Published online Apr 18, 2023. doi: 10.13105/wjma.v11.i4.92
Peer-review started: December 23, 2022
First decision: February 21, 2023
Revised: March 11, 2023
Accepted: March 29, 2023
Article in press: March 29, 2023
Published online: April 18, 2023
Processing time: 111 Days and 22.4 Hours
Diabetes is a major global public health issue. The prevalence of type 1 diabetes is comparatively static, as hereditary and genetic causes are involved, while type 2 diabetes (T2D) prevalence is increasing day by day. T2D is associated with chronic complications, including diabetic neuropathy (DN), nephropathy, retinopathy, and other complications like diabetic foot. DN is the main complication of both types of diabetes. DN can be diagnosed by routine laboratory tests, microalbuminuria > 300 mg/24 h, and a gradual decrease in glomerular filtration rate. As the appearance of microalbuminuria is a late manifestation, an early marker for renal damage is needed. Lipocalin-2, also known as neutrophil gelatinase-associated lipocalin (NGAL), is a small protein purified from neutrophil granules and a good marker for kidney disease. NGAL is a transporter protein responsible for many physiological processes, such as inflammation, generation of the immune response, and metabolic homeostasis. NGAL has been reported to depict the early changes in renal damage when urine microalbumin is still undetecable. Therefore, elucidating the role of NGAL in detecting DN and understanding its mechanism can help establish it as a potential early marker for DN.
Core Tip: Diabetic nephropathy (DN) is a chronic complication of diabetes. The mainstay markers for kidney injury are a gradual decrease in glomerular filtration rate and microalbuminuria. Microalbuminuria appears late in DN; thus, new biomarkers are required. Different researchers highlighted the role of lipocalin-2 (NGAL) in the early detection of nephropathy before the appearance of microalbumin in urine. In this review, we briefly describe the role of NGAL in various diseases and cancers and detail its role as an early biomarker in DN.
- Citation: Dahiya K, Prashant P, Dhankhar R, Dhankhar K, Kumar S, Vashist S. Lipocalin-2 as a biomarker for diabetic nephropathy. World J Meta-Anal 2023; 11(4): 92-101
- URL: https://www.wjgnet.com/2308-3840/full/v11/i4/92.htm
- DOI: https://dx.doi.org/10.13105/wjma.v11.i4.92
Diabetic nephropathy (DN) is a chronic complication of diabetes, and it affects more than 40% of both type 1 diabetes (T1D) and type 2 diabetes (T2D) cases and may lead to end-stage renal disease as reported worldwide. DN can be diagnosed clinically based on a gradual decrease in glomerular filtration rate (GFR) and an increase in urine albumin > 300 mg/24 h, which is shown to be associated with cardiovascular complications. An early diagnostic and prognostic marker is still needed to detect DN early for better treatment outcomes and predictive value[1,2].
The current diagnostic markers for DN, i.e., microalbuminuria and serum creatinine levels, have questionable reliability even when specific indicators like creatinine clearance or ratio of creatinine and albumin in 24-hour urine samples are used. Microalbuminuria can be associated with other physiological and pathological conditions such as exercise, diet, infections, and dehydration[3]. Serum creatinine levels vary according to age, gender, hydration, muscle mass, and kidney conditions, and are often elevated later in advancing disease processes. Therefore, the reliability of these markers in early renal damage detection is questionable[4-10].
The characteristics of a biomarker shall be considered to determine its usefulness. Its measurement should be easy and accurate, and results should be reproducible. It should also indicate an early renal injury, and the response to the treatment, cost-effectiveness, and availability should be taken into account. It should be able to be applied to a large population and augment the disease's clinical diagnosis and prognosis[11].
The commonly used markers for acute kidney injury (AKI) and renal dysfunctions are plenty, which may be extrapolated to DN. The biomarkers for oxidative stress include 8-hydroxy-2'-deoxyguanine (8-OHdG) as a novel but controversial marker for DNA damage; pentosidine, 2,4-dinitrophenylhydrazine, and advanced oxidation protein products for protein injury; and F2-α prostaglandin and 4-hydroxy-2-nonenal for lipid injury. The glutathione-s-transferase, an enzyme-like protein, is a marker for the glutathione antioxidant system. Some other biomarkers of inflammation, like cytokines and a variety of chemokines, are essential biomarkers for AKI and kidney dysfunction and include interleukin-8 (IL-8), tumor necrosis factor-α (TNF-α), monocyte chemoattractant protein-1 (MCP-1), and interferon-inducible protein-10 (IP-10). The renin-angiotensin-aldosterone system biomarkers are also used as kidney injury markers[12-14].
Biomarkers for damage to glomerular filtration membranes include urinary mRNA levels of podocin, synaptopodin, and nephrin. The levels of basement membrane injury markers like type IV collagen are substantially higher before microalbuminuria and and serum creatinine abnormality appear[15,16]. The biomarkers for endothelial cell injury, like vascular endothelial growth factor, von Willebrand factor (vWF), and intercellular adhesion molecule-1(ICAM-1), are found raised in patients with DN[17-20]. The biomarkers for mesangial expansion and fibrosis are also crucial, as DN is seen with extracellular matrix alterations and mesangial expansion, e.g., transforming growth factor-β1 (TGF-β1) and pigment epithelial-derived factor[21,22]. The alteration of renal function is associated with glomerular and renal tubular dysfunction[23]. Transferrin, ceruloplasmin, and immunoglobulin G are early biomarkers for glomerular dysfunction. The renal tubular dysfunction markers include α-1 microglobulin, retinol-binding protein 4, lipocalin-2, N-acetyl-β-D-glucosidase, kidney injury molecule-1, and heart-type fatty acid binding protein[24-27].
