highest prevalence of arrhythmic events was in the H/M range 1.30 to 1.39 and the highest H/M in a subject who experienced a fatal arrhythmic event was 1.60. Noteworthy, only 5 arrhythmic events occurred in the 191 subjects with H/M ratio ≥ 1.6; of these, 2 of 137 subjects with no ICD experienced self-limited episodes of ventricular tachycardia, whereas 3 of 54 subjects with ICD had device activations (2 antitachycardia pacing, 1 direct current shock). These findings confirm a previous observation that abnormal MIBG uptake and washout is associated with increased incidence of sudden cardiac death and appropriate ICD discharges[154,155]. Post-hoc analyses of ADMIRE-HF study demonstrated that MIBG imaging has prognostic value across a spectrum of LVEF[156] and that the combination of myocardial sympathetic innervation status by MIBG imaging and scar quantification by resting MPS provides risk stratification in patients with HF for the incidence of ventricular arrhythmia, in particular in patients with nonischemic cardiomyopathy[157].
| Risk level | Predictors combination |
| Very high | H/M ratio < 1.6 |
| LVEF < 30% | |
| BNP > 140 ng/L | |
| High | H/M ratio < 1.6 |
| LVEF ≥ 30% | |
| BNP ≤ 140 ng/L | |
| Low | H/M ratio ≥ 1.6 |
| LVEF < 30% | |
| BNP > 140 ng/L | |
| Very low | H/M ratio ≥ 1.6 |
| LVEF ≥ 30% | |
| BNP ≤ 140 ng/L |
It must be outlined that a cut-off point of 1.60 for H/M ratio, as found in the ADMIRE study for poor prognosis[150], may be too high for selecting patients with severe LV dysfunction who would benefit from ICD therapy. Preliminary data obtained in 47 consecutive patients with HF in NYHA functional class II or III, LVEF ≤ 35%, optimum pharmacological treatment, and class I indication for ICD who had undergone prior MIBG scintigraphy showed that only 5 patients had late H/M ratio > 1.6[158]. Nevertheless, the multivariable analysis, including QRS duration, creatinine levels, treatment with angiotensin converting enzyme inhibitors/angiotensin II receptor antagonists, and early and late H/M ratios < 1.38 (median value), demonstrated an association between late H/M ratio ≤ 1.38 and creatinine levels and an increased risk of experiencing cardiac events at follow-up.
The presence of an innervation/perfusion mismatch, in which a peri-infarction zone of sympathetic denervation extends beyond the area of myocardial scar, as well as the presence of regional inhomogeneity of adrenergic innervation, also seems promising for identifying patients with HF at risk for sudden cardiac death. Hayashi et al[159] followed 40 patients with prior myocardial infarction, sustained ventricular arrhythmia and ICD placement for 2 years with serial noninvasive electrophysiological studies. In their study, the extent of innervation/perfusion mismatch was related to long-term variability in induced arrhythmias and was predictive of the emergence of spontaneous ventricular tachycardia or fibrillation. In patients with ventricular tachycardia and without underlying CAD, regional sympathetic denervation has been detected by MIBG imaging, whereas it was absent in patients without ventricular tachycardia[160].
The potential role of PET imaging has been evaluated in a small study[161] performed at the time of defibrillator implantation with an innervation tracer (11C-hydroxyephedrine) imaging followed by electrophysiological mapping in patients with a history of sustained ventricular tachycardia or sudden cardiac death referred for placement of ICD. Regions of reduced hydroxyephedrine retention were detectable in each patient. The effective refractory period in areas of myocardium that demonstrated reduced hydroxyephedrine retention was longer than in areas of myocardium demonstrating normal retention. The PAREPET (Prediction of ARrhythmic Events with Positron Emission Tomography) study was designed to test the hypothesis that quantifying inhomogeneity in myocardial sympathetic innervation could identify patients at highest risk for sudden cardiac death[162]. This study prospectively enrolled 204 subjects with ischemic cardiomyopathy (LVEF ≤ 35%) eligible for primary prevention with ICD. PET was used to quantify myocardial sympathetic denervation, perfusion and viability. The primary end-point was sudden cardiac death defined as arrhythmic death or ICD discharge for ventricular fibrillation or ventricular tachycardia > 240 bpm. The results have been recently published[163]. After 4.1 years follow-up, cause-specific sudden cardiac death was 16.2%. Multivariate predictors of sudden cardiac death were PET sympathetic denervation, LV end-diastolic volume index, creatinine and no angiotensin inhibition. The study indicates that, in ischemic cardiomyopathy, sympathetic denervation assessed by PET predicts sudden cardiac death independently of LVEF and infarct volume. This may provide an improved approach for the identification of patients most likely to benefit from an ICD. Despite, some limitations[164], the PAREPET results are important because they show that cardiac PET innervation imaging is feasible in large clinical projects and that the presence of viable but denervated myocardium is harmful for the heart rhythm. Prospective trials where imaging is combined with image-test driven therapy are now the next steps.
In patients with HF, nuclear cardiology techniques are valuable for establishing the etiology and the severity of the disease and may also be useful to guide CRT and select patients for ICD. Prospective trials where imaging is combined with image-test driven therapy are needed to better define the role of nuclear cardiology for guiding device therapy in patients with HF.
P- Reviewers: Chen SJ, Liu T S- Editor: Ma YJ L- Editor: Roemmele A E- Editor: Wang CH
| 1. | de Jong MC, Genders TS, van Geuns RJ, Moelker A, Hunink MG. Diagnostic performance of stress myocardial perfusion imaging for coronary artery disease: a systematic review and meta-analysis. Eur Radiol. 2012;22:1881-1895. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 2. | Beller GA, Heede RC. SPECT imaging for detecting coronary artery disease and determining prognosis by noninvasive assessment of myocardial perfusion and myocardial viability. J Cardiovasc Transl Res. 2011;4:416-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 3. | Cuocolo A, Petretta M, Acampa W, De Falco T. Gated SPECT myocardial perfusion imaging: the further improvements of an excellent tool. Q J Nucl Med Mol Imaging. 2010;54:129-144. [PubMed] |
| 4. | Acampa W, Petretta MP, Daniele S, Perrone-Filardi P, Petretta M, Cuocolo A. Myocardial perfusion imaging after coronary revascularization: a clinical appraisal. Eur J Nucl Med Mol Imaging. 2013;40:1275-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Petretta M, Cuocolo A. Prediction models for risk classification in cardiovascular disease. Eur J Nucl Med Mol Imaging. 2012;39:1959-1969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127:e6-e245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2893] [Cited by in RCA: 3393] [Article Influence: 282.8] [Reference Citation Analysis (0)] |
| 7. | Curtis LH, Whellan DJ, Hammill BG, Hernandez AF, Anstrom KJ, Shea AM, Schulman KA. Incidence and prevalence of heart failure in elderly persons, 1994-2003. Arch Intern Med. 2008;168:418-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 277] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 8. | Roger VL, Weston SA, Redfield MM, Hellermann-Homan JP, Killian J, Yawn BP, Jacobsen SJ. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292:344-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1172] [Cited by in RCA: 1197] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 9. | McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Køber L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Rønnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A; ESC Committee for Practice Guidelines. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787-1847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3411] [Cited by in RCA: 3554] [Article Influence: 273.4] [Reference Citation Analysis (0)] |
| 10. | Gheorghiade M, Sopko G, De Luca L, Velazquez EJ, Parker JD, Binkley PF, Sadowski Z, Golba KS, Prior DL, Rouleau JL. Navigating the crossroads of coronary artery disease and heart failure. Circulation. 2006;114:1202-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 272] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 11. | Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PW, Woo YJ; American Heart Association Advocacy Coordinating Committee; Stroke Council; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Arteriosclerosis; Thrombosis and Vascular. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123:933-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2162] [Cited by in RCA: 2282] [Article Influence: 163.0] [Reference Citation Analysis (0)] |
| 12. | Levy WC, Mozaffarian D, Linker DT, Sutradhar SC, Anker SD, Cropp AB, Anand I, Maggioni A, Burton P, Sullivan MD. The Seattle Heart Failure Model: prediction of survival in heart failure. Circulation. 2006;113:1424-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1435] [Cited by in RCA: 1572] [Article Influence: 82.7] [Reference Citation Analysis (0)] |
| 13. | Gorodeski EZ, Chu EC, Chow CH, Levy WC, Hsich E, Starling RC. Application of the Seattle Heart Failure Model in ambulatory patients presented to an advanced heart failure therapeutics committee. Circ Heart Fail. 2010;3:706-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 14. | Felker GM, Shaw LK, O’Connor CM. A standardized definition of ischemic cardiomyopathy for use in clinical research. J Am Coll Cardiol. 2002;39:210-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 495] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 15. | O’Connor CM, Whellan DJ, Wojdyla D, Leifer E, Clare RM, Ellis SJ, Fine LJ, Fleg JL, Zannad F, Keteyian SJ. Factors related to morbidity and mortality in patients with chronic heart failure with systolic dysfunction: the HF-ACTION predictive risk score model. Circ Heart Fail. 2012;5:63-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 165] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 16. | Aaronson KD, Cowger J. Heart failure prognostic models: why bother. Circ Heart Fail. 2012;5:6-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Kashani A, Barold SS. Significance of QRS complex duration in patients with heart failure. J Am Coll Cardiol. 2005;46:2183-2192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 257] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 18. | Doval HC, Nul DR, Grancelli HO, Perrone SV, Bortman GR, Curiel R. Randomised trial of low-dose amiodarone in severe congestive heart failure. Grupo de Estudio de la Sobrevida en la Insuficiencia Cardiaca en Argentina (GESICA). Lancet. 1994;344:493-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 606] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 19. | Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240-e327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 795] [Cited by in RCA: 1569] [Article Influence: 130.8] [Reference Citation Analysis (0)] |
| 20. | Bleeker GB, Schalij MJ, Molhoek SG, Verwey HF, Holman ER, Boersma E, Steendijk P, Van Der Wall EE, Bax JJ. Relationship between QRS duration and left ventricular dyssynchrony in patients with end-stage heart failure. J Cardiovasc Electrophysiol. 2004;15:544-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 284] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 21. | Young JB, Abraham WT, Smith AL, Leon AR, Lieberman R, Wilkoff B, Canby RC, Schroeder JS, Liem LB, Hall S, Wheelan K; Multicenter InSync ICD Randomized Clinical Evaluation (MIRACLE ICD) Trial Investigators. Combined cardiac resynchronization and implantable cardioversion defibrillation in advanced chronic heart failure: the MIRACLE ICD Trial. JAMA. 2003;289:2685-2694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1231] [Cited by in RCA: 1214] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 22. | Shamim W, Francis DP, Yousufuddin M, Varney S, Pieopli MF, Anker SD, Coats AJ. Intraventricular conduction delay: a prognostic marker in chronic heart failure. Int J Cardiol. 1999;70:171-178. [PubMed] |
| 23. | Xiao HB, Roy C, Fujimoto S, Gibson DG. Natural history of abnormal conduction and its relation to prognosis in patients with dilated cardiomyopathy. Int J Cardiol. 1996;53:163-170. [PubMed] |
| 24. | Gasparini M, Bocchiardo M, Lunati M, Ravazzi PA, Santini M, Zardini M, Signorelli S, Passardi M, Klersy C; BELIEVE Investigators. Comparison of 1-year effects of left ventricular and biventricular pacing in patients with heart failure who have ventricular arrhythmias and left bundle-branch block: the Bi vs Left Ventricular Pacing: an International Pilot Evaluation on Heart Failure Patients with Ventricular Arrhythmias (BELIEVE) multicenter prospective randomized pilot study. Am Heart J. 2006;152:155.e1-155.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 242] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 25. | Ritter P, Delnoy PP, Padeletti L, Lunati M, Naegele H, Borri-Brunetto A, Silvestre J. A randomized pilot study of optimization of cardiac resynchronization therapy in sinus rhythm patients using a peak endocardial acceleration sensor vs. standard methods. Europace. 2012;14:1324-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 26. | Cazeau S, Leclercq C, Lavergne T, Walker S, Varma C, Linde C, Garrigue S, Kappenberger L, Haywood GA, Santini M, Bailleul C, Daubert JC; Multisite Stimulation in Cardiomyopathies (MUSTIC) Study Investigators. Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N Engl J Med. 2001;344:873-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1988] [Cited by in RCA: 1834] [Article Influence: 76.4] [Reference Citation Analysis (0)] |
| 27. | Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, Kocovic DZ, Packer M, Clavell AL, Hayes DL, Ellestad M, Trupp RJ, Underwood J, Pickering F, Truex C, McAtee P, Messenger J; MIRACLE Study Group. Multicenter InSync Randomized Clinical Evaluation. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346:1845-1853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3658] [Cited by in RCA: 3500] [Article Influence: 152.2] [Reference Citation Analysis (0)] |
| 28. | Auricchio A, Stellbrink C, Sack S, Block M, Vogt J, Bakker P, Huth C, Schöndube F, Wolfhard U, Böcker D, Krahnefeld O, Kirkels H; Pacing Therapies in Congestive Heart Failure (PATH-CHF) Study Group. Long-term clinical effect of hemodynamically optimized cardiac resynchronization therapy in patients with heart failure and ventricular conduction delay. J Am Coll Cardiol. 2002;39:2026-2033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 716] [Cited by in RCA: 676] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 29. | Auricchio A, Stellbrink C, Butter C, Sack S, Vogt J, Misier AR, Böcker D, Block M, Kirkels JH, Kramer A, Huvelle E; Pacing Therapies in Congestive Heart Failure II Study Group; Guidant Heart Failure Research Group. Clinical efficacy of cardiac resynchronization therapy using left ventricular pacing in heart failure patients stratified by severity of ventricular conduction delay. J Am Coll Cardiol. 2003;42:2109-2116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 258] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 30. | Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, Carson P, DiCarlo L, DeMets D, White BG, DeVries DW, Feldman AM; Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) Investigators. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140-2150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4324] [Cited by in RCA: 4151] [Article Influence: 197.7] [Reference Citation Analysis (0)] |
| 31. | Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L; Cardiac Resynchronization-Heart Failure (CARE-HF) Study Investigators. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539-1549. [PubMed] |
| 32. | Linde C, Daubert C, Abraham WT, St John Sutton M, Ghio S, Hassager C, Herre JM, Bergemann TL, Gold MR. Impact of ejection fraction on the clinical response to cardiac resynchronization therapy in mild heart failure. Circ Heart Fail. 2013;6:1180-1189. [PubMed] |
| 33. | Bleeker GB, Schalij MJ, Holman ER, Steendijk P, van der Wall EE, Bax JJ. Cardiac resynchronization therapy in patients with systolic left ventricular dysfunction and symptoms of mild heart failure secondary to ischemic or nonischemic cardiomyopathy. Am J Cardiol. 2006;98:230-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 34. | Leclercq C, Kass DA. Retiming the failing heart: principles and current clinical status of cardiac resynchronization. J Am Coll Cardiol. 2002;39:194-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 322] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 35. | Sutton MS, Keane MG. Reverse remodelling in heart failure with cardiac resynchronisation therapy. Heart. 2007;93:167-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 36. | Bax JJ, Abraham T, Barold SS, Breithardt OA, Fung JW, Garrigue S, Gorcsan J, Hayes DL, Kass DA, Knuuti J. Cardiac resynchronization therapy: Part 1--issues before device implantation. J Am Coll Cardiol. 2005;46:2153-2167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 311] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 37. | Dickstein K, Vardas PE, Auricchio A, Daubert JC, Linde C, McMurray J, Ponikowski P, Priori SG, Sutton R, van Veldhuisen DJ; Committee for Practice Guidelines of the European Society of Cardiology; ESC Committee for Practice Guidelines (CPG). 2010 focused update of ESC Guidelines on device therapy in heart failure: an update of the 2008 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure and the 2007 ESC Guidelines for cardiac and resynchronization therapy. Developed with the special contribution of the Heart Failure Association and the European Heart Rhythm Association. Eur J Heart Fail. 2010;12:1143-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 120] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 38. | Khan NK, Goode KM, Cleland JG, Rigby AS, Freemantle N, Eastaugh J, Clark AL, de Silva R, Calvert MJ, Swedberg K. Prevalence of ECG abnormalities in an international survey of patients with suspected or confirmed heart failure at death or discharge. Eur J Heart Fail. 2007;9:491-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 39. | Baldasseroni S, Opasich C, Gorini M, Lucci D, Marchionni N, Marini M, Campana C, Perini G, Deorsola A, Masotti G, Tavazzi L, Maggioni AP; Italian Network on Congestive Heart Failure Investigators. Left bundle-branch block is associated with increased 1-year sudden and total mortality rate in 5517 outpatients with congestive heart failure: a report from the Italian network on congestive heart failure. Am Heart J. 2002;143:398-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 534] [Cited by in RCA: 508] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 40. | Clark AL, Goode K, Cleland JG. The prevalence and incidence of left bundle branch block in ambulant patients with chronic heart failure. Eur J Heart Fail. 2008;10:696-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 41. | Brignole M, Auricchio A, Baron-Esquivias G, Bordachar P, Boriani G, Breithardt OA, Cleland J, Deharo JC, Delgado V, Elliott PM, Gorenek B, Israel CW, Leclercq C, Linde C, Mont L, Padeletti L, Sutton R, Vardas PE; ESC Committee for Practice Guidelines (CPG), Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Kirchhof P, Blomstrom-Lundqvist C, Badano LP, Aliyev F, Bänsch D, Baumgartner H, Bsata W, Buser P, Charron P, Daubert JC, Dobreanu D, Faerestrand S, Hasdai D, Hoes AW, Le Heuzey JY, Mavrakis H, McDonagh T, Merino JL, Nawar MM, Nielsen JC, Pieske B, Poposka L, Ruschitzka F, Tendera M, Van Gelder IC, Wilson CM. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Eur Heart J. 2013;34:2281-2329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1437] [Cited by in RCA: 1473] [Article Influence: 122.8] [Reference Citation Analysis (0)] |
| 42. | Tracy CM, Epstein AE, Darbar D, Dimarco JP, Dunbar SB, Estes NA, Ferguson TB, Hammill SC, Karasik PE, Link MS. 2012 ACCF/AHA/HRS focused update of the 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2012;60:1297-1313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 257] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 43. | Bax JJ, Van der Wall EE, Schalij MJ. Cardiac resynchronization therapy for heart failure. N Engl J Med. 2002;347:1803-1804; author reply 1803-1804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 44. | Bax JJ, Bleeker GB, Marwick TH, Molhoek SG, Boersma E, Steendijk P, van der Wall EE, Schalij MJ. Left ventricular dyssynchrony predicts response and prognosis after cardiac resynchronization therapy. J Am Coll Cardiol. 2004;44:1834-1840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 823] [Cited by in RCA: 785] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 45. | Yu CM, Fung JW, Zhang Q, Chan CK, Chan YS, Lin H, Kum LC, Kong SL, Zhang Y, Sanderson JE. Tissue Doppler imaging is superior to strain rate imaging and postsystolic shortening on the prediction of reverse remodeling in both ischemic and nonischemic heart failure after cardiac resynchronization therapy. Circulation. 2004;110:66-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 454] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 46. | Yu CM, Zhang Q, Chan YS, Chan CK, Yip GW, Kum LC, Wu EB, Lee PW, Lam YY, Chan S. Tissue Doppler velocity is superior to displacement and strain mapping in predicting left ventricular reverse remodelling response after cardiac resynchronisation therapy. Heart. 2006;92:1452-1456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 73] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 47. | Leclercq C, Faris O, Tunin R, Johnson J, Kato R, Evans F, Spinelli J, Halperin H, McVeigh E, Kass DA. Systolic improvement and mechanical resynchronization does not require electrical synchrony in the dilated failing heart with left bundle-branch block. Circulation. 2002;106:1760-1763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 328] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 48. | Achilli A, Sassara M, Ficili S, Pontillo D, Achilli P, Alessi C, De Spirito S, Guerra R, Patruno N, Serra F. Long-term effectiveness of cardiac resynchronization therapy in patients with refractory heart failure and “narrow” QRS. J Am Coll Cardiol. 2003;42:2117-2124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 241] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 49. | Mollema SA, Bleeker GB, van der Wall EE, Schalij MJ, Bax JJ. Usefulness of QRS duration to predict response to cardiac resynchronization therapy in patients with end-stage heart failure. Am J Cardiol. 2007;100:1665-1670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 79] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 50. | Yancy CW, McMurray JJ. ECG--still the best for selecting patients for CRT. N Engl J Med. 2013;369:1463-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 51. | Ruschitzka F, Abraham WT, Singh JP, Bax JJ, Borer JS, Brugada J, Dickstein K, Ford I, Gorcsan J, Gras D, Krum H, Sogaard P, Holzmeister J; the EchoCRT Study Group. Cardiac-resynchronization therapy in heart failure with a narrow QRS complex. N Engl J Med. 2013;369:1395-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 578] [Cited by in RCA: 620] [Article Influence: 51.7] [Reference Citation Analysis (0)] |
| 52. | Strickberger SA, Conti J, Daoud EG, Havranek E, Mehra MR, Piña IL, Young J; Council on Clinical Cardiology Subcommittee on Electrocardiography and Arrhythmias and the Quality of Care and Outcomes Research Interdisciplinary Working Group; Heart Rhythm Society. Patient selection for cardiac resynchronization therapy: from the Council on Clinical Cardiology Subcommittee on Electrocardiography and Arrhythmias and the Quality of Care and Outcomes Research Interdisciplinary Working Group, in collaboration with the Heart Rhythm Society. Circulation. 2005;111:2146-2150. [PubMed] |
| 53. | Botvinick EH, O’Connell JW, Badhwar N. Imaging synchrony. J Nucl Cardiol. 2009;16:846-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 138] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 54. | Bax JJ, Gorcsan J. Echocardiography and noninvasive imaging in cardiac resynchronization therapy: results of the PROSPECT (Predictors of Response to Cardiac Resynchronization Therapy) study in perspective. J Am Coll Cardiol. 2009;53:1933-1943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 140] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 55. | Delgado V, Bax JJ. Assessment of systolic dyssynchrony for cardiac resynchronization therapy is clinically useful. Circulation. 2011;123:640-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 56. | Richardson M, Freemantle N, Calvert MJ, Cleland JG, Tavazzi L; CARE-HF Study Steering Committee and Investigators. Predictors and treatment response with cardiac resynchronization therapy in patients with heart failure characterized by dyssynchrony: a pre-defined analysis from the CARE-HF trial. Eur Heart J. 2007;28:1827-1834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 111] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 57. | Faletra FF, Conca C, Klersy C, Klimusina J, Regoli F, Mantovani A, Pasotti E, Pedrazzini GB, De Castro S, Moccetti T. Comparison of eight echocardiographic methods for determining the prevalence of mechanical dyssynchrony and site of latest mechanical contraction in patients scheduled for cardiac resynchronization therapy. Am J Cardiol. 2009;103:1746-1752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 58. | Chung ES, Leon AR, Tavazzi L, Sun JP, Nihoyannopoulos P, Merlino J, Abraham WT, Ghio S, Leclercq C, Bax JJ. Results of the Predictors of Response to CRT (PROSPECT) trial. Circulation. 2008;117:2608-2616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1585] [Cited by in RCA: 1522] [Article Influence: 89.5] [Reference Citation Analysis (0)] |
| 59. | van Bommel RJ, Bax JJ, Abraham WT, Chung ES, Pires LA, Tavazzi L, Zimetbaum PJ, Gerritse B, Kristiansen N, Ghio S. Characteristics of heart failure patients associated with good and poor response to cardiac resynchronization therapy: a PROSPECT (Predictors of Response to CRT) sub-analysis. Eur Heart J. 2009;30:2470-2477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 157] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 60. | Park JH, Negishi K, Grimm RA, Popovic Z, Stanton T, Wilkoff BL, Marwick TH. Echocardiographic predictors of reverse remodeling after cardiac resynchronization therapy and subsequent events. Circ Cardiovasc Imaging. 2013;6:864-872. [PubMed] |
| 61. | Bilchick KC, Dimaano V, Wu KC, Helm RH, Weiss RG, Lima JA, Berger RD, Tomaselli GF, Bluemke DA, Halperin HR. Cardiac magnetic resonance assessment of dyssynchrony and myocardial scar predicts function class improvement following cardiac resynchronization therapy. JACC Cardiovasc Imaging. 2008;1:561-568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 164] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 62. | Boogers MM, Van Kriekinge SD, Henneman MM, Ypenburg C, Van Bommel RJ, Boersma E, Dibbets-Schneider P, Stokkel MP, Schalij MJ, Berman DS. Quantitative gated SPECT-derived phase analysis on gated myocardial perfusion SPECT detects left ventricular dyssynchrony and predicts response to cardiac resynchronization therapy. J Nucl Med. 2009;50:718-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 110] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 63. | Gorcsan J, Oyenuga O, Habib PJ, Tanaka H, Adelstein EC, Hara H, McNamara DM, Saba S. Relationship of echocardiographic dyssynchrony to long-term survival after cardiac resynchronization therapy. Circulation. 2010;122:1910-1918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 139] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 64. | Hara H, Oyenuga OA, Tanaka H, Adelstein EC, Onishi T, McNamara DM, Schwartzman D, Saba S, Gorcsan J. The relationship of QRS morphology and mechanical dyssynchrony to long-term outcome following cardiac resynchronization therapy. Eur Heart J. 2012;33:2680-2691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 65. | Lardo AC, Abraham TP, Kass DA. Magnetic resonance imaging assessment of ventricular dyssynchrony: current and emerging concepts. J Am Coll Cardiol. 2005;46:2223-2228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 92] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 66. | Truong QA, Singh JP, Cannon CP, Sarwar A, Nasir K, Auricchio A, Faletra FF, Sorgente A, Conca C, Moccetti T. Quantitative analysis of intraventricular dyssynchrony using wall thickness by multidetector computed tomography. JACC Cardiovasc Imaging. 2008;1:772-781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 67. | Henneman MM, Chen J, Dibbets-Schneider P, Stokkel MP, Bleeker GB, Ypenburg C, van der Wall EE, Schalij MJ, Garcia EV, Bax JJ. Can LV dyssynchrony as assessed with phase analysis on gated myocardial perfusion SPECT predict response to CRT. J Nucl Med. 2007;48:1104-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 229] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 68. | Van Kriekinge SD, Nishina H, Ohba M, Berman DS, Germano G. Automatic global and regional phase analysis from gated myocardial perfusion SPECT imaging: application to the characterization of ventricular contraction in patients with left bundle branch block. J Nucl Med. 2008;49:1790-1797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 69. | Kerwin WF, Botvinick EH, O’Connell JW, Merrick SH, DeMarco T, Chatterjee K, Scheibly K, Saxon LA. Ventricular contraction abnormalities in dilated cardiomyopathy: effect of biventricular pacing to correct interventricular dyssynchrony. J Am Coll Cardiol. 2000;35:1221-1227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 216] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 70. | Toussaint JF, Lavergne T, Kerrou K, Froissart M, Ollitrault J, Darondel JM, Alonso C, Diebold B, Le Heuzey JY, Guize L. Basal asynchrony and resynchronization with biventricular pacing predict long-term improvement of LV function in heart failure patients. Pacing Clin Electrophysiol. 2003;26:1815-1823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 71. | Riedlbauchová L, Brunken R, Jaber WA, Popová L, Patel D, Lánská V, Civello K, Cummings J, Burkhardt JD, Saliba W. The impact of myocardial viability on the clinical outcome of cardiac resynchronization therapy. J Cardiovasc Electrophysiol. 2009;20:50-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 72. | Friehling M, Soman P. Newer applications of nuclear cardiology in systolic heart failure: detecting coronary artery disease and guiding device therapy. Curr Heart Fail Rep. 2011;8:106-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 73. | Cooke CD, Garcia EV, Cullom SJ, Faber TL, Pettigrew RI. Determining the accuracy of calculating systolic wall thickening using a fast Fourier transform approximation: a simulation study based on canine and patient data. J Nucl Med. 1994;35:1185-1192. [PubMed] |
| 74. | Ritt P, Vija H, Hornegger J, Kuwert T. Absolute quantification in SPECT. Eur J Nucl Med Mol Imaging. 2011;38 Suppl 1:S69-S77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 135] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 75. | van der Veen BJ, Al Younis I, Ajmone-Marsan N, Westenberg JJ, Bax JJ, Stokkel MP, de Roos A. Ventricular dyssynchrony assessed by gated myocardial perfusion SPECT using a geometrical approach: a feasibility study. Eur J Nucl Med Mol Imaging. 2012;39:421-429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 76. | Chen J, Henneman MM, Trimble MA, Bax JJ, Borges-Neto S, Iskandrian AE, Nichols KJ, Garcia EV. Assessment of left ventricular mechanical dyssynchrony by phase analysis of ECG-gated SPECT myocardial perfusion imaging. J Nucl Cardiol. 2008;15:127-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 77. | O’Connell JW, Schreck C, Moles M, Badwar N, DeMarco T, Olgin J, Lee B, Tseng Z, Kumar U, Botvinick EH. A unique method by which to quantitate synchrony with equilibrium radionuclide angiography. J Nucl Cardiol. 2005;12:441-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 67] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 78. | Chen J, Garcia EV, Bax JJ, Iskandrian AE, Borges-Neto S, Soman P. SPECT myocardial perfusion imaging for the assessment of left ventricular mechanical dyssynchrony. J Nucl Cardiol. 2011;18:685-694. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 79. | Chen J, Garcia EV, Folks RD, Cooke CD, Faber TL, Tauxe EL, Iskandrian AE. Onset of left ventricular mechanical contraction as determined by phase analysis of ECG-gated myocardial perfusion SPECT imaging: development of a diagnostic tool for assessment of cardiac mechanical dyssynchrony. J Nucl Cardiol. 2005;12:687-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 308] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 80. | Trimble MA, Borges-Neto S, Honeycutt EF, Shaw LK, Pagnanelli R, Chen J, Iskandrian AE, Garcia EV, Velazquez EJ. Evaluation of mechanical dyssynchrony and myocardial perfusion using phase analysis of gated SPECT imaging in patients with left ventricular dysfunction. J Nucl Cardiol. 2008;15:663-670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 81. | Adelstein EC, Saba S. Scar burden by myocardial perfusion imaging predicts echocardiographic response to cardiac resynchronization therapy in ischemic cardiomyopathy. Am Heart J. 2007;153:105-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 191] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 82. | Ypenburg C, Schalij MJ, Bleeker GB, Steendijk P, Boersma E, Dibbets-Schneider P, Stokkel MP, van der Wall EE, Bax JJ. Impact of viability and scar tissue on response to cardiac resynchronization therapy in ischaemic heart failure patients. Eur Heart J. 2007;28:33-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 305] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 83. | Adelstein EC, Tanaka H, Soman P, Miske G, Haberman SC, Saba SF, Gorcsan J. Impact of scar burden by single-photon emission computed tomography myocardial perfusion imaging on patient outcomes following cardiac resynchronization therapy. Eur Heart J. 2011;32:93-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 141] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 84. | Cho GY, Song JK, Park WJ, Han SW, Choi SH, Doo YC, Oh DJ, Lee Y. Mechanical dyssynchrony assessed by tissue Doppler imaging is a powerful predictor of mortality in congestive heart failure with normal QRS duration. J Am Coll Cardiol. 2005;46:2237-2243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 104] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 85. | Schuster I, Habib G, Jego C, Thuny F, Avierinos JF, Derumeaux G, Beck L, Medail C, Franceschi F, Renard S. Diastolic asynchrony is more frequent than systolic asynchrony in dilated cardiomyopathy and is less improved by cardiac resynchronization therapy. J Am Coll Cardiol. 2005;46:2250-2257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 86. | Verbrugge FH, Verhaert D, Grieten L, Dupont M, Rivero-Ayerza M, De Vusser P, Van Herendael H, Reyskens R, Vandervoort P, Tang WH. Revisiting diastolic filling time as mechanistic insight for response to cardiac resynchronization therapy. Europace. 2013;15:1747-1756. [PubMed] |
| 87. | Shanks M, Bertini M, Delgado V, Ng AC, Nucifora G, van Bommel RJ, Borleffs CJ, Holman ER, van de Veire NR, Schalij MJ. Effect of biventricular pacing on diastolic dyssynchrony. J Am Coll Cardiol. 2010;56:1567-1575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 88. | Boogers MJ, Chen J, Veltman CE, van Bommel RJ, Mooyaart EA, Al Younis I, van der Hiel B, Dibbets-Schneider P, van der Wall EE, Schalij MJ. Left ventricular diastolic dyssynchrony assessed with phase analysis of gated myocardial perfusion SPECT: a comparison with tissue Doppler imaging. Eur J Nucl Med Mol Imaging. 2011;38:2031-2039. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 89. | Chen J, Kalogeropoulos AP, Verdes L, Butler J, Garcia EV. Left-ventricular systolic and diastolic dyssynchrony as assessed by multi-harmonic phase analysis of gated SPECT myocardial perfusion imaging in patients with end-stage renal disease and normal LVEF. J Nucl Cardiol. 2011;18:299-308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 90. | Schindler TH, Schelbert HR, Quercioli A, Dilsizian V. Cardiac PET imaging for the detection and monitoring of coronary artery disease and microvascular health. JACC Cardiovasc Imaging. 2010;3:623-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 315] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 91. | Parker MW, Iskandar A, Limone B, Perugini A, Kim H, Jones C, Calamari B, Coleman CI, Heller GV. Diagnostic accuracy of cardiac positron emission tomography versus single photon emission computed tomography for coronary artery disease: a bivariate meta-analysis. Circ Cardiovasc Imaging. 2012;5:700-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 184] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 92. | Tillisch J, Brunken R, Marshall R, Schwaiger M, Mandelkern M, Phelps M, Schelbert H. Reversibility of cardiac wall-motion abnormalities predicted by positron tomography. N Engl J Med. 1986;314:884-888. [PubMed] |
| 93. | Yamagishi H, Akioka K, Hirata K, Sakanoue Y, Takeuchi K, Yoshikawa J, Ochi H. A reverse flow-metabolism mismatch pattern on PET is related to multivessel disease in patients with acute myocardial infarction. J Nucl Med. 1999;40:1492-1498. [PubMed] |
| 94. | Zanco P, Desideri A, Mobilia G, Cargnel S, Milan E, Celegon L, Buchberger R, Ferlin G. Effects of left bundle branch block on myocardial FDG PET in patients without significant coronary artery stenoses. J Nucl Med. 2000;41:973-977. [PubMed] |
| 95. | Nowak B, Sinha AM, Schaefer WM, Koch KC, Kaiser HJ, Hanrath P, Buell U, Stellbrink C. Cardiac resynchronization therapy homogenizes myocardial glucose metabolism and perfusion in dilated cardiomyopathy and left bundle branch block. J Am Coll Cardiol. 2003;41:1523-1528. [PubMed] |
| 96. | Inoue N, Takahashi N, Ishikawa T, Sumita S, Kobayashi T, Matsushita K, Matsumoto K, Taima M, Shimura M, Uchino K. Reverse perfusion-metabolism mismatch predicts good prognosis in patients undergoing cardiac resynchronization therapy: a pilot study. Circ J. 2007;71:126-131. [PubMed] |
| 97. | Birnie D, de Kemp RA, Tang AS, Ruddy TD, Gollob MH, Guo A, Williams K, Thomson K, DaSilva JN, Beanlands RS. Reduced septal glucose metabolism predicts response to cardiac resynchronization therapy. J Nucl Cardiol. 2012;19:73-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 98. | Lehner S, Uebleis C, Schüßler F, Haug A, Kääb S, Bartenstein P, Van Kriekinge SD, Germano G, Estner H, Hacker M. The amount of viable and dyssynchronous myocardium is associated with response to cardiac resynchronization therapy: initial clinical results using multiparametric ECG-gated [18F]FDG PET. Eur J Nucl Med Mol Imaging. 2013;40:1876-1883. [PubMed] |
| 99. | Uebleis C, Ulbrich M, Tegtmeyer R, Schuessler F, Haserueck N, Siebermair J, Becker C, Nekolla S, Cumming P, Bartenstein P. Electrocardiogram-gated 18F-FDG PET/CT hybrid imaging in patients with unsatisfactory response to cardiac resynchronization therapy: initial clinical results. J Nucl Med. 2011;52:67-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 100. | Böhm M, La Rosée K, Schwinger RH, Erdmann E. Evidence for reduction of norepinephrine uptake sites in the failing human heart. J Am Coll Cardiol. 1995;25:146-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 159] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 101. | Flotats A, Carrió I, Agostini D, Le Guludec D, Marcassa C, Schäfers M, Somsen GA, Unlu M, Verberne HJ; EANM Cardiovascular Committee; European Council of Nuclear Cardiology. Proposal for standardization of 123I-metaiodobenzylguanidine (MIBG) cardiac sympathetic imaging by the EANM Cardiovascular Committee and the European Council of Nuclear Cardiology. Eur J Nucl Med Mol Imaging. 2010;37:1802-1812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 243] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 102. | Haider N, Baliga RR, Chandrashekhar Y, Narula J. Adrenergic excess, hNET1 down-regulation, and compromised mIBG uptake in heart failure poverty in the presence of plenty. JACC Cardiovasc Imaging. 2010;3:71-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 103. | Chirumamilla A, Travin MI. Cardiac applications of 123I-mIBG imaging. Semin Nucl Med. 2011;41:374-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 104. | Perrone-Filardi P, Paolillo S, Dellegrottaglie S, Gargiulo P, Savarese G, Marciano C, Casaretti L, Cecere M, Musella F, Pirozzi E. Assessment of cardiac sympathetic activity by MIBG imaging in patients with heart failure: a clinical appraisal. Heart. 2011;97:1828-1833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 105. | Schroeder C, Jordan J. Norepinephrine transporter function and human cardiovascular disease. Am J Physiol Heart Circ Physiol. 2012;303:H1273-H1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 106. | Liang CS. Cardiac sympathetic nerve terminal function in congestive heart failure. Acta Pharmacol Sin. 2007;28:921-927. [PubMed] |
| 107. | Carrió I. Cardiac neurotransmission imaging. J Nucl Med. 2001;42:1062-1076. [PubMed] |
| 108. | Agostini D, Carrio I, Verberne HJ. How to use myocardial 123I-MIBG scintigraphy in chronic heart failure. Eur J Nucl Med Mol Imaging. 2009;36:555-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 109. | Veltman CE, Boogers MJ, Meinardi JE, Al Younis I, Dibbets-Schneider P, Van der Wall EE, Bax JJ, Scholte AJ. Reproducibility of planar (123)I-meta-iodobenzylguanidine (MIBG) myocardial scintigraphy in patients with heart failure. Eur J Nucl Med Mol Imaging. 2012;39:1599-1608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 110. | Pellegrino T, Petretta M, De Luca S, Paolillo S, Boemio A, Carotenuto R, Petretta MP, di Nuzzo C, Perrone-Filardi P, Cuocolo A. Observer reproducibility of results from a low-dose 123I-metaiodobenzylguanidine cardiac imaging protocol in patients with heart failure. Eur J Nucl Med Mol Imaging. 2013;40:1549-1557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 111. | Estorch M, Carrió I, Berná L, López-Pousa J, Torres G. Myocardial iodine-labeled metaiodobenzylguanidine 123 uptake relates to age. J Nucl Cardiol. 1995;2:126-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 112. | Chen J, Garcia EV, Galt JR, Folks RD, Carrio I. Optimized acquisition and processing protocols for I-123 cardiac SPECT imaging. J Nucl Cardiol. 2006;13:251-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 113. | Treglia G, Stefanelli A, Bruno I, Giordano A. Clinical usefulness of myocardial innervation imaging using Iodine-123-meta-iodobenzylguanidine scintigraphy in evaluating the effectiveness of pharmacological treatments in patients with heart failure: an overview. Eur Rev Med Pharmacol Sci. 2013;17:56-68. [PubMed] |
| 114. | Matsui T, Tsutamoto T, Maeda K, Kusukawa J, Kinoshita M. Prognostic value of repeated 123I-metaiodobenzylguanidine imaging in patients with dilated cardiomyopathy with congestive heart failure before and after optimized treatments--comparison with neurohumoral factors. Circ J. 2002;66:537-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 115. | Scholtens AM, Braat AJ, Tuinenburg A, Meine M, Verberne HJ. Cardiac sympathetic innervation and cardiac resynchronization therapy. Heart Fail Rev. 2013;Epub ahead of print. [PubMed] |
| 116. | Simantirakis EN, Prassopoulos VK, Chrysostomakis SI, Kochiadakis GE, Koukouraki SI, Lekakis JP, Karkavitsas NS, Vardas PE. Effects of asynchronous ventricular activation on myocardial adrenergic innervation in patients with permanent dual-chamber pacemakers; an I(123)-metaiodobenzylguanidine cardiac scintigraphic study. Eur Heart J. 2001;22:323-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 117. | Erol-Yilmaz A, Verberne HJ, Schrama TA, Hrudova J, De Winter RJ, Van Eck-Smit BL, De Bruin R, Bax JJ, Schalij MJ, Wilde AA. Cardiac resynchronization induces favorable neurohumoral changes. Pacing Clin Electrophysiol. 2005;28:304-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 53] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 118. | Gould PA, Kong G, Kalff V, Duffy SJ, Taylor AJ, Kelly MJ, Kaye DM. Improvement in cardiac adrenergic function post biventricular pacing for heart failure. Europace. 2007;9:751-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 119. | Tanaka H, Tatsumi K, Fujiwara S, Tsuji T, Kaneko A, Ryo K, Fukuda Y, Matsumoto K, Shigeru M, Yoshida A. Effect of left ventricular dyssynchrony on cardiac sympathetic activity in heart failure patients with wide QRS duration. Circ J. 2012;76:382-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 120. | Josephson M, Wellens HJ. Implantable defibrillators and sudden cardiac death. Circulation. 2004;109:2685-2691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 128] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 121. | Adabag AS, Luepker RV, Roger VL, Gersh BJ. Sudden cardiac death: epidemiology and risk factors. Nat Rev Cardiol. 2010;7:216-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 287] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 122. | Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, Stevenson LW, Sweeney MO, Smith SC, Jacobs AK, Adams CD, Anderson JL, Buller CE, Creager MA, Ettinger SM, Faxon DP, Halperin JL, Hiratzka LF, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura RA, Ornato JP, Page RL, Riegel B, Tarkington LG, Yancy CW; American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices); American Association for Thoracic Surgery; Society of Thoracic Surgeons. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices) developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;51:e1-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1189] [Cited by in RCA: 1116] [Article Influence: 65.6] [Reference Citation Analysis (0)] |
| 123. | Dagres N, Hindricks G. Risk stratification after myocardial infarction: is left ventricular ejection fraction enough to prevent sudden cardiac death. Eur Heart J. 2013;34:1964-1971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 119] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 124. | Turakhia MP. Sudden cardiac death and implantable cardioverter-defibrillators. Am Fam Physician. 2010;82:1357-1366. [PubMed] |
| 125. | Bryant J, Brodin H, Loveman E, Clegg A. Clinical effectiveness and cost-effectiveness of implantable cardioverter defibrillators for arrhythmias: a systematic review and economic evaluation. Int J Technol Assess Health Care. 2007;23:63-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 126. | Das M. Indications for ICD and cardiac resynchronization therapy for prevention of sudden cardiac death. Expert Rev Cardiovasc Ther. 2009;7:181-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 127. | Atwater BD, Daubert JP. Implantable cardioverter defibrillators: risks accompany the life-saving benefits. Heart. 2012;98:764-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 128. | Sanders GD, Kong MH, Al-Khatib SM, Peterson ED. Cost-effectiveness of implantable cardioverter defibrillators in patients & gt; or=65 years of age. Am Heart J. 2010;160:122-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 129. | Moss AJ, Greenberg H, Case RB, Zareba W, Hall WJ, Brown MW, Daubert JP, McNitt S, Andrews ML, Elkin AD; Multicenter Automatic Defibrillator Implantation Trial-II (MADIT-II) Research Group. Long-term clinical course of patients after termination of ventricular tachyarrhythmia by an implanted defibrillator. Circulation. 2004;110:3760-3765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 452] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 130. | Buxton AE, Lee KL, Hafley GE, Pires LA, Fisher JD, Gold MR, Josephson ME, Lehmann MH, Prystowsky EN; MUSTT Investigators. Limitations of ejection fraction for prediction of sudden death risk in patients with coronary artery disease: lessons from the MUSTT study. J Am Coll Cardiol. 2007;50:1150-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 271] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 131. | Aljaroudi WA, Hage FG, Hermann D, Doppalapudi H, Venkataraman R, Heo J, Iskandrian AE. Relation of left-ventricular dyssynchrony by phase analysis of gated SPECT images and cardiovascular events in patients with implantable cardiac defibrillators. J Nucl Cardiol. 2010;17:398-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 132. | Braunwald E. Research advances in heart failure: a compendium. Circ Res. 2013;113:633-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 133. | Akutsu Y, Kaneko K, Kodama Y, Li HL, Kawamura M, Asano T, Tanno K, Shinozuka A, Gokan T, Kobayashi Y. The significance of cardiac sympathetic nervous system abnormality in the long-term prognosis of patients with a history of ventricular tachyarrhythmia. J Nucl Med. 2009;50:61-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 134. | Scott PA, Sterns LD, Tang AS. Do patients at high risk of nonsudden cardiac death benefit from prophylactic ICD therapy. Curr Opin Cardiol. 2012;27:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 135. | Stein KM. Noninvasive risk stratification for sudden death: signal-averaged electrocardiography, nonsustained ventricular tachycardia, heart rate variability, baroreflex sensitivity, and QRS duration. Prog Cardiovasc Dis. 2008;51:106-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 136. | Stecker EC, Chugh SS. Prediction of sudden cardiac death: next steps in pursuit of effective methodology. J Interv Card Electrophysiol. 2011;31:101-107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 137. | Buxton AE. Risk stratification for sudden death in patients with coronary artery disease. Heart Rhythm. 2009;6:836-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 138. | Morishima I, Sone T, Tsuboi H, Mukawa H, Uesugi M, Morikawa S, Takagi K, Niwa T, Morita Y, Murakami R. Risk stratification of patients with prior myocardial infarction and advanced left ventricular dysfunction by gated myocardial perfusion SPECT imaging. J Nucl Cardiol. 2008;15:631-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 139. | Hoque A, Maaieh M, Longaker RA, Stoddard MF. Exercise echocardiography and thallium-201 single-photon emission computed tomography stress test for 5- and 10-year prognosis of mortality and specific cardiac events. J Am Soc Echocardiogr. 2002;15:1326-1334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 140. | Piccini JP, Horton JR, Shaw LK, Al-Khatib SM, Lee KL, Iskandrian AE, Borges-Neto S. Single-photon emission computed tomography myocardial perfusion defects are associated with an increased risk of all-cause death, cardiovascular death, and sudden cardiac death. Circ Cardiovasc Imaging. 2008;1:180-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 141. | Piccini JP, Starr AZ, Horton JR, Shaw LK, Lee KL, Al-Khatib SM, Iskandrian AE, O’Connor CM, Borges-Neto S. Single-photon emission computed tomography myocardial perfusion imaging and the risk of sudden cardiac death in patients with coronary disease and left ventricular ejection fraction& gt; 35%. J Am Coll Cardiol. 2010;56:206-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 142. | Assomull RG, Prasad SK, Lyne J, Smith G, Burman ED, Khan M, Sheppard MN, Poole-Wilson PA, Pennell DJ. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J Am Coll Cardiol. 2006;48:1977-1985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 845] [Cited by in RCA: 856] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 143. | Gulati A, Jabbour A, Ismail TF, Guha K, Khwaja J, Raza S, Morarji K, Brown TD, Ismail NA, Dweck MR. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA. 2013;309:896-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 775] [Cited by in RCA: 887] [Article Influence: 73.9] [Reference Citation Analysis (0)] |
| 144. | Volders PG. Novel insights into the role of the sympathetic nervous system in cardiac arrhythmogenesis. Heart Rhythm. 2010;7:1900-1906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 145. | Tan AY, Verrier RL. The role of the autonomic nervous system in cardiac arrhythmias. Handb Clin Neurol. 2013;117:135-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 146. | Gerson MC, Abdallah M, Muth JN, Costea AI. Will imaging assist in the selection of patients with heart failure for an ICD. JACC Cardiovasc Imaging. 2010;3:101-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 147. | Merlet P, Valette H, Dubois-Randé JL, Moyse D, Duboc D, Dove P, Bourguignon MH, Benvenuti C, Duval AM, Agostini D. Prognostic value of cardiac metaiodobenzylguanidine imaging in patients with heart failure. J Nucl Med. 1992;33:471-477. [PubMed] |
| 148. | Agostini D, Verberne HJ, Burchert W, Knuuti J, Povinec P, Sambuceti G, Unlu M, Estorch M, Banerjee G, Jacobson AF. I-123-mIBG myocardial imaging for assessment of risk for a major cardiac event in heart failure patients: insights from a retrospective European multicenter study. Eur J Nucl Med Mol Imaging. 2008;35:535-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 156] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 149. | Kasama S, Toyama T, Sumino H, Nakazawa M, Matsumoto N, Sato Y, Kumakura H, Takayama Y, Ichikawa S, Suzuki T. Prognostic value of serial cardiac 123I-MIBG imaging in patients with stabilized chronic heart failure and reduced left ventricular ejection fraction. J Nucl Med. 2008;49:907-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 77] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 150. | Jacobson AF, Senior R, Cerqueira MD, Wong ND, Thomas GS, Lopez VA, Agostini D, Weiland F, Chandna H, Narula J; ADMIRE-HF Investigators. Myocardial iodine-123 meta-iodobenzylguanidine imaging and cardiac events in heart failure. Results of the prospective ADMIRE-HF (AdreView Myocardial Imaging for Risk Evaluation in Heart Failure) study. J Am Coll Cardiol. 2010;55:2212-2221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 672] [Cited by in RCA: 652] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 151. | Kuwabara Y, Tamaki N, Nakata T, Yamashina S, Yamazaki J. Determination of the survival rate in patients with congestive heart failure stratified by ¹²³I-MIBG imaging: a meta-analysis from the studies performed in Japan. Ann Nucl Med. 2011;25:101-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 152. | Nakata T, Nakajima K, Yamashina S, Yamada T, Momose M, Kasama S, Matsui T, Matsuo S, Travin MI, Jacobson AF. A pooled analysis of multicenter cohort studies of (123)I-mIBG imaging of sympathetic innervation for assessment of long-term prognosis in heart failure. JACC Cardiovasc Imaging. 2013;6:772-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 161] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 153. | Jacobson AF, Lombard J, Banerjee G, Camici PG. 123I-mIBG scintigraphy to predict risk for adverse cardiac outcomes in heart failure patients: design of two prospective multicenter international trials. J Nucl Cardiol. 2009;16:113-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 154. | Nagahara D, Nakata T, Hashimoto A, Wakabayashi T, Kyuma M, Noda R, Shimoshige S, Uno K, Tsuchihashi K, Shimamoto K. Predicting the need for an implantable cardioverter defibrillator using cardiac metaiodobenzylguanidine activity together with plasma natriuretic peptide concentration or left ventricular function. J Nucl Med. 2008;49:225-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 96] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 155. | Tamaki S, Yamada T, Okuyama Y, Morita T, Sanada S, Tsukamoto Y, Masuda M, Okuda K, Iwasaki Y, Yasui T. Cardiac iodine-123 metaiodobenzylguanidine imaging predicts sudden cardiac death independently of left ventricular ejection fraction in patients with chronic heart failure and left ventricular systolic dysfunction: results from a comparative study with signal-averaged electrocardiogram, heart rate variability, and QT dispersion. J Am Coll Cardiol. 2009;53:426-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 175] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 156. | Shah AM, Bourgoun M, Narula J, Jacobson AF, Solomon SD. Influence of ejection fraction on the prognostic value of sympathetic innervation imaging with iodine-123 MIBG in heart failure. JACC Cardiovasc Imaging. 2012;5:1139-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 157. | Sood N, Al Badarin F, Parker M, Pullatt R, Jacobson AF, Bateman TM, Heller GV. Resting perfusion MPI-SPECT combined with cardiac 123I-mIBG sympathetic innervation imaging improves prediction of arrhythmic events in non-ischemic cardiomyopathy patients: sub-study from the ADMIRE-HF trial. J Nucl Cardiol. 2013;20:813-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 158. | García-González P, Cozar-Santiago P, Fabregat-Andrés O, Sánchez-Jurado R, Estornell-Erill J, Ridocci-Soriano F. Evaluation of Cardiac (123)I-MIBG Imaging in Patients With Severe Left Ventricular Dysfunction and Indication for Implantable Cardioverter Defibrillator. Rev Esp Cardiol. 2013;66:1000-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 159. | Hayashi M, Kobayashi Y, Morita N, Iwasaki YK, Ohmura K, Atarashi H, Katoh T, Takano T. Clinical significance and contributing factors of long-term variability in induced ventricular tachyarrhythmias. J Cardiovasc Electrophysiol. 2003;14:1049-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 160. | Mitrani RD, Klein LS, Miles WM, Hackett FK, Burt RW, Wellman HN, Zipes DP. Regional cardiac sympathetic denervation in patients with ventricular tachycardia in the absence of coronary artery disease. J Am Coll Cardiol. 1993;22:1344-1353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 102] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 161. | Calkins H, Allman K, Bolling S, Kirsch M, Wieland D, Morady F, Schwaiger M. Correlation between scintigraphic evidence of regional sympathetic neuronal dysfunction and ventricular refractoriness in the human heart. Circulation. 1993;88:172-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 57] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 162. | Fallavollita JA, Luisi AJ, Michalek SM, Valverde AM, deKemp RA, Haka MS, Hutson AD, Canty JM. Prediction of arrhythmic events with positron emission tomography: PAREPET study design and methods. Contemp Clin Trials. 2006;27:374-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 163. | Fallavollita JA, Heavey BM, Luisi AJ, Michalek SM, Baldwa S, Mashtare TL, Hutson AD, Dekemp RA, Haka MS, Sajjad M. Regional myocardial sympathetic denervation predicts the risk of sudden cardiac arrest in ischemic cardiomyopathy. J Am Coll Cardiol. 2014;63:141-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 320] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 164. | Bengel FM, Thackeray JT. Altered cardiac innervation predisposes to ventricular arrhythmia: targeted positron emission tomography identifies risk in ischemic cardiomyopathy. J Am Coll Cardiol. 2014;63:150-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |