Published online Apr 18, 2023. doi: 10.13105/wjma.v11.i4.125
Peer-review started: October 31, 2022
First decision: January 17, 2023
Revised: January 27, 2023
Accepted: March 29, 2023
Article in press: March 29, 2023
Published online: April 18, 2023
Processing time: 179 Days and 7.3 Hours
Recent studies have indicated the use of baricitinib in coronavirus disease 2019 (COVID-19) patients. However, the use of baricitinib in COVID-19 patients is unclear so far.
To determine the precise role of baricitinib in the mortality of COVID-19 patients.
The relevant studies were searched in PubMed, Google scholar, and Clinical trials registries till July 13, 2021 and sorted out based on inclusion and exclusion criteria. The quality of studies was assessed using Newcastle-Ottawa Scale. A random-effect model was used, and the pooled estimate was calculated as the odds ratio with a 95% confidence interval using Rev Man 5.
A total of 11 studies (4 observational and 7 clinical trials) were found relevant for analysis. The overall estimate measure in terms of odds ratio for observational studies was 0.42 [0.11, 1.67], whereas for clinical trials it was 0.37 [0.09, 1.46], indicating a non-significant reduction in COVID-19 patient deaths in the baricitinib group versus the non-baricitinib group.
More studies are required to confirm the role of baricitinib in the deaths of COVID-19 patients.
Core Tip: Emerging reports have indicated the use of baricitinib in hospitalized coronavirus disease 2019 (COVID-19) patients. However, the use of baricitinib in COVID-19 patients is unclear so far. Current study aimed to find out the exact association of baricitinib in the mortality of COVID-19 patients.
- Citation: Thakur M, Babu A, Khatik GL, Datusalia AK, Khatri R, Kumar A. Role of baricitinib in COVID-19 patients: A systematic review and meta-analysis. World J Meta-Anal 2023; 11(4): 125-133
- URL: https://www.wjgnet.com/2308-3840/full/v11/i4/125.htm
- DOI: https://dx.doi.org/10.13105/wjma.v11.i4.125
According to the World Health Organization (WHO), multiple pneumonia episodes of unknown cause were reported in the central Metropolitan area of Wuhan in December 2019 in China. The causal infection was later identified as a novel coronavirus, tentatively termed coronavirus disease 2019 (COVID-19). This virus has been causing havoc on public health across the world since its outbreak in December 2019. More than 2000 incidents of COVID-19 infection were reported as of January 26, 2020, the majority of which were individuals living in or traveling Wuhan. The WHO claimed the COVID-19 pandemic was a Public Health Emergency of International Concern on January 30, 2020[1]. The cases of infection were highly associated with the seafood market in Wuhan[2]. Chinese officials announced 2835 confirmed cases in 2020, with 81 deaths. The causal agent has been identified as a novel coronavirus, COVID-19, a pathogen linked to severe acute respiratory syndrome (SARS), was quickly identified as the cause (SARS-CoV) by Chinese officials[3,4]. Coronaviruses (CoV) belongs to the family “coronaviridae”. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is thought to be spread directly from bats to humans or by a single or several host species[3,5]. The treatment is based on the symptoms of the patients. Various classes of drugs are repurposed and are being used in the management of this infection.
Janus Kinase Inhibitors (JAKi) are also one of the repurposed drugs which are being used in the management of hospitalized COVID-19 patients due to their anti-inflammatory (inhibition of IL-6) and anti-viral effects (inhibit the entry of virus)[6]. Baricitinib is one of the JAKi approved for the treatment of rheumatoid arthritis. It has been observed that most SARS-CoV-2 infected patients were died due to cytokine storms, specifically the excess release of IL-6. Thus, baricitinib might be useful in the reduction of deaths of COVID-19 patients[7,8]. Meta-analysis is one of the quantitative analyses that help in clinical decision-making. The results of the individual studies are pooled and integrated using suitable statistical procedures[9-11]. In the current study, we performed a systematic review of clinical studies to determine the role of baricitinib in the deaths of COVID-19 hospitalised patients.
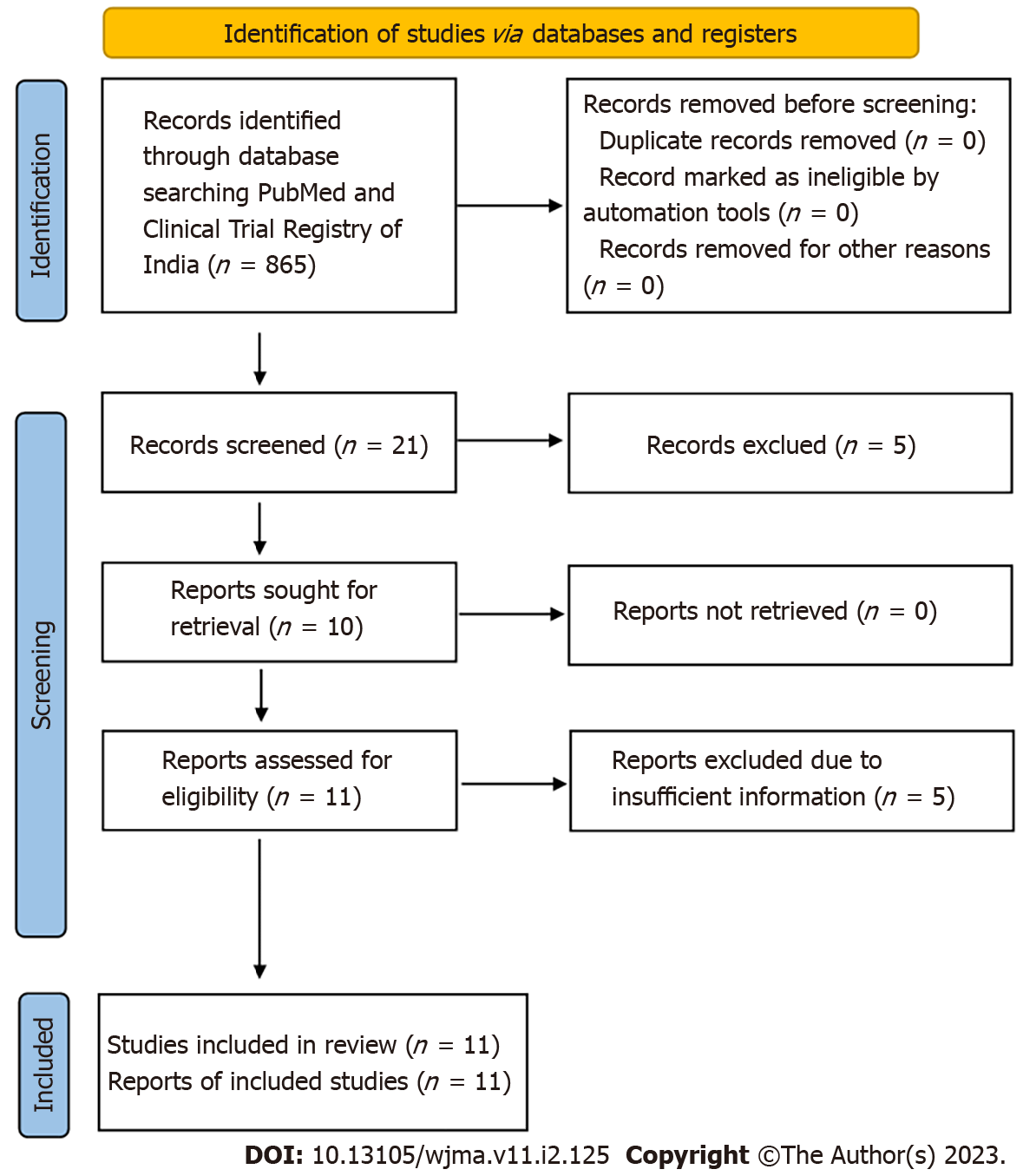
The study was conducted in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines (Figure 1). The study is registered with the International prospective register of systematic reviews (PROSPERO, Registration number: CRD42021281366).
A search was conducted in PubMed, Google scholar, and Clinical trial registry for observational, randomized, and non-randomized controlled studies, cohort studies, and comparative cross-sectional studies with the following search strategies: “baricitinib”, OR “immunosuppressants”, OR “anti-rheumatoid”, OR “Janus kinase inhibitor” OR “Disease-modifying antirheumatic drug” AND “COVID-19” OR “Coronavirus” OR “Acute respiratory distress syndrome” OR “SARS-CoV-2”. The references of included studies were screened to boost the search.
Two reviewers (MT and AB) separately screened all the titles and abstracts as per the inclusion and exclusion criteria. The studies were included if participants were on baricitinib therapy, with all age groups, and all sexes. The case reports, case series, narrative review, systematic review, meta-analysis, studies of poor quality as per standard scale were excluded. The reviewers (MT and AB) separately screened the full-text studies for final inclusion. In the case of conflicts over the inclusion, the third reviewer (AK) was consulted.
The quality assessment of eligible observational studies was done using Newcastle-Ottawa Scale whereas quality assessment of clinical trials was done using NIH quality assessment scale for quality assessment of controlled intervention studies. The assessment was done by two reviewers (MT and AB) separately. The disagreement among authors was resolved after a discussion with four reviewers (GLK, AKD, RK, and AK). The studies were categorized into three categories, i.e., good, fair, and poor quality.
The data was extracted from studies by two reviewers (MT and AB) in an excel sheet. The information includes the name of the first author with publication year, the country where the study has been conducted, gender, study design, the total number of subjects, number of subjects, and deaths in baricitinib, non-baricitinib group.
The sensitivity analysis was done to check the effect of high or low sample size on the outcome to address the degree of heterogeneity.
RevMan 5 was used for all of the analyses. Using a random-effect model, the overall estimate was calculated as an odds ratio with 95% confidence intervals. Cochrane Q and I square statistics were used to calculate study heterogeneity.
We found 865 articles after the initial search. After primarily screening of titles, 21 relevant articles were found. Further, based on the screening of abstracts, 16 were retrieved, out of which 05 articles were excluded due to insufficient information. Finally, 11 articles[11-22] were included for qualitative and quantitative analysis. Figure 1 depicts the selection of articles. The full-text or secondary screening with bibliography searches yielded no additional articles for inclusion. Out of the 11 studies, 4 were observational studies whereas the remaining 7 studies were clinical trials. The four studies were conducted in Italy, two in Spain, one in Italy and Spain, and one each at, Omaha, Bangladesh, Germany, Wuhan. The characteristics of included observational studies were compiled in Table 1 whereas the characteristics of included clinical trials were compiled in Table 2.
| Ref. | Country | Study design | Sample size | Sex | Baricitinib group | Non-baricitinib group | |||
| Male | Female | Number of patients | Death | Number of patients | Death | ||||
| Hasan et al[14], 2021 | Bangladesh | Cohort study | 238 | 159 | 79 | 122 | 4 | 116 | 7 |
| Stebbing et al[15], 2021 | Italy, Spain | Observational | 790 | 438 | 352 | 37 | 1 | 142 | 47 |
| Rodriguez-Garcia et al[16], 2020 | Spain | Cohort study | 112 | 78 | 34 | 62 | 5 | 50 | 2 |
| Rosas et al[19], 2020 | Spain | Case control study | 29 | 20 | 9 | 12 | 2 | 17 | 6 |
| Ref. | Country | Study design | Sample size | Sex | Baricitinib group | Non-baricitinib group | |||
| Male | Female | Number of patients | Death | Number of patients | Death | ||||
| Bronte et al[13], 2020 | Italy | Clinical trial | 76 | 38 | 38 | 20 | 1 | 56 | 25 |
| Kalil et al[12], 2020 | Omaha | Clinical trial | 1033 | 652 | 381 | 515 | 32 | 518 | 52 |
| Cantini et al[17], 2020 | Germany | Clinical trial | 24 | 20 | 4 | 12 | 0 | 12 | 0 |
| Cantini et al[18], 2020 | Italy | Clinical trial | 191 | 119 | 72 | 113 | 0 | 78 | 7 |
| Cao et al[20],2020 | Wuhan | Clinical trial | 41 | 24 | 17 | 20 | 0 | 21 | 3 |
| D'Alessio et al[21],2021 | Italy | Clinical trial | 75 | 52 | 23 | 32 | 3 | 43 | 0 |
| Giudice et al[22], 2020 | Italy | Clinical trial | 17 | 13 | 4 | 7 | 1 | 10 | 1 |
All observational studies on the Newcastle-Ottawa Scale were found to be of good to fair quality based on their scores in the selection, comparability, and outcome subscales. Three of the four studies were of high quality, while the fourth was of fair quality (Table 3). According to the NIH quality assessment scale, 5 studies were of good quality, while the remaining two were of fair quality (Table 4).
| No. | Ref. | Type of study | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Q12 | Q13 | Q14 | Quality of the study |
| 1 | Bronte et al[13], 2020 | Clinical trial | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Good |
| 2 | Kalil et al[12], 2020 | Clinical trial | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | NR | Yes | Yes | Yes | Yes | Yes | Good |
| 3 | Cantini et al[17], 2020 | Clinical trial | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Good |
| 4 | Cantini et al[18], 2020 | Clinical trial | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | NO | Yes | Yes | Fair |
| 5 | Cao et al[20], 2020 | Clinical trial | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Good |
| 6 | D'Alessio et al[21], 2021 | Clinical trial | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Fair |
| 7 | Giudice et al[22], 2020 | Clinical trial | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | NO | Yes | Yes | NO | Yes | NR | Good |
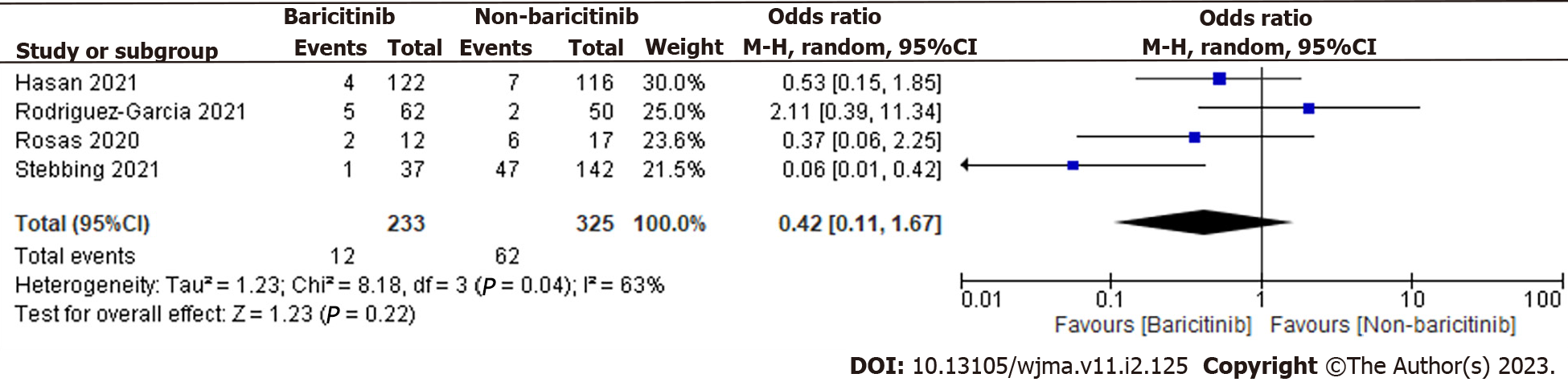
A total of 558 patients were found in selected 4 observational studies. 233 of the 558 coronavirus disease 2019 (COVID-19) cases were taking baricitinib, while the remaining 325 were not. The overall estimate was 0.42 [0.11, 1.67], indicating that the baricitinib group had a non-significant reduction in COVID-19 patient deaths compared to the non-baricitinib group (Figure 2).
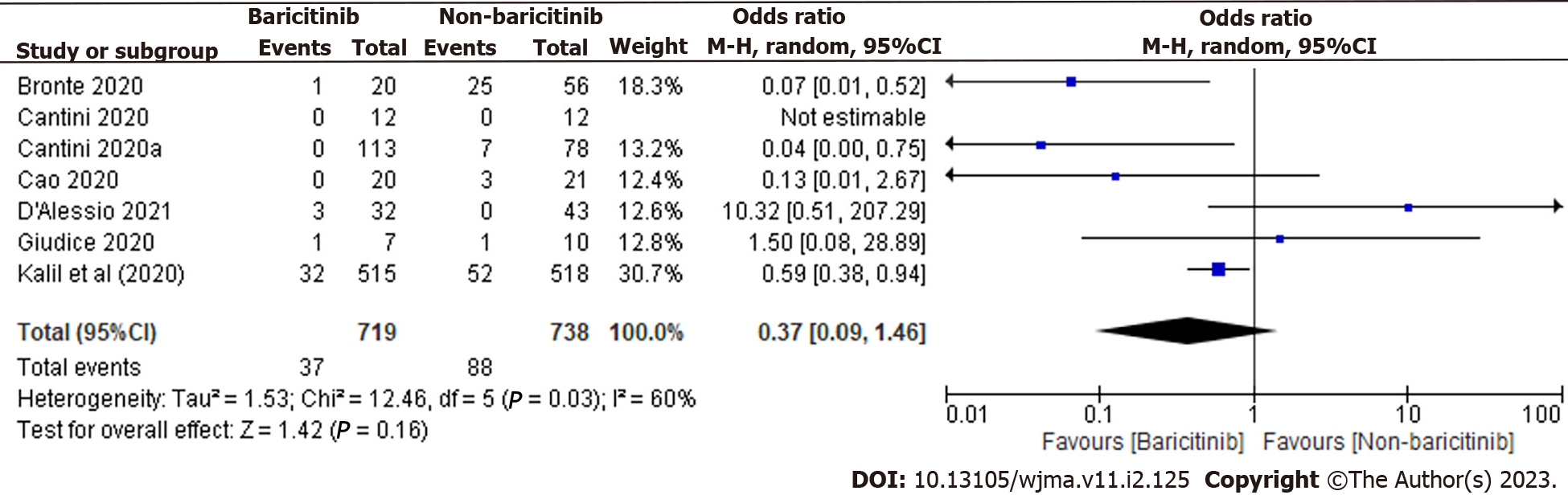
In total, 1457 patients were found in 7 clinical trials. 719 of the 1457 COVID-19 cases were taking baricitinib, while the remaining 738 were not. The overall estimate was 0.37 [0.09, 1.46], indicating that the baricitinib group had a non-significant reduction in COVID-19 patient deaths compared to the non-baricitinib group (Figure 3).
The I2 (90%) and chi2 statics have shown high heterogeneity among studies.
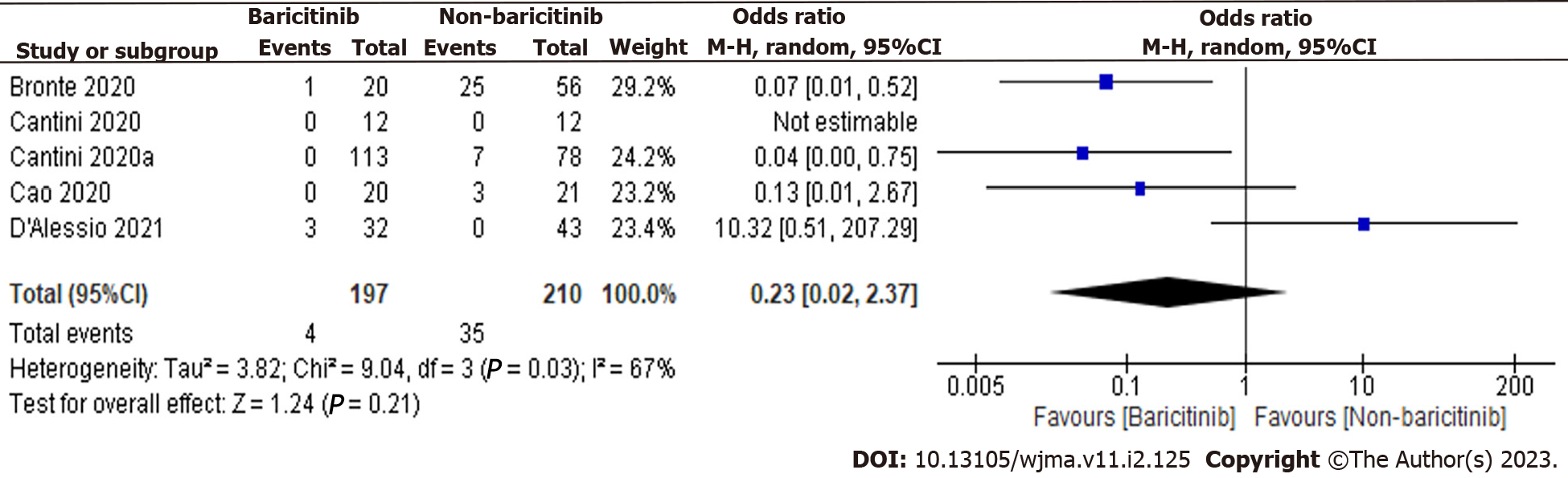
We have analyzed the forest plots of both observational and clinical trials and found that there is a study with high and low sample sizes, particularly in clinical trials. Therefore, analysis was also done again to check the effect of these studies on the outcome. The studies with a high and low sample sizes i.e., Kalil et al[12] and Giudice et al[22], were excluded, and analysis was done again. The overall estimate was 0.23 [0.02, 2.37], indicating a non-significant reduction in COVID-19 patient deaths in the baricitinib group versus the non-baricitinib group (Figure 4). Overall, results were not affected by the studies with high and low sample sizes.
The current analysis was done to find out the role of baricitinib in the reduction of deaths of COVID-19 hospitalized patients. To the best of our knowledge, very few meta-analyses have been done so far on the use of baricitinib in COVID-19 treatment. Recently, Chen et al[23], have performed a meta-analysis of 11 studies and reported the safety and efficacy of JAK-inhibitors including baricitinib in COVID-19 patients. Another JAK inhibitor i.e., ruxolitinib is also used in hospitalized patients. The meta-analysis results of Wijaya et al[24], have demonstrated a significant clinical improvement and decrease in the risk of mortality of COVID-19 patients. The potential of baricitinib in the reduction of deaths of hospitalized COVID 19 patients is also indicated by a meta-analysis conducted by Walz et al[25]. Recently, Putman et al[26], have also performed a meta-analysis to find out the efficacy of anti-rheumatoid therapy, including baricitinib and steroids for the treatment of COVID-19. However, number of available studies regarding the use of baricitinib in COVID-19 patients at that time was very less. The already published meta-analysis have also analyzed different design of studies together which make less valid conclusion. In the current meta-analysis, we have analyzed observational and clinical trials separately. However, the results of both observational and clinical trials have shown the non-significant deaths of COVID-19 hospitalized patients in the baricitinib group as compared to non-baricitinib group. Further, the sensitivity analysis results have also shown no effect of outliers on the outcome.
In conclusion, more research is needed to draw a valid conclusion about the use of baricitinib in the reduction of COVID-19 patient deaths.
More research is needed to draw a valid conclusion about the use of baricitinib in the reduction of coronavirus disease 2019 (COVID-19) patient deaths.
More research is needed to confirm the role of baricitinib in COVID-19 patient deaths.
A total of 11 studies (4 observational and 7 clinical trials) were found relevant for analysis. The overall estimate measure in terms of odds ratio for observational studies was 0.42 [0.11, 1.67], whereas for clinical trials it was 0.37 [0.09, 1.46], indicating a non-significant reduction in COVID-19 patient deaths in the baricitinib group versus the non-baricitinib group. The degree of heterogeneity among studies was also discovered to be high.
The study was conducted as per the PRISMA guideline using RevMan 5 software.
To investigate the role of baricitininb in the reduction of COVID-19 patient deaths.
Can baricitinib reduce the deaths of COVID-19 patients?
Emerging reports have indicated the use of baricitinib in hospitalized COVID-19 patients. However, the use of baricitinib in COVID-19 patients is unclear so far.
The authors are thankful to Vice-Chancellor, Prof. R.K. Goyal, Delhi Pharmaceutical Sciences & Research University, New Delhi, and Director, National Institute of Pharmaceutical Education and Research, Raebareli, India for their constant encouragement, motivation, and provision of the necessary resources to carry out this work.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pharmacology and pharmacy
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Badri M, Iran; Mohammadi M, Iran S-Editor: Liu JH L-Editor: A P-Editor: Yu HG
| 1. | Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, Bi Y, Ma X, Zhan F, Wang L, Hu T, Zhou H, Hu Z, Zhou W, Zhao L, Chen J, Meng Y, Wang J, Lin Y, Yuan J, Xie Z, Ma J, Liu WJ, Wang D, Xu W, Holmes EC, Gao GF, Wu G, Chen W, Shi W, Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565-574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8473] [Cited by in RCA: 7586] [Article Influence: 1517.2] [Reference Citation Analysis (0)] |
| 2. | Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14869] [Cited by in RCA: 12964] [Article Influence: 2592.8] [Reference Citation Analysis (1)] |
| 3. | Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270-273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15248] [Cited by in RCA: 14094] [Article Influence: 2818.8] [Reference Citation Analysis (1)] |
| 4. | Backer JA, Klinkenberg D, Wallinga J. Incubation period of 2019 novel coronavirus (2019-nCoV) infections among travellers from Wuhan, China, 20-28 January 2020. Euro Surveill. 2020;25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1082] [Cited by in RCA: 915] [Article Influence: 183.0] [Reference Citation Analysis (0)] |
| 5. | Lam TT, Jia N, Zhang YW, Shum MH, Jiang JF, Zhu HC, Tong YG, Shi YX, Ni XB, Liao YS, Li WJ, Jiang BG, Wei W, Yuan TT, Zheng K, Cui XM, Li J, Pei GQ, Qiang X, Cheung WY, Li LF, Sun FF, Qin S, Huang JC, Leung GM, Holmes EC, Hu YL, Guan Y, Cao WC. Identifying SARS-CoV-2-related coronaviruses in Malayan pangolins. Nature. 2020;583:282-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1019] [Cited by in RCA: 1198] [Article Influence: 239.6] [Reference Citation Analysis (0)] |
| 6. | Mehta P, Ciurtin C, Scully M, Levi M, Chambers RC. JAK inhibitors in COVID-19: the need for vigilance regarding increased inherent thrombotic risk. Eur Respir J. 2020;56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 7. | Spinelli FR, Conti F, Gadina M. HiJAKing SARS-CoV-2? Sci Immunol. 2020;5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 8. | Napolitano M, Fabbrocini G, Patruno C. Reply: Potential role of Janus kinase inhibitors in COVID-19. J Am Acad Dermatol. 2020;83:e65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Srivastava R, Kumar A. Use of aspirin in reduction of mortality of COVID-19 patients: A meta-analysis. Int J Clin Pract. 2021;75:e14515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 10. | Thakur M, Datusalia AK, Kumar A. Use of steroids in COVID-19 patients: A meta-analysis. Eur J Pharmacol. 2022;914:174579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 11. | Sharma R, Kumar A, Majeed J, Thakur AK, Aggarwal G. Drugs acting on the renin-angiotensin-aldosterone system (RAAS) and deaths of COVID-19 patients: a systematic review and meta-analysis of observational studies. Egypt Heart J. 2022;74:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |
| 12. | Kalil AC, Patterson TF, Mehta AK, Tomashek KM, Wolfe CR, Ghazaryan V, Marconi VC, Ruiz-Palacios GM, Hsieh L, Kline S, Tapson V, Iovine NM, Jain MK, Sweeney DA, El Sahly HM, Branche AR, Regalado Pineda J, Lye DC, Sandkovsky U, Luetkemeyer AF, Cohen SH, Finberg RW, Jackson PEH, Taiwo B, Paules CI, Arguinchona H, Erdmann N, Ahuja N, Frank M, Oh MD, Kim ES, Tan SY, Mularski RA, Nielsen H, Ponce PO, Taylor BS, Larson L, Rouphael NG, Saklawi Y, Cantos VD, Ko ER, Engemann JJ, Amin AN, Watanabe M, Billings J, Elie MC, Davey RT, Burgess TH, Ferreira J, Green M, Makowski M, Cardoso A, de Bono S, Bonnett T, Proschan M, Deye GA, Dempsey W, Nayak SU, Dodd LE, Beigel JH; ACTT-2 Study Group Members. Baricitinib plus Remdesivir for Hospitalized Adults with Covid-19. N Engl J Med. 2021;384:795-807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1159] [Cited by in RCA: 1327] [Article Influence: 331.8] [Reference Citation Analysis (1)] |
| 13. | Bronte V, Ugel S, Tinazzi E, Vella A, De Sanctis F, Canè S, Batani V, Trovato R, Fiore A, Petrova V, Hofer F, Barouni RM, Musiu C, Caligola S, Pinton L, Torroni L, Polati E, Donadello K, Friso S, Pizzolo F, Iezzi M, Facciotti F, Pelicci PG, Righetti D, Bazzoni P, Rampudda M, Comel A, Mosaner W, Lunardi C, Olivieri O. Baricitinib restrains the immune dysregulation in patients with severe COVID-19. J Clin Invest. 2020;130:6409-6416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 214] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 14. | Hasan MJ, Rabbani R, Anam AM, Huq SMR, Polash MMI, Nessa SST, Bachar SC. Impact of high dose of baricitinib in severe COVID-19 pneumonia: a prospective cohort study in Bangladesh. BMC Infect Dis. 2021;21:427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 15. | Stebbing J, Sánchez Nievas G, Falcone M, Youhanna S, Richardson P, Ottaviani S, Shen JX, Sommerauer C, Tiseo G, Ghiadoni L, Virdis A, Monzani F, Rizos LR, Forfori F, Avendaño Céspedes A, De Marco S, Carrozzi L, Lena F, Sánchez-Jurado PM, Lacerenza LG, Cesira N, Caldevilla Bernardo D, Perrella A, Niccoli L, Méndez LS, Matarrese D, Goletti D, Tan YJ, Monteil V, Dranitsaris G, Cantini F, Farcomeni A, Dutta S, Burley SK, Zhang H, Pistello M, Li W, Romero MM, Andrés Pretel F, Simón-Talero RS, García-Molina R, Kutter C, Felce JH, Nizami ZF, Miklosi AG, Penninger JM, Menichetti F, Mirazimi A, Abizanda P, Lauschke VM. JAK inhibition reduces SARS-CoV-2 liver infectivity and modulates inflammatory responses to reduce morbidity and mortality. Sci Adv. 2021;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 169] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 16. | Rodriguez-Garcia JL, Sanchez-Nievas G, Arevalo-Serrano J, Garcia-Gomez C, Jimenez-Vizuete JM, Martinez-Alfaro E. Baricitinib improves respiratory function in patients treated with corticosteroids for SARS-CoV-2 pneumonia: an observational cohort study. Rheumatology (Oxford). 2021;60:399-407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 95] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 17. | Cantini F, Niccoli L, Matarrese D, Nicastri E, Stobbione P, Goletti D. Baricitinib therapy in COVID-19: A pilot study on safety and clinical impact. J Infect. 2020;81:318-356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 317] [Cited by in RCA: 309] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 18. | Cantini F, Niccoli L, Nannini C, Matarrese D, Natale MED, Lotti P, Aquilini D, Landini G, Cimolato B, Pietro MAD, Trezzi M, Stobbione P, Frausini G, Navarra A, Nicastri E, Sotgiu G, Goletti D. Beneficial impact of Baricitinib in COVID-19 moderate pneumonia; multicentre study. J Infect. 2020;81:647-679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 131] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 19. | Rosas J, Liaño FP, Cantó ML, Barea JMC, Beser AR, Rabasa JTA, Adsuar FM, Auli BV, López IF, Sainz AMG, Ramis PE, Pérez LR, Rebollo MLN, Lorido RH, Escolar LG; COVID19-HMB Group. Experience With the Use of Baricitinib and Tocilizumab Monotherapy or Combined, in Patients With Interstitial Pneumonia Secondary to Coronavirus COVID19: A Real-World Study. Reumatol Clin (Engl Ed). 2020;18:150-156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 20. | Cao Y, Wei J, Zou L, Jiang T, Wang G, Chen L, Huang L, Meng F, Wang N, Zhou X, Luo H, Mao Z, Chen X, Xie J, Liu J, Cheng H, Zhao J, Huang G, Wang W, Zhou J. Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): A multicenter, single-blind, randomized controlled trial. J Allergy Clin Immunol. 2020;146:137-146.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 344] [Cited by in RCA: 331] [Article Influence: 66.2] [Reference Citation Analysis (0)] |
| 21. | D'Alessio A, Del Poggio P, Bracchi F, Cesana G, Sertori N, Di Mauro D, Fargnoli A, Motta M, Giussani C, Moro P, Vitale G, Giacomini M, Borra G. Low-dose ruxolitinib plus steroid in severe SARS-CoV-2 pneumonia. Leukemia. 2021;35:635-638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 22. | Giudice V, Pagliano P, Vatrella A, Masullo A, Poto S, Polverino BM, Gammaldi R, Maglio A, Sellitto C, Vitale C, Serio B, Cuffa B, Borrelli A, Vecchione C, Filippelli A, Selleri C. Combination of Ruxolitinib and Eculizumab for Treatment of Severe SARS-CoV-2-Related Acute Respiratory Distress Syndrome: A Controlled Study. Front Pharmacol. 2020;11:857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 96] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 23. | Chen CX, Wang JJ, Li H, Yuan LT, Gale RP, Liang Y. JAK-inhibitors for coronavirus disease-2019 (COVID-19): a meta-analysis. Leukemia. 2021;35:2616-2620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 24. | Wijaya I, Andhika R, Huang I, Purwiga A, Budiman KY, Bashari MH, Reniarti L, Roesli RMA. The use of Janus Kinase inhibitors in hospitalized patients with COVID-19: Systematic review and meta-analysis. Clin Epidemiol Glob Health. 2021;11:100755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 25. | Walz L, Cohen AJ, Rebaza AP, Vanchieri J, Slade MD, Dela Cruz CS, Sharma L. JAK-inhibitor and type I interferon ability to produce favorable clinical outcomes in COVID-19 patients: a systematic review and meta-analysis. BMC Infect Dis. 2021;21:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 26. | Putman M, Chock YPE, Tam H, Kim AHJ, Sattui SE, Berenbaum F, Danila MI, Korsten P, Sanchez-Alvarez C, Sparks JA, Coates LC, Palmerlee C, Peirce A, Jayatilleke A, Johnson SR, Kilian A, Liew J, Prokop LJ, Murad MH, Grainger R, Wallace ZS, Duarte-García A; COVID-19 Global Rheumatology Alliance. Antirheumatic Disease Therapies for the Treatment of COVID-19: A Systematic Review and Meta-Analysis. Arthritis Rheumatol. 2021;73:36-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |