Published online Dec 18, 2024. doi: 10.13105/wjma.v12.i4.95755
Revised: September 7, 2024
Accepted: September 30, 2024
Published online: December 18, 2024
Processing time: 238 Days and 17.2 Hours
Positron emission tomography/computed tomography (PET/CT) with radio
Core Tip: Fibroblast activation protein inhibitors (FAPI) has been a protagonist in the most recent research in the field of nuclear medicine and oncology, demonstrating surprising diagnostic versatility with a dizzying rise, due to its successful labeling with radioisotopes (Ga68 and F18). In the comparative clinical studies that have been carried out to date, it appears that FAPI is superior to fluorodeoxyglucose in oncological diagnostic performance, according to certain advantages it has, mainly related to a better lesion-to-background ratio, lower radiation dose, non-dependence on glucose level or preparatory diet, low brain uptake and its promising use as a theragnostic. FAPI positron emission tomography/computed tomography constitutes a great challenge to address for nuclear medicine and current oncology, which is approaching our present by leaps and bounds, possibly marking a new route in the approach and treatment of oncological diseases.
- Citation: Hernandez-Hidalgo N, Cortes G, Ortega-Anaya K, Varela H. Fibroblast activation protein inhibitors positron emission tomography/computed tomography: Review of the literature. World J Meta-Anal 2024; 12(4): 95755
- URL: https://www.wjgnet.com/2308-3840/full/v12/i4/95755.htm
- DOI: https://dx.doi.org/10.13105/wjma.v12.i4.95755
Since 2018, fibroblast activation protein inhibitors (FAPI) has been a protagonist in the most recent research in the field of nuclear medicine and oncology, demonstrating surprising diagnostic versatility with a dizzying rise, due to its successful labeling with radioisotopes (Ga68 and F18), initially for studies positron emission tomography (PET) through FAP-02, FAP-03 and currently with FAPI-46, in this way, emerges as a different and in some aspects better alternative to the traditional PET with 18F-Fluorodeoxyglucose (18F-FDG). In the comparative clinical studies that have been carried out to date, it appears that FAPI is superior to FDG in oncological diagnostic performance, according to certain advantages it has, mainly related to a better lesion-to-background ratio (LBR), lower radiation dose, non-dependence on glucose level or preparatory diet, low brain uptake and its promising use as a theragnostic[1,2].
However, it should be noted that the initial clinical trials have lacked sufficiently large patient samples and FAPI has not been compared with what would be the gold standard (pathology), so far and on the other hand, expression has been found of FAPI in other non-malignant conditions similar to those seen in FDG studies, especially related to inflammatory changes and fibrotic diseases that are found in practically any tissue (alveolar process, thyroid, gastrointestinal tract, heart, osteo-articular, kidneys, lungs)[2]. However, FAPI PET/computed tomography (CT) constitutes a great challenge to address for nuclear medicine and current oncology, which is approaching our present by leaps and bounds, possibly marking a new route in the approach and treatment of oncological diseases.
A review of the literature was carried out by searching for articles in the PubMed, Science Direct and Google Scholar databases with what was published in relation to the topic until January 2024, without language restriction; there were no exclusion criteria. As this diagnostic tool is relatively new and is constantly being researched, there are not many articles prior to 2019. The MeSH terms used were FAPI PET/CT. All articles were reviewed by the 4 authors of the article and the manuscript was prepared together in a duration of six months.
FAPI, refers to a group of quinoline-based compounds that bind to the enzymatic portion of the fibroblast activation protein (FAP)[3]. FAP is a type II transmembrane serine protease, member of the proplyl peptidase family and also contains dipeptidyl peptidase IV. This protein is overexpressed in cancer-associated fibroblasts (CAF), cells found in about 90% of epithelial carcinomas, making up to half of the stromal mass in these tumors and being involved in tumor growth processes, immune suppression and cancer invasion[4].
These CAF also modulate the tumor microenvironment through the release of different cytokines[5]. They are differentiated and adapted cells that can come from multiple cell lineages, which is why they are highly genetically and phenotypically heterogeneous. CAF are larger than the rest of the fibroblasts; however, the difference it is above all a functional distinction[6].
It is also important to note that CAF are the main producers of type I collagen in the tumor stroma; the increase in the production of this type of collagen decreases the effectiveness of chemotherapeutic agents by reducing its uptake in the tumor tissue, so therapies aimed at reducing the number of CAF (e.g. therapy with radioisotopes linked to FAPI) could improve tumor susceptibility to chemotherapy[7].
FAP is overexpressed in CAF, but has little or no expression in normal fibroblasts, benign lesions and precancerous tissues, being a white marker with favorable properties that improve the localization of diagnostic or therapeutic tracers with low background activity[8]. Apart from almost 90% of different epithelial tumors and their metastases, FAP can also be seen in the cervical stroma, healing wounds, fibrosis, arthritis and transiently during embryonic development. This protease has dipeptidase and endopeptidase functions and also collagenase activity, which is why it is believed to be as
In tumor cells FAP plays multiple biological roles, it promotes the invasion and migration of tumor cells by partici
Given all these characteristics and its homogeneity of expression in CAF, FAP has represented great interest for some time in terms of the development of therapeutic strategies, from inhibitors of its enzymatic action such as talabostat, which did not show adequate results in phase II studies[10], protoxins that were FAP ligands and that when proteolyzed released cytotoxic substances but caused a bystander effect in neighboring cells and toxicity due to the unprocessed protoxin and even immunotherapy with specific antibodies but with limited therapeutic effect and sometimes severe adverse effects. These antibodies were also used linked to radioisotopes for diagnosis; however, they have slow plasma clearance, showing high background activity and limiting sensitivity in small lesions[6].
The FAPI that have shown the most satisfactory results for imaging diagnosis and treatment, linked to radioisotopes, are monomers based on quinoline, a chemical compound formed by the fusion of a benzene ring and another pyridine ring which is found in many alkaloids that have a biological function, therefore it has been the basis for the development of multiple medications and that in the case of these monomers that bind to a chelating agent and a radioactive isotope to compose the radiopharmaceutical, allow its binding to FAP in a highly specific manner, with rapid cellular interna
In research carried out on models in mice and later in humans, a high expression of FAP was demonstrated in tumors associated with CAFs, this motivated the development of specific inhibition FAP molecules, which have been radiolabeled for diagnostic use with positron emitters, through the use of DOTA chelators and others, thus emerging radiopharmaceuticals such as Ga68-FAPI 02 and Ga68-FAPI 04. These radiotracers show high intratumoral uptake with rapid renal clearance and low concentration in normal organs and tissues such as the liver or brain (except for tissues with active damage, remodeling or inflammation), considerably improving the LBR, which greatly favors the diagnostic performance of the test[3,12]. FAPI-02 and FAPI-04 present a tumor accumulation of up to 50% in 1 to 3 h post-injection and recently, FAPI-46 demonstrated a longer internalization time which allows it to have a theragnostic approach. On the other hand, FAPI has also been successfully radiolabeled with F18 (FAPI-74/NOTA), which would provide some advantages in costs and ease of programming[3].
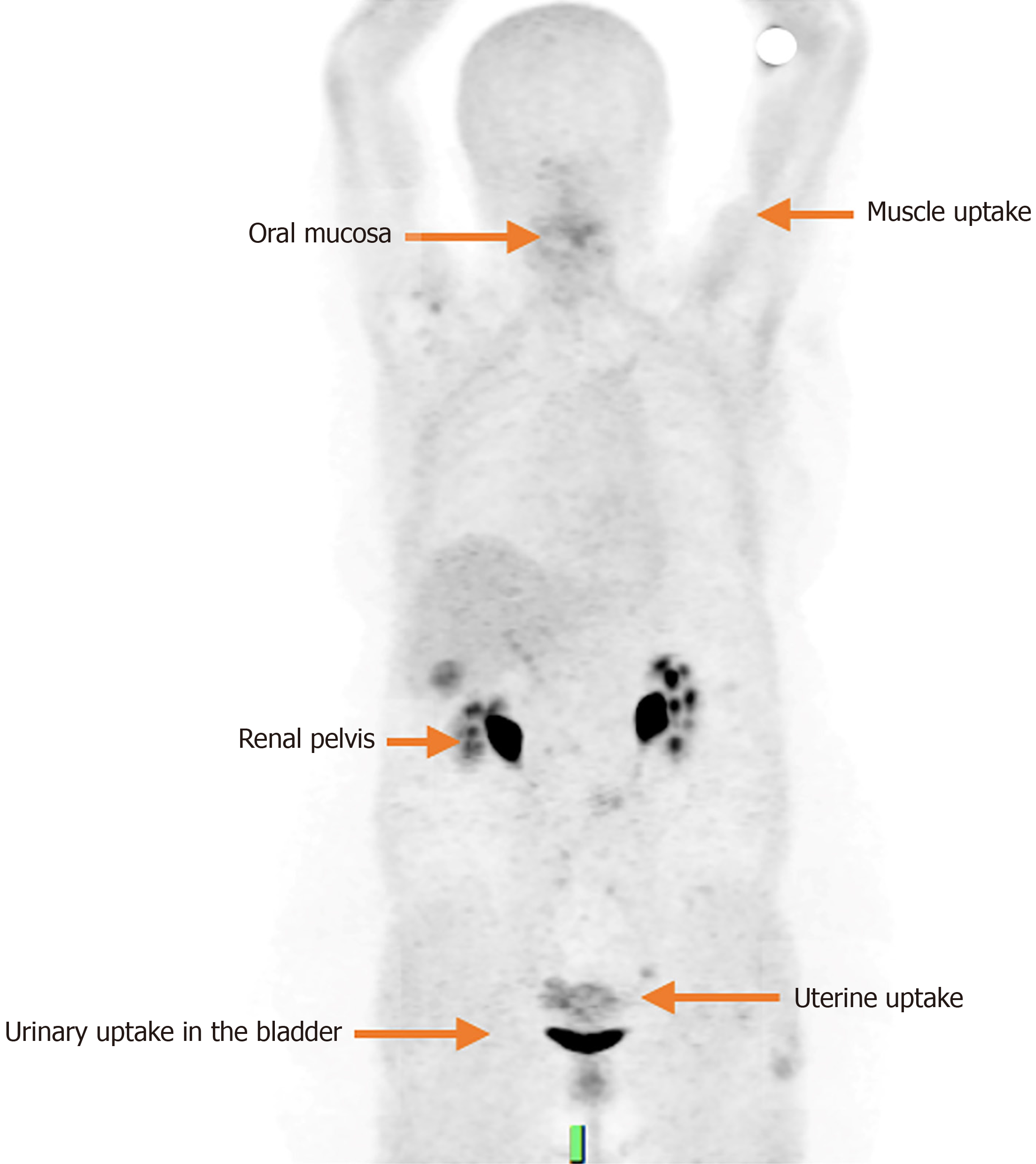
The main elimination route of FAPI is urinary, therefore, it has a high concentration in the kidneys, ureters and bladder; There is also biliary excretion, although to a lesser extent, with very low activity observed in the hepatobiliary system. Other low intensity physiological uptakes have been seen in the salivary glands, thyroid, spleen, bone marrow, uterus and pancreas, and minimal in the brain, parotids, lungs, oral mucosa, myocardium and muscles[3,12] (Figure 1).
The doses used in the different studies vary in ranges from 100 to 370 MBq with excellent dosimetry rates (up to 1.6 mSv/100 mBq) and 3 images are obtained: 10 minutes post-injection (before emptying the bladder), at 1 and at 3 h[1].
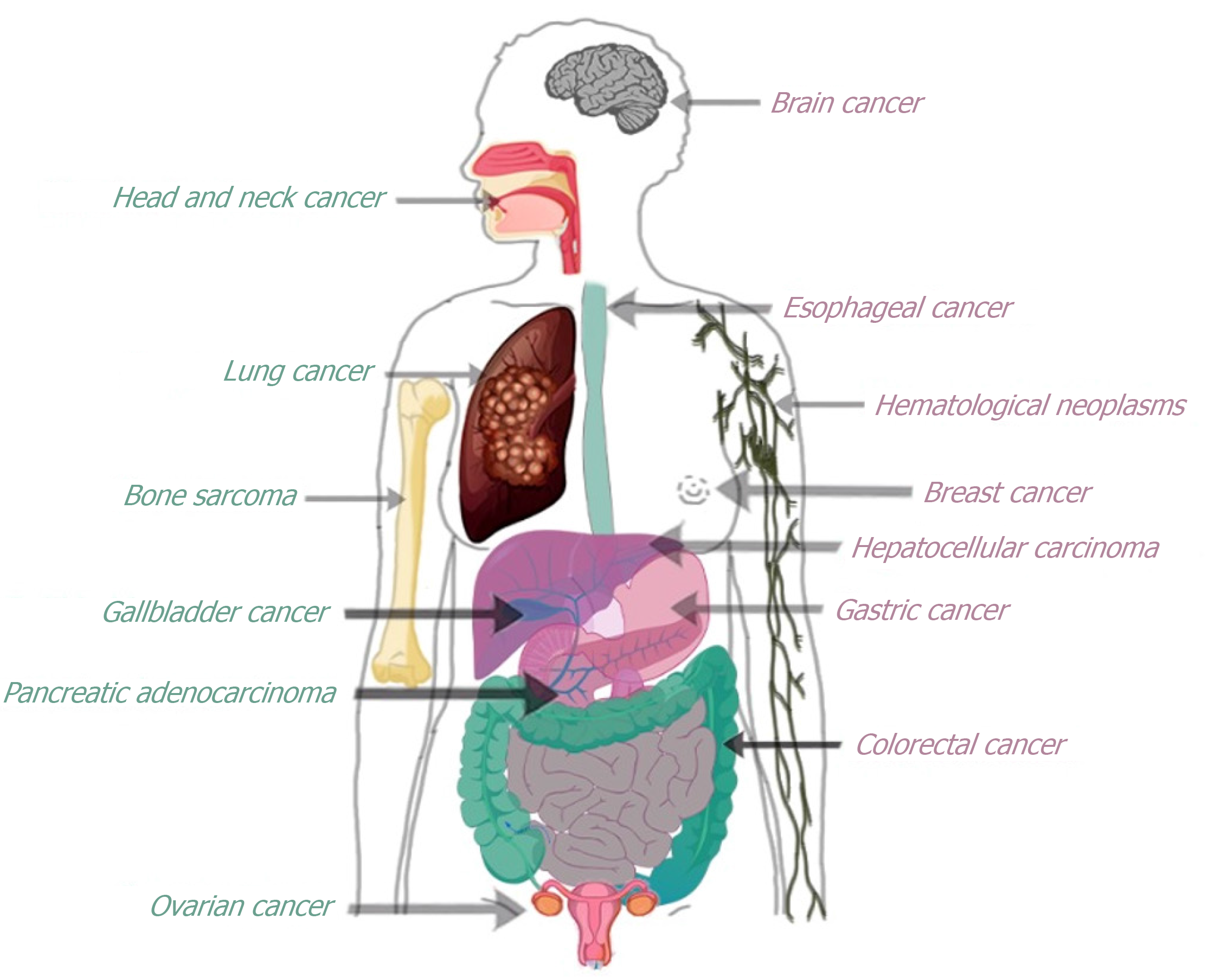
Various studies have demonstrated the usefulness of FAPI-PET/CT as a highly promising imaging technique in a variety of malignant tumors (Figure 2), being valuable for tumor detection and staging. Some of them are described below
Brain cancer:18F-FDG is restricted in brain diseases due to the physiological uptake that conditions the identification of the tumor. FAPI has a little physiological background uptake in the brain, allowing the finding of some brain tumors[13]. Benign tumors and also low-grade astrocytoma apparently do not have FAP expression. In glial tumors, the existence of FAP overexpression can be associated with a poor prognosis and the levels correlate with the grade[13]. Röhrich et al[14] included 18 patients with gliomas (five IDH-mutated gliomas and 13 IDH-wild-type glioblastomas); they found elevated levels of uptake in high-grade gliomas, but not in low-grade IDH-mutated gliomas, which could allow non-invasive diagnostic differentiation.
Head and neck cancer: Initial studies have shown high FAPI uptake in malignant lesions and low background activity in healthy tissue (e.g., salivary glands)[13]. Linz et al[15] studied 10 patients with oral squamous cell carcinoma who underwent FAPI and 18F-FDG PET/CT; both studies demonstrated similar levels of uptake in the primary tumors, as well as similar sensitivity and specificity in cervical lymph node metastases. Lesions showing FAP expression were confirmed by immunohistochemistry.
Head and neck cancers require future research since the results published so far are not conclusive in determining the diagnostic accuracy of FAPI images[13].
Lung cancer: Histologically is classified in small cell lung cancer (SCLC) and non-SCLC (NSCLC). 18F-FDG is used to systematically diagnose and stage in NSCLC. PET/CT with 68Ga-FAPI demonstrates high uptake of the intratumoral radiotracer, low uptake in normal tissue and rapid elimination, which provides adequate visibility of the tumor and a better LBR[16,17]. A prospective analysis of 28 patients with histologically confirmed NSCLC compared the performance of 68Ga-FAPI and 18F-FDG PET/CT for both primary tumors and metastases, demonstrating that 68Ga-FAPI provides better lesion visualization and staging accuracy than 18F-FDG in NSCLC, they further found that 68Ga-FAPI was able to detect lymph node metastases than 18F-FDG PET/CT, yielding higher standardized uptake value (SUVmax) and LBR values for these metastases. Furthermore, the analyzes also revealed that 68Ga-FAPI is superior to PET/CT with 18F-FDG for the detection of liver, pleural and bone metastases, which is attributable to the reduced physiological uptake of the radiotracer of 68Ga-FAPI.
Regrettable, they found that 68Ga-FAPI had a low sensitivity for detecting adrenal metastases, inferring that these lesions might not have significant fibrotic activity. However, 18F-FDG has low specificity for diagnosing adrenal metastases, requiring complementary studies for an accurate diagnosis[17].
Esophageal cancer: In a study Ristau et al[18] investigated the role of FAPI-PET/CT in 7 patients with esophageal cancer, demonstrating high tumor uptake and low background activity, facilitating the delineation of tumor volume and consequently a particular benefit for surgery planning radiotherapy[18]. These findings were confirmed by Zhao et al[19] in 21 patients with recently diagnosed esophageal cancer, who underwent 18F-FDG and 68Ga-FAPI PET/CT. FAPI images showed a good SUVmax, LBR, and superior target volume delineation[19,20].
Gastric cancer: Zhang et al[21] performed 68Ga-FAPI-04 and 18F-FDG PET/CT on 25 patients with gastric cancer (GC), where they compared the diagnostic accuracy of the two images for staging and restaging. 68Ga-FAPI-04 PET/CT proved to be more efficient in detecting primary tumors [18/19 (94.74%) vs 13/19 (68.42%)], lymph node metastases [75/77 (97.40%) vs 32/77 (41.56%)] and distant metastases [275/283 (97.17%) vs 122/283 (43.11%)], being similar to the results found by Kuten et al[22] and Jiang et al[23]. 68Ga-FAPI-04 PET/CT also upstaged or restaged tumor in 5/25 patients and down staged or restaged tumor in 2/25 patients. Therefore, 68Ga-FAPI-04 PET/CT is superior to 18F-FDG PET/CT for the detection of primary tumors, lymph nodes, and distant metastases in patients with GC. 68Ga-FAPI-04 PET/CT corrected clinical stage in seven patients; furthermore, negative 68Ga-FAPI-04 PET/CT expression in patients with residual gastritis is useful to distinguish it from high 18F-FDG uptake caused by inflammation.
A prospective study comparing PET/CT with FAPI and FDG in patients with primary gastric adenocarcinoma demonstrated that FAPI was superior to FDG in detecting primary gastric tumors, showing a 100% detection rate compared to 50% for FDG[22]. It was also superior in detecting peritoneal carcinomatosis, with a 100% detection rate vs none[22].
In a study of 38 patients with GC, including 31 adenocarcinomas and seven signet ring cell carcinomas, the sensitivity of Ga-FAPI-04 and 18F-FDG for diagnosing primary GC was 100% and 82% respectively. Four cases of adenocarcinoma and three cases of signet ring cell carcinoma were not detected by 18F-FDG PET/CT. This may be due to the high FDG uptake in the intestinal wall and other abdominal organs[23].
These studies indicate that 68Ga-FAPI PET/CT has advantages over 18F-FDG in detecting primary GC, lymph node metastasis, and peritoneal metastasis of GC, largely because 68Ga-FAPI has better contrast between the lesion and the background and provides a more detailed tumor profile. However, when the tumor invades other tissues, a fibrotic reaction may occur, causing severe fibrosis and possible false-positive uptake of 68Ga-FAPI, so other clinical and imaging data should be explored to avoid false positives[24].
Colorectal cancer: Colorectal cancer (CRC) exhibits overexpression of FAP in neoplastic cells and tumor stroma and is a marker observed in the early stages of CRC development. A poor prognosis, higher grade, stage, and invasion have been correlated with FAP levels[13]. Some studies have shown that 68Ga-FAPI-04 improved tumor staging in patients with CRC due to a favorable LBR and low uptake of the radiotracer in the gastrointestinal tract. Mucinous/signet ring carci
Gallbladder cancer: Is the most common cancer of the biliary system[3]. Few studies have studied the role of FAPI in the diagnosis of malignancies of the biliary system. Kratochwil et al[25] performed 68Ga-FAPI PET/CT in 80 patients with 28 different tumor entities and found one of the highest SUVmax values in cholangiocarcinoma (mean SUVmax > 12)[25].
Hepatocellular carcinoma: Hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (ICC) are the two most common types of primary liver carcinoma. The liver accounts for 75.7% of all synchronous metastatic cancers[24]. A study demonstrated the high diagnostic accuracy of 68Ga-FAPI PET/CT in the detection of primary liver tumors (both HCC and ICC) in all differentiated lesions[26]. They prospectively compared the diagnostic performance of FAPI PET/CT compared to FDG PET/CT in 20 patients with suspicious intrahepatic lesions and found that FAPI was more sensitive (100% vs 58.8%), although both with a specificity of 100%. All typical intrahepatic malignancies had high 68Ga-FAPI uptake but heterogeneous 18F-FDG uptake. Furthermore, combined with lower background uptake, the significantly higher tumor contrast of 68Ga-FAPI allowed for a more confident diagnosis compared to 18F-FDG[27].
Pancreatic ductal adenocarcinoma: In patients with pancreatic cancer 18F-FDG PET/CT is currently used, however, it has some limitations, such as when the patients have hyperglycemia and FDG can be competitively inhibited, demonstrating a low uptake and the possibility of false negatives results. In pancreatic cancer 18F-FDG has low sensitivity and specificity in the diagnosis[13]. In contrast, FAP expression is elevated in PDAC and has been correlated with prognosis.
A retrospective analysis was performed in 19 patients with PDAC, demonstrating high FAPI uptake in primary PDAC, in lymph nodes and distant metastases, while healthy tissues have negligible background activity, leading to an excellent LBR for PDAC[28].
68Ga-FAPI is also a good marker for fibroblast-mediated inflammatory responses, therefore non-specific fibrosis induced by inflammation can also cause positive uptake of 68Ga-FAPI-04 and can therefore be confused with an indicator of tumor recurrence[24].
Breast cancer: Kömek et al[29] analyzed prospectively 20 patients with histopathologically confirmed primary or recurrent breast cancer (BC), in addition to their metastases, all underwent 18F-FDG and FAPI PET/CT, demonstrating that FAPI is superior to FDG detecting the primary tumor with its high sensitivity, high SUVmax and high LBR. In lymph nodes, lung and bone metastases SUVmax values were significantly higher in FAPI images than in FDG, except for liver lesions that did not demonstrate significant differences[29].
Ovarian cancer: Primary ovarian malignancies are mainly classified into two groups: Epithelial and non-epithelial. Among them, 95% of ovarian cancer (OC) originates from ovarian epithelial cells called epithelial OC (EOC). High-grade serous carcinomas represent up to 70% of EOC, while low-grade serous carcinoma represent up to 5%, and the other three primary histological types of EOC are endometrioid, mucinous, and clear cells[30].
18F-FDG PET/CT is used to detect early recurrence of OC before lesions are visible on CT or clinical symptoms appear, especially in patients with elevated CA-125 levels. However, due to its physiological distribution, 18F-FDG accumulates in the intestine and is then excreted through the urinary tract, which may interfere with optimal evaluation of primary pelvic tumors[24].
Previous studies determined the presence of PAF in 97% of all OCs and showed classification among PAF levels with poor clinical prognosis, resistance to chemotherapy and shorter time to recurrence[13]. Furthermore, due to the intense uptake of FAPI-PET/CT, it could be established as a potential therapeutic target, since FAP expression is absent in healthy ovaries[13]. In a study that compared 68Ga-FAPI-04 and 18F-FDG PET/CT to evaluate the diagnostic performance of ovarian malignancies, it was shown that 68Ga-FAPI-04 was more sensitive than 18F-FDG (100% vs 78%) to detect primary ovarian tumors, and also has greater potential in the detection of peritoneal carcinomatosis[30].
FAPI PET/CT could replace or complement FDG-PET/CT in some cases. In different tumor types, studies have shown that FAPI shows superior diagnostic efficacy, more accurate detection, and lower background activity compared with 18F-FDG PET/CT, especially in BC, HCC, ICC, GC, and pancreatic cancer. In contrast, FAPI has not demonstrated superiority over FDG for the diagnosis of hematological malignancies. However, the role of FAPI PET/CT needs to be further explored in larger prospective clinical trials to define its diagnostic role.
| 1. | Dendl K, Schlittenhardt J, Staudinger F, Kratochwil C, Altmann A, Haberkorn U, Giesel FL. The Role of Fibroblast Activation Protein Ligands in Oncologic PET Imaging. PET Clin. 2021;16:341-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 2. | Bentestuen M, Al-Obaydi N, Zacho HD. FAPI-avid nonmalignant PET/CT findings: An expedited systematic review. Semin Nucl Med. 2023;53:694-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 3. | Gilardi L, Airò Farulla LS, Demirci E, Clerici I, Omodeo Salè E, Ceci F. Imaging Cancer-Associated Fibroblasts (CAFs) with FAPi PET. Biomedicines. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 38] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 4. | Zhao L, Niu B, Fang J, Pang Y, Li S, Xie C, Sun L, Zhang X, Guo Z, Lin Q, Chen H. Synthesis, Preclinical Evaluation, and a Pilot Clinical PET Imaging Study of (68)Ga-Labeled FAPI Dimer. J Nucl Med. 2022;63:862-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 105] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 5. | Lindner T, Loktev A, Altmann A, Giesel F, Kratochwil C, Debus J, Jäger D, Mier W, Haberkorn U. Development of Quinoline-Based Theranostic Ligands for the Targeting of Fibroblast Activation Protein. J Nucl Med. 2018;59:1415-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 609] [Article Influence: 87.0] [Reference Citation Analysis (0)] |
| 6. | Altmann A, Haberkorn U, Siveke J. The Latest Developments in Imaging of Fibroblast Activation Protein. J Nucl Med. 2021;62:160-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 174] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 7. | Loeffler M, Krüger JA, Niethammer AG, Reisfeld RA. Targeting tumor-associated fibroblasts improves cancer chemotherapy by increasing intratumoral drug uptake. J Clin Invest. 2006;116:1955-1962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 534] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 8. | Huang R, Pu Y, Huang S, Yang C, Yang F, Pu Y, Li J, Chen L, Huang Y. FAPI-PET/CT in Cancer Imaging: A Potential Novel Molecule of the Century. Front Oncol. 2022;12:854658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 50] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 9. | Li M, Younis MH, Zhang Y, Cai W, Lan X. Clinical summary of fibroblast activation protein inhibitor-based radiopharmaceuticals: cancer and beyond. Eur J Nucl Med Mol Imaging. 2022;49:2844-2868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 76] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 10. | Narra K, Mullins SR, Lee HO, Strzemkowski-Brun B, Magalong K, Christiansen VJ, McKee PA, Egleston B, Cohen SJ, Weiner LM, Meropol NJ, Cheng JD. Phase II trial of single agent Val-boroPro (Talabostat) inhibiting Fibroblast Activation Protein in patients with metastatic colorectal cancer. Cancer Biol Ther. 2007;6:1691-1699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 205] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 11. | Ferreira D, Murie V, Santos T, Vieira P, Clososki G. Recentes avancos NA Functionalizacao Seletiva De Quinolinas. Quím Nova. 2021;44:599-615. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 12. | Meyer C, Dahlbom M, Lindner T, Vauclin S, Mona C, Slavik R, Czernin J, Haberkorn U, Calais J. Radiation Dosimetry and Biodistribution of (68)Ga-FAPI-46 PET Imaging in Cancer Patients. J Nucl Med. 2020;61:1171-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 165] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 13. | Dendl K, Koerber SA, Kratochwil C, Cardinale J, Finck R, Dabir M, Novruzov E, Watabe T, Kramer V, Choyke PL, Haberkorn U, Giesel FL. FAP and FAPI-PET/CT in Malignant and Non-Malignant Diseases: A Perfect Symbiosis? Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 95] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 14. | Röhrich M, Loktev A, Wefers AK, Altmann A, Paech D, Adeberg S, Windisch P, Hielscher T, Flechsig P, Floca R, Leitz D, Schuster JP, Huber PE, Debus J, von Deimling A, Lindner T, Haberkorn U. IDH-wildtype glioblastomas and grade III/IV IDH-mutant gliomas show elevated tracer uptake in fibroblast activation protein-specific PET/CT. Eur J Nucl Med Mol Imaging. 2019;46:2569-2580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 107] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 15. | Linz C, Brands RC, Kertels O, Dierks A, Brumberg J, Gerhard-Hartmann E, Hartmann S, Schirbel A, Serfling S, Zhi Y, Buck AK, Kübler A, Hohm J, Lapa C, Kircher M. Targeting fibroblast activation protein in newly diagnosed squamous cell carcinoma of the oral cavity - initial experience and comparison to [(18)F]FDG PET/CT and MRI. Eur J Nucl Med Mol Imaging. 2021;48:3951-3960. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 16. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64436] [Article Influence: 16109.0] [Reference Citation Analysis (176)] |
| 17. | Wu J, Deng H, Zhong H, Wang T, Rao Z, Wang Y, Chen Y, Zhang C. Comparison of (68)Ga-FAPI and (18)F-FDG PET/CT in the Evaluation of Patients With Newly Diagnosed Non-Small Cell Lung Cancer. Front Oncol. 2022;12:924223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 18. | Ristau J, Giesel FL, Haefner MF, Staudinger F, Lindner T, Merkel A, Schlittenhardt J, Kratochwil C, Choyke PL, Herfarth K, Debus J, Haberkorn U, Koerber SA. Impact of Primary Staging with Fibroblast Activation Protein Specific Enzyme Inhibitor (FAPI)-PET/CT on Radio-Oncologic Treatment Planning of Patients with Esophageal Cancer. Mol Imaging Biol. 2020;22:1495-1500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 19. | Zhao L, Chen S, Chen S, Pang Y, Dai Y, Hu S, Lin L, Fu L, Sun L, Wu H, Chen H, Lin Q. (68)Ga-fibroblast activation protein inhibitor PET/CT on gross tumour volume delineation for radiotherapy planning of oesophageal cancer. Radiother Oncol. 2021;158:55-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 20. | Pang Y, Zhao L, Luo Z, Hao B, Wu H, Lin Q, Sun L, Chen H. Comparison of (68)Ga-FAPI and (18)F-FDG Uptake in Gastric, Duodenal, and Colorectal Cancers. Radiology. 2021;298:393-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 209] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 21. | Zhang S, Wang W, Xu T, Ding H, Li Y, Liu H, Huang Y, Liu L, Du T, Zhao Y, Chen Y, Qiu L. Comparison of Diagnostic Efficacy of [(68)Ga]Ga-FAPI-04 and [(18)F]FDG PET/CT for Staging and Restaging of Gastric Cancer. Front Oncol. 2022;12:925100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 22. | Kuten J, Levine C, Shamni O, Pelles S, Wolf I, Lahat G, Mishani E, Even-Sapir E. Head-to-head comparison of [68Ga]Ga-FAPI-04 and [18F]-FDG PET/CT in evaluating the extent of disease in gastric adenocarcinoma. Eur J Nucl Med Mol Imaging. 2022;49:743-750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 79] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 23. | Jiang D, Chen X, You Z, Wang H, Zhang X, Li X, Ren S, Huang Q, Hua F, Guan Y, Zhao J, Xie F. Comparison of [(68) Ga]Ga-FAPI-04 and [(18)F]-FDG for the detection of primary and metastatic lesions in patients with gastric cancer: a bicentric retrospective study. Eur J Nucl Med Mol Imaging. 2022;49:732-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 71] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 24. | Yang T, Ma L, Hou H, Gao F, Tao W. FAPI PET/CT in the Diagnosis of Abdominal and Pelvic Tumors. Front Oncol. 2021;11:797960. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 25. | Kratochwil C, Flechsig P, Lindner T, Abderrahim L, Altmann A, Mier W, Adeberg S, Rathke H, Röhrich M, Winter H, Plinkert PK, Marme F, Lang M, Kauczor HU, Jäger D, Debus J, Haberkorn U, Giesel FL. (68)Ga-FAPI PET/CT: Tracer Uptake in 28 Different Kinds of Cancer. J Nucl Med. 2019;60:801-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 997] [Article Influence: 166.2] [Reference Citation Analysis (0)] |
| 26. | Shi X, Xing H, Yang X, Li F, Yao S, Zhang H, Zhao H, Hacker M, Huo L, Li X. Fibroblast imaging of hepatic carcinoma with (68)Ga-FAPI-04 PET/CT: a pilot study in patients with suspected hepatic nodules. Eur J Nucl Med Mol Imaging. 2021;48:196-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 90] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 27. | Shi X, Xing H, Yang X, Li F, Yao S, Congwei J, Zhao H, Hacker M, Huo L, Li X. Comparison of PET imaging of activated fibroblasts and (18)F-FDG for diagnosis of primary hepatic tumours: a prospective pilot study. Eur J Nucl Med Mol Imaging. 2021;48:1593-1603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 109] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 28. | Röhrich M, Naumann P, Giesel FL, Choyke PL, Staudinger F, Wefers A, Liew DP, Kratochwil C, Rathke H, Liermann J, Herfarth K, Jäger D, Debus J, Haberkorn U, Lang M, Koerber SA. Impact of (68)Ga-FAPI PET/CT Imaging on the Therapeutic Management of Primary and Recurrent Pancreatic Ductal Adenocarcinomas. J Nucl Med. 2021;62:779-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 152] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 29. | Kömek H, Can C, Güzel Y, Oruç Z, Gündoğan C, Yildirim ÖA, Kaplan İ, Erdur E, Yıldırım MS, Çakabay B. (68)Ga-FAPI-04 PET/CT, a new step in breast cancer imaging: a comparative pilot study with the (18)F-FDG PET/CT. Ann Nucl Med. 2021;35:744-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 101] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 30. | Zheng W, Liu L, Feng Y, Wang L, Chen Y. Comparison of 68 Ga-FAPI-04 and fluorine-18-fluorodeoxyglucose PET/computed tomography in the detection of ovarian malignancies. Nucl Med Commun. 2023;44:194-203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 23] [Article Influence: 11.5] [Reference Citation Analysis (0)] |