Published online Sep 18, 2023. doi: 10.13105/wjma.v11.i6.277
Peer-review started: February 28, 2023
First decision: March 24, 2023
Revised: May 17, 2023
Accepted: June 16, 2023
Article in press: June 16, 2023
Published online: September 18, 2023
Processing time: 196 Days and 14.8 Hours
Percutaneous endoscopic gastrostomy (PEG) and percutaneous radiological gastrostomy (PRG) are minimally invasive techniques commonly used for prolonged enteral nutrition. Despite safe, both techniques may lead to complications, such as bleeding, infection, pain, peritonitis, and tube-related complications. The literature is unclear on which technique is the safest.
To establish which approach has the lowest complication rate.
A database search was performed from inception through November 2022, and comparative studies of PEG and PRG were selected following the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines. All included studies compared the two techniques directly and provided absolute values of the number of complications. Studies with pediatric populations were excluded. The primary outcome of this study was infection and bleeding. Pneumonia, peritonitis, pain, and mechanical complications were secondary outcomes. The risk of bias was assessed using the Cochrane risk-of-bias tool for randomized trials (RoB2) and we used The Risk of Bias in Nonrandomized Studies (ROBINS-I) to analyze the retrospective studies. We also performed GRADE analysis to assess the quality of evidence. Data on risk differences and 95% confidence intervals were obtained using the Mantel-Haenszel test.
Seventeen studies were included, including two randomized controlled trials and fifteen retrospective cohort studies. The total population was 465218 individuals, with 273493 having undergone PEG and 191725 PRG. The only outcome that showed a significant difference was tube related complications in retrospective studies favoring PEG (95%CI: 0.03 to 0.08; P < 0.00001), although this outcome did not show significant difference in randomized studies (95%CI: -0.07 to 0.04; P = 0.13). There was no difference in the analyses of the following outcomes: infection in retrospective (95%CI: -0.01 to 0.00; P < 0.00001) or randomized (95%CI: -0.06 to 0.04; P = 0.44) studies; bleeding in retrospective (95%CI: -0.00 to 0.00; P < 0.00001) or randomized (95%CI: -0.06 to 0.02; P = 0.43) studies; pneumonia in retrospective (95%CI: -0.04 to 0.00; P = 0.28) or randomized (95%CI: -0.09 to 0.11; P = 0.39) studies; pain in retrospective (95%CI: -0.05 to 0.02; P < 0.00001) studies; peritonitis in retrospective (95%CI: -0.02 to 0.01; P < 0.0001) studies.
PEG has lower levels of tube-related complications (such as dislocation, leak, obstruction, or breakdown) when compared to PRG.
Core Tip: Gastrostomy is a routine and preferred feeding route in patients who require enteral nutrition for prolonged period. This metanalysis compared percutaneous endoscopic gastrostomy and percutaneous radiological gastrostomy multiple outcomes, such as bleeding, infection, pneumonia, pain, and tube-related complications. Based on this meta-analysis, gastrostomy technique is related to a lower complication rate of tube-related complications and thus, should be preferred. Costs, devices availability, personal and local experience as well as patients preference should be considered when choose the best technique.
- Citation: dos Santos ESV, de Oliveira GHP, de Moura DTH, Hirsch BS, Trasolini RP, Bernardo WM, de Moura EGH. Endoscopic vs radiologic gastrostomy for enteral feeding: A systematic review and meta-analysis. World J Meta-Anal 2023; 11(6): 277-289
- URL: https://www.wjgnet.com/2308-3840/full/v11/i6/277.htm
- DOI: https://dx.doi.org/10.13105/wjma.v11.i6.277
Patients unable to tolerate oral intake for a prolonged period have an indication for an alternative route of enteral feeding, such as gastrostomy[1]. Gastrostomy involves connecting the stomach to an outflow in the skin with a tube, providing an alimentary route.
The first gastrostomy was performed in the 19th century, and Stamm's technique, surgical gastrostomy described in 1894, was long considered standard for performing a prolonged enteric access. The surgical technique became less performed with the emergence of the endoscopic technique. The method of percutaneous endoscopic gastrostomy (PEG) was first used in 1980 by Gauderer and Ponsky[2]. The technique was developed as a minimally invasive feeding route for neurologically impaired patients.
In 1981, percutaneous radiologic gastrostomy (PRG) was described[3], expanding the options available. This was an important development for scenarios such as head and neck tumors, where endoscopy is sometimes not an option, due to upper obstruction.
Endoscopic and radiological gastrostomy are both considered effective, safe and minimally invasive[4,5]. The preferred method is often based on specialist opinion or institution preference. We aim to perform a systematic review of the literature and meta-analysis to establish which approach has the lowest complication rate.
This study was performed in conformity with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines[6] and was registered in the International Prospective Register of Systematic Reviews (PROSPERO) under the file number CRD42022377213.
The electronic databases searched were MEDLINE (via PubMed), Embase, Scopus, LILACS, the Cochrane Library (via BVS), and Google Scholar from inception until November 2022. The search was performed with the following mesh terms: [(Gastrostomy or Gastrostomies) and (Endoscopic)].
The selection criteria were studies that contained patients undergoing gastrostomy, that compared the two interventions (PEG and PRG) and that included the following outcomes: Bleeding, infection, pain, peritonitis, tube-related complications with their results in absolute values.
Eligibility assessment was performed independently and standardized by 2 authors according to PRISMA guidelines[6]. Discrepancies between reviewers were resolved by consensus. A third reviewer was consulted in case of disagree
Case reports, reviews and letters were excluded. Studies that exclusively analyzed patients under 18 years of age, compared other techniques or did not consider the desired outcomes were excluded. Studies with the pediatric population were excluded because of anatomical differences with the adult population and consequently different complications.
To assess the quality of eligible studies we used The Risk of Bias in Nonrandomized Studies (ROBINS-I)[7] to analyze the comparative studies and the Cochrane risk-of-bias tool for randomized trials (RoB2)[8] to analyze the randomized studies. The quality of evidence was assessed using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) criteria using the GRADE pro Guideline Development Tool software (Mc Master University, Ontario, Canada)[9].
The randomized controlled trials (RCT) studies were analyzed separately from the observational studies since they have different levels of evidence. This allowed us to compare the outcomes separately and to make a global analysis of the results.
The analysis was performed using Review Manager (RevMan 5.4) from the Cochrane Informatics & Knowledge Management Department website. Risk differences for dichotomous variables were computed using a fixed-effects model and the respective forest and funnel plots were obtained. Data on risk differences and the 95% confidence intervals (CI) for each outcome were calculated using the Mantel-Haenszel test. Inconsistency (heterogeneity) was qualified and reported using the Chi-squared (Chi2) and Higgins methods and was termed I2. I2 values > 50% were considered to indicate substantial heterogeneity. We performed an analysis using a funnel plot to identify possible outliers. If the sample became homogeneous after excluding possible outliers, the studies were permanently excluded. We used random effects to reduce the influence of heterogeneity on the final result[10]. Outcome measures are described as the mean difference or risk difference (RD), with their corresponding 95%CI.
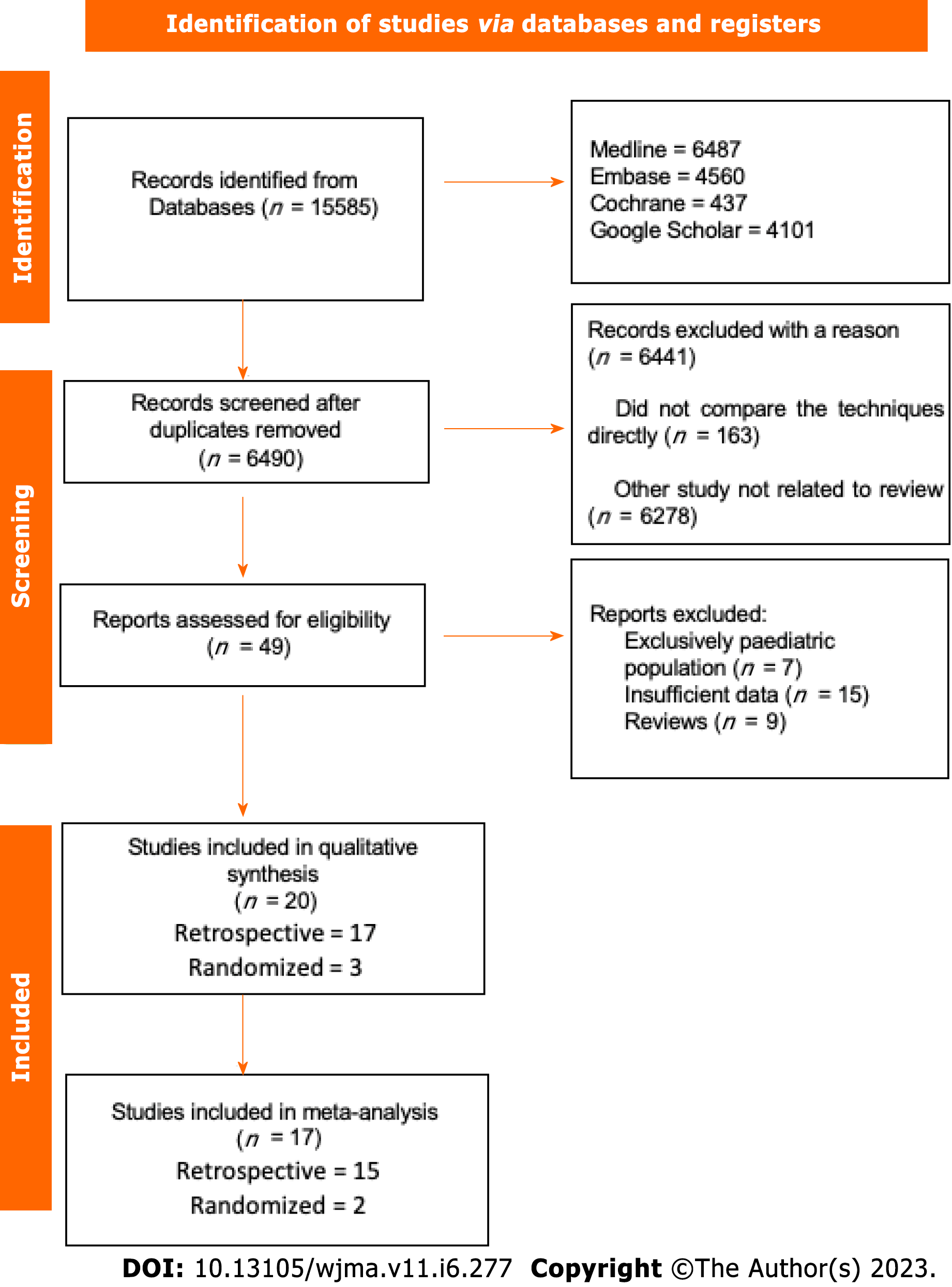
The initial search showed 15585 results, after removing the duplicate articles, 6490 remained. A total of twenty studies passed the screening stage and were included in qualitative synthesis, seventeen studies met criteria to be included in the metanalysis, two were prospective randomized studies and fifteen were retrospective cohort studies. The search strategy can be visualized in the following diagram (Figure 1).
Seventeen studies were included in the systematic review, including two RCTs, one prospective, and 14 retrospective cohort studies. A total of 465218 individuals, with 273493 received PEG and 191725 PRG. The characteristics of the studies can be seen in Table 1[11-27]. Early outcomes were analyzed.
| Ref. | Country | Design | Period | PEG (N) | RIG (N) | Mean age PEG | Mean age RIG | Single (S) or Multicenter (M) |
| Hoffer et al[11], 1999 | United States | Randomized | 1993-1994 | 69 | 66 | 58.2 | 51.9 | S |
| Möller et al[12], 1999 | Sweden | Retrospective | 1990-1994 | 12 | 94 | 48 | 64 | S |
| Laasch et al[13], 2002 | United Kingdom | Prospective | 2000-2002 | 50 | 50 | 73 | 68 | M (3) |
| Silas et al[14], 2005 | United States | Retrospective | 1997-2001 | 177 | 193 | 68 | 63 | S |
| Rustom et al[15], 2006 | United Kingdom | Retrospective | 2002-2005 | 40 | 28 | 63.6 | 64.8 | S |
| Galaski et al[16], 2009 | Canada | Retrospective | 2004-2005 | 30 | 44 | 55 | 65 | S |
| La Nauze et al[17], 2012 | Australia | Retrospective | 2007-2009 | 80 | 97 | 61 | 61 | S |
| Rio et al[18], 2010 | United Kingdom | Retrospective | 1999-2006 | 21 | 122 | 64 | 64 | S |
| Lewis et al[19], 2014 | United Kingdom | Randomized | 2012-2013 | 34 | 31 | 73 | 71 | S |
| ProGas Study Group[20], 2015 | United Kingdom | Retrospective | 2010-2014 | 121 | 163 | 64.2 | 63.6 | M (24) |
| Vidhya et al[21], 2018 | Australia | Retrospective | 2013-2015 | 85 | 52 | 65 | 64 | S |
| Park et al[22], 2019 | South Korea | Retrospective | 2010-2015 | 324 | 94 | 66 | 66.2 | M (5) |
| Strijbos et al[23], 2019 | Netherlands | Retrospective | 2008-2016 | 291 | 469 | 66 | 66.2 | S |
| Lainez et al[24], 2020 | Spain | Retrospective | 2019 | 25 | 23 | 63.98 | 62.41 | S |
| Maasarani et al[25], 2020 | United States | Retrospective | 2004-2014 | 232164 | 26477 | NI | NI | M |
| Kohli et al[26], 2020 | United States | Retrospective | 2014-2017 | 16384 | 154007 | 53.7 | 67.2 | M |
| Kohli et al[27], 2021 | United States | Retrospective | 2011-2021 | 23566 | 9715 | 70.7 | 69.6 | M |
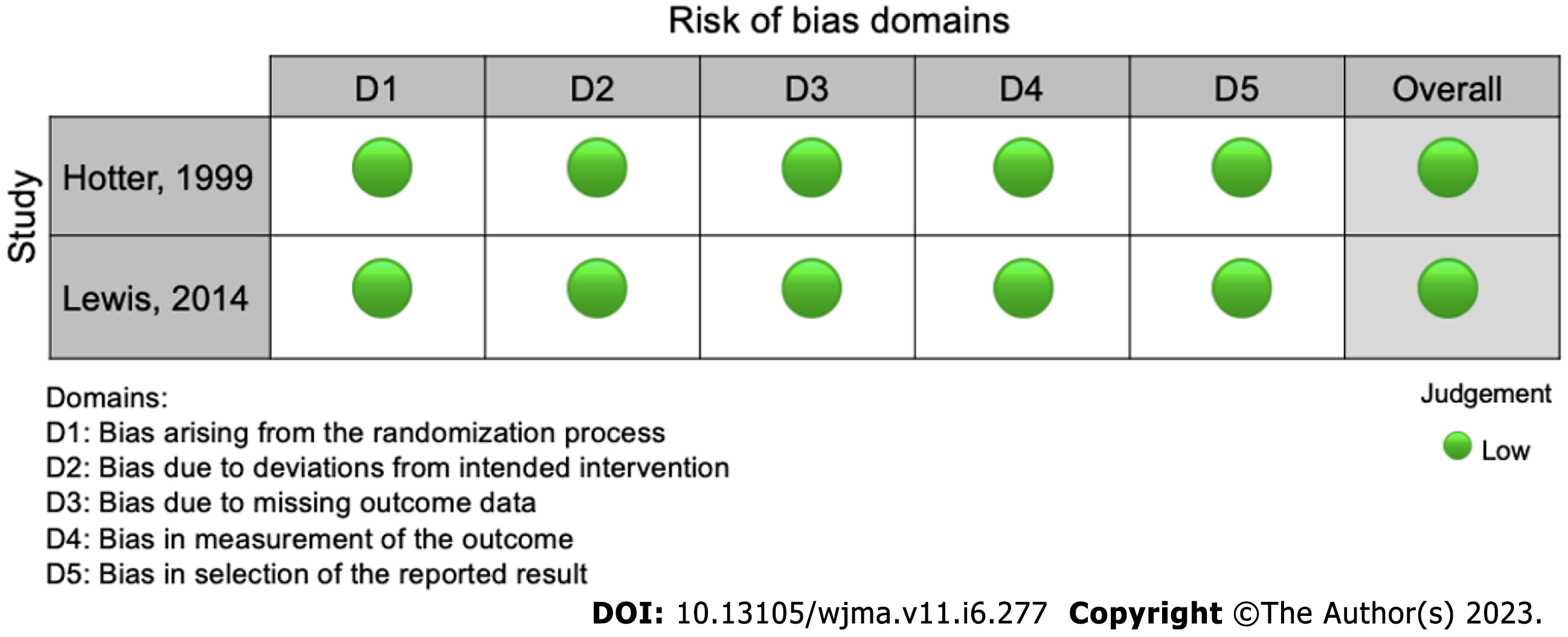
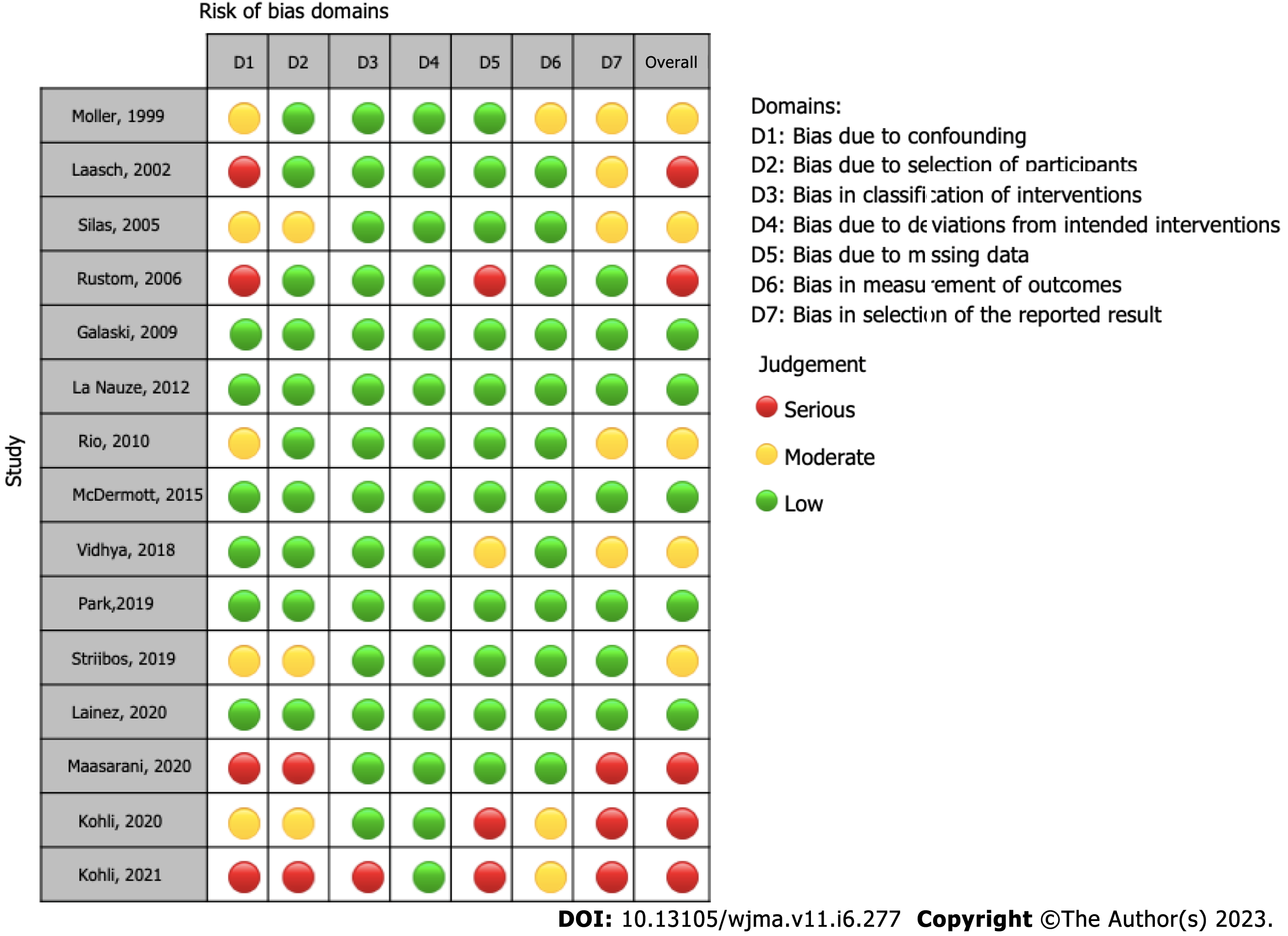
The ROBINS-I and ROB-2 scoring system were used to evaluate risk of bias for observational[12-18,20-27] and randomized studies[11,19], respectively (Table 1). We identified a low risk of bias in the two RCT studies (Figure 2), and a strong methodological quality. As for the observational studies, we note that 5 of them present serious risk of bias[13,15,25,27] and 5 moderate risk[12,14,18,21,23], mostly due to issues in the dissemination of results (Figure 3).
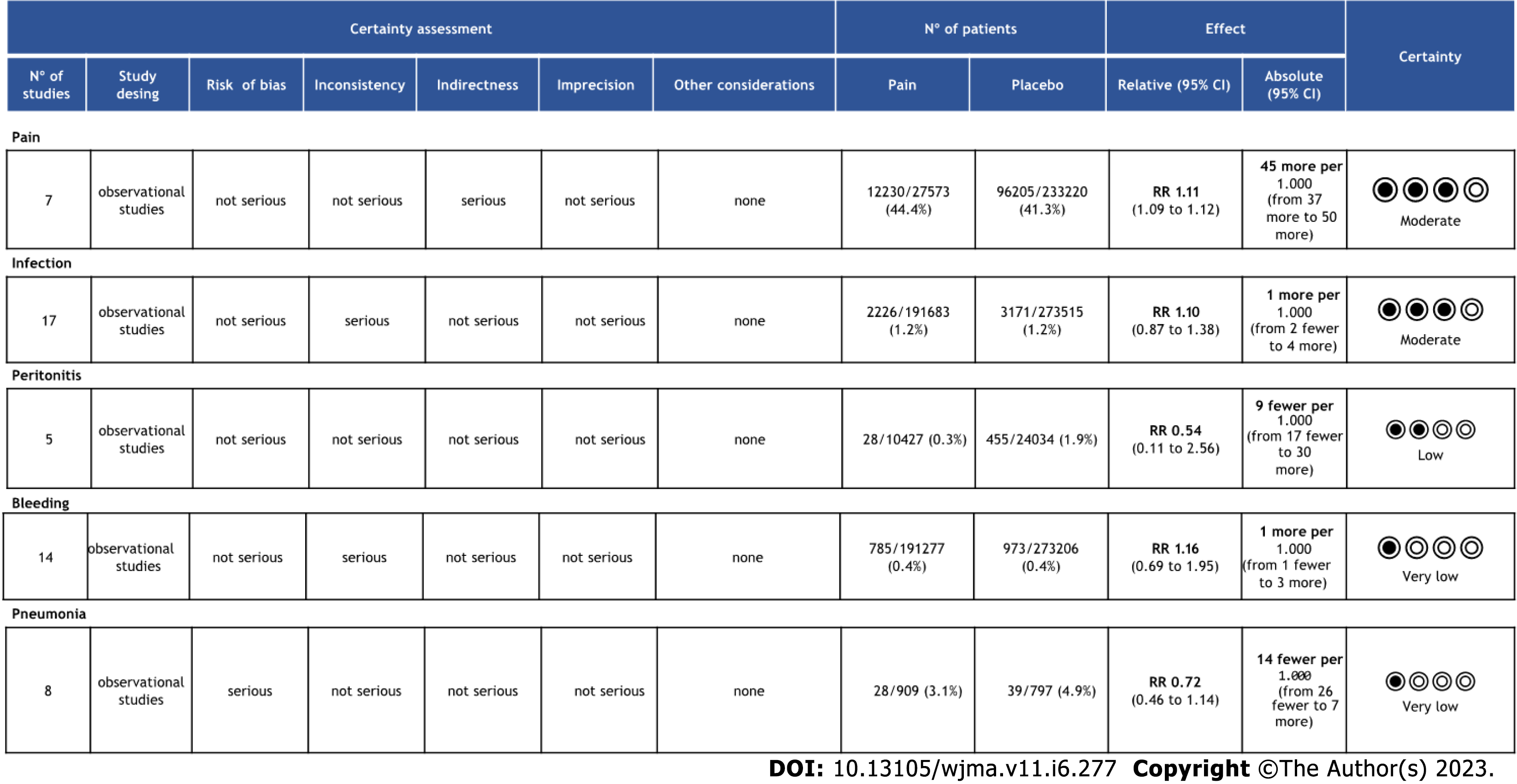
The objective criteria of GRADE analysis to evaluate the quality of evidence identified moderate certainty for pain and infection, low certainty for peritonitis and very low certainty for bleeding and pneumonia (Figure 4).
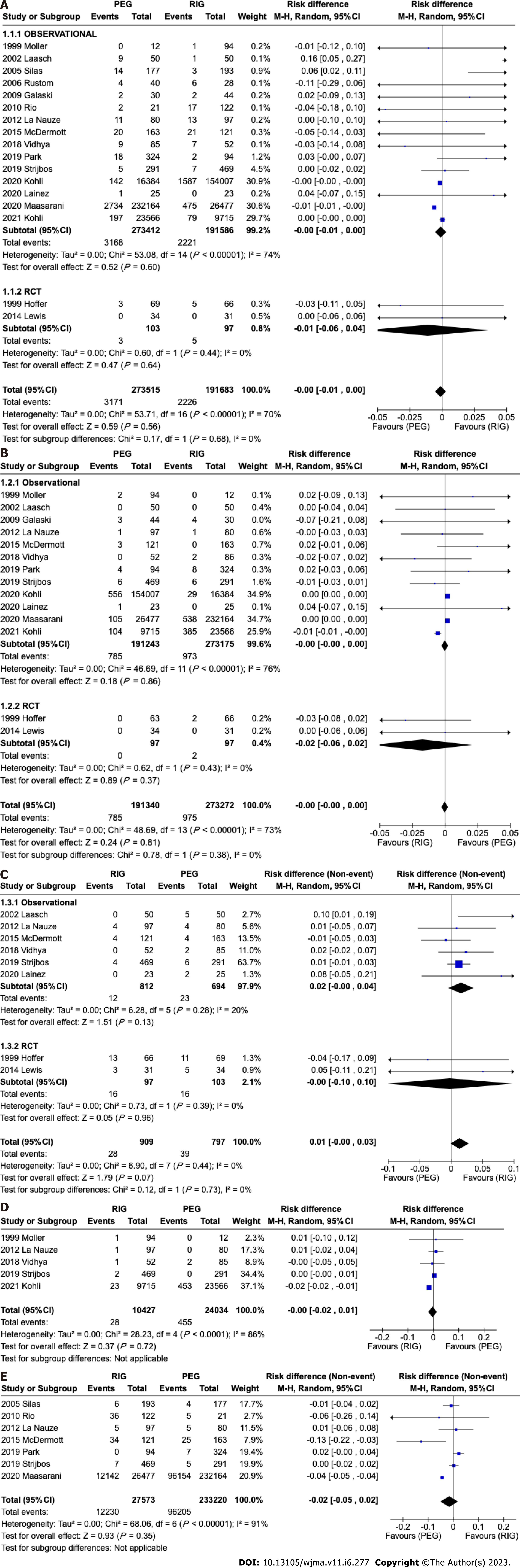
A total of 465198 patients from 17 studies[12-27] were analyzed. There was no difference in the incidence of infection in retrospective (95%CI: -0.01 to 0.00; P < 0.00001; I2 = 74%) or randomized (95%CI: -0.06 to 0.04; P = 0.68; I2 = 0%) studies. In the overall analysis there was no difference in the meta-analysis of observational and RCT studies combined (95%CI: -0.01 to 0.00; P = 0.56; I2 = 70%) (Figure 5A).
A total of 464618 patients from fourteen[11-13,16,17,19-27] studies were analyzed. There was no difference in the incidence of bleeding in observational studies (95% CI: -0.00 to 0.00; P < 0.00001; I2 = 76%) or RCTs (95%CI: -0.06 to 0.02; P = 0.43; I2 = 0%). In the overall analysis there was no difference in the meta-analysis of observational and RCT studies combined (95%CI: -0.00 to 0.00); P = 0.81; I2 = 73%) (Figure 5B).
A total of 1796 patients from eight[11,13,17,19-21,23,24] studies were analyzed. There was no difference in the incidence of pneumonia in comparative studies (95%CI: -0.00 to 0.04; P = 0.28; I2 = 20%) or RCT (95%CI: -0.10 to 0.10; P = 0.39; I2 = 0%) studies. In the overall analysis there was no difference in the meta-analysis of observational and RCT studies combined (95%CI: -0.00 to 0.03; P = 0.44; I2 = 0%) (Figure 5C).
A total of 34461 patients from five[12,17,21,23,27] were analyzed. There was no difference in the incidence of peritonitis in retrospective (95%CI: -0.02 to 0.01; P < 0.0001; I2 = 86%) studies. It was not possible to evaluate the peritonitis outcome in RCT studies because this outcome was not included in these studies (Figure 5D).
A total of 260793 patients from seven[14,17,18,20,22,23,25] studies were analyzed. There was no difference in the incidence of pain in retrospective (95%CI: -0.05 to 0.02; P < 0.00001; I2 = 91%) studies. It was not possible to evaluate the pain outcome in RCT studies because this outcome was not included in these studies (Figure 5E).
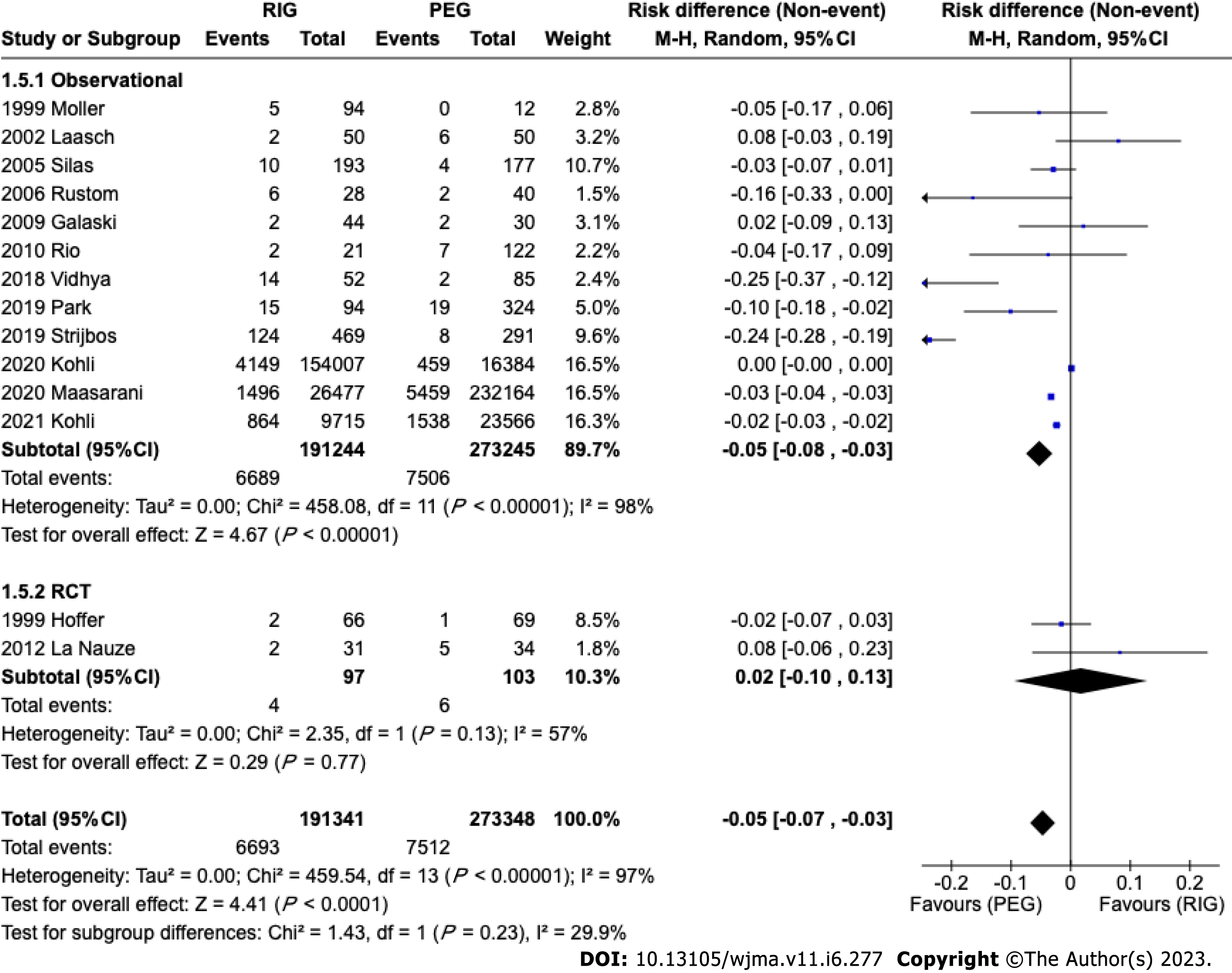
A total of 464689 patients from 14 studies[11-19,21-23,25,26] were analyzed. This analysis showed a significant difference in tube related complications in observational studies favoring PEG (95%CI: -0.03 to -0.08; P < 0.00001), although there was no significant difference in randomized studies (95%CI: -0.07 to 0.04; P = 0.13). In the global analysis there was a difference, favoring PEG (95%CI: -0.07 to -0.03; P < 0.00001) (Figure 6).
This meta-analysis shows that both PEG and PRG techniques are similar in terms of safety profile, except potentially in tube-related complications, which was higher for PRG in observational studies (Evidence 2A). We included 20 studies in this review (3 randomized and 17 comparative studies) and 17 in our meta-analysis, totaling 465218 individuals, with 273493 undergoing PEG and 191725 undergoing PRG. While other metanalyses compared these 2 approaches[28-34], this analysis is unique as it includes the largest number of adult patients and also separates RCT and observational studies providing further insight. This approach follows Cochrane recommendations and thus provides for a more reliable comparison. Additionally, we separated all adverse events, including pain and pneumonia, which have not been individually analyzed to date. The adverse effects chosen were based on previous publications showing the most frequent complications related to the method[4].
The three most common techniques for performing gastrostomy are endoscopic, radiologic, and surgical. Although surgical gastrostomy was the first described approach, it is now less used due to its invasiveness. A meta-analysis including RCT (evidence 1A) comparing endoscopic and surgical techniques demonstrated a lower number of minor complications for endoscopic procedures[35].
Until now, there is no consensus regarding the superiority of either endoscopic or radiologic gastrostomy. Our results clarify that both approaches are similar in terms of safety as shown in our meta-analysis including only RCTs. Furthermore, a recent RCT including 42 patients comparing the two techniques[36], showed similar results to this meta-analysis. Unfortunately, this RCT was not included due to a lack of data available in the published manuscript, despite our attempt to contact the author.
Local infection is a common adverse outcome of gastrostomy. For this reason, the American Society for Gastrointestinal Endoscopy[37] and the Society for Interventional Radiology[38,39] recommends administering periprocedural antibiotics. The studies utilized in this meta-analysis did not expressly state if antibiotics were administered or not, but as this is a common practice, it was likely used. Our meta-analysis did not demonstrate a significant difference regarding infection in both RCT and non-RCT analysis.
In previous publications[26,27], it has been stated that patients undergoing PEG have a higher rate of bleeding since PEG is preferentially performed in patients with diseases requiring antiplatelets or anticoagulants such as stroke and vascular dementia[27,40]. We expected to prove this hypothesis, however, this meta-analysis demonstrated a low risk of bleeding due to the gastrostomy procedure, without a statistically significant difference between PEG and PRG in both RCT and observational studies. Data on antiplatelet and/or anticoagulant medications among patients who bled were not available.
This study showed no significant difference in the incidence of pneumonia. In previous studies it was observed that gastrostomy compared to nasogastric feeding has a lower incidence of pneumonia, however, this complication is a major cause of mortality in patients undergoing gastrostomy[16]. It is important to state that we were not able to evaluate gastrostomy and gastrojejunostomy separately due to a lack of data. Gastrojejunostomy is associated with a theoretically lower rate of reflux and pneumonia[11,19].
Pain and peritonitis are complex outcomes to measure objectively. Since the definition of these outcomes differs in several studies[13,14,17,18,20-25]. There was no statistical difference between the two methods in our study.
In the analyzed studies, the types, brands, and sizes of tubes were not differentiated. This heterogeneity may influence the results of this analysis. The meta-analysis of observational studies demonstrated a statistically significant difference in the incidence of tube-related complications of a PEG and PRG, such as dislocation, leak, obstruction, or breakdown, showing a higher incidence in PRG. In the RCT meta-analysis, there was no difference. However, the observational studies included 464489 patients versus 200 patients from RCT studies and this should be considered if the RCTs were underpowered to detect a small difference between the techniques. A difference may be expected due to the size difference between endoscopic and radiological techniques. PEG is usually performed using 20FR or 24FR tubes whereas PRG uses 14-16 FR[41]. The size of the gastrostomy ostium influences the incidence of migration; a smaller caliber is associated with a higher incidence of migration and obstruction. The feeding tube can become blocked due to various reasons, such as the accumulation of food formula, medications, or debris. Smaller tubes increase the probability of the tube becoming blocked. Leaks can occur around the insertion site or through the tube itself, which can cause skin irritation and infection, so if the size of the skin insertion is larger than the tube caliber there is a greater chance of leakage.
Tube-related complications are usually associated with longer hospital stays, the need for further procedures, and potentially increased costs[16,33,42]. Evaluating costs is challenging since procedure cost varies significantly between countries. A study comparing the two techniques published in 2009 showed that the costs of the procedures are also different, with PEGs being 43% more expensive than PRGs[16] but the costs are related only to the procedure and not to the overall cost. In Brazil, PEG has a low cost, being more cost-effective than a CT scan. Although few studies provide information regarding costs, this information would be useful, given that these procedures are performed on a large scale worldwide[11,16].
The strengths of this study include a large number of patients from different continents, dedicated analysis of RCT data, use of a validated quality assessment tool, and application of the GRADE process to assess the quality of our data.
Although systematic review and meta-analysis represent the most thorough assessment of available evidence comparing the risks of PEG and PRG, our study has limitations as discussed above. Most data was gathered from observational studies. Additionally, lack of data on tube size, antibiotic, and anticoagulant use, indications for the gastrostomy procedure, and inclusion of both gastrostomy and gastrojejunostomy all limit understanding of potential nuances that differentiate PEG from PRG.
In summary, both approaches are safe. Thus, individual evaluation is required considering several factors including local and personal experience, device availability, cost, and patient preference.
PEG and PRG present a similar safety profile. However, PRG is associated with a slightly higher rate of tube-related complications, potentially related to the small caliber of the gastrostomy tube.
Gastrostomy feeding is superior to nasogastric tube feeding when medium to long-term enteral feeding (≥ 4 wk) is indicated. The optimal technique for long-term enteral feeding is not yet well established. Therefore, we performed a meta-analysis comparing the two methods.
This paper motivation is to demonstrate which technique for performing a gastrostomy has the lowest incidence rate of adverse events.
The aim of the paper is to compare the technique of endoscopic gastrostomy (PEG) and gastrostomy via interventional radiology (PRG) and establish which technique is the safest for the patient.
Comparative studies of PEG and PRG were selected. Included studies had outcomes such as infection, bleeding, pneumonia, pain, peritonitis and tube related complications. The risk of bias and quality of evidence were assessed. The analysis was performed using Review Manager (RevMan 5.4) from the Cochrane Informatics & Knowledge Management Department website.
Seventeen studies were included, with a total of 465218 patients. The only outcome that showed a significant difference was tube-related complications in retrospective studies favoring PEG (95%CI: 0.03 to 0.08; P < 0.00001), although this outcome did not show significant difference in randomized studies (95%CI: -0.07 to 0.04; P = 0.13). There was no difference in the analyses of the following outcomes: Infection in retrospective (95%CI: -0.01 to 0.00; P < 0.00001) or randomized (95%CI: -0.06 to 0.04; P = 0.44) studies; bleeding in retrospective (95%CI: -0.00 to 0.00; P < 0.00001) or randomized (95%CI: -0.06 to 0.02; P = 0.43) studies; pneumonia in retrospective (95%CI: -0.04 to 0.00; P = 0.28) or randomized (95%CI: -0.09 to 0.11; P = 0.39) studies; pain in retrospective (95%CI: -0.05 to 0.02; P < 0.00001) studies; peritonitis in retrospective (95%CI: -0.02 to 0.01; P < 0.0001) studies.
The study concluded that RIG has a higher incidence of tube-related complications than PEG. This difference is probably associated with the caliber of the tubes used. There was no statistical difference in the other outcomes evaluated.
This study aimed to determine which technique is safer for the patient, and both methods proved to be safe. We can conclude that the choice of technique depends on the type of patient, the experience of the service, the cost, and the availability of the method.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Homan M, Slovenia; Konishi H, Japan S-Editor: Liu JH L-Editor: A P-Editor: Zhao S
| 1. | Gramlich L, Kichian K, Pinilla J, Rodych NJ, Dhaliwal R, Heyland DK. Does enteral nutrition compared to parenteral nutrition result in better outcomes in critically ill adult patients? A systematic review of the literature. Nutrition. 2004;20:843-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 365] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 2. | Gauderer MW, Ponsky JL, Izant RJ Jr. Gastrostomy without laparotomy: a percutaneous endoscopic technique. J Pediatr Surg. 1980;15:872-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1408] [Cited by in RCA: 1299] [Article Influence: 28.9] [Reference Citation Analysis (1)] |
| 3. | Preshaw RM. A percutaneous method for inserting a feeding gastrostomy tube. Surg Gynecol Obstet. 1981;152:658-660. [PubMed] |
| 4. | Rahnemai-Azar AA, Rahnemaiazar AA, Naghshizadian R, Kurtz A, Farkas DT. Percutaneous endoscopic gastrostomy: indications, technique, complications and management. World J Gastroenterol. 2014;20:7739-7751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 416] [Cited by in RCA: 337] [Article Influence: 30.6] [Reference Citation Analysis (8)] |
| 5. | Leeds JS, McAlindon ME, Grant J, Robson HE, Lee FK, Sanders DS. Survival analysis after gastrostomy: a single-centre, observational study comparing radiological and endoscopic insertion. Eur J Gastroenterol Hepatol. 2010;22:591-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 47203] [Article Influence: 2950.2] [Reference Citation Analysis (0)] |
| 7. | Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan AW, Churchill R, Deeks JJ, Hróbjartsson A, Kirkham J, Jüni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schünemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JP. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7683] [Cited by in RCA: 10888] [Article Influence: 1209.8] [Reference Citation Analysis (2)] |
| 8. | Cochrane HandbookforSystematicReviewsofInterventionsversion6.0 Internet]. London (UK); c2019 [Cited 2021 Apr 16]. Available from: https://training.cochrane.org/handbook. |
| 9. | Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ; GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11058] [Cited by in RCA: 14941] [Article Influence: 878.9] [Reference Citation Analysis (0)] |
| 10. | Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4895] [Cited by in RCA: 6906] [Article Influence: 345.3] [Reference Citation Analysis (0)] |
| 11. | Hoffer EK, Cosgrove JM, Levin DQ, Herskowitz MM, Sclafani SJ. Radiologic gastrojejunostomy and percutaneous endoscopic gastrostomy: a prospective, randomized comparison. J Vasc Interv Radiol. 1999;10:413-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 52] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Möller P, Lindberg CG, Zilling T. Gastrostomy by various techniques: evaluation of indications, outcome, and complications. Scand J Gastroenterol. 1999;34:1050-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Laasch HU, Wilbraham L, Bullen K, Marriott A, Lawrance JA, Johnson RJ, Lee SH, England RE, Gamble GE, Martin DF. Gastrostomy insertion: comparing the options--PEG, RIG or PIG? Clin Radiol. 2003;58:398-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 81] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Silas AM, Pearce LF, Lestina LS, Grove MR, Tosteson A, Manganiello WD, Bettmann MA, Gordon SR. Percutaneous radiologic gastrostomy versus percutaneous endoscopic gastrostomy: a comparison of indications, complications and outcomes in 370 patients. Eur J Radiol. 2005;56:84-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 107] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 15. | Rustom IK, Jebreel A, Tayyab M, England RJ, Stafford ND. Percutaneous endoscopic, radiological and surgical gastrostomy tubes: a comparison study in head and neck cancer patients. J Laryngol Otol. 2006;120:463-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 87] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 16. | Galaski A, Peng WW, Ellis M, Darling P, Common A, Tucker E. Gastrostomy tube placement by radiological versus endoscopic methods in an acute care setting: a retrospective review of frequency, indications, complications and outcomes. Can J Gastroenterol. 2009;23:109-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 17. | La Nauze RJ, Collins K, Lyon S, Bailey M, Kemp W, Nyulasi I, Roberts SK. Outcomes of percutaneous endoscopic gastrostomy vs radiologically inserted gastrostomy tube insertion at a tertiary hospital. e-SPEN J. 2012;7:e144-e148. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Rio A, Ellis C, Shaw C, Willey E, Ampong MA, Wijesekera L, Rittman T, Nigel Leigh P, Sidhu PS, Al-Chalabi A. Nutritional factors associated with survival following enteral tube feeding in patients with motor neurone disease. J Hum Nutr Diet. 2010;23:408-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Lewis S, Jackson S, Latchford A. Randomized Study of Radiologic vs Endoscopic Placement of Gastrojejunostomies in Patients at Risk of Aspiration Pneumonia. Nutr Clin Pract. 2014;29:498-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | ProGas Study Group. Gastrostomy in patients with amyotrophic lateral sclerosis (ProGas): a prospective cohort study. Lancet Neurol. 2015;14:702-709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 116] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 21. | Vidhya C, Phoebe D, Dhina C, Jayne S, Robert F. Percutaneous endoscopic gastrostomy (PEG) versus radiologically inserted gastrostomy (RIG): A comparison of outcomes at an Australian teaching hospital. Clin Nutr ESPEN. 2018;23:136-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | Park SK, Kim JY, Koh SJ, Lee YJ, Jang HJ, Park SJ; Small Intestine and Nutrition Research Group of the Korean Association for the Study of Intestinal Diseases (KASID). Complications of percutaneous endoscopic and radiologic gastrostomy tube insertion: a KASID (Korean Association for the Study of Intestinal Diseases) study. Surg Endosc. 2019;33:750-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 23. | Strijbos D, Keszthelyi D, Gilissen LPL, Lacko M, Hoeijmakers JGJ, van der Leij C, de Ridder RJJ, de Haan MW, Masclee AAM. Percutaneous endoscopic versus radiologic gastrostomy for enteral feeding: a retrospective analysis on outcomes and complications. Endosc Int Open. 2019;7:E1487-E1495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 24. | Lainez LM, Florencio Ojeda L, Ternero Fonseca J, Maraver Zamora M, Rebollo P, M. I. Percutaneous endoscopic gastrostomy (PEG) vs radiologic percutaneous gastrostomy (RPG): comparison of the results in our center in the last year. Clinical Nutrition ESPEN, 2020; 40: 678. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 25. | Maasarani S, Khalid SI, Creighton C, Manatis-Lornell AJ, Wiegmann AL, Terranella SL, Skertich NJ, DeCesare L, Chan EY. Outcomes following percutaneous endoscopic gastrostomy versus fluoroscopic procedures in the Medicare population. Surg Open Sci. 2021;3:2-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 26. | Kohli DR, Kennedy KF, Desai M, Sharma P. Safety of endoscopic gastrostomy tube placement compared with radiologic or surgical gastrostomy: nationwide inpatient assessment. Gastrointest Endosc. 2021;93:1077-1085.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 27. | Kohli DR, Kennedy KF, Desai M, Sharma P. Comparative Safety of Endoscopic vs Radiological Gastrostomy Tube Placement: Outcomes From a Large, Nationwide Veterans Affairs Database. Am J Gastroenterol. 2021;116:2367-2373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 28. | Bravo JG, Ide E, Kondo A, de Moura DT, de Moura ET, Sakai P, Bernardo WM, de Moura EG. Percutaneous endoscopic versus surgical gastrostomy in patients with benign and malignant diseases: a systematic review and meta-analysis. Clinics (Sao Paulo). 2016;71:169-178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Strijbos D, Keszthelyi D, Bogie RMM, Gilissen LPL, Lacko M, Hoeijmakers JGJ, van der Leij C, de Ridder R, de Haan MW, Masclee AAM. A Systematic Review and Meta-Analysis on Outcomes and Complications of Percutaneous Endoscopic Versus Radiologic Gastrostomy for Enteral Feeding. J Clin Gastroenterol. 2018;52:753-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 30. | Mohamed Elfadil O, Linch FB, Seegmiller SL, Hurt RT, Mundi MS, Neisen MJ. Safety and effectiveness of radiologic and endoscopic percutaneous gastrostomy placement: A randomized study. JPEN J Parenter Enteral Nutr. 2022;46:1808-1817. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 31. | Wollman B, D'Agostino HB, Walus-Wigle JR, Easter DW, Beale A. Radiologic, endoscopic, and surgical gastrostomy: an institutional evaluation and meta-analysis of the literature. Radiology. 1995;197:699-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 294] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 32. | Grant DG, Bradley PT, Pothier DD, Bailey D, Caldera S, Baldwin DL, Birchall MA. Complications following gastrostomy tube insertion in patients with head and neck cancer: a prospective multi-institution study, systematic review and meta-analysis. Clin Otolaryngol. 2009;34:103-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 164] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 33. | Burkitt P, Carter LM, Smith AB, Kanatas A. Outcomes of percutaneous endoscopic gastrostomy and radiologically inserted gastrostomy in patients with head and neck cancer: a systematic review. Br J Oral Maxillofac Surg. 2011;49:516-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Yuan TW, He Y, Wang SB, Kong P, Cao J. Technical success rate and safety of radiologically inserted gastrostomy versus percutaneous endoscopic gastrostomy in motor neuron disease patients undergoing: A systematic review and meta-analysis. J Neurol Sci. 2020;410:116622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 35. | Yang B, Shi X. Percutaneous endoscopic gastrostomy versus fluoroscopic gastrostomy in amyotrophic lateral sclerosis (ALS) sufferers with nutritional impairment: A meta-analysis of current studies. Oncotarget. 2017;8:102244-102253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 36. | Lim JH, Choi SH, Lee C, Seo JY, Kang HY, Yang JI, Chung SJ, Kim JS. Thirty-day mortality after percutaneous gastrostomy by endoscopic versus radiologic placement: a systematic review and meta-analysis. Intest Res. 2016;14:333-342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 37. | ASGE Standards of Practice Committee; Khashab MA, Chithadi KV, Acosta RD, Bruining DH, Chandrasekhara V, Eloubeidi MA, Fanelli RD, Faulx AL, Fonkalsrud L, Lightdale JR, Muthusamy VR, Pasha SF, Saltzman JR, Shaukat A, Wang A, Cash BD. Antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc. 2015;81:81-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 242] [Article Influence: 24.2] [Reference Citation Analysis (2)] |
| 38. | Itkin M, DeLegge MH, Fang JC, McClave SA, Kundu S, d'Othee BJ, Martinez-Salazar GM, Sacks D, Swan TL, Towbin RB, Walker TG, Wojak JC, Zuckerman DA, Cardella JF; Society of Interventional Radiology; American Gastroenterological Association Institute; Canadian Interventional Radiological Association; Cardiovascular and Interventional Radiological Society of Europe. Multidisciplinary practical guidelines for gastrointestinal access for enteral nutrition and decompression from the Society of Interventional Radiology and American Gastroenterological Association (AGA) Institute, with endorsement by Canadian Interventional Radiological Association (CIRA) and Cardiovascular and Interventional Radiological Society of Europe (CIRSE). Gastroenterology. 2011;141:742-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 124] [Article Influence: 8.9] [Reference Citation Analysis (1)] |
| 39. | Chehab MA, Thakor AS, Tulin-Silver S, Connolly BL, Cahill AM, Ward TJ, Padia SA, Kohi MP, Midia M, Chaudry G, Gemmete JJ, Mitchell JW, Brody L, Crowley JJ, Heran MKS, Weinstein JL, Nikolic B, Dariushnia SR, Tam AL, Venkatesan AM. Adult and Pediatric Antibiotic Prophylaxis during Vascular and IR Procedures: A Society of Interventional Radiology Practice Parameter Update Endorsed by the Cardiovascular and Interventional Radiological Society of Europe and the Canadian Association for Interventional Radiology. J Vasc Interv Radiol. 2018;29:1483-1501.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 105] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 40. | Azzopardi N, Ellul P. Pneumonia and mortality after percutaneous endoscopic gastrostomy insertion. Turk J Gastroenterol. 2013;24:109-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 41. | Shin JH, Park AW. Updates on percutaneous radiologic gastrostomy/gastrojejunostomy and jejunostomy. Gut Liver. 2010;4 Suppl 1:S25-S31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 42. | Barkmeier JM, Trerotola SO, Wiebke EA, Sherman S, Harris VJ, Snidow JJ, Johnson MS, Rogers WJ, Zhou XH. Percutaneous radiologic, surgical endoscopic, and percutaneous endoscopic gastrostomy/gastrojejunostomy: comparative study and cost analysis. Cardiovasc Intervent Radiol. 1998;21:324-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 90] [Article Influence: 3.3] [Reference Citation Analysis (0)] |