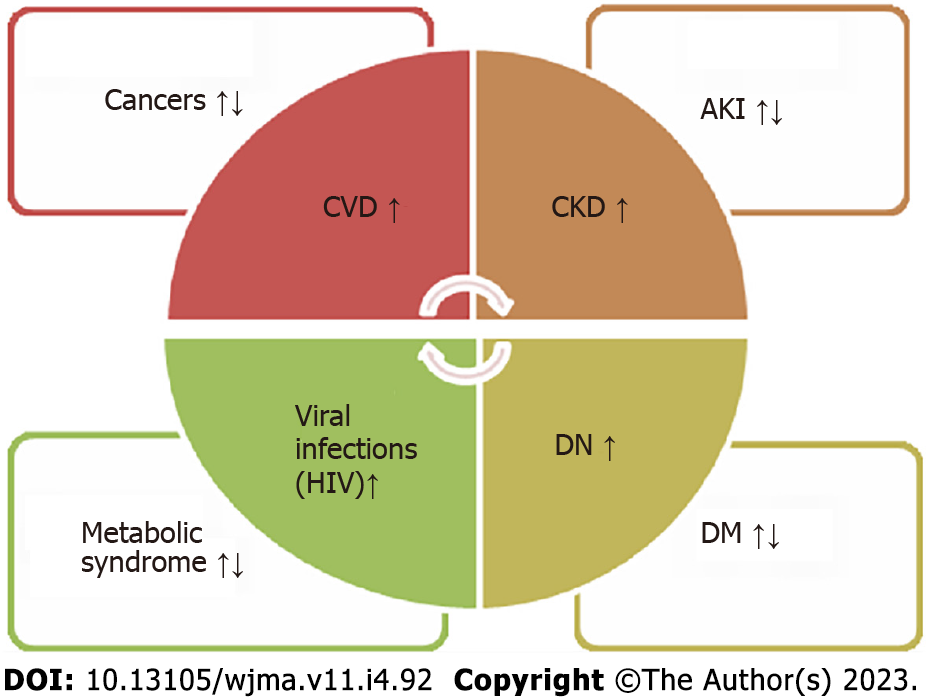
Neutrophil gelatinase-associated lipocalin (NGAL), also known as lipocalin-2, is a small protein purified from neutrophil granules and is considered a good marker for AKI and kidney disease. It belongs to the lipocalin family and is encoded by the lipocalin-2 (LCN2) gene on chromosome 9[28-30]. NGAL is a transporter protein responsible for many physiological processes, such as inflammation, the generation of an immune response, and metabolic homeostasis. Several studies have reported the role of lipocalin-2 in renal diseases, suggesting its role as a novel biomarker for acute renal injury and chronic kidney disorders. A few studies have also demonstrated its inverse relation with serum creatinine in T1D and T2D, although albuminuria was undetectable in these patients. In patients with DN, NGAL levels were significantly higher in serum and urine, which correlated with the estimated glomerular filtration rate (eGFR) inversely (Figure 1). However, these patients did not have albuminuria, implicating the potential role of NGAL as a diagnostic biomarker for DN[29-33].
NGAL is expressed in several body tissues, including the kidney, liver, lungs, trachea, small intestine, bone marrow, prostate, non-neoplastic breast tissue, macrophages, and fat tissues. Expression of NGAL is seen in fetal skin in the epidermis as early as the 20th week of intrauterine life and later concentrated around hair follicles only[32,34].
The normal concentration of NGAL in serum averages 20 ng/mL, while in urine also, it is 20 ng/mL. Its low molecular weight and positive charge make it undergo filtration, so renal clearance is seen as the primary regulator of the concentration of NGAL[35,36].
As part of transport proteins, lipocalin-2 is also seen in many physiological conditions of the body involved in the innate immune response. It is generated through neutrophil degranulation and thus, released at the site of bacterial infection for bacterial sequestration. Iron transport is another role of NGAL as it is accumulated in the cytoplasm, and iron-responsive genes are stimulated in response to this increased concentration. Apo-NGAL is responsible for transporting chelated iron from the inside to the extracellular matrix. Apo-NGAL binds to the 24p3 receptor and internalizes to bind with the cellular siderophore, thus transporting it out of the cell. It signalizes the apoptotic cascade to start due to the expression of the pro-apoptotic protein Bim. The initiation of programmed cell death, whether under normal or abnormal circumstances, depends on the Bim protein. Its activation is precisely regulated at various levels to ensure its proper functioning. Bim is essential in preventing autoimmunity during normal immune responses; however, excessive activation can lead to chronic inflammation and tumor development. In nerve cells, the overexpression of Bim can result in neurodegenerative diseases such as Alzheimer's and Parkinson's diseases. On the other hand, cancer cells typically inhibit Bim expression from facilitating their proliferation and metastasis[29,37-44].
The low molecular weight of NGAL makes it easily filterable through the glomerulus and later reabsorbed in the proximal tubules. If renal tubular damage starts, the reabsorption changes, and thus, excretion of NGAL starts early; epithelial damage thus results in increased NGAL concentration in serum and urine[45].
Several inflammatory and metabolic disorders are seen with altered concentrations of NGAL. Inflammatory conditions like pancreatitis, meningitis, psoriasis, and myocarditis are seen with increased NGAL expression. In certain autoimmune diseases like psoriasis, NGAL mRNA levels were found raised ten times or more. NGAL levels have been reported to be considerably higher in viral infective diseases but markedly lower in human immunodeficiency virus-infected patients who were not receiving therapy than in healthy controls[46,47]. Higher levels of NGAL were found to be associated with anemia independent of eGFR and other parameters like myeloperoxidase and high-sensitivity C-reactive protein[36].
The possible role of NGAL in various cancer models has been studied and is suggested to be both beneficial and detrimental. The nuclear factor kappa B (NF-κB) signaling pathway regulates the transcription of NGAL, and the mitogen-activated protein kinase (MAPK) pathway may cooperate with NF-κB to upregulate the expression of NGAL. Moreover, epigenetic modifications might significantly initiate NGAL expression in tumor cells[28,48-56]. It may explain the increased levels of NGAL in most cancers. It remains to identify the specific molecular forms of NGAL (in serums and cells) associated with a particular cancer type (solid or liquid)[48]. Functionally, NGAL appears to exhibit all the significant events of tumorigenesis, including tumor proliferation, tumor cell survival, distant migration, local invasion, tumor angiogenesis, and resistance to anti-cancer drugs[57]. NGAL protein and mRNA levels are quantitatively measured in body fluids like blood, urine, and tissues and found overexpressed in various cancers like ovarian, endometrial, bladder, liver, breast, brain, lung, pancreatic, colorectal, and several other solid tumors[48-50,54]. The NGAL complex may help assess tumor stage in endometrial cancers before surgical treatment. The NGAL complex is found in blood tumor cells in patients with different types of leukemia[55,58-60].
In metabolic diseases, including obesity, kidney disorders, and pre-eclamptic subjects, NGAL levels were significantly higher in animal models and obese human subjects[61-63]. T2D is characterized by inflammatory processes in the whole body, resulting in endothelial dysfunction. Pro-inflammatory cytokines such as IL-1, IL-6, and TNF-α, as well as chemokines and adhesion molecules, have been shown to contribute to vascular complications in T2D. In T1D, an early predictive role of NGAL as a biomarker for nephropathy and incipient cardiovascular morbidity before and independent of microalbuminuria has been observed[6].
The plasma NGAL (pNGAL) is filtered by the glomerulus and can be almost reabsorbed in the proximal tubules. The chance of detection of urinary NGAL (uNGAL) and pNGAL in animal and human subjects with renal injury has led to evaluating NGAL as an early noninvasive biomarker in human acute and chronic kidney injury in numerous research studies. Lipocalin-2 is, therefore, one of the most promising early, next-generation biomarkers for AKI. Glomerular basement thickening and mesangial expansion have been reported in several studies. The pathogenesis of DN is associated with glomerular and renal tubular interstitial injury. The primary mechanism of NGAL clearance from the blood is via megalin-dependent endocytosis in the proximal tubules of the kidney. Therefore, urinary excretion of NGAL is only expected when there is proximal renal tubular injury which prevents NGAL reabsorption or increased de novo NGAL synthesis. The NGAL protein secreted into the urine from the distal nephron segments is predominantly monomeric and differs from the dimeric NGAL originating from neutrophils. The overexpression of NGAL in the distal tubules and its rapid secretion into the urinary tract align with its role as an antimicrobial strategy. Furthermore, recent evidence suggests that NGAL may also promote cell survival and proliferation, given the documented apoptotic cell death in distal nephron segments in various animal and human models of AKI[6,28,62]
Various proteomic and transcriptomic studies have identified NGAL as one of the most upregulated genes (LCN2 gene) and one of the most highly induced proteins in the kidney very early in the course of acute kidney disease in animal as well as human models[63,64]. NGAL is a novel marker for the diagnosis of DN. It is a marker for kidney injury or any other condition affecting the functions of the kidneys. Early diagnosis of AKI is often challenging and complicated, as suitable early markers for renal damage and kidney function are scarce. NGAL, being an early marker of AKI, overcomes such limitations and seems to demonstrate its role in the diagnosis at an early stage[65,66].
Various studies have reported increased urinary and serum levels of NGAL in AKI. NGAL as an early biomarker to diagnose DN, even earlier to incipient nephropathy, can be seen as a tubular injury marker. Both pNGAL and uNGAL can predict early tubular damage and can be used as a noninvasive tool for diagnosing, staging, and monitoring progressing DN[67]. Subclinical and early kidney injury can be seen in children with T1D with normal renal function. The pNGAL and uNGAL derangement, low-range albuminuria, and normal eGFR can indicate early kidney injury even in optimal glycaemic control. pNGAL and uNGAL in these changes result from tubular injury[68].
In non-terminal chronic kidney disease, NGAL can be used as a novel, independent renal predictor of CKD progression along with the severity of the renal disease. The urinary NGAL can be used as a marker for the early detection of DN, and its mean value has been observed to correlate with the degree of renal impairment. The parallel elevation in uNGAL with disease severity or with increasing stages of CKD supports the hypothesis of active tubular production, excluding a passive consequence of reduced renal clearance capacity. Urinary NGAL has been reported to correlate positively with urine albumin/creatinine ratio, duration of diabetes, hemoglobin A1C, and dyslipidemia. As the positive urine NGAL results were found even in normoalbuminuric patients, uNGAL can be used as an early biomarker for DN in normoalbuminuric patients, especially those with long-standing and uncontrolled diabetes[28,69-72]. Urinary NGAL levels may help monitor the status and treatment of diverse renal diseases reflecting defects in the glomerular filtration barrier, proximal tubule reabsorption, and distal nephrons[34].
It was appreciated that uNGAL is produced in response to ischemia, toxins, or inflammation in the tubular epithelial cells. For each 300 ng/mL increase in uNGAL, an increased risk for the resultant outcome of CKD (due to T1D and T2D) progression, end-stage kidney disease, or death in CKD patients is seen. Urinary NGAL of the microalbuminuric group increased way higher than the normoalbuminuric group[73-75].
The plasma levels of NGAL and IGFBP4 have been appreciated to be higher in patients with DN. Regular follow-up and monitoring before the symptomatic presentation of DN can be carried out with serial monitoring of uNGAL levels, but defining the baseline concentration of NGAL in patients is required[76-78].
The uNGAL may be a more specific marker of active renal tubular epithelial damage and tubulointerstitial inflammation, whereas pNGAL may be more indicative of the renal (and possibly extra-renal) vasculature state, including glomerular filtration ability. Increased level of NGAL as an endogenous filtration biomarker in type 2 diabetic patients is considered a predictive biomarker for early detection of DN. The uNGAL was found to be higher in patients with microalbuminuria than normoalbuminuria, especially in those with long-standing, uncontrolled diabetes and dyslipidemia[79-82]. The serum NGAL (sNGAL) showed an excellent diagnostic value comparable to uNGAL[83].
Urinary NGAL has a positive association with microalbuminuria and can be a noninvasive tool for diagnosing and monitoring the progression of DN. Urinary NGAL measurement is more sensitive than microalbumin, detecting early renal involvement in patients with diabetes mellitus. The uNGAL and creatinine ratio (uNCR) might prove promising in identifying cases with a high clinical suspicion of diabetic kidney disease and in patients with confirmatory biopsy. T2D patients with increased uNCR may have worse outcomes and higher chances of DN complications. However, pNGAL rises markedly with the reduction in GFR, resulting in many false positive inclusions of AKI in chronic patients. So along with eGFR, the uNGAL and plasma brain natriuretic peptide should be used in chronic kidney disease patients to assess AKI[69,84,85].
The increase in uNGAL and cystatin-C levels was directly proportional to microalbuminuria in diabetic patients. T2D patients with early DN had high uNGAL and cystatin-C levels. NGAL reflects tubular damage, and nitric oxide may be used as an angiogenic and oxidative stress marker. Using specific biomarkers along with NGAL can increase its diagnostic efficacy in differentiating renal causes from other clinical conditions[85-88]. uNGAL may be a more specific marker for active renal tubular epithelial damage and tubulointerstitial inflammation, whereas pNGAL may be more indicative of the renal (and possibly extra-renal) vasculature state, including glomerular filtration ability[89].
However, some studies have shown that exosomal-NGAL (NGAL-E) is a better marker than free-NGAL in T1D. NGAL was present in subjects' urinary enriched extracellular vesicle fraction (NGAL-E); however, NGAL-E did not correlate with glycated hemoglobin and albumin/creatinine ratio in the early stages[90].
NGAL was readily detected in the urine after anti-neoplastic drug administration in a dose- and duration-dependent manner. By comparison, uNGAL excretion following cisplatin administration was quantified within 96 h of drug administration so that it can be used as an early marker of kidney injury in cancer subjects very early, showing its efficacy as an early marker in other pathologies leading to renal dysfunction[91,92].
A metanalytical study also concluded that NGAL is a potential diagnostic marker for patients with DN and that its diagnostic value for microalbuminuria and macroalbuminuria is superior to that for microalbuminuria alone[93]. Several studies collectively and strongly support using NGAL as a biomarker for predicting AKI. However, the lack of published studies that adhere to diagnostic study guidelines, heterogeneity in AKI definition, the lack of uniformly applicable cut-off values, and variability in the performance of commercially available NGAL assays are big challenges to establish its role concretely[94]. The specificity and sensitivity of NGAL were found to be moderate to excellent in various studies in various conditions, including indoor and outdoor patients, as a good predictor of AKI[10,63,95-98]. Although some limitations are reported, NGAL (sNGAL and uNGAL) can be prognostic of renal damage even in the case of subclinical or modest renal damage that can only be diagnosed by creatinine studies late in the course of the disease[99].
The studies reported in the present review describe the role of NGAL in nephropathy, particularly DN. Early detection of renal changes is vital for diagnostic and prognostic purposes. NGAL is an important renal dysfunction marker. Although its role in other conditions like infections, metabolic disorders, and cancers is already established, its function in nephropathy is also promising, as it increases significantly before other usual markers appear in the urine and blood.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, general and internal
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Miao MS, China; Singh Y, United States S-Editor: Liu JH L-Editor: Wang TQ P-Editor: Yu HG
| 1. | De Fronzo RA. Diabetic Nephropathy: Etiologic and Therapeutic Considerations. Diabetes Rev. 1995;3:510-564. |
| 2. | Haneda M, Utsunomiya K, Koya D, Babazono T, Moriya T, Makino H, Kimura K, Suzuki Y, Wada T, Ogawa S, Inaba M, Kanno Y, Shigematsu T, Masakane I, Tsuchiya K, Honda K, Ichikawa K, Shide K; Joint Committee on Diabetic Nephropathy. A new Classification of Diabetic Nephropathy 2014: a report from Joint Committee on Diabetic Nephropathy. J Diabetes Investig. 2015;6:242-246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 147] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 3. | Tapp RJ, Shaw JE, Zimmet PZ, Balkau B, Chadban SJ, Tonkin AM, Welborn TA, Atkins RC. Albuminuria is evident in the early stages of diabetes onset: results from the Australian Diabetes, Obesity, and Lifestyle Study (AusDiab). Am J Kidney Dis. 2004;44:792-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 95] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | Satirapoj B. Tubulointerstitial Biomarkers for Diabetic Nephropathy. J Diabetes Res. 2018;2018:2852398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 5. | Tuttle KR, Bakris GL, Bilous RW, Chiang JL, de Boer IH, Goldstein-Fuchs J, Hirsch IB, Kalantar-Zadeh K, Narva AS, Navaneethan SD, Neumiller JJ, Patel UD, Ratner RE, Whaley-Connell AT, Molitch ME. Diabetic kidney disease: a report from an ADA Consensus Conference. Diabetes Care. 2014;37:2864-2883. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 753] [Cited by in RCA: 786] [Article Influence: 71.5] [Reference Citation Analysis (0)] |
| 6. | Papadopoulou-Marketou N, Chrousos GP, Kanaka-Gantenbein C. Diabetic nephropathy in type 1 diabetes: a review of early natural history, pathogenesis, and diagnosis. Diabetes Metab Res Rev. 2017;33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 168] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 7. | Qiu X, Liu C, Ye Y, Li H, Chen Y, Fu Y, Liu Z, Huang X, Zhang Y, Liao X, Liu H, Zhao W, Liu X. The diagnostic value of serum creatinine and cystatin c in evaluating glomerular filtration rate in patients with chronic kidney disease: a systematic literature review and meta-analysis. Oncotarget. 2017;8:72985-72999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Alaini A, Malhotra D, Rondon-Berrios H, Argyropoulos CP, Khitan ZJ, Raj DSC, Rohrscheib M, Shapiro JI, Tzamaloukas AH. Establishing the presence or absence of chronic kidney disease: Uses and limitations of formulas estimating the glomerular filtration rate. World J Methodol. 2017;7:73-92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | Delanaye P, Cavalier E, Pottel H. Serum Creatinine: Not So Simple! Nephron. 2017;136:302-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 216] [Article Influence: 27.0] [Reference Citation Analysis (1)] |
| 10. | Devarajan P. Neutrophil gelatinase-associated lipocalin (NGAL): a new marker of kidney disease. Scand J Clin Lab Invest Suppl. 2008;241:89-94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 272] [Cited by in RCA: 246] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 11. | Tesch GH. Review: Serum and urine biomarkers of kidney disease: A pathophysiological perspective. Nephrology (Carlton). 2010;15:609-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 12. | Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6145] [Cited by in RCA: 6195] [Article Influence: 258.1] [Reference Citation Analysis (0)] |
| 13. | Cooper ME. Pathogenesis, prevention, and treatment of diabetic nephropathy. Lancet. 1998;352:213-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 339] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 14. | Forbes JM, Coughlan MT, Cooper ME. Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes. 2008;57:1446-1454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 815] [Cited by in RCA: 861] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 15. | Reddy GR, Kotlyarevska K, Ransom RF, Menon RK. The podocyte and diabetes mellitus: is the podocyte the key to the origins of diabetic nephropathy? CurrOpinNephrolHypertens. 2008;17:32-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Wang G, Lai FM, Lai KB, Chow KM, Li KT, Szeto CC. Messenger RNA expression of podocyte-associated molecules in the urinary sediment of patients with diabetic nephropathy. Nephron ClinPract. 2007;106:c169-c179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Jensen T. Increased plasma concentration of von Willebrand factor in insulin dependent diabetics with incipient nephropathy. BMJ. 1989;298:27-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Yu Y, Suo L, Yu H, Wang C, Tang H. Insulin resistance and endothelial dysfunction in type 2 diabetes patients with or without microalbuminuria. Diabetes Res ClinPract. 2004;65:95-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 1.9] [Reference Citation Analysis (1)] |
| 19. | Hirano T, Ookubo K, Kashiwazaki K, Tajima H, Yoshino G, Adachi M. Vascular endothelial markers, von Willebrand factor and thrombomodulin index, are specifically elevated in type 2 diabetic patients with nephropathy: comparison of primary renal disease. ClinChimActa. 2000;299:65-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Fang YH, Zhang JP, Zhou SX, Zheng JF, Yu YW, Yan SG, Fan WK, Cheng YS. [Relationship between serum vWF and PAF in type 2 diabetic patients and diabetic nephropathy]. Di Yi Jun Yi Da XueXueBao. 2005;25:729-731. [PubMed] |
| 21. | Tamaki K, Okuda S. Role of TGF-beta in the progression of renal fibrosis. ContribNephrol. 2003;139:44-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Xie F. Significance of serum and urinary TGF-β1 to the early diagnosis of diabetic nephropathy. Strait Pharmaceutical J. 2009;21:145-146. |
| 23. | Narita T, Sasaki H, Hosoba M, Miura T, Yoshioka N, Morii T, Shimotomai T, Koshimura J, Fujita H, Kakei M, Ito S. Parallel increase in urinary excretion rates of immunoglobulin G, ceruloplasmin, transferrin, and orosomucoid in normoalbuminuric type 2 diabetic patients. Diabetes Care. 2004;27:1176-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 69] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Hong CY, Hughes K, Chia KS, Ng V, Ling SL. Urinary alpha1-microglobulin as a marker of nephropathy in type 2 diabetic Asian subjects in Singapore. Diabetes Care. 2003;26:338-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 89] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 25. | Salem MA, el-Habashy SA, Saeid OM, el-Tawil MM, Tawfik PH. Urinary excretion of n-acetyl-beta-D-glucosaminidase and retinol binding protein as alternative indicators of nephropathy in patients with type 1 diabetes mellitus. Pediatr Diabetes. 2002;3:37-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Bagshaw SM, Bellomo R. Early diagnosis of acute kidney injury. CurrOpinCrit Care. 2007;13:638-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Han WK, Wagener G, Zhu Y, Wang S, Lee HT. Urinary biomarkers in the early detection of acute kidney injury after cardiac surgery. Clin J Am SocNephrol. 2009;4:873-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 291] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 28. | Bolignano D, Donato V, Coppolino G, Campo S, Buemi A, Lacquaniti A, Buemi M. Neutrophil gelatinase-associated lipocalin (NGAL) as a marker of kidney damage. Am J Kidney Dis. 2008;52:595-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 431] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 29. | Kjeldsen L, Johnsen AH, Sengeløv H, Borregaard N. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J BiolChem. 1993;268:10425-10432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 844] [Cited by in RCA: 889] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 30. | Gharishvandi F, Kazerouni F, Ghanei E, Rahimipour A, Nasiri M. Comparative assessment of neutrophil gelatinase-associated lipocalin (NGAL) and cystatin C as early biomarkers for early detection of renal failure in patients with hypertension. Iran Biomed J. 2015;19:76-81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 31. | Chan P, Simon-Chazottes D, Mattei MG, Guenet JL, Salier JP. Comparative mapping of lipocalin genes in human and mouse: the four genes for complement C8 gamma chain, prostaglandin-D-synthase, oncogene-24p3, and progestagen-associated endometrial protein map to HSA9 and MMU2. Genomics. 1994;23:145-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Cowland JB, Borregaard N. Molecular characterization and pattern of tissue expression of the gene for neutrophil gelatinase-associated lipocalin from humans. Genomics. 1997;45:17-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 453] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 33. | Flower DR. The lipocalin protein family: structure and function. Biochem J. 1996;318 (Pt 1):1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1222] [Cited by in RCA: 1247] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 34. | Kuwabara T, Mori K, Mukoyama M, Kasahara M, Yokoi H, Saito Y, Yoshioka T, Ogawa Y, Imamaki H, Kusakabe T, Ebihara K, Omata M, Satoh N, Sugawara A, Barasch J, Nakao K. Urinary neutrophil gelatinase-associated lipocalin levels reflect damage to glomeruli, proximal tubules, and distal nephrons. Kidney Int. 2009;75:285-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 221] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 35. | Paragas N, Qiu A, Hollmen M, Nickolas TL, Devarajan P, Barasch J. NGAL-Siderocalin in kidney disease. BiochimBiophysActa. 2012;1823:1451-1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 36. | Shrestha K, Borowski AG, Troughton RW, Klein AL, Tang WH. Association between systemic neutrophil gelatinase-associated lipocalin and anemia, relative hypochromia, and inflammation in chronic systolic heart failure. Congest Heart Fail. 2012;18:239-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 37. | Akerstrom B, Flower DR, Salier JP. Lipocalins: unity in diversity. BiochimBiophysActa. 2000;1482:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 196] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 38. | Yan L, Borregaard N, Kjeldsen L, Moses MA. The high molecular weight urinary matrix metalloproteinase (MMP) activity is a complex of gelatinase B/MMP-9 and neutrophil gelatinase-associated lipocalin (NGAL). Modulation of MMP-9 activity by NGAL. J BiolChem. 2001;276:37258-37265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 504] [Cited by in RCA: 542] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 39. | Goetz DH, Holmes MA, Borregaard N, Bluhm ME, Raymond KN, Strong RK. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol Cell. 2002;10:1033-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 984] [Cited by in RCA: 1019] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 40. | Miethke M, Skerra A. Neutrophil gelatinase-associated lipocalin expresses antimicrobial activity by interfering with L-norepinephrine-mediated bacterial iron acquisition. Antimicrob Agents Chemother. 2010;54:1580-1589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 41. | Devireddy LR, Gazin C, Zhu X, Green MR. A cell-surface receptor for lipocalin 24p3 selectively mediates apoptosis and iron uptake. Cell. 2005;123:1293-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 522] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 42. | Liu Z, Petersen R, Devireddy L. Impaired neutrophil function in 24p3 null mice contributes to enhanced susceptibility to bacterial infections. J Immunol. 2013;190:4692-4706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 43. | Schroll A, Eller K, Feistritzer C, Nairz M, Sonnweber T, Moser PA, Rosenkranz AR, Theurl I, Weiss G. Lipocalin-2 ameliorates granulocyte functionality. Eur J Immunol. 2012;42:3346-3357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 112] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 44. | La Manna G, Ghinatti G, Tazzari PL, Alviano F, Ricci F, Capelli I, Cuna V, Todeschini P, Brunocilla E, Pagliaro P, Bonsi L, Stefoni S. Neutrophil gelatinase-associated lipocalin increases HLA-G(+)/FoxP3(+) T-regulatory cell population in an in vitro model of PBMC. PLoS One. 2014;9:e89497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 45. | Schmidt-Ott KM, Mori K, Li JY, Kalandadze A, Cohen DJ, Devarajan P, Barasch J. Dual action of neutrophil gelatinase-associated lipocalin. J Am SocNephrol. 2007;18:407-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 525] [Cited by in RCA: 566] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 46. | Nomura I, Gao B, Boguniewicz M, Darst MA, Travers JB, Leung DY. Distinct patterns of gene expression in the skin lesions of atopic dermatitis and psoriasis: a gene microarray analysis. J Allergy ClinImmunol. 2003;112:1195-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 258] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 47. | Wang Y, Lam KS, Kraegen EW, Sweeney G, Zhang J, Tso AW, Chow WS, Wat NM, Xu JY, Hoo RL, Xu A. Lipocalin-2 is an inflammatory marker closely associated with obesity, insulin resistance, and hyperglycemia in humans. ClinChem. 2007;53:34-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 439] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 48. | Chakraborty S, Kaur S, Guha S, Batra SK. The multifaceted roles of neutrophil gelatinase associated lipocalin (NGAL) in inflammation and cancer. BiochimBiophysActa. 2012;1826:129-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 292] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 49. | Lippi G, Meschi T, Nouvenne A, Mattiuzzi C, Borghi L. Neutrophil gelatinase-associated lipocalin in cancer. AdvClinChem. 2014;64:179-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 50. | Yang J, Moses MA. Lipocalin 2: a multifaceted modulator of human cancer. Cell Cycle. 2009;8:2347-2352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 103] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 51. | Candido S, Maestro R, Polesel J, Catania A, Maira F, Signorelli SS, McCubrey JA, Libra M. Roles of neutrophil gelatinase-associated lipocalin (NGAL) in human cancer. Oncotarget. 2014;5:1576-1594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 52. | Liao CJ, Huang YH, Au HK, Wang LM, Chu ST. The cancer marker neutrophil gelatinase-associated lipocalin is highly expressed in human endometrial hyperplasia. MolBiol Rep. 2012;39:1029-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 53. | Ricci S, Bruzzese D, DI Carlo A. Evaluation of MMP-2, MMP-9, TIMP-1, TIMP-2, NGAL and MMP-9/NGAL complex in urine and sera from patients with bladder cancer. Oncol Lett. 2015;10:2527-2532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 54. | Kaur S, Chakraborty S, Baine MJ, Mallya K, Smith LM, Sasson A, Brand R, Guha S, Jain M, Wittel U, Singh SK, Batra SK. Potentials of plasma NGAL and MIC-1 as biomarker(s) in the diagnosis of lethal pancreatic cancer. PLoS One. 2013;8:e55171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 55. | Monisha J, Roy NK, Padmavathi G, Banik K, Bordoloi D, Khwairakpam AD, Arfuso F, Chinnathambi A, Alahmadi TA, Alharbi SA, Sethi G, Kumar AP, Kunnumakkara AB. NGAL is Downregulated in Oral Squamous Cell Carcinoma and Leads to Increased Survival, Proliferation, Migration and Chemoresistance. Cancers (Basel). 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 56. | Devarajan P. Neutrophil gelatinase-associated lipocalin: new paths for an old shuttle. Cancer Ther. 2007;5:463-470. [PubMed] |
| 57. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51728] [Cited by in RCA: 47087] [Article Influence: 3363.4] [Reference Citation Analysis (5)] |
| 58. | Bouchet S, Bauvois B. Neutrophil Gelatinase-Associated Lipocalin (NGAL), Pro-Matrix Metalloproteinase-9 (pro-MMP-9) and Their Complex Pro-MMP-9/NGAL in Leukaemias. Cancers (Basel). 2014;6:796-812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 59. | Kamiguti AS, Lee ES, Till KJ, Harris RJ, Glenn MA, Lin K, Chen HJ, Zuzel M, Cawley JC. The role of matrix metalloproteinase 9 in the pathogenesis of chronic lymphocytic leukaemia. Br J Haematol. 2004;125:128-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 60. | Villalva C, Sorel N, Bonnet ML, Guilhot J, Mayeur-Rousse C, Guilhot F, Chomel JC, Turhan AG. Neutrophil gelatinase-associated lipocalin expression in chronic myeloid leukemia. Leuk Lymphoma. 2008;49:984-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 61. | Zhao P, Elks CM, Stephens JM. The induction of lipocalin-2 protein expression in vivo and in vitro. J BiolChem. 2014;289:5960-5969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 62. | Filiopoulos V, Biblaki D, Vlassopoulos D. Neutrophil gelatinase-associated lipocalin (NGAL): a promising biomarker of contrast-induced nephropathy after computed tomography. Ren Fail. 2014;36:979-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 63. | Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, Barasch J, Devarajan P. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am SocNephrol. 2003;14:2534-2543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1227] [Cited by in RCA: 1286] [Article Influence: 61.2] [Reference Citation Analysis (0)] |
| 64. | Yuen PS, Jo SK, Holly MK, Hu X, Star RA. Ischemic and nephrotoxic acute renal failure are distinguished by their broad transcriptomic responses. Physiol Genomics. 2006;25:375-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 69] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 65. | Gazareena SS, Korani MAER, Tawfeek AR, Omar TA, Dwidar GIEA. Role of urinary neutrophil gelatinase-associated lipocalin in diabetic and nondiabetic patients with nephropathy. Menoufia Med J. 2021;34:135-140. [DOI] [Full Text] |
| 66. | Ronco C. N-GAL: diagnosing AKI as soon as possible. Crit Care. 2007;11:173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 67. | Kaul A, Behera MR, Rai MK, Mishra P, Bhaduaria DS, Yadav S, Agarwal V, Karoli R, Prasad N, Gupta A, Sharma RK. Neutrophil Gelatinase-associated Lipocalin: As a Predictor of Early Diabetic Nephropathy in Type 2 Diabetes Mellitus. Indian J Nephrol. 2018;28:53-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 68. | Sołtysiak J, Skowrońska B, Fichna P, Stankiewicz W, Lewandowska-Stachowiak M, Ostalska-Nowicka D, Zachwieja J. Neutrophil gelatinase-associated lipocalin and Cathepsin L as early predictors of kidney dysfunction in children with type 1 diabetes. Endokrynol Pol. 2014;65:479-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 69. | Padmini PJ, Ashok V. Urine neutrophil gelatinase-associated lipocalin as an early biochemical marker of microalbuminuria in predicting early kidney damage in patients with type 2 diabetes mellitus. UkrBiochem J. 2021;93:6. [DOI] [Full Text] |
| 70. | Patel ML, Sachan R, Verma A, Kamal R, Gupta KK. Neutrophil gelatinase-associated lipocalin as a biomarker of disease progression in patients with chronic kidney disease. Indian J Nephrol. 2016;26:125-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 71. | Forghani MS, Khezrian F, Khezrian S, Ghafoori S, Saed L, Rahmani K. Urinary neutrophil gelatinaseassociatedlipocalin in early detection of diabetic nephropathy; a pilot study. J Renal InjPrev. 2020;9:23. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 72. | Hafez MH, El-Mougy FA, Makar SH, Abd El Shaheed S. Detection of an earlier tubulopathy in diabetic nephropathy among children with normoalbuminuria. Iran J Kidney Dis. 2015;9:126-131. [PubMed] |
| 73. | Quang TH, Nguyet MP, Thao DP, Thi MH, Phuong Thi Dam L, Thi HH, Van AP, Luong TC, Tuyet MNT, Duy QD, Nhu BD, Duc TN. Evaluation of Urinary Neutrophil Gelatinase Associated Lipocalin and Kidney Injury Molecule-1 as Diagnostic Markers for Early Nephropathy in Patients with Type 2 Diabetes Mellitus. Diabetes MetabSyndrObes. 2020;13:2199-2207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 74. | Lobato GR, Lobato MR, Thomé FS, Veronese FV. Performance of urinary kidney injury molecule-1, neutrophil gelatinase-associated lipocalin, and N-acetyl-β-D-glucosaminidase to predict chronic kidney disease progression and adverse outcomes. Braz J Med Biol Res. 2017;50:e6106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 75. | Nielsen SE, Schjoedt KJ, Astrup AS, Tarnow L, Lajer M, Hansen PR, Parving HH, Rossing P. Neutrophil Gelatinase-Associated Lipocalin (NGAL) and Kidney Injury Molecule 1 (KIM1) in patients with diabetic nephropathy: a cross-sectional study and the effects of lisinopril. Diabet Med. 2010;27:1144-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 76. | Veiga G, Alves B, Perez M, Alcantara LV, Raimundo J, Zambrano L, Encina J, Pereira EC, Bacci M, Murad N, Fonseca F. NGAL and SMAD1 gene expression in the early detection of diabetic nephropathy by liquid biopsy. J ClinPathol. 2020;73:713-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 77. | Ali H, Abu-Farha M, Alshawaf E, Devarajan S, Bahbahani Y, Al-Khairi I, Cherian P, Alsairafi Z, Vijayan V, Al-Mulla F, Al Attar A, Abubaker J. Association of significantly elevated plasma levels of NGAL and IGFBP4 in patients with diabetic nephropathy. BMC Nephrol. 2022;23:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 78. | Smertka M, Chudek J. Using NGAL as an early diagnostic test of acute kidney injury. Ren Fail. 2012;34:130-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 79. | Şen S, ÖzalpKızılay D, Taneli F, Özen Ç, Ertan P, Özunan İ, Yıldız R, Ersoy B. Urinary NGAL is a Potential Biomarker for Early Renal Injury in Insulin Resistant Obese Non-diabetic Children. J Clin Res PediatrEndocrinol. 2021;13:400-407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 80. | Lima C, Fatima VM, Macedo E. Neutrophil Gelatinase-Associated Lipocalin as a Promising Biomarker in Acute Kidney Injury. In: Kumar, V, Salgado, AA, Athari, SS. editors. Inflammation in the 21st Century. London: IntechOpen; 2020. [DOI] [Full Text] |
| 81. |
Shael SK, Rasheed MK, Saeedi SM. Role of Serum β Trace Protein and Neutrophil Gelatinase Associated Lipocalin in Early Diabetic Nephropathy in Type 2 Diabetes of Iraqi Patients.
J Res Med Dent Sci. 2020;8 526-533.Available from: |
| 82. | Elbana KA, Bakr HG, Fekry A, Elkot MS. Urinary Neutrophil Gelatinase-Associated Lipocalin as an early marker for diagnosis of diabetic nephropathy in T2DM patients and its correlation to albumin creatinine ratio. Annals of RSCB. 2021;25:13517 Available from: www.annalsofrscb.ro/index.php/journal/article/view/8158. |
| 83. | Lacquaniti A, Donato V, Pintaudi B, Di Vieste G, Chirico V, Buemi A, Di Benedetto A, Arena A, Buemi M. "Normoalbuminuric" diabetic nephropathy: tubular damage and NGAL. ActaDiabetol. 2013;50:935-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 84. | Duan S, Lu F, Song D, Zhang C, Zhang B, Xing C, Yuan Y. Current Challenges and Future Perspectives of Renal Tubular Dysfunction in Diabetic Kidney Disease. Front Endocrinol (Lausanne). 2021;12:661185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 85. | Sueud T, Hadi NR, Abdulameer R, Jamil DA, Al-Aubaidy HA. Assessing urinary levels of IL-18, NGAL and albumin creatinine ratio in patients with diabetic nephropathy. Diabetes MetabSyndr. 2019;13:564-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 86. | Toson el-SA, Waly S, Omran MM. Neutrophil gelatinase-associated lipocalin and oxidative stress markers based –scores to improve the diagnostic accuracy of chronic kidney diseases. JBAAR. 2021;5:44-55. [DOI] [Full Text] |
| 87. | Donadio C. Effect of glomerular filtration rate impairment on diagnostic performance of neutrophil gelatinase-associated lipocalin and B-type natriuretic peptide as markers of acute cardiac and renal failure in chronic kidney disease patients. Crit Care. 2014;18:R39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 88. | Vijay S, Hamide A, Senthilkumar GP, Mehalingam V. Utility of urinary biomarkers as a diagnostic tool for early diabetic nephropathy in patients with type 2 diabetes mellitus. Diabetes MetabSyndr. 2018;12:649-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 89. |
Greco M, Chiefari E, Mirabelli M, Salatino A, Tocci V, Cianfrone P et al Plasma or Urine Neutrophil Gelatinase-Associated Lipocalin (NGAL): Which Is Better at Detecting Chronic Kidney Damage in Type 2 Diabetes? Endocrines 2022; 3:175-186 [DOI:10.
Greco M,Chiefari E, Mirabelli M, Salatino A, Tocci V, Cianfrone P et al Plasma or Urine Neutrophil Gelatinase-Associated Lipocalin (NGAL): Which Is Better at Detecting Chronic Kidney Damage in Type 2 Diabetes? |
| 90. | Ugarte F, Santapau D, Gallardo V, Garfias C, Yizmeyián A, Villanueva S, Sepúlveda C, Rocco J, Pasten C, Urquidi C, Cavada G, San Martin P, Cano F, Irarrázabal CE. Urinary Extracellular Vesicles as a Source of NGAL for Diabetic Kidney Disease Evaluation in Children and Adolescents With Type 1 Diabetes Mellitus. Front Endocrinol (Lausanne). 2021;12:654269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 91. | Seibert FS, Heringhaus A, Pagonas N, Rudolf H, Rohn B, Bauer F, Timmesfeld N, Trappe HJ, Babel N, Westhoff TH. Biomarkers in the prediction of contrast media induced nephropathy - the BITCOIN study. PLoS One. 2020;15:e0234921. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 92. | Mishra J, Mori K, Ma Q, Kelly C, Barasch J, Devarajan P. Neutrophil gelatinase-associated lipocalin: a novel early urinary biomarker for cisplatin nephrotoxicity. Am J Nephrol. 2004;24:307-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 382] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 93. | Chen B, Li Y, Liu Y, Zang C, Wu M, Xu Z. Diagnostic value of neutrophil gelatinase-associated lipocalin in diabetic nephropathy: a meta-analysis. Ren Fail. 2019;41:489-496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 94. | Haase-Fielitz A, Haase M, Devarajan P. Neutrophil gelatinase-associated lipocalin as a biomarker of acute kidney injury: a critical evaluation of current status. Ann ClinBiochem. 2014;51:335-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 188] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 95. | Prabhu A, Sujatha DI, Ninan B, Vijayalakshmi MA. Neutrophil gelatinase associated lipocalin as a biomarker for acute kidney injury in patients undergoing coronary artery bypass grafting with cardiopulmonary bypass. Ann VascSurg. 2010;24:525-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 96. | Parikh CR, Coca SG, Thiessen-Philbrook H, Shlipak MG, Koyner JL, Wang Z, Edelstein CL, Devarajan P, Patel UD, Zappitelli M, Krawczeski CD, Passik CS, Swaminathan M, Garg AX; TRIBE-AKI Consortium. Postoperative biomarkers predict acute kidney injury and poor outcomes after adult cardiac surgery. J Am SocNephrol. 2011;22:1748-1757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 419] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 97. | Nickolas TL, O'Rourke MJ, Yang J, Sise ME, Canetta PA, Barasch N, Buchen C, Khan F, Mori K, Giglio J, Devarajan P, Barasch J. Sensitivity and specificity of a single emergency department measurement of urinary neutrophil gelatinase-associated lipocalin for diagnosing acute kidney injury. Ann Intern Med. 2008;148:810-819. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 538] [Cited by in RCA: 494] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 98. | Shapiro NI, Trzeciak S, Hollander JE, Birkhahn R, Otero R, Osborn TM, Moretti E, Nguyen HB, Gunnerson K, Milzman D, Gaieski DF, Goyal M, Cairns CB, Kupfer K, Lee SW, Rivers EP. The diagnostic accuracy of plasma neutrophil gelatinase-associated lipocalin in the prediction of acute kidney injury in emergency department patients with suspected sepsis. Ann Emerg Med. 2010;56:52-59.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 99. | Sachan R, Patel M, Gaurav A, Gangwar R, Sachan P. Correlation of serum neutrophil gelatinase associated lipocalin with disease severity in hypertensive disorders of pregnancy. Adv Biomed Res. 2014;3:223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